Abstract
Objective
To evaluate the evidence concerning systemic pharmacological treatments for SSc digital ulcers (DUs) to inform the development of evidence-based treatment guidelines.
Methods
A systematic literature review of seven databases was performed to identify all original research studies of adult patients with SSc DUs. Randomized controlled trials (RCTs) and prospective longitudinal observational studies (OBSs) were eligible for inclusion. Data were extracted, applying the patient, intervention, comparison, outcome framework, and risk of bias (RoB) was assessed. Due to study heterogeneity, narrative summaries were used to present data.
Results
Forty-seven studies that evaluated the treatment efficacy or safety of pharmacological therapies were identified among 4250 references. Data from 18 RCTs of 1927 patients and 29 OBSs of 661 patients, at various RoB (total 2588 patients) showed that i.v. iloprost, phosphodiesterase-5 inhibitors and atorvastatin are effective for the treatment of active DUs. Bosentan reduced the rate of future DUs in two RCTs (moderate RoB) and eight OBSs at low to high RoB. Two small studies (moderate RoB) indicate that Janus kinase inhibitors may be effective for the treatment of active DUs, otherwise there are no data to support the use of immunosuppression or anti-platelet agents in the management of DUs.
Conclusion
There are several systemic treatments, across four medication classes, that are effective therapies for the management of SSc DUs. However, a lack of robust data means it is not possible to define the optimal treatment regimen for SSc DUs. The relatively low quality of evidence available has highlighted further areas of research need.
Keywords: SSc (scleroderma), digital ulcers, management, pharmacotherapy, systemic treatment
Rheumatology key messages.
Therapeutic strategies for the management of SSc DUs vary significantly.
Bosentan can prevent future DUs and iloprost, phosphodiesterase-5 inhibitors and atorvastatin can treat active DUs.
Limited high-quality data exist to support DU management guidelines, highlighting areas of future research need.
Introduction
Digital ulcers (DUs) affect approximately half of patients with SSc, resulting in significant pain and disability [1, 2]. DUs, particularly those that develop on the fingertips, are believed to be the result of peripheral microvascular ischaemia [3]. There is now a wide range of drug therapies available for the prevention and treatment of new DUs. A primary aim of the World Scleroderma Foundation (WSF) Digital Ulcer Working Group is to develop evidence-based treatment recommendations to optimize DU management in clinical practice. In this context, we present the findings of a systematic literature review (SLR) to investigate the efficacy and safety data of systemic pharmacological therapies for SSc DUs.
Methods
This SLR was performed in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) checklist [4]. A systematic literature search of PubMed, MEDLINE (OVID), Embase (OVID), Web of Science, Cochrane Library, Emcare (OVID) and Academic Search Premier databases from inception to 26 August 2022 was performed. Data from unpublished studies or conference abstracts and proceedings were not included in this review. We sought to identify original research studies of adult populations with SSc DUs treated with systemic pharmacological treatment. A patient, intervention, comparison, outcome (PICO) model was applied to the research questions and search strategy, which are detailed in Supplementary Data S1 and S2, available at Rheumatology online.
Based on the PICO framework, studies were eligible for inclusion if they included adult (age ≥18 years) patients with definite SSc and reported DU outcomes as either a primary or secondary endpoint. Prospective studies, of any design, were included. Outcomes of interest were the treatment of active DUs, including number of DUs and healing rates of DUs, as well as prevention of new DUs and treatment safety data. Only manuscripts published in English were included in the final review.
All abstracts were independently screened by two reviewers (L.R. and N.M.). The full text of all eligible citations was then independently assessed by the same reviewers (see Supplementary Data S3 for all full text articles excluded) and relevant study data extracted according to a prespecified template (Supplementary Data S4, both available at Rheumatology online). Any disagreement between reviewers was resolved by consensus. Due to the extensive interstudy heterogeneity in relation to both methodology and outcome measurement, meta-analysis of results was not possible and narrative summaries were used to present the data. The risk of bias (RoB) of randomized controlled trials (RCTs) was assessed using the Cochrane RoB tool [5] and the RoB in Non-Randomized Studies of Interventions (ROBINS-I) tool [6] was applied to observational studies (OBSs). RoB assessment was performed independently by two authors (L.R. and N.M.). All disagreements were resolved by consensus.
Results
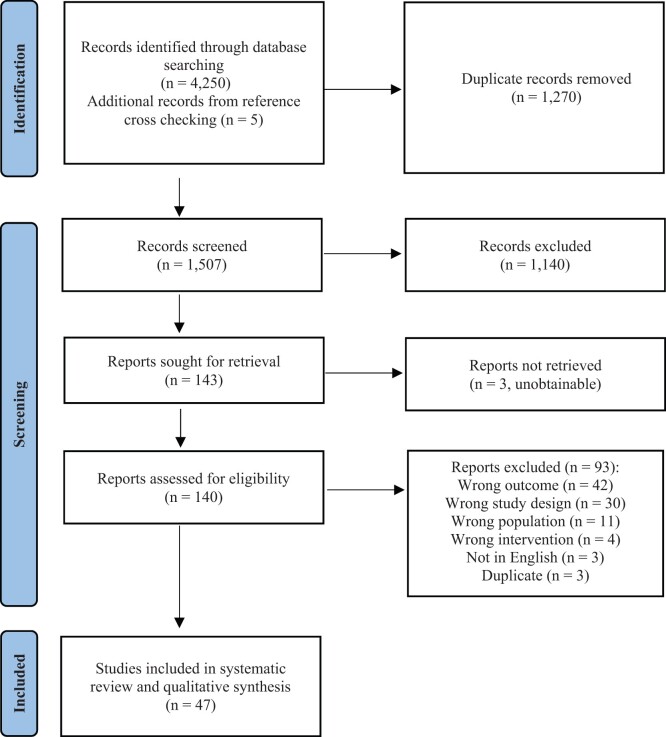
The literature search identified 4250 records. After deduplication, 1507 titles and abstracts were screened and 47 articles were eligible for inclusion (Fig. 1). Of these, 18 were RCTs and 29 were prospective OBSs (8 cohort studies, 21 prospective case series). There was a total of 2588 patients studied, of whom 1539 (60.16%) had a DU at the baseline study visit. In 16 (34%) studies, DU was the secondary endpoint (Table 1). Ten (21%) studies did not explicitly state the primary endpoint of interest. A range of pharmacotherapies were evaluated in a various number of studies; with endothelin receptor antagonists (ERAs; n = 13), phosphodiesterase-5 inhibitors (PDE5is; n = 6), prostacyclin analogues (n = 12), calcium channel blockers (CCBs; n = 2), atorvastatin (n = 2), antiplatelet agents (n = 2), immunosuppressive or immunomodulatory agents (n = 5) and other (n = 4). One study evaluated i.v. iloprost in combination with bosentan. One study compared the use of i.v. prostacyclin analogues and local therapy with botulinum toxin A and compared the costs for each therapy. There was no formal health economic analysis of any therapy for DUs presented in any study. An overview of the main study characteristics is shown in Table 1, with additional details of RCTs provided in Table 2. Additional details of OBSs and RoB assessments of all studies are shown in Supplementary Tables S1 and S2, available at Rheumatology online.
Figure 1.
PRISMA flowchart of study selection
Table 1.
