Abstract
Fluorescence microscopic methods have been used to characterize the cell cycle of Bacillus subtilis at four different growth rates. The data obtained have been used to derive models for cell cycle progression. Like that of Escherichia coli, the period required by B. subtilis for chromosome replication at 37°C was found to be fairly constant (although a little longer, at about 55 min), as was the cell mass at initiation of DNA replication. The cell cycle of B. subtilis differed from that of E. coli in that changes in growth rate affected the average cell length but not the width and also in the relative variability of period between termination of DNA replication and septation. Overall movement of the nucleoid was found to occur smoothly, as in E. coli, but other aspects of nucleoid behavior were consistent with an underlying active partitioning machinery. The models for cell cycle progression in B. subtilis should facilitate the interpretation of data obtained from the recently introduced cytological methods for imaging the assembly and movement of proteins involved in cell cycle dynamics.
The bacterial cell cycle incorporates two important, discontinuous processes, DNA replication and cell division or septation. Many of the genes and proteins involved in these processes have been identified and characterized previously. In general, they are highly conserved across the eubacterial subkingdom, though much of what is known has been derived from studies of Escherichia coli (recently reviewed in detail in references 18, 26, and 29). During each cell cycle, the chromosome, usually in the form of a single circular molecule, needs to undergo a single complete round of replication. Regulation is mainly exerted at the level of initiation, and over a range of growth rates, this seems to occur when the cell attains a critical mass, relative to the number of copies of the origin of replication (oriC) it contains. Originally, this “initiation mass” (Mi) was thought to be constant (5), but more recent measurements suggest that it decreases slightly with increasing growth rate (52).
Following initiation, DNA replication proceeds bidirectionally from oriC. At all reasonably rapid growth rates (doubling time, <100 min), the time taken to replicate the chromosome (the C period) is more or less constant, at about 40 min. When the forks meet, in the terminus region, diametrically opposite to oriC on the E. coli chromosome map, termination occurs. This is followed by decatenation of the interlinked daughter chromosomes and, if necessary, resolution of chromosome dimers which may have arisen by homologous recombination.
During the remainder of the cycle, the chromosomes need to move apart (partition) so that when division occurs, they are segregated into the daughter cells. The mechanism of partitioning is poorly understood (reviewed in reference 50). One school of thought supposes that the nucleoids are moved apart by a relatively passive mechanism, mediated by the many transient interactions that occur between the DNA and the cell envelope, as a result of the coupled transcription and translation of secreted proteins. An alternative view is that partitioning is effected by an active, specific partitioning machinery, perhaps analogous to the mitotic apparatus of eukaryotic cells.
The period between the termination of DNA replication and septation, sometimes known as the D period in E. coli, is also relatively constant, at about 20 min (18). During this period, the cell prepares for division by assembling a multicomponent “divisome” comprising several proteins. The major component of this assembly is a tubulin-like protein, FtsZ, which forms a ring at the site of septation that is thought to contract to bring about division (reviewed in reference 26). The extent to which the division and DNA replication cycles are coupled or interconnected is not yet clear (34).
Aside from E. coli, little work has been done on cell division in any other bacterium except Bacillus subtilis. An important advantage of B. subtilis for cell cycle studies lies in its ability to sporulate. This occurs in response to starvation, and it begins with a reorganization of the cell cycle to produce a modified, asymmetric cell division (10, 45). Thus, the mechanisms controlling both the timing and positioning of the septum are under inducible control. The few studies that have been done on cell cycle progression in vegetatively growing B. subtilis (e.g., see references 2, 19, and 32) have detected only small differences from the E. coli paradigm. The most overt of these lies in the tendency of sister B. subtilis cells to remain connected in cell chains for a significant but variable period after formation of the division septum (19, 36). Thus, the D period of B. subtilis, as formally defined, is highly variable.
Recently, the subcellular localization of many proteins involved in cell division of both E. coli and B. subtilis has been determined by application of newly developed cytological methods (reviewed in reference 42). In the case of B. subtilis, interpretation of the results of these experiments would be greatly facilitated by a better knowledge of cell cycle progression in this organism. Several of the methods routinely used to measure cell cycle parameters in E. coli are unsuitable for B. subtilis because of the tendency of the cells of the latter to form chains. However, this problem can be overcome by the use of fluorescence microscopy (15). This work describes an analysis of cell cycle progression of B. subtilis under four different growth conditions. Several similarities and differences between cell cycle dynamics in B. subtilis and E. coli have been detected. The availability of four different well-characterized growth conditions should be of considerable use for future studies of the regulation and subcellular assembly of the machineries responsible for DNA replication, chromosome partitioning, and cell division.
MATERIALS AND METHODS
Bacterial strains.
B. subtilis SG38 trpC2 amyE (11) was used for all the experiments described in this work. Strain L5481 dnaB19(Ts) xin-15 flaD1 (49) was used for the internal standard of DNA content in the digital image analysis. Strain 1.5 (trpC2 spoIIAC1 [12]) was used to prepare chromosomal DNA for use as a standard for the quantitative DNA-DNA hybridization.
Media and growth conditions.
S medium contained (NH4)2SO4 (0.2% w/v), K2HPO4 (1.4%), KH2PO4 (0.6%), sodium citrate (0.1%), Mg2SO4 (0.02%), MnSO4 (0.056%), and glucose (0.5%). TS medium was S medium supplemented with l-glutamate (0.5%) and Difco yeast extract (0.001%). CH medium (which contains 10% casein hydrolysate) was as specified in detail previously (32a; modified as described in reference 35). CHG medium was CH medium containing glucose (0.5%). All media were supplemented with tryptophan (20 μg/ml). A single colony of SG38 was inoculated into 5 ml of each medium, diluted 5,000-fold into a further 5 ml, and incubated overnight at 30°C. For S medium, the overnight culture was supplemented with 15 μl of CH medium to prevent sporulation. In the morning, each culture was diluted back in fresh prewarmed medium to an optical density at 600 nm (OD600) of 0.1. The cultures were incubated with shaking at 37°C until they reached an OD600 of 0.7 to 0.8, at which point they were harvested for DNA analysis or fixed for microscopy. A plot of log OD600 against time was used to ensure that the cultures were growing exponentially when harvested (not shown).
Measurement of cell length, cell width, nucleoid length, and DNA content by digital image analysis.
The microscopic methods of Hauser and Errington (15) were used. Briefly, the cells were fixed with ethanol and the DNA was stained with DAPI (4′,6-diamidino-2-phenylindole). Phase contrast and epifluorescence images of fields of cells were captured and analyzed electronically. To randomize the sampling, all cells within a field, except those in aggregates were analyzed. Cell length and cell width were measured directly from images. The DNA content of nucleoids in individual cells was measured by its fluorescence intensity relative to that of standard nucleoids containing single complete chromosomes. By this method, DNA content is underestimated because the DNA is more condensed in the sample nucleoids than in the standard nucleoids. The approximate extent of this underestimation is given by the ratio of the maximum pixel brightness of the nucleoid to that of the standard nucleoids (15). This ratio (1.2 to 1.5) was calculated for each field of cells from the mean maximum pixel brightness of all of the nucleoids (standard deviation, ∼10%) and was used as a correction factor for the DNA content measurements. The average of the measurements obtained did not differ significantly from the average DNA content of the population measured by a direct chemical method (see below). The images in Fig. 5 were obtained as described by Glaser et al. (13).
