Abstract
Extensive literature has explored the beneficial effects of music in age-related cognitive disorders (ACD), but limited knowledge exists regarding its impact on gene expression. We analyzed transcriptomes of ACD patients and healthy controls, pre-post a music session (n = 60), and main genes/pathways were compared to those dysregulated in mild cognitive impairment (MCI) and Alzheimer’s disease (AD) as revealed by a multi-cohort study (n = 1269 MCI/AD and controls). Music was associated with 2.3 times more whole-genome gene expression, particularly on neurodegeneration-related genes, in ACD than in controls. Co-expressed gene-modules and pathways analysis demonstrated that music impacted autophagy, vesicle and endosome organization, biological processes commonly dysregulated in MCI/AD. Notably, the data indicated a strong negative correlation between musically-modified genes/pathways in ACD and those dysregulated in MCI/AD. These findings highlight the compensatory effect of music on genes/biological processes affected in MCI/AD, providing insights into the molecular mechanisms underlying the benefits of music on these disorders.
Subject terms: Gene expression, Sequencing
Introduction
What is the genuine impact of musical stimuli on human beings? Music, as an artistic manifestation, creatively blends sounds and silences to craft compositions encompassing elements of melody, timbre, pitch, and rhythm. It serves as a means of human expression and aesthetic communication, offering a profound impact on the emotional, cognitive, and physiological aspects of individuals’ experiences. In the realm of physics, sound is however, a vibration (mechanical disturbance) that propagates through the air or other media as an acoustic wave. The multifaceted influence of music on brain plasticity, autobiographical memory, and emotions represents a complex phenomenon that requires comprehensive scientific exploration. While numerous studies have been conducted in the field of neuroscience and cognitive sciences1, there is still limited understanding of the underlying biochemical changes responsible for the observed effects. To bridge this gap, investigating genes and their expression under the stimulus of music constitutes an innovative approach to understanding the molecular mechanisms that underpin its beneficial effects on health. Despite the significant advancements in omics sciences over the past two decades, only four discrete attempts have been made to analyze the impact of music on transcriptomes2. Kanduri et al.3 observed that exposure to 20 min of classical music led to the differential expression of 45 genes in musically experienced participants; however, no significant results were detected in musically inexperienced participants4; they also identified the involvement of GATA2, a gene previously linked to musical aptitude5. The same research group further examined the transcriptomic changes occurring in professional musicians by analyzing their pre- and post-performance transcriptomes following a 2-h concert6; that study revealed up-regulated pathways related to dopaminergic neurotransmission, motor behavior, neuronal plasticity, and neurocognitive functions (e.g., learning and memory)6. Two further studies7,8 identified microRNAs that were up- and down-regulated of both listeners and professional musicians, building upon previous findings3,6. Navarro et al.1 used publicly available transcriptomic data from Alzheimer’s disease (AD) patients cross-compared to music-related genes and found a notable commonality of these genes with those altered in AD patients. None of all these studies analyzed the direct impact of music in age-related cognitive disorder (ACD) patients.
The aforementioned studies shed light on the intricate relationship between music and gene expression3–6. However, further empirical research is needed to gain a comprehensive understanding of the specific molecular mechanisms underlying the association between music and gene expression2. The current investigation aligns with the concept of musical sensogenomics2 and serves as the initial effort to analyze the impact of music on the transcriptomes of individuals with ACD. In contrast to previous attempts, this study has been meticulously designed to replicate an ecological environment, that is, resembling the natural or real-life conditions in which music is typically experienced by individuals, thereby minimizing the potential influence of confounding factors2.
Methods
Participants, RNA isolation and RNA-Seq analysis
Under the frame of the Sensogenomics project (https://sensogenomics.com) and for the purpose of the present project, an experimental concert of classical music with a duration of 50 min was organized on June 14, 2022, in the ‘Auditorio de Galicia’ located in the city of Santiago of Compostela (Galicia, Spain); see details in Supplementary Note. The audience included, among others, patients diagnosed with mild cognitive impairment (MCI) and dementia (henceforth referred to as the age-related cognitive disorder [ACD] cohort), and a healthy control cohort; Supplementary Note.
We collected capillary blood samples using a procedure of capillary puncture before (Time Point 1 or TP1) and after (Time Point 2 or TP2) exposure to music (Fig. 1), including 16 ACD patients (aged 64–93, mean 81.8), and 14 healthy donors (aged 18–74; mean 48.2); Table 1. Sample collection was adapted to administratively accessible population and procedures. RNA was isolated using the PAXgene blood miRNA extraction kit (Qiagen) adapting buffer volumes to the amount of capillary blood collected (100–200 μl). Sequencing was performed using a NovaSeq 6000 System (100 paired end; ~ 70 M reads/ sample); Supplementary Note.
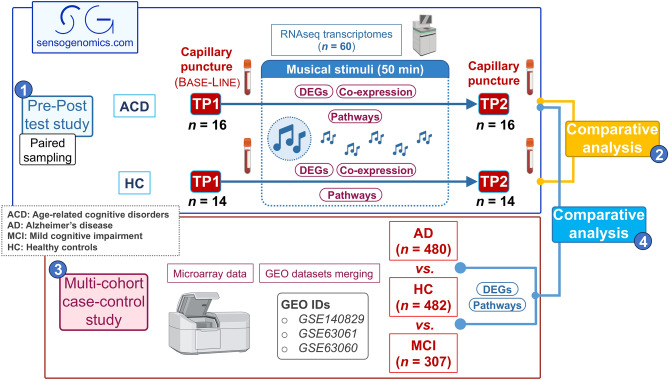
Figure 1.
Scheme showing the overall experimental and analytical design. Numbers in blue circles indicate the four different blocks of analysis carried out in the present study: (1) Pre–Post test study comparing transcriptomes before and after listening to music (same subjects) from ACD patients and healthy controls separately; (2) comparative analysis between transcriptomic response to music in ACD and healthy donors; (3) multi-cohort case–control study to detect MCI/AD-related genes and pathways; and (4) comparative analysis to study the impact of the musical stimuli on the expression of genes and pathways altered in MCI/AD patients.
Table 1.
Demographic characteristics of donors.
| Healthy controls | ACD patients | |
|---|---|---|
| Gender | ||
| Female | 12 (85.7%) | 10 (62.5%) |
| Age | ||
| Mean (SD) | 48.2 (18.0) | 81.8 (9.2) |
| Range | 18–74 | 64–93 |
| Disease condition | ||
| MCI | – | 8 (50.0%) |
| AD | – | 2 (12.5%) |
| Non-AD dementia | – | 6 (37.5%) |
Statistical analysis
DESeq29 R package was used to normalize counts and carry out the differential expression (DE) analysis after removing lowly expressed genes. We first carried out a pre-post study analysis by comparing samples collected before the musical stimuli (baseline; TP1) to samples collected after the musical stimuli (TP2) from the same healthy controls and ACD patients. To isolate transcriptomic changes exclusively related to the musical stimuli, we used a paired-samples design accounting for patient-to-patient differences with respect to TP1. For the Principal Component Analysis (PCA), we defined the transcriptome displacement (TD) score to quantitatively measure the ‘global displacement of transcriptomes’ from TP1 to TP2 as reflected in the Euclidian space of the plot. TD is the mean of the absolute values of the differences between individual i projections from TP1 and TP2 coordinates on the j different principal components (e.g., PC1 and PC2); TD is computed for the two cohorts, e.g., in ACD patients: TDACD,i,j = mean (|TP1ACD,i,j–TP2ACD,i,j|). We additionally estimated the specific weight of the main genes driving the variability in PC1 (top and bottom 10% of the loading range in PC1) using PCAtools10.
In a different comparative analysis, we evaluated the effect of listening to music in ACD patients with differentially expressed genes (DEGs) and pathways (DEPs) that are altered in MCI and AD patients when these are compared to controls. For this purpose, data from three independent microarray datasets analyzing MCI and AD patients and healthy controls were downloaded from Gene Expression Omnibus (GEO) accession numbers GSE140829, GSE63061 and GSE63060 (case–control study). By using this multi-cohort strategy, we aimed at having a statistically robust age-matched group of AD/MCI patients and controls to contrast them with our findings. After an initial quality control of each dataset separately, and the removal of outliers, the whole multi-cohort dataset was composed of gene expression data from 307 MCI patients (mean age = 75.1 [SD = 7.2]), 480 AD patients (mean age = 75.1 [SD = 7.1]) and 482 age-matched healthy controls (mean age = 73.8 [SD = 6.3]) (Fig. 1). Processing and merging of the data as well as the case–control DE analysis between AD and controls was carried out as explained elsewhere1. STRING database11 was used to build the Protein–Protein Interaction (PPI) network of the shared DEGs obtained from AD versus controls (multi-cohort study), and from TP2 versus TP1 in ACD patients (Pre/Post test study). Visualization and pathways functional enrichment of the PPI was carried out with Cytoscape software12.
