Abstract
Background
Disseminated intravascular coagulation (DIC) worsens the prognosis of septic shock and contributes to multiple organ failure. To date, no data linking DIC and acute kidney injury (AKI) occurrence, severity, and evolution in this setting are available. We aimed at analyzing the association between AKI occurrence, severity and evolution in patients with septic shock-induced DIC. In a prospective monocentric cohort study, consecutive patients, 18 years and older, admitted in the ICU of Strasbourg University Hospital in the setting of systemic hypotension requiring vasopressor related to an infection, without history of terminal chronic kidney disease were eligible. AKI was defined according to the KDIGO classification. DIC diagnosis was based on the International Society on Thrombosis and Haemostasis (ISTH) score. Evolution of AKI was evaluated through the composite endpoint of major adverse kidney events. Only patients with DIC that occurred before or at the time of AKI diagnosis were considered. Univariate and multivariate analysis were performed to determine factors associated with renal outcomes.
Results
350 patients were included, of whom 129 experienced DIC. Patients with DIC were more seriously ill (median SAPS II 64 vs. 56, p < 0.001), and had higher 28-day mortality (43.3% vs. 26.2%, p < 0.001). AKI was more frequent in patients with DIC (86.8% vs. 74.2%, p < 0.005), particularly for the more severe stage of AKI [KDIGO 3 in 58.1% of patients with DIC vs. 30.8% of patients without DIC, p < 0.001, AKI requiring renal replacement therapy (RRT) in 47.3% of patients with DIC vs. 21.3% of patients without DIC, p < 0.001]. After adjustment for confounding factors, DIC occurrence remained associated with the risk of having the more severe stage of AKI with an odds ratio (OR) of 2.74 [IC 95% (1.53–4.91), p < 0.001], and with the risk of requiring RRT during the ICU stay [OR 2.82 (1.53–5.2), p < 0.001].
Conclusion
DIC appears to be strongly associated with the risk of developing the more severe form of AKI (stage 3 of the KDIGO classification, RRT requirement), even after adjustment for severity and other relevant factors.
Supplementary Information
The online version contains supplementary material available at 10.1186/s13613-023-01216-8.
Keywords: Disseminated intravascular coagulation, Sepsis, Septic shock, Acute kidney injury, Acute kidney disease
Background
Acute kidney injury (AKI) in the intensive care unit (ICU) is associated with a higher mortality rate, with a close relationship between AKI severity and death [1, 2]. AKI has also been reported to be associated with worse long-term outcome: patients who experienced AKI during ICU stay are at higher risk of developing chronic kidney disease (CKD) and major cardiovascular events [3, 4].
Beyond common factors associated with AKI in ICU, septic shock remains the main trigger of AKI [5]. However, pathophysiology of sepsis-induced AKI is complex and still poorly understood. While it certainly involves factors like inflammation, oxidative stress, microvascular dysfunction, and tubular epithelial cell insult [6, 7], disseminated intravascular coagulation (DIC) might also contribute to AKI development. DIC results from an excessive activation of coagulation, along with a defect in anticoagulant and fibrinolytic regulatory systems [8, 9], and thus leads to disseminated microthrombosis, which might contribute to organ dysfunction during septic shock [10, 11]. As 30–40% of patients with septic shock develop DIC, which is associated with severity and increased mortality rate [12, 13], it might be a considerable contributor to AKI development.
However, few studies investigated the association between DIC and AKI. A higher rate of AKI has been suggested in septic shock patients with DIC, but this possible association was not analyzed with adjustment of confounding variables, mainly patient severity [14, 15].
This study, therefore aimed at investigating the association between DIC and AKI among patients with septic shock with a special emphasis on AKI occurrence, severity, and evolution.
Methods
Design of the study
A prospective cohort study was conducted between July 2013 and March 2019, in an ICU of Strasbourg University Hospital (France).
Criteria for inclusions were patients aged 18 years and older, admitted to the ICU for septic shock according to the Sepsis-2 definition were screened. Patients had to be included in the 12 h following vasopressor initiation. Patients with moribund status at screening phase, or patients with limitation of life-sustaining therapies at admission have been excluded. Patients under legal protection (inability to provide consent, incarceration…) have been excluded. Patients already enrolled in the present study have been excluded in case of readmission, and patients who developed shock later during the ICU stay were not screened.
Furthermore, patients with a history of terminal chronic kidney disease (stage 5 of the chronic kidney disease (CKD) definition [16], under chronic renal replacement therapy or kidney transplant recipients) were excluded. To delineate the association between DIC and AKI and its severity, patients in whom DIC was diagnosed after AKI reached its higher stage were excluded.
Data collection
The following data were prospectively recorded: age, sex, body mass index, significant medical history, and the Charlson index [17]. Acute condition was characterized by: severity with the SAPSII and SOFA scores [18, 19], hemodynamic parameters (mean arterial pressure, urine output), source of infection, nosocomial or community-acquired infections and available microbiological data were collected. We registered all the supporting therapies required during the ICU stay [mechanical ventilation, vasopressors, fluid therapy, renal replacement therapy (RRT)] and their duration. Mortality was assessed at the end of ICU and hospital stay, and at day 28.
Definitions
AKI occurrence and severity were defined according to the Kidney Disease Improving Global Outcome (KDIGO) classification [20]. Stage 1 (KDIGO1) was considered when serum creatinine increased by at least 26 µmol/L from baseline creatinine or 1.5 to 1.9 times from the baseline creatine, or if urine output was below 0.5 mL/kg/h for 6–12 h. Stage 2 (KDIGO2) was reached if serum creatinine increased to more than twofold from baseline or if urine output was less than 0.5 mL/kg/h for at least 12 h. Stage 3 (KDIGO 3) represented the most severe form of AKI and was defined as an increase in serum creatinine to more than threefold from baseline, or upper than 354 µmol/L, or need for RRT or urine output below 0.3 mL/kg/h for at least 24 h or anuria for at least 12 h.
-
Baseline serum creatinine: for each patient, when available, pre-admission serum creatinine was considered within a time period of a minimum of 7 days and a maximum of 1 year from hospital admission. If unavailable, when serum creatinine was elevated at admission, baseline serum creatinine was extrapolated using the MDRD formula [21] assuming that baseline estimated Glomerular Filtration Rate (GFR) is 75 mL/min/1.73 m2, as suggested by guidelines [20]. Patients were considered with CKD when baseline GFR was below 60 mL/min/1.73 m2.
To describe the evolution of AKI, we collected as far as possible criteria for acute kidney disease (AKD), that was defined as a persistent AKI (stage 1 or higher of the KDIGO classification) for more than 7 days. Furthermore, at the end of the hospital stay, criteria for the composite endpoint for Major Adverse Kidney Event (MAKE) at the end of hospitalization were collected for each patient. MAKE was defined as the composite criteria of death, need for RRT or worsened kidney function (a twofold increase in serum creatinine level from baseline) at the end of hospital stay [22].