Summary of characteristics of all included studies.
| Drug, median (range), year of publication | RCTs |
OBSs |
||||||
|---|---|---|---|---|---|---|---|---|
| Patients with DUs at baseline, n | Studies with DU primary endpoint, n (%) | Primary endpoint | Follow-up, months, median (range) | Patients with DUs at baseline, n | Studies with DU primary endpoint, n (%) | Primary endpoint | Follow-up, months, median (range) | |
| 9 | 1/1 (100) | NR [8] | 4.6 | 4 | 1/1 (100) | Daily RP frequency and number of DUs [7] | 1.4 | |
|
94 | 1/3 (33) | 2.3 (1.4–2.8) | 38 | 2/3 (66) | 5.2 (3–16.2) | ||
| 819 | 3/3 (100) | 3.7 (3.7–7.4) | 242 | 8/10 (80) | 12 (5.5–36) | |||
|
280b | 3/6 (50) | 3.7 (2.1–12) | 96b | 3/6 (50) | 4.15 (0–36.5) | ||
| 10 | 0/1 (0) | Change in general SSc [40] | 12 | 0 | 0/1 (0) | Suppression of ADP-dependent platelet activation [41] | 12 | |
| 45 | 0/1 (0) | Vascular function [42] | 4 | 3 | 0/1 (0) | Effects on RP [43] | 24 | |
| 26 | 0/2 (0) | 12 | 80 | 1/5 (20) | 14.8 (1–42) | |||
|
17 | 1/1 (100) | Change in net ulcer burden [51] | 7.4 | – | – | – | |
|
– | – | – | 6 | 0/1 (0) | Pharmacokinetics [52] | 6 | |
|
– | – | – | 10 | 0/1 (0) | Fewer new DU [53] | 48 | |
DUAL 1 and 2 studies considered one study as results published in a single publication.
One study [Vayssairat (RCT), n = 107; Rademaker (observational), n = 13] did not state the number of patients with DUs at baseline.
Agents studied: baricitinib, cyclophosphamide, methotrexate, N-acetylcysteine, plasmapheresis and tofacitinib.
Post hoc analysis of Scleroderma Lung Study data set.
ADP: adenosine diphosphate; NP: not published; NR: not reported.
Table 2.
Characteristics of included RCTs of systemic pharmacological treatment
| Author [ref], year | Participants (n); baseline characteristics | Intervention | Study design | Inclusion criteria | Definition of DU | Definition of ulcer healing | Concomitant therapies | Primary outcome | Results |
|---|---|---|---|---|---|---|---|---|---|
| CCBs | |||||||||
| Meyrick Thomas [8], 1987 | 9 | Nifedipine: 10 mg TDS; 6 weeks therapy, 20 weeks observation | Double-blind, placebo-controlled, crossover study | SSc and RP | Not stated | Not stated | None | Not stated | 33% of patients developed new DUs on treatment, 66% of patients developed new DUs on treatment (NS) |
| PDE5i | |||||||||
| Hachulla [10], 2016 | 83; dcSSc: 47.0%, mean age 49.3 years, mean disease duration 6.9 years | Sildenafil: 20 mg TDS for 12 weeks | Double-blind, placebo-controlled RCT | At least one ischaemic DU present on fingers distal to PIP | Break in skin with loss of epithelialization of distal finger surface of ischaemic origin not located over calcifications or extensor surfaces | Complete re-epithelialization |
|
Time to healing and healing rate |
|
| Andrigueti [11], 2017 | 41 (4 patients with active DUs at baseline); dcSSc: 34.15%, mean age 44.69 years, mean disease duration 3.2 years | Sildenafil: 100 mg/day; 8 weeks of treatment and 2 weeks post-treatment follow-up | Double-blind, placebo-controlled RCT | ≥1 RP attack/day during week before recruitment despite RP treatment | Not stated | Not stated |
|
Changes in finger blood flow | 75% of patients with baseline DUs randomized to treatment; all patients had complete healing of DUs after 8 weeks with no new DUs recorded |
| Shenoy [9], 2010a | 24; dcSSc: 75%, mean age 36.87 years, mean disease duration 6.8 years | Tadalafil: 20 mg alternate days for 6 weeks | Double-blind, placebo-controlled, crossover study | ≥4 RP attacks per week in 2 weeks preceding recruitment despite vasodilator therapy | Breach in surface epithelium at finger or toe pulps at or distal to PIP joints | Not stated |
|
Raynaud’s condition score | One new DU during treatment vs 13 new DUs during placebo treatment (P < 0.001) |
| ERAs | |||||||||
| Khanna [20], 2016 (DUAL 1 and 2 studies) | 554; dcSSc 55.78%, mean age 50.4 years, mean disease duration 10.4 years | Macitentan: 3 mg vs 10 mg vs placebo for 16 weeks | Double-blind, placebo-controlled RCT | ≥1 visible, active DU located at or distal to PIP that developed or worsened within 8 weeks and a history of additional DUs within 6–12 months of screening | Definition not stated but investigators provided with training to assess DUs prior to participating in study | Complete re-epithelialization of DU regardless of residual pain |
|
Cumulative number of new DUs from baseline to week 16 | No difference between groups—no reduction in the number of new DUs with treatment |
| Korn [19], 2004 (RAPIDS-1 study) | 122; dcSSc 37.7%, mean age 51.8 years, mean disease duration 109.6 months | Bosentan: 62.5 mg BD for 4 weeks increased to 125 mg BD for 12 weeks | Prospective double-blind, placebo-controlled RCT | Documented DU within 12 months | Loss of surface epithelialization and not including fissures or cracks or areas of calcinotic extrusion, DU either at or distal to PIP |
|
Oral vasodilating medication and oral medications for RP | Number of new DUs over 16 weeks | Reduced number of new ulcers with treatment 1.4/patient vs 2.7/patient (P < 0.01); no difference in healing rates |
| Matucci-Cerinic [18], 2011 (RAPIDS-2 study) | 188; dcSSc 43.62%, mean age 49.5 years, mean disease duration 8.7 years | Bosentan: 62.5 mg BD for 4 weeks increased to 125 mg BD for remainder of study; total treatment duration 24 weeks + 8 week post-treatment follow-up period | Prospective double-blind, placebo-controlled RCT | At least 1 active DU (considered cardinal ulcer) | Painful area ≥2 mm in diameter with visible depth and loss of dermis, amenable to healing and in a location judged to be compatible with vascular lesion (volar surface of digit distal to PIP crease) | Complete epithelialization regardless of residual pain |
|
Mean number of new DUs and time to healing of cardinal ulcer |
|
| Prostacyclin analogues | |||||||||
| Seibold [36], 2017 | 148; dcSSc 35.14%, mean age 48.77 years, mean disease duration 10.5 years | Treprostinil (oral): 16 mg BD or maximally tolerated dose for 20 weeks | Double-blind, placebo-controlled RCT; randomization stratified by number of active DUs at baseline | At least one active DU at baseline | Area with visually discernible depth and a loss of continuity of epithelial coverage distal to PIP, volar to equator of finger and not localized to IP creases, triggered by trauma with no osteomyelitis or subtending calcinosis | Not stated |
|
Change in net DU burden | Mean change in DU burden: treprostinil 0.43 vs placebo −0.10 (P = 0.20). No difference in percentage of patients who reached various threshold reductions of net DU burden. No effect on healing time or DU prevention |
| Wigley [31], 1994 | 126 (73 with digital lesions); mean age 48.9 years, mean disease duration: 8.65 years | Iloprost (i.v.): 5 consecutive days of 0.5–2 ng/kg/min for 6 h/day, 9 weeks follow-up | Double-blind, placebo-controlled RCT | SSc with RP | Digital lesions included ulcer, fissure or paronychia | Reduction in the number of baseline finger lesions by at least 50% | No other vasodilator therapy permitted | Total number of RP attacks, duration of RP attack and RP severity score | No significant difference between number of patients improved over 9 week period (P > 0.05) |
| Vayssairat [37], 1999 | 107; mean age 52 years, mean disease duration 5.9 years | Beraprost sodium (oral): 60 μg TDS for 6 months | Double-blind, placebo-controlled RCT; block randomization | History of DU within 3 years that healed at least 1 month before inclusion | Not stated | Not stated | Usual care with CCB, corticosteroids, D-penicillamine, colchicine continued | Percentage of patients with new DU |
|
| Denton [38], 2017 | 74 (14.9% had DU at baseline); dcSSc 35.1%, mean age 52.6 years, mean disease duration 9.0 years | Selexipag: 1600 μg BD or maximally tolerated dose; 2–4 week single-blind placebo run-in, 8 week treatment, 30 day post-treatment follow-up | Double-blind, placebo-controlled RCT | ≥7 RP attacks on ≥5 different days during baseline week and ≥80% eDiary compliance | Not stated | Not stated |
