Abstract
Intestinal fibrosis is a common complication of inflammatory bowel disease. There is still a lack of effective drugs for the prevention or treatment of intestinal fibrosis. Heat shock protein 47 (HSP47) plays a key role in the development of intestinal fibrosis. In this study we investigated the therapeutic potential and underlying mechanisms of fraxinellone, a degraded limonoid isolated from the root bark of Dictamnus dasycarpus, in the treatment of intestinal fibrosis. Intestinal fibrosis was induced in mice by dextran sodium sulfate (DSS) treatment. DDS-treated mice were administered fraxinellone (7.5, 15, 30 mg·kg−1·d−1, i.g.) for 45 days. We showed that fraxinellone administration dose-dependently alleviated DSS-induced intestinal impairments, and reduced the production of intestinal fibrosis biomarkers such as α-smooth muscle actin (SMA), collagen I, hydroxyproline, fibronectin and laminin, and cytokines such as TGF-β, TNF-α and IL-β. We then established in vitro intestinal fibrosis cell models in SW480 and HT-29 cells, and demonstrated that treatment with fraxinellone (3, 10, 30 μM) significantly relieved TGF-β-induced fibrosis responses by inhibiting the TGF-β/Smad2/3 signaling pathway. Molecular docking suggested that the fraxinellone might disrupt the interaction between HSP47 and collagen, which was confirmed by coimmunoprecipitation experiments. SPR analysis showed that fraxinellone had a high affinity for HSP47 with a Kd value of 3.542 × 10−5 M. This study provides a new example of HSP47-collagen intervention by a natural compound and has important implications for the clinical treatment of inflammation-induced issue fibrosis.
Keywords: intestinal fibrosis, inflammatory bowel disease, fraxinellone, HSP47, collagen, antifibrotic drugs
Introduction
Intestinal fibrosis is a common and serious complication in people with inflammatory bowel disease (IBD) and is the result of chronic inflammation [1]. Thirty percent of patients with Crohn’s disease have fibrosis-related diseases, and ulcerative colitis patients also have some degree of fibrosis [2, 3]. The elements of the fibrotic process mainly include the effector cells of the host, the offensive factors that initiate the pathway, the end products deposited in the extracellular space, and the complex network of molecular and cellular inflammatory mediators, characterized by the accumulation of extracellular matrix (ECM)-related proteins [1, 4]. Transforming growth factor (TGF)-β, especially the TGF-β1 subtype, is the most recognized regulator of fibrosis in the intestine and extraintestinal organs [5]. When TGF-β binds to TGF-β receptor type 1 (TGFβR1) and TGFβR2, it initiates the phosphorylation of Smad2/3/4 for further signaling, which is counterregulated by Smad7 [6]. Stimulation of TGF-β can lead to upregulation of fibrotic markers such as α-smooth muscle actin (SMA), collagen I, and connective tissue growth factor (CTGF) [7]. The fibrosis process can cause organ dysfunction and affect human health.
At present, the clinical treatment of intestinal fibrosis is limited to surgical intervention and endoscopic balloon dilation, and there are no approved therapeutic drugs [8–10]. Most drugs and biological therapies can relieve inflammatory lesions and related symptoms but do not have a direct antifibrotic effect. The latest studies have shown that antibodies against the IL-36 receptor reduce chronic intestinal inflammation, inhibitors against TNF-like cytokine 1 A (TL1A) can be used to treat other organ fibrosis, and oncostatin M, a cytokine that induces fibrosis with monoclonal GSK2330811, also provides an attractive strategy, but these are far from sufficient for the clinical treatment of intestinal fibrosis [11, 12]. In short, there is still a lack of understanding of the mechanism of intestinal fibrosis at this stage, and there is still a lack of specific anti-fibrosis therapy, so it is urgent to find new therapeutic targets and methods.
Heat shock protein 47 (HSP47) is a collagen-specific chaperone present in the endoplasmic reticulum (ER) encoded by the SERPINH1 gene that belongs to the serine protease inhibitor (serpin) family but does not inhibit serine proteases [13]. Unlike other chaperones with broad substrate specificity, such as HSP60, HSP70, and HSP90, HSP47 is induced only by heat shock and specifically binds to procollagens [14]. Collagen I is a typical fibril-forming collagen consisting of one α2 chain and two α1 chains, each of which are cotranslationally inserted into the ER [13]. HSP47 can bind and stabilize the triple helix form of procollagen and prevent the lateral aggregation of procollagen in the triple helix form in the ER [15]. The Asp385 site of HSP47 can form a salt bridge with collagen, and Leu381 and Tyr383 are essential for the hydrophobic interaction between HSP47 and collagen [16]. HSP47, as a chaperone in the collagen synthesis pathway, can inhibit the local expansion of procollagen and inhibit the aggregation of procollagen after triple helix formation, which is essential for collagen secretion, fibril formation and deposition in the ECM [17]. HSP47 is involved in the deterioration of fibrosis and can be used as a therapeutic target for fibrotic diseases. Inhibition of HSP47 has been shown to successfully mitigate fibrosis in preclinical models of fibrosis, but its clinical safety, tolerability, and efficacy remain to be evaluated [18].
Fraxinellone is a degraded limonoid isolated from the root bark of Dictamnus dasycarpus [19]. It was initially characterized as a selective blocker of voltage-dependent Ca2+ channels that protects nerves by inhibiting calcium influx in rat cortical cells [20]. In addition, fraxinellone has antibacterial, insecticidal, anti-inflammatory, and anticancer activities [21]. It has been shown to reduce the overactivation of hepatic stellate cells by reducing CUG-binding protein 1 (CUGBP1) levels and to alleviate liver fibrosis significantly [22]. It has a similar role in the treatment of renal fibrosis [23]. However, the effect of fraxinellone on intestinal fibrosis is unknown.
In this study, we found that fraxinellone can alleviate intestinal fibrosis by inhibiting the TGF-β/Smad2/3 signaling pathway and directly acting on the HSP47-collagen complex, which provides new ideas and methods for the clinical treatment of intestinal fibrosis.
Materials and methods
Chemicals and reagents
Fraxinellone (F405738) was purchased from Aladdin (Shanghai, China), with a purity of 98% by HPLC analysis. Dextran sulfate sodium (DSS, molecular weight: 36–50 kDa, ICN16011010) was obtained from MP Biomedicals (Aurora, OH, USA). Antibodies against smooth muscle actin (sc-53142), COL1A1 (sc-293182), fibronectin (sc-8422) and CTGF (sc-365970) were purchased from Santa Cruz (Santa Cruz, CA, USA). Anti-E-cadherin antibody (ab231303) was purchased from Abcam (Cambridge, UK). Anti-N-cadherin (13116), anti-Vimentin (46173) and anti-p-Smad2/3 (8828) antibodies were purchased from Cell Signaling Technology (Danvers, MA, USA). Antibodies against GAPDH (M20006) and GFP (P30010) were purchased from Abmart (Shanghai, China). Anti-HSP47 antibody (A2517) was purchased from ABclonal (Wuhan, China). A hydroxyproline assay kit (A030-2-1) was provided by Nanjing Jiancheng Bioengineering Institute (Nanjing, China). Recombinant human TGF-β1 (100-21) was purchased from PeproTech Inc. (Cranbury, NJ, USA). Col003 (T10860) was purchased from Topscience Co., Ltd. (Shanghai, China). ELISA kits for mouse fibronectin (CSB-E04552m), laminin (CSB-E04645m) were provided by Huamei Biotech Co., Ltd. (Wuhan, China). ELISA kits for mouse TNF-α (1217202) and IL-1β (1210122) were provided by Dakewe Biotech Co., Ltd. (Beijing, China).