DIC diagnosis was based on the International Society on Thrombosis and Haemostasis (ISTH) score [23], and, accordingly, a score upper or equal to 5 was retained for the diagnosis of DIC. Biological tests for the diagnosis of DIC were performed at ICU admission and on a daily basis until day 7.
Nephrotoxic drugs: all the potential nephrotoxic drugs administered before ICU admission and before AKI occurrence during the ICU stay, among a predefined list, based on a previous work [24] were identified in patient’s record (see Additional file 1).
Ethical concerns
The design of the study was approved by the ethic committee of Strasbourg (Comité de Protection des Personnes N° 12/35, DC-2012-1633). Before inclusion, written informed consent was obtained from all patients. If patients were unable to provide informed consent, it was obtained from their next of kin or another surrogate decision-maker, as appropriate. Post hoc consent was obtained as soon as possible in these patients.
Statistic
Quantitative data were expressed as mean, standard deviation, median, and interquartile range (IQR) for parametric and non-parametric distributions, respectively, and were compared using Student’s t-tests or Wilcoxon rank-sum tests as appropriate. Qualitative variables were compared using χ2 or Fisher’s exact test.
To identify factors associated with AKI occurrence, severity, and evolution (MAKE criteria at the end of hospital stay), as odds ratios, logistical regression models were performed. In the first step, univariate analyses were conducted for every baseline characteristic variable, independently of each other. In the second step, multivariate models were built using variables with clinical relevance and/or with p-value < 0.2 in univariate analysis. When some covariates were strongly correlated, the most associated was kept in the multivariate model. Some continuous variables (norepinephrine and fluid administration) were transformed into qualitative variables according to clinically relevant values.
As AKI occurrence and severity in the ICU depends on mortality and ICU length of stay, we performed a survival analysis in ICU free from stage 3 AKI occurrence in patients with and without DIC (DIC diagnosis was considered as baseline) during the first 7 days. Survival without stage 3 AKI was estimated by the Kaplan–Meier method and was compared between the two groups with the use of a log-rank test.
Results
Baseline characteristics
From the 437 patients of the initial cohort, 350 patients were eligible and included in the study. Reasons for patient exclusion are presented in the flowchart (Fig. 1). Baseline characteristics are presented in Table 1.
Fig. 1.
Flowchart of the study
Table 1.
Baseline characteristics of the cohort, according to the occurrence of disseminate intravascular coagulation
| Characteristics | Total (n = 350) | Patients with DIC (n = 129) | Patients without DIC (n = 221) | p |
|---|---|---|---|---|
| Age (years), mean ± sd | 67 ± 14 | 66 ± 15 | 68 ± 13 | 0.115 |
| Med [IQR] | 69 [60–77] | 68 [57–77] | 70 [60–77] | 0.179 |
| Male sex, n (%) | 227 (64.9) | 76 (58.9) | 151 (68.3) | 0.075 |
| SAPS II (points), mean ± sd | 61 ± 19 | 64 ± 20 | 59 ± 18 | 0.011 |
| Med [IQR] | 58 [48–73] | 64 [49–78] | 56 [47–69] | 0.010 |
| SOFA (points), mean ± sd | 11 ± 3 | 13 ± 3 | 10 ± 3 | < 0.001 |
| Med [IQR] | 11 [9–13] | 13 [10–15] | 9 [8–12] | < 0.001 |
| Preexisting conditions, n (%) | ||||
| Chronic hypertension | 206 (58.9) | 67 (51.9) | 139 (62.9) | 0.044 |
| Diabetes | 103 (29.4) | 28 (21.7) | 75 (33.9) | 0.015 |
| Chronic heart failure | 23 (6.6) | 9 (7.0) | 14 (6.3) | 0.815 |
| Ischemic heart disease | 27 (7.7) | 7 (5.4) | 20 (9.0) | 0.220 |
| Chronic kidney disease | 49 (14.0) | 9 (7.0) | 40 (18.1) | 0.004 |
| Liver cirrhosis | 14 (4.0) | 11 (8.5) | 3 (1.4) | < 0.001 |
| Chronic obstructive pulmonary disease | 65 (18.6) | 16 (12.4) | 49 (22.2) | 0.023 |
| Cancer | 65 (18.6) | 18 (13.9) | 47 (21.3) | 0.090 |
| Charlson index, mean ± sd | 2.6 ± 3.0 | 2.7 ± 2.9 | 2.6 ± 2.9 | 0.847 |
| Med [IQR] | 1 [0–4] | 2 [1–4] | 2 [1–4] | 0.227 |
| Basal serum creatinine (µmol/L), mean ± sd | 83.3 ± 35.8 | 77.6 ± 24.9 | 87.5 ± 40.5 | 0.013 |
| Med [IQR] | 76 [62–93] | 75 [61–91] | 78.2 [63–95] | 0.137 |
| Back calculated, n (%) | 106 (30.3) | 47 (36.4) | 59 (26.7) | 0.056 |
| Infection, n (%) | ||||
| Source of infection | ||||
| Lung | 123 (35.1) | 41 (31.8) | 82 (37.1) | 0.314 |
| Abdominal | 53 (15.1) | 25 (19.4) | 28 (12.7) | 0.091 |
| Urinary tract | 72 (20.6) | 27 (20.9) | 45 (20.4) | 0.899 |
| Bloodstream infection | 90 (25.7) | 38 (29.5) | 52 (23.5) | 0.221 |
| Other/unknown | 90 (25.7) | 28 (21.7) | 62 (28.1) | 0.190 |
| Nosocomial infection | 31 (8.9) | 9 (7.0) | 22 (10.0) | 0.344 |
| Immunosuppression | 39 (11.1) | 13 (10.1) | 26 (11.8) | 0.628 |
| Involved bacteria | ||||
| Cocci gram positive | 122 (34.9) | 40 (31.0) | 82 (37.1) | 0.248 |
| Bacillus gram negative | 156 (44.6) | 64 (49.6) | 92 (41.6) | 0.147 |
| Other/unknown | 72 (20.6) | 25 (19.4) | 47 (21.3) | 0.673 |
| Organ-support at inclusion | ||||
| Norepinephrine, n (%) | 346 (98.9) | 126 (97.7) | 220 (99.5) | 0.112 |
| Epinephrine, n (%) | 36 (10.3) | 18 (14.0) | 18 (8.1) | 0.084 |
| Dobutamine, n (%) | 66 (18.9) | 26 (20.2) | 40 (18.1) | 0.635 |
| Norepinephrine highest dosea (µg/kg/min), mean ± sd | 1.0 ± 1.0 | 1.3 ± 1.4 | 0.8 ± 0.8 | < 0.001 |
| Med [IQR] | 0.6 [0.3–1.2] | 0.9 [0.4–1.9] | 0.5 [0.3–0.9] | < 0.001 |
| Fluid therapy before inclusion (liters), mean ± sd | 0.8 ± 1.0 | 0.9 ± 0.9 | 0.8 ± 1.0 | 0.330 |
| Med [IQR] | 0.5 [0–1.3] | 0.8 [0–1.5] | 0.4 [0–1.0] | 0.102 |
| Invasive mechanical ventilation, n (%) | 306 (87.4) | 120 (93.0) | 186 (84.2) | 0.016 |
| Mean diuresis at day 1 (mL/kg/h), mean ± sd | 0.7 ± 0.8 | 0.7 ± 0.8 | 0.8 ± 0.8 | 0.294 |
| Med [IQR] | 0.5 [0.2–1] | 0.5 [0.2–0.9] | 0.5 [0.2–1.1] | 0.283 |
Bolditalic characters were proposed to illustrate values who reached statistical significancy, i.e. p < 0.05
Data are expressed as mean ± standard deviation (sd), or median (Med) with interquartile range (IQR) for quantitative variables and as number and percentages for qualitative variables [n (%)]. The Chi-square test was used for qualitative data. Quantitative data were compared by t-test for mean comparison and Mann–Whitney test for median comparison
DIC, disseminated intravascular coagulation; SAPS II, Simplified Acute Physiologic Score II; SOFA, Sequential Organ Failure Assessment
aNorepinephrine highest dose was collected during the first 24 h after the beginning of infusion
Included patients were predominantly males, a median age of 69 years old. Patients were seriously ill with a median SAPS II of 58 (48–73) points. Cardiovascular history was the most frequent comorbidity. Baseline serum creatinine was unavailable in 106 patients (30.3%) and was extrapolated through MDRD equation calculation as planned by the study design. Infections responsible for septic shock were mainly community-acquired ones. All patients required vasopressor treatment, with norepinephrine as the first-used agent, and most of them (n = 306, 87.4%) required mechanical ventilation.
