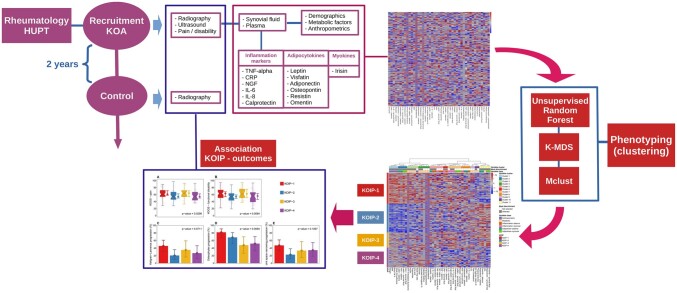
Abstract
Objectives
Osteoarthritis has been the subject of abundant research in the last years with limited translation to the clinical practice, probably due to the disease’s high heterogeneity. In this study, we aimed to identify different phenotypes in knee osteoarthritis (KOA) patients with joint effusion based on their metabolic and inflammatory profiles.
Methods
A non-supervised strategy based on statistical and machine learning methods was applied to 45 parameters measured on 168 female KOA patients with persistent joint effusion, consecutively recruited at our hospital after a monographic OA outpatient visit. Data comprised anthropometric and metabolic factors and a panel of systemic and local inflammatory markers. The resulting clusters were compared regarding their clinical, radiographic and ultrasound severity at baseline and their radiographic progression at two years.
Results
Our analyses identified four KOA inflammatory phenotypes (KOIP): a group characterized by metabolic syndrome, probably driven by body fat and obesity, and by high local and systemic inflammation (KOIP-1); a metabolically healthy phenotype with mild overall inflammation (KOIP-2); a non-metabolic phenotype with high inflammation levels (KOIP-3); and a metabolic phenotype with low inflammation and cardiovascular risk factors not associated with obesity (KOIP-4). Of interest, these groups exhibited differences regarding pain, functional disability and radiographic progression, pointing to a clinical relevance of the uncovered phenotypes.
Conclusion
Our results support the existence of different KOA phenotypes with clinical relevance and differing pathways regarding their pathophysiology and disease evolution, which entails implications in patients’ stratification, treatment tailoring and the search of novel and personalized therapies.
Keywords: knee osteoarthritis, phenotype, inflammatory, metabolism, clinical severity, machine learning
Graphical abstract
Rheumatology key messages.
Despite the abundant research conducted on knee osteoarthritis, there is a lack of translational results.
Using non-supervised machine learning techniques, we identified four KOA phenotypes.
The uncovered phenotypes exhibited differences in clinical severity and radiographic progression.
Introduction
OA is the most common form of arthritis worldwide in older adults [1]. Knee OA (KOA) is the most prevalent and the major contributor to OA socioeconomic burden, causing a significant degree of pain and disability [2]. Several studies have evaluated factors associated with clinical severity and radiographic progression in KOA [3–5], including age, obesity, cardiovascular conditions [6], cytokines in plasma and synovial fluid [7, 8], and molecular determinants of cartilage degradation [9]. Numerous sex-related differences have been reported among KOA affected subjects, not only regarding prevalence, but also metabolic conditions, inflammatory factors and levels of pain and function disability [10, 11]. Despite this abundant research, KOA pathophysiology is currently not well understood, the available therapeutic options are limited and, as of the date of writing, there is no specific therapy with a disease-modifying effect [12].
An explanation for this lack of translational results is the great variability across OA patients regarding clinical presentation, exhibit of risk factors and prognosis. Based on these observations, it has been proposed that OA does not correspond to a single entity, but to a multifaceted and heterogeneous syndrome consisting of different subgroups (phenotypes), possibly with specific pathophysiologic traits (endotypes) [13]. The identification of these phenotypes entails clinical implications of high relevance, as they might improve patients’ stratification, enable a personalized choice of treatment and a more accurate selection for clinical trials, provide insight into their pathophysiology and generate new hypotheses for future research, especially on targets for novel therapeutic options [14].
Following this hypothesis, various groups aimed their research at the characterization of OA phenotypes, most of them in a hypothesis-driven manner based on one or a few selected features (top-down phenotyping) [15]. In a completely different approach, a few works attempted to identify OA phenotypes by uncovering the patterns and clusters present in patients’ data using statistical and machine learning techniques in a totally unsupervised manner (step-up phenotyping) [15]. This approach has been used in a great variety of data, including clinical parameters [16, 17], transcriptomic [18, 19], metabolomics [20, 21] and other biochemical markers [22, 23]. Although all these works suggested the existence of OA phenotypes characterized by features of a different nature, their association with severity and progression and, especially, their clinical applicability was very limited. In agreement with findings previously published [10, 11], some of these studies reported important sex-specificities, suggesting that OA phenotyping should be conducted for women and men separately [23].
Despite these efforts, there is as of now no consensus on a comprehensive classification of OA with clinical relevance. In KOA, the existence of an inflammatory clinical phenotype characterized by the presence of synovitis and higher levels of pain, functional disability and rate of progression is widely acknowledged [24, 25]. In our study, a data-driven approach was used to identify phenotypes in 168 KOA patients with persistent joint effusion from a prospective KOA cohort. To do so, anthropometric parameters, metabolic factors and systemic and local inflammatory markers were analysed using well-established statistical and machine learning methods in a non-supervised manner. To assess their clinical relevance, differences across phenotypes were assessed regarding pain, functional disability, ultrasound and radiographic severity and progression.
Methods
Patients’ description
The subjects of this study are part of a prospective cohort of patients that includes men and women with primary knee osteoarthritis (KOA) and persistent joint effusion. The cohort includes 202 KOA patients consecutively recruited after an outpatient visit in our Rheumatology Service from October 2013 to April 2018. At present, the cohort includes 171 women, three of which were excluded from our study due to their high number of missing values in the parameters used for clustering (>10%, five variables), leaving 168 female KOA patients for our analysis (Table 1). We focused the present work on female patients to homogenize the study sample, as previous works have reported numerous sex-related differences in OA regarding metabolic conditions, inflammatory factors and levels of pain and function disability [10, 11]. An exhaustive description of the inclusion and exclusion criteria can be found in the Supplementary Information available at Rheumatology online.
Table 1.
Main baseline patients’ characteristics
| Kellgren–Lawrence |
||||||
|---|---|---|---|---|---|---|
| All | Grade 1 | Grade 2 | Grade 3 | Grade 4 | ||
| 168 (100%) | 19 (11.3%) | 65 (38.7%) | 78 (46.4%) | 6 (3.6%) | ||
| Age at recruitment | 69.1 [50.9, 83.0] | 69.2 [55.7, 80.8] | 68.7 [51.7, 83] | 69.0 [50.9, 81.4] | 74.1 [52.0, 77.4] | |
| Disease evolution time (months) | 48 [4, 200] | 36 [6, 130] | 48 [4, 200] | 48 [6, 150] | 57 [6, 135] | |
| Obesity | 94 (56.0%) | 12 (63.2%) | 32 (49.2%) | 45 (57.7%) | 5 (83.3%) | |
| Physical exercise | None | 61 (36.3%) | 6 (31.6%) | 24 (36.9%) | 27 (34.6%) | 4 (66.7%) |
| Sporadic | 51 (30.4%) | 5 (26.3%) | 19 (29.2%) | 26 (33.3%) | 1 (16.7%) | |
| Moderate | 46 (27.4%) | 5 (26.3%) | 20 (30.8%) | 20 (25.6%) | 1 (16.7%) | |
| Vigorous | 10 (6.0%) | 3 (15.8%) | 2 (3.1%) | 5 (6.4%) | 0 (0.0%) | |
| Diabetes mellitus | 18 (10.7%) | 1 (5.3%) | 8 (12.3%) | 9 (11.5%) | 0 (0.0%) | |
| Arterial hypertension | 92 (54.8%) | 8 (42.1%) | 35 (53.8%) | 47 (60.3%) | 2 (33.3%) | |
| Dyslipidaemia | 68 (40.5%) | 4 (21.1%) | 29 (44.6%) | 33 (42.3%) | 2 (33.3%) | |
| ATP III metabolic syndrome | 61 (36.3%) | 8 (42.1%) | 24 (36.9%) | 28 (35.9%) | 1 (16.7%) | |
| KOOS—pain (reversed, 0–100) | 58 [28, 97] | 58 [29, 87] | 58 [33, 89] | 58 [28, 97] | 58 [42, 92] | |
| KOOS—symptoms (reversed, 0–100) | 57 [11, 96] | 62 [19, 82] | 57 [11, 96] | 56 [19, 96] | 67 [37, 79] | |
| KOOS—functional disability (reversed, 0–100) | 58 [19, 94] | 59 [22, 79] | 57 [25, 93] | 58 [19, 94] | 60 [29, 72] | |
| Joint effusion (mm) | 9.1 [4.5, 19.1] | 8.6 [5.8, 14.1] | 9.1 [4.5, 15.2] | 9.2 [4.5, 19.1] | 9.7 [8.10, 12.30] | |
Demographic, anthropometric, metabolic, radiographic and clinical factors, for all the KOA patients included in the study and stratified by Kellgren–Lawrence (KL) staging. All subjects are female patients diagnosed with symptomatic primary knee osteoarthritis (KOA) with persistent joint effusion. Continuous parameters are described with their median and ranges (minimum and maximum values), while absolute frequencies and percentages are displayed for categorical variables. No missing values were observed for any of variables displayed in the table (n = 168).