Animal experiments
Male C57BL/6 mice were purchased from the College of Veterinary Medicine Yangzhou University (Institute of Comparative Medicine, Yangzhou, China). C57BL/6 male mice (6–8 weeks old) were randomized into five groups: control (n = 5), DSS (n = 11), DSS + fraxinellone 7.5 mg/kg (n = 5), DSS + fraxinellone 15 mg/kg (n = 5), and DSS + fraxinellone 30 mg/kg (n = 5). Mice were grown in an SPF-grade animal room with a 12:12 h light-dark cycle and controlled temperature (21 ± 2 °C). Except for the control group, the mice in the other four groups were fed 2.0% (w/v) DSS for five consecutive days, and then DSS was replaced with water for the next ten consecutive days. With this as one cycle, three cycles were performed. At the same time, the DSS + fraxinellone 7.5 mg/kg, DSS + fraxinellone 15 mg/kg and DSS + fraxinellone 30 mg/kg groups were given fraxinellone (7.5, 15, 30 mg/kg, i.g.) once a day, respectively. In the DSS group, three mice were sacrificed at the end of the first and second cycles. At the completion of three cycles, all mice were euthanized. Mouse serum and colorectal tissue were collected for subsequent experiments. During the mouse experiment, the body weight and disease activity index (DAI) scores were recorded every three days. The scoring criteria for DAI refer to previously published literature [24]. The following parameters were used for calculation: a) hematochezia (0 points for no bleeding, 2 points for slight bleeding, 4 points for severe bleeding); b) diarrhea (normal is 0 points, loose stool is 2 points, watery diarrhea is 4 points). Sections of colorectal tissue were stained with hematoxylin and eosin (H&E), and histological scoring parameters were as follows: 1, adhesions; 2, stricture formation; 3, bleeding; 4, stool blood; 5, edema; 6, presence of mucus; 7, ulcers; 8, erythema; and 9, diarrhea. The highest score is nine points, indicating that the colon is accompanied by severe inflammation. The animal study was reviewed and approved by the Nanjing University Animal Care and Use Committee (NJU-ACUC).
ELISA
ELISA experiments were performed according to the manufacturer’s instructions (Huamei Biotech Co., Ltd., Wuhan, China and Dakewe Biotech Co., Ltd., Beijing, China).
Real-time PCR
Real-time quantitative PCR was performed with a Bio-Rad CFX96 Touch™ real-time PCR detection system (Bio-Rad, Hercules, CA, USA). The amplification conditions were performed as described previously [25]. The primer sequences used in this study are listed in Supplementary Table S1.
Western blot
Whole protein lysates were collected from cells or tissues, isolated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), and electrotransferred to polyvinylidene fluoride (PVDF) membranes (Millipore, Billerica, MA, USA). Finally, different antibodies were incubated according to the molecular weight of target proteins for Western blotting.
Immunofluorescence assay
Paraffin-embedded tissue sections were deparaffinized, rehydrated, subjected to antigen retrieval, blocked, and then incubated overnight at 4 °C with antibodies against α-SMA or collagen I. After washing with PBST, slides were exposed to Alexa Fluor 488-conjugated anti-mouse IgG or Alexa Fluor 594-conjugated anti-mouse IgG (Thermo Fisher Scientific, MA, USA), and finally, cell nuclei were stained with DAPI (Beyotime Biotechnology, Shanghai, China). Images were acquired using a confocal laser-scanning microscope (Olympus FV1000, Olympus, Japan) and analyzed by the Olympus Fluview Ver1.7b viewer and ImageJ.
Cell culture
The SW480 cell line, HT-29 cell line and HEK293T cell line were obtained from the National Collection of Authenticated Cell Cultures (Shanghai, China). SW480 cells were cultured in RPMI- 1640 medium supplemented with 10% fetal bovine serum (FBS) and penicillin-streptomycin solution (1×) under an atmosphere of 5% (v/v) CO2 at 37 °C. HT-29 and HEK293T cells were cultured in DMEM containing 10% FBS and penicillin-streptomycin solution (1×) under an atmosphere of 5% (v/v) CO2 at 37 °C. To induce cellular fibrosis models, SW480 and HT-29 cells were stimulated with 10 ng/mL TGF-β for 48 h and fraxinellone (3, 10, 30 μM) for 24 h.
Cell cycle assay
Cell cycle assays were performed according to the instructions of the Cell Cycle and Apoptosis Analysis Kit manufacturer (Yeasen, Shanghai, China).
Cell transfection assay
Human HSP47 WT-EGFP and HSP47 Mut (Y383A and D385A)-EGFP plasmids were obtained from General Biology (Chuzhou, China). Human HSP47 WT-EGFP and HSP47 Mut (Y383A and D385A)-EGFP plasmids were transfected into HEK293T cells by Exfect Transfection Reagent (Vazyme, Nanjing, China) for subsequent experiments.
Coimmunoprecipitation
A total of 1 × 108 cells were collected and lysed with cell lysis buffer. Cell lysates were immunoprecipitated with anti-GFP antibody and protein A/G-Sepharose. Subsequently, samples were analyzed by Western blotting.
Molecular docking
The structure of fraxinellone was drawn by ChemDraw. The dimensional structure of HSP47 (PDB ID: 4AU2, 3ZHA) was obtained from the RCSB PDB website (https://www.rcsb.org/). The CDOCKER algorithm of Discovery Studio was used for molecular docking of fraxinellone on HSP47.
Cellular thermal shift assay
After HEK293T cells transfected with human HSP47 WT-EGFP or HSP47 Mut-EGFP plasmid were incubated with DMSO, fraxinellone (30 μM) or Col003 (30 μM) for 2 h, the cells were collected and resuspended in PBS. The cell suspension was divided into 10 aliquots, heated at the indicated temperatures, and then analyzed by Western blotting.
Microscale thermophoresis assay
HEK293T cells were transiently transfected with human HSP47 WT-EGFP or HSP47 Mut-EGFP plasmid for 24 h and then lysed with lysis buffer. Fraxinellone or Col003 was gradually diluted from 1 mM and added to an aliquot of cell lysate. They were mixed and sucked into Monolith NT.115 capillary tubes (Nanotemper Technologies). Finally, samples were detected by Monolith NT.115 (Nanotemper Technologies).