FIG. 5.
Compilation of representative images of the different cell types found in four different growth media S (A), TS (B), CH (C), and CHG (D). DAPI fluorescence images are overlaid on phase-contrast images. The nucleoids are the brightly stained objects within the dark cell bodies. All of the images are at the same magnification. Scale bar, 2 μm.
Preparation of chromosomal DNA, probes, and filter hybridization.
The cell samples were lysed and chromosomal DNA was extracted as described previously (15). For preparation of the standard DNA from strain 1.5, a 150-ml culture of the strain was induced to sporulate, as described by Partridge and Errington (35). Twenty-two hours after the initiation of sporulation, the sporlets (representing about 95% of the population) were harvested and isolated by differential centrifugation, as described by Magill and Setlow (27). The sporlets were lysed and their DNA was purified as described for the DNA samples from vegetative cells. The chromosomes of sporlets are generated by a mechanism similar to that of spores (41, 55) and so are expected, like spores (16), to contain single completed chromosomes, but their DNA is much easier to extract than that of mature spores. The concentrations of the chromosomal DNA stocks were estimated by absorbance at 260 nm, and then they were diluted in TE buffer (10 mM Tris-HCl, 1 mM EDTA, pH 7.5) to a final concentration of 50 μg/ml. For filter hybridization (17), the DNA stocks were further diluted in denaturing buffer (1 N NaOH, 3 M NaCl in TE buffer), such that 20 μl contained 0.15 μg of DNA. (Samples of 0.05 μg of DNA were also hybridized to check that the probe was saturating.) The DNA was applied to a nylon membrane with a dot blot apparatus, denatured with 0.5 M NaOH–1.5 M NaCl (2 min), and then neutralized with 0.5 M Tris-Cl–1.5 M NaCl, pH 7.5 (5 min). The filter was briefly immersed (5 s) in 2× SSPE (1× SSPE is 0.18 M NaCl, 10 mM NaH2PO4, and 1 mM EDTA [pH 7.7]) and baked at 80°C for 120 min. After soaking in 6× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate), prehybridization was for 2 h at 42°C in 5× SSPE, 50% (vol/vol) formamide, 0.001% (wt/vol) sodium dodecyl sulfate (SDS), 0.001% (wt/vol) Ficoll, 0.001% (wt/vol) polyvinylpyrrolidine, and sonicated veal thymus DNA (0.1 mg/ml). Hybridization was done in the same buffer containing the appropriate labelled probe. Table 1 shows the primers used in the PCRs to generate the 3-kbp probes from various regions of the B. subtilis chromosome. The probes were radiolabelled by using the Random Primed DNA Labelling Kit (Boehringer Mannheim) and [α-32P]dATP (Amersham). Following hybridization, the filters were washed twice for 15 min (ambient temperature) in buffer 1 (2× SSC, 0.1% SDS) and once for 30 min (65°C) in buffer 2 (5× SSC, 0.1% SDS). The membrane was cut into rectangles, each containing a single DNA dot, and placed into 2 ml of H2O in a scintillation vial. Samples were counted for 5 min in the energy window for 3H (Cerenkov effect).
TABLE 1.
PCR primer sequences
| Probe location | Name | Sequence |
|---|---|---|
| cge | 8365 | 5′-CAAACGATGCCGATTCAGACG-3′ |
| 8366 | 5′-GAAATCGTTCGCATGCAGCG-3′ | |
| spoIIIJ | 8369 | 5′-CACAGTCACAATTCCCCCGTG-3′ |
| 8370 | 5′-CATATCCACATGCGTGATTTTAGGGGC-3′ | |
| rtp | 8371 | 5′-GTGATCCCGGCAATCACAAGCGAGG-3′ |
| 8372 | 5′-GCTTCGAGCCCCGCTGCTGTCG-3′ | |
| dnaA | 8406 | 5′-CTGCCTTTGCGGCGACGGCGTG-3′ |
| 8376 | 5′-CGATATCTACAAGTTCCTGG-3′ |
Determination of DNA content in cultures.
The biochemical quantitation of the total DNA content of cultures was as described previously (15). The cell concentration was determined by directly counting ethanol fixed cells in a Petroff-Hausser counting chamber (average of three estimations). Each estimation was based on the average number of cells in 20 ruled squares, each with a volume of 50 pl.
Calculation of the cell length distributions.
The distribution of cells as a function of cell length was modelled according to a newly derived model developed for this work (see the Appendix). The major difference between the new model and the Collins-Richmond approach (4) is that we assume that the spectra of lengths at birth and septation do not overlap significantly. It is known that in E. coli there is a narrow distribution of cell lengths at septation, and overlap is, therefore, unlikely. This allows the probability of a cell having a given length at birth to be calculated directly by using equation 8 in the Appendix; no iteration is required.
In order to use this equation, we assume that the length probability distribution at birth is a Gaussian distribution with average l̄ and variance (Δl)2:
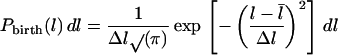 |
where l is the cell length. Similarly, we assume that the length probability distribution at septation is a Gaussian distribution with average 2l̄ and variance 2(Δl)2:
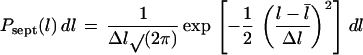 |
Thus, there are only two parameters in the model: the average length at birth, l̄, and the normalized variance of the birth probability distribution, (Δl/l̄)2. With normalized variances of the birth probability distribution in the range 0.01 to 0.20, it is found that equation 8 predicts that there is a 57% ± 1% probability of finding a cell with length ≤1.4 regardless of the value of the normalized variance. Thus, the experimentally measured length distribution can be examined to find the length, lC, at which the probability of any cell having length ≤lC is 57%. Then, the average length at birth, l̄, is (lC/1.4). This leaves the normalized variance of the birth probability distribution as the only free parameter of the model, which may be varied to attempt a variety of fits to the experimental data.
Use of the cell length distributions derived with this new model as the initial input to the iterative Collins-Richmond method (4) revealed no significant discrepancies between the two methods.
Determination of average DNA content at any point in the cell cycle.
We assume that an “ideal” cell is born with the average birth length, l̄, and with average DNA content at birth, yo chromosome equivalents, and that it septates (after a period of time equal to the generation time, τ) with length equal to the average septation length, 2l̄, and average DNA content, 2yo chromosome equivalents. Migration of DNA replication forks are assumed to occur linearly with time, at a rate determined by the C period, τC. If no chromosomal growth occurs between termination of replication and initiation (i.e., τ ≥ τC), then the DNA content of an ideal cell as a function of time, y(t), can be calculated as follows:
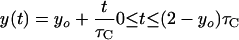 |
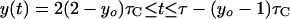 |
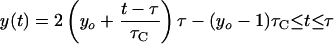 |
If dichotomous replication occurs (i.e., τC/2 ≤ τ ≤ τC), then:
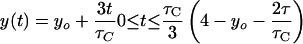 |
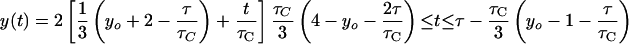 |
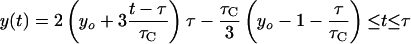 |
Calculation of average cell length at nucleoid transition.