ATP III: Adult Treatment Panel III; KOOS: knee injury and osteoarthritis outcome scores (reversed scores).
All subjects signed an informed consent authorizing the collection of samples and data for their use in the context of KOA studies. The project was evaluated and approved by the ethical committee of our centre (CEIm Parc Taulí) with approval number 2015/539, and was conducted according to the national and international ethical guidelines (Ethical Standards, Declaration of Helsinki).
Samples
Samples from plasma and joint fluid were systematically extracted at recruitment from all patients in the cohort. Synovial fluid was obtained by aspiration (13.5 mean and 9 ml median) and analysed to discard the presence of inflammatory fluid (joint cell count <2500 cells) and microcrystals. Collected samples were appropriately centrifuged and stored at –80°C, until their use for quantifications. Blood extractions and the arthrocentesis were performed at the same day and in fasting conditions.
Data collection
Baseline information regarding demographics, anthropometric and metabolic factors were systematically collected from the patients in the cohort at time of recruitment (Supplementary Table S1, available at Rheumatology online). Blood determinations related to metabolic syndrome were assessed as per clinical practice. Synovial and plasma samples were evaluated by ELISA for a set of 13 selected cytokines, in order to assess their local and systemic inflammatory profiles (Supplementary Table S1, available at Rheumatology online). In total, a comprehensive panel of 45 parameters clinically relevant for OA were available for the phenotyping analysis, (Supplementary Table S1, available at Rheumatology online), which displayed evident structures of correlation among them (Supplementary Fig. S1, available at Rheumatology online).
Baseline KOA severity outcomes included: pain, functional disability and symptoms levels as measured by the Knee injury and Osteoarthritis Outcome Scores (KOOS, in reversed order); ultrasound measurements of joint effusion and synovial tissue thickness (mm); Kellgren–Lawrence (KL) radiographic stage; and OARSI atlas lecture, including osteophytes assessment and joint space narrowing (JSN). To assess their radiographic progression at two years, most of the patients (n = 143, 85%) also underwent a radiographic evaluation during the follow-up. (Supplementary Table S1, available at Rheumatology online). Of the 24 patients without a follow-up radiography, 13 had undergone knee prosthesis surgery (one in baseline KL-1, three in KL-2, seven in KL-3 and two in KL-4). The remaining 11 patients (7%) represent losses in the follow-up.
Clustering analyses for phenotype discovery
A clustering analysis based on well established statistical and machine learning methods was carried out on 45 variables including anthropometric, metabolic and systemic and local inflammatory factors, which were selected from the information available in our KOA cohort for their clinical relevance in OA (see previous sections and Supplementary Table S1, available at Rheumatology online). All 45 variables participated in the clustering analyses, and no explicit feature selection was performed prior to the clustering procedure. Of note, this approach was completely unsupervised and no variable expressing KOA severity or progression was involved in the clustering process, which consisted of four steps: (i) missing values were imputed by Recursively Subtracted Empirical Orthogonal Functions analysis [26]; (ii) pairwise patients’ dissimilarities were estimated by unsupervised Random Forest (RF) [27]; (iii) a Kruskal's Non-metric Multidimensional Scaling (K-MDS) was applied to the resulting dissimilarity matrix; and (iv) components derived from K-MDS underwent a model-based clustering analysis based on mixture of Gaussian distributions (Mclust) [28]. Results for the two first K-MDS components showed the most evident data structure as they involved a high number of clusters (four) according to Bayes Information Criterion (BIC) (Supplementary Fig. S2, available at Rheumatology online). These clusters showed a strong statistical significance according to a likelihood ratio test (Supplementary Table S2, available at Rheumatology online), and remarkable robustness as measured by a number of stability indexes that included Jaccard, adjusted Rand, Fowlkes–Mallows and j-score, and normalized mutual information (Supplementary Table S3, available at Rheumatology online). Hence, this configuration was chosen to define the knee osteoarthritis inflammatory phenotypes (KOIP) and used in downstream analyses (Supplementary Fig. S3, available at Rheumatology online).
Clustering results were graphically represented using heatmaps at the patient and the cluster level. To facilitate the interpretation of the results, the 45 clustering variables were grouped by their similarity across patients using a hierarchical clustering and a correlation-like distance based on Spearman correlation (SC), Phi coefficients and Glass rank biserial correlations (GRBCorr).
To assist the interpretation of the clusters, a supervised RF classifier was trained to predict patients’ KOIP membership. In these analyses, variables contributing the most to KOIP definitions were identified using VSURF [29], a sequential selection procedure based on RF. Finally, and to explore clusters’ sex-specificity, male patients with available data (n = 23) were assigned to the KOIP groups using a supervised RF model trained with data from the female patients (Supplementary Information available at Rheumatology online).
Statistical analysis
Continuous parameters were described by their medians, median absolute deviations and ranges, while categorical variables were summarized using absolute frequencies and percentages. Associations with KOIP groups and outcomes were assessed using non-parametric methods, namely Kruskal–Wallis and Mann–Whitney tests for continuous variables, and Fisher's tests for categorical variables. All data analyses were conducted with R [30] (see Supplementary Information available at Rheumatology online).
Results
Phenotype discovery
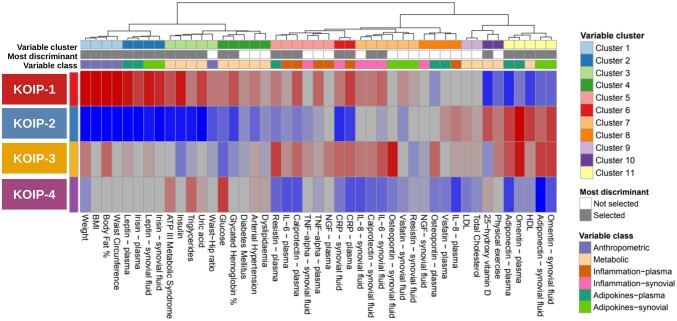
A clustering analysis was performed on data from 168 women included in a cohort of primary KOA patients with joint effusion (Table 1). Data consisted of a comprehensive panel of 45 variables systematically collected in the cohort and selected for their clinical relevance in OA (Supplementary Table S1, available at Rheumatology online). Based on machine learning techniques and objective statistical criteria for model selection, our methodology identified four robust patient clusters (Supplementary Figs S2 and S3, Supplementary Tables S2 and S3, available at Rheumatology online) that displayed clearly differing profiles regarding their anthropometric, metabolic and inflammatory features. Hence, these clusters were used to define four KOA inflammatory phenotypes (KOIP) whose characteristics are detailed in Fig. 1 and Table 2 (see also Supplementary Fig. S4, available at Rheumatology online).