Among the 350 patients included, 129 patients developed DIC, while 221 did not. DIC occurred at day 1 in 92 patients, at day 2 for 22 patients, at day 3 for 7 and between day 4 and 7 for 8 patients. Coagulation tests at admission and at DIC diagnosis are presented in Additional file 2: Table S1. No other DIC provider than sepsis was present in patients with DIC.
Patients with DIC were more seriously ill
As shown in Table 1, although patients with DIC were less comorbid, their severity scores (SOFA and SAPSII) at ICU admission were higher. There were no differences in the source of infection, communautary or nosocomial nature of infection and microbiological findings between patients with or without DIC. As a consequence of higher severity, patients with DIC required higher doses of norepinephrine and more frequently invasive mechanical ventilation.
Among the 129 patients with DIC, 11 suffered from cirrhosis, of whom 6 met the ISTH criteria for DIC without the prothrombin time component. Among the remaining five patients, a decrease of at least 30% in platelet count over time was taken into consideration as an additional criteria [25]. Similarly, for patients treated with oral anticoagulation with vitamin K antagonists, DIC diagnosis was retained only if they met the ISTH criteria without the prothrombin time component and a positive JAAM 2016 score.
Patients with DIC had higher mortality- and AKI-rates
Patients with DIC had a higher mortality rate when compared to patients without DIC (43.3% vs. 26.2%, respectively, p < 0.01, for in-ICU mortality).
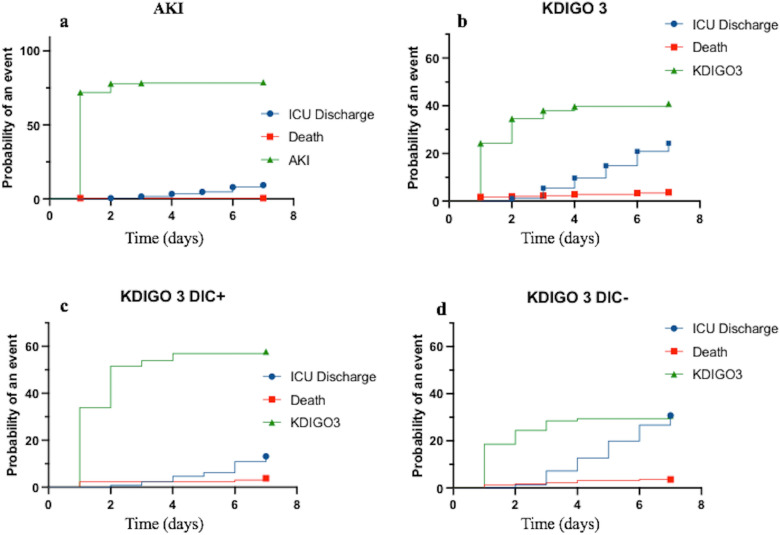
AKI occurred in 276 patients (78.9%). In 256 patients, AKI began within the first 24 h following ICU admission, and reached the higher stage at day 1 for 185 patients, at day 2 for 62 patients, at day 3 for 13 patients and between day 4 and 7 for the remaining 16 patients. AKI was more frequent in patients with DIC: 112 (86.8%) vs. 164 (74.2%) in patients without DIC (p = 0.005) (see Fig. 2 and Additional file 3: Fig. S1a). AKI and DIC occurred at the same day in 95 of the 112 patients.
Fig. 2.
Cumulative incidence functions of competing events: acute kidney injury in the ICU (a), AKI KDIGO3 in the ICU (b), ICU death and ICU discharge. b AKI KDIGO unstratified and according to DIC status (DIC [KDIGO3 DIC +] c and no DIC [KDIGO3 DIC −] d). AKI, acute kidney injury; DIC, disseminated intravascular coagulation; KDIGO, Kidney Disease Improving Global Outcome (KDIGO 3: threefold increase from creatinine baseline or creatinine > 354 µmol/L; or Renal replacement Therapy or urine output < 0.3 mL/kg/h during 24 h or anuria during more than 12 h)
Patients with DIC were more likely to develop severe AKI and fulfill MAKE criteria
Patients with DIC were more likely to develop severe AKI, reaching stage 3 of the KDIGO classification in 73/129 patients with DIC (58.1%) versus 68/221 patients without DIC (30.8%), respectively (p < 0.001) (see Fig. 2 and Additional file 3: Fig. S1b). AKI stage 3 and DIC occurred at the same day in 57 of the 73 patients.
Among patients with stage 3 AKI, 108 required RRT during their ICU stay (61/129 patients with DIC (47.3%) vs. 47/221 patients without DIC (21.3%), p < 0.001).
We found that the higher the ISTH score, the higher the proportion of patients with stage 3 AKI (p < 0.001) (Fig. 3). The association between ISTH score and other outcomes is presented in Additional file 2: Table S2.
Fig. 3.