|
|
0.2 new DUs per patient in placebo group vs 0.4 in treatment group, all baseline DUs in treatment group healed, 5/8 baseline DUs in placebo group healed (not significant) |
| Kawald [39], 2008 | 50; dcSSc 30%, mean age 50.65 years, mean disease duration 4.55 years | Iloprost (i.v.): high (0.5–2.0 ng/kg/min) vs low (0.5 ng/kg/min) dose for 21 days once or twice per year | Randomised, open-label, placebo-controlled study | Severe RP or new active DU distal to MCP | Loss of both epidermis and dermis in the area of at least 2 mm diameter at distal phalanx | Not stated |
|
Healing of DU | No significant difference between groups. High vs low dose: 63 vs 64 DU pre-treatment decreased to 15 vs 25 DU post-treatment |
| Wigley [29], 1992 | 35 (11 with DUs); dcSSc 37.14%, mean age 46.67 years |
|
Double-blind, placebo-controlled RCT | ≥8 symptomatic RP episodes per week without vasoactive treatment | Digital cutaneous lesions included ulcers, fissures and paronychia | Healing of all lesions observed at baseline | Corticosteroids, immunosuppressants, D-penicillamine, NSAIDs permitted |
|
|
| Antiplatelet agents | |||||||||
| Beckett [40], 1984 | 41; disease onset within 3 years |
|
|
Onset of SSc symptoms within 3 years | Finger ulcers | Not stated |
|
Improvement or worsening of general SSc | No benefit from treatment. No change in DU status over follow-up |
| Statins | |||||||||
| Abou-Raya [42], 2008 | 84; dcSSc 27.38%, mean age: 48.6 years, mean disease duration 6.7 years | Atorvastatin: 40 mg daily for 4 months | Double-blind, placebo-controlled study | RP and history of DUs within past 12 months despite vasodilator therapy | Loss of surface epithelium at or distal to PIP | Not stated |
|
Vascular function and endothelial injury | −26% change in the number of DUs (P < 0.001) in favour of statin, mean number of DUs per patient 1.6 vs 2.5 (P = 0.003) in favour of statin |
| Immunosuppression and other therapies | |||||||||
| Au [45], 2010 | 158 (12 patients with DU); dcSSc 59.5%, mean age 48.5 years, mean disease duration 3.1 years | Cyclophosphamide (oral): 2 mg/kg as tolerated for 12 months | Double-blind, placebo-controlled RCT | Disease duration <7 years, ILD on HRCT | Digital dip ulcer distal to DIP joint | Not stated | Glucocorticoids (26.6%) | Number of patients with DU | No change in DU number over 52 weeks |
| Karalilova [44], 2021 | 66 (14 patients with DU); mean age 48.36 years, mean disease duration 34.62 years | Tofacitinib 5 mg BD vs methotrexate 10 mg/week; 52 weeks of follow-up | Pilot, randomized treatment study (single-blinded) | Definite SSc ≥24 weeks duration | Not stated | Not stated |
|
Change in mRSS and safety data |
|
| Nagaraja [51], 2019 | 17; dcSSc 47%, mean age 51 years, mean disease duration: 10.4 years | Riociguat: 2.5 mg TDS or maximally tolerated dose, for 16 weeks + 16 week open-label extension phase for participants with active DU or recurrence of DU at end of treatment | Double-blind, randomized, proof-of-concept trial, placebo controlled | At least 1 visible active ischaemic DU or painful indeterminate DU distal to PIP that developed or worsened within 8 weeks prior |
|
Not stated |
|
Change from baseline to week 16 net ulcer burden (total number of DUs at assessment) Designed to capture cumulative ulcer burden | No significant difference with riociguat treatment for DU |
ACEI: angiotensin-converting enzyme inhibitor; ADP: adenosine diphosphate; ARB: angiotensin receptor antagonist; BD: twice daily; CREST: calcinosis, Raynaud’s phenomenon, oesophageal dysmotility, sclerodactyly, telangiectasia syndrome; HR: hazard ratio; IP: interphalangeal; mRSS: modified Rodnan Skin Score; NS: not significant; PAH: pulmonary arterial hypertension; PVR: peripheral vascular resistance; TDS: three times daily.
Study included one patient with mixed connective tissue disease.
Only studies that recruited patients with definite SSc, generally according to either the 1980 ACR criteria [54] or 2013 ACR/EULAR classification criteria for SSc [55], were included in the review. The only study that evaluated the efficacy of tadalafil presented the results of one patient with MCTD along with those of the patients with SSc [9]. Given the significant phenotypic overlap of patients with MCTD and SSc and this was the only study available evaluating tadalafil, the results of this study were included.
Twenty-six (55%) studies specified a definition of DU. Of these studies, two early studies included skin fissures in the study definition [28, 29]. There was no consistent definition of DU applied across all studies, however, a DU was commonly considered to be a loss of surface epithelialization. The location of ulcers most commonly specified was a lesion at or distal to the proximal interphalangeal joint, most commonly on the volar aspect of the hand, and in more recent studies, specification that the lesion not be located over areas of calcification. The location of DUs and identification of areas of calcification were the only strategies applied in studies to attempt to identify ischaemic DU. The reliability of such methods was not tested in any included study. The location of DUs was not consistently reported across all studies. A healed DU was consistently reported to be present once complete re-epithelialization had occurred [10, 15–20, 30].
CCBs
Two small studies (one RCT and one OBS) with a total of 13 patients suggested a reduction in the number of DUs with nifedipine [7, 8]. Neither study specified a definition of DU. Headache and dizziness, flushing and peripheral oedema were all reported as side effects of CCB therapy [7, 8].
PDE5 inhibitor
Accelerated complete healing of active DUs with sildenafil was demonstrated in one small RCT at a moderate RoB, however, a definition of DU or ulcer healing was not stated along with the study results [11]. Two placebo-controlled RCTs with a clear definition of DU but at a moderate RoB [9, 10] demonstrated a reduction in the number of new DUs. Results of OBSs, all at a moderate RoB, indicated improved healing of DUs with sildenafil [12–14]. However, treatment efficacy across all uncontrolled OBSs was difficult to determine due to the high use of vasoactive concomitant medications such as CCBs, PDE5is and i.v. iloprost, which have either demonstrated or hypothesized efficacy in the treatment of DUs. Between 0 and 12% of patients ceased treatment with PDE5i (Table 3) [9–13]. The most common side effects of PDE5i were headache, flushing, pain, nasopharyngitis, palpitations, upper gastrointestinal tract symptoms and peripheral oedema [9–13, 15].
Table 3.
Adverse events from systemic pharmacological treatment of SSc DUsa
| Treatment | Headache/dizziness, % | Flushing, % | Pain, % | Nasopharyngitis, % | Paraesthesia, % | Palpitations, % | Upper GI symptomsb, % | Diarrhoea, % | Peripheral oedema, % | Anaemia, % | Elevated LFTs, % | Infection, % |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CCB | 11–50 [7, 8] | 50 [7] | NR | NR | NR | NR | NR | NR | 75 [7] | NR | NR | NR |
| PDE5i | 1–38 [9–11, 13] | 8–19 [9, 11, 12, 15] | 13 [9] | 29 [9] | NR | 9–25 [12, 15] | 9–13 [11, 12] | NR | 9–19 [12, 15] | NR | NR | NR |
| ERA | 2–20 [15, 16, 20, 23] | 12–50 [15, 16] | 6–7 [17, 19] | 10–13 [20, 23] | 30 [16] | NR | 20 [16] | 9–11 [17, 19, 20] | 10–75 [15–18, 20, 26] | 4–18 [15, 17, 20, 23, 24] | 4–43 [15–21, 23, 24, 27] | NR |
| Prostacyclin analogues | 12–100 [29, 31, 36–39] | 11–50 [29, 31, 36, 38, 39] | 11–23 [31, 36, 38] | NR | NR | NR | 4–78 [29, 31, 36, 38, 39] | 20–52 [31, 36, 38] | NR | NR | NR | NR |
| Antiplatelet agents | NR | NR | NR | NR | NR | NR | 5 [40] | NR | NR | NR | NR | NR |
| N-acetylcysteine | 2–23 [48, 49] | 4–14 [48, 49] | 14 [49] | NR | 14 [49] | NR | NR | 5 [49] | 9 [49] | NR | NR | NR |
| Baricitinib | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | 10 (n = 1) [50] |
| Methotrexate | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | 6.06 [44] | NR |
Data are presented for therapies where treatment-related adverse events were reported.