The percentage of the cell population in each nucleoid state class (mononucleate monolobed, mononucleate bilobed, binucleate monolobed, binucleate bilobed, and tetranucleate monolobed) was calculated directly from the measured data. The cell length at which the transition between any two consecutive nucleoid states occurs was estimated by calculating (from the best fits [see the Appendix] shown in Fig. 1) the cell length at which the cumulative probability of a cell having a cell length less than the transition length is equal to the cumulative probability of a cell existing in the preceding state.
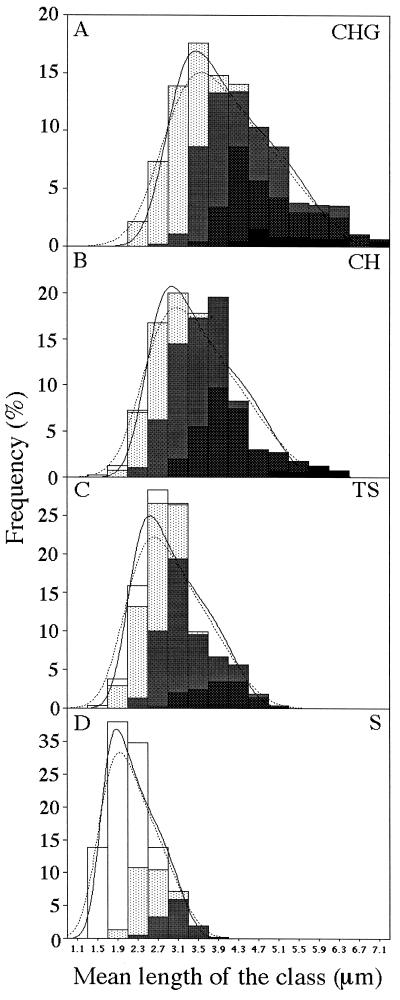
FIG. 1.
Characterization of exponentially growing cells in CHG (A), CH (B), TS (C), and S (D) media by digital image analysis. The cells were categorized according to cell length (frequency within the population) and to nucleoid conformation and number (lightest to darkest bars, monucluceate, mononucleate bilobed, binucleate, binucleate bilobed, and tri- or tetranucleate, respectively). The solid and dotted lines show ideal cell length distributions calculated as described in the Appendix, using values for the normalized variance of the birth probability distributions, (Δl/l̄)2, of 0.03 and 0.06, respectively. Data are from >400 individual cells from each medium.
RESULTS AND DISCUSSION
Measurement of cell cycle parameters in four different media.
To determine the average cell cycle for B. subtilis cells growing in various media, affording generation times between 30 and 73 min, we used the light microscopic methods of Hauser and Errington (15). Some of the data for one medium, CH, were described previously (15). In Fig. 1 and 2, data from 200 or more individual cells growing in each of the four different media are summarized. For each cell the state of the nucleoids (number and conformation) were compared with cell length and DNA content per cell (see Materials and Methods). To check the accuracy of the fluorimetric DNA estimations, we measured the DNA concentration of each culture by a direct chemical method and, having determined the cell number by direct microscopic counting, calculated the average DNA content per cell. As shown in Table 2, the values obtained differed only by about 10% from the average DNA content per cell obtained by fluorescence microscopy. An error of this magnitude would have little effect on the cell cycle modelling described below.
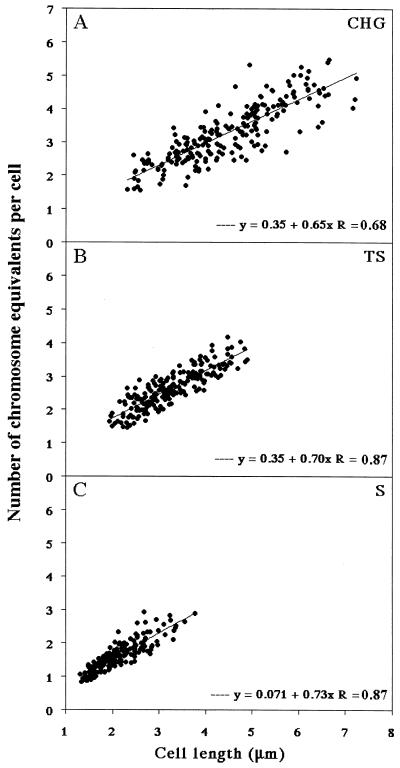
FIG. 2.
Relationship between DNA content and cell length for populations of cells grown in CHG (A), TS (B), and S (C) media. DNA content (expressed as chromosome equivalents per cell) was measured and plotted against cell length. The standard regression line for each plot is shown. Data are from about 200 individual cells from each medium.
TABLE 2.
Summary of measured and calculated cell cycle parameters for B. subtilis SG38 at different growth rates
| Cell cycle parameter | Culture medium
|
|||
|---|---|---|---|---|
| S | TS | CH | CHG | |
| Generation time (min) | 73 | 58 | 40 | 30 |
| Avg cell length (μm) | 2.2 | 3.1 | 3.6 | 4.5 |
| Avg cell width (μm)a | 0.81 (0.093) | 0.83 (0.077) | 0.86 (0.072) | 0.81 (0.075) |
| Avg DNA content/ cell (G)b | ||||
| Microscopic | 1.6 | 2.6 | 2.9c | 3.3 |
| Chemical | 1.5 | 2.3 | NDe | 3.1 |
| C period (min) | 58 | 53 | 50 | 52 |
| Birth | ||||
| Length (μm) | 1.6 | 2.2 | 2.6 | 3.0 |
| DNA content (G) | 1.3 | 1.7 | 2.0 | 2.3 |
| Initiation of DNA replication | ||||
| Length (μm) | 2.8 | 2.7 | 4.8 | 4.4 |
| Time (min) | 58 | 19 | 37 | 17 |
| DNA content (G) | 2 | 2 | 3.6 | 3.2 |
| Li (μm)f | 1.4 | 1.4 | 1.3 | 1.1 |
| Termination of DNA replication | ||||
| Length (μm) | 2.4 | 2.6 | 2.9 | 3.7 |
| Time (min) | 43 | 14 | 7 | 9 |
| DNA content (G) | 2 | 2 | 2.4 | 2.8 |
| D* period (min) | 30 | 34 | 34 | 21 |
| Nucleoid separation | ||||
| Length (μm) | 3.0 | 3.0 | 3.0 | 3.7 |
| Time (min) | 63 | 26 | 9 | 10 |
| DNA content (G) | 2.2 | 2.3 | 2.5 | 2.9 |
| Nucleoid bilobation | ||||
| Length (μm) | 2.6 | 2.3 | 4.1d | 4.8d |
| Time (min) | 49 | 2.6 | 26d | 21d |
| DNA content (G) | 2.0 | 1.8 | 3.2d | 3.5d |
Values in parentheses are standard deviations.