Biological pathways were inferred directly from gene expression data using Gene Set Variation Analysis (GSVA) R package13 and Quantitative Set Analysis for Gene Expression (QuSAGE) R package14; see details in Supplementary Note. In QuSAGE, to explore the most differentially activated pathways between ACD and healthy donors in response to music, we consider conservative filters of pathway activity (|PA| in ACD +|PA| in controls > 0.05) and statistical significance (P-value < 0.05). Heatmaps and rain cloud plots were built using ComplexHeatmap15 and Raincloudplots16, respectively. The Wilcoxon test was used to assess statistical significance between patient groups, and the Spearman test (ρ representing the Spearman’s coefficient) was used to compute correlation values. We investigated clusters of co-expressed genes potentially correlated to the musical stimuli in both the ACD and control groups, separately. Gene expression data normalized and corrected for patient-to-patient differences was used to build a signed weighted correlation network with the Weighted Gene Co-expression Network Analysis (WGCNA) R package17. Only genes that showed the most variant expression values between samples (the top 75% with the highest variance) were included in the analysis. See Supplementary Note for more information on co-expression analysis.
Adjustment for multiple test was carried out using the FDR method by Benjamini-Hochberg18 (henceforth ‘FDR’ refers to adjusted P-value). All statistical analyses were performed using the statistical software R v. 4.2.219.
Ethics approval and consent to participate
Written informed consent was obtained from all the participants in the present study. The Ethics Committee of Xunta de Galicia approved the present project (Registration code: 2020/021), and the study was conducted in accordance with guidelines of the Helsinki Declaration.
Results
Global impact of music on the human transcriptome
We first aimed at quantifying the global effect of music on the transcriptomes of the two groups of donors separately. ACD patients exposed to music showed 2.3 times more DEGs (n = 2605) than controls (n = 1148); Table 2. Moreover, while the proportion up-regulated/down-regulated DEGs was balanced in ACD patients (1315/1290 = 1.02), it was significantly lower in controls (528/620 = 0.85). This differential pattern of gene expression is very clear in a density plot of log2 fold-change (log2FC) values of the DEGs in both groups (Fig. 2A): the median in ACD patients is displaced towards positive log2FC values (0.10) compared to the median in controls (− 0.11). Among the DEGs, the proportion of protein coding genes was higher in ACD patients than in controls (1.89 in ACD patients vs. 1.24 in controls); Table 2.
Table 2.
Biotypes of the DEGs observed in the control and the ACD patient cohorts. Biotypes definitions are available at https://ensembl.org/info/genome/genebuild/biotypes.html.
| Biotype | Controls | ACD patients | ||||
|---|---|---|---|---|---|---|
| n | nUR | nDR | n | nUR | nDR | |
| IG C gene | 2 | 2 | – | – | – | – |
| IG V gene | 1 | – | 1 | 5 | 1 | 4 |
| IG V pseudogene | 1 | 1 | – | – | – | – |
| lncRNA | 140 | 81 | 59 | 243 | 60 | 183 |
| miRNA | 1 | 1 | – | – | – | – |
| Misc RNA | 3 | 2 | 1 | 9 | – | 9 |
| Mt rRNA | 1 | – | 1 | – | – | – |
| Processed pseudogene | 37 | 21 | 16 | 52 | 12 | 40 |
| Protein coding | 921 | 392 | 529 | 2,192 | 1,204 | 988 |
| rRNA pseudogene | – | – | – | 2 | – | 2 |
| snoRNA | 1 | 1 | – | – | – | – |
| snRNA | 4 | 1 | 3 | 3 | 1 | 2 |
| TEC | 14 | 9 | 5 | 32 | 1 | 31 |
| TR C gene | – | – | – | 2 | 2 | – |
| TR J gene | 1 | 1 | – | 2 | 2 | – |
| TR V gene | – | – | – | 12 | 12 | – |
| Transcribed processed pseudogene | 2 | 1 | 1 | 11 | 3 | 8 |
| Transcribed unitary pseudogene | 3 | 3 | – | 4 | 2 | 2 |
| Transcribed unprocessed pseudogene | 11 | 9 | 2 | 20 | 12 | 8 |
| Translated processed pseudogene | – | – | – | 1 | 1 | – |
| Unitary pseudogene | – | – | – | 2 | – | 2 |
| Unprocessed pseudogene | 4 | 3 | 1 | 9 | 1 | 8 |
| No data | 1 | – | 1 | 4 | 1 | 3 |
| Total | 1,148 | 528 | 620 | 2,605 | 1,315 | 1,290 |
UR Up-regulated; DR Down-regulated.
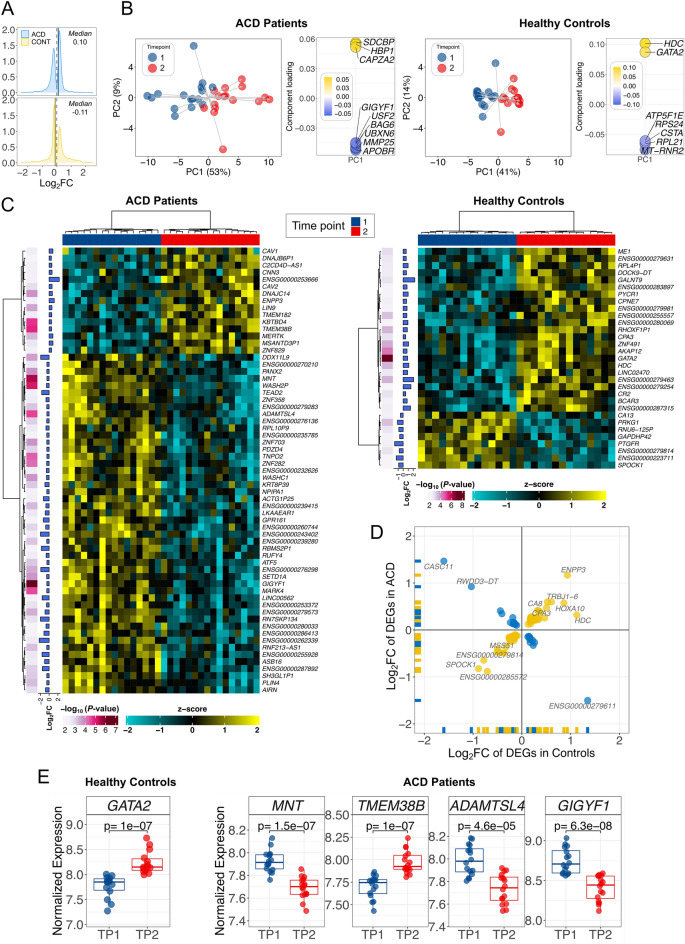
Figure 2.
Global transcriptome patterns and differential expression analysis in ACD and healthy controls. (A) Density plots of log2FC; (B) Principal Component Analysis (left) and the genes that play the main role in the topology of the PC1 (right). (C) Heatmaps of the most remarkable DEGs (P-values < 0.005 and |log2FC|> 0.5) in ACD patients and healthy controls when contrasting the two time points. (D) Correlation of DEGs in the two cohorts. (E) DEGs showing the most extreme and significant differences in ACD patients and controls.
PCA (Fig. 2B) showed the displacement of transcriptomes in TP2 with respect to TP1, revealing also the remarkable differences existing between ACD donors and healthy controls. The main pattern emerging from these plots is the clear differentiation existing between time points; especially marked in the first principal component (PC1; accounting for 53% and 41% of the variation in ACD patients and controls, respectively). The genes GATA2 (in controls) and GIGYF1 (in ACD patients) showed the strongest contribution to PC1, suggesting a key role in the observed differentiation between the groups at TP2. (Fig. 2B). The displacement of individual transcriptomes was more pronounced in ACD patients than in controls (PC1: TDACD;1 = 7.5; TDCO;1 = 4.6; PC2: TDACD;2 = 2.5 [9% of the variation]; TDCO;2 = 1.8 [14%]). In good agreement with the PCA, the heatmap representations of DEGs (P-values < 0.005 and |log2FC|> 0.5) show the main differentiation in both cohorts comes from TP1 vs. TP2 (Fig. 2C). Heatmap representations of DEGs confirmed that the main differentiation between ACD patients and controls occurred between TP1 and TP2 (Supplementary Figure S1). A comparison of the common genes that are DEGs in the two cohorts, indicates that most of them (59/84; 70%) show a positively correlated expression pattern (ρ = 0.95, P-value = 2.2 × 10–16), and only a few genes (25/84; 30%) appear to be negatively correlated (ρ = 0.98, P-value = 6.5 × 10–7); therefore, resulting in a positive overall correlation value (ρ = 0.5, P-value = 2.2 × 10–6); Fig. 2D.
While there is only one gene surpassing the multiple test threshold of significance in healthy controls, namely, GATA2 (up-regulated in TP2; P-value = 1.0 × 10–7), there are 328 genes surpassing the multiple test threshold in ACD cases. Among the DEGs that show the most extreme and significant expression changes before and after musical stimuli in ACD patients are the down-regulated genes GIGYF1 (P-value = 6.3 × 10–8), and MNT (P-value = 1.5 × 10–7), ADAMTSL4 (P-value = 4.6 × 10–5) and the up-regulated gene TMEM38B (P-value = 1.0 × 10–7) (Fig. 2E). A full list of significant DEGs is provided in Supplementary Table S1.