Figure 1.
Results at the group level of the phenotype discovery analyses performed on data from 168 female patients of primary knee osteoarthritis (KOA) with persistent joint effusion. Cells show the median (continuous variables) or averages (numerically coded categorical variables) of the standardized (centered and scaled) values of anthropometric, metabolic and systemic and local inflammatory factors involved in the clustering analysis. Red indicates high, blue represents low, and colour intensity expresses more extreme values. Values of binary variables were previously converted to numeric format, where 1 indicated presence and 0 represented absence of the corresponding feature. Physical exercise (four categories) was also converted to numeric format and treated as ordinal. Colour intensities were saturated approximately to percentiles 5% and 95% of the overall values distribution. Patient clusters were derived from a machine learning (ML)-based strategy that used unsupervised random forest (RF), Kruskal’s non-metric multidimensional scaling (KMDS) and Gaussian finite mixture models for model-based clustering (Mclust), which selected the optimal number of clusters with objective statistical criteria (Bayes information criterion, BIC). Variables were grouped by a hierarchical clustering using Ward agglomerative method and non-parametric correlation-like measurements, namely: Spearman correlation (continuous vs continuous or ordinal variables), Phi coefficient (binary vs binary variables) and Glass rank biserial correlation (continuous/ordinal vs binary variables). Variables with the highest discriminative power of patients’ clusters were identified using a sequential selection procedure based on random forests (VSURF—interpretation mode) and highlighted in the corresponding annotation bar (Most discriminant). ATP III: Adult Treatment Panel III; HDL: high-density lipoprotein; KOA: knee osteoarthritis; KOIP: knee osteoarthritis inflammatory phenotype; LDL: low-density lipoprotein; NGF: nerve growth factor
Table 2.
Characterization of knee osteoarthritis inflammatory phenotypes (KOIP)
| N | All | KOIP-1 | KOIP-2 | KOIP-3 | KOIP-4 | P-value | ||
|---|---|---|---|---|---|---|---|---|
| (%Miss.) | 168 (100%) | 55 (32.7%) | 51 (30.4%) | 27 (16.1%) | 35 (20.8%) | |||
| Weight (kg) | 168 (0.0%) | 74.00 (10.90) | 84.20 (8.15) | 64.40 (7.12) | 76.90 (7.56) | 71.90 (5.49) | <0.0001 | |
| Body mass index | 168 (0.0%) | 31.04 (4.97) | 35.56 (3.49) | 26.60 (2.54) | 30.78 (3.39) | 31.27 (2.86) | <0.0001 | |
| Body fat percentage (%) | 167 (0.6%) | 41.60 (5.49) | 46.05 (4.00) | 36.40 (3.56) | 43.60 (3.26) | 42.00 (2.82) | <0.0001 | |
| Waist circumference (cm) | 167 (0.6%) | 101.00 (11.12) | 110.75 (7.78) | 91.00 (7.41) | 100.50 (6.67) | 101.00 (5.93) | <0.0001 | |
| Leptin—plasma (pg/mL) | 167 (0.6%) | 36688.11 (19582.18) | 54392.71 (25049.17) | 23566.59 (10682.27) | 39809.19 (18942.95) | 35530.72 (13628.15) | <0.0001 | |
| Irisin—plasma (ng/mL) | 166 (1.2%) | 707.78 (256.30) | 859.25 (107.21) | 437.22 (211.14) | 727.20 (174.17) | 726.02 (212.84) | <0.0001 | |
| Leptin—synovial fluid (pg/mL) | 168 (0.0%) | 36160.71 (20839.58) | 54016.11 (21012.50) | 23126.79 (9402.28) | 39506.85 (20839.78) | 34888.07 (15821.33) | <0.0001 | |
| Irisin—synovial fluid (ng/mL) | 165 (1.8%) | 695.95 (366.25) | 938.23 (228.26) | 422.07 (289.34) | 701.88 (366.00) | 686.28 (341.25) | <0.0001 | |
| ATP III metabolic syndrome | 168 (0.0%) | 61 (36.3%) | 33 (60.0%) | 2 (3.9%) | 9 (33.3%) | 17 (48.6%) | <0.0001 | |
| Insulin (microU/mL) | 168 (0.0%) | 10.61 (5.95) | 15.09 (5.47) | 7.20 (3.08) | 8.94 (2.49) | 11.07 (5.99) | <0.0001 | |
| Triglycerides (mg/dL) | 168 (0.0%) | 114.50 (49.67) | 133.00 (56.34) | 83.00 (25.20) | 110.00 (31.13) | 134.00 (48.93) | <0.0001 | |
| Uric acid (mg/dL) | 168 (0.0%) | 4.75 (1.56) | 5.60 (1.48) | 3.70 (0.74) | 4.80 (1.63) | 4.90 (1.19) | <0.0001 | |
| Waist–hip ratio | 167 (0.6%) | 0.92 (0.06) | 0.94 (0.07) | 0.89 (0.06) | 0.91 (0.07) | 0.92 (0.04) | <0.0001 | |
| Glucose (mg/dL) | 168 (0.0%) | 85.00 (13.34) | 90.00 (11.86) | 80.00 (7.41) | 80.00 (8.90) | 93.00 (14.83) | <0.0001 | |
| Glycated haemoglobin (%) | 168 (0.0%) | 5.70 (0.44) | 5.90 (0.44) | 5.50 (0.30) | 5.60 (0.44) | 5.70 (0.30) | <0.0001 | |
| Diabetes mellitus | 168 (0.0%) | 18 (10.7%) | 10 (18.2%) | 2 (3.9%) | 2 (7.4%) | 4 (11.4%) | 0.2069 | |
| Arterial hypertension | 168 (0.0%) | 92 (54.8%) | 37 (67.3%) | 18 (35.3%) | 16 (59.3%) | 21 (60.0%) | 0.0182 | |
| Dyslipidaemia | 168 (0.0%) | 68 (40.5%) | 26 (47.3%) | 16 (31.4%) | 9 (33.3%) | 17 (48.6%) | 0.3675 | |
| Resistin—plasma (pg/mL) | 168 (0.0%) | 2115.23 (772.55) | 2389.10 (775.51) | 1891.16 (542.59) | 2657.56 (991.28) | 1874.89 (579.99) | 0.0001 | |
| Interleukin 6—plasma (pg/mL) | 167 (0.6%) | 2.01 (1.89) | 2.28 (2.17) | 1.55 (1.48) | 2.45 (1.49) | 1.27 (1.18) | 0.0149 | |
| Calprotectin—plasma (ng/mL) | 168 (0.0%) | 694.27 (257.02) | 851.11 (329.44) | 613.63 (208.62) | 800.12 (272.14) | 588.54 (149.27) | <0.0001 | |
| Tumor necrosis factor alpha—synovial fluid (pg/mL) | 155 (7.7%) | 9.02 (4.17) | 9.09 (5.62) | 8.14 (4.40) | 9.90 (3.08) | 8.90 (2.56) | 0.1239 | |
| Tumor necrosis factor alpha—plasma (pg/mL) | 167 (0.6%) | 6.37 (1.97) | 7.18 (2.49) | 6.18 (2.47) | 6.70 (2.81) | 6.08 (1.35) | 0.0997 | |
| Nerve growth factor—plasma (pg/mL) | 167 (0.6%) | 1.52 (0.51) | 1.52 (0.41) | 1.52 (0.38) | 1.69 (0.60) | 1.52 (0.59) | 0.2699 | |
| C-reactive protein—synovial fluid (mg/L) | 167 (0.6%) | 1.23 (0.85) | 1.52 (0.86) | 0.78 (0.58) | 1.61 (1.24) | 0.98 (0.67) | <0.0001 | |
| C-reactive protein—plasma (mg/L) | 168 (0.0%) | 4.26 (3.64) | 6.87 (3.43) | 2.59 (1.83) | 6.00 (4.53) | 2.90 (2.01) | <0.0001 | |
| Interleukin 8 - synovial fluid (pg/mL) | 165 (1.8%) | 6.21 (4.55) | 8.00 (6.32) | 6.21 (4.53) | 8.21 (9.04) | 3.92 (2.69) | 0.0012 | |
| Calprotectin—synovial fluid (ng/mL) | 164 (2.4%) | 576.06 (506.81) | 763.04 (539.41) | 599.99 (640.78) | 759.60 (560.50) | 420.07 (269.98) | 0.0006 | |
| Interleukin 6—synovial fluid (pg/mL) | 167 (0.6%) | 116.33 (142.96) | 208.03 (227.13) | 91.67 (107.28) | 222.45 (208.76) | 71.56 (74.70) | 0.0012 | |
| Osteopontin—synovial fluid (ng/mL) | 168 (0.0%) | 47.95 (45.78) | 48.55 (46.67) | 46.75 (44.66) | 103.82 (103.73) | 36.02 (28.09) | 0.0013 | |