Surface plasmon resonance
HSP47 WT or HSP47 Mut Human Recombinant Protein (Absin, Shanghai, China) was immobilized on an activated Series S Sensor Chip CM5 chip, and a gradient concentration of fraxinellone solution was prepared. The Biacore T200 molecular interaction analysis system (GE Healthcare, Uppsala, Sweden) was used to measure the change in response value when the gradient concentration of fraxinellone solution flowed through the chip, and the kinetic constant of the interaction between small molecules and proteins was obtained.
Statistical analysis
The results are presented as the mean ± SEM, and each experiment included at least three replicates. Data were statistically evaluated by Student’s t test or one-way analysis of variance (ANOVA) followed by Dunnett’s test. P < 0.05 was considered significant.
Results
Fraxinellone dose-dependently alleviated DSS-induced pathological impairment in mice
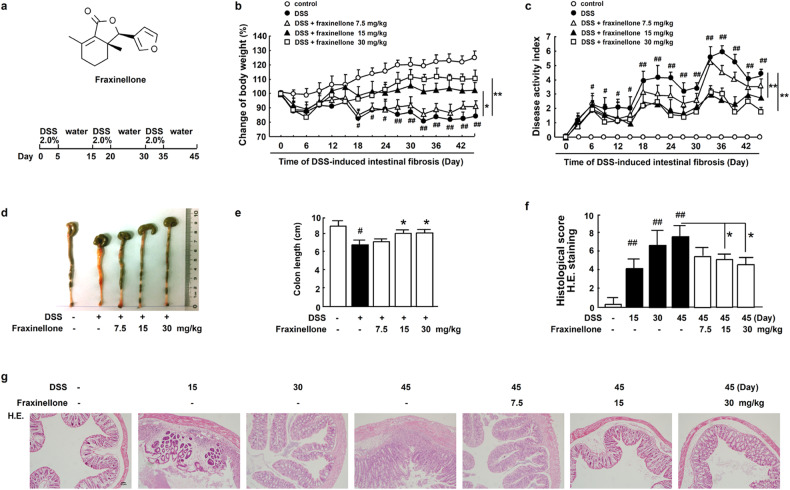
To explore the effect of fraxinellone treatment on intestinal fibrosis, we used fraxinellone to treat dextran sulfate sodium (DSS)-induced mouse models of intestinal fibrosis. The chemical structure of fraxinellone and the schematic diagram of this experiment are shown in Fig. 1a. The body weight of the model group mice was significantly reduced, and fraxinellone treatment alleviated it in a dose-dependent manner (Fig. 1b). Fraxinellone inhibited DSS-induced elevation of the disease activity index (DAI) (Fig. 1c). In addition, colon length was significantly shortened during the progression of intestinal fibrosis, and fraxinellone restored colon length in a dose-dependent manner (Fig. 1d, e). Hematoxylin and eosin (H&E) staining showed that DSS induced a large number of inflammatory infiltrates in the colon, and with prolonged DSS stimulation time, colon inflammation became increasingly serious, and fraxinellone relieved colon inflammation in a dose-dependent manner (Fig. 1f, g).
Fig. 1. Fraxinellone dose-dependently alleviated DSS-induced pathological impairment in mice.
a Chemical structure of fraxinellone and schematic overview of the intestinal fibrosis model. b Body weights and (c) DAI of each group during the experimental process. d Colorectal tissue lesions were photographed and recorded. e Changes in colon length. f–g Hematoxylin and eosin (H&E) staining and histological score of colorectal tissue sections. Scale bar, 50 µm. Data are presented as the mean ± SEM (n = 3–5 per group). #P < 0.05, ##P < 0.01 vs. control group, *P < 0.05, **P < 0.01 vs. DSS model group.
Fraxinellone treatment inhibited DSS-induced fibrosis in mice
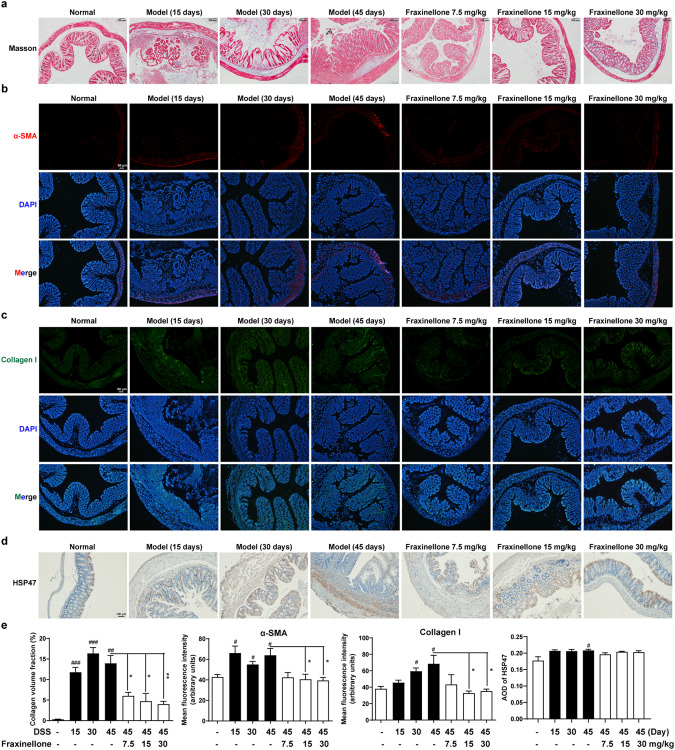
Then, through Masson staining, we found that fraxinellone can reduce collagen deposition (Fig. 2a). α-Smooth muscle actin (SMA) is a marker of myofibroblast activation, collagen I is one of the main collagen fibers of intestinal fibrosis, and they are important biomarkers of fibrosis [26]. Therefore, we used immunofluorescence experiments to detect α-SMA and collagen I levels in the colon, and the results showed that during fibrosis, α-SMA and collagen I expression levels increased, while fraxinellone significantly reduced α-SMA and collagen I levels (Fig. 2b, c). In addition, the expression levels of HSP47, a key collagen-specific chaperone, increased during fibrosis, but fraxinellone had no significant effect on its expression levels (Fig. 2d, e). These results suggest that fraxinellone can alleviate intestinal fibrosis in mice but has no significant effect on HSP47 protein levels.
Fig. 2. Fraxinellone treatment inhibited DSS-induced fibrosis in mice.
a Masson staining of colorectal tissue sections, Scale bar, 100 µm. b, c Immunofluorescence staining of α-SMA and Collagen I, Scale bar, 50 µm. d Colon tissue sections from each group were stained for HSP47. Scale bar, 100 µm. e Statistical analysis of (a–d). Data are presented as the mean ± SEM (n = 3–5 per group). #P < 0.05, ##P < 0.01, ###P < 0.001 vs. control group, *P < 0.05, **P < 0.01 vs. DSS model group.