Music effect on key neurological-related pathways
GSVA was carried out to better understand the main biological pathways representing the differences observed between the expression of genes in patients and controls when exposed to music. GSVA yielded 556 DEPs in ACD patients, and 512 DEPs in healthy controls, 49 and 38 of them were related to neurobiological processes in ACD patients and controls, respectively (Supplementary Table S2). Interestingly, we found that music impacts key pathways so strongly that the patterns generated allow to perfectly discriminate transcriptome profiles by their time points (see heatmaps in Supplementary Figure S2). Most of the statistically significant DEPs involved in neuro-biological processes are exclusive to one of the two cohorts analyzed (see details in Supplementary Table S2; Supplementary Figure S2).
Remarkably, the top DEPs observed in the music-stimulated ACD cohort are closely related to pathways that are typically dysregulated in these neurodegenerative disorders. This observation is particularly noteworthy if we bear in mind that the DEPs were inferred using a paired-sampling design, which essentially measures the displacement of the transcriptome from TP1 to TP2. Among these pathways are the over-activated “regulation of amyloid beta clearance” (log2FC = 0.24; FDR = 0.0095), “L glutamate import across plasma membrane” (log2FC = 0.43; FDR = 6.1 × 10–7), and the “sphingomyelin biosynthetic process” (log2FC = 0.33; FDR = 0.0099); but also the under-activated “positive regulation of glutamate secretion” (log2FC = − 0.34; FDR = 0.004) (Supplementary Figure S2. In addition, we have observed that the pathway “dopamine biosynthetic process” was also activated by the musical stimuli in ACD donors (log2FC = 0.41; FDR = 0.0014).
In controls, there are a few DEPs that are closely related to neurological processes and neurotransmission, such as the down-regulated “negative regulation of oxidative stress induced neuron death” (log2FC = − 0.33; FDR = 0.0001), “neuron death in response to oxidative stress” (log2FC = − 0.24; FDR = 0.0033), and the up-regulated “regulation of neurotransmitter transport” (log2FC = − 0.16; FDR = 0.0081) (Supplementary Figure S2). Nevertheless, it is also noticeable that the activation of the pathway “L glutamate import across plasma membrane”, observed in ACD patients in response to music, stands in contrast with the under-activation observed in healthy controls (log2FC = − 0.25; FDR = 0.0045); Supplementary Figure S3; Supplementary Table S2.
QuSAGE allows to evaluate the pathways showing the most extreme differences in activation (pathway activation; PA) between patients and controls following the musical stimuli; Supplementary Table S2 and Supplementary Figure S4A. As in the case of GSVA analysis, the observed measurements from this paired-sampling QuSAGE analysis (TP1 vs. TP2), must be attributed primarily to the action of the musical stimuli impacting the phenotype condition (ACD and healthy controls).
In good agreement with GSVA, the pathway that shows the highest PA in ACD patients is “negative regulation of amyloid beta clearance”, while it is virtually inactive in healthy controls (PAACD = 0.14; PAHC = 0.01; P-value = 0.0032; Supplementary Figure S4B). Another pathway showing major differentiation between these groups is “regulation of L glutamate import across plasma membrane”, being the second most activated in ACD patients (PAACD = 0.14; PAHC = − 0.06; P-value = 0.0025); its related pathway “L-glutamate import across plasma membrane” shows even a more statistically significant difference between ACD patients and controls (PAACD = 0.09; PAHC = − 0.05; P-value = 0.0016), Supplementary Table S2. QuSAGE also reveals important differences in the impact of the musical stimuli between ACD patients and controls in dopamine related pathways: the “dopamine biosynthetic process” is significantly more activated in ACD than in controls (PAACD = 0.083; PAHC = − 0.01; P-value = 0.027), the same applies to the “positive regulation of dopamine receptor signaling pathway” (PAACD = 0.12; PAHC = − 0.03; P-value = 0.039); Supplementary Table S2. Also interesting is the recurrent presence of pathways related to sphingolipids metabolism: “cellular sphingolipid homeostasis” (PAACD = − 0.06; PAHC = 0.04; P-value = 0.005), “regulation of sphingolipid biosynthetic process” (PAACD = − 0.02; PAHC = 0.07; P-value = 0.005), “positive regulation of sphingolipid biosynthetic process” (PAACD = − 0.03; PAHC = 0.13; P-value = 0.0076), “sphingomyelin biosynthetic process” (PAACD = 0.08; PAHC = − 0.02; P-value = 0.0098), “sphingomyelin translocation” (PAACD = − 0.11; PAHC = 0.04; P-value = 0.013), and sphingomyelin catabolic process (PAACD = − 0.04; PAHC = 0.07; P-value = 0.042); Supplementary Table S2.
Other notable neurology-related pathways showing important differences in expression between patients and controls are: “negative regulation of oxidative stress induced neuron death” (PAACD = 0.05; PAHC = − 0.05; being the most statistically significant value, namely, P-value = 0.0001), “trans synaptic signaling modulating synaptic transmission” (PAACD = 0.07; PAHC = − 0.04; P-value = 0.0063), “neuronal action potential propagation” (PAACD = − 0.009; PAHC = 0.04; P-value = 0095), and “neuron death in response to oxidative stress” (PAACD = 0.03; PAHC = − 0.03; P-value = 0.002).
Co-expression modules driven by music modulate autophagy in ACDs
After filtering out genes with lower variability, a co-expression network analysis was conducted using the ACD and the control datasets (4283 and 4250 genes, respectively).
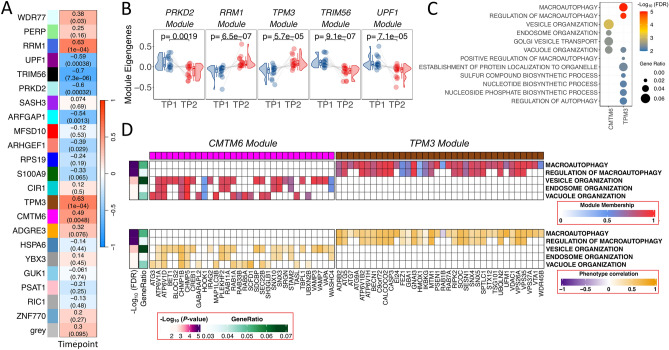
Interestingly, the WGCNA analysis revealed 22 clusters of co-expressed genes from ACD gene expression data, with 9 of them significantly correlated with the musical stimuli (Fig. 3A; Supplementary Figure S5A; Supplementary Figure S5B). Multiple test correction was applied (FDR < 0.05), resulting in 7 statistically significant modules (Supplementary Table S3), 3 of them positively correlated (RRM1 [royalblue], TPM3 [saddlebrown] and CMTM6 [magenta]) and 4 negatively correlated (TRIM56 [black], UPF1 [darkmagenta], PRKD2 [paleturquoise] and ARFGAP1 [cyan]) with the music stimulus. This global over-activation or under-activation in TP2 compared to TP1 is also notable when representing eigengene values for individual samples; the results are consistent with the correlation values, with modules PRKD2, RRM1, TPM3, TRIM56 and UPF1 exhibiting the most significant differences in individual expression patterns (Fig. 3B). The TRIM56 module showed the highest correlation (negative) with the musical stimuli (R = − 0.70) and the lowest P-value (7.3 × 10–6), followed by TPM3 (R = 0.63; P-value = 1.0 × 10–4) and RRM1 (R = 0.63; P-value = 1.0 × 10–4); both indicating a global activation pattern. The influence of core genes, measured as module membership (MM), was evaluated, and 6 out of the 7 modules showed a high statistically significant correlation between individual gene MM and individual gene correlation with musical stimuli, indicating functional relatedness; the strongest link was detected for the TPM3 (R = 0.56; P-value = 1.5 × 10–63) and TRIM56 (R = 0.83; P-value = 1.2 × 10–55) modules (Supplementary Figure S5C).
Figure 3.
Co-expression analysis in ACD patients. (A) Correlation values heatmap obtained from WGCNA module analysis of co-expressed genes in ACD patients. Upper value corresponds to the individual correlation value of the module with musical stimuli. P-values of these correlations are shown in brackets (B) Raincloud plots of differences in individual samples eigengenes between TP1 and TP2 from modules showing the largest statistical significance in ACD patients. (C) Top biological processes detected from most statistically significant modules. Modules with significant pathways are named as the hub gene names CMTM6 and TPM3. (D) Module membership (MM) and phenotype correlation (musical stimuli) for genes from the most significant pathways obtained from the modules CMTM6 and TPM3.
Pathway analysis of the genes in the modules identified statistically significant pathways in modules ‘TPM3’ and ‘CMTM6’ (Supplementary Table S3; Fig. 3C). The ‘TPM3’ module was associated with macroautophagy (FDR = 1.15 × 10–5), while ‘CMTM6’ was linked to vesicle, endosome and vacuole organization (FDR = 0.003). Shared genes between these modules and pathways have higher MM values and showed a high correlation with the musical stimuli, indicating functional relatedness, as also suggested by the proximity observed for the pathways in the hierarchical clustering tree (Fig. 3C, 3D, S5B).
In the control dataset, correlations between musical stimuli and modules of co-expressed genes were not found to be statistically significant after multiple test correction (see Supplementary Note for additional results).