| Visfatin—synovial fluid (ng/mL) | 168 (0.0%) | 2.07 (0.96) | 2.28 (0.97) | 1.88 (0.76) | 2.11 (0.89) | 1.90 (1.13) | 0.5075 | |
| Resistin—synovial fluid (pg/mL) | 168 (0.0%) | 1480.20 (1186.82) | 1692.08 (1292.53) | 1390.64 (1251.31) | 1242.23 (1180.45) | 1329.84 (1125.35) | 0.4210 | |
| Nerve growth factor—synovial fluid (pg/mL) | 163 (3.0%) | 2.26 (0.65) | 2.26 (1.09) | 2.26 (0.63) | 2.20 (0.48) | 2.20 (0.90) | 0.0380 | |
| Osteopontin—plasma (ng/mL) | 168 (0.0%) | 13.71 (6.07) | 12.52 (6.59) | 14.12 (6.40) | 16.40 (10.18) | 12.10 (4.37) | 0.1872 | |
| Visfatin—plasma (ng/mL) | 168 (0.0%) | 4.01 (0.99) | 4.02 (1.20) | 4.23 (1.12) | 4.01 (0.54) | 3.74 (1.01) | 0.2810 | |
| Interleukin 8—plasma (pg/mL) | 168 (0.0%) | 3.19 (1.93) | 3.21 (1.78) | 3.79 (2.07) | 3.52 (2.59) | 2.30 (1.28) | 0.0152 | |
| Low-density lipoprotein (mg/dL) | 168 (0.0%) | 121.50 (34.84) | 123.00 (38.55) | 128.00 (34.10) | 126.00 (23.72) | 113.00 (28.17) | 0.2931 | |
| Total cholesterol (mg/dL) | 168 (0.0%) | 208.00 (32.62) | 208.00 (32.62) | 211.00 (29.65) | 213.00 (28.17) | 200.00 (32.62) | 0.1780 | |
| 25-hydroxy vitamin D (ng/mL) | 167 (0.6%) | 19.75 (11.05) | 13.80 (7.12) | 25.63 (10.50) | 17.55 (11.81) | 21.30 (8.90) | <0.0001 | |
| Physical exercise | None | 168 (0.0%) | 61 (36.3%) | 29 (52.7%) | 12 (23.5%) | 6 (22.2%) | 14 (40.0%) | 0.0525 |
| Sporadic | 51 (30.4%) | 18 (32.7%) | 16 (31.4%) | 8 (29.6%) | 9 (25.7%) | |||
| Moderate | 46 (27.4%) | 6 (10.9%) | 18 (35.3%) | 11 (40.7%) | 11 (31.4%) | |||
| Vigorous | 10 (6.0%) | 2 (3.6%) | 5 (9.8%) | 2 (7.4%) | 1 (2.9%) | |||
| Adiponectin—plasma (ng/mL) | 168 (0.0%) | 16406.09 (9301.69) | 11544.55 (6280.30) | 22357.78 (10366.17) | 21707.37 (7352.75) | 11562.39 (5390.11) | <0.0001 | |
| Omentin—plasma (pg/mL) | 168 (0.0%) | 26859.10 (17845.88) | 22317.93 (13067.24) | 44069.15 (26688.23) | 41837.49 (19372.98) | 22449.96 (11219.76) | <0.0001 | |
| High-density lipoprotein (mg/dL) | 168 (0.0%) | 60.65 (13.79) | 53.30 (11.12) | 68.90 (13.49) | 63.20 (9.93) | 58.00 (17.05) | <0.0001 | |
| Adiponectin—synovial fluid (ng/mL) | 168 (0.0%) | 2420.01 (1470.78) | 1937.73 (1263.00) | 3047.42 (1271.50) | 3372.91 (2111.49) | 1501.12 (1186.51) | <0.0001 | |
| Omentin—synovial fluid (pg/mL) | 165 (1.8%) | 4292.68 (3586.44) | 3073.42 (2125.52) | 6648.21 (4252.38) | 6943.02 (4134.11) | 2787.84 (2109.62) | <0.0001 | |
Cells show medians and median absolute deviations (continuous) and absolute frequencies and percentages (categorical) for the 45 variables used in the clustering analysis within each KOIP and in the overall series.
Statistical significance was assessed using a Kruskal–Wallis (continuous) or a Fisher’s test for contingency tables (categorical variables).
%Miss.: percentage of missing values; KOIP: knee osteoarthritis inflammatory phenotype; N: number of observations.
KOIP-1 represented a fat-driven metabolic inflammatory phenotype (55 subjects, 32.7%). Compared with the rest of the patients, this phenotype showed classic hallmarks of metabolic syndrome (MetS) driven by body fat and obesity, such as: higher weight, BMI, body fat, waist circumference and waist–hip ratio; high prevalence of diabetes, arterial hypertension, dyslipidaemia and MetS; increased values of insulin, triglycerides, uric acid, glucose and percentage of glycated haemoglobin; and low high-density lipoprotein (HDL), vitamin D and physical activity. Its inflammatory profile was characterized by the highest levels of leptin and irisin, in contrast to decreased values of adiponectin and omentin, both in plasma and in synovial fluid. Typically, their patients also displayed higher than average values of some inflammatory factors at the local and systemic level, namely IL-6, CRP, calprotectin and resistin, as well as increased values of plasma tumour necrosis factor alpha (TNF-alpha) and synovial interleukin-8 (IL-8) and visfatin.
KOIP-2 defined a metabolically healthy and mild-inflammatory phenotype (51 subjects, 30.4%). Subjects in this group represented a mirrored picture of the KOIP-1 cluster, as they presented the lowest expressions of the metabolic and obesity features listed above, the lowest levels of leptin and irisin, and increased values of adiponectin and omentin, both in plasma and in synovial fluid. In this phenotype, the expression for the rest of systemic and local inflammatory markers remained around the cohort’s average or below, except for plasma IL-8 and visfatin. Patients in the KOIP-2 group were among the most physically active and presented high levels of vitamin D and HDL.
KOIP-3 depicted a non-metabolic and high-inflammatory phenotype (27 subjects, 16.1%). Although their subjects presented relatively high levels of weight and body fat content, this group was characterized by intermediate values of BMI and average or lower levels and presence of conditions related to MetS and individual cardiovascular risk factors, such as diabetes, glucose, glycated haemoglobin, dyslipidaemia, triglycerides, uric acid and waist–hip ratio. Their patients also displayed intermediate levels of leptin and irisin, high expression of adiponectin and omentin and the highest levels of osteopontin, both systemically and locally. Regarding the rest of cytokines, and compared with KOIP-1, KOIP-3 subjects showed increased levels of synovial TNF-alpha and plasma nerve grow factor (NGF), lower expression of synovial visfatin and resistin, and a similar profile for the rest of factors. Their vitamin D levels were low, and they were physically active compared to the series average.
KOIP-4 represented a metabolic low-inflammatory phenotype (35 subjects, 20.8%). This group displayed average values of leptin, irisin and anthropometric factors related with obesity (weight, BMI, body fat, waist perimeter and waist–hip ratio). Their patients, though, showed a high frequency and levels of classic cardiovascular risk factors, especially MetS, triglycerides and glucose, and higher prevalence of arterial hypertension and dyslipidaemia than the cohort’s average. Despite that, this phenotype showed low average expression in all the cytokines of our panel, both in plasma and in synovial fluid.