Upper GI symptoms: nausea, vomiting and reflux.
GI: gastrointestinal; LFTs: liver function tests; NR: not reported.
ERAs
Effective prevention of new DUs with bosentan, as compared with placebo, has been demonstrated in two RCTs, with careful attention paid to the clinical definition of DU in both studies [18, 19]. Of note, this finding was not replicated in two RCTs of macitentan compared with placebo, albeit in a milder DU population with a much lower new ulcer rate in the placebo patients than in the pivotal trials of bosentan [20]. Observational data of low to high RoB has supported this finding, with long-term bosentan treatment being associated with a reduction in the overall number of DUs, including in those patients with DUs despite treatment with i.v. iloprost [17, 21–24, 53]. OBSs have suggested improved healing of DUs with bosentan [16, 17, 21–25] and ambrisentan [26, 27], however, results of four RCTs with a moderate RoB showed that ERAs have no effect on healing rates of active DUs [18–20]. Observational data at a moderate RoB compared ERA therapy with PDE5i, showing no difference in time to healing with either treatment, but a reduced risk of development of new DUs with ERA as compared with PDE5i treatment [15]. Treatment discontinuation due to adverse events occurred in 4–20% of patients across both RCTs and OBSs of ERAs (Table 3) [9, 10, 12, 18, 20, 21, 51]. Headache, flushing, nausea, peripheral oedema and abnormal liver function tests (LFTs) with ERA therapy were the most commonly reported adverse events. All changes in LFTs were reversible with the withdrawal of therapy [15–21, 23, 24, 27].
Prostacyclin analogues
No study has evaluated i.v. iloprost compared with placebo with DU healing as a primary endpoint. Two RCTs at a moderate RoB have evaluated i.v. iloprost for the management of RP and assessed DU healing as a secondary endpoint, with inconsistent application of definitions of DU and other finger lesions applied between studies. One study that included 11 patients at baseline with DUs showed improved DU healing in the treatment arm [29]. A follow-up study that included 73 participants with baseline digital cutaneous lesions (both DUs and skin fissures) failed to show a significant improvement in DU healing over 9 weeks [31]. Observational data, rated as having moderate to high RoB, suggests an improvement in DU healing following treatment with oral or i.v. prostacyclin analogues [28, 32–35]. However, no observational study included an appropriate control group, therefore true treatment efficacy is difficult to determine. No placebo-controlled RCT of oral prostacyclin analogues has shown treatment efficacy of this class of medication [36–38]. One small study, at high RoB, compared the direct costs of treating refractory DUs with i.v. prostaglandin analogues compared with botulinum toxin A injections [30]. The costs of the systemic i.v. therapy was significantly higher than those of local therapy with botulinum toxin A injections. The efficacy of both medications was not directly compared, so it is not possible to draw conclusions as to whether the clinical efficacy of either therapy is superior to further inform any cost-effectiveness analysis. Adverse effects of treatment were a near-universal finding in patients treated with prostacyclin analogues, however, they led to treatment cessation in a minority (3–24%) of patients [29, 32, 34, 37, 38]. A serious adverse event of ongoing clinical consequence was reported in one study—a central retinal vein thrombosis that was possibly attributed to iloprost therapy [34]. All other documented adverse events due to prostacyclin analogue therapy, such as such as headache, dizziness, flushing and gastrointestinal symptoms, were reversible upon cessation of the medication.
Antiplatelet agents
No change in DU burden was observed in a placebo-controlled RCT of dipyramidole and aspirin [40], and one pilot observational study of clopidogrel suggested there may be an increased rate of endothelial dysfunction and DUs with clopidogrel use [41]. Upper gastrointestinal symptoms were reported in a single study as a result of treatment, otherwise antiplatelet agents were well tolerated [40].
Statins
One RCT at a moderate RoB showed improved healing with the use of atorvastatin in addition to standard vasodilator therapy [42]. However, DUs were evaluated as a secondary endpoint in this study. No adverse events from statin use were reported in any study.
Immunomodulatory and other agents
There has been no placebo-controlled RCTs of immunosuppressive therapies with DUs as a primary endpoint. The results of a small randomized study comparing the use of tofacitinib and low-dose oral methotrexate for treatment of SSc suggested tofacitinib was associated with a higher frequency of DU healing over 12 months [44]. A post hoc analysis of the Scleroderma Lung Study showed that cyclophosphamide as compared with placebo was not associated with a decrease in the number of DUs [45]. Observational data from four studies at a high RoB suggested improvement in DU healing with plasmapheresis [46, 47] and N-acetylcysteine infusions [48, 49]. One small study of riociguat, at a moderate RoB, applied a strict definition of DU but failed to show any treatment efficacy of riociguat and one-third of patients ceased treatment during the trial [51]. Adverse events were only variably reported in studies of immunomodulatory agents, however, infection was only rarely reported [50]. The use of N-acetylcysteine was associated with headache, flushing, pain, paraesthesia, diarrhoea and peripheral oedema [48, 49].
Health-related quality of life (HRQoL) and patient-reported function outcomes
Twenty-two (47%) studies variably presented findings of the effects of DU treatment on patient HRQoL and function (Table 4). Ten studies reported changes in pain scores following successful DU treatment, with decreased pain scores recorded in 6 (60%) studies [12, 14, 27, 42]. Improved 36-item Short Form (SF-36) physical component scores were reported in one of the two studies that recorded SF-36 scores [9]. Positive effects on overall patient function with treatment of DUs was not commonly observed. Only 6 of 18 (33%) studies that reported global function demonstrated any improvement in global function with treatment of DUs. Despite effective prevention of new DUs, improved function was not observed in either of the bosentan RCTs [18, 19]. Half of the patients who achieved clinical control of active DUs in an observational study had a clinically significant improvement in function after 12 months of treatment with bosentan [21]. However, this finding was not observed in any randomized studies of bosentan [18, 19]. Three further studies showed a statistically significant improvement in HAQ Disability Index (HAQ-DI) scores (one each for PDE5i, ERA and atorvastatin) [9, 14, 42], but the change did not reach the threshold of minimal clinically important difference of the HAQ-DI [56, 57].
Table 4.
Summary of reported HRQoL and functional outcomes
| Therapy/study | HRQoL |
Function |
|||
|---|---|---|---|---|---|
| Pain | SF-36 | Other | HAQ-DI | Other | |
| PDE5i | |||||
| Hachulla [10] | No change | NR | NR | No change | No change in CHFS |
| Shenoy [9] | Improved | Improved | NR | Improved | NR |
| Brueckner [12] | Improved | NR | NR | No change | Improved VAS for daily activity |
| Della Rossa [14] | Improved | NR | NR | Improved | NR |
| ERA | |||||
| Khanna [20] | No change | NR | NR | No change | NR |
| Korn [19] | NR | NR | NR | No changea | NR |
| Matucci-Cerinic [18] | No change | NR | NR | No change | NR |
| Mouthon [21] | NR | No change | NR | Improved | CHFS improved |
| Kucuksahin [25] | NR | NR | NR | Improved | NR |
| de la Pena-Lefebvre [23] | NR | NR | NR | No change | Improved hand flexion |
| Kuhn [16] | NR | NR | NR | No change | NR |
| Parisi [27] | Improved | NR | NR | No change | NR |
| Prostacyclin analogues | |||||
| Seibold [36] | No change | NR | NR | No change | CHFS improved |
| Wigley [31] | NR | NR | NR | NR | No change MSHAQ |
| Vayssairat [37] | NR | NR | Improved overall well being | NR | NR |
| Bettoni [34] | NR | NR | NR | No change | NR |
| Shenavandeh [30] | Improved | NR | NR | NR | NR |
| Antiplatelet agents | |||||
| Ntelis [41] | NR | NR | NR | No change | NR |
| Atorvastatin | |||||
| Abou-Raya [42] | Improved | NR | NR | Improved | NR |
| Kuwana [43] | NR | NR | No change AIMS2 | No change | NR |
| Riociguat | |||||
| Nagaraja [51] | NR | NR | No change PROMIS-29 | No change | NR |
| Baricitinib | |||||
| Hou [50] | NR | NR | Improved overall disease severity | NR | Reduced DU impact |
Individual HAQ component scores and hand function improved in treatment group, no change in overall HAQ-DI scores.