Music elicits expression in the opposite direction to MCI/AD in patients
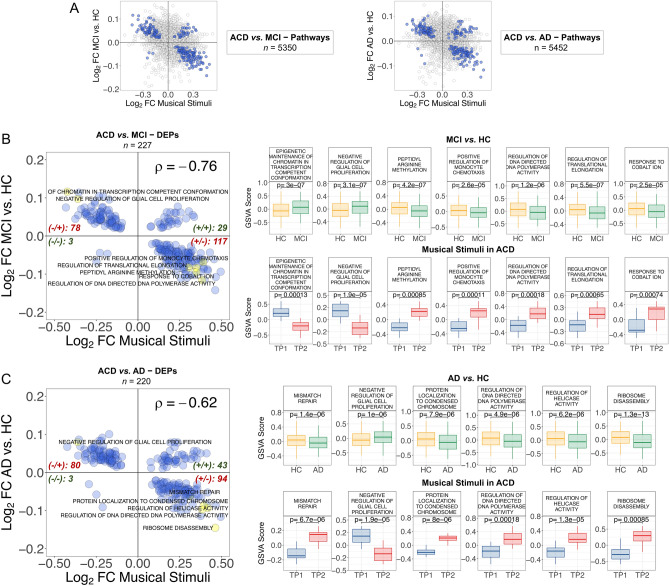
We cross-compared the genes and pathways emerging from the contrast ‘MCI versus controls’, with the genes and pathways emerging from the comparison ‘TP1 versus TP2’ in ACD patients; this comparison yielded 16,743 overlapping genes (FDR significant DEGs = 207; henceforth DEGsMCI) and 5350 overlapping pathways (FDR significant DEPs = 227; henceforth DEPsMCI) (Fig. 4A). We also conducted a cross-comparison of DEGs and DEPs that are altered in AD patients (compared to controls) with those emerging from musical stimuli in ACD patients. The analysis revealed 16,721 overlapping genes, including 131 FDR significant DEGs (DEGsAD), and 5,352 overlapping pathways, including 220 FDR significant DEPs (DEGsAD); Fig. 5A.
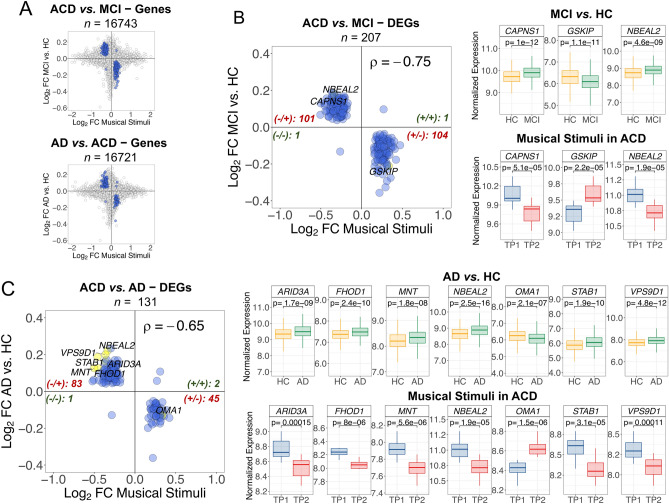
Figure 4.
Correlation patterns of genes between the comparisons ‘ACD patients in TP1 versus TP2’ to (i) ‘MCI versus healthy controls’, and (ii) ‘AD versus healthy controls’. (A) Dots indicate log2FC values for genes shared between the comparisons; blue dots are DEGs with significant FDR < 0.05. (B) FDR DEGsMCI; (C) FDR DEGsAD. For (B) and (C): only top DEG names are displayed (yellow points); these top elements were identified by first selecting the best 50 FDR values and top 50 higher |log2FC| from each comparison separately; next selecting the shared elements between the two comparisons. For the top DEGs we also show boxplots of (normalized) expression. Spearman correlation values and the number of genes plotted in each quadrant are indicated. For all the correlation values, P-value < 2.2 × 10–16. MCI Mild cognitive impairment; AD Alzheimer’s disease; HC Healthy controls; ACD Age-related cognitive disorder.
Figure 5.
Correlation patterns of pathways between the comparisons ‘ACD patients in TP1 versus TP2′ to (i) ‘MCI versus healthy controls’, and (ii) ‘AD versus healthy controls’. (A) Dots indicate log2FC values for pathways shared between the comparisons; blue dots are DEPs with significant FDR < 0.05. (B) FDR DEPsMCI; (C) FDR DEPsAD. For (B) and (C): only top DEP names are displayed (yellow points); these top elements were identified by first selecting the best 50 FDR values and top 50 higher |log2FC| from each comparison separately; next selecting the shared elements between the two comparisons. For the top DEGs we also show boxplots of GSVA score. Spearman correlation values and the number of genes plotted in each quadrant are indicated. For all the correlation values, P-value < 2.2 × 10–16. MCI Mild cognitive impairment; AD Alzheimer’s disease; HC Healthy controls; ACD Age-related cognitive disorder.
Of the total FDR DEGsMCI, 99% of them (205/207) showed log2FC that are negatively correlated with musically-stimulated DEGs (n = 205; ρ = − 0.65, P-value = 2.2 × 10–16), the overall correlation being significantly negative (n = 207; ρ = − 0.75, P-value = 2.2 × 10–16); Fig. 4B, Supplementary Table S4. Two of the most remarkable genes were up-regulated in MCI (CAPNS1 and NBEAL2), and one was down-regulated (GSKIP); Supplementary Table S4. The PPI-network and downstream enrichment analysis of the proteins coded by the top 207 genes (Supplementary Figure S6A; Supplementary Table S4) shows interesting biological connections between these genes; these connections are more clearly visible in the enrichment map (Supplementary Figure S6A). Thus, the most interesting biological process emerging from these genes is the “regulation of macroautophagy” (FDR = 0.0057); Supplementary Figure S6A, Supplementary Table S4.
As seen with the MCI comparison, we observed a major proportion of FDR DEGsAD (97.7%; 128/131) with negatively correlated log2FC (n = 128; ρ = − 0.68, P-value = 2.2 × 10–16); the overall correlation being also negative (n = 131; ρ = − 0.65, P-value = 2.2 × 10–16); Fig. 4C. The most remarkable DEGsAD were all up-regulated in AD (FHOD1, MNT, STAB1, NBEAL2, ARID3A and VPS9D1), except for one (OMA1), which showed down-regulation in AD with respect to control samples (OMA1); Supplementary Table S4. We further explored the PPI-network and downstream enrichment analysis of the proteins coded by these top 131 genes (Supplementary Figure S6B; Supplementary Table S4); the enrichment map of this network (Supplementary Figure S6B) indicates that the more important processes related to these genes are inter-connected within pathways related to G-alpha receptors (FDR = 0.0361), AD (FDR = 0.0063), and NRAGE cell death (FDR = 0.0361 and 0.0417); Supplementary Table S4.
The compensatory effect of the musical stimuli on the expression of genes involved in MCI is also evident when comparing log2FC of DEPsMCI compared to musically-stimulated DEPs (Fig. 5B, Supplementary Table S4). A main proportion of the FDR shared DEPs showed negatively correlated log2FC values (85.9%; n = 195; ρ = − 0.90, P-value = 2.2 × 10–16); the global log2FC correlation pattern being therefore negative (n = 227; ρ = − 0.76, P-value = 2.2 × 10–16); Fig. 5B. Among the top pathways in MCI (and down-regulated under the music stimuli), are the up-regulated “epigenetic maintenance of chromatin in transcription competent conformation” and “negative regulation of glial cell proliferation”. Additionally, the down-regulated processes included “peptidyl arginine methylation”, “positive regulation of monocyte chemotasis”, “regulation of DNA directed DNA polymerase activity”, “regulation of translational elongation”, and “response to cobalt ion” (Fig. 5B, Supplementary Table S4).
We additionally examined the offset effect of the musical stimuli on the expression of pathways involved in AD, by comparing DEPsAD with musically-stimulated DEPs (Fig. 5C, Supplementary Table S4). In 79.1% (174/220) of the FDR DEPsAD, log2FC values were negatively correlated (ρ = − 0.88, P-value = 2.2 × 10–16); this trend dominates the overall correlation value for all shared DEPs (n = 220; ρ = − 0.62, P-value = 2.2 × 10–16); Fig. 5C. Among the top pathways that are up-regulated in AD, and therefore down-regulated after listening to music in ACD, there are processes involved in DNA repair and replication, such as “mismatch repair”, “regulation of helicase activity” and “regulation of DNA directed DNA polymerase activity”, and others related to protein translation, namely “ribosome disassembly” (Fig. 5C, Supplementary Table S4). Interestingly, the only top pathway that is over-activated in AD (P-value = 1.4 × 10–5) and under-activated after musical stimuli in ACD patients (P-value = 5.1 × 10–5) was the “negative regulation of glial cell proliferation”.
The FDR DEGsMCI and DEPsMCI (n = 207 and n = 227) as well as FDR DEGsAD and DEPsAD (n = 131 and n = 220, respectively) observed in the comparisons above, overlap in 114 DEGs and 147 DEPs; both genes and pathways also have highly correlated log2FC values (ρ [DEGs] = 0.88 and ρ [DEPs] = 0.94; Supplementary Figure S7). This probably reflects the fact that MCI is often an early or transitional stage between aging and AD20.