Subjects across KOIP groups did not significantly differ in terms of age or evolution time of their disease although, in median, KOIP-3 subjects were slightly younger (2.2 years) and they had been diagnosed more recently (12 months) compared with the overall series. Subjects in KOIP-4 showed the longest disease evolution (12 months more than the whole patients set, in median). A supervised RF classifier trained to predict KOIP membership achieved a high overall accuracy (89%), with classification errors across KOIP groups ranging from 4% to 17%. A sequential procedure selected up to 27 predictors (out of the 45 analysed) as the most informative for KOIP classification while retaining a similar accuracy in all groups (87%) (Supplementary Table S4, available at Rheumatology online). With exploratory purposes, and although the number of male patients in our KOA cohort available for these analyses did not allow for conducting a proper phenotyping analysis (n = 23), we observed that the KOIP profiles described for women were only partially reproduced in the male patients of our KOA cohort (Supplementary Fig. S5, available at Rheumatology online).
Association of KOIP groups with clinical outcomes
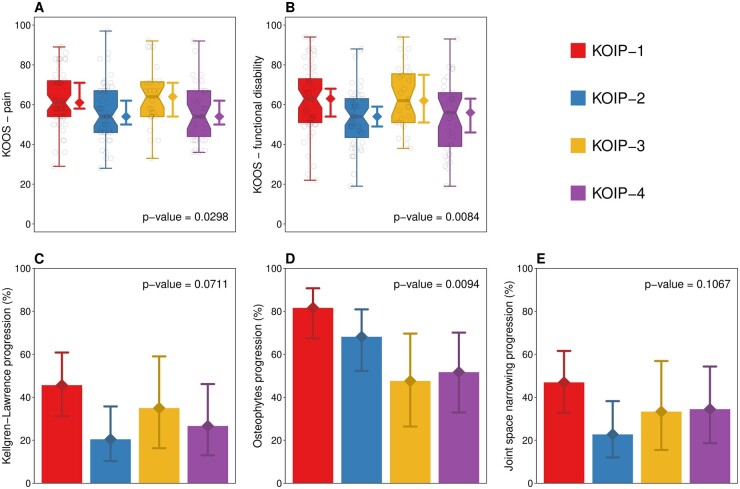
The identified KOIP groups were evaluated for their association with different outcomes, including clinical (KOOS, reversed scale), radiographic and ultrasound severity at baseline and radiographic progression at two years (Fig. 2, Table 3). KOIP-1 and KOIP-3 groups displayed the highest baseline pain across phenotypes, which were statistical significant in the case of KOIP-1 (7 points increase in median compared with KOIP-2 and KOIP-4, P-values = 0.0217 and 0.0327) and close to the significance threshold for KOIP-3 (10 points more than KOIP-2 and KOIP-4 in median, P-values = 0.0566 and 0.0535), respectively (Fig. 2, Table 3 and Supplementary Table S5, available at Rheumatology online). The same pattern was observed for functional disability where, in median, KOIP-1 and KOIP-3 displayed 8 and 9 KOOS points more than subjects in the KOIP-2 group (P-values = 0.0033 and 0.0106) and 7 and 6 more points than KOIP-4 subjects (P-values = 0.0595 and 0.0735), respectively (Fig. 2, Table 3 and Supplementary Table S6, available at Rheumatology online). Regarding radiographic assessments, KOIP-1 showed the highest rates of progression in all criteria considered, which included Kellgren–Lawrence (KL) (45.7%), formation of new osteophytes (81.6%) and joint space narrowing (JSN) (46.9%) (Fig. 2, Table 3 and Supplementary Tables S7–S9, available at Rheumatology online). Although the homogeneity test was non-significant for KL and JSN, these rates were significantly higher compared with the KOIP-2 group for KL (25.2% increase, P-value = 0.0143) and JSN (24.2% increase, P-value = 0.0178), and for osteophytes progression when compared with KOIP-3 (34.0% increase, P-value = 0.0080) and KOIP-4 (29.9%, P-value = 0.0093). Importantly, KOIP-1 and KOIP-3 showed both a high progression and a high baseline radiographic severity according to KL and JSN, although baseline differences were not statistically significant across phenotypes for any of the criteria considered (Fig. 2, Table 3). KOIP-3 and KOIP-4 showed similar patterns and rates of radiographic progression (Fig. 2, Table 3). No significant differences were found between phenotypes for KOOS symptoms or ultrasound severity (Table 3).
Figure 2.
Association of knee osteoarthritis inflammatory phenotypes (KOIP) with clinical severity and radiographic progression. Panels (A) and (B) represent boxplots showing the distribution of knee injury and osteoarthritis outcome scores (KOOS, reversed scores) across KOIP groups for pain (A) and functional disability (B). Panels (C), (D) and (E) display the percentage of patients whose disease progressed after a two-year follow-up according to different radiographic criteria, namely Kellgren–Lawrence (KL) stage (C), formation of new osteophytes (D) and increase of joint space narrowing (JSN) (E). Diamond-shaped points and segments show group medians (A and B), percentages (C, D and E) and their 95% CI. P-values are derived from an Kruskal–Wallis test (A, B) or a Fisher’s test for contingency tables (C, D and E). KOIP: knee osteoarthritis inflammatory phenotype; KOOS: knee injury and osteoarthritis outcome scores (reversed scores)
Table 3.
Association of knee osteoarthritis inflammatory phenotypes (KOIP) with severity and progression
| N | All | KOIP-1 | KOIP-2 | KOIP-3 | KOIP-4 | P-value | ||
|---|---|---|---|---|---|---|---|---|
| (%Miss) | 168 (100%) | 55 (32.7%) | 51 (30.4%) | 27 (16.1%) | 35 (20.8%) | |||
| KOOS—pain (reversed, 0–100) | 168 (0.0%) | 58.00 (16.31) | 61.00 (14.83) | 54.00 (14.83) | 64.00 (11.86) | 54.00 (17.79) | 0.0298 | |
| KOOS—symptoms (reversed, 0–100) | 168 (0.0%) | 57.00 (17.79) | 62.00 (17.79) | 56.00 (8.90) | 57.00 (19.27) | 62.00 (19.27) | 0.7737 | |
| KOOS—functional disability (reversed, 0–100) | 168 (0.0%) | 58.00 (15.57) | 63.00 (17.79) | 54.00 (13.34) | 62.00 (17.79) | 56.00 (19.27) | 0.0084 | |
| Joint effusion (mm) | 168 (0.0%) | 9.05 (2.45) | 9.30 (2.52) | 8.70 (2.37) | 9.40 (2.67) | 8.80 (1.33) | 0.1545 | |
| Synovial tissue thickness (mm) | 163 (3%) | 4.20 (1.78) | 4.50 (2.00) | 4.20 (1.78) | 4.40 (1.78) | 4.10 (1.63) | 0.6986 | |
| Kellgren–Lawrence radiographic grade | 1 | 168 (0.0%) | 19 (11.3%) | 5 (9.1%) | 4 (7.8%) | 3 (11.1%) | 7 (20.0%) | 0.1255 |
| 2 | 65 (38.7%) | 19 (34.5%) | 25 (49.0%) | 7 (25.9%) | 14 (40.0%) | |||
| 3 | 78 (46.4%) | 26 (47.3%) | 22 (43.1%) | 16 (59.3%) | 14 (40.0%) | |||
| 4 | 6 (3.6%) | 5 (9.1%) | 0 (0.0%) | 1 (3.7%) | 0 (0.0%) | |||
| Osteophytes score | 167 (0.6%) | 3.00 (2.97) | 3.00 (2.97) | 3.00 (2.97) | 4.00 (2.97) | 4.00 (2.97) | 0.9087 | |
| Joint space narrowing | 0 | 168 (0.0%) | 63 (37.5%) | 15 (27.3%) | 22 (43.1%) | 9 (33.3%) | 17 (48.6%) | 0.1391 |
| 1 | 32 (19.0%) | 13 (23.6%) | 11 (21.6%) | 4 (14.8%) | 4 (11.4%) | |||
| 2 | 42 (25.0%) | 10 (18.2%) | 12 (23.5%) | 9 (33.3%) | 11 (31.4%) | |||
| 3 | 26 (15.5%) | 14 (25.5%) | 4 (7.8%) | 5 (18.5%) | 3 (8.6%) | |||
| 4 | 5 (3.0%) | 3 (5.5%) | 2 (3.9%) | 0 (0.0%) | 0 (0.0%) | |||
| Kellgren–Lawrence radiographic progression | 140 (16.7%) | 45 (32.1%) | 21 (45.7%) | 9 (20.5%) | 7 (35.0%) | 8 (26.7%) | 0.0709 | |
| Osteophytes radiographic progression | 143 (14.9%) | 95 (66.4%) | 40 (81.6%) | 30 (68.2%) | 10 (47.6%) | 15 (51.7%) | 0.0093 | |
| Joint space narrowing radiographic progression | 143 (14.9%) | 50 (35.0%) | 23 (46.9%) | 10 (22.7%) | 7 (33.3%) | 10 (34.5%) | 0.1110 | |
Cells show median and median absolute deviation (continuous) and absolute frequencies and percentages (categorical) for outcomes within each KOIP and in the overall series, including clinical, radiographic an ultrasound severity at baseline and radiographic progression.