G, the DNA content of a single completed chromosome.
Data from reference 15.
Transition from binucleate to binucleate bilobed state.
ND, not done.
Cell length per copy of oriC.
The mean cell size of the population increased with increasing growth rate (Table 2), in agreement with previous studies (37, 38). In all four media, the shapes of the cell length frequency distributions, with a high proportion of small newborn cells and a decrease in frequency with increasing cell size, were generally characteristic of an asynchronously growing population dividing by binary fission (20, 21). In all media, the longest cells were always more than twice the length of the smallest. This probably arises through slight variations in cell length at septation, as described previously for E. coli (20, 39, 53). Apparently, the smallest cells at birth have a longer subsequent cell cycle and vice versa for the longer cells (22).
As with cell size, the mean DNA content increased with increasing growth rate. The plot of DNA content against cell length gave a linear relationship in all media (Fig. 2 and reference 15), indicating that within each population DNA replication is tightly coupled to cell growth. The regression lines had small intercept values in accordance with the expectation that both DNA content and cell length tended to be halved at septation. Interestingly, the regression lines in all four media were not significantly different, suggesting that the ratio of DNA content to cell length is constant, at least over this range of growth conditions. This was unexpected, because it suggested that B. subtilis compensates for an increased growth rate solely by increasing its length rather than by altering both length and width, as is the case for E. coli (7). Indeed, we were unable to detect significant changes in cell width across the range of growth rates tested (Table 2), in agreement with previous reports (40, 47). This observation has important implications for measurement of the Mi for DNA replication, as discussed below.
Measurement of the B. subtilis C period.
The ratios of chromosomal markers close to oriC and terC in the various cultures were determined by quantitative DNA-DNA hybridization (Table 3), and these values were used to calculate C periods (Table 3). The results were similar to each other, from 50 to 58 min, and were close to previously published values (8). It is interesting that the C period of B. subtilis is significantly longer than that of E. coli (about 42 min over this range of generation times [18]). Thus, DNA replication forks in B. subtilis apparently migrate at only about four-fifths of the rate in E. coli.
TABLE 3.
Calculation of the DNA replication (C) period
| Medium | Relative hybridization (cpm) witha:
|
Avg ratio (R) of oriC/terC | log R/log 2 (N)b | Generation time (T) (min) | C period (N × T) (min)c | |||
|---|---|---|---|---|---|---|---|---|
|
oriC marker
|
terC marker
|
|||||||
| spoIIIJ (360°) | dnaA (0°) | rtp (178°) | cge (181°) | |||||
| S | 0.45 (407) | 0.43 (810) | 0.29 (189) | 0.22 (351) | 1.73 | 0.79 | 73 | 58 ± 13% |
| TS | 1.4 (1,282) | 1.1 (1,998) | 0.72 (463) | 0.63 (1,179) | 1.9 | 0.92 | 58 | 53 ± 11% |
| CH | 3.9 (3,587) | 4.6 (5,473) | 1.7 (1,143) | 1.9 (5,492) | 2.4 | 1.26 | 40 | 50 ± 10% |
| CHG | 1.7 (1,545) | 1.3 (2,460) | 0.5 (317) | 0.4 (757) | 3.3 | 1.72 | 30 | 52 ± 11% |
Hybridization of the probe to the test DNA, relative to that of the control DNA from sporlets of strain 1.5. Averages of three independent experiments are given. Data in parentheses represent the average counts per minute for the test DNA samples.
From reference 46.
Percentages are standard deviations.
Cell cycle models.
To derive models for cell cycle progression we needed to determine, in addition to the generation time and C period, the average length and DNA content at birth. For the cell length at birth, the data described above were treated with an algorithm developed for this purpose, which is described above and in the Appendix. The solid and broken lines shown in Fig. 1 show predicted ideal cell length distributions, using two possible values for the only free parameter, the normalized variance of the birth probability distribution (0.03 and 0.06). As shown, variation of this parameter had little effect on the shape of the plot. For the purposes of all subsequent analyses, a value of 0.03 was used.
Values for the average lengths at birth and septation (assumed to be twice that at birth) calculated in this way are shown in Table 2. Cell size at birth and, hence, septation increased with increasing growth rate. This was in accordance with previous descriptions of the E. coli cell cycle except that, as discussed above, both length and width vary (and indeed maintain a fairly constant ratio in E. coli [7]). The main factor responsible for the marked effects of growth rate on the average dimensions of bacterial cells may be the relative constancy of the C period. Because initiation occurs at a relatively fixed cell mass irrespective of the growth rate (see below) and division can occur only after a round of DNA replication has been completed, rapidly growing cells reach a much greater size at division than more slowly growing cells.
The average DNA content of newborn cells (Table 2) was determined directly from the regression lines in Fig. 2 and the results of Hauser et al. (15), and as expected, this content increased with increasing growth rate. The relationships between cell length, DNA content and time were calculated from the various measured and derived parameters (see Materials and Methods), allowing models for progression through the B. subtilis cell cycle at the four different growth rates to be derived (Fig. 3).
FIG. 3.
Models for cell cycle events progression in B. subtilis growing at four different rates. Shown are the major events during the cell cycle: birth (b), initiation (i), termination (t), binucleation (bi), and septation (s). Dots indicate the initiation of DNA replication. The cell length is shown on the scale below the models.
Initiation of replication.
The initiation of DNA replication is a key event in the bacterial cell cycle. The point in the cell cycle at which initiation occurred was calculated from the average DNA content at birth and the C period (Table 2). In all cases the observed DNA content of cells at the initiation length was consistent with the predicted value (compare Fig. 1 and 2 with Table 2). For cells in which the generation time was greater than or equal to the C period (S or TS media), initiation of DNA replication occurred in fully replicated chromosomes. The DNA content of such cells was two chromosome equivalents rather than one, because the products of the previous round of DNA replication did not segregate into separate cells until later in the cycle. It is interesting to compare this with E. coli, in which slow-growing cells (e.g., doubling time, 70 min) tend to initiate DNA replication soon after birth. Termination then occurs soon enough in the cycle for the nascent sister chromosomes to segregate immediately at the forthcoming division (18). B. subtilis thus tends to maintain a higher chromosomal copy number than E. coli.
For cells with a generation time of less than the C period, new rounds of replication began before the previous rounds had terminated, resulting in dichotomous (multifork) replication. Therefore, the DNA content at initiation of replication was usually greater than two. Moreover, at the growth rates sustained by CH or CHG media, the cells in which initiation occurred would divide twice before the round of DNA replication terminated and the newly formed sister chromosomes could segregate. Thus, in these media, initiation occurred in much larger cells containing four replication origins (Table 2).