Discussion
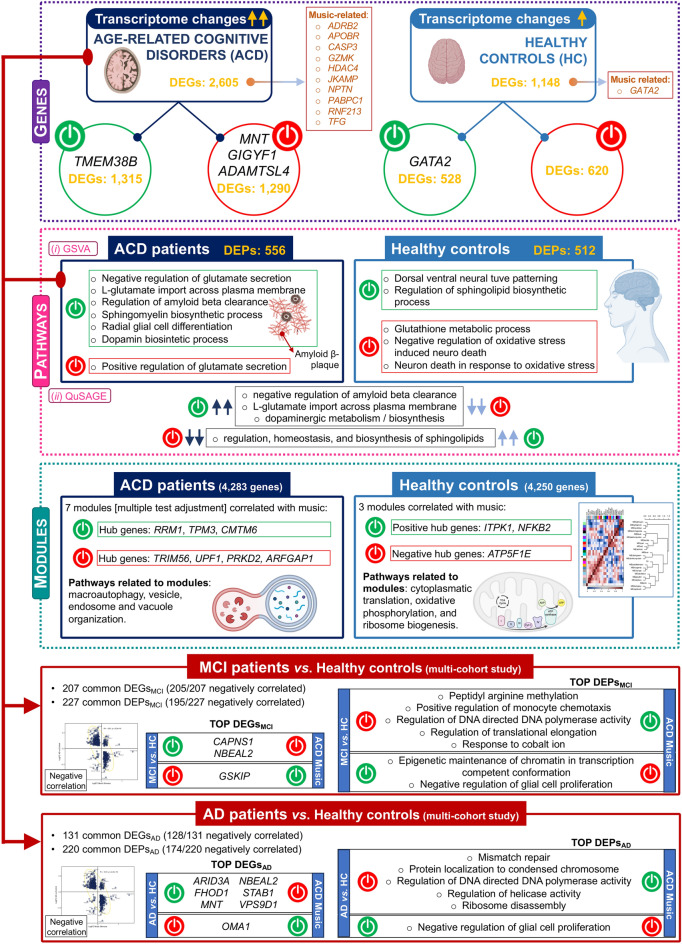
Music exerts a profound influence on human brains, affecting emotions, activating the reward system, and inducing changes in brain anatomy. However, research on the impact of music on gene expression is still in its nascent stages. The present study represents the first attempt to analyze the effect of music on the transcriptome of ACD patients. Capillary blood samples were collected from ACD patients and healthy donors using a minimally invasive procedure before and after a 50-min concert of classical musical stimuli in an ecologically valid environment. Subsequently, we performed RNA sequencing on these samples, allowing for the analysis of gene expression information for over 35,000 transcripts. The analysis focused on differential expression between the two time points and cohorts, as well as the contextualization of gene expression changes within the framework of genes and pathways that exhibit dysregulated expression in MCI and AD (Fig. 6).
Figure 6.
Summary of the most outstanding features related to the impact of musical stimuli on gene expression in the studied cohorts. The figure was built using Biorender resources (https://biorender.com/).
The findings of the present study revealed several noteworthy observations. Firstly, the number of DEGs is more than double in ACD patients compared to healthy controls. Secondly, on average, the transcriptomes of ACD patients quantitatively deviate to a greater extent before and after the music stimuli compared to controls. Additionally, there was a trend towards up-regulation of DEGs in ACD patients, while healthy donors exhibited the opposite effect. These global gene expression changes warrant further investigation, particularly considering the potential massive impact of music’s effect on transcriptomes, which may surpass previous conceptions. Notably, neurologists have posited that music affects almost all regions of the brain21; in parallel, our study indicated a high impact of music on our transcriptomes. It would be of particular interest to explore the extent to which this effect underlies the widely held belief that music can help manage AD and other medical conditions1.
Several genes displayed extreme DE when comparing time points. GIGYF1, which is associated with Autism Spectrum Disorder (ASD) and indirectly linked to AD, as well as ADAMTSL4, involved in the metabolism of amyloidogenic peptides in AD, were both down-regulated in ACD patients. In contrast, GATA2, previously reported to be associated with music7,22, was up-regulated in controls; Supplementary Note. Pathway analysis revealed important insight into the biological processes impacted by music stimuli. The metabolism of L-glutamate was found to be activated in ACD compared to controls; this pathway is known to play a role in the pathogenesis of AD at early stages23. The data suggest that musical stimuli could contribute to managing L-glutamate in the brains of AD patients, which aligns with traditional pharmacological therapies targeting the same pathway24. Furthermore, DE of sphingolipid metabolism was observed between ACD and healthy individuals; these complex molecules have been found to be associated with neurodegenerative processes25. Music stimuli also impact the regulation of the Aβ peptides in ACD patients. Accumulation of Aβ in the brain is an early toxic event in AD pathogenesis; our study showed that music stimuli targeted the regulation of Aβ with FC more than 15 times greater in ACD patients compared to controls; Supplementary Note. Moreover, several articles have found a link between dopaminergic pathways, memory, and music26,27; our results are in line with these previous findings showing that dopamine-related pathways are activated in ACD patients after the musical stimuli.
Analysis of co-expression networks identified clusters of correlated genes showing significant patterns of global activation (positively correlated) or inhibition (negatively correlated) after listening to music. In agreement with analysis of individual genes, musical stimuli target important pathways involved in the pathogenesis of MCI and AD. Functional analysis of the genes in these modules (especially the ‘TPM3’ and ‘CMTM6’ modules) in ACD patients pointed out the music-mediated activation of biological processes including macroautophagy, vesicle transport, vacuole, and autophagosome organization. Autophagy is a key mechanism to maintain cellular homeostasis by facilitating the clearance of long-lived proteins, including toxic protein aggregates28, and is essential for maintaining a proper axonal homeostasis29.
The present study provides compelling evidence indicating that musical stimuli in ACD patients elicit the expression of a notable number of genes and pathways in a direction contrary to that observed in individuals with MCI and AD conditions (Fig. 4). Several genes stand out as expressing in an opposite direction, including: (1) CALPNS1 (Calpain Small Subunit 1), which encodes a protein crucial in various biological processes, namely: proliferation, migration, and autophagy, and has been implicated in neurodegenerative processes30; (2) NBEAL2 (Neurobeachin Like 2), previously identified as DE in the brains of individuals with AD31; (3) GSKIP (GSK3B interacting protein), known to play a role in Tau phosphorylation, a key protein involved in the pathogenesis of AD; (4) STAB1 (stabilin 1), associated with anxiety32 and impaired cognition33; (5) OMA1 (OMA1 Zinc Metallopeptidase), which encodes a protease that plays a critical role in maintaining mitochondrial homeostasis34,35 (also, impaired processing of OPA1, one of the main substrates of OMA1, has been reported in post-mortem brains of AD patients36,37); and (6) FHOD1 (Formin Homology 2 Domain Containing 1), a gene involved in the regulation of actin cytoskeleton organization or gene transcription, and found to be over-expressed in extracellular vesicles of AD patients38. An enrichment map of these highly negatively correlated genes reveals their involvement in MCI- and AD-associated pathways, particularly the “regulation of macroautophagy” (when comparing MCI patients to controls) and the “NRAGE cell death” (when comparing AD patients to controls); Supplementary Figure S6. The “receptor of advanced glycation end-products” (RAGE) pathway, including its N-truncated isoform NRAGE, has been implicated in neurotoxic and inflammatory cascades of neurodegenerative processes39,40; Supplementary Note.
The impact of music on gene expression related to MCI and AD reveals also a significant compensatory effect when examining DEPs. Notably, several crucial pathways involved in DNA repair/damage response and protein synthesis exhibited remarkable involvement. Previous studies have linked deficiencies in DNA protective mechanisms to age-related neurodegenerative disorders such as Parkinson’s disease and AD41–43. Down-regulation of genes involved in the DNA repairing system has been associated with AD44–46. Similarly, adequate protein synthesis plays a crucial role in establishing and maintaining long-term memory, while dysfunction in protein homeostasis, synthesis, and ribosomes represents characteristic features of AD47,48. Among ACD patients, music-induced down-regulation was observed in the glial cell proliferation pathway. Glial cells, encompassing microglia, astrocytes, and oligodendrocytes, are non-neuronal cells in the central/ peripheral nervous system (CNS/PNS), crucial for supporting neuronal functions, providing insulation, participating in immune responses, and promoting homeostasis. Dysregulation of glial cells has been associated with AD; however, their specific roles remain unclear49. Some researchers suggest that increased activation and proliferation of microglia and astrocytes have neuroprotective effects in AD50,51, facilitating Aβ clearance52–55 and restricting plaque growth56–58. Conversely, others propose that microgliosis or astrogliosis processes contribute to AD pathogenesis59–63. Additionally, the involvement of oligodendrocytes has been investigated due to their role in myelin production and maintenance64. Neuronal demyelination, a hallmark of AD, suggests a potential role for oligodendrocyte loss in this pathological feature65,66.
There are several limitations to this study. Firstly, although the study’s population-based nature aimed to minimize noise originating from stimuli other than music, the experimental design is susceptible to potential confounding effects that may be unavoidable. Secondly, our findings need to be investigated and validated in other ecological settings. Thirdly, the applied sampling strategy does not allow an investigation into the long-term effects of music on gene expression, or its potential connections to the benefits attributed to music in patients with MCI and AD; Supplementary Note.