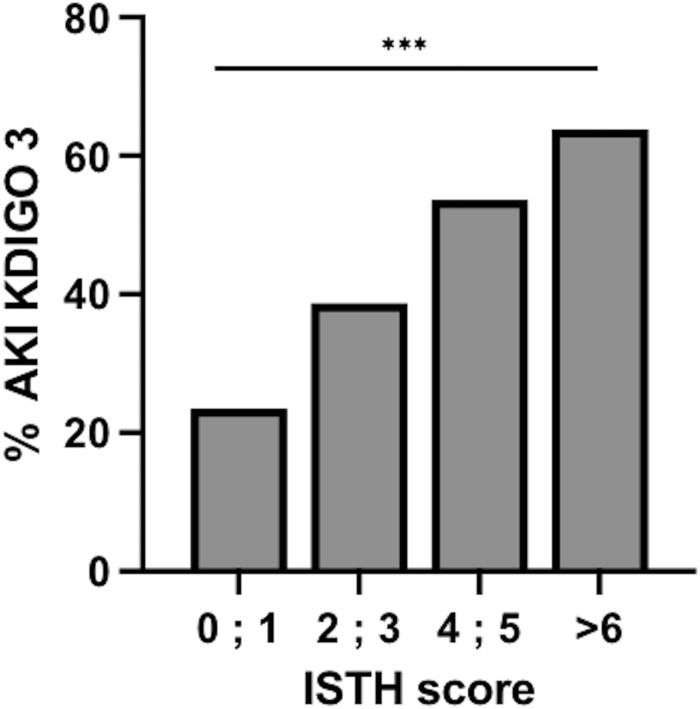
Proportion of patients with acute kidney injury stage 3 of the KDIGO classification according to the value of the ISTH score. AKI, acute kidney injury; ISTH, International Society on Thrombosis and Haemostasis; KDIGO, Kidney Disease Improving Global Outcome
The Kaplan–Meier curve analysis assessing the likelihood of developing stage 3 AKI of the KDIGO classification showed a higher risk for patients with DIC as compared to those without DIC, displaying a hazard ratio of 2.05 (1.48–2.85), p < 0.001 (log-rank test) (Fig. 4) (data were censored for deaths and leaving alive from ICU).
Fig. 4.
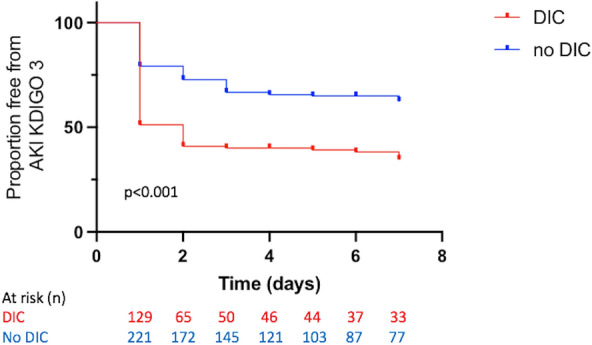
Probability of survival without acute kidney injury stage 3 of the KDIGO classification. AKI, acute kidney injury; DIC, disseminated intravascular coagulation; KDIGO, Kidney Disease Improving Global Outcome. Kaplan–Meier curves of the probability of survival without AKI reaching the stage 3 of the KDIGO classification during the first 7 days, according to the presence of DIC or not. p < 0.001 between groups (log-rank test)
AKD was assessed in 236 patients, as those who died before day 7 after admission were not eligible for this evaluation. Among patients with available renal function evaluation after day 7, 74 experienced AKD (31.4%), of whom 34 had DIC, and 40 had not DIC [34/75 (45.3%) vs. 40/161 (24.8%), p = 0.002 χ2 test]. At the end of hospital stay, 71 patients (55%) with DIC fulfilled the MAKE criteria as compared to 78 patients (35.3%) without DIC (p < 0.001).
After adjustment for cofounders, DIC remained significantly associated with AKI severity and need for renal replacement therapy
After adjustment for cofounders, DIC was still significantly associated with a higher risk of KDIGO 3 AKI, with an odds ratio (OR) of 2.74 [IC 95% (1.53–4.91), p < 0.001] (Table 2). Other factors significantly associated with KDIGO3 AKI were a history of CKD [OR 2.42 (1.03–5.68), p = 0.043], higher SAPS II [OR 1.06 (1.04–1.07), per 1 point increment p < 0.001] and age [OR 0.97 (0.95–0.99), p = 0.011]. An association between DIC and RRT requirement was also found [OR 2.83 (1.53–5.20), p < 0.001] (Table 3). When considering AKI—whatever its stage—DIC occurrence was no longer associated with AKI occurrence after multivariate analysis, nor with MAKE at the end of the hospital stay (Additional file 2: Tables S3 and 4).
Table 2.
Univariate and multivariate analysis of factors associated with acute kidney injury meeting the criteria for the stage 3 of the KDIGO classification
| Univariate analysis | Multivariate analysis | |||||
|---|---|---|---|---|---|---|
| OR | IC 95% | p | OR | IC 95% | p | |
| DIC | 3.12 | 1.99–4.91 | < 0.001 | 2.74 | 1.53–4.91 | < 0.001 |
| Age (per 1-year increment) | 1.0 | 0.99–1.02 | 0.645 | 0.97 | 0.95–0.99 | 0.011 |
| Male sex | 0.96 | 0.62–1.50 | 0.865 | – | – | – |
| SAPS II (per 1pt increment) | 1.06 | 1.05–1.08 | < 0.001 | 1.06 | 1.04–1.07 | < 0.001 |
| SOFA (per 1pt increment) | 1.44 | 1.31–1.57 | < 0.001 | – | – | – |
| Chronic hypertension | 1.80 | 1.16–2.81 | 0.009 | 1.75 | 0.95–3.20 | 0.071 |
| Diabetes | 1.56 | 0.98–2.48 | 0.059 | 1.41 | 0.77–2.60 | 0.265 |
| Chronic heart failure | 1.35 | 0.58–3.16 | 0.483 | – | – | – |
| CKD | 1.78 | 0.97–3.26 | 0.063 | 2.42 | 1.03–5.68 | 0.043 |
| Liver cirrhosis | 2.71 | 0.89–8.27 | 0.079 | 1.00 | 0.26–3.91 | 0.996 |
| Cancer | 0.68 | 0.38–1.22 | 0.194 | 0.52 | 0.25–1.11 | 0.091 |
| COPD | 0.82 | 0.47–1.42 | 0.475 | – | – | – |
| Source of infection | ||||||
| Lung | 1.15 | 0.74–1.80 | 0.532 | – | – | – |
| Abdominal | 1.77 | 0.99–3.19 | 0.056 | 1.66 | 0.80–3.43 | 0.175 |
| Urinary tract | 0.90 | 0.53–1.53 | 0.703 | – | – | – |
| Bloodstream infection | 0.79 | 0.48–1.29 | 0.349 | – | – | – |
| Nosocomial infection | 0.78 | 0.36–1.68 | 0.525 | – | – | – |
| Immunosuppression | 1.13 | 0.58–2.22 | 0.713 | – | – | – |
| Cocci gram positive | 0.78 | 0.49–1.22 | 0.269 | – | – | - |
| Bacillus gram negative | 1.49 | 0.97–2.28 | 0.071 | 1.47 | 0.86–2. 45 | 0.161 |
| Organ-support at inclusion | ||||||
| Epinephrine | 6.06 | 2.67–13.73 | < 0.001 | 1.99 | 0.71–5.55 | 0.187 |
| Dobutamine | 1.71 | 1.00–2.92 | 0.052 | 1.28 | 0.63–2.59 | 0.494 |
| Norepinephrine > 1 µg/kg/min at day 1 | 3.18 | 1.99–5.07 | < 0.001 | 1.31 | 0.72–2.39 | 0.372 |
| Fluid before inclusion > 1L | 5.57 | 1.21–17.18 | 0.025 | 4.34 | 0.82–23.1 | 0.085 |
| Invasive mechanical ventilation | 5.13 | 2.11–12.50 | < 0.001 | 2.50 | 0.86–7.27 | 0.092 |
| Nephrotoxic drugs, yes | 0.52 | 0.19–1.43 | 0.207 | 0.77 | 0.18–3.23 | 0.724 |
| Nephrotoxic drugs | 1.06 | 0.89–1.25 | 0.514 | – | – | – |
Bolditalic characters were proposed to illustrate values who reached statistical significancy, i.e. p < 0.05
CKD, chronic kidney disease; COPD, chronic obstructive pulmonary disease; DIC, disseminated intravascular coagulation; OR, odds ratio; SAPS II, Simplified Acute Physiologic Score II; SOFA, Sequential Organ Failure Assessment
Table 3.