Statistical significance was assessed using a Kruskal–Wallis test (continuous) or a Fisher’s test for contingency tables (categorical variables).
%Miss.: percentage of missing values; KOIP: knee osteoarthritis inflammatory phenotype; KOOS: knee injury and osteoarthritis outcome scores (reversed scores); N: number of observations.
Discussion
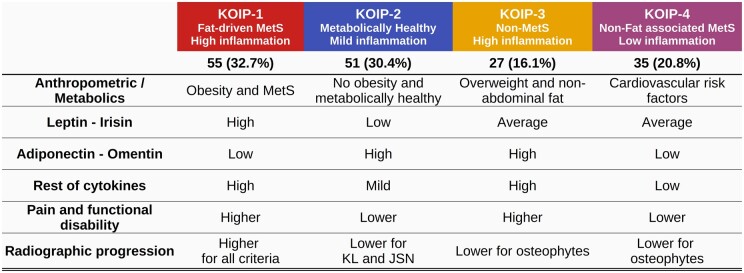
In this study, we aimed to identify different phenotypes of KOA characterized by their anthropometric and metabolic traits and their systemic and local inflammatory profiles. To do so, we applied a non-supervised approach to data from a homogeneous and tightly controlled cohort of female KOA patients with persistent joint effusion, using well-established machine learning techniques and objective statistical criteria for model selection. At present, the existence of a general so-called inflammatory phenotype that includes patients presenting synovitis is widely accepted [31, 32]. Of note, all the subjects studied in our work fall into this category and, hence, they suffered from higher levels of pain, functional disability and probability of progression compared with the non-inflammatory phenotype. Despite this sample homogeneity, our methodology identified four robust clusters of patients defining four KOA inflammatory phenotypes (KOIP, Fig. 3), which drastically differed in their anthropometric, metabolic and inflammatory profiles, presented substantial differences in clinical severity and suggested different rates of radiographic progression. These results point to differential pathways across these phenotypes regarding pathophysiology and disease evolution (endotypes) and, therefore, implications in treatment tailoring that, in line with previously established hypotheses provide a possible explanation for the current lack of translational results [12, 33–35].
Figure 3.
Knee osteoarthritis inflammatory phenotypes (KOIP). The table reflects the KOIP characteristics regarding anthropometric, metabolic and inflammatory profiles, as well as clinical severity and radiographic progression. JSN: joint space narrowing; KL: Kellgren–Lawrence; KOIP: knee osteoarthritis inflammatory phenotype; MetS: metabolic syndrome
KOIP-1 patients represented the most severe inflammatory phenotype, as shown by their highest levels of pain, functional disability and radiographic stage, and their less favourable evolution according to three different radiographic criteria. We hypothesize that KOIP-1 constitutes what has been previously described as a metabolic osteoarthritis subgroup, whose disease is mediated by a low-grade systemic inflammation promoted by metabolic factors possibly driven by body fat and obesity [36, 37]. Most of the cytokines contributing to the KOIP-1 definition had been previously linked to KOA severity [38]. The identification of this phenotype might be of relevance in clinical practice, to distinguish patients likely to benefit from therapies targeting their metabolic condition [39].
The KOIP-2 group includes patients with a healthy metabolic profile and mild overall inflammation. Their levels of pain and functional disability were the lowest in our series, though were still high compared with patients of a non-inflammatory phenotype [40]. In our data, KOIP-2 showed the lowest proportion of radiographic progression according to KL and JSN criteria. In contrast, their patients frequently suffered from osteophytes formation during their follow-up, a radiographic feature whose prognosis value and relation with cartilage impairment has raised some controversy [41, 42]. Interestingly, the divergence observed in the pattern of radiographic progression suggests the existence of differential mechanisms for the evolution of the disease across these phenotypes. Because the healthy metabolic profile exhibited by KOIP-2 patients probably offers protection against the disease’s severity and progression, alternative explanations are needed to clarify the determinants of their KOA onset and inflammatory presentation, which might be related to the mild levels of local inflammation observed in these patients.
KOIP-3 patients are characterized by low or average presence for most metabolic factors and increased values for some plasma and synovial cytokines. Their patients depict a specific inflammatory phenotype that, in contrast to the KOIP-1 group, seems not to be associated to metabolic factors. Similarly to KOIP-1, though, their levels of pain, functional disability and KL radiographic progression were among the highest in our series which, in both groups, was consistent with elevated levels of inflammatory cytokines such as IL-6, IL-8, CRP and calprotectin. These traits correspond to what might be defined as pure inflammatory phenotype, and a selection of KOIP-3 patients might be of interest in clinical trials designed to test a new generation of inflammation-targeting drugs, some of them currently ongoing [43].
KOIP-4 is a metabolic phenotype characterized by cardiovascular risk factors not associated with body fat distribution or obesity and by low inflammation in all markers. Compared with the overall series, their levels of pain and functional disability were relatively low and similar to those in the KOIP-2 group. This phenotype deserves a special consideration as, although all their patients fell into the clinical definition of inflammatory subtype, their cytokines levels were substantially lower than those observed in the rest of KOIP groups. This suggests that KOIP-4 describes a phenotype of patients whose disease is not only mediated by chronic low-grade inflammation linked to MetS as suggested in previous works [44] but also, by different pathways involving the direct action of these cardiovascular conditions [45, 46]. Although different mechanisms related to cardiovascular risk factors have been proposed for KOA onset and severity, none of them provide an explanation for an inflammatory presentation of the disease [47, 48]. Regarding its clinical applicability, patients from this phenotype might benefit from a tight control of their cardiovascular-related comorbidities.
A high number of parameters (27 out of 45) were needed to discriminate the KOIP groups in our data, indicating that these phenotypes were defined by a complex combination of various metabolic, anthropometric and inflammatory factors, rather than fully characterized by a limited number of these features. However, their implementation in clinical practice would require a panel composed of a small number of biomarkers. In this regard, the use of Omics data derived from high-throughput technologies offers a great potential to identify KOIP-specific biomarkers in the very near future [49], and it is currently an ongoing line of research in our group. Another limitation of our study is that phenotyping was conducted on a homogeneous sample of patients with inflammatory (joint effusion) KOA, and all were recruited from a single hospital, potentially limiting the extrapolation of its results. Although the sample size (168) was large enough to uncover these underlying phenotypes, future studies involving multiple centres and a larger sample size are mandatory to further confirm our findings. Our study was focused on women, as they represented the vast majority of our cohort and several sex-specificities had been reported regarding prevalence, metabolic and inflammatory conditions, and levels of pain and disability [10, 11]. An analysis with exploratory purposes showed that the uncovered KOIP groups were female-specific to a substantial extent, as their features could not be fully reproduced in male patients. Although this result is in agreement with previous works [23], they don’t allow for strong conclusions to be derived due to the low number of men in our cohort with available data for these analyses (n = 23). An important strength of our study is the exhaustive availability of data, which were systematically collected in the context of a prospective cohort specifically and accurately designed to study the determinants of KOA severity and progression. These data allowed a complete characterization of patients regarding features known for their relevance in KOA, and distinguishes our study from previously published works.