Genes and pathways related to the central nervous system (CNS) exhibit the highest sensitivity to musical stimuli, which is expected given that sight and hearing serve as natural entry points for such stimuli. Furthermore, the transcriptomes of ACD patients display greater sensitivity to music and undergo more significant changes compared to healthy individuals. The observation that many genes/pathways influenced by music exhibit an opposite direction of expression and regulation in comparison to those altered in MCI/AD warrants careful attention in future studies, particularly in exploring the potential therapeutic implications of music for neurodegenerative diseases. Validating the current findings in larger and independent cohorts is imperative. Additionally, expanding the study to include other disorders i.e., mental-related disorders, ASD, brain damage, etc., would provide a broader understanding of the effects of music on gene expression. Further research is necessary to fully comprehend the implications of these findings for human health1.
Supplementary Information
Acknowledgements
We would like to kindly acknowledge all participants in the present study, the AGADEA association of Alzheimer disease patients, and the musicians of SANARTE, who kindly agreed to participate in this project. We would also like to acknowledge the Real Filharmonía de Galicia (www.rfgalicia.org; and Sabela García Fonte in particular), and the Auditorio de Galicia for their support.
We would like to thank the contribution of all members of the Sensogenomics Working Group: Antonio Salas Ellacuriaga—PI, Federico Martinón-Torres—PI; Laura Navarro Ramón—Coordinator.
GenPoB/GenVip—Instituto de Investigación Sanitaria (IDIS) (alphabetic order) Alba Camino Mera, Albert Padín Villar, Alberto Gómez Carballa, Alejandro Pérez López, Alicia Carballal Fernández, Ana Cotovad Bellas, Ana Isabel Dacosta Urbieta, Narmeen Mallah, Ana María Pastoriza Mourelle, Ana María Senín Ferreiro, Andrés Muy Pérez, Antía Rivas Oural, Antonio Justicia Grande, Antonio Piñeiro García, Anxela Cristina Delgado García, Belén Mosquera Pérez, Blanca Díaz Esteban, Carlos Durán Suárez, Carmen Curros Novo, Carmen Gómez Vieites, Carmen Rodríguez-Tenreiro Sánchez, Celia Varela Pájaro, Claudia Navarro Gonzalo, Cristina Serén Trasorras, Cristina Talavero González, Einés Monteagudo Vilavedra, Estefanía Rey Campos, Esther Montero Campos, Fernando Álvez González, Fernando Caamaño Viñas, Francisco García Iglesias, Gloria Viz Rodríguez, Hugo Alberto Tovar Velasco, Irene Álvarez Rodríguez, Irene García Zuazola, Irene Rivero Calle, Iria Afonso Carrasco, Isabel Ferreirós Vidal, Isabel Lista García, Isabel Rego Lijo, Iván Prieto Gómez, Iván Quintana Cepedal, Jacobo Pardo Seco, Jesús Eirís Puñal, José Gómez Rial, José Manuel Fernández García, José María Martinón Martínez, Julia Cela Mosquera, Julia García Currás, Julián Montoto Louzao, Lara Martínez Martínez, Laura Navarro Marrón, Lidia Piñeiro Rodríguez, Lorenzo Redondo Collazo, Lúa Castelo Martínez, Lucía Company Arciniegas, Luis Crego Rodríguez, Luisa García Vicente, Manuel Vázquez Donsión, María Dolores Martínez García, María Elena Gamborino Caramés, María Elena Sobrino Fernández, María José Currás Tuala, María Martínez Leis, María Soledad Vilas Iglesias, María Sol Rodriguez Calvo, María Teresa Autran García, Marina Casas Pérez, Marta Aldonza Torres, Marta Bouzón Alejandro, Marta Lendoiro Fuentes, Miriam Ben García, Miriam Cebey López, Montserrat López Franco, Narmeen Mallah, Natalia García Sánchez, Natalia Vieito Perez, Patricia Regueiro Casuso, Ricardo Suárez Camacho, Rita García Fernández, Rita Varela Estévez, Rosaura Picáns Leis, Ruth Barral Arca, Sandra Carnota Antonio, Sandra Viz Lasheras, Sara Pischedda, Sara Rey Vázquez, Sonia Marcos Alonso, Sonia Serén Fernández, Susana Rey García, Vanesa Álvarez Iglesias, Victoria Redondo Cervantes, Vanesa Álvarez Iglesias, Wiktor Dominik Nowak, Xabier Bello Paderne, Xabier Mazaira López
Nursing volunteers (alphabetic order) Alejandra Fernández Méndez, Ana Isabel Abadín Campaña, Ana María León Caamaño, Ana María Buide Illobre, Ángeles Mera Cores, Carmen Nieves Vastro, Carolina Suarez Crego, Concepción Rey Iglesias, Cristina Candal Regueira, Dolores Barreiro Puente, Elvira Rodríguez Rodríguez, Eugenia González Budiño, Eva Rey Álvarez, Fernando Rodríguez Gerpe, Gemma Albela Silva, Isabel Castro Pérez, Isabel Domínguez Ríos, José Ángel Fernández de la Iglesia, José Cruces Vázquez, José Luis Cambeiro Quintela, José Ramón Magariños Iglesias, Julia Rey Brandariz, Julio Abel Fernández López, Luisa García Vicente, Manuel González Lito, Manuel González Lijó, Manuela Pérez Rivas, Margarita Turnes Paredes, María Aurora Méndez López, María Begoña Tomé Arufe, María Campos Torres, María del Carmen Baloira Nogueira, María del Carmen García juan, María Esther Moricosa García, María Luz Chao Jarel, María Martínez Leis, María Mercedes Jiménez Santos, María Salomé Buide Illobre, María Victoria López Pereira, Mercedes Jorge González, Mercedes Isolina Rodríguez Rodríguez, Miren Payo Puente, Natalia Carter Domínguez, Olga María Reyes González, Pilar Mera Rodríguez, Purificación Sebio Brandariz, Salomé Quintáns lago, Yolanda Rodríguez Taboada, María Pereira Grau.
Other volunteers (alphabetic order) Alba Arias Gómez, Alejandro Moreno Díaz, Ana Arca Marán, Astro González Guirado, Brais García Iglesias, Carlos Sánchez Rubín, Carmen Otero de Andrés, Clara Pérez Errazquin Barrera, Claudia Rey Posse, Cristina Rojas García, Eduardo Xavier Giménez Bargiela, Elena Gloria Morales García, Fabio Izquierdo García Escribano, Gabriel Guisande García, Jaime López Martín, Lara Pais Ramiro, Lucía Rico Montero, Luís Estévez Martínez, Manuel Estévez Casal, María Aránzazu Palomino Caño, María Rubio Valdés, Marisol Nogales Benítez, Miryam Tilve Pérez, Nuria Villar Muiños, Pablo Del Cerro Rodríguez, Pablo Pozuelo Martínez Cardeñoso, Salma Ouahabi El Ouahabi, Santiago Vázquez Calvache.
Author contributions
A.S., F.M.-T. and L.N. conceived and coordinated the study; A.S. and A.G.C. analyzed the data and wrote the first draft; J.P.-S., X.B., S.P., S.V.-L., A.C.-M., M.-J.C., I.F., N.M.; S.R.-V., L.R., A.D.-U., F.C.-V., I.R.-C., C.R.-T., F.M.-T., A.S., contributed to logistics and sample and data collection; All the authors revised and approved the final version of the text. All participants have given permission for publication of the project’s findings.
Funding
This study received support from GAIN: Grupos con Potencial de Crecimiento [IN607B 2020/08, (A.S.)] and Grupos de Referencia Competitiva [IN607A2023/02 (A.S.), IIN607A2021/05 (F.M-T)].
Data availability
The authors confirm that data supporting the findings of this study are available in Gene Expression Omnibus—NCBI (GEO; https://www.ncbi.nlm.nih.gov/geo/) under accession number GSE239282.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-023-48094-5.
References
- 1.Navarro L, Gomez-Carballa A, Pischedda S, Montoto-Louzao J, Viz-Lasheras S, Camino-Mera A, Hinault T, Martinon-Torres F, Salas A. Sensogenomics of music and Alzheimer’s disease: An interdisciplinary view from neuroscience, transcriptomics, and epigenomics. Front. Aging Neurosci. 2023;15:1063536. doi: 10.3389/fnagi.2023.1063536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Navarro L, Martinón-Torres F, Salas A. Sensogenomics and the biological background underlying musical stimuli: Perspectives for a new era of musical research. Genes. 2021;12(9):1454. doi: 10.3390/genes12091454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kanduri C, Raijas P, Ahvenainen M, Philips AK, Ukkola-Vuoti L, Lahdesmaki H, Jarvela I. The effect of listening to music on human transcriptome. PeerJ. 2015;3:e830. doi: 10.7717/peerj.830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Järvelä, I. Genomics studies on musical aptitude, music perception, and practice. Ann. N. Y. Acad. Sci.1423, 82–91 (2018). [DOI] [PubMed]
- 5.Oikkonen J, Järvelä I. Genomics approaches to study musical aptitude. BioEssays. 2014;36(11):1102–1108. doi: 10.1002/bies.201400081. [DOI] [PubMed] [Google Scholar]
- 6.Kanduri C, Kuusi T, Ahvenainen M, Philips AK, Lahdesmaki H, Jarvela I. The effect of music performance on the transcriptome of professional musicians. Sci. Rep. 2015;5:9506. doi: 10.1038/srep09506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nair PS, Kuusi T, Ahvenainen M, Philips AK, Jarvela I. Music-performance regulates microRNAs in professional musicians. PeerJ. 2019;7:e6660. doi: 10.7717/peerj.6660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nair, P. S., Raijas, P., Ahvenainen, M., Philips, A. K., Ukkola-Vuoti, L., Jarvela, I. Music-listening regulates human microRNA expression. Epigenetics16(5), 554–566 (2021). [DOI] [PMC free article] [PubMed]
- 9.Love MI, Huber W, Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014;15(12):550. doi: 10.1186/s13059-014-0550-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Blighe K, Lun A: PCAtools: PCAtools: Everything principal components analysis. R package version 2.10.0 edn (2022).