Univariate and multivariate analysis of factors associated with renal replacement therapy requirement during the ICU stay
| Univariate analysis | Multivariate analysis | |||||
|---|---|---|---|---|---|---|
| OR | IC 95% | p | OR | IC 95% | p | |
| DIC | 3.32 | 2.07–5.33 | < 0.001 | 2.82 | 1.53–5.20 | 0.001 |
| Male sex | 0.89 | 0.55–1.42 | 0.62 | – | – | – |
| Age, (per 1-year increment) | 1.01 | 0.99–1.02 | 0.364 | 0.97 | 0.95–1 | 0.048 |
| SAPS II (per 1pt increment) | 1.06 | 1.04–1.08 | < 0.001 | 1.06 | 1.04–1.07 | < 0.001 |
| SOFA (per 1pt increment) | 1.44 | 1.31–1.58 | < 0.001 | – | – | – |
| Chronic hypertension | 2.05 | 1.26–3.32 | 0.004 | 2.16 | 1.11–4.20 | 0.023 |
| Diabetes | 1.57 | 0.97–2.56 | 0.068 | 1.36 | 0.73–2.54 | 0.337 |
| Chronic heart failure | 1.21 | 0.50–2.95 | 0.674 | – | – | – |
| CKD | 1.67 | 0.90–3.11 | 0.106 | 1.70 | 0.74–3.90 | 0.208 |
| Liver cirrhosis | 4.31 | 1.41–13.18 | 0.01 | 2.01 | 0.50–7.89 | 0.325 |
| Cancer | 0.64 | 0.33–1.21 | 0.168 | 0.55 | 0.25–1.24 | 0.15 |
| COPD | 0.99 | 0.56–1.78 | 0.986 | – | – | – |
| Source of infection | ||||||
| Lung | 0.84 | 0.52–1.36 | 0.474 | – | – | – |
| Abdominal | 1.74 | 0.95–3.18 | 0.071 | 1.58 | 0.76–3.31 | 0.221 |
| Urinary tract | 0.83 | 0.47–1.48 | 0.526 | – | – | – |
| Bloodstream infection | 0.71 | 0.41–1.21 | 0.208 | – | – | – |
| Nosocomial infection | 0.76 | 0.33–1.76 | 0.525 | – | – | – |
| Immunosuppression | 1.14 | 0.56–2.31 | 0.723 | – | – | – |
| Cocci gram positive | 0.59 | 0.36–0.97 | 0.037 | 0.53 | 0.29–0.97 | 0.04 |
| Bacillus gram negative | 1.37 | 0.87–2.16 | 0.173 | 1.15 | 0.62–1.96 | 0.747 |
| Organ-support at inclusion | ||||||
| Epinephrine | 4.77 | 2.31–9.83 | < 0.001 | 1.32 | 0.52–3.36 | 0.556 |
| Dobutamine | 1.6 | 0.92–2.79 | 0.097 | 1.12 | 0.55–2.31 | 0.75 |
| Norepinephrine > 1 µg/kg/min at day 1 | 3.75 | 2.31–6.08 | < 0.001 | 0.57 | 0.31–1.07 | 0.079 |
| Fluid before inclusion > 1L | 2.31 | 0.73–7.35 | 0.155 | 0.67 | 0.37–1.22 | 0.193 |
| Nephrotoxic drugs, yes | 0.73 | 0.26–2.07 | 0.557 | – | – | – |
| Nephrotoxic drugs | 1.09 | 0.91–1.33 | 0.336 | 1.02 | 0.80–1.30 | 0.871 |
Bolditalic characters were proposed to illustrate values who reached statistical significancy, i.e. p < 0.05
CKD, chronic kidney disease; COPD, chronic obstructive pulmonary disease; DIC, disseminated intravascular coagulation; OR, hazard ratio; SAPS II, Simplified Acute Physiologic Score II; SOFA, Sequential Organ Failure Assessment
Discussion
In this prospective cohort of septic shock patients, DIC was associated with a near threefold increased risk of developing the more severe stage of AKI (KDIGO3) and of requiring RRT, which highly suggest that DIC worsens the prognosis of septic shock patients and contributes to multiple organ failure and its severity. However, after adjustment, DIC was no longer associated with AKI or MAKE criteria.
These findings are of interest. Indeed, if DIC occurrence was previously reported to be an independent factor associated with both severity and mortality in patients with septic shock [11, 12, 26, 27], the link between AKI and DIC had been poorly investigated. In a retrospective cohort of patients with septic shock caused by intra-abdominal infection, Xu et al. [28] showed that some coagulation biomarkers (aPTT, prothrombin time, and D-dimers) on ICU admission were significantly associated with AKI after multivariable logistic regression analysis, suggesting that coagulation activation might play a role in AKI development. Another retrospective study suggested the link between DIC and AKI, as biomarkers of endothelial injury (such as soluble thrombomodulin, E-selectin, protein C, and plasminogen activator inhibitor-1) were associated with AKI occurrence [29]. In this study, DIC was associated with AKI in univariate analysis, but no adjustment was made with cofounders, and the timing of DIC and AKI is not described.
In acute and chronic renal disease, the coagulation system and coagulation protease-dependent signaling might be altered [30]. Coagulation regulators and receptors both play a pivotal role in hemostasis and non-hemostatic functions in the kidneys. It has indeed been shown that coagulation proteases are able to alter the function of renal cells via protease-activated receptors (PARs) and co-receptors, while activated protein C would have nephroprotective effects that are at least partly independent of its anticoagulant function. It is therefore not surprising that excessive coagulation activation might alter renal function.
In a monocentric retrospective study including 582 critically ill patients, overt-DIC was associated with AKI occurrence in univariate analysis, but not in multivariate analysis, and DIC was associated with higher mortality in multivariate analysis [31]. Other studies highlighted this association [14, 15, 32]; however, none of these studies provided clear information regarding: (1) the timing of AKI development and (2) AKI severity. The design of our study is therefore original regarding these two points. Indeed, we have tried to assess the early course of both AKI and DIC during septic shock. With this in mind, we have included patients who met AKI and DIC criteria at the same timeframe.