In E. coli growing at a reasonably rapid rate (e.g., more than one doubling per h), the ratio of the cell mass at initiation of DNA replication (Mi) to the number of chromosome origins (Ni) is constant (see reference 5) or decreases slightly with increasing growth rate (see reference 52). Given the relatively constant width of B. subtilis cells (see above), cell length should be approximately proportional to cell mass. It is interesting that for three of the four media used, S, TS, and CH, the Li (length at initiation, per copy of oriC) was fairly constant (Table 2), whereas in CHG medium a slightly smaller Li was obtained. Thus, it appears that the initiation mass behaves similarly in B. subtilis and E. coli.
Termination of DNA replication.
The bidirectional replication of the B. subtilis chromosome ends when the two replication forks meet in the terminus region, located approximately opposite to the replication origin (57). The timing of termination and the cell length at which this occurs are determined by the time of initiation of replication and the duration of the C period (18). Therefore, it varied considerably from medium to medium (Table 2). For more slowly growing cells (with a generation time less than or equal to the C period) termination should coincide with the cell’s attaining a DNA content of two chromosome equivalents. Thus, in cells growing in TS medium, in which the C period is more or less equal to the generation time, initiation and termination should occur almost simultaneously. In S medium, in which the generation time is well in excess of the C period, a significant gap should occur between termination of one round of replication and initiation of the next. During this interval the DNA content should remain constant at two chromosome equivalents, until the initiation mass is achieved. However, we did not detect a clear gap in the S medium cell cycle, presumably either because of the natural variation in the population of cells or the relative insensitivity of our measurements. For cell populations growing in CH and CHG with generation times less than the C period, termination of replication occurred after the succeeding cell cycle had started and, hence, at a DNA content in excess of two chromosome equivalents.
The D* period.
In E. coli it has been shown that there is a relatively fixed interval (the D period) between the termination of a round of DNA replication and cell separation (18). Measurement of this period in B. subtilis is more complicated because septation and cell separation do not coincide; indeed the time between these two events varies markedly under different growth conditions (19). It seems that most of the variation lies in the time taken to hydrolyze the thick peptidoglycan layer (typical of gram-positive bacteria) that binds the sister cells together after septation. We have therefore found it useful to use the term D* for the period between termination of DNA replication and formation of the corresponding division septum (15). Our present data show (Table 2) that the D* period was relatively constant in three of the media tested but that, as was the case for the Li, it was somewhat reduced in CHG medium. The possibility that the link between termination of DNA replication and septation is less tight in B. subtilis than in E. coli is supported by the observation (7a, 43, 56) that a delay in DNA replication in B. subtilis has much less of an effect on septation than that in E. coli (3).
Nucleoid separation.
While measuring cell length and DNA content, we also recorded nucleoid appearance and number per cell (Fig. 1). At all growth rates tested, the cells showed either one or two discrete nucleoids (i.e., were mononucleate or binucleate), with the exception of a few of the largest cells in CH or CHG. In general, it appeared from the distributions shown in Fig. 1 that the transition from one to two nucleoids occurred progressively later in the cell cycle as the growth rate increased. Thus, in S medium, the cells were mononucleate throughout most of the cycle, whereas in CH and CHG, the cells were mainly binucleate. The average point of transition from the mononucleate to the binucleate state was determined for each medium as described in Materials and Methods. In the faster-growing cells (CH or CHG medium), binucleation occurred at or soon after termination, in accordance with previous observations for E. coli (6). However, in more slowly growing cells (S or TS medium), there was a significant delay between the two events. Interestingly, nucleoid separation (binucleation) occurred at a cell length of about 3 μm in both of these media, which was similar to the length at binucleation in CH medium (Table 2). It seemed possible that the failure to observe nucleoid separation in cells of less than 3.0 μm might reflect the minimum length of the nucleoid. To determine the length of a nucleoid corresponding to a single unreplicated chromosome we used a dnaB(Ts) mutant. At the nonpermissive temperature, this mutant completes ongoing rounds of DNA replication but does not initiate new rounds (49), so the cells arrest with single completed chromosomes. (Such cells were used as a standard for the DNA content measurements discussed above.) The average length of the nucleoid in these standard cells was 1.5 ± 0.25 μm (200 nucleoids measured). This value suggests an obvious explanation for our failure to detect nucleoid separation in cells of <3.0 μm.
Nucleoid length.
Woldringh and coworkers have argued that chromosome partitioning is a relatively passive process driven by multiple transient associations between the DNA and the cell envelope, mediated by the coupled transcription and translation of secreted proteins (summarized in reference 54). In support of this view, they showed that in E. coli the nucleoids increase in length smoothly and continuously during growth (48). To test whether nucleoid expansion was also continuous in B. subtilis, we measured the total length of the nucleoid in the images of cells grown in the four different media and plotted these relative to cell length (Fig. 4). As for E. coli, a clear linear relationship was obtained in all four media. Thus, overall movement of the nucleoid in both organisms appears to be continuous.
FIG. 4.
Relationship between nuclear length and cell length over a range of growth rates for cells growing in CHG (A), CH (B), TS (C), and S (D) media. For each plot standard regression lines are shown. For binucleate cells, nuclear length corresponds to the sum of the two nuclei. Data are from 200 cells from each medium.
Despite the evidence for gradual overall movement of the nucleoid, there is increasing support for the existence of more-active, mitosis-like partitioning mechanisms in bacteria (1, 13, 24, 25, 30, 33, 51). How can the passive and active models for nucleoid partitioning be reconciled? We suggest that both mechanisms are used by the cell. The active, mitosis-like partitioning apparatus serves to ensure that the oriC regions of newly replicated sister chromosomes are driven apart by a significant distance early in the DNA replication cycle (13, 24, 44, 51). The passive mechanism would tend to move the bulk of each sister nucleoid into a position more or less centered on the fixed oriC region, this movement being facilitated by extension of the cell envelope. Thus, the active and passive mechanisms of chromosome partitioning need not be mutually exclusive.
Nucleoid bilobation.
Support for an underlying active partitioning mechanism was obtained from the observation of cell cycle-associated changes in nucleoid morphology. Figure 5 shows a compilation of images of cells at different points in the cell cycle, from each of the four different media. In general, small (i.e., newly separated) nucleoids had a relatively uniform oval shape. However, larger nucleoids tended to appear dumbbell shaped or bilobed. The frequency of bilobation was greater at higher growth rates: at the lowest growth rate in S medium, only 20% of the nucleoids were bilobed, whereas at the highest growth rate tested (CHG medium) most of the nucleoids were bilobed (Fig. 1). The time of transition to a bilobed state was measured for all four cell populations and related to cell length and DNA content, as shown in Table 2. For cells growing with doubling times greater than the C period, the nucleoids became bilobed shortly before they divided to produce two separate nucleoids in a binucleate cell. It is possible that in these cells the bilobed appearance was due to the presence of two completed but still overlapping nucleoids. As discussed above, complete nucleoid separation is probably delayed as a function of the overall dimensions of the cell at these low growth rates. However, in larger cells, growing with generation times of less than the C period, bilobation occurred relatively early in the cell cycle and well before termination of DNA replication. From the average DNA contents at the transition to the bilobed state (Table 2) it can be deduced (46) that the ongoing rounds of DNA replication would have progressed to about 60 (CH) or 75% (CHG). We suggest that in these faster-growing cells, at least, the bilobed conformation is a consequence of the active separation of the partially replicated daughter chromosomes. If so, the data indicate that this partitioning process is in progress or complete well before the round of DNA replication finishes. The bilobed state probably reflects the active separation of the oriC regions of the daughter chromosome segments, which is mediated in part by the Spo0J-dependent chromosome partitioning system (13, 24, 25, 51).