- 11.Szklarczyk D, Gable AL, Nastou KC, Lyon D, Kirsch R, Pyysalo S, Doncheva NT, Legeay M, Fang T, Bork P, et al. The STRING database in 2021: Customizable protein-protein networks, and functional characterization of user-uploaded gene/measurement sets. Nucleic Acids Res. 2021;49(D1):D605–D612. doi: 10.1093/nar/gkaa1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shannon P, Markiel A, Ozier O, Baliga NS, Wang JT, Ramage D, Amin N, Schwikowski B, Ideker T. Cytoscape: A software environment for integrated models of biomolecular interaction networks. Genome Res. 2003;13(11):2498–2504. doi: 10.1101/gr.1239303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hänzelmann S, Castelo R, Guinney J. GSVA: Gene set variation analysis for microarray and RNA-seq data. BMC Bioinform. 2013;14:7. doi: 10.1186/1471-2105-14-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Meng H, Yaari G, Bolen CR, Avey S, Kleinstein SH. Gene set meta-analysis with quantitative set analysis for gene expression (QuSAGE) PLoS Comput. Biol. 2019;15(4):e1006899. doi: 10.1371/journal.pcbi.1006899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gu Z, Eils R, Schlesner M. Complex heatmaps reveal patterns and correlations in multidimensional genomic data. Bioinformatics. 2016;32(18):2847–2849. doi: 10.1093/bioinformatics/btw313. [DOI] [PubMed] [Google Scholar]
- 16.Allen M, Poggiali D, Whitaker K, Marshall TR, Kievit RA. Raincloud plots: A multi-platform tool for robust data visualization. Wellcome Open Res. 2019;4:63. doi: 10.12688/wellcomeopenres.15191.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Langfelder P, Horvath S. WGCNA: An R package for weighted correlation network analysis. BMC Bioinform. 2008;9:559. doi: 10.1186/1471-2105-9-559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J. R. Stat. Soc. B. 1995;57:289–300. [Google Scholar]
- 19.The R Core Team: R: A Language and Enviroment for Statistical Computing (R Foundation for Statistical Computing, Vienna, 2019).
- 20.Morris JC, Cummings J. Mild cognitive impairment (MCI) represents early-stage Alzheimer’s disease. J. Alzheimer’s Dis. 2005;7(3):235–239. doi: 10.3233/JAD-2005-7306. [DOI] [PubMed] [Google Scholar]
- 21.Vuust P, Heggli OA, Friston KJ, Kringelbach ML. Music in the brain. Nat. Rev. Neurosci. 2022;23(5):287–305. doi: 10.1038/s41583-022-00578-5. [DOI] [PubMed] [Google Scholar]
- 22.Oikkonen J, Huang Y, Onkamo P, Ukkola-Vuoti L, Raijas P, Karma K, Vieland VJ, Jarvela I. A genome-wide linkage and association study of musical aptitude identifies loci containing genes related to inner ear development and neurocognitive functions. Mol.s Psychiatry. 2015;20(2):275–282. doi: 10.1038/mp.2014.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Esposito Z, Belli L, Toniolo S, Sancesario G, Bianconi C, Martorana A. Amyloid beta, glutamate, excitotoxicity in Alzheimer’s disease: Are we on the right track? CNS Neurosci. Ther. 2013;19(8):549–555. doi: 10.1111/cns.12095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Conway ME. Alzheimer’s disease: Targeting the glutamatergic system. Biogerontology. 2020;21(3):257–274. doi: 10.1007/s10522-020-09860-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hannun YA, Obeid LM. Sphingolipids and their metabolism in physiology and disease. Nat. Rev. Mol. Cell Biol. 2018;19(3):175–191. doi: 10.1038/nrm.2017.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Emanuele E, Boso M, Cassola F, Broglia D, Bonoldi I, Mancini L, Marini M, Politi P. Increased dopamine DRD4 receptor mRNA expression in lymphocytes of musicians and autistic individuals: Bridging the music-autism connection. Neuro Endocrinol. Lett. 2010;31(1):122–125. [PubMed] [Google Scholar]
- 27.Wise RA. Dopamine, learning and motivation. Nat. Rev. Neurosci. 2004;5(6):483–494. doi: 10.1038/nrn1406. [DOI] [PubMed] [Google Scholar]
- 28.Dikic I, Elazar Z. Mechanism and medical implications of mammalian autophagy. Nat. Rev. Mol. Cell Biol. 2018;19(6):349–364. doi: 10.1038/s41580-018-0003-4. [DOI] [PubMed] [Google Scholar]
- 29.Komatsu M, Wang QJ, Holstein GR, Friedrich VL, Jr, Iwata J, Kominami E, Chait BT, Tanaka K, Yue Z. Essential role for autophagy protein Atg7 in the maintenance of axonal homeostasis and the prevention of axonal degeneration. Proc. Natl. Acad. Sci. USA. 2007;104(36):14489–14494. doi: 10.1073/pnas.0701311104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mahaman YAR, Huang F, Kessete Afewerky H, Maibouge TMS, Ghose B, Wang X. Involvement of calpain in the neuropathogenesis of Alzheimer’s disease. Med. Res. Rev. 2019;39(2):608–630. doi: 10.1002/med.21534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li X, Wang H, Long J, Pan G, He T, Anichtchik O, Belshaw R, Albani D, Edison P, Green EK, et al. Systematic analysis and biomarker study for Alzheimer’s disease. Sci. Rep. 2018;8(1):17394. doi: 10.1038/s41598-018-35789-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nagel M, Jansen PR, Stringer S, Watanabe K, de Leeuw CA, Bryois J, Savage JE, Hammerschlag AR, Skene NG, Munoz-Manchado AB, et al. Meta-analysis of genome-wide association studies for neuroticism in 449,484 individuals identifies novel genetic loci and pathways. Nat. Genet. 2018;50(7):920–927. doi: 10.1038/s41588-018-0151-7. [DOI] [PubMed] [Google Scholar]
- 33.Liguori M, Nuzziello N, Simone M, Amoroso N, Viterbo RG, Tangaro S, Consiglio A, Giordano P, Bellotti R, Trojano M. Association between miRNAs expression and cognitive performances of pediatric multiple sclerosis patients: A pilot study. Brain Behav. 2019;9(2):e01199. doi: 10.1002/brb3.1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Anand R, Wai T, Baker MJ, Kladt N, Schauss AC, Rugarli E, Langer T. The i-AAA protease YME1L and OMA1 cleave OPA1 to balance mitochondrial fusion and fission. J. Cell Biol. 2014;204(6):919–929. doi: 10.1083/jcb.201308006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kaser M, Kambacheld M, Kisters-Woike B, Langer T. Oma1, a novel membrane-bound metallopeptidase in mitochondria with activities overlapping with the m-AAA protease. J. Biol. Chem. 2003;278(47):46414–46423. doi: 10.1074/jbc.M305584200. [DOI] [PubMed] [Google Scholar]
- 36.Manczak M, Calkins MJ, Reddy PH. Impaired mitochondrial dynamics and abnormal interaction of amyloid beta with mitochondrial protein Drp1 in neurons from patients with Alzheimer’s disease: Implications for neuronal damage. Hum. Mol. Genet. 2011;20(13):2495–2509. doi: 10.1093/hmg/ddr139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang X, Su B, Lee HG, Li X, Perry G, Smith MA, Zhu X. Impaired balance of mitochondrial fission and fusion in Alzheimer’s disease. J. Neurosci. 2009;29(28):9090–9103. doi: 10.1523/JNEUROSCI.1357-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nielsen JE, Honore B, Vestergard K, Maltesen RG, Christiansen G, Boge AU, Kristensen SR, Pedersen S. Shotgun-based proteomics of extracellular vesicles in Alzheimer’s disease reveals biomarkers involved in immunological and coagulation pathways. Sci. Rep. 2021;11(1):18518. doi: 10.1038/s41598-021-97969-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Deane R, Singh I, Sagare AP, Bell RD, Ross NT, LaRue B, Love R, Perry S, Paquette N, Deane RJ, et al. A multimodal RAGE-specific inhibitor reduces amyloid beta-mediated brain disorder in a mouse model of Alzheimer disease. J. Clin. Investig. 2012;122(4):1377–1392. doi: 10.1172/JCI58642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ramasamy R, Vannucci SJ, Yan SS, Herold K, Yan SF, Schmidt AM. Advanced glycation end products and RAGE: A common thread in aging, diabetes, neurodegeneration, and inflammation. Glycobiology. 2005;15(7):16R–28R. doi: 10.1093/glycob/cwi053. [DOI] [PubMed] [Google Scholar]