Fraxinellone treatment reduced the levels of fibrosis biomarkers and cytokines
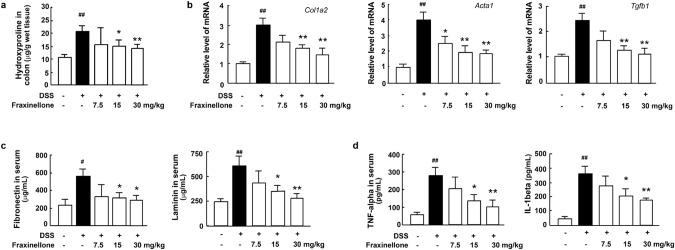
Hydroxyproline is an amino acid unique to collagen, so we characterized collagen levels by measuring the amount of hydroxyproline in colonic tissue. The results showed that fraxinellone significantly inhibited the DSS-induced increase in hydroxyproline levels (Fig. 3a). At the same time, the expression of the Col1a2, Acta1 and Tgfb1 genes in the model group induced by DSS increased significantly, and the expression of these genes could be downregulated by fraxinellone therapy in a dose-dependent manner (Fig. 3b). In addition to collagen, noncollagen glycoproteins such as fibronectin and laminin are also important components of the extracellular matrix (ECM) and play a key role in the fibrosis process [27]. We found that the serum levels of fibronectin, laminin and the inflammatory factors TNF-α and IL-1β in the model group also increased significantly, and fraxinellone treatment significantly reversed their elevation (Fig. 3c, d).
Fig. 3. Fraxinellone treatment reduced the levels of fibrosis biomarkers and cytokines.
a The level of colon hydroxyproline. b The mRNA expression levels of Col1a2, Acta1, and Tgfb1. c Serum fibronectin and laminin levels. d The levels of serum TNF-alpha and IL-1beta. Data are presented as the mean ± SEM (n = 5 per group). #P < 0.05, ##P < 0.01 vs. control group, *P < 0.05, **P < 0.01 vs. DSS model group.
TGF-β induced the formation of cell fibrosis models in vitro
The main feature of intestinal fibrosis is excessive deposition of ECM by activated myofibroblasts in the submucosa and muscle layers [28]. The myofibroblasts that produce ECM are mainly derived from epithelial cells, fibroblasts, etc. [28]. At present, most fibrosis research focuses on the activation of fibroblasts but ignores the irreplaceable role played by epithelial cells. During fibrosis, intestinal epithelial cells can not only directly transdifferentiate into myofibroblasts but also release key signals for myofibroblast differentiation through EMT [29]. The EMT process causes epithelial cells to lose their polarity and tight junctions and become mesenchymal cells, producing a large amount of ECM deposited in the intestine [30]. Studies have shown that approximately one-third of FSP1 (fibroblast marker) fibroblasts are derived from intestinal epithelial cells [31]. This evidence suggests that we should focus on the mechanism by which fraxinellone regulates intestinal epithelial cells.
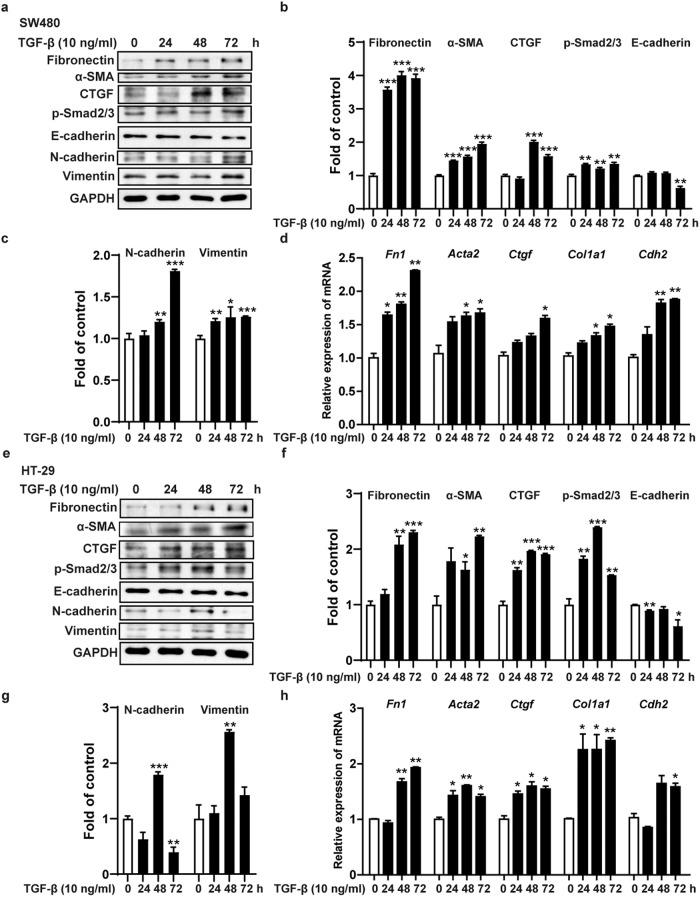
SW480 and HT-29 cells are colorectal cancer cells with epithelial properties. To further explore the mechanism by which fraxinellone alleviates intestinal fibrosis, we first used TGF-β to stimulate SW480 and HT-29 cells to establish in vitro intestinal fibrosis cell models. TGF-β is the main molecular mediator of the fibrosis process and can phosphorylate and activate Smad2/3 signaling and promote the transcription of target genes involved in fibrosis reactions [32]. Western blot experiments showed that TGF-β activated Smad2/3 signaling in SW480 and HT-29 cells, promoted the expression of the fibrosis biomarkers fibronectin, α-SMA and connective tissue growth factor (CTGF) and the mesenchymal cell markers N-cadherin and vimentin, and reduced the expression of the epithelial cell marker E-cadherin (Fig. 4a–c, e–g). At the same time, TGF-β facilitated the transcription of the Fn1, Acta2, Ctgf, Col1a1 and Cdh2 genes in SW480 and HT-29 cells (Fig. 4d, h).
Fig. 4. TGF-β induced the formation of cell fibrosis models in vitro.
a–c, e–g Immunoblot analysis of fibronectin, α-SMA, CTGF, p-Smad2/3, E-cadherin, N-cadherin, Vimentin and GAPDH. d, h The mRNA expression levels of Fn1, Acta2, Ctgf, Col1a1 and Cdh2. Data are presented as the mean ± SEM. *P < 0.05, **P < 0.01, ***P < 0.001 vs. 0 h.