It is interesting that Wake and coworkers have demonstrated that commitment to septation in germinating spores of B. subtilis can occur when the first round of chromosome replication has progressed about 70% to completion (28, 56). It is therefore possible that initiation of septation is coupled in some way to nucleoid bilobation. This could be consistent with the proposal that initiation of septation could be triggered by a DNA-free zone in the cell, the so-called “negative nucleoid effect” of Mulder and Woldringh (31).
There is presently much interest in following the subcellular localization and assembly of proteins involved in cell division and chromosome partitioning in B. subtilis (see, for example, references 9, 13, 14, 23, and 25). The detailed descriptions we have presented for cell cycle progression in B. subtilis at four different growth rates should be of considerable use in these studies.
ACKNOWLEDGMENTS
M.E.S. and P.M.H. contributed approximately equally to this work.
We thank R. G. Wake for helpful comments on the manuscript.
This work was supported by grants from the Biotechnology and Biological Sciences Research Council of the United Kingdom. P.H.M. was supported by fellowships from the Swiss National Fund for Scientific Research and EMBO.
Appendix
Cell cycle model. (i) Basic assumptions. In order to predict the probability that a given cell has a length inside the range l to l + dl, ρ (l) dl, four pieces of information are needed. First, the rate of increase in the population with time is required. Assuming that the rate of growth of the population is exponential with time, the number of cells that exist at time t is given by:
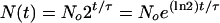 |
1 |
where No is the number of cells at time (t) zero and τ is the generation time. Since cells divide in two at septation, it follows that the number of cells born in the time interval t′ to t′ + dt′ is:
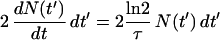 |
2 |
Second, the rate of growth of the length of a given cell with time is required. Assuming that the length of a cell grows exponentially with time, then at time t, the length of a cell born with length l′ at time t′ is:
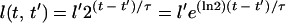 |
3 |
In addition, the probability that a cell is born with a length between l and l + dl, Pbirth(l) dl is required probability that a cell septates with a length between l and l + dl, Psept(l) dl is required.
In this model two further assumptions are made that are the key difference between this model and earlier ones, e.g., the Collins-Richmond approach (4). (v) There is no significant overlap between the birth and septation probabilities. (vi) The probability of a cell septating at any length is not dependent on its length at birth. These two assumptions enable the cell length distribution to be calculated directly from the model parameters unlike in the Collins-Richmond approach, where an iterative method is needed. It can be shown that the distributions calculated from this model fit the Collins-Richmond equation (4) well, thereby justifying these additional approximations.
(ii) Model derivation. The number of cells born with a length in the interval l′ to l′ + dl′ in the time interval t′ to t′ + dl′ is:
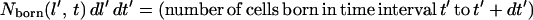 |
4 |
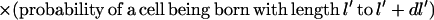 |
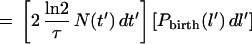 |
Provided that they do not septate, all the cells born with length l′ at time t′ will grow to length l at time t while those cells born with length l′ + dl′ at time t′ will grow to length l + dl at time t (where dl = (∂l/∂l′) dl′). Thus, the number of cells with length l to l + dl that exist at time t which were born in the time interval t′ to t + dl′ is:
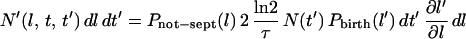 |
5 |
where 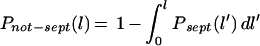 is the probability that a given cell can grow to a length l without septating. This equation implicitly assumes that there is a negligible overlap between the birth and septation probabilities and that the probability of a cell septating is not dependent on its birth length as indicated above.
is the probability that a given cell can grow to a length l without septating. This equation implicitly assumes that there is a negligible overlap between the birth and septation probabilities and that the probability of a cell septating is not dependent on its birth length as indicated above.
By integrating over all possible birth times, we obtain the total number of cells with length l to l + dl that exist at time t:
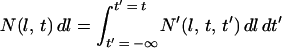 |
 |
6 |
Hence, the probability that the length of a given cell is l to l + dl is:
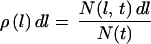 |
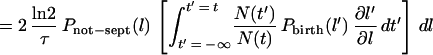 |
7 |
The derivation of this equation has made no assumptions about the rate of growth of cell length or about the birth or septation probabilities. However, if the cells grow exponentially with time, as assumed above (equation 3), it follows that N(t′)/N(t) = l′/l (by using equation 1), ∂l′/∂l = l′/l, and dt′ = (τ/ln2)(dl′/l′). Hence, equation 7 becomes:
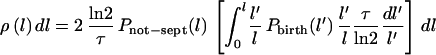 |
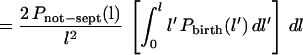 |
8 |
REFERENCES
- 1.Begg K J, Donachie W D. Experiments on chromosome separation and positioning in Escherichia coli. New Biol. 1991;3:475–486. [PubMed] [Google Scholar]
- 2.Burdett I D J, Kirkwood T B L, Whalley J B. Growth kinetics of individual Bacillus subtilis cells and correlation with nucleoid extension. J Bacteriol. 1986;167:219–230. doi: 10.1128/jb.167.1.219-230.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Burton P, Holland I B. Two pathways of division inhibition in UV-irradiated E. coli. Mol Gen Genet. 1983;189:128–132. doi: 10.1007/BF00330334. [DOI] [PubMed] [Google Scholar]
- 4.Collins J F, Richmond M H. Rate of growth of Bacillus cereus between divisions. J Gen Microbiol. 1962;28:15–23. doi: 10.1099/00221287-28-1-15. [DOI] [PubMed] [Google Scholar]
- 5.Donachie W D. Relationship between cell size and time of initiation of DNA replication. Nature. 1968;219:1077–1079. doi: 10.1038/2191077a0. [DOI] [PubMed] [Google Scholar]