AIMS2: Arthritis Impact Measurement Scales; CHFS: Cochin hand function score; MSHA: Modified Stanford Health Assessment Questionnaire; NR: not reported; SHAQ-DI: Scleroderma Health Assessment Questionnaire Disability Index; VAS: visual analogue scale.
Discussion
A key finding of our SLR is that while treatment of active DUs was shown to be successful using iloprost, PDE5i and perhaps atorvastatin, the data are not very robust. There is stronger evidence for the impact of systemic pharmacological therapies for prevention compared with the treatment of active DUs. Data from both RCTs and OBSs support the use of ERAs, particularly bosentan, for the prevention of future DUs. There is an absence of robust evidence from large placebo-controlled RCTs to support the use of any systemic therapy for the treatment of active DUs. Small trials have shown the efficacy of i.v. iloprost, PDE5i and atorvastatin for the management of active DUs. However, the role of statins in the management of SSc DUs remains unresolved, as atorvastatin has not been evaluated in a study with DUs as a primary endpoint and previous data have shown no positive effect from atorvastatin on peripheral blood flow in patients with SSc [58]. Additional observational data suggest that CCBs, plasmapheresis, N-acetylcysteine and ketanserin may have a role in the management of active DUs. The strength of any recommendation to support the use of i.v. iloprost is limited by an absence of data from dedicated RCTs adequately powered to evaluate treatment efficacy for this therapy for DUs rather than RP. Much of the data used to justify various treatment strategies for SSc DUs are drawn from studies of RP that are underpowered to truly demonstrate treatment efficacy for DU outcomes. Although CCBs are generally recommended as a first-line agent in the management of digital vasculopathy in SSc [59], there is a paucity of evidence regarding the role CCBs in the prevention and/or treatment of DUs.
Future dedicated studies of management strategies for SSc DUs, with DU endpoints as their primary outcomes, are needed to develop a stronger evidence base to inform the management of active DUs. Newer agents are commonly ‘added on’ to an undefined standard of care. However, it is arguable there is no established standard of care for management of SSc DUs, nor is there evidence to support this add-on therapeutic strategy. A major challenge remains the interpretation of true treatment effect of any agent under investigation, given the confounding effects of background systemic and local therapies. The role of combination therapy or ‘step-up’ treatment, akin to the treat-to-target approach applied to many other inflammatory rheumatological conditions, is little studied. Currently there have been no head-to-head trials to demonstrate superior efficacy or the equivalence of one treatment compared with another. Future studies are also required to better understand the relationship between a reduction in burden of DUs and patient function and formal health economic analysis is required to establish the cost-effectiveness of various DU treatment strategies. Our results have shown that reduced DU burden, indicated by either improved healing or prevention of further DUs, is associated with an improvement in pain, but does not necessarily correlate with improved function.
To date, studies have not examined the impact on the non-pharmacological management of DUs, including lifestyle modifications such as smoking cessation. The contribution of smoking and macrovascular disease with DUs remains unresolved, with conflicting results from observational data [60–64]. The role of aggressive management of important comorbidities such as cardiovascular disease and diabetes mellitus as an adjunct to specific SSc DU therapies is undefined. Local pharmaceutical therapies in addition to local surgical procedures may also offer therapeutic benefit in the management of SSc DUs and is the topic of another SLR. A recent consensus statement from the Arthritis and Collaboration Hub Study Group highlighted both the importance to patients of understanding these management strategies and the absence of high-quality evidence to guide treatment decisions in the management of peripheral vascular manifestations of SSc [65].
The search methodology employed In this study was consciously inclusive to capture as many studies as possible evaluating systemic therapies for SSc DUs. However, only studies published in the English language were evaluated and therefore it is possible that pertinent studies published in other languages were excluded. The significant heterogeneity among study methodologies and outcome measures used limits the comparisons that can be drawn between multiple studies. There is yet to be a universally adopted definition of DU in clinical studies or a definition of what constitutes DU healing. It is critical that a specific and universal definition of ‘active’ DU is applied across studies and a careful distinction is made between ischaemic, traumatic and calcinotic ulcers. Vasoactive medications are unlikely to be effective in the healing of non-ischaemic ulcers. Therefore, if non-ischaemic ulcer types are not excluded from clinical trials, the lack of success of novel therapeutic strategies may be the result of study methodological limitations and inappropriate participant recruitment rather than true inefficacy of a novel treatment.
Furthermore, there remains a lack of consensus as to the optimal study duration and primary endpoints of DU RCTs required to establish treatment efficacy. Frequently, methods for randomization pertaining to DUs were unreported and it was unclear whether DU outcomes were analysed according to prespecified statistical analyses. Clinical experience indicates that healing of individual DUs can be slow and any study that aims to measure therapeutic efficacy over a period of weeks may be too brief a time period to demonstrate any treatment effect. Improvement in pain management and hand function remain important clinical outcomes for patients, and the absence of long-term observation following therapeutic intervention has resulted in a knowledge gap regarding the long-term symptomatic and functional benefits, or otherwise, of pharmacological intervention for SSc DUs. Arguably, an improved understanding of the natural history of DUs and, in particular, the expected time to healing of individual lesions may provide the rationale for a longer duration of future therapeutic studies. Longer treatment duration and longer trial observation periods may provide much needed data to guide treatment decisions for active DUs.
The lack of standardization regarding background therapies and inclusion of local therapies likely confounds the results of the included studies, making it challenging to draw robust conclusions about the efficacy of any therapy. The challenge in measuring the treatment success of peripheral vascular manifestations of SSc is well recognized [66] and there are historical and ongoing efforts to develop robust outcome measures for clinical trial design, including a clinical definition of DU and RCT endpoints [67]. There remains limited evidence to draw upon to support the development of new treatment recommendations of SSc DUs and our study has highlighted areas for future research (Table 5).
Table 5.
Research agenda
|
|
|
|
|
|
|
|
|
|
In conclusion, there is evidence from RCTs to support the use of i.v. iloprost, PDE5i and atorvastatin in the management of active DUs and bosentan for the prevention of future DUs. The use of Janus kinase inhibitors to manage DUs requires further investigation. These results will be used to support the development of the WSF-endorsed recommendations for the management of SSc DUs. However, there are important methodological limitations to many previous studies of SSc DUs, including the need to clearly define the clinical presentation of DUs, the lack of validated endpoints and that conclusions regarding new therapeutics are frequently drawn from analysis of trials’ secondary endpoints. Our data have highlighted areas of significant need for future research to improve our management of this important disease manifestation.
Supplementary Material
Contributor Information
Laura Ross, Department of Medicine and Rheumatology, University of Melbourne at St Vincent’s Hospital, Melbourne, Victoria, Australia.
Nancy Maltez, Department of Medicine, Division of Rheumatology, Ottawa Hospital, University of Ottawa, Ottawa, Ontario, Canada.
Michael Hughes, Department of Rheumatology, Northern Care Alliance NHS Foundation Trust, Salford Care Organisation, Salford, UK; Division of Musculoskeletal and Dermatological Sciences, University of Manchester, Manchester, UK.
Jan W Schoones, Directorate of Research Policy (formerly Walaeus Library), Leiden University Medical Center, Leiden, The Netherlands.
Murray Baron, Division of Rheumatology, Jewish General Hospital, McGill University, Montreal, Quebec, Canada.