In conclusion, our work identified four groups of KOA female patients with joint effusion that showed differential profiles of anthropometric, metabolic and inflammatory factors, displayed substantial differences in clinical severity and suggested implications in radiographic progression. Our results support the view of KOA as a multifaceted and heterogeneous syndrome consisting of different phenotypes with differing pathways regarding their pathophysiology and disease evolution. If confirmed in larger series of patients, that these findings would entail important implications in research and in clinical practice, as they might boost patients’ stratification, the design of personalized therapies and the search for novel treatments.
Supplementary Material
Acknowledgements
We thank all the team of nurses who helped in performing blood extractions and with the accuracy of sample storage and conservation until its utilization. We greatly appreciate the support from the Research Unit in I3PT headed by Néstor Albiñana, especially Maria Nieves Gómez Gerique and Inmaculada Carmona Hernández, who collaborated in the laboratory procedures. We also thank all the Rheumatology staff of Hospital Universitari Parc Taulí who referred candidate patients for recruitment and follow-up and actively collaborated in the functioning of health assistance so that the authors could contribute to the crafting of the study.
Contributor Information
Joan Calvet, Rheumatology Department, Parc Taulí Hospital Universitari, Institut d'Investigació i Innovació Parc Taulí (I3PT-CERCA), Sabadell, Spain; Departament de Medicina, Universitat Autónoma de Barcelona (UAB), Barcelona, Spain.
María García-Manrique, Rheumatology Department, Parc Taulí Hospital Universitari, Institut d'Investigació i Innovació Parc Taulí (I3PT-CERCA), Sabadell, Spain; Departament de Medicina, Universitat Autónoma de Barcelona (UAB), Barcelona, Spain.
Antoni Berenguer-Llergo, Rheumatology Department, Biostatistics and Bioinformatics, Institut d'Investigació i Innovació Parc Taulí (I3PT-CERCA), Sabadell, Spain.
Cristóbal Orellana, Rheumatology Department, Parc Taulí Hospital Universitari, Institut d'Investigació i Innovació Parc Taulí (I3PT-CERCA), Sabadell, Spain.
Silvia Garcia Cirera, Rheumatology Department, Parc Taulí Hospital Universitari, Institut d'Investigació i Innovació Parc Taulí (I3PT-CERCA), Sabadell, Spain.
Maria Llop, Rheumatology Department, Parc Taulí Hospital Universitari, Institut d'Investigació i Innovació Parc Taulí (I3PT-CERCA), Sabadell, Spain.
Carlos Galisteo Lencastre, Rheumatology Department, Parc Taulí Hospital Universitari, Institut d'Investigació i Innovació Parc Taulí (I3PT-CERCA), Sabadell, Spain.
Marta Arévalo, Rheumatology Department, Parc Taulí Hospital Universitari, Institut d'Investigació i Innovació Parc Taulí (I3PT-CERCA), Sabadell, Spain.
Cristina Aymerich, Rheumatology Department, Parc Taulí Hospital Universitari, Institut d'Investigació i Innovació Parc Taulí (I3PT-CERCA), Sabadell, Spain.
Rafael Gómez, Rheumatology Department, Parc Taulí Hospital Universitari, Institut d'Investigació i Innovació Parc Taulí (I3PT-CERCA), Sabadell, Spain.
Néstor Albiñana Giménez, Scientific-Technical Unit, Institut d'Investigació i Innovació Parc Taulí (I3PT-CERCA) (UAB), Sabadell, Spain.
Jordi Gratacós, Rheumatology Department, Parc Taulí Hospital Universitari, Institut d'Investigació i Innovació Parc Taulí (I3PT-CERCA), Sabadell, Spain; Departament de Medicina, Universitat Autónoma de Barcelona (UAB), Barcelona, Spain.
Supplementary material
Supplementary material is available at Rheumatology online.
Data availability
All the code used in the analyses is available upon reasonable request.
Contribution statement
J.C., M.G.-M., A.B.-L. and J.G. contributed to the conception and design of the study. M.G.M., C.O., S.G.C., M.L., M.A. and C.G.L. contributed to the acquisition of data. C.A., R.G. and N.A.G. contributed to the blood sample extraction, processing, storage and analysis performing. J.C., M.G.M., A.B.-L., M.L. and M.A. contributed to the analysis and interpretation of data. J.C. is the paper’s guarantor. All authors contributed to drafting the article or revising it critically for relevant intellectual content. All authors gave the final approval of the version to be submitted.
Funding
This research leading to these results has received support from the Societat Catalana de Reumatologia Official Grants 2015–2016-2017–2018-2019 and the grant for original research projects from Sociedad Española de sReumatología 2017. Dr Joan Calvet was awarded by an intensification grant from I3PT in 2021 and Sociedad Española de Reumatología 2022. This research was partially funded by CERCA Programme / Generalitat de Catalunya.
Disclosure statement: The authors have declared no conflicts of interest.
References
- 1. Hunter DJ, Bierma-Zeinstra S.. Osteoarthritis. Lancet 2019;393:1745–59. [DOI] [PubMed] [Google Scholar]
- 2. Jackson J, Iyer R, Mellor J, Wei W.. The burden of pain associated with osteoarthritis in the hip or knee from the patient's perspective: a multinational cross-sectional study. Adv Ther 2020;37:3985–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wesseling J, Bierma-Zeinstra SM, Kloppenburg M, Meijer R, Bijlsma JW.. Worsening of pain and function over 5 years in individuals with ‘early’ OA is related to structural damage: data from the Osteoarthritis Initiative and CHECK (Cohort Hip & Cohort Knee) study. Ann Rheum Dis 2015;74:347–53. [DOI] [PubMed] [Google Scholar]
- 4. Bastick AN, Runhaar J, Belo JN, Bierma-Zeinstra SM.. Prognostic factors for progression of clinical osteoarthritis of the knee: a systematic review of observational studies. Arthritis Res Ther 2015;17:152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Schiphof D, Runhaar J, Waarsing JH. et al. The clinical and radiographic course of early knee and hip osteoarthritis over 10 years in CHECK (Cohort Hip and Cohort Knee). Osteoarthr Cartil 2019;27:1491–500. [DOI] [PubMed] [Google Scholar]
- 6. Sellam J, Rat AC, Fellahi S. et al. Pain in women with knee and/or hip osteoarthritis is related to systemic inflammation and to adipose tissue dysfunction: cross-sectional results of the KHOALA cohort. Semin Arthritis Rheum 2021;51:129–36. [DOI] [PubMed] [Google Scholar]
- 7. Calvet J, Orellana C, Albiñana Giménez N. et al. Differential involvement of synovial adipokines in pain and physical function in female patients with knee osteoarthritis. A cross-sectional study. Osteoarthr Cartil 2018;26:276–84. [DOI] [PubMed] [Google Scholar]
- 8. Kardos D, Marschall B, Simon M. et al. Investigation of cytokine changes in osteoarthritic knee joint tissues in response to hyperacute serum treatment. Cells 2019;8:824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bihlet AR, Byrjalsen I, Bay-Jensen AC. et al. Associations between biomarkers of bone and cartilage turnover, gender, pain categories and radiographic severity in knee osteoarthritis. Arthritis Res Ther 2019;21:203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Srikanth VK, Fryer JL, Zhai G. et al. A meta-analysis of sex differences prevalence, incidence and severity of osteoarthritis. Osteoarthr Cartil 2005;13:769–81. [DOI] [PubMed] [Google Scholar]
- 11. Peshkova M, Lychagin A, Lipina M. et al. Gender-related aspects in osteoarthritis development and progression: a review. Int J Mol Sci 2022;23:2767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Oo WM, Little C, Duong V, Hunter DJ.. The development of disease-modifying therapies for osteoarthritis (DMOADs): the evidence to date. Drug Des Devel Ther 2021;15:2921–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Mobasheri A, Saarakkala S, Finnilä M. et al. Recent advances in understanding the phenotypes of osteoarthritis. F1000Res 2019;8:F1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Henrotin Y. Osteoarthritis in year 2021: biochemical markers. Osteoarthr Cartil 2022;30:237–48. [DOI] [PubMed] [Google Scholar]