- 6.Donachie W D, Begg K J. Chromosome partition in Escherichia coli requires postreplication protein synthesis. J Bacteriol. 1989;171:5405–5409. doi: 10.1128/jb.171.10.5405-5409.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Donachie W D, Robinson A C. Cell division: parameter values and the process. In: Neidhardt F C, Ingraham J L, Low K B, Magasanik B, Schaechter M, Umbarger H E, editors. Escherichia coli and Salmonella typhimurium: cellular and molecular biology. Washington, D.C: American Society for Microbiology; 1987. pp. 1578–1593. [Google Scholar]
- 7a.Donachie W D, Martin D T M, Begg K J. Independence of cell division and DNA replication in Bacillus subtilis. Nat New Biol. 1971;231:274–276. doi: 10.1038/newbio231274a0. [DOI] [PubMed] [Google Scholar]
- 8.Dunn G, Jeffs P, Mann N H, Torgersen D M, Young M. The relationship between DNA replication and the induction of sporulation in Bacillus subtilis. J Gen Microbiol. 1978;108:189–195. [Google Scholar]
- 9.Edwards D H, Errington J. The Bacillus subtilis DivIVA protein targets to the division septum and controls the site specificity of cell division. Mol Microbiol. 1997;24:905–915. doi: 10.1046/j.1365-2958.1997.3811764.x. [DOI] [PubMed] [Google Scholar]
- 10.Errington J. Bacillus subtilis sporulation: regulation of gene expression and control of morphogenesis. Microbiol Rev. 1993;57:1–33. doi: 10.1128/mr.57.1.1-33.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Errington J, Mandelstam J. Use of a lacZ gene fusion to determine the dependence pattern of sporulation operon spoIIA in spo mutants of Bacillus subtilis. J Gen Microbiol. 1986;132:2967–2976. doi: 10.1099/00221287-132-11-2967. [DOI] [PubMed] [Google Scholar]
- 12.Errington J, Mandelstam J. Use of a lacZ gene fusion to determine the dependence pattern and the spore compartment expression of sporulation operon spoVA in spo mutants of Bacillus subtilis. J Gen Microbiol. 1986;132:2977–2985. doi: 10.1099/00221287-132-11-2977. [DOI] [PubMed] [Google Scholar]
- 13.Glaser P, Sharpe M E, Raether B, Perego M, Ohlsen K, Errington J. Dynamic, mitotic-like behaviour of a bacterial protein required for accurate chromosome partitioning. Genes Dev. 1997;11:1160–1168. doi: 10.1101/gad.11.9.1160. [DOI] [PubMed] [Google Scholar]
- 14.Harry E J, Wake R G. The membrane-bound cell division protein DivIB is localized to the division site in Bacillus subtilis. Mol Microbiol. 1997;25:275–283. doi: 10.1046/j.1365-2958.1997.4581822.x. [DOI] [PubMed] [Google Scholar]
- 15.Hauser P M, Errington J. Characterization of cell cycle events during the onset of sporulation in Bacillus subtilis. J Bacteriol. 1995;177:3923–3931. doi: 10.1128/jb.177.14.3923-3931.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hauser P M, Karamata D. A method for the determination of bacterial spore DNA content based on isotopic labelling, spore germination and diphenylamine assay; ploidy of spores of several Bacillus species. Biochimie. 1992;74:723–733. doi: 10.1016/0300-9084(92)90145-5. [DOI] [PubMed] [Google Scholar]
- 17.Hauser P M, Karamata D. Characterization of the chromosomes of Bacillus subtilis merodiploid strains by quantitative DNA-DNA hybridization. Microbiology. 1994;140:1605–1611. doi: 10.1099/13500872-140-7-1605. [DOI] [PubMed] [Google Scholar]
- 18.Helmstetter C E. Timing of synthetic activities in the cell cycle. In: Neidhardt F C, Curtiss III R, Ingraham J L, Lin E C C, Low K B, Magasanik B, Reznikoff W S, Riley M, Schaechter M, Umbarger H E, editors. Escherichia coli and Salmonella: cellular and molecular biology. Washington, D.C: American Society for Microbiology; 1996. pp. 1627–1639. [Google Scholar]
- 19.Holmes M, Rickert M, Pierucci O. Cell division cycle of Bacillus subtilis: evidence of variability in period D. J Bacteriol. 1980;142:254–261. doi: 10.1128/jb.142.1.254-261.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Koch A L. Does the initiation of chromosome replication regulate cell division? Adv Microb Physiol. 1977;16:49–98. doi: 10.1016/s0065-2911(08)60047-8. [DOI] [PubMed] [Google Scholar]
- 21.Koch A L, Schaechter M. A model for statistics of the cell division process. J Gen Microbiol. 1962;29:435–454. doi: 10.1099/00221287-29-3-435. [DOI] [PubMed] [Google Scholar]
- 22.Koppes L J H, Woldringh C L, Nanninga N. Size variations and correlation of different cell cycle events in slow-growing Escherichia coli. J Bacteriol. 1978;134:423–433. doi: 10.1128/jb.134.2.423-433.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Levin P A, Losick R. Transcription factor Spo0A switches the localization of the cell division protein FtsZ from a medial to a bipolar pattern in Bacillus subtilis. Genes Dev. 1996;10:478–488. doi: 10.1101/gad.10.4.478. [DOI] [PubMed] [Google Scholar]
- 24.Lewis P J, Errington J. Direct evidence for active segregation of oriC regions of the Bacillus subtilis chromosome and co-localization with the Spo0J partitioning protein. Mol Microbiol. 1997;25:945–954. doi: 10.1111/j.1365-2958.1997.mmi530.x. [DOI] [PubMed] [Google Scholar]
- 25.Lin D C-H, Levin P A, Grossman A D. Bipolar localization of a chromosome partitioning protein in Bacillus subtilis. Proc Natl Acad Sci USA. 1997;94:4721–4726. doi: 10.1073/pnas.94.9.4721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lutkenhaus J, Mukherjee A. Cell division. In: Neidhardt F C, Curtiss III R, Ingraham J L, Lin E C C, Low K B, Magasanik B, Reznikoff W S, Riley M, Schaechter M, Umbarger H E, editors. Escherichia coli and Salmonella: cellular and molecular biology. Washington, D.C: American Society for Microbiology; 1996. pp. 1615–1626. [Google Scholar]
- 27.Magill N G, Setlow P. Properties of purified sporlets produced by spoII mutants of Bacillus subtilis. J Bacteriol. 1992;174:8148–8151. doi: 10.1128/jb.174.24.8148-8151.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McGinness T, Wake R G. Division septation in the absence of chromosome termination in Bacillus subtilis. J Mol Biol. 1979;134:251–264. doi: 10.1016/0022-2836(79)90035-4. [DOI] [PubMed] [Google Scholar]
- 29.Messer W, Weigel C. Initiation of chromosome replication. In: Neidhardt F C, Curtiss III R, Ingraham J L, Lin E C C, Low K B, Magasanik B, Reznikoff W S, Riley M, Schaechter M, Umbarger H E, editors. Escherichia coli and Salmonella: cellular and molecular biology. Washington, D.C: American Society for Microbiology; 1996. pp. 1579–1601. [Google Scholar]
- 30.Mohl D A, Gober J W. Cell cycle-dependent polar localization of chromosome partitioning proteins in Caulobacter crescentus. Cell. 1997;88:675–684. doi: 10.1016/s0092-8674(00)81910-8. [DOI] [PubMed] [Google Scholar]