Fraxinellone relieves cellular fibrosis by inhibiting TGF-β/Smad2/3 signaling
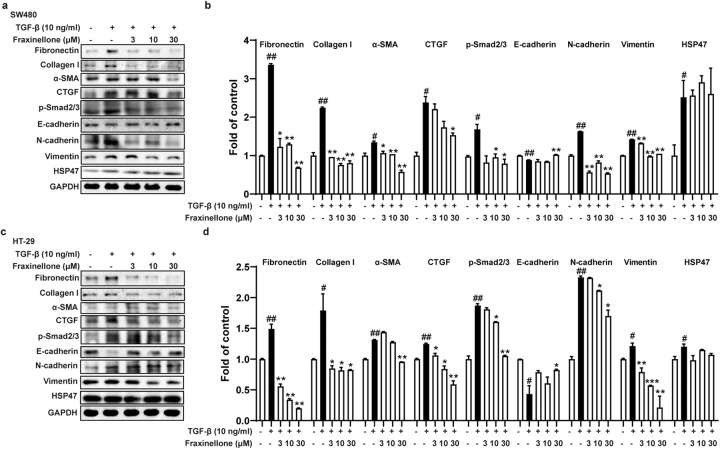
Next, we treated SW480 and HT-29 cells stimulated by TGF-β using fraxinellone. As shown in Fig. 5, fraxinellone significantly inhibited TGF-β-induced Smad2/3 signaling activation and increased the protein levels of the fibrosis biomarkers fibronectin, collagen I, α-SMA, and CTGF and the mesenchymal cell markers N-cadherin and vimentin in a dose-dependent manner. In addition, fraxinellone markedly promoted the expression of the epithelial cell marker E-cadherin but had no significant effect on the expression of HSP47.
Fig. 5. Fraxinellone relieved cellular fibrosis by inhibiting TGF-β/Smad2/3 signaling.
a–d Western blot and statistical analysis of fibronectin, collagen I, α-SMA, CTGF, p-Smad2/3, E-cadherin, N-cadherin, vimentin, HSP47 and GAPDH. Data are presented as the mean ± SEM. #P < 0.05, ##P < 0.01 vs. control, *P < 0.05, **P < 0.01, ***P < 0.001 vs. TGF-β.
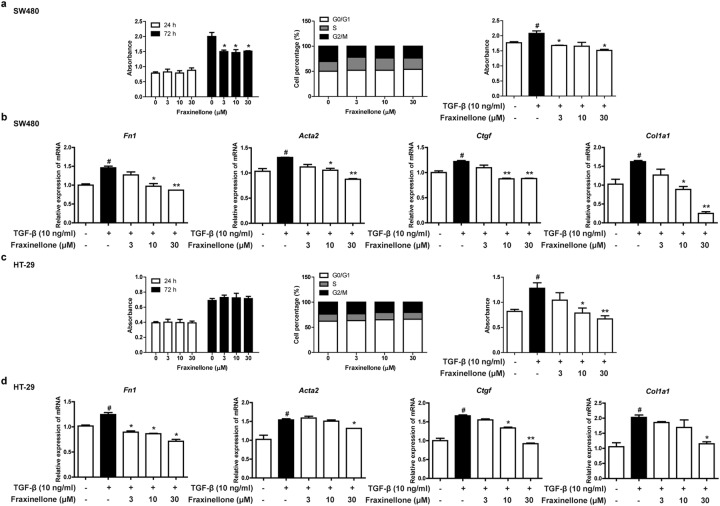
When SW480 and HT-29 cells were treated with fraxinellone alone for 24 h in the absence of TGF-β stimulation, it had no significant effect on their proliferation and cell cycle compared to the control group (Fig. 6a, c). However, when SW480 and HT-29 cells were treated with fraxinellone and TGF-β, fraxinellone inhibited TGF-β-induced cell proliferation (Fig. 6a, c). In addition, quantitative real-time PCR results showed the inhibitory effect of fraxinellone on the transcription level of fibrosis biomarkers (Fn1, Acta2, Ctgf, Col1a1) (Fig. 6b, d). These results reveal that fraxinellone can alleviate intestinal fibrosis by downregulating TGF-β/Smad2/3 signaling.
Fig. 6. Fraxinellone reduced the transcription of cellular fibrosis biomarkers.
a, c The effect of fraxinellone (0, 3, 10, 30 µM) on cell viability and the cell cycle with or without TGF-β (10 ng/mL). b, d The mRNA expression levels of Fn1, Acta2, Ctgf and Col1a1. Data are presented as the mean ± SEM. #P < 0.05 vs. control, *P < 0.05, **P < 0.01 vs. TGF-β.
Fraxinellone interfered with the HSP47-collagen complex by directly binding to HSP47
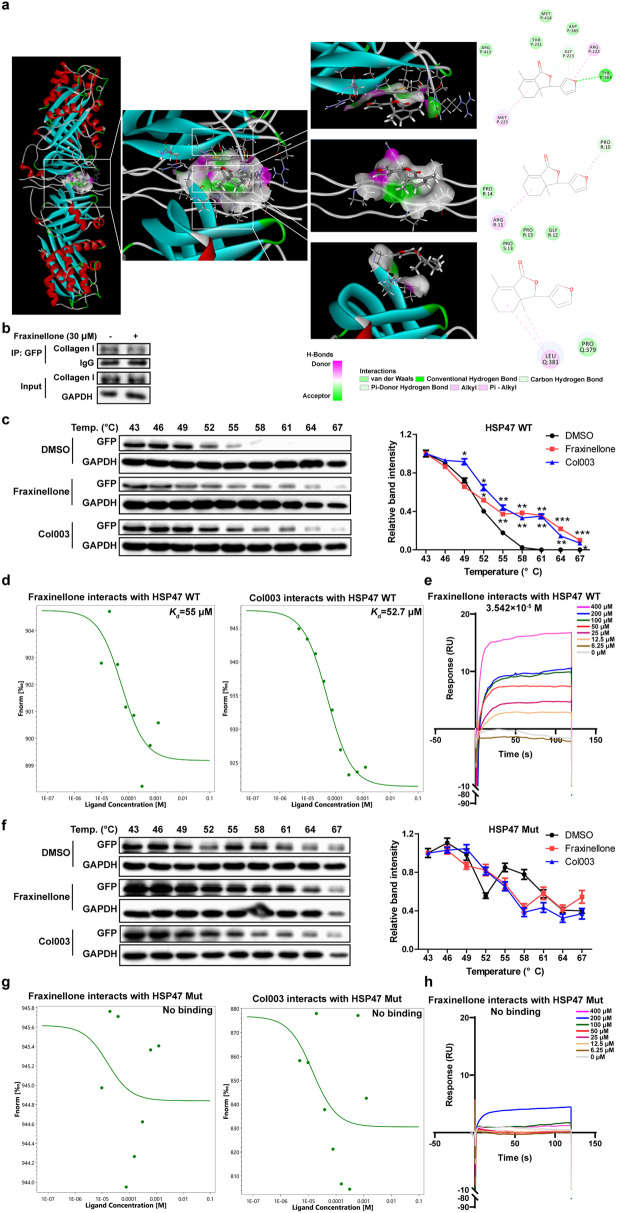
In addition to the classical TGF-β/Smad2/3 signaling pathway, an increasing number of studies have shown the importance of HSP47 in the fibrosis process. As a collagen-specific chaperone involved in collagen processing and secretion, HSP47 is closely related to abnormal collagen deposition in the ECM and is a promising therapeutic target for fibrosis [33]. Although fraxinellone does not significantly affect the expression of HSP47, it is unknown whether it affects the function of HSP47. We predicted the binding of fraxinellone to HSP47 (PDB ID: 3ZHA, -Cdocker interaction energy = 29.9859 kcal/mol) by molecular docking (Fig. 7a). Moreover, the interaction sites between fraxinellone and HSP47 overlapped with those between collagen and HSP47. This suggested that fraxinellone may disrupt the interaction between HSP47 and collagen, which was eventually confirmed by coimmunoprecipitation experiments (Fig. 7b). The Tyr383 and Asp385 sites of HSP47 are critical for its binding to collagen. Next, we constructed human HSP47-WT and HSP47-Mut (Y383A and D385A) plasmids, transfected them into HEK293T cells, prepared human HSP47-WT and HSP47-Mut recombinant proteins, used Col003 (HSP47 inhibitor) as a positive control, and proved that fraxinellone can directly bind to HSP47-WT rather than HSP47-Mut through a cellular thermal shift assay, microscale thermophoresis and surface plasmon resonance (SPR) (Fig. 7c–h). The SPR results showed that fraxinellone had a high affinity for HSP47 with an equilibrium dissociation constant (Kd) value of 3.542 × 10−5 M (Fig. 7e). These results illustrate that fraxinellone can interfere with the HSP47-collagen complex by directly binding to HSP47 and that the Tyr383 and Asp385 sites of HSP47 are important for the interaction between HSP47 and fraxinellone.