- 15. Berenbaum F. Deep phenotyping of osteoarthritis: a step forward. Ann Rheum Dis 2019;78:3–5. [DOI] [PubMed] [Google Scholar]
- 16. Nelson AE, Keefe TH, Schwartz TA. et al. Biclustering reveals potential knee OA phenotypes in exploratory analyses: data from the Osteoarthritis Initiative. PLoS One 2022;17:e0266964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Carlesso LC, Segal NA, Frey-Law L. et al. Pain susceptibility phenotypes in those free of knee pain with or at risk of knee osteoarthritis: the multicenter osteoarthritis study. Arthritis Rheumatol 2019;71:542–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Steinberg J, Southam L, Fontalis A. et al. Linking chondrocyte and synovial transcriptional profile to clinical phenotype in osteoarthritis. Ann Rheum Dis 2021;80:1070–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Coutinho de Almeida R, Mahfouz A, Mei H. et al. Identification and characterization of two consistent osteoarthritis subtypes by transcriptome and clinical data integration. Rheumatology 2021;60:1166–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Werdyani S, Liu M, Zhang H. et al. Endotypes of primary osteoarthritis identified by plasma metabolomics analysis. Rheumatology 2021;60:2735–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Carlson AK, Rawle RA, Wallace CW. et al. Characterization of synovial fluid metabolomic phenotypes of cartilage morphological changes associated with osteoarthritis. Osteoarthr Cartil 2019;27:1174–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. van Spil WE, Jansen NW, Bijlsma JW. et al. Clusters within a wide spectrum of biochemical markers for osteoarthritis: data from CHECK, a large cohort of individuals with very early symptomatic osteoarthritis. Osteoarthr Cartil 2012;20:745–54. [DOI] [PubMed] [Google Scholar]
- 23. Angelini F, Widera P, Mobasheri A. et al. Osteoarthritis endotype discovery via clustering of biochemical marker data. Ann Rheum Dis 2022;81:666–75. [DOI] [PubMed] [Google Scholar]
- 24. Deveza LA, Nelson AE, Loeser RF.. Phenotypes of osteoarthritis: current state and future implications. Clin Exp Rheumatol 2019;37(Suppl 120):64–72. [PMC free article] [PubMed] [Google Scholar]
- 25. Dell'Isola A, Allan R, Smith SL, Marreiros SS, Steultjens M.. Identification of clinical phenotypes in knee osteoarthritis: a systematic review of the literature. BMC Musculoskelet Disord 2016;17:425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Taylor ML, Wenzel M, Schroeter J.. On the sensitivity of field reconstruction and prediction using empirical orthogonal functions derived from grappy data. J Climate 2013;26:9194–205. [Google Scholar]
- 27. Breiman L. Random forests. Mach Learn 2013;45:5–32. [Google Scholar]
- 28. Fraley C, Raftery E.. Model-based clustering, discriminant analysis and density estimation. J Am Stat Assoc 2002;97:611–31. [Google Scholar]
- 29. Genuer R, Poggi JM, Tuleau-Malot C.. Variable selection using random forests. Pattern Recogn Lett 2010;31:2225–36. [Google Scholar]
- 30. RC Team. R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing, 2020. https://www.R-project.org/.
- 31. Sellam J, Berenbaum F.. The role of synovitis in pathophysiology and clinical symptoms of osteoarthritis. Nat Rev Rheumatol 2010;6:625–35. [DOI] [PubMed] [Google Scholar]
- 32. Mathiessen A, Conaghan PG.. Synovitis in osteoarthritis: current understanding with therapeutic implications. Arthritis Res Ther 2017;19:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Oo WM, Hunter DJ.. Repurposed and investigational disease-modifying drugs in osteoarthritis (DMOADs). Ther Adv Musculoskelet Dis 2022;14:1759720X221090297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Deveza LA, Loeser RF.. Is osteoarthritis one disease or a collection of many? Rheumatology 2018;57(Suppl 4):iv34–iv42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Zhang XX, He SH, Liang X. et al. Aging, cell senescence, the pathogenesis and targeted therapies of osteoarthritis. Front Pharmacol 2021;12:728100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Courties A, Berenbaum F, Sellam J.. The phenotypic approach to osteoarthritis: a look at metabolic syndrome-associated osteoarthritis. Joint Bone Spine 2019;86:725–30. [DOI] [PubMed] [Google Scholar]
- 37. Berenbaum F, Walker C.. Osteoarthritis and inflammation: a serious disease with overlapping phenotypic patterns. Postgrad Med 2020;132:377–84. [DOI] [PubMed] [Google Scholar]
- 38. Schadler P, Lohberger B, Thauerer B. et al. The association of blood biomarkers and body mass index in knee osteoarthritis: a cross-sectional study. Cartilage 2022;13:19476035211069251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Conrozier T. How to treat osteoarthritis in obese patients? Curr Rheumatol Rev 2020;16:99–104. [DOI] [PubMed] [Google Scholar]
- 40. Kittelson AJ, Stevens-Lapsley JE, Schmiege SJ.. Determination of pain phenotypes in knee osteoarthritis: a latent class analysis using data from the osteoarthritis initiative study. Arthritis Care Res 2016;68:612–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. MacKay J, Guermazi A, Kwoh CK. et al. MRI-defined osteophyte presence and concomitant cartilage damage in knees with incident tibiofemoral osteoarthritis: data from the pivotal osteoarthritis initiative magnetic resonance imaging analyses (POMA) study. Arthritis Care Res 2022;74:1513–19. [DOI] [PubMed] [Google Scholar]
- 42. Roemer FW, Guermazi A, Niu J. et al. Prevalence of magnetic resonance imaging-defined atrophic and hypertrophic phenotypes of knee osteoarthritis in a population-based cohort. Arthritis Rheum 2012;64:429–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Schieker M, Conaghan PG, Mindeholm L. et al. Effects of interleukin-1β inhibition on incident hip and knee replacement: exploratory analyses from a randomized, double-blind, placebo-controlled trial. Ann Intern Med 2020;173:509–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Berenbaum F, Griffin TM, Liu-Bryan R.. Metabolic Regulation of Inflammation in Osteoarthritis. Arthritis Rheumatol 2017;69:9–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Ching K, Houard X, Berenbaum F, Wen C.. Hypertension meets osteoarthritis - revisiting the vascular aetiology hypothesis. Nat Rev Rheumatol 2021;17:533–49. [DOI] [PubMed] [Google Scholar]
- 46. Schett G, Kleyer A, Perricone C. et al. Diabetes is an independent predictor for severe osteoarthritis: results from a longitudinal cohort study. Diabetes Care 2013;36:403–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Chowdhury T, Bellamkonda A, Gousy N, Roy DP.. The association between diabetes mellitus and osteoarthritis: does diabetes mellitus play a role in the severity of pain in osteoarthritis? Cureus 2022;14:e21449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Shin D. Association between metabolic syndrome, radiographic knee osteoarthritis, and intensity of knee pain: results of a national survey. J Clin Endocrinol Metab 2014;99:3177–83. [DOI] [PubMed] [Google Scholar]
- 49. Ratneswaran A, Rockel JS, Kapoor M.. Understanding osteoarthritis pathogenesis: a multiomics system-based approach. Curr Opin Rheumatol 2020;32:80–91. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All the code used in the analyses is available upon reasonable request.