Key Teaching Points.
-
•
Atrial arrhythmias in patients with transplanted heart may be challenging to treat and ablate.
-
•
To help achieve a successful ablation outcome, a thorough understanding of the surgical anatomy and the characterization of the arrhythmic substrate/mechanism are pivotal.
-
•
In this scenario, high-density mapping systems can represent a valuable tool.
Introduction
Atrial tachycardia (AT) after heart transplantation occurs in nearly 10% of orthotopic heart transplant recipients, with atrial flutter (AFL) being the most common.1,2
However, AT may occur in the recipient as well as in the donor atria. Catheter ablation of these ATs is an effective treatment, but it can be challenging because of the complexity of atrial scar and distorted atrial anatomy and substrate.
Case report
A 65-year-old man was referred for catheter ablation of recurrent symptomatic AT.
Twelve years earlier, owing to nonischemic dilated cardiomyopathy, he underwent orthotopic cardiac transplant with a standard biatrial anastomosis technique. The patient had a known right bundle branch block intraventricular electrical conduction delay and no history of significant rejection, tachyarrhythmia, sinus node dysfunction, or atrioventricular block.
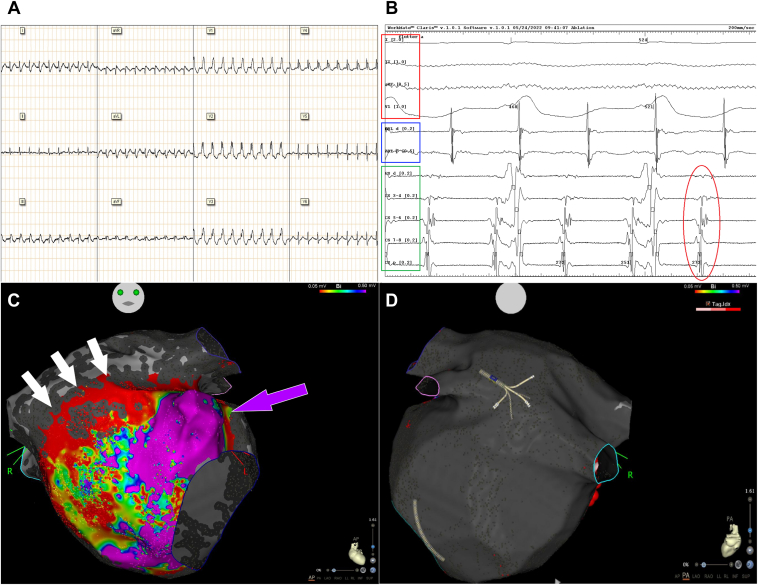
The 12-lead electrocardiogram obtained during arrhythmia suggested the diagnosis of AT/AFL (Figure 1A). Transthoracic echocardiography showed no systolic dysfunction. The coronary angiography did not reveal any sign of cardiac allograft vasculopathy and the results of histological analysis of multiple ventricular endomyocardial biopsies were normal.
Figure 1.
A: Twelve-lead electrocardiogram (ECG) recorded during atrial tachycardia. B: Pattern of the left atrial electrical activation recorded with a 6F 10-pole catheter positioned inside the coronary sinus (bottom, green box, CSd distal dipole electrogram [EGM], CSp proximal dipole EGM). The absence of a clear distal-to-proximal or proximal-to-distal left atrial activation suggested the presence of a left atrial/septal tachycardia circuit (red circle). Upper 4 channels (red box): surface ECG leads D1, D2, aVF, and V1, respectively. Middle 2 channels (blue box): ablation catheter distal (ABL d) and proximal (ABL p) EGM, recording the right atrial electrical activation. C: Electroanatomic map anteroposterior view of recipient’s left atrial cuff showing the presence of an anterolateral area with normal voltage (violet arrow), while abnormal electrical activity was found in the anteroseptal area (red to yellow and green spots) and in the cranial area corresponding to the suture line between the donor’s and recipient’s atrial cuffs (white arrows, wide red area). D: Electroanatomic map posteroanterior view of the recipient’s left atrium showing the presence of a massive enlargement of the camera with absence of any electrical activity (gray area).
Owing to the recurrence of the tachyarrhythmia despite 2 electrical cardioversions and antiarrhythmic drug therapy with amiodarone, flecainide, and beta-blocker, an electrophysiological study was indicated. The pattern of atrial activation recorded with a 6F 10-pole catheter positioned inside the coronary sinus during tachycardia suggested the presence of a septal/left-sided tachycardia circuit (Figure 1B), which was then confirmed by the electroanatomic map of the right atrium performed with a close-spaced, multipolar steerable catheter (PentaRay NAV®; Biosense Webster Inc, Irvine, CA; 2 mm interelectrode spacing distance, 1 mm electrode size).