- 31.Mulder E, Woldringh C L. Actively replicating nucleoids influence positioning of division sites in Escherichia coli filaments forming cells lacking DNA. J Bacteriol. 1989;171:4303–4314. doi: 10.1128/jb.171.8.4303-4314.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nanninga N, Koppes L J H, de Vries-Tijssen F C. The cell cycle of Bacillus subtilis as studied by electron microscopy. Arch Microbiol. 1979;123:173–181. doi: 10.1007/BF00446817. [DOI] [PubMed] [Google Scholar]
- 32a.Nicholson W L, Setlow P. Sporulation, germination and outgrowth. In: Harwood C R, Cutting S M, editors. Molecular biological methods for Bacillus. J. Chichester, United Kingdom: Wiley and Sons; 1990. pp. 391–450. [Google Scholar]
- 33.Niki H, Jaffe A, Imamura R, Ogura T, Hiraga S. The new gene mukB codes for a 177 kd protein with coiled-coil domains involved in chromosome partitioning of E. coli. EMBO J. 1991;10:183–193. doi: 10.1002/j.1460-2075.1991.tb07935.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nordström K, Bernander R, Dasgupta S. The Escherichia coli cell cycle: one cycle or multiple independent processes that are co-ordinated? Mol Microbiol. 1991;5:769–774. doi: 10.1111/j.1365-2958.1991.tb00747.x. [DOI] [PubMed] [Google Scholar]
- 35.Partridge S R, Errington J. The importance of morphological events and intercellular interactions in the regulation of prespore-specific gene expression during sporulation in Bacillus subtilis. Mol Microbiol. 1993;8:945–955. doi: 10.1111/j.1365-2958.1993.tb01639.x. [DOI] [PubMed] [Google Scholar]
- 36.Paulton R J L. Nuclear and cell division in filamentous bacteria. Nat New Biol. 1971;231:271–274. doi: 10.1038/newbio231271a0. [DOI] [PubMed] [Google Scholar]
- 37.Sargent M G. Control of cell length in Bacillus subtilis. J Bacteriol. 1975;123:7–19. doi: 10.1128/jb.123.1.7-19.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schaechter M, Maaloe O, Kjelgaard N O. Dependency on medium and temperature on cell size and chemical composition during balanced growth of Salmonella typhimurium. J Gen Microbiol. 1958;19:592–606. doi: 10.1099/00221287-19-3-592. [DOI] [PubMed] [Google Scholar]
- 39.Schaechter M, Williamson J P, Hood J R, Koch A L. Growth, cell and nuclear divisions in some bacteria. J Gen Microbiol. 1962;29:421–434. doi: 10.1099/00221287-29-3-421. [DOI] [PubMed] [Google Scholar]
- 40.Sedgwick E G, Paulton R J L. Dimension control in bacteria. Can J Microbiol. 1974;20:231–236. doi: 10.1139/m74-036. [DOI] [PubMed] [Google Scholar]
- 41.Setlow B, Magill N, Febbroriello P, Nakhimovsky L, Koppel D E, Setlow P. Condensation of the forespore nucleoid early in sporulation of Bacillus species. J Bacteriol. 1991;173:6270–6278. doi: 10.1128/jb.173.19.6270-6278.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shapiro L, Losick R. Protein localization and cell fate in bacteria. Science. 1997;276:712–718. doi: 10.1126/science.276.5313.712. [DOI] [PubMed] [Google Scholar]
- 43.Sharpe M E, Errington J. Postseptational chromosome partitioning in bacteria. Proc Natl Acad Sci USA. 1995;92:8630–8634. doi: 10.1073/pnas.92.19.8630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sharpe, M. E., and J. Errington. 1997. Unpublished results.
- 45.Stragier P, Losick R. Molecular genetics of sporulation in Bacillus subtilis. Annu Rev Genet. 1996;30:297–341. doi: 10.1146/annurev.genet.30.1.297. [DOI] [PubMed] [Google Scholar]
- 46.Sueoka N, Yoshikawa H. The chromosome of Bacillus subtilis. I: theory of marker frequency analysis. Genetics. 1965;52:747–757. doi: 10.1093/genetics/52.4.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Trueba F J, Woldringh C L. Changes in cell diameter during the division cycle of Escherichia coli. J Bacteriol. 1980;121:13–19. doi: 10.1128/jb.142.3.869-878.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.van Helvoort J M L M, Woldringh C L. Nucleoid partitioning in Escherichia coli during steady state growth and upon recovery from chloramphenicol treatment. Mol Microbiol. 1994;13:577–583. doi: 10.1111/j.1365-2958.1994.tb00452.x. [DOI] [PubMed] [Google Scholar]
- 49.Viret J-F. Etude des corrélations entre la synthèse de DNA et celle de l’enveloppe bactérienne chez B. subtilis. Ph.D. thesis. Lausanne, Switzerland: University of Lausanne; 1984. [Google Scholar]
- 50.Wake R G, Errington J. Chromosome partitioning in bacteria. Annu Rev Genet. 1995;29:41–67. doi: 10.1146/annurev.ge.29.120195.000353. [DOI] [PubMed] [Google Scholar]
- 51.Webb C D, Teleman A, Gordon S, Straight A, Belmont A, Lin D C-H, Grossman A D, Wright A, Losick R. Bipolar localization of the replication origin regions of chromosomes in vegetative and sporulating cells of B. subtilis. Cell. 1997;88:667–674. doi: 10.1016/s0092-8674(00)81909-1. [DOI] [PubMed] [Google Scholar]
- 52.Wold S, Skarstad K, Steen H B, Stokke T, Boye E. The initiation mass for DNA replication in Escherichia coli K-12 is dependent on growth rate. EMBO J. 1994;13:2097–2102. doi: 10.1002/j.1460-2075.1994.tb06485.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Woldringh C L. Morphological analysis of nuclear separation and cell division during the life cycle of Escherichia coli. J Bacteriol. 1976;125:248–257. doi: 10.1128/jb.125.1.248-257.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Woldringh C L, Jensen P R, Westerhoff H V. Structure and partitioning of bacterial DNA: determined by a balance of compaction and expansion forces? FEMS Microbiol Lett. 1995;131:235–242. doi: 10.1111/j.1574-6968.1995.tb07782.x. [DOI] [PubMed] [Google Scholar]
- 55.Wu L J, Errington J. Bacillus subtilis SpoIIIE protein required for DNA segregation during asymmetric cell division. Science. 1994;264:572–575. doi: 10.1126/science.8160014. [DOI] [PubMed] [Google Scholar]
- 56.Wu L J, Franks A H, Wake R G. Replication through the terminus region of the Bacillus subtilis chromosome is not essential for the formation of a division septum that partitions the DNA. J Bacteriol. 1995;177:5711–5715. doi: 10.1128/jb.177.19.5711-5715.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yoshikawa H, Wake R G. Initiation and termination of chromosome replication. In: Sonenshein A L, Hoch J A, Losick R, editors. Bacillus subtilis and other gram-positive bacteria: biochemistry, physiology, and molecular genetics. Washington, D.C: American Society for Microbiology; 1993. pp. 507–528. [Google Scholar]