Lorinda Chung, Department of Rheumatology, Stanford University School of Medicine and Palo Alto VA Health Care System, Palo Alto, CA, USA.
Dilia Giuggioli, Department of Rheumatology, Division of Rheumatology, University of Modena and Reggio Emilia, Policlinico of Modena, Modena, Italy.
Pia Moinzadeh, Department of Dermatology and Venereology, University Hospital Cologne, Cologne, Germany.
Yossra A Suliman, Department of Rheumatology and Rehabilitation, Assiut University Hospital, Assiut, Egypt.
Corrado Campochiaro, Department of Immunology, Rheumatology, Allergy and Rare Diseases, Raffaele Hospital, Vita-Salute San Raffaele Università, Milan, Italy.
Yannick Allanore, Department of Rheumatology, Cochin Hospital, AP-HP, Paris Descartes University, Paris, France.
Christopher P Denton, Centre for Rheumatology and Connective Tissue Diseases, Royal Free Hospital, London, UK; UCL Division of Medicine, University College London, London, UK.
Oliver Distler, Department of Rheumatology, University Hospital Zurich, University of Zurich, Zurich, Switzerland.
Tracy Frech, Department of Medicine, Division of Rheumatology and Immunology, Vanderbilt University Medical Center, Veterans Affairs Medical Center, Nashville, TN, USA.
Daniel E Furst, Department of Rheumatology and Medicine, University of California, Los Angeles, Los Angeles, CA, USA.
Dinesh Khanna, Department of Rheumatology, University of Michigan, Ann Arbor, MI, USA.
Thomas Krieg, Department of Rheumatology, Division of Rheumatology, University of Modena and Reggio Emilia, Policlinico of Modena, Modena, Italy.
Masataka Kuwana, Department of Allergy and Rheumatology, Nippon Medical School, Tokyo, Japan.
Marco Matucci-Cerinic, Department of Experimental and Clinical Medicine, Division of Rheumatology, Careggi University Hospital, University of Florence, Florence, Italy.
Janet Pope, Schulich School of Medicine and Dentistry, University of Western Ontario, London, Ontario, Canada.
Alessia Alunno, Department of Life, Health and Environmental Sciences, University of L’Aquila, L’Aquila, Italy; Internal Medicine and Nephrology Division, ASL1 Avezzano-Sulmona-L'Aquila, L'Aquila, Italy.
Supplementary material
Supplementary material is available at Rheumatology online.
Data availability
The study search protocol and data dictionary are available with publication. Individual study data are not available.
Funding
This work was supported by the World Scleroderma Foundation Digital Ulcer Working Group.
Disclosure statement: M.H. has received speaking fees from Actelion Pharmaceuticals, Eli Lilly and Pfizer, outside of the submitted work, and is a member of a Data and Safety Monitoring Board for Certa Therapeutics. L.C. has served as an advisor and steering committee member for Eicos Sciences and has received consulting fees from Mitsubishi Tanabe, Genentech, Kyverna and Jasper. P.M. has received speaking fees from Actelion Pharmaceuticals and Boehringer Ingelheim. Y.A. has received consulting fees from Boehringer Ingelheim and Sanofi, payment or honoraria from Boehringer Ingelheim and participated in data safety or advisory boards for Boehringer Ingelheim, Menarini, Chemomab, Curzion, Medseni and Sanofi. C.P.D. has received grants from GlaxoSmithKline, Inventiva, CSL Behring, Servier and Arxx Therapeutics and consulting fees from GlaxoSmithKline, Janssen, Bayer, Sanofi, Inventiva, Boehringer Ingelheim, Roche, CSL Behring, Corbus and Acceleron. O.D. has a consultancy relationship with and/or has received research funding from and/or has served as a speaker for the following companies in the area of potential treatments for SSc and its complications in the last 3 calendar years: AbbVie, Acceleron, Alcimed, Amgen, AnaMar, Arxx, AstraZeneca, Baecon, Blade, Bayer, Boehringer Ingelheim, Corbus, CSL Behring, Galapagos, Glenmark, Horizon, Inventiva, Kymera, Lupin, Medscape, Miltenyi Biotec, Mitsubishi Tanabe, MSD, Novartis, Prometheus, Roivant, Sanofi and Topadur and holds a patent: ‘mir-29 for the treatment of systemic sclerosis’ (US8247389, EP2331143). T.F. has held a paid leadership role with the Scleroderma Clinical Trials Consortium. D.E.F. has received grants or contracts from Amgen, Corbus, CSL Behring, Galapagos, Gilead, GlaxoSmithKline, Horizon, Kadmon, Novartis, Pfizer, Roche/Genentech and Talaris; consulting fees from Amgen, Corbus, Galapagos, Horizon, Kadmon, Pfizer and Talaris and payment or honoraria from CME. D.K. has received consulting fees from Actelion Pharmaceuticals, Acceleron, Amgen, Bayer, Boehringer Ingelheim, Chemomab, CSL Behring, Genentech/Roche, Horizon, Paracrine Cell Therapy, Mitsubishi Tanabe and Prometheus and holds stock or stock options in Eicos Sciences. T.K. is a World Scleroderma Foundation board member, Edith Busch Foundation advisory board member and German Scleroderma Foundation board member. M.K. has received speaker fees from AbbVie, Asahi-Kasei, Astellas, Boehringer Ingelheim, Chugai, Eisai, Nippon Shinyaku, Ono Pharmaceuticals and Tanabe-Mitsubishi; consultant fees from AstraZeneca, Boehringer Ingelheim, Chugai, Corbus, GlaxoSmithKline, Horizon, Mochida, Kissei and grant/research support from Boehringer Ingelheim, MBL and Ono Pharmaceuticals. M.M.C. has received grants from Actelion Pharmaceuticals and consulting fees from Actelion Pharmaceuticals, Biogen, Bayer, Boehringer Ingelheim, CSL Behring and Eli Lilly. The remaining authors have declared no conflicts of interest.