After left atrial (LA) access was obtained by means of intracardiac echocardiography–guided transseptal puncture, detailed high-density mapping of both native and donor LA cuffs was performed using the PentaRay catheter. The anterior wall of the donor’s atrium showed extensive low-voltage areas, whereas low-amplitude complex atrial fractionated electrograms corresponding to an area of slow conduction were identified along the anastomotic suture line connecting the donor’s left atrium with the remnants of the recipient’s left atrium (Figure 1C and Supplemental Figure 1), while no sign of electrical activity was detected in the recipient’s massively enlarged left atrium (Figure 1D). The electrical activation map of the donor’s LA cuff was consistent with the presence of a clockwise macroreentrant tachycardia with a protected isthmus along the LA suture lines by the roof (Supplemental Figure 2, Supplemental Video 1).
Radiofrequency ablation was then delivered using a 3.5 mm irrigated-tip catheter (ThermoCool SmartTouch® D-F; Biosense Webster Inc; maximum power 35 W, ablation index target 500) in the donor’s LA cuff in its anteroseptal superior aspect, where a linear lesion was deployed in a caudocranial fashion (Supplemental Figure S1, red dots), obtaining slowing of AT cycle length and, eventually, sinus rhythm restoration (Supplemental Figure 3).
During a 1-year follow-up period the patient remained free of symptoms and maintained stable sinus rhythm, as documented with periodic electrocardiograms and 24-hour Holter monitoring, without any antiarrhythmic medication.
Discussion
In heart transplant recipients atrial fibrillation is rarely seen (although associated with a poor prognosis),3 and typical counterclockwise cavotricuspid isthmus–dependent right AFL represents the most frequent atrial arrhythmia,1,2 but more complex ATs may be encountered as well. In the latter case, a thorough understanding of the surgical anatomy with a clear illustration of the scar substrate and electrical activation patterns by 3-dimensional high-density electroanatomic mapping may allow to identify the mechanisms of these often-complex ATs and help to achieve a successful ablation outcome.
In our patient a subtle form of graft dysfunction (ie, confined only to the atrial tissue) may have underlain the development of diffuse patchy scars, which acted as the proarrhythmic substrate. In this scenario, the proposed mechanism of arrhythmia is that the presence in the donor’s atrium of both tissue (eg, patchy scars) and electrical (eg, anisotropic conduction or refractory period inhomogeneity) abnormalities could have played a role in the development of the proarrhythmic substrate.
At a macroscopic scale, the diseased tissue embedded in the donor’s atrial cuff constituted part of the AT macroreentrant circuit, while the suture lines by the roof and the surrounding atrial tissue harbored a protected isthmus of the AT. Targeting the latter area with the ablation lesions terminated the tachycardia.
Schratter and colleagues4 reported the case of a patient with 3 different macroreentrant right ATs running at the same time, which were mapped by means of a high-density mapping linear catheter; notably, in this patient the donor’s and recipient’s atria were electrically dissociated.
Shi and colleagues5 offered a review of the existing literature and described the case of a patient who underwent orthotopic heart transplant with biatrial anastomosis technique and developed multiple ATs: a focal one arising from the donor’s right atrium, another microreentrant AT in the donor’s left atrium, and a macroreentrant perimitral AFL. All these arrhythmias were successfully ablated after careful high-density mapping.
While in the above-mentioned reports electrical dissociation between donors’ and recipients’ atria was described, strikingly in our case any electrical activity in the recipient’s massively enlarged atrial cuff was completely absent and the AT circuit was confined to the donor’s relatively small anterior atrial wall.
Moreover, the patient’s complex electrical atrial activity and AT circuit would have been impossible to ascertain in detail without the use of a high-density mapping catheter: electrical signals with very small amplitude were barely detected with the PentaRay catheter even with the gain set to the highest values and would have otherwise been missed using a conventional mapping catheter (eg, with greater interelectrode spacing distance or electrode sizing) or the ablation catheter (Supplemental Figure 1).
Conclusion
This case report highlights the value of using a multipolar high-density mapping catheter for high-resolution mapping of extremely diseased atrial tissues, where very low-amplitude electrical signals can be missed, especially when, as in this case, large amounts of unexcitable tissue owing to extensive scar are present.