Fig. 7. Fraxinellone interfered with the HSP47-collagen complex by directly binding to HSP47.
a Molecular docking analysis of fraxinellone and the HSP47-collagen complex. b Proteins were isolated and immunoprecipitated with an antibody against GFP. c The effect of fraxinellone and Col003 on the thermal stability of human HSP47 WT. d The binding ability between human HSP47 WT and fraxinellone or Col003. e Dose response curves for a gradient dilution of fraxinellone binding to HSP47-WT were measured by SPR. f The effect of fraxinellone and Col003 on the thermal stability of the human HSP47 Mut. g The binding ability between human HSP47 Mut and fraxinellone or Col003. h Dose response curves for a gradient dilution of fraxinellone binding to HSP47-Mut were measured by SPR. Data are presented as the mean ± SEM. *P < 0.05, **P < 0.01 vs. DMSO.
Discussion
Intestinal fibrosis, which afflicts many IBD patients, is a serious and common complication caused by the response of intestinal tissue to chronic inflammation [34]. It is characterized by excessive ECM deposition, scarring of various tissues, impaired function and organ damage [35]. There are currently no approved antifibrotic therapies for IBD, so there is an urgent need to find new therapeutic targets and approaches.
Fraxinellone is a natural compound isolated from the root bark of Dictamnus dasycarpus that has been found to have antibacterial, insecticidal, anti-inflammatory, and anticancer activities [19, 36, 37]. The role of fraxinellone in the treatment of hepatic and renal fibrosis has also been demonstrated [22, 23], but its effect on intestinal fibrosis is unknown. Our study found that fraxinellone can effectively relieve DSS-induced intestinal fibrosis in mice and reduce the levels of fibrosis biomarkers and inflammatory factors (Figs. 1–3).
In the process of intestinal fibrosis, myofibroblasts cause excessive deposition of ECM, and myofibroblasts are mainly derived from fibroblasts, epithelial cells, etc [28, 38]. Fibroblasts can be produced by epithelial cell transformation, and epithelial-mesenchymal and endothelial-mesenchymal transformation can lead to fibrosis [39, 40]. Most fibrosis research focuses on the activation of fibroblasts but neglects the irreplaceable role of epithelial cells. Our study focused on the effect of fraxinellone on the pathological changes of epithelial cells during intestinal fibrosis and demonstrated that fraxinellone can reduce the level of fibrosis biomarkers and inhibit EMT in intestinal epithelial cells by downregulating the TGF-β/Smad2/3 signaling pathway (Figs. 4–6).
In addition, we found that fraxinellone can also affect collagen processing and secretion through HSP47. As a collagen-specific chaperone, HSP47 plays a critical role in the intracellular processing of collagen [41]. Its Asp385 site can form a salt bridge with collagen, and Leu381 and Tyr383 are essential for hydrophobic interactions between HSP47 and collagen [16, 42]. HSP47 is a very promising therapeutic target for fibrotic diseases, but its application in the treatment of intestinal fibrosis is still insufficient. At present, only Col003 has been reported to interfere with the HSP47-collagen complex, and it can alleviate cardiac fibrosis by inhibiting HSP47, but its role in intestinal fibrosis has not been reported [43]. Our study revealed for the first time that fraxinellone can directly bind to HSP47 and destroy the HSP47-collagen complex (Fig. 7). Furthermore, as a natural compound, fraxinellone is safer and easier to use in clinical practice than Col003.
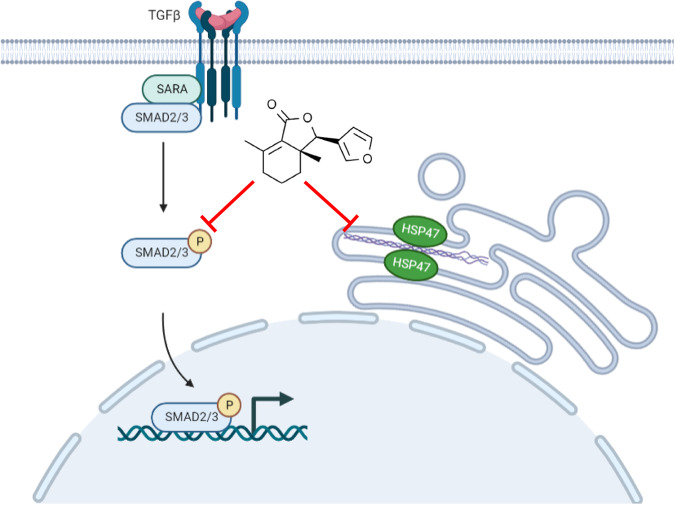
In conclusion, our study is the first to evaluate the safety and efficacy of fraxinellone treatment in intestinal fibrosis, elucidate its molecular mechanism (Fig. 8), and provide new drug candidates and targets for the clinical treatment of intestinal fibrosis.
Fig. 8.
Schematic diagram of the mechanism by which fraxinellone relieves intestinal fibrosis.
Supplementary information
Acknowledgements
This work was supported by the National Natural Science Foundation of China (Nos. 82073910, 82173871, 21937005), the Open Fund of State Key Laboratory of Pharmaceutical Biotechnology, Nanjing University, China (Grant No. KF-GN-202101), and Fundamental Research Funds for the Central University (021414380503).