From a pathophysiological point of view, it is now admitted that AKI related to septic shock is a multifactorial disease, not only attributable to kidney hypoperfusion. AKI associated with sepsis involves multiple mechanisms, including oxidative stress, inflammation, tubular cell adaptation to injury, renal hemodynamic alterations, and microcirculation dysfunction [7, 33]. Kidney microcirculation dysfunction during sepsis is related to both an alteration in renal blood flow [34] and to endothelial injury [35, 36], particularly in peritubular capillaries [37]. As a result, endothelial biomarkers, such as soluble thrombomodulin for example, were reported to be independent predictive biomarkers for AKI [29], even if this must be tempered as soluble thrombomodulin is excreted by kidneys. Such interplay between AKI, and endothelial dysfunction reinforces the hypothesis that AKI and DIC might be related.
DIC indeed results from excessive activation of coagulation pathways associated with vascular endothelial damage, and hypofibrinolysis [10, 38], that ultimately results in disseminated microthrombi formation that impairs microcirculation. Thus, DIC represents a major contributor to the development or worsening of organ failures [10, 11]. However, histopathologic features do not support such association. Indeed, in post-mortem renal biopsies of 19 patients with septic shock, arteriolar thromboses were found only in 4 patients, without relationship with the presence of DIC or not [39].
The present study also suffers from some shortcomings: being monocentric, the generalization of the findings is unsure. The main limitation in the present study is that, despite the design of our study, DIC and AKI occurred at the same time in more than 80% of the population, and in 76% of the patients with AKI KDIGO3 and DIC.
Elsewhere, the high incidence of AKI (78.9%) limits the external validity of our study. This high incidence might, however, be explained by the severity of the included patients (median SAPS II 61 points), and by the definition of AKI cases, strictly according to the KDIGO definition, taking into account urine output criteria. Indeed, it has been reported in a large multicenter observational cohort that taken into account or not the diuresis component result in a large difference in the number of patients with sepsis-induced AKI diagnosis, with differences in general and renal evolution [40]. In addition, the epidemiology of sepsis-induced AKI is poorly reported [41]. However, in a post hoc analysis of the ProCESS trial, AKI occurred in 69% of septic shock patients during the first 7 days after admission in the ICU, closest to our findings, with lower severity (29.7% of patients with AKI KDIGO 3) [42]. This high incidence of AKI in patients with high severity scores might explain the absence of association between AKI and DIC after adjustment for cofounders, as a result of insufficient statistical power.
Unfortunately, renal evolution at day 7 was missing in 33% of the cohort, which precludes any conclusion with regard to renal evolution. To deal with this concern, we have collected criteria for MAKE classification, which was reported to be a relevant composite criterion to illustrate clinically meaningful adverse outcomes following AKI: new hemodialysis, death and persistent impaired renal function (with variable range for such definition).
The association between DIC and patients who fulfilled the MAKE criteria was not found after adjustment, but the time of this evaluation (end of hospitalization) is probably too early for a relevant evaluation of renal function evolution in patients who experienced septic shock.
Lastly, we have chosen the ISTH score [23] for DIC definition whereas other score such as the Japanese Association for Acute Medicine-DIC (JAAM-DIC) [43] were proposed to allow an earlier recognition of DIC in the setting of sepsis. If the optimal score for DIC diagnosis remain a matter of debate [44], today, the ISTH score is recommended by learned societies [45].
Conclusion
In this prospective cohort of septic shock patients, DIC was strongly associated with the risk of KDIGO3 AKI, even after adjustment for severity and other relevant factors. Of course, this study cannot establish the causality of such relationship. If DIC indeed plays a role in the pathogenesis of AKI among patients with septic shock, forthcoming studies focusing on DIC should likely incorporate AKI as a significant outcome. Similarly, research endeavors investigating AKI within the context of sepsis and its risk factors should perhaps consider DIC as a potential contributing factor, a consideration that has not been addressed so far.
Supplementary Information
Additional file 1. List of nephrotoxic drugs collected in patient's medical record.
Additional file 2. Table S1: Results of the coagulation test at inclusion and at diagnosis of disseminated intravascular coagulation. Table S2: Main outcomes of included patients, according to the value of the ISTH score. Table S3: Univariate and multivariate analysis of factors associated with Acute Kidney Injury occurrence during the ICU stay. Table S4: Univariate and multivariate analysis of factors associated with Major Adverse Kidney Events at the end of the hospital stay.
Additional file 3. Fig. S1: a Acute Kidney Injury occurrence according to the presence of disseminated intravascular coagulation. b Stages of Acute Kidney Injury (KDIGO classification), according to the presence of disseminated intravascular coagulation. AKI, acute kidney injury; DIC, disseminated intravascular coagulation; KDIGO, Kidney disease Improving global outcome (KDIGO 1: increase of 26.5 µmol/L from baseline creatinine or 1.5–1.9 fold increase from baseline or urine output < 0.5 mL/kg/h during 6–12 h; KDIGO 2, 2–2.9 fold increase creatinine from baseline or urine output < 0.5 mL/h during at least 12 h; KDIGO 3, 3 fold increase from creatinine baseline or creatinine > 354 µmol/L, or Renal replacement Therapy or urine output < 0.3 mL/kg/h during 24 h or anuria during more than 12 h).
Acknowledgements
Not applicable.
Author contributions
JH, HM, FM and JD were major contributors in writing the manuscript. SL, AM, AC, JK and JD collected data. JD and FS performed the statistical analysis. All authors read and approved the final version of the manuscript.
Funding
Not applicable.