Acknowledgments
The authors are grateful to Alessandro Zanuoli and Jovana Janjic for their outstanding support during the procedure and image editing process.
Funding Sources
The authors received an unrestricted grant from Johnson & Johnson to cover the publication fees.
Disclosures
None of the authors (LDM, MC, LR, CN, VC, CC) has any conflict of interest to declare.
Footnotes
Supplementary data associated with this article can be found in the online version at https://doi.org/10.1016/j.hrcr.2023.07.013.
Appendix. Supplementary Data
Antero-posterior (AP) view of the electro-anatomic map of donor’s left atrial cuff showing the line of RF lesions deployed (red dots) to achieve sinus rhythm. Notably, the local atrial activation recorded with a high-density mapping catheter (PentaRay NAV®, Biosense Webster Inc) shows the presence of low-amplitude fragmented signals (left green box, red arrow), which were barely visible with the 3.5-mm irrigated tip ablation catheter positioned in the same location (upper left white box). MAP 1,2 and MAP 3,4: closed-spaced multipolar mapping catheter EGM. ABL d and ABL p: ablation catheter distal (ABL d) and proximal (ABL p) EGM. Note that in MAP and ABL EGM gain set is similar (2 mV/cm).
AP view of the left atrium electro-anatomic map showing the hypothesized circuit of the macro-reentrant AT involving the anteroseptal aspect of the donor’s atrial cuff (white circle).
Intracardiac EGM recordings obtained during RF lesion deployment showing AT cycle prolongation from 275 to 305 ms (red boxes) just before the interruption of the arrhythmia (light blue empty arrow). EGM channels arrangement as in Main Figure, panel B.
Antero-posterior view of the patient’s LA showing the propagation map of the depolarization wavefront during the tachycardia. The zone of slow conduction in the protected isthmus is highlighted (flashing circles in the anteroseptal red area).
References
- 1.Vaseghi M., Boyle N.G., Kedia R., et al. Supraventricular tachycardia after orthotopic cardiac transplantation. J Am Coll Cardiol. 2008;51:2241–2249. doi: 10.1016/j.jacc.2008.02.065. [DOI] [PubMed] [Google Scholar]
- 2.Joglar J., Wan E.Y., Chung M.K., et al. Management of arrhythmias after heart transplant. Circ Arrhythm Electrophysiol. 2021;14 doi: 10.1161/CIRCEP.120.007954. [DOI] [PubMed] [Google Scholar]
- 3.Ferretto S., Giuliani I., Sanavia T., et al. Atrial fibrillation after orthotopic heart transplantation: pathophysiology and clinical impact. Int J Cardiol Heart Vasc. 2021;32 doi: 10.1016/j.ijcha.2020.100710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schratter A., Schirripa V., Kosiuk J., et al. Electroanatomical high-density mapping of different tachycardias in the right atrium after heart transplantation. HeartRhythm Case Rep. 2016;2:517–520. doi: 10.1016/j.hrcr.2016.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shi R., Chen Z., Mantziari L., et al. Multiple atrial tachycardias after orthotopic heart transplantation: a case report and literature review. HeartRhythm Case Rep. 2018;4:538–541. doi: 10.1016/j.hrcr.2018.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Antero-posterior (AP) view of the electro-anatomic map of donor’s left atrial cuff showing the line of RF lesions deployed (red dots) to achieve sinus rhythm. Notably, the local atrial activation recorded with a high-density mapping catheter (PentaRay NAV®, Biosense Webster Inc) shows the presence of low-amplitude fragmented signals (left green box, red arrow), which were barely visible with the 3.5-mm irrigated tip ablation catheter positioned in the same location (upper left white box). MAP 1,2 and MAP 3,4: closed-spaced multipolar mapping catheter EGM. ABL d and ABL p: ablation catheter distal (ABL d) and proximal (ABL p) EGM. Note that in MAP and ABL EGM gain set is similar (2 mV/cm).
AP view of the left atrium electro-anatomic map showing the hypothesized circuit of the macro-reentrant AT involving the anteroseptal aspect of the donor’s atrial cuff (white circle).
Intracardiac EGM recordings obtained during RF lesion deployment showing AT cycle prolongation from 275 to 305 ms (red boxes) just before the interruption of the arrhythmia (light blue empty arrow). EGM channels arrangement as in Main Figure, panel B.
Antero-posterior view of the patient’s LA showing the propagation map of the depolarization wavefront during the tachycardia. The zone of slow conduction in the protected isthmus is highlighted (flashing circles in the anteroseptal red area).