References
- 1. Matucci-Cerinic M, Krieg T, Guillevin L. et al. Elucidating the burden of recurrent and chronic digital ulcers in systemic sclerosis: long-term results from the DUO Registry. Ann Rheum Dis 2016;75:1770–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hughes M, Allanore Y, Chung L. et al. Raynaud phenomenon and digital ulcers in systemic sclerosis. Nat Rev Rheumatol 2020;16:208–21. [DOI] [PubMed] [Google Scholar]
- 3. Hughes M, Murray A, Denton CP, Herrick AL.. Should all digital ulcers be included in future clinical trials of systemic sclerosis-related digital vasculopathy? Med Hypotheses 2018;116:101–4. [DOI] [PubMed] [Google Scholar]
- 4. Liberati A, Altman DG, Tetzlaff J. et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. Br Med J 2009;339:b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Higgins JPT, Savovic J, Page MJ, Elbers RG, Sterne JAC.. Chapter 8: assessing risk of bias in a randomized trial. In: Higgins JPT, Thomas J, Chandler J. et al. , eds. Cochrane handbook for systematic reviews of interventions version 6.3. London: Cochrane, 2022. [Google Scholar]
- 6. Sterne JAC, Hernan MA, McAleenan A, Reeves BC, Higgins JPT.. Chapter 25: assessing risk of bias in a non-randomized study. Higgins JPT, Thomas J, Chandler J. et al. , eds. Cochrane handbook for systematic reviews of interventions version 6.3. London: Cochrane, 2022. [Google Scholar]
- 7. Kahan A, Amor B, Menkes CJ, Weber S.. Nifedipine in digital ulceration in scleroderma. Arthritis Rheum 1983;26:809. [DOI] [PubMed] [Google Scholar]
- 8. Meyrick Thomas RH, Rademaker M, Grimes SM. et al. Nifedipine in the treatment of Raynaud’s phenomenon in patients with systemic sclerosis. Br J Dermatol 1987;117:237–41. [DOI] [PubMed] [Google Scholar]
- 9. Shenoy PD, Kumar S, Jha LK. et al. Efficacy of tadalafil in secondary Raynaud’s phenomenon resistant to vasodilator therapy: a double-blind randomized cross-over trial. Rheumatology (Oxford) 2010;49:2420–8. [DOI] [PubMed] [Google Scholar]
- 10. Hachulla E, Hatron PY, Carpentier P. et al. Efficacy of sildenafil on ischaemic digital ulcer healing in systemic sclerosis: the placebo-controlled SEDUCE study. Ann Rheum Dis 2016;75:1009–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Andrigueti FV, Ebbing PCC, Arismendi MI, Kayser C.. Evaluation of the effect of sildenafil on the microvascular blood flow in patients with systemic sclerosis: a randomised, double-blind, placebo-controlled study. Clin Exp Rheumatol 2017;35(Suppl 106):S151–8. [PubMed] [Google Scholar]
- 12. Brueckner CS, Becker MO, Kroencke T. et al. Effect of sildenafil on digital ulcers in systemic sclerosis: analysis from a single centre pilot study. Ann Rheum Dis 2010;69:1475–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kumar U, Sankalp G, Sreenivas V, Kaur S, Misra D.. Prospective, open-label, uncontrolled pilot study to study safety and efficacy of sildenafil in systemic sclerosis-related pulmonary artery hypertension and cutaneous vascular complications. Rheumatol Int 2013;33:1047–52. [DOI] [PubMed] [Google Scholar]
- 14. Della Rossa A, Doveri M, D’Ascanio A. et al. Oral sildenafil in skin ulcers secondary to systemic sclerosis. Scand J Rheumatol 2011;40:323–5. [DOI] [PubMed] [Google Scholar]
- 15. Chang SH, Jun JB, Lee YJ. et al. A clinical comparison of an endothelin receptor antagonist and phosphodiesterase-5 inhibitors for treating digital ulcers of systemic sclerosis. Rheumatology (Oxford) 2021;60:5814–9. [DOI] [PubMed] [Google Scholar]
- 16. Kuhn A, Haust M, Ruland V. et al. Effect of bosentan on skin fibrosis in patients with systemic sclerosis: a prospective, open-label, non-comparative trial. Rheumatology (Oxford) 2010;49:1336–45. [DOI] [PubMed] [Google Scholar]
- 17. Hamaguchi Y, Sumida T, Kawaguchi Y. et al. Safety and tolerability of bosentan for digital ulcers in Japanese patients with systemic sclerosis: prospective, multicenter, open-label study. J Dermatol 2017;44:13–7. [DOI] [PubMed] [Google Scholar]
- 18. Matucci-Cerinic M, Denton CP, Furst DE. et al. Bosentan treatment of digital ulcers related to systemic sclerosis: results from the RAPIDS-2 randomised, double-blind, placebo-controlled trial. Ann Rheum Dis 2011;70:32–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Korn JH, Mayes M, Matucci Cerinic M. et al. Digital ulcers in systemic sclerosis: prevention by treatment with bosentan, an oral endothelin receptor antagonist. Arthritis Rheum 2004;50:3985–93. [DOI] [PubMed] [Google Scholar]
- 20. Khanna D, Denton CP, Merkel PA. et al. Effect of macitentan on the development of new ischemic digital ulcers in patients with systemic sclerosis: DUAL-1 and DUAL-2 randomized clinical trials. JAMA 2016;315:1975–88. [DOI] [PubMed] [Google Scholar]
- 21. Mouthon L, Carpentier PH, Lok C. et al. Controlling the digital ulcerative disease in systemic sclerosis is associated with improved hand function. Semin Arthritis Rheum 2017;46:759–66. [DOI] [PubMed] [Google Scholar]
- 22. Nagai Y, Hasegawa M, Hattori T. et al. Bosentan for digital ulcers in patients with systemic sclerosis. J Dermatol 2012;39:48–51. [DOI] [PubMed] [Google Scholar]
- 23. de la Pena-Lefebvre PG, Rubio SR, Exposito MV. et al. Long-term experience of bosentan for treating ulcers and healed ulcers in systemic sclerosis patients. Rheumatology (Oxford) 2008;47:464–6. [DOI] [PubMed] [Google Scholar]
- 24. Tsifetaki N, Botzoris V, Alamanos Y. et al. Bosentan for digital ulcers in patients with systemic sclerosis: a prospective 3-year followup study. J Rheumatol 2009;36:1550–2. [DOI] [PubMed] [Google Scholar]
- 25. Küçükşahin O, Yildizgören MT, Gerede DM, Maraş Y, Erten Ş.. Bosentan for digital ulcers in patients with systemic sclerosis: single center experience. Arch Rheumatol 2016;31:229–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Chung L, Ball K, Yaqub A, Lingala B, Fiorentino D.. Effect of the endothelin type A-selective endothelin receptor antagonist ambrisentan on digital ulcers in patients with systemic sclerosis: results of a prospective pilot study. J Am Acad Dermatol 2014;71:400–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Parisi S, Peroni CL, Laganà A. et al. Efficacy of ambrisentan in the treatment of digital ulcers in patients with systemic sclerosis: a preliminary study. Rheumatology (Oxford) 2013;52:1142–4. [DOI] [PubMed] [Google Scholar]
- 28. Rademaker M, Thomas RH, Provost G. et al. Prolonged increase in digital blood flow following iloprost infusion in patients with systemic sclerosis. Postgrad Med J 1987;63:617–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Wigley FM, Seibold JR, Wise RA, McCloskey DA, Dole WP.. Intravenous iloprost treatment of Raynaud's phenomenon and ischemic ulcers secondary to systemic sclerosis. J Rheumatol 1992;19:1407–14. [PubMed] [Google Scholar]
- 30. Shenavandeh S, Sepaskhah M, Dehghani S, Nazarinia M.. A 4-week comparison of capillaroscopy changes, healing effect, and cost-effectiveness of botulinum toxin-A vs prostaglandin analog infusion in refractory digital ulcers in systemic sclerosis. Clin Rheumatol 2022;41:95–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Wigley F, Wise RA, Seibold JR. et al. Intravenous iloprost infusion in patients with Raynaud phenomenon secondary to systemic sclerosis: a mulicenter, placebo-controlled, double-blind study. Ann Intern Med 1994;120:199–206. [DOI] [PubMed] [Google Scholar]
- 32. Zachariae H, Halkier-Sorensen L, Bjerring P, Heickendorff L.. Treatment of ischaemic digital ulcers and prevention of gangrene with intravenous iloprost in systemic sclerosis. Acta Derm Venereol 1996;76:236–8. [DOI] [PubMed] [Google Scholar]
- 33. Del Papa N, Vitali C, Bilia S. et al. Selexipag may be effective in inducing digital ulcer healing in patients with systemic sclerosis. Clin Exp Rheumatol 2020;38(Suppl 125):S181–2. [PubMed] [Google Scholar]
- 34. Bettoni L, Geri A, Airo P. et al. Systemic sclerosis therapy with iloprost: a prospective observational study of 30 patients treated for a median of 3 years. Clin Rheumatol 2002;21:244–50. [DOI] [PubMed] [Google Scholar]
- 35. Shah AA, Schiopu E, Chatterjee S. et al. The recurrence of digital ulcers in patients with systemic sclerosis after discontinuation of oral treprostinil. J Rheumatol 2016;43:1665–71. [DOI] [PubMed] [Google Scholar]