- 41.Lovell MA, Xie C, Markesbery WR. Decreased base excision repair and increased helicase activity in Alzheimer’s disease brain. Brain Res. 2000;855(1):116–123. doi: 10.1016/S0006-8993(99)02335-5. [DOI] [PubMed] [Google Scholar]
- 42.Gabbita SP, Lovell MA, Markesbery WR. Increased nuclear DNA oxidation in the brain in Alzheimer’s disease. J. Neurochem. 1998;71(5):2034–2040. doi: 10.1046/j.1471-4159.1998.71052034.x. [DOI] [PubMed] [Google Scholar]
- 43.Myung NH, Zhu X, Kruman II, Castellani RJ, Petersen RB, Siedlak SL, Perry G, Smith MA, Lee HG. Evidence of DNA damage in Alzheimer disease: Phosphorylation of histone H2AX in astrocytes. Age. 2008;30(4):209–215. doi: 10.1007/s11357-008-9050-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kanungo J. DNA-PK deficiency in Alzheimer’s disease. J Neurol. Neuromed. 2016;1(3):17–22. doi: 10.29245/2572.942X/2016/3.1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Suberbielle E, Djukic B, Evans M, Kim DH, Taneja P, Wang X, Finucane M, Knox J, Ho K, Devidze N, et al. DNA repair factor BRCA1 depletion occurs in Alzheimer brains and impairs cognitive function in mice. Nat Commun. 2015;6:8897. doi: 10.1038/ncomms9897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Weissman L, Jo DG, Sorensen MM, de Souza-Pinto NC, Markesbery WR, Mattson MP, Bohr VA. Defective DNA base excision repair in brain from individuals with Alzheimer’s disease and amnestic mild cognitive impairment. Nucleic Acids Res. 2007;35(16):5545–5555. doi: 10.1093/nar/gkm605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hernandez-Ortega K, Garcia-Esparcia P, Gil L, Lucas JJ, Ferrer I. Altered machinery of protein synthesis in Alzheimer’s: From the nucleolus to the ribosome. Brain Pathol. 2016;26(5):593–605. doi: 10.1111/bpa.12335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ding Q, Markesbery WR, Chen Q, Li F, Keller JN. Ribosome dysfunction is an early event in Alzheimer’s disease. J. Neurosci. 2005;25(40):9171–9175. doi: 10.1523/JNEUROSCI.3040-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhou Y, Song WM, Andhey PS, Swain A, Levy T, Miller KR, Poliani PL, Cominelli M, Grover S, Gilfillan S, et al. Human and mouse single-nucleus transcriptomics reveal TREM2-dependent and TREM2-independent cellular responses in Alzheimer’s disease. Nat. Med. 2020;26(1):131–142. doi: 10.1038/s41591-019-0695-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kobayashi E, Nakano M, Kubota K, Himuro N, Mizoguchi S, Chikenji T, Otani M, Mizue Y, Nagaishi K, Fujimiya M. Activated forms of astrocytes with higher GLT-1 expression are associated with cognitive normal subjects with Alzheimer pathology in human brain. Sci. Rep. 2018;8(1):1712. doi: 10.1038/s41598-018-19442-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Saha P, Sarkar S, Paidi RK, Biswas SC. TIMP-1: A key cytokine released from activated astrocytes protects neurons and ameliorates cognitive behaviours in a rodent model of Alzheimer’s disease. Brain Behav. Immun. 2020;87:804–819. doi: 10.1016/j.bbi.2020.03.014. [DOI] [PubMed] [Google Scholar]
- 52.El Khoury J, Toft M, Hickman SE, Means TK, Terada K, Geula C, Luster AD. Ccr2 deficiency impairs microglial accumulation and accelerates progression of Alzheimer-like disease. Nat. Med. 2007;13(4):432–438. doi: 10.1038/nm1555. [DOI] [PubMed] [Google Scholar]
- 53.Katsouri L, Birch AM, Renziehausen AWJ, Zach C, Aman Y, Steeds H, Bonsu A, Palmer EOC, Mirzaei N, Ries M, et al. Ablation of reactive astrocytes exacerbates disease pathology in a model of Alzheimer’s disease. Glia. 2020;68(5):1017–1030. doi: 10.1002/glia.23759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mildner A, Schlevogt B, Kierdorf K, Bottcher C, Erny D, Kummer MP, Quinn M, Bruck W, Bechmann I, Heneka MT, et al. Distinct and non-redundant roles of microglia and myeloid subsets in mouse models of Alzheimer’s disease. J. Neurosci. 2011;31(31):11159–11171. doi: 10.1523/JNEUROSCI.6209-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wang Y, Cella M, Mallinson K, Ulrich JD, Young KL, Robinette ML, Gilfillan S, Krishnan GM, Sudhakar S, Zinselmeyer BH, et al. TREM2 lipid sensing sustains the microglial response in an Alzheimer’s disease model. Cell. 2015;160(6):1061–1071. doi: 10.1016/j.cell.2015.01.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Meyer-Luehmann M, Spires-Jones TL, Prada C, Garcia-Alloza M, de Calignon A, Rozkalne A, Koenigsknecht-Talboo J, Holtzman DM, Bacskai BJ, Hyman BT. Rapid appearance and local toxicity of amyloid-beta plaques in a mouse model of Alzheimer’s disease. Nature. 2008;451(7179):720–724. doi: 10.1038/nature06616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bolmont T, Haiss F, Eicke D, Radde R, Mathis CA, Klunk WE, Kohsaka S, Jucker M, Calhoun ME. Dynamics of the microglial/amyloid interaction indicate a role in plaque maintenance. J. Neurosci. 2008;28(16):4283–4292. doi: 10.1523/JNEUROSCI.4814-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Grathwohl SA, Kalin RE, Bolmont T, Prokop S, Winkelmann G, Kaeser SA, Odenthal J, Radde R, Eldh T, Gandy S, et al. Formation and maintenance of Alzheimer’s disease beta-amyloid plaques in the absence of microglia. Nat. Neurosci. 2009;12(11):1361–1363. doi: 10.1038/nn.2432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chun H, Im H, Kang YJ, Kim Y, Shin JH, Won W, Lim J, Ju Y, Park YM, Kim S, et al. Severe reactive astrocytes precipitate pathological hallmarks of Alzheimer’s disease via H(2)O(2)(-) production. Nat. Neurosci. 2020;23(12):1555–1566. doi: 10.1038/s41593-020-00735-y. [DOI] [PubMed] [Google Scholar]
- 60.Liddelow SA, Guttenplan KA, Clarke LE, Bennett FC, Bohlen CJ, Schirmer L, Bennett ML, Munch AE, Chung WS, Peterson TC, et al. Neurotoxic reactive astrocytes are induced by activated microglia. Nature. 2017;541(7638):481–487. doi: 10.1038/nature21029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Spangenberg E, Severson PL, Hohsfield LA, Crapser J, Zhang J, Burton EA, Zhang Y, Spevak W, Lin J, Phan NY, et al. Sustained microglial depletion with CSF1R inhibitor impairs parenchymal plaque development in an Alzheimer’s disease model. Nat. Commun. 2019;10(1):3758. doi: 10.1038/s41467-019-11674-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Spangenberg EE, Lee RJ, Najafi AR, Rice RA, Elmore MR, Blurton-Jones M, West BL, Green KN. Eliminating microglia in Alzheimer’s mice prevents neuronal loss without modulating amyloid-beta pathology. Brain J. Neurol. 2016;139(Pt 4):1265–1281. doi: 10.1093/brain/aww016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wright AL, Zinn R, Hohensinn B, Konen LM, Beynon SB, Tan RP, Clark IA, Abdipranoto A, Vissel B. Neuroinflammation and neuronal loss precede Abeta plaque deposition in the hAPP-J20 mouse model of Alzheimer’s disease. PLoS One. 2013;8(4):e59586. doi: 10.1371/journal.pone.0059586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Desai MK, Sudol KL, Janelsins MC, Mastrangelo MA, Frazer ME, Bowers WJ. Triple-transgenic Alzheimer’s disease mice exhibit region-specific abnormalities in brain myelination patterns prior to appearance of amyloid and tau pathology. Glia. 2009;57(1):54–65. doi: 10.1002/glia.20734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mitew S, Kirkcaldie MT, Halliday GM, Shepherd CE, Vickers JC, Dickson TC. Focal demyelination in Alzheimer’s disease and transgenic mouse models. Acta Neuropathol. 2010;119(5):567–577. doi: 10.1007/s00401-010-0657-2. [DOI] [PubMed] [Google Scholar]
- 66.Bartzokis G. Alzheimer’s disease as homeostatic responses to age-related myelin breakdown. Neurobiol. Aging. 2011;32(8):1341–1371. doi: 10.1016/j.neurobiolaging.2009.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The authors confirm that data supporting the findings of this study are available in Gene Expression Omnibus—NCBI (GEO; https://www.ncbi.nlm.nih.gov/geo/) under accession number GSE239282.