Availability of data and materials
Datasets analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethical approval and consent to participate
The design of the study was approved by the ethic committee of Strasbourg (Comité de Protection des Personnes N° 12/35, DC-2012-1633). Before inclusion, written informed consent was obtained from all patients. If patients were unable to provide informed consent, it was obtained from their next of kin or another surrogate decision-maker, as appropriate. Post hoc consent was obtained as soon as possible in these patients.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Bagshaw SM, Lapinsky S, Dial S, Arabi Y, Dodek P, Wood G, et al. Acute kidney injury in septic shock: clinical outcomes and impact of duration of hypotension prior to initiation of antimicrobial therapy. Intens Care Med. 2009;35(5):871–881. doi: 10.1007/s00134-008-1367-2. [DOI] [PubMed] [Google Scholar]
- 2.Metnitz PGH, Krenn CG, Steltzer H, Lang T, Ploder J, Lenz K, et al. Effect of acute renal failure requiring renal replacement therapy on outcome in critically ill patients. Crit Care Med. 2002;30(9):2051–2058. doi: 10.1097/00003246-200209000-00016. [DOI] [PubMed] [Google Scholar]
- 3.Coca SG, Singanamala S, Parikh CR. Chronic kidney disease after acute kidney injury: a systematic review and meta-analysis. Kidney Int. 2012;81(5):442–448. doi: 10.1038/ki.2011.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wu VC, Wu CH, Huang TM, Wang CY, Lai CF, Shiao CC, et al. Long-term risk of coronary events after AKI. J Am Soc Nephrol. 2014;25(3):595–605. doi: 10.1681/ASN.2013060610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Uchino S, Kellum JA, Bellomo R, Doig GS, Morimatsu H, Morgera S, et al. Acute renal failure in critically ill patients: a multinational, multicenter study. JAMA. 2005;294(7):813–818. doi: 10.1001/jama.294.7.813. [DOI] [PubMed] [Google Scholar]
- 6.Bellomo R, Kellum JA, Ronco C, Wald R, Martensson J, Maiden M, et al. Acute kidney injury in sepsis. Intens Care Med. 2017;43(6):816–828. doi: 10.1007/s00134-017-4755-7. [DOI] [PubMed] [Google Scholar]
- 7.Gomez H, Ince C, De Backer D, Pickkers P, Payen D, Hotchkiss J, et al. a unified theory of sepsis-induced acute kidney injury: inflammation, microcirculatory dysfunction, bioenergetics, and the tubular cell adaptation to injury. Shock. 2014;41(1):3–11. doi: 10.1097/SHK.0000000000000052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Helms J, Iba T, Connors JM, Gando S, Levi M, Meziani F, et al. How to manage coagulopathies in critically ill patients. Intensive Care Med. 2023;49(3):273–290. doi: 10.1007/s00134-023-06980-6. [DOI] [PubMed] [Google Scholar]
- 9.da Cruz DB, Helms J, Aquino LR, Stiel L, Cougourdan L, Broussard C, et al. DNA-bound elastase of neutrophil extracellular traps degrades plasminogen, reduces plasmin formation, and decreases fibrinolysis: proof of concept in septic shock plasma. FASEB J. 2019;33(12):14270–14280. doi: 10.1096/fj.201901363RRR. [DOI] [PubMed] [Google Scholar]
- 10.Delabranche X, Helms J, Meziani F. Immunohaemostasis: a new view on haemostasis during sepsis. Ann Intens Care. 2017;7(1):117. doi: 10.1186/s13613-017-0339-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hartemink KJ, Hack CE, Groeneveld ABJ. Relation between coagulation/fibrinolysis and lactate in the course of human septic shock. J Clin Pathol. 2010;63(11):1021–1026. doi: 10.1136/jcp.2010.079707. [DOI] [PubMed] [Google Scholar]
- 12.Gando S, Shiraishi A, Yamakawa K, Ogura H, Saitoh D, Fujishima S, et al. Role of disseminated intravascular coagulation in severe sepsis. Thromb Res. 2019;178:182–188. doi: 10.1016/j.thromres.2019.04.025. [DOI] [PubMed] [Google Scholar]
- 13.Okabayashi K, Wada H, Ohta S, Shiku H, Nobori T, Maruyama K. Hemostatic markers and the sepsis-related organ failure assessment score in patients with disseminated intravascular coagulation in an intensive care unit. Am J Hematol. 2004;76(3):225–229. doi: 10.1002/ajh.20089. [DOI] [PubMed] [Google Scholar]
- 14.Delabranche X, Quenot JP, Lavigne T, Mercier E, François B, Severac F, et al. Early detection of disseminated intravascular coagulation during septic shock: a multicenter prospective study. Crit Care Med. 2016;44(10):e930–939. doi: 10.1097/CCM.0000000000001836. [DOI] [PubMed] [Google Scholar]
- 15.Dhainaut JF, Yan SB, Joyce DE, Pettila V, Basson B, Brandt JT, et al. Treatment effects of drotrecogin alfa (activated) in patients with severe sepsis with or without overt disseminated intravascular coagulation1. J Thromb Haemost. 2004;2(11):1924–1933. doi: 10.1111/j.1538-7836.2004.00955.x. [DOI] [PubMed] [Google Scholar]
- 16.KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney International Supplements. 2013 Jan;3(1):1–150. [DOI] [PubMed]
- 17.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 18.Le Gall JR, Lemeshow S, Saulnier F. A new simplified acute physiology score (SAPS II) based on a European/North American multicenter study. JAMA. 1993;270(24):2957–2963. doi: 10.1001/jama.1993.03510240069035. [DOI] [PubMed] [Google Scholar]
- 19.Vincent JL, Moreno R, Takala J, Willatts S, De Mendonça A, Bruining H, et al. The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. On behalf of the Working Group on Sepsis-Related Problems of the European Society of Intensive Care Medicine. Intensive Care Med. 1996;22(7):707–10. [DOI] [PubMed]
- 20.Ad-hoc working group of ERBP, Fliser D, Laville M, Covic A, Fouque D, Vanholder R, et al. A European Renal Best Practice (ERBP) position statement on the Kidney Disease Improving Global Outcomes (KDIGO) clinical practice guidelines on acute kidney injury: part 1: definitions, conservative management and contrast-induced nephropathy. Nephrol Dial Transpl. 2012;27(12):4263–72. [DOI] [PMC free article] [PubMed]
- 21.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, Feldman HI, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Palevsky PM, Molitoris BA, Okusa MD, Levin A, Waikar SS, Wald R, et al. Design of clinical trials in acute kidney injury: report from an NIDDK workshop on trial methodology. CJASN. 2012;7(5):844–850. doi: 10.2215/CJN.12791211. [DOI] [PubMed] [Google Scholar]
- 23.Taylor FB, Toh CH, Hoots WK, Wada H, Levi M, Scientific Subcommittee on Disseminated Intravascular Coagulation (DIC) of the International Society on Thrombosis and Haemostasis (ISTH). Towards definition, clinical and laboratory criteria, and a scoring system for disseminated intravascular coagulation. Thromb Haemost. 2001;86(5):1327–30. [PubMed]