- 36. Seibold JR, Wigley FM, Schiopu E. et al. Digital ulcers in SSc treated with oral treprostinil: a randomized, double-blind, placebo-controlled study with open-label follow-up. J Scleroderma Relat Dis 2017;2:42–9. [Google Scholar]
- 37. Vayssairat M. Preventive effect of an oral prostacyclin analog, beraprost sodium, on digital necrosis in systemic sclerosis. French Microcirculation Society Multicenter Group for the Study of Vascular Acrosyndromes. J Rheumatol 1999;26:2173–8. [PubMed] [Google Scholar]
- 38. Denton CP, Hachulla E, Riemekasten G. et al. Efficacy and safety of selexipag in adults with Raynaud’s phenomenon secondary to systemic sclerosis: a randomized, placebo-controlled, phase II study. Arthritis Rheumatol 2017;69:2370–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kawald A, Burmester GR, Huscher D, Sunderkötter C, Riemekasten G.. Low versus high-dose iloprost therapy over 21 days in patients with secondary Raynaud’s phenomenon and systemic sclerosis: a randomized, open, single-center study. J Rheumatol 2008;35:1830–7. [PubMed] [Google Scholar]
- 40. Beckett VL, Conn DL, Fuster V. et al. Trial of platelet-inhibiting drug in scleroderma. Double-blind study with dipyridamole and aspirin. Arthritis Rheum 1984;27:1137–43. [DOI] [PubMed] [Google Scholar]
- 41. Ntelis K, Gkizas V, Filippopoulou A. et al. Clopidogrel treatment may associate with worsening of endothelial function and development of new digital ulcers in patients with systemic sclerosis: results from an open label, proof of concept study. BMC Musculoskelet Disord 2016;17:213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Abou-Raya A, Abou-Raya S, Helmii M.. Statins: potentially useful in therapy of systemic sclerosis-related Raynaud's phenomenon and digital ulcers. J Rheumatol 2008;35:1801–8. [PubMed] [Google Scholar]
- 43. Kuwana M, Okazaki Y, Kaburaki J.. Long-term beneficial effects of statins on vascular manifestations in patients with systemic sclerosis. Mod Rheumatol 2009;19:530–5. [DOI] [PubMed] [Google Scholar]
- 44. Karalilova RV, Batalov ZA, Sapundzhieva TL, Matucci-Cerinic M, Batalov AZ.. Tofacitinib in the treatment of skin and musculoskeletal involvement in patients with systemic sclerosis, evaluated by ultrasound. Rheumatol Int 2021;41:1743–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Au K, Mayes MD, Maranian P. et al. Course of dermal ulcers and musculoskeletal involvement in systemic sclerosis patients in the scleroderma lung study. Arthritis Care Res (Hoboken) 2010;62:1772–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Dau PC, Kahaleh MB, Sagebiel RW.. Plasmapheresis and immunosuppressive drug therapy in scleroderma. Arthritis Rheum 1981;24:1128–36. [DOI] [PubMed] [Google Scholar]
- 47. Mascaro G, Cadario G, Bordin G. et al. Plasma exchange in the treatment of nonadvanced stages of progressive systemic sclerosis. J Clin Apher 1987;3:219–25. [DOI] [PubMed] [Google Scholar]
- 48. Rosato E, Borghese F, Pisarri S, Salsano F.. The treatment with N-acetylcysteine of Raynaud’s phenomenon and ischemic ulcers therapy in sclerodermic patients: a prospective observational study of 50 patients. Clin Rheumatol 2009;28:1379–84. [DOI] [PubMed] [Google Scholar]
- 49. Sambo P, Amico D, Giacomelli R. et al. Intravenous N-acetylcysteine for treatment of Raynaud’s phenomenon secondary to systemic sclerosis: a pilot study. J Rheumatol 2001;28:2257–62. [PubMed] [Google Scholar]
- 50. Hou Z, Su X, Han G. et al. JAK1/2 inhibitor baricitinib improves skin fibrosis and digital ulcers in systemic sclerosis. Front Med (Lausanne) 2022;9:859330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Nagaraja V, Spino C, Bush E. et al. A multicenter randomized, double-blind, placebo-controlled pilot study to assess the efficacy and safety of riociguat in systemic sclerosis-associated digital ulcers. Arthritis Res Ther 2019;21:202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Klimiuk PS, Kay EA, Mitchell WS. et al. Ketanserin: an effective treatment regimen for digital ischaemia in systemic sclerosis. Scand J Rheumatol 1989;18:107–11. [DOI] [PubMed] [Google Scholar]
- 53. Trombetta AC, Pizzorni C, Ruaro B. et al. Effects of longterm treatment with bosentan and iloprost on nailfold absolute capillary number, fingertip blood perfusion, and clinical status in systemic sclerosis. J Rheumatol 2016;43:2033–41. [DOI] [PubMed] [Google Scholar]
- 54. American Rheumatology Association. Preliminary criteria for the classification of systemic sclerosis (scleroderma). Subcommittee for scleroderma criteria of the American Rheumatism Association Diagnostic and Therapeutic Criteria Committee. Arthritis Rheum 1980;23:581–90. [DOI] [PubMed] [Google Scholar]
- 55. van den Hoogen F, Khanna D, Fransen J. et al. 2013 classification criteria for systemic sclerosis: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Ann Rheum Dis 2013;72:1747–55. [DOI] [PubMed] [Google Scholar]
- 56. Sekhon S, Pope J, Canadian Scleroderma Research Group,Baron M, The minimally important difference in clinical practice for patient-centered outcomes including health assessment questionnaire, fatigue, pain, sleep, global visual analog scale, and SF-36 in scleroderma. J Rheumatol 2010;37:591–8. [DOI] [PubMed] [Google Scholar]
- 57. Khanna D, Furst DE, Hays RD. et al. Minimally important difference in diffuse systemic sclerosis: results from the D-penicillamine study. Ann Rheum Dis 2006;65:1325–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Sadik HY, Moore TL, Vail A. et al. Lack of effect of 8 weeks atorvastatin on microvascular endothelial function in patients with systemic sclerosis. Rheumatology (Oxford) 2010;49:990–6. [DOI] [PubMed] [Google Scholar]
- 59. Kowal-Bielecka O, Fransen J, Avouac J. et al. Update of EULAR recommendations for the treatment of systemic sclerosis. Ann Rheum Dis 2017;76:1327–39. [DOI] [PubMed] [Google Scholar]
- 60. Luders S, Friedrich S, Ohrndorf S. et al. Detection of severe digital vasculopathy in systemic sclerosis by colour Doppler sonography is associated with digital ulcers. Rheumatology (Oxford) 2017;56:1865–73. [DOI] [PubMed] [Google Scholar]
- 61. Motegi S, Toki S, Hattori T. et al. No association of atherosclerosis with digital ulcers in Japanese patients with systemic sclerosis: evaluation of carotid intima-media thickness and plaque characteristics. J Dermatol 2014;41:604–8. [DOI] [PubMed] [Google Scholar]
- 62. Blagojevic J, Abignano G, Avouac J. et al. Use of vasoactive/vasodilating drugs for systemic sclerosis (SSc)-related digital ulcers (DUs) in expert tertiary centres: results from the analysis of the observational real-life DeSScipher study. Clin Rheumatol 2020;39:27–36. [DOI] [PubMed] [Google Scholar]
- 63. Jaeger VK, Valentini G, Hachulla E. et al. Brief report: smoking in systemic sclerosis: a longitudinal European Scleroderma Trials and Research Group Study. Arthritis Rheumatol 2018;70:1829–34. [DOI] [PubMed] [Google Scholar]
- 64. Khimdas S, Harding S, Bonner A. et al. Associations with digital ulcers in a large cohort of systemic sclerosis: results from the Canadian Scleroderma Research Group registry. Arthritis Care Res (Hoboken) 2011;63:142–9. [DOI] [PubMed] [Google Scholar]
- 65. Stocker JK, Schouffoer AA, Spierings J. et al. Evidence and consensus-based recommendations for non-pharmacological treatment of fatigue, hand function loss, Raynaud's phenomenon and digital ulcers in patients with systemic sclerosis. Rheumatology (Oxford) 2022;61:1476–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Pauling JD. The challenge of establishing treatment efficacy for cutaneous vascular manifestations of systemic sclerosis. Expert Rev Clin Immunol 2018;14:431–42. [DOI] [PubMed] [Google Scholar]
- 67. Maltez N, Hughes M, Brown E. et al. Developing a core set of outcome measure domains to study Raynaud’s phenomenon and digital ulcers in systemic sclerosis: report from OMERACT 2020. Semin Arthritis Rheum 2021;51:640–3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The study search protocol and data dictionary are available with publication. Individual study data are not available.