Author contributions
JW, XFW, and QX designed research. JW, MB, CZ, NA, and LW performed research and analyzed data. XNW, RHD, YS, XDW, XFW, ZYY, and QX contributed new reagents. JW and XFW wrote the paper. All of the authors have approved the final manuscript.
Competing interests
The authors declare no competing interests.
Contributor Information
Xu-dong Wu, Email: xudongwu@nju.edu.cn.
Xue-feng Wu, Email: wuxf@nju.edu.cn.
Qiang Xu, Email: molpharm@163.com.
Supplementary information
The online version contains supplementary material available at 10.1038/s41401-023-01143-1.
References
- 1.Bamias G, Pizarro TT, Cominelli F. Immunological regulation of intestinal fibrosis in inflammatory bowel disease. Inflamm Bowel Dis. 2022;28:337–49. doi: 10.1093/ibd/izab251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Silverstein MD, Loftus EV, Sandborn WJ, Tremaine WJ, Feagan BG, Nietert PJ, et al. Clinical course and costs of care for Crohn’s disease: Markov model analysis of a population-based cohort. Gastroenterology. 1999;117:49–57. doi: 10.1016/S0016-5085(99)70549-4. [DOI] [PubMed] [Google Scholar]
- 3.Gordon IO, Agrawal N, Willis E, Goldblum JR, Lopez R, Allende D, et al. Fibrosis in ulcerative colitis is directly linked to severity and chronicity of mucosal inflammation. Aliment Pharmacol Ther. 2018;47:922–39. doi: 10.1111/apt.14526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Henderson NC, Rieder F, Wynn TA. Fibrosis: from mechanisms to medicines. Nature. 2020;587:555–66. doi: 10.1038/s41586-020-2938-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vallance BA, Gunawan MI, Hewlett B, Bercik P, Van Kampen C, Galeazzi F, et al. TGF-beta1 gene transfer to the mouse colon leads to intestinal fibrosis. Am J Physiol Gastrointest Liver Physiol. 2005;289:G116–28. doi: 10.1152/ajpgi.00051.2005. [DOI] [PubMed] [Google Scholar]
- 6.Letterio JJ, Roberts AB. Regulation of immune responses by TGF-beta. Annu Rev Immunol. 1998;16:137–61. doi: 10.1146/annurev.immunol.16.1.137. [DOI] [PubMed] [Google Scholar]
- 7.Amamou A, Yaker L, Leboutte M, Bole-Feysot C, Savoye G, Marion-Letellier R. Dietary AhR ligands have no anti-fibrotic properties in TGF-beta1-stimulated human colonic fibroblasts. Nutrients. 2022;14:3253. [DOI] [PMC free article] [PubMed]
- 8.Wang Y, Zhang Y, Lu B, Xi J, Ocansey DKW, Mao F, et al. hucMSC-Ex alleviates IBD-associated intestinal fibrosis by inhibiting ERK phosphorylation in intestinal fibroblasts. Stem Cells Int. 2023;2023:2828981. doi: 10.1155/2023/2828981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pallotta N, Barberani F, Hassan NA, Guagnozzi D, Vincoli G, Corazziari E. Effect of infliximab on small bowel stenoses in patients with Crohn’s disease. World J Gastroenterol. 2008;14:1885–90. doi: 10.3748/wjg.14.1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Calabrese E, Petruzziello C, Onali S, Condino G, Zorzi F, Pallone F, et al. Severity of postoperative recurrence in Crohn’s disease: correlation between endoscopic and sonographic findings. Inflamm Bowel Dis. 2009;15:1635–42. doi: 10.1002/ibd.20948. [DOI] [PubMed] [Google Scholar]
- 11.Scheibe K, Kersten C, Schmied A, Vieth M, Primbs T, Carle B, et al. Inhibiting interleukin 36 receptor signaling reduces fibrosis in mice with chronic intestinal inflammation. Gastroenterology. 2019;156:1082–97. doi: 10.1053/j.gastro.2018.11.029. [DOI] [PubMed] [Google Scholar]
- 12.D’Haens G, Rieder F, Feagan BG, Higgins PDR, Panes J, Maaser C, et al. Challenges in the pathophysiology, diagnosis, and management of intestinal fibrosis in inflammatory bowel disease. Gastroenterology. 2022;162:26–31. doi: 10.1053/j.gastro.2019.05.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ito S, Nagata K. Biology of Hsp47 (Serpin H1), a collagen-specific molecular chaperone. Semin Cell Dev Biol. 2017;62:142–51. doi: 10.1016/j.semcdb.2016.11.005. [DOI] [PubMed] [Google Scholar]
- 14.Niwa T, Kanamori T, Ueda T, Taguchi H. Global analysis of chaperone effects using a reconstituted cell-free translation system. Proc Natl Acad Sci USA. 2012;109:8937–42. doi: 10.1073/pnas.1201380109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhu J, Xiong G, Fu H, Evers BM, Zhou BP, Xu R. Chaperone Hsp47 drives malignant growth and invasion by modulating an ECM gene network. Cancer Res. 2015;75:1580–91. doi: 10.1158/0008-5472.CAN-14-1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Widmer C, Gebauer JM, Brunstein E, Rosenbaum S, Zaucke F, Drogemuller C, et al. Molecular basis for the action of the collagen-specific chaperone Hsp47/SERPINH1 and its structure-specific client recognition. Proc Natl Acad Sci USA. 2012;109:13243–7. doi: 10.1073/pnas.1208072109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Abd El-Fattah EE, Zakaria AY. Targeting HSP47 and HSP70: promising therapeutic approaches in liver fibrosis management. J Transl Med. 2022;20:544. doi: 10.1186/s12967-022-03759-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bellaye PS, Burgy O, Bonniaud P, Kolb M. HSP47: a potential target for fibrotic diseases and implications for therapy. Expert Opin Ther Targets. 2021;25:49–62. doi: 10.1080/14728222.2021.1861249. [DOI] [PubMed] [Google Scholar]
- 19.Bailly C, Vergoten G. Fraxinellone: From pesticidal control to cancer treatment. Pestic Biochem Physiol. 2020;168:104624. doi: 10.1016/j.pestbp.2020.104624. [DOI] [PubMed] [Google Scholar]
- 20.Yoon JS, Yang H, Kim SH, Sung SH, Kim YC. Limonoids from Dictamnus dasycarpus protect against glutamate-induced toxicity in primary cultured rat cortical cells. J Mol Neurosci. 2010;42:9–16. doi: 10.1007/s12031-010-9333-1. [DOI] [PubMed] [Google Scholar]
- 21.Wu XF, Ouyang ZJ, Feng LL, Chen G, Guo WJ, Shen Y, et al. Suppression of NF-kappaB signaling and NLRP3 inflammasome activation in macrophages is responsible for the amelioration of experimental murine colitis by the natural compound fraxinellone. Toxicol Appl Pharmacol. 2014;281:146–56. doi: 10.1016/j.taap.2014.10.002. [DOI] [PubMed] [Google Scholar]
- 22.Wu X, Wu X, Ma Y, Shao F, Tan Y, Tan T, et al. CUG-binding protein 1 regulates HSC activation and liver fibrogenesis. Nat Commun. 2016;7:13498. doi: 10.1038/ncomms13498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zheng B, Yuan M, Wang S, Tan Y, Xu Y, Ye J, et al. Fraxinellone alleviates kidney fibrosis by inhibiting CUG-binding protein 1-mediated fibroblast activation. Toxicol Appl Pharmacol. 2021;420:115530. doi: 10.1016/j.taap.2021.115530. [DOI] [PubMed] [Google Scholar]
- 24.Wang J, Shi K, Li S, Chen L, Liu W, Wu X, et al. Meisoindigo attenuates dextran sulfate sodium-induced experimental colitis via its inhibition of TAK1 in macrophages. Int Immunopharmacol. 2021;101:108239. doi: 10.1016/j.intimp.2021.108239. [DOI] [PubMed] [Google Scholar]
- 25.Wang J, Shi K, An N, Li S, Bai M, Wu X, et al. Direct Inhibition of GSDMD by PEITC Reduces Hepatocyte Pyroptosis and Alleviates Acute Liver Injury in Mice. Front Immunol. 2022;13:825428. doi: 10.3389/fimmu.2022.825428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lu NH, Zhao HQ, Jing M, Liu X, Ren CZ, Liu XF, et al. The pharmacodynamic active components study of Tibetan medicine Gentianopsis paludosa on ulcerative colitis fibrosis. Int Immunopharmacol. 2017;46:163–9. doi: 10.1016/j.intimp.2017.01.001. [DOI] [PubMed] [Google Scholar]
- 27.Daulagala AC, Kourtidis A. ECM Substrates Impact RNAi localization at adherens junctions of colon epithelial cells. Cells 2022;11:3740. [DOI] [PMC free article] [PubMed]
- 28.Vieujean S, Hu S, Bequet E, Salee C, Massot C, Bletard N, et al. Potential role of epithelial endoplasmic reticulum stress and anterior gradient protein 2 homologhomologue in Crohn’s disease fibrosis. J Crohns Colitis. 2021;15:1737–50. doi: 10.1093/ecco-jcc/jjab061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yu M, Wu H, Wang J, Chen X, Pan J, Liu P, et al. Vitamin D receptor inhibits EMT via regulation of the epithelial mitochondrial function in intestinal fibrosis. J Biol Chem. 2021;296:100531. doi: 10.1016/j.jbc.2021.100531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wenxiu J, Mingyue Y, Fei H, Yuxin L, Mengyao W, Chenyang L, et al. Effect and mechanism of TL1A expression on epithelial-mesenchymal transition during chronic colitis-related intestinal fibrosis. Mediators Inflamm. 2021;2021:5927064. doi: 10.1155/2021/5927064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Flier SN, Tanjore H, Kokkotou EG, Sugimoto H, Zeisberg M, Kalluri R. Identification of epithelial to mesenchymal transition as a novel source of fibroblasts in intestinal fibrosis. J Biol Chem. 2010;285:20202–12. doi: 10.1074/jbc.M110.102012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Derynck R, Zhang YE. Smad-dependent and Smad-independent pathways in TGF-beta family signalingsignalling. Nature. 2003;425:577–84. doi: 10.1038/nature02006. [DOI] [PubMed] [Google Scholar]
- 33.Cai H, Sasikumar P, Little G, Bihan D, Hamaia SW, Zhou A, et al. Identification of HSP47 binding site on native collagen and its implications for the development of HSP47 inhibitors. Biomolecules. 2021;11:983. [DOI] [PMC free article] [PubMed]
- 34.Rieder F, Fiocchi C. Intestinal fibrosis in IBD–a dynamic, multifactorial process. Nat Rev Gastroenterol Hepatol. 2009;6:228–35. doi: 10.1038/nrgastro.2009.31. [DOI] [PubMed] [Google Scholar]
- 35.Wang J, Lin S, Brown JM, van Wagoner D, Fiocchi C, Rieder F. Novel mechanisms and clinical trial endpoints in intestinal fibrosis. Immunol Rev. 2021;302:211–27. doi: 10.1111/imr.12974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shi J, Sun S, Xing S, Huang C, Huang Y, Wang Q, et al. Fraxinellone inhibits progression of glioblastoma by regulating via regulating the SIRT3 signaling pathway. Biomed Pharmacother. 2022;153:113416. doi: 10.1016/j.biopha.2022.113416. [DOI] [PubMed] [Google Scholar]
- 37.Kim MJ, Bae GS, Jo IJ, Choi SB, Kim DG, Jung HJ, et al. Fraxinellone inhibits inflammatory cell infiltration during acute pancreatitis by suppressing inflammasome activation. Int Immunopharmacol. 2019;69:169–77. doi: 10.1016/j.intimp.2019.01.043. [DOI] [PubMed] [Google Scholar]
- 38.Wu F, Shao Q, Hu M, Zhao Y, Dong R, Fang K, et al. Wu-Mei-Wan ameliorates chronic colitis-associated intestinal fibrosis through inhibiting fibroblast activation. J Ethnopharmacol. 2020;252:112580. doi: 10.1016/j.jep.2020.112580. [DOI] [PubMed] [Google Scholar]
- 39.Lovisa S, Genovese G, Danese S. Role of Epithelial-to-mesenchymal transition in inflammatory bowel disease. J Crohns Colitis. 2019;13:659–68. doi: 10.1093/ecco-jcc/jjy201. [DOI] [PubMed] [Google Scholar]
- 40.Bataille F, Rohrmeier C, Bates R, Weber A, Rieder F, Brenmoehl J, et al. Evidence for a role of epithelial mesenchymal transition during pathogenesis of fistulae in Crohn’s disease. Inflamm Bowel Dis. 2008;14:1514–27. doi: 10.1002/ibd.20590. [DOI] [PubMed] [Google Scholar]
- 41.Miyamura T, Sakamoto N, Kakugawa T, Taniguchi H, Akiyama Y, Okuno D, et al. Small molecule inhibitor of HSP47 prevents pro-fibrotic mechanisms of fibroblasts in vitro. Biochem Biophys Res Commun. 2020;530:561–5. doi: 10.1016/j.bbrc.2020.07.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Abraham ET, Oecal S, Morgelin M, Schmid PWN, Buchner J, Baumann U, et al. Collagen’s primary structure determines collagen:HSP47 complex stoichiometry. J Biol Chem. 2021;297:101169. doi: 10.1016/j.jbc.2021.101169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Xie S, Xing Y, Shi W, Zhang M, Chen M, Fang W, et al. Cardiac fibroblast heat shock protein 47 aggravates cardiac fibrosis post myocardial ischemia-reperfusion injury by encouraging ubiquitin specific peptidase 10 dependent Smad4 deubiquitination. Acta Pharm Sin B. 2022;12:4138–53. doi: 10.1016/j.apsb.2022.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.