Abstract
Diabetic peripheral neuropathy (DPN) is a common complication of diabetes, which has yet no curable medication. Neuroinflammation and mitochondrial dysfunction are tightly linked to DPN pathology. G-protein-coupled receptor 40 (GPR40) is predominantly expressed in pancreatic β-cells, but also in spinal dorsal horn and dorsal root ganglion (DRG) neurons, regulating neuropathic pain. We previously have reported that vincamine (Vin), a monoterpenoid indole alkaloid extracted from Madagascar periwinkle, is a GPR40 agonist. In this study, we evaluated the therapeutic potential of Vin in ameliorating the DPN-like pathology in diabetic mice. Both STZ-induced type 1 (T1DM) and db/db type 2 diabetic (T2DM) mice were used to establish late-stage DPN model (DPN mice), which were administered Vin (30 mg·kg-1·d-1, i.p.) for 4 weeks. We showed that Vin administration did not lower blood glucose levels, but significantly ameliorated neurological dysfunctions in DPN mice. Vin administration improved the blood flow velocities and blood perfusion areas of foot pads and sciatic nerve tissues in DPN mice. We demonstrated that Vin administration protected against sciatic nerve myelin sheath injury and ameliorated foot skin intraepidermal nerve fiber (IENF) density impairment in DPN mice. Moreover, Vin suppressed NLRP3 inflammasome activation through either β-Arrestin2 or β-Arrestin2/IκBα/NF-κB signaling, improved mitochondrial dysfunction through CaMKKβ/AMPK/SIRT1/PGC-1α signaling and alleviated oxidative stress through Nrf2 signaling in the sciatic nerve tissues of DPN mice and LPS/ATP-treated RSC96 cells. All the above-mentioned beneficial effects of Vin were abolished by GPR40-specific knockdown in dorsal root ganglia and sciatic nerve tissues. Together, these results support that pharmacological activation of GPR40 as a promising therapeutic strategy for DPN and highlight the potential of Vin in the treatment of this disease.
Keywords: diabetic peripheral neuropathy, vincamine, GPR40, dorsal root ganglia, sciatic nerve
Introduction
Diabetic peripheral neuropathy (DPN) is a common complication of diabetes and the leading cause of diabetic foot ulcers (DFU), afflicting more than two-thirds of diabetic patients [1, 2]. DPN is clinically characterized by pain, paraesthesia, and numbness [3]. Unfortunately, current available therapeutic strategy can only alleviate the symptoms or delay the progression of DPN [4].
Pathologically, neuroinflammation plays an important role in DPN occurrence and development, and varied pro-inflammatory cytokines including TNF-α and IL-6 have been determined in the plasma of DPN model mice and patients [1]. In addition, mitochondrial dysfunction is also tightly linked to DPN pathology, in that inner mitochondrial membrane potential (MMP) is depolarized and respiratory chain activity is reduced in DRG neurons of diabetic mice [5], while MMP depolarization is associated with induction of reactive oxygen species (ROS) that is contributable to oxidative burden [6, 7]. Moreover, neuroinflammation and mitochondrial dysfunction are also severely related to the destruction of microvasculature supply to the peripheral nerves that correlate closely with DPN progression.
G protein-coupled receptor 40 (GPR40) is a seven-transmembrane domain receptor that is predominantly expressed in pancreatic β-cells and activated by medium to long-chain free fatty acids (FFAs) [8, 9]. GPR40 was recognized as a potent regulator of glucose homeostasis and its agonist is believed to be promising for anti-type 2 diabetes mellitus (T2DM) drug discovery [10]. In addition, GPR40 is also found to be expressed in spinal dorsal horn (SDH) and dorsal root ganglion (DRG) neurons, participating in the regulation of neuropathic pain [8]. It was reported that ω-3 FAs suppressed inflammation and improved neurological dysfunctions through GPR40 [10, 11]. Given that DPN is a common complication of diabetes with malfunctional pain-related behaviors by major clinical manifestations and that GPR40 regulation is linked to inflammation and neurological dysfunctions, we speculate that GPR40 regulation may be implicated in DPN pathology and GPR40 activation may provide beneficial effects on DPN.
Vincamine (Vin, Fig. 1a) is a monoterpenoid indole alkaloid extracted from Madagascar periwinkle [12] and was reported to be a GPR40 activator in our previous work [13]. Clinically, Vin is being used to treat cerebrovascular diseases by improving the cognitive impairment of the patients based on its capability in increasing blood flow to the brain and oxygen consumption [12, 13]. Thus, by considering that the already obtained pharmacokinetics and safety data of Vin may greatly save the development cycle and cost in new drug discovery based on Vin as a lead compound, we here performed the study to evaluate the potential of Vin as a GPR40 activator in ameliorating the DPN-like pathology in mice.
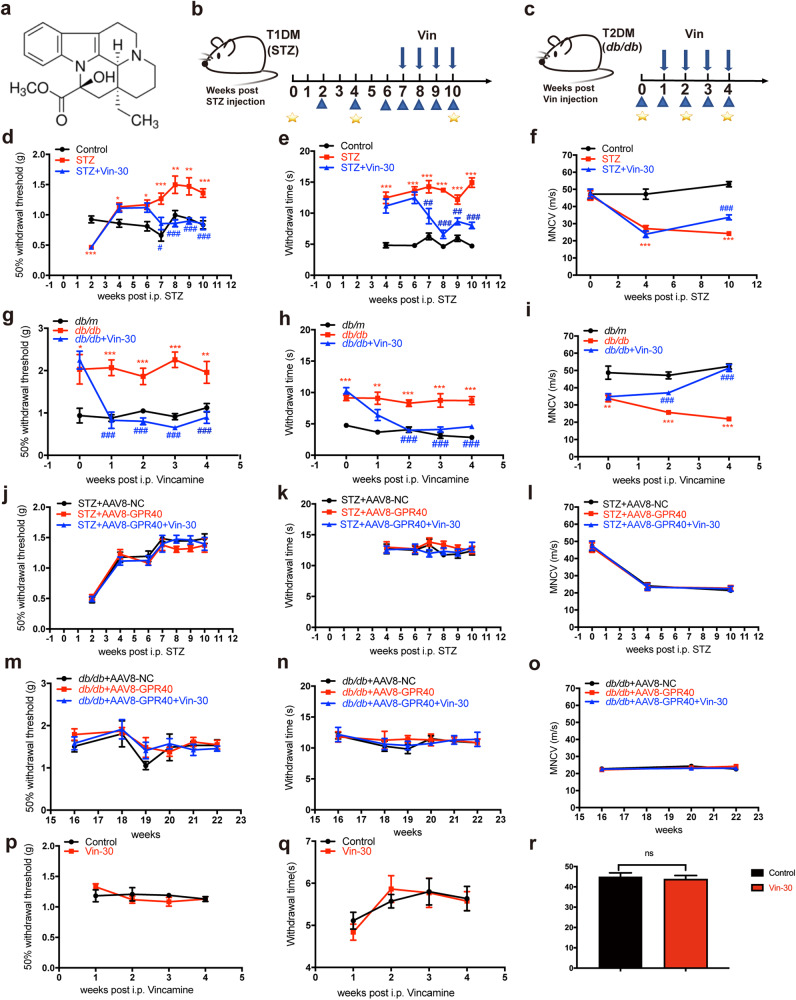
Fig. 1. Vin improved neurological dysfunctions in DPN mice through GPR40.
a Structure of Vin. b, c Animal experimental schedule for STZ and db/db mice. Blue arrows above axis indicated Vin injection, blue triangles below axis indicated behavioral tests (tactile allodynia and thermal sensitivity), and yellow five-pointed stars below axis indicated motor nerve conduction velocity (MNCV). d–i Vin-30 improved neurological dysfunctions as assayed against (d, g) tactile allodynia, (e, h) thermal response test and (f, i) motor nerve conduction velocity (MNCV) in (d–f) STZ and (g–i) db/db mice (n = 8 per group). j–o Vin-30 rendered no impacts on neurological functions in AAV8-GPR40 injected DPN mice (n = 8 per group). p–r Vin had no impacts on (p) tactile allodynia, (q) thermal sensitivity or (r) MNCV in non-diabetic mice (n = 8 per group). All values were presented as mean ± SEM, *P < 0.05, **P < 0.01, ***P < 0.001 vs Control, db/m, STZ + AAV8-NC, db/db + AAV8-NC mice (Student’s t test); #P < 0.05, ##P < 0.01, ###P < 0.001 vs STZ or db/db mice (One-way ANOVA with Dunnett’s post-hoc test).