- 24.Ehrmann S, Helms J, Joret A, Martin-Lefevre L, Quenot JP, Herbrecht JE, et al. Nephrotoxic drug burden among 1001 critically ill patients: impact on acute kidney injury. Ann Intens Care. 2019;9(1):106. doi: 10.1186/s13613-019-0580-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wada H, Takahashi H, Uchiyama T, Eguchi Y, Okamoto K, Kawasugi K, et al. The approval of revised diagnostic criteria for DIC from the Japanese Society on Thrombosis and Hemostasis. Thromb J. 2017;15:17. doi: 10.1186/s12959-017-0142-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vallet B. Bench-to-bedside review: endothelial cell dysfunction in severe sepsis: a role in organ dysfunction? Crit Care. 2003;7(2):130–138. doi: 10.1186/cc1864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fourrier F, Chopin C, Goudemand J, Hendrycx S, Caron C, Rime A, et al. Septic shock, multiple organ failure, and disseminated intravascular coagulation. Compared patterns of antithrombin III, protein C, and protein S deficiencies. Chest. 1992;101(3):816–23. [DOI] [PubMed]
- 28.Xu Z, Cheng B, Fu S, Liu X, Xie G, Li Z, et al. Coagulative biomarkers on admission to the ICU predict acute kidney injury and mortality in patients with septic shock caused by intra-abdominal infection. Infect Drug Resist. 2019;12:2755–2764. doi: 10.2147/IDR.S218592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Katayama S, Nunomiya S, Koyama K, Wada M, Koinuma T, Goto Y, et al. Markers of acute kidney injury in patients with sepsis: the role of soluble thrombomodulin. Crit Care. 2017;21(1):229. doi: 10.1186/s13054-017-1815-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Madhusudhan T, Kerlin BA, Isermann B. The emerging role of coagulation proteases in kidney disease. Nat Rev Nephrol. 2016;12(2):94–109. doi: 10.1038/nrneph.2015.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhi DY, Lin J, Zhuang HZ, Dong L, Ji XJ, Guo DC, et al. Acute kidney injury in critically ill patients with sepsis: clinical characteristics and outcomes. J Invest Surg. 2019;32(8):689–696. doi: 10.1080/08941939.2018.1453891. [DOI] [PubMed] [Google Scholar]
- 32.Delabranche X, Boisramé-Helms J, Asfar P, Berger A, Mootien Y, Lavigne T, et al. Microparticles are new biomarkers of septic shock-induced disseminated intravascular coagulopathy. Intens Care Med. 2013;39(10):1695–1703. doi: 10.1007/s00134-013-2993-x. [DOI] [PubMed] [Google Scholar]
- 33.De Vriese AS. Prevention and treatment of acute renal failure in sepsis. J Am Soc Nephrol. 2003;14(3):792–805. doi: 10.1097/01.ASN.0000055652.37763.F7. [DOI] [PubMed] [Google Scholar]
- 34.Tiwari MM, Brock RW, Megyesi JK, Kaushal GP, Mayeux PR. Disruption of renal peritubular blood flow in lipopolysaccharide-induced renal failure: role of nitric oxide and caspases. Am J Physiol Renal Physiol. 2005;289(6):F1324–1332. doi: 10.1152/ajprenal.00124.2005. [DOI] [PubMed] [Google Scholar]
- 35.Umbro I, Gentile G, Tinti F, Muiesan P, Mitterhofer AP. Recent advances in pathophysiology and biomarkers of sepsis-induced acute kidney injury. J Infect. 2016;72(2):131–142. doi: 10.1016/j.jinf.2015.11.008. [DOI] [PubMed] [Google Scholar]
- 36.Schrier RW, Wang W. Acute renal failure and sepsis. N Engl J Med. 2004;351(2):159–169. doi: 10.1056/NEJMra032401. [DOI] [PubMed] [Google Scholar]
- 37.Wu L, Tiwari MM, Messer KJ, Holthoff JH, Gokden N, Brock RW, et al. Peritubular capillary dysfunction and renal tubular epithelial cell stress following lipopolysaccharide administration in mice. Am J Physiol Renal Physiol. 2007;292(1):F261–268. doi: 10.1152/ajprenal.00263.2006. [DOI] [PubMed] [Google Scholar]
- 38.Iba T, Levi M, Thachil J, Levy JH. Disseminated intravascular coagulation: the past, present, and future considerations. Semin Thromb Hemost. 2022;48(8):978–987. doi: 10.1055/s-0042-1756300. [DOI] [PubMed] [Google Scholar]
- 39.Lerolle N, Nochy D, Guérot E, Bruneval P, Fagon JY, Diehl JL, et al. Histopathology of septic shock induced acute kidney injury: apoptosis and leukocytic infiltration. Intens Care Med. 2010;36(3):471–478. doi: 10.1007/s00134-009-1723-x. [DOI] [PubMed] [Google Scholar]
- 40.White KC, Serpa-Neto A, Hurford R, Clement P, Laupland KB, See E, et al. Sepsis-associated acute kidney injury in the intensive care unit: incidence, patient characteristics, timing, trajectory, treatment, and associated outcomes. A multicenter, observational study. Intensive Care Med [Internet]. 2023 July 11 [cited 2023 Aug 2]; Available from: 10.1007/s00134-023-07138-0. [DOI] [PMC free article] [PubMed]
- 41.Peerapornratana S, Manrique-Caballero CL, Gómez H, Kellum JA. Acute kidney injury from sepsis: current concepts, epidemiology, pathophysiology, prevention and treatment. Kidney Int. 2019;96(5):1083–1099. doi: 10.1016/j.kint.2019.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kellum JA, Chawla LS, Keener C, Singbartl K, Palevsky PM, Pike FL, et al. The effects of alternative resuscitation strategies on acute kidney injury in patients with septic Shock. Am J Respir Crit Care Med. 2016;193(3):281–287. doi: 10.1164/rccm.201505-0995OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Iba T, Di Nisio M, Thachil J, Wada H, Asakura H, Sato K, et al. Revision of the Japanese Association for Acute Medicine (JAAM) disseminated intravascular coagulation (DIC) diagnostic criteria using antithrombin activity. Crit Care. 2016;20(1):287. doi: 10.1186/s13054-016-1468-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Iba T, Umemura Y, Watanabe E, Wada T, Hayashida K, Kushimoto S, et al. Diagnosis of sepsis‐induced disseminated intravascular coagulation and coagulopathy. Acute Med Surg. 2019 Apr;ams2.411. [DOI] [PMC free article] [PubMed]
- 45.Iba T, Levy JH, Yamakawa K, Thachil J, Warkentin TE, Levi M. Proposal of a two-step process for the diagnosis of sepsis-induced disseminated intravascular coagulation. J Thromb Haemost. 2019;17(8):1265–1268. doi: 10.1111/jth.14482. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1. List of nephrotoxic drugs collected in patient's medical record.
Additional file 2. Table S1: Results of the coagulation test at inclusion and at diagnosis of disseminated intravascular coagulation. Table S2: Main outcomes of included patients, according to the value of the ISTH score. Table S3: Univariate and multivariate analysis of factors associated with Acute Kidney Injury occurrence during the ICU stay. Table S4: Univariate and multivariate analysis of factors associated with Major Adverse Kidney Events at the end of the hospital stay.
Additional file 3. Fig. S1: a Acute Kidney Injury occurrence according to the presence of disseminated intravascular coagulation. b Stages of Acute Kidney Injury (KDIGO classification), according to the presence of disseminated intravascular coagulation. AKI, acute kidney injury; DIC, disseminated intravascular coagulation; KDIGO, Kidney disease Improving global outcome (KDIGO 1: increase of 26.5 µmol/L from baseline creatinine or 1.5–1.9 fold increase from baseline or urine output < 0.5 mL/kg/h during 6–12 h; KDIGO 2, 2–2.9 fold increase creatinine from baseline or urine output < 0.5 mL/h during at least 12 h; KDIGO 3, 3 fold increase from creatinine baseline or creatinine > 354 µmol/L, or Renal replacement Therapy or urine output < 0.3 mL/kg/h during 24 h or anuria during more than 12 h).
Data Availability Statement
Datasets analyzed during the current study are available from the corresponding author on reasonable request.