In the current work, we reported that Vin targeted GPR40 to efficiently ameliorate the pathology of late-stage DPN mice with hypoalgesia. The underlying mechanisms were intensively investigated by assay against the diabetic mice (including both treptozotocin (STZ)-induced type 1 (T1DM) and db/db type 2 diabetic (T2DM) mice) with in vivo GPR40 knockdown via injection of adeno associated virus (AAV)8-GPR40-RNAi. Our results have highly supported that GPR40 activation may show promise as a therapeutic strategy for DPN and highlighted the potential of Vin in the treatment of this disease.
Materials and methods
Materials
Vincamine (Vin) was purchased from Absin (Shanghai, China). NF-κB inhibitor (pyrrolidinedithiocarbamate ammonium, PDTC), AMPK inhibitor (Compound C), Nrf2 inhibitor (ML385), GPR40 inhibitor (GW1100) and GPR40 activators (GW9508 and TAK-875) were purchased from MedChemExpress (Shanghai, China). FFAR1 ligand (γ-linolenic acid, γ-LA) was purchased from Sigma-Aldrich (St. Louis, MO, USA). CaMKKβ inhibitor (STO-69) was purchased from TargetMol (Shanghai, China). LKB1 inhibitor (radicicol) was purchased from Aladdin (Shanghai, China). β-Arrestin2 siRNA was purchased from Genomeditech (Shanghai, China) and GPR40 siRNA was purchased from Sangon (Shanghai, China).
Animals
Mice were kept in SPF facility and received humane care. All mice experiments were approved by the Institutional Animal Care and Use Committees at Nanjing University of Chinese Medicine. All animals were maintained in compliance with the Regulations for the Administration of Affairs Concerning Experimental Animals made by the Ministry of Science and Technology of China. All animal-related experiments followed the Institutional Ethical Guidelines on Animal Care of Nanjing University of Chinese Medicine (Ethics Numbers: 202103A020 and 202109A007).
Seven-week-old male C57BL/6J mice were purchased from Beijing Vital River Laboratory Animal Technology Co., Ltd (Beijing, China), and 17-week-old male BKS Cg-m+/+Leprdb/J (db/db) mice were purchased from Model Animal Research Center of Nanjing University (Nanjing, China). By considering that loss of sensation caused by late-stage DPN is a high-risk factor to diabetic foot ulcers (DFUs) [14], we constructed the late-stage DPN mice model with hypoalgesia according to the published reports [15, 16].
T1DM mice with DPN
T1DM mice with DPN were prepared by published approach [16]. Briefly, 8-week-old male C57BL/6J mice were intraperitoneally injected with a single 150 mg/kg STZ (Sigma-Aldrich). Non-diabetic mice (Control, age-matched C57BL/6 mice as control in T1DM mice related assay) were injected with the same volume of vehicle buffer (Na-Citrate Buffer, pH 4.5). T1DM mice (characterized by absolute insulin deficiency) were defined with blood glucose level over 16 mmol/L and T1DM mice with DPN (STZ) (characterized by pain, paraesthesia and numbness) were defined at 12 weeks of age (4 weeks after STZ injection) through behavioral tests (increases in 50% paw withdrawal threshold and thermal response latencies and a decrease in MNCV).
T2DM mice with DPN
Eighteen-week-old db/db mice were treated as T2DM mice (characterized by relative lack of insulin) with DPN (db/db) (characterized by pain, paraesthesia and numbness, and defined through behavioral tests) [15]. Age-matched heterozygotes mice with nonpenetrant genotype (db/m) were used as control in db/db mice related assay.
GPR40-specific knockdown in diabetic mice
Adeno associated virus (AAV)8-GPR40-RNAi (AAV8-GPR40) and its negative control vector (AAV8-NC) were purchased from Shanghai Genechem Co., Ltd. STZ mice and db/db mice were both injected with AAV8-GPR40 or AAV8-NC to tibialis anterior and gastrocnemius muscles (1.1 × 1011 vector genomes/mouse). GPR40 knockdown efficiency of mice was detected 2 weeks after AAV injection by Western blot assay (Supplementary Fig. S1).
AAV8-NC rendered no influences on DPN pathology in mice
Notably, AAV8-NC had no effects on the key proteins of p-NF-κB, NLRP3, COXIV or NDUFS3 in either STZ or db/db mice (Supplementary Fig. S2 and S3), while rendered no influences on the tactile allodynia, thermal sensitivity or motor nerve conduction velocity in db/db mice (Supplementary Fig. S4 and Supplementary Tables S1–S3). Given that the pathology of STZ mice was much similar to that of db/db mice [16, 17] and that the proteins of p-NF-κB, NLRP3, COXIV and NDUFS3 are highly related to inflammation and mitochondrial function in either STZ or db/db mice, we suggested that AAV8-NC had no influences on the DPN-like pathology in mice.
Animal administration
Vin was dissolved in physiological saline with 4% DMSO (Solarbio) and 4% Tween-80 (Solarbio). Non-diabetic C57BL/6 mice, STZ mice, db/db mice, AAV8-GPR40 injected STZ (STZ + AAV8-GPR40) or db/db (db/db + AAV8-GPR40) mice were daily administered with 30 mg/kg of Vin (Vin-30, in vivo Vin concentration was set up according to our previous report [13]) by intraperitoneal injection for 4 weeks (n = 12 per group). Control and db/m mice (n = 12 per group) were administered with the same volume of vehicle buffer as that for DPN mice.
Tactile allodynia and thermal sensitivity determination
Tactile allodynia
The hind paw of mice was allowed to touch Von Frey filaments (Ugo Basile, Italy) for measuring tactile allodynia as previously described [18]. Briefly, mice were placed under transparent plastic boxes and stood on a metal mesh floor with hind paw full contact for at least 15 min to suit surroundings before testing. Then hind paws were touched by diffident Von Frey filaments for 6–8 s to confirm the stimulus response while the filament was bent. A removal of hind paw withdrawal to von frey filament application was recorded as a positive response. The area tested included the distribution of the sciatic nerve in mid-plantar left hind paw. A 50% paw withdrawal threshold was calculated by the recorded values and analyzed by GraphPad Prism 8 (GraphPad, San Diego, California, USA).
Thermal sensitivity
Heat sensitivity was assessed as previously described [19]. Briefly, mice were placed under transparent plastic boxes and stood on the platform of the thermal testing apparatus (Ugo Basile) and adapted to surroundings for at least 15 min. The paw withdrawal latency was recorded from the onset of the irradiation (lamp 40 W, distance lamp to paw 40 mm) to the withdrawal of the hind paw at 25 °C by a movable radiant heat source. The average withdrawal time of three times was used for statistical analysis.
Electrophysiology test
Motor nerve conduction velocity was measured as previously described [6]. Briefly, mice were anesthetized and placed on a heating pad maintained at 37 °C to provide thermal support. The sciatic nerve was stimulated proximally at the sciatic notch and distally at the ankle by a supramaximal stimulus (3 V) of 0.05 ms duration. The motor nerve conduction velocity (MNCV) was recorded by measuring the latency from the stimulus artifact to the onset of the negative M-wave deflection of the compound muscle action potentials recorded from the first interosseous muscle and divided by the distance (in millimeters) between the electrodes.
Regional blood flow velocity and perfused blood vessel area assessments
After 4 weeks of Vin administration, mice were anesthetized with isoflurane (anesthesia machine, RWD, China), and a constant temperature heating pad was applied to make uniform temperatures during measurements. The real-time regional blood flow velocity and perfused blood vessel area in foot pads and sciatic nerve tissues were detected by Laser Speckle Contrast Imaging/LSCI (RFLSI Pro, RWD, China). The time for measurement per mouse was about 2 min.
Enzyme-linked immunosorbent assay (ELISA) and biochemical measurements
Levels of inflammatory cytokines TNF-α and IL-6 in serum of mice were detected by ELISA using commercial kits (Nanjing Jiancheng Bioengineering Institute).
Serum insulin level of mice was detected by ELISA using commercial kits (Mercodia, Sweden).
Activity of SOD and levels of GSH and MDA in serum of mice were determined using commercial kits by manufacturer’s protocols (Nanjing Jiancheng Bioengineering Institute).
Triglyceride (TG) and total cholesterol (TC) levels in serum of mice were determined using commercial kits by manufacturer’s protocols (Nanjing Jiancheng Bioengineering Institute).
Cell culture
hGPR40-CHO cells
CHO cells were stably transfected to express hGPR40 and cultured in Ham’s F12 medium supplemented with 10% FBS (Gibco), 0.4 mg/mL Geneticin (YEASEN, Shanghai, China) and 0.25 mg/mL Zeocin (Invitrogen, USA).
RSC96 cells
Rat SCs (RSC96) were cultured in DMEM (high glucose) (Hyclone, USA) containing 10% FBS, 100 U/mL penicillin and 100 mg/mL streptomycin (PS).
DRG neurons isolated and cultured from adult mice
Isolation of primary DRG neurons from mice was performed by published approach [5]. Briefly, mice were anesthetized and the spinal cord was removed after the spinal canal severed with micro-scissors. DRG tisssues were collected into a 35 mm petri dish with 2 mL ice-cold serum-free medium by using micro-scissors under a microscop. Then, DRG tisssues were transferred to the dissociating medium solution and incubated for 30 min at 37 °C with an atmosphere of 5% CO2. DRG neurons were isolated after digestion and plated onto the glass sides coated with poly-d-L-ornithine hydrobromide (PDL; Solarbio, Beijing, China) and laminin (Sigma-Aldrich) coated. Neurons were grown in defined Ham’s F12 (Gibco, Grand Island, USA) medium supplemented with 2% B-27 (Gibco), 100 U/mL penicillin and 100 mg/mL streptomycin (PS; Gibco). In all studies, neurons from age-matched control mice were cultured with 10 nM insulin (Sigma-Aldrich) and 10 mM D-glucose (Sangon), and neurons from diabetic mice (including T1DM and T2DM mice) were cultured with 25 mM D-glucose without insulin.
GPR40 activity assay
Activation of the compound on GPR40 was denoted by the level of Ca2+ flow change in hGPR40-CHO cells according to the published approach [13]. Briefly, hGPR40-CHO cells were cultured in a petri dish with a diameter of 10 cm. When cells were grown to 80% confluence, 3 mL (for each 10 cm plate) of PBS-EDTA was added. After centrifugation (1000 rpm, 5 min), cells were resuspended at a concentration of 5 × 105 cells/mL in BSA (Solarbio) medium, followed by incubation with coelenterazine h (YEASEN) (final concentration at 5 μM) in the dark for 3–5 h with continuous stirring. After incubation, hGPR40-CHO cells were seeded into 96-well plates. The cells were incubated for at least 30 min before measurement, and Ca2+ dynamics in cells were observed when HBSS buffer containing test compounds were added into cells automatically by FlexStation 3 (Molecular Devices, Sunnyvale, CA, USA).
Mitochondrial function assay
Mitochondrial membrane potential (MMP) assay
DRG neurons from mice were cultured in 96-well plates overnight, followed by staining with JC-1 dyes for MMP detection by published approach [20]. Fluorescent data were detected by Microplate Reader (Molecular Devices).
Mitochondrial respiration assay
Mitochondrial bioenergetic function was evaluated by using an XF24 Analyzer (Seahorse Biosciences, Billerica, MA, USA) according to the published approach [21]. DRG neurons from mice were seeded and cultured with Ham’s F-12 medium in specialized 24-well microplates for monitoring oxygen consumption rate (OCR) in real time. One hour before the assay, the culture medium (Ham’s F-12 medium) was changed to basal medium (unbuffered Dulbecco’s modified Eagle’s medium (pH 7.4)) supplemented with 1 mM pyruvate (Gibco), 2 mM L-glutamine (Sigma-Aldrich) and 10 mM D-glucose. In the assay, oligomycin (1 mM), carbonyl cyanide4-(trifluoromethoxy) phenylhydrazone (FCCP, 1 mM) and a combination of rotenone (1 mM) with antimycin A (1 mM) were injected sequentially through ports in the Seahorse Flux Pak cartridges. Each loop was started by mixing for 3 min and then delayed for 2 min, and OCR was measured for 3 min. This assay was allowed to measure the oxygen consumption rate (OCR), basal respiration, maximal respiration, ATP production and spare respiratory capacity of DRG neurons.
Immunofluorescence assay
For immunofluorescence assay against tissue samples from control and DPN mice, the plantar skin of the hind paw tissues, DRG tissues and sciatic nerves were kept in 4% polyformaldehyde (Solarbio) and then made into paraffin blocks by paraffin-embedded immunohistochemistry assay (IHC-P). Paraffin blocks of plantar skin were cut into 6 mm-thick sections, paraffin blocks of DRG tissues were cut into 5 mm-thick sections and paraffin blocks of sciatic nerves were cut into 2 mm-thick sections. Primary antibody (diluted by 3% goat serum and 0.5% Triton X-100 for 1:1000) was incubated overnight at 4 °C, followed by treatment of secondary antibody for 1 h. The immunocytochemistry staining images were captured by using fluorescence microscope (Leica, Germany).
For immunofluorescence assay against cell samples, RSC96 cells were washed three times with PBS and fixed in 4% paraformaldehyde at room temperature for 30 min. The cells were then incubated with PBST (0.3% Triton X-100) for 5 min and blocked by PBS (5% BSA) at room temperature for 1 h, followed by incubation with the primary antibodies (1:500) overnight at 4 °C. The cells were washed three times with PBS and incubated with corresponding fluorescent secondary antibodies (anti-rabbit and goat anti-mouse; dilution 1:500) at 37 °C for 1 h in dark. The nuclei were stained by using Dapi (Solarbio) or Hoechst33342 (Solarbio). The images were acquired by using a Leica DMI8 microscope.
Antibodies for immunofluorescence assay were provided in Supplementary Table S4.
Histological staining
Sciatic nerve tissues were kept in 4% polyformaldehyde and then made into paraffin blocks by paraffin-embedded immunohistochemistry assay (IHC-P). Paraffin blocks of sciatic nerves were cut into 2 mm-thick sections. Subsequently, slices were stained with luxol fast blue (LFB) (Biossci, Wuhan, China) in 37 °C overnight. The next day, the excess stain was rinsed off with 95% alcohol, and the sections were then rinsed in distilled water. On completion of rinsing, the sections were differentiated with 0.05% lithium carbonate (Solarbio) for 10 s, and the differentiation was terminated by rinsing in distilled water. Subsequently, continued differentiation in 70% alcohol was carried out until the gray and white matter were distinguished. Finally, the sections were rinsed, dehydrated, cleaned, and mounted. LFB-stained tissue sections were examined with fluorescence microscopy (Leica) after histological staining.
Paraffin tissue sections were pasted on glass slides, and then dried in a 37 °C oven. After that, the glass slides were rinsed using 0.01 mol/L PBS and then placed in a luxol fast blue (LFB) solution in a 37 °C oven overnight. The next day, the excess stain was rinsed off with 95% alcohol, and the sections were then rinsed in distilled water. On completion of rinsing, the sections were differentiated with 0.05% lithium carbonate for 10 s, and the differentiation was terminated by rinsing in distilled water. Subsequently, continued differentiation in 70% alcohol was carried out until the gray and white matter were distinguished. Finally, the sections were rinsed, dehydrated, cleaned, and mounted.
Western blot assay
Cells or tissues were lysed in the buffer (Beyotime, Shanghai, China) according to the manufacturer’s instructions. Protein concentrations were determined with the Micro BCA Kit (Beyotime). Total proteins of cells or tissues were separated by SDS-PAGE and transferred from gel to nitrocellulose membrane (Cytiva, USA) and incubated with skimmed milk for over 30 min. Primary antibodies were then incubated with nitrocellulose membrane at 4 °C overnight, and the membranes were washed three times and incubated with secondary antibodies for 2 h at room temperature. The targeted protein bands were detected by Western ECL Substrate (Bio-Rad, USA). Total extracellular regulated protein kinase (ERK) was used as the loading control instead of GAPDH due to the better stability of ERK in DRG tissues [5, 22]. Antibodies for Western blot were provided in Supplementary Table S4.
Co-immunoprecipitation assay
RSC96 cells were lysed in NP40 lysis buffer (Beyotime) according to the manufacturer’s instructions. Cell lysates were centrifuged at 14,000 × g for 15 min and the supernatants were subsequently collected. Proteins of RSC96 cells were immunoprecipitated with NLRP3 antibody (1 μg antibody per 100 μg of total protein) followed by incubation with Protein G Agarose (Beyotime). The proteins eluted from the beads were prepared for Western blotting.
Small-interfering RNA (siRNA) transfection
Rat SCs (RSC96) cells were seeded into 24-well plates (5 × 102 cells/well). After overnight culture, the cells were transfected with siRNA against β-Arrestin2 and GPR40 for 24 h using Lipofectamine 2000 (Invitrogen) according to the manufacturer’s protocols, and non-targeting siRNA was used as a negative control (NC). Interference efficiency was detected by Western blot assay.
Quantitative real-time PCR (qRT-PCR) assay
Total mRNA in sciatic nerve tissues from control and DPN mice was extracted by using TRIzol reagent (TaKaRa Bio Inc., Shiga, Japan) according to the protocols of commercial kits. Total RNA was reverse-transcribed into cDNA by using Reverse-PCR kit (TaKaRa Bio Inc). qRT-PCR assay was performed by SYBR green PCR core reagent kit for mRNA quantity detection (TaKaRa Bio Inc). The thermal cycling condition was 95 °C for 10 s followed by 40 cycles of amplification at 95 °C for 10 s, 60 °C for 20 s, 72 °C for 10 s and 80 °C for 1 s, then keeping at 72 °C for 10 min.
PCR primer sequences were provided in Supplementary Table S5.
Statistical analysis
Data were expressed as mean ± SEM. Student’s t test was used to analyze values of two experimental groups, and one-way ANOVA with Dunnett’s post-hoc test was used to compare values of three or more experimental groups by GraphPad Prism 8.0 Software.
Results
Vin acted as a GPR40 agonist
Vin enhanced intracellular Ca2+ through GPR40
Vin was reported to be a GPR40 activator with EC50 value at 6.28 μM in our previous work [13]. Here, we further verified the activation of Vin on GPR40. As illustrated in Supplementary Fig. S5a, b, both GW9508 (GPR40 activator [23]) and Vin enhanced intracellular Ca2+ level, while GW1100 (GPR40 inhibitor [24]) deprived either GW9508 or Vin of its above capability in hGPR40-CHO cells. These results demonstrated that Vin enhanced intracellular Ca2+ through GPR40.
Vin bound to GPR40 with the binding site distinct from that of orthosteric FFA
To investigate the binding site of GPR40 for Vin, we detected the agonistic activity of Vin against GPR40 in the presence of FFAR1 ligand γ-linolenic acid (γ-LA) by Ca2+ flux assay. As shown in Supplementary Fig. S5c–f, like TAK-875, a reported allosteric modulator of GPR40 [25], Vin could amplify the agonistic activity of γ-LA against GPR40, implying that Vin bound to GPR40 with the binding site distinct from that of orthosteric FFA.
Thus, all results indicated that Vin acted as an allosteric modulator of GPR40.
Vin ameliorated neurological dysfunctions in DPN mice through GPR40
As shown in Fig. 1d–i, compared with control mice, DPN mice exhibited increases in 50% paw withdrawal threshold and thermal response latencies and a decrease in MNCV, indicative of the typical neurological dysfunctions in DPN mice. Notably, Vin improved all above-mentioned neurological dysfunctions in DPN mice (Fig. 1d–i) but had no impacts on any of these neurological dysfunctions in AAV8-GPR40 injected DPN mice (Fig. 1j–o). In addition, Vin had no effects on any of those neurological dysfunctions in non-diabetic mice (Fig. 1p–r).
Since Vin was previously found to lower blood glucose in T2DM mice [13], we here also investigated the potential effect of Vin on blood glucose homeostasis in DPN mice. The results indicated that Vin had no effects on body weight or blood glucose in DPN mice or AAV8-GPR40 injected DPN mice (Supplementary Fig. S6). These results thus indicated that unlike the case in early-stage DPN model mice, the blood glucose of late-stage DPN model mice was too high to be reversed by hypoglycemic agents, which is consistent with the published reports [26, 27]. Moreover, our results further supported that the ameliorative effect of Vin on DPN was independent of its blood glucose regulation. Similarly, it was also found that DPN mice exhibited metabolic abnormalities including TG (Supplementary Fig. S7a and c), TC (Supplementary Fig. S7b and d) and insulin levels (Supplementary Fig. S8), which were not reversed by Vin treatment.
Thus, all results implied that Vin ameliorated neurological dysfunctions in DPN mice through GPR40.
Vin improved peripheral vascular dysfunctions in DPN mice through GPR40
Vascular supply correlates tightly neurophysiological events and intracapillary oxygen saturation, which are highly related to DPN pathology [28, 29]. In addition, Vin is clinically used as a vasodilator [29, 30]. With these facts, we investigated the potential amelioration of Vin on vascular impairment in DPN mice.
Laser Speckle Contrast Imaging results indicated that the blood flow velocities and blood perfusion areas of foot pads (Fig. 2a–c and Fig. 2g–i) and sciatic nerve tissues (Fig. 2d–f and Fig. 2j–l) were all decreased in DPN mice compared with those in control mice. Notably, Vin ameliorated the above-mentioned impairments in DPN mice except the blood flow velocity of sciatic nerve tissues in STZ mice, possibly due to more severe DPN-like pathology in STZ mice compared with that in db/db mice [5]. Moreover, Vin failed to ameliorate any of those impairments in AAV8-GPR40 injected DPN mice (Fig. 2m–x).
Fig. 2. Vin improved peripheral vascular dysfunctions through GPR40.
a, d Representative images and (b, c, e, f) their quantifications of regional blood flow velocities and perfusion areas of (a–c) foot pads or (d–f) sciatic nerve tissues in STZ mice. Vin-30 increased blood perfusion area and flow velocity of foot pads, and increased blood perfusion area but had no impacts on blood flow velocity of sciatic nerve tissues in STZ mice (n = 3–6 per group). g, j Representative images and (h, i, k, l) their quantifications of blood flow velocities and perfusion areas of (g–i) foot pads and (j–l) sciatic nerve tissues in db/db mice. Vin-30 increased blood perfusion areas and flow velocities in db/db mice (n = 3–6 per group). m, p, s, v Representative images and (n, o, q, r, t, u, w, x) their quantifications of regional blood flow velocities and blood perfusion areas of (m–o, s–u) foot pads and (p–r, v–x) sciatic nerve tissues in AAV8-GPR40 injected (m, r) STZ and (s–x) db/db mice. Vin-30 rendered no effects on blood perfusion areas and flow velocities in AAV8-GPR40 injected DPN mice (n = 3–5 per group). Flow velocity and perfusion area were shown as different colors as blue, green, yellow, orange, and red represented from low to high. All values were presented as mean ± SEM, ***P < 0.001 vs Control or db/m mice (Student’s t test); ##P < 0.01, ###P < 0.001 vs STZ or db/db mice (One-way ANOVA with Dunnett’s post-hoc test).
Thus, all results indicated that Vin improved peripheral vascular dysfunctions in DPN mice through GPR40.
Vin enhanced intraepidermal nerve fiber density and improved myelin sheath injury in DPN mice through GPR40
IENFs density
Immunofluorescence staining assay results demonstrated that the fibers (labelled by PGP9.5 [5]) number of foot pads was downregulated in DPN mice compared with that in control mice, and Vin upregulated such a number in DPN mice (Fig. 3a–d).
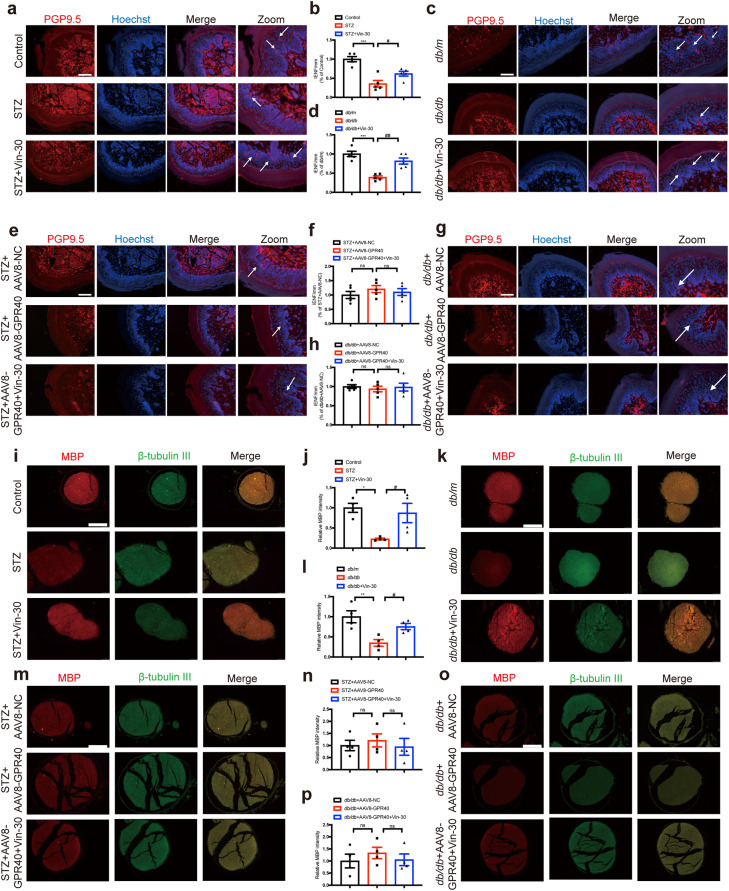
Fig. 3. Vin enhanced intraepidermal nerve fiber density and myelin sheath injury in DPN mice through GPR40.
a–d Representative images of intraepidermal nerve fiber (IENF) and their quantification results demonstrated that Vin-30 attenuated the DPN-induced IENF loss of hind paw plantar skin in (a, b) STZ and (c, d) db/db mice (n = 6 per group). Images displaying PGP9.5 (red) and Hoechst (blue) staining of hind paw plantar skin paraffin section. Scale bar: 50 μm. e–h Vin-30 rendered no effects on IENF loss in AAV8-GPR40 injected (e, f) STZ and (g, h) db/db mice (n = 6 per group). Images displaying PGP9.5 (red) and Hoechst (blue) staining of hind paw plantar skin paraffin section. Scale bar: 50 μm. i–l Representative images of MBP and their quantification results demonstrated that Vin-30 ameliorated myelin sheath injury of sciatic nerve tissues in (i, j) STZ and (k, l) db/db mice (n = 4 per group). Images displaying β-tubulin III (green), MBP (red) and Hoechst (blue) staining of sciatic nerve paraffin section. Scale bar: 50 μm. m–p Vin-30 rendered no effects on myelin sheath injury in AAV8-GPR40 injected (m, n) STZ or (o, p) db/db mice (n = 4 per group). Images displaying β-tubulin III (green), MBP (red) and Hoechst (blue) staining of sciatic nerve paraffin section. Scale bar: 50 μm. All values were presented as mean ± SEM, *P < 0.05, **P < 0.01, ***P < 0.001 vs Control or db/m mice (Student’s t test); #P < 0.05, ##P < 0.01 vs STZ or db/db mice (One-way ANOVA with Dunnett’s post-hoc test).
Myelin basic protein (MBP) staining assay
Given that MBP is the main protein component of the myelin sheath and that fluorescence intensity of MBP could be used as a marker of myelin sheath injury [31], immunofluorescence staining assay against MBP was here performed. The results indicated that the fluorescence intensity of MBP of sciatic nerve tissues was downregulated in DPN mice compared with that in control mice, and Vin upregulated fluorescence intensity of MBP in DPN mice (Fig. 3i–l).
Luxol Fast Blue staining assay
Luxol dye binds and stains the myelin sheath blue, leaving a clear background with an unstained axon and other structures [32]. As shown in Supplementary Fig. S9a, b, the structure of myelin sheath was regular and uniform with an obvious outline in control mice. In contrast, the myelinated nerve fibers were arranged irregularly with myelin sheath swelling or shrinking, dissolution loss in DPN mice, and Vin treatment reversed such myelin sheath injury in DPN mice.
Notably, Vin had no impacts on IENFs of foot pads (Fig. 3e–h), MBP fluorescence intensity (Fig. 3m–p) or myelin sheath morphology (Supplementary Fig. S9c, d) of sciatic nerve tissues in AAV8-GPR40 injected DPN mice.
Thus, all results demonstrated that Vin enhanced intraepidermal nerve fiber density and myelin sheath thickness in DPN mice through GPR40.
Vin targeted GPR40 to suppress NLRP3 inflammasome activation through either β-Arrestin2 or β-Arrestin2/IκBα/NF-κB pathway signaling
Vin suppressed inflammation in DPN mice through GPR40
Given the tight linkage of inflammation to DPN pathology [33–35], we investigated the potential amelioration of Vin on inflammation in DPN mice.
Western blot results indicated that Vin downregulated pro-inflammatory factors IL-1β and TNF-α, and pro-inflammatory enzyme iNOS of both DRG (Supplementary Fig. S10a–d and Supplementary Fig. S11i–l) and sciatic nerve tissues (Fig. 4a–d and Fig. 5e–h) in DPN mice. In addition, ELISA results also demonstrated that Vin suppressed the levels of pro-inflammatory factors TNF-α and IL-6 in serum of DPN mice (Fig. 5a–d). Notably, Vin had no influences on any of the above-mentioned inflammatory factors in AAV8-GPR40 injected DPN mice (Fig. 4e–h, Supplementary Fig. S10e–h, Fig. 5i–l and Supplementary Fig. S11m–p). All results thus demonstrated that Vin suppressed inflammation in DPN mice through GPR40.
Fig. 4. Vin alleviated inflammation in DPN mice involving GPR40/β-Arrestin2/NLRP3 pathway.
a, c Western blot with (b, d) quantification assay results demonstrated that Vin-30 increased the protein levels of GPR40 and β-Arrestin2, and reduced the protein levels of NLRP3, ASC, Cleaved caspase-1 and IL-1β of sciatic nerve tissues in DPN mice (STZ, db/db. n = 3 per group). e, g Western blot with (f, h) quantification assay results indicated that Vin-30 had no effects on GPR40/β-Arrestin2/NLRP3 pathway in DPN mice (STZ, db/db. n = 3 per group). i Western blot with (j) quantification assay results demonstrated Vin (2, 5, 10 μM) treatment increased the expression levels of GPR40 and β-Arrestin2, but reduced the expression levels of NLRP3, ASC, Cleaved caspase-1 and IL-1β in LPS (1 μg/mL)/ATP (3 mM) treated RSC96 cells (n = 3). k Western blot with (l) quantification assay results demonstrated that Vin (2, 5, 10 μM) treatment had no effects on GPR40/β-Arrestin2/NLRP3 signaling in LPS (1 μg/mL)/ATP (3 mM) treated RSC96 cells with β-Arrestin2 siRNA transfected (n = 3). m Cell lysates of RSC96 cells treated with LPS (1 μg/mL)/ATP (3 mM) and Vin (10 μM) were immunoprecipitated with anti-NLRP3 antibody, and samples were then analyzed by immunoblotting. Vin (10 μM) increased NLRP3 binding to β-Arrestin2. All values were presented as mean ± SEM, *P < 0.05, **P < 0.01, ***P < 0.001 vs Control, db/m, STZ + AAV8-NC, db/db + AAV8-NC mice or DMSO group (Student’s t test); #P < 0.05, ##P < 0.01, ###P < 0.001 vs STZ, db/db mice or LPS/ATP group (One-way ANOVA with Dunnett’s post-hoc test).
Fig. 5. Vin alleviated inflammation in DPN mice involving GPR40/β-Arrestin2/IκBα/NF-κB/NLRP3 pathway.
a–d Enzyme-linked immunosorbent assay result against the serum indicated that Vin-30 decreased (a, c) TNF-α and (b, d) IL-6 levels in DPN mice (STZ, db/db; n = 4–9 per group). e, g Western blot with (f, h) quantification assay results demonstrated that Vin-30 decreased the levels of p-IκBα, p-NF-κB, iNOS and TNF-α of sciatic nerve tissues in DPN mice (STZ, db/db; n = 3 per group). i–l Vin-30 had no effects on p-IκBα, p-NF-κB, iNOS or TNF-α protein level of sciatic nerve tissues in (i, j) STZ + AAV8-GPR40 or (k, l) db/db + AAV8-GPR40 mice (n = 3 per group). m Western blot with (n) quantification assay results demonstrated Vin (2, 5, 10 μM) reduced the levels of p-IκBα, p-NF-κB, iNOS and TNF-α in LPS (1 μg/mL)/ATP (3 mM) treated RSC96 cells (n = 3). o Western blot with (p) quantification assay results demonstrated Vin (2, 5, 10 μM) treatment had no effects on the level of p-IκBα, p-NF-κB, iNOS or TNF-α in LPS (1 μg/mL)/ATP (3 mM) treated RSC96 cells with β-Arrestin2 siRNA transfection (n = 3). q–t RT-PCR results indicated that Vin (10 μM) failed to affect NLRP3 inflammasome activation with PDTC (5 μM) treatment (n = 6–12). All values were presented as mean ± SEM, *P < 0.05, **P < 0.01, ***P < 0.001 vs Control, db/m, STZ + AAV8-NC, db/db + AAV8-NC mice or DMSO group (Student’s t test); #P < 0.05, ##P < 0.01, ###P < 0.001 vs STZ, db/db mice or LPS/ATP group (One-way ANOVA with Dunnett’s post-hoc test); &P < 0.05, &&P < 0.01, &&&P < 0.001 vs PDTC group (Student’s t test).
Vin repressed NLRP3 inflammasome activation in DPN mice through GPR40
Given that activation of NLRP3 inflammasome, a complex composed of sensor molecule NLRP3, adaptor protein ASC and Cleaved-caspase 1 [36], may result in IL-1β that is tightly implicated in DPN pathology [32, 36], NLRP3 related assays were next performed.
Western blot assay results indicated that the protein levels of NLRP3, ASC, Cleaved caspase-1 and IL-1β were all upregulated in sciatic nerve tissues of DPN mice (Fig. 4a–d) and LPS/ATP-treated RSC96 cells (Fig. 4i, j) compared with those of control mice or vehicle-treated RSC96 cells, and Vin decreased all those protein levels in sciatic nerve tissues of DPN mice (Fig. 4a–d) and LPS/ATP-treated RSC96 cells (Fig. 4i, j). Vin had no impacts on the levels of those above-mentioned proteins in the sciatic nerve tissues of AAV8-GPR40 injected DPN mice (Fig. 4e–h). All results thus implied that Vin repressed NLRP3 inflammasome activation in DPN mice through GPR40.
Notably, Western blot results indicated that NLRP3 was rarely expressed in DRG tissues (Supplementary Fig. S10), NLRP3 inflammasome related assay was thus not performed in DRG neurons or tissues of DPN mice in the current work.
GPR40/β-Arrestin2/NLRP3 pathway was involved in Vin-mediated suppression of NLRP3 inflammasome activation
β-Arrestin2 as a key regulatory protein in GPR40 signaling is linked to NLRP3 inflammasome activation in the central nervous system (CNS) [10], and Western blot results (Fig. 4a–d, Fig. 4i, j) indicated that both β-Arrestin2 and GPR40 protein levels were decreased in sciatic nerve tissues of DPN mice and LPS/ATP-treated RSC96 cells compared with those of control mice or vehicle-treated RSC96 cells, and Vin increased both protein levels in the sciatic nerve tissues of DPN mice or LPS/ATP-treated RSC96 cells.
Notably, Western blot assay results demonstrated that small interfering RNA (siRNA) targeting β-Arrestin2 (si-β-Arrestin2) deprived Vin of its capability in inhibiting NLRP3, ASC, Cleaved-caspase 1 and IL-1β in LPS/ATP-treated RSC96 cells (Fig. 4k, l), which implied that β-Arrestin2 was required for Vin-mediated suppression of NLRP3 inflammasome activation.
Moreover, Co-immunoprecipitation assay results indicated that NLRP3 could pull-down β-Arrestin2 in LPS/ATP-treated RSC96 cells, indicative of the binding of β-Arrestin2 to NLRP3, while Vin increased NLRP3/β-Arrestin2 binding (Fig. 4m).
Together, all results demonstrated that GPR40/β-Arrestin2/NLRP3 pathway was involved in Vin-mediated suppression of inflammation.
GPR40/β-Arrestin2/IκBα/NF-κB/NLRP3 pathway was involved in Vin-mediated suppression of inflammation
Given the fact that NF-κB functions potently in regulating iNOS, TNF-α and IL-6 [3, 35, 37], while NF-κB protein is predominantly restricted to the cytosol by associating with members of IκB family before activation, Western blot assay was performed. The results indicated that the protein levels of p-IκBα, p-NF-κB, iNOS and TNF-α were all increased in sciatic nerve tissues of DPN mice (Fig. 5e–h) or LPS/ATP-treated RSC96 cells (Fig. 5m, n) compared with those of control mice or in vehicle-treated RSC96 cells, while Vin decreased all those protein levels of sciatic nerve tissues in DPN mice or LPS/ATP-treated RSC96 cells. Notably, Vin had no impacts on any of the protein levels of the sciatic nerve tissues in AAV8-GPR40 injected DPN mice (Fig. 5i–l).
Thus, all results implied that GPR40/IκBα/NF-κB pathway was involved in Vin-mediated suppression of inflammation in DPN mice.
Moreover, Western blot assay results demonstrated that si-β-Arrestin2 deprived Vin of its inhibitory capability against p-IκBα, p-NF-κB, TNF-α and iNOS in LPS/ATP treated RSC96 cells (Fig. 5o, p), thus implying that β-Arrestin2 is required for NF-κB activation. Notably, PDTC (NF-κB inhibitor [24]) could efficiently deprive Vin of its capability in inhibiting NLRP3 inflammasome activation (Fig. 5q–t).
In addition, Vin had no effects on the protein expression levels of β-Arrestin2, p-IκBα, p-NF-κB or NLRP3 in LPS/ATP treated RSC96 cells with GPR40 siRNA (small interfering RNA targeting GPR40) transfected (Supplementary Fig. S12).
Taken together, all results demonstrated that Vin targeted GPR40 to suppress NLRP3 inflammasome activation through either β-Arrestin2 or β-Arrestin2/IκBα/NF-κB pathway signaling.
Vin improved mitochondrial dysfunction in DPN mice through GPR40/CaMKKβ/AMPK/SIRT1/PGC-1α pathway
Vin improved mitochondrial dysfunction in DPN mice through GPR40
Given that mitochondrial dysfunction is implicated in DPN pathology [6, 20, 38], mitochondrial respiration in DRG neurons was evaluated by an XF24 Analyzer. The results indicated that the levels of oxygen consumption rate (OCR), basal respiration, maximum respiration, spare capacity, and ATP production of DRG neurons were all downregulated in DPN mice compared with those in control mice, while Vin upregulated all parameters of DRG neurons in DPN mice (Fig. 6a–j). Notably, Vin lost its above-mentioned ameliorative effects in AAV8-GPR40 injected DPN mice (Fig. 6k–t).
Fig. 6. Vin improved mitochondrial respiration impairments in DRG neurons from DPN mice through GPR40.
a, f Oxygen consumption rate (OCR) was measured at basal level with sequential addition of oligomycin (1 μM), FCCP (1 μM) and rotenone (1 μM) + antimycin A (AA; 1 μM) to DRG neurons from DPN mice (STZ, db/db). The levels of (b, g) basal respiration, (c, h) maximal respiration, (d, i) spare respiration capacity and (e, j) ATP production were measured (n = 3–7). The results indicated that Vin improved mitochondrial respiration impairments in DPN mice. k–t Vin-30 had no impacts on (k, p) OCR, (l, q) basal respiration, (m, r) maximal respiration, (n, s) spare respiration capacity or (o, t) ATP production in AAV8-GPR40 injected DPN mice (n = 3–6 per group). All values were presented as mean ± SEM, *P < 0.05, **P < 0.01, ***P < 0.001 vs Control or db/m mice (Student’s t test); ##P < 0.01, ###P < 0.001 vs STZ or db/db mice (One-way ANOVA with Dunnett’s post-hoc test).
In addition, MMP was also detected in DRG neurons by staining with JC-1 dyes. The results (Fig. 7a, b) indicated that MMP level was downregulated in DRG neurons from DPN mice compared with those from control mice, and Vin upregulated the MMP level of DRG neurons in DPN mice. Notably, Vin failed to affect MMP level in AAV8-GPR40 injected DPN mice (Fig. 7c, d).
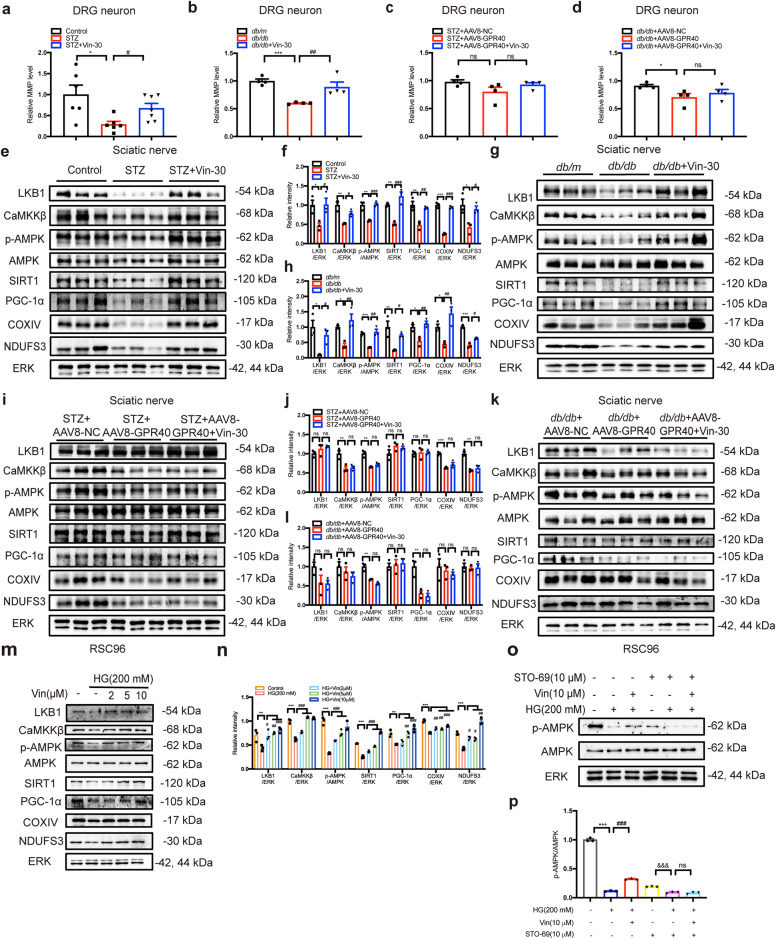
Fig. 7. Vin improved mitochondrial dysfunction of DPN mice through GPR40/CaMKKβ/AMPK/SIRT1/PGC-1α pathway.
a, b Vin-30 enhanced MMP level of DRG neurons in (a) STZ and (b) db/db mice (n = 4–7 per group). c, d Vin-30 failed to affect MMP level of DRG neurons in AAV8-GPR40 injected DPN mice (n = 4 per group). e, g Western blot with (f, h) quantification assay results demonstrated that Vin-30 increased the expression levels of LKB1, CaMKKβ, p-AMPK, SIRT1, PGC-1α, NDUFS3 and COXIV of sciatic nerve tissues in (e, f) STZ and (g, h) db/db mice (n = 3 per group). i–l Vin-30 had no effects on LKB1 or CaMKKβ/AMPK/SIRT1/PGC-1α signaling in AAV8-GPR40 injected DPN mice (n = 3 per group). m Western blot with (n) quantification results demonstrated Vin (2, 5, 10 μM) treatment increased the levels of LKB1, CaMKKβ, p-AMPK, SIRT1, PGC-1α, NDUFS3 and COXIV in high glucose (HG; 200 mM)-treated RSC96 cells (n = 3). o Western blot with (p) quantification results demonstrated STO-69 (10 μM) deprived Vin (10 μM) of its capability in regulating AMPK (n = 3). All values were presented as mean ± SEM, *P < 0.05, **P < 0.01, ***P < 0.001 vs Control, db/m, STZ + AAV8-NC, db/db + AAV8-NC mice or DMSO group (Student’s t test); &&&P < 0.001 vs STO-69 group (Student’s t test); #P < 0.05, ##P < 0.01, ###P < 0.001 vs STZ, db/db mice or HG group (One-way ANOVA with Dunnett’s post-hoc test).
Moreover, it was found that Vin enhanced the expressions of mitochondrial complexes I (NDUFS3) and IV (COXIV) of sciatic nerve (Fig. 7e–h) and DRG tissues (Supplementary Fig. S13a–d) in DPN mice, but had no impacts on either complex of sciatic nerve (Fig. 7i–l) or DRG tissues (Supplementary Fig. S13e–h) in AAV8-GPR40 injected DPN mice.
Thus, all results demonstrated that Vin improved mitochondrial dysfunction in DPN mice through GPR40.
Vin ameliorated mitochondrial dysfunction through GPR40/CaMKKβ/AMPK/SIRT1/PGC-1α pathway in DPN mice
AMPK/SIRT1/PGC-1α signaling functions potently in sensing oxidative stress and mitochondrial function [39–41], and Western blot results also indicated that Vin increased the levels of p-AMPK, SIRT1 and PGC-1α of both sciatic nerve (Fig. 7e–h) and DRG tissues (Supplementary Fig. S13a–d) in DPN mice. Notably, Vin had no impacts on any of these protein levels in AAV8-GPR40 injected DPN mice (Fig. 7i–l and Supplementary Fig. S13e–h).
Considering that AMPK can be regulated by two upstream kinases CaMKKβ and LKB1 [42–45], we next detected the protein levels of these two kinases in both sciatic nerve and DRG tissues of mice. Western blot assay results indicated that Vin upregulated the protein levels of both CaMKKβ and LKB1 of sciatic nerve (Fig. 7e–h) or DRG tissues (Supplementary Fig. S13a–d) in DPN mice. Moreover, cell-based assay results also verified that Vin upregulated the protein levels of LKB1, CaMKKβ, p-AMPK, SIRT1 and PGC-1α in high glucose (HG, 200 mM)-treated RSC96 cells (Fig. 7m, n). Notably, STO-69 (CaMKKβ inhibitor [46]) deprived Vin of its capability in regulating p-AMPK (Fig. 7o, p), indicating that CaMKKβ is required for Vin-mediated AMPK activation. However, radicicol as a LKB1 inhibitor [45] failed to deprive Vin of its capability in regulating p-AMPK (Supplementary Fig. S13i, j), indicating that LKB1 was not required for Vin-mediated AMPK activation. In addition, Vin had no effects on CaMKKβ/AMPK/SIRT1/PGC-1α pathway in AAV8-GPR40 injected DPN mice (Fig. 7i–l; Supplementary Fig. S13e–h).
In addition, Vin had no effects on the protein expression levels of CaMKKβ, p-AMPK, SIRT1 or PGC-1α in HG treated RSC96 cells with GPR40 siRNA transfected (Supplementary Fig. S14).
Together, all results demonstrated that Vin improved mitochondrial dysfunction in DPN mice through GPR40/CaMKKβ/AMPK/SIRT1/PGC-1α pathway.
Vin suppressed oxidative stress in DPN mice through GPR40/Nrf2 pathway
Vin reduced oxidative stress level through GPR40
As indicated in Fig. 8a–d, the serum of DPN mice exhibited downregulation of GSH level and upregulation of MDA level compared with that of control mice, while Vin antagonized these two levels in DPN mice as indicated by the results from the serum of Vin-treated DPN mice.
Fig. 8. Vin improved oxidative stress through GPR40/Nrf2 pathway.
a, c Vin-30 increased GSH level of serum in (a) STZ and (c) db/db mice (n = 4–6 per group). b, d Vin-30 decreased MDA level of serum in (b) STZ and (d) db/db mice (n = 4–6 per group). e, g Immunofluorescence and (f, h) quantification assay results demonstrated that Vin-30 decreased 8-OHdG fluorescence intensity of DRG tissues in DPN mice (n = 5–6 per group). Images displaying β-tubulin III (green), 8-OHdG (red) and Dapi (blue) staining of DRG paraffin section. Scale bar: 50 μm. i, k Immunofluorescence and (j, l) quantification assay results demonstrated that Vin-30 rendered no impacts on 8-OHdG fluorescence intensity of DRG tissues in AAV8-GPR40 injected DPN mice (n = 3–5 per group). Scale bar: 50 μm. q, s Immunofluorescence and (r, t) quantification assay results demonstrated that Vin-30 promoted Nrf2 nuclear translocation of DRG neurons in DPN mice (STZ, db/db, n = 3–5 per group). Images displaying β-tubulin III (green), Nrf2 (red) and Dapi (blue) staining of DRG paraffin section. Scale bar: 50 μm. u, w Immunofluorescence and (v, x) quantification assay results demonstrated that Vin-30 rendered no impacts on the Nrf2 nuclear translocation of DRG neurons in AAV8-GPR40 injected DPN mice (n = 3–5 per group). Scale bar: 50 μm. m, n RT-PCR results indicated that Vin-30 upregulated Nrf2 mRNA level of sciatic nerve tissues in (m) STZ and (n) db/db mice (n = 4 per group). o, p Vin-30 failed to affect Nrf2 mRNA level in AAV8-GPR40 injected DPN mice (n = 4–5 per group). y Proposed schematic diagram illustrating the mechanisms underlying the improvement of Vin on DPN-like pathology in mice. All values were presented as mean ± SEM, *P < 0.05, **P < 0.01, ***P < 0.001 vs Control or db/m (Student’s t test); #P < 0.05, ###P < 0.001 vs STZ or db/db mice (One-way ANOVA with Dunnett’s post-hoc test).
Besides, 8-hydroxy-2deoxyguanosine (8-OHdG) is a biomarker of mitochondrial DNA (mtDNA) oxidative damage [47], and our data indicated that Vin reduced 8-OHdG fluorescence intensity of DRG tissues in DPN mice (Fig. 8e–h). Notably, Vin had no impacts on 8-OHdG in AAV8-GPR40 injected DPN mice (Fig. 8i–l).
Vin treatment suppressed oxidative stress in DPN mice through GPR40/Nrf2 pathway
RT-PCR results indicated that Vin upregulated Nrf2 mRNA level of sciatic nerve tissues in DPN mice but had no impacts on Nrf2 mRNA level in AAV8-GPR40 injected DPN mice (Fig. 8m–p). In addition, immunofluorescence results also demonstrated that Vin promoted Nrf2 nuclear translocation level of DRG neurons in DPN mice (Fig. 8q–t) but had no impacts on Nrf2 nuclear translocation in AAV8-GPR40 injected DPN mice (Fig. 8u–x). Moreover, as shown in Supplementary Fig. S15, Vin promoted Nrf2 nuclear translocation in HG treated RSC96 cells but had no impacts on Nrf2 nuclear translocation with GPR40 siRNA transfected.
Thus, all results indicated that Vin suppressed oxidative stress in DPN mice through GPR40/Nrf2 pathway.
Vin targeted GPR40 to ameliorate mitochondrial dysfunction and oxidative stress involving AMPK/Nrf2/NF-κB signaling
Next, the relationships among mitochondrial dysfunction, oxidative stress and inflammation were also studied by using AMPK inhibitor Compound C [48] and Nrf2 inhibitor ML385 [49]. As shown in Supplementary Fig. S16, Compound C deprived Vin (10 μM) of its capability in regulating p-NF-κB protein expression and Nrf2 nuclear translocation in RSC96 cells, and ML385 deprived Vin (10 μM) of its capability in regulating p-NF-κB protein expression in RSC96 cells. All results implied that AMPK is required for Nrf2/NF-κB signaling in response to Vin-mediated GPR40 activation.
Thus, all results indicated that Vin targeted GPR40 to ameliorate mitochondrial dysfunction and oxidative stress involving AMPK/Nrf2/NF-κB signaling.
Discussion
DPN is a common complication of diabetes lack of curable treatment [50], and it is full of challenges to develop effective anti-DPN medication [51]. Here, we determined that Vin as a GPR40 agonist efficiently improved DPN-like pathology in mice, and our results have supported that GPR40 activation may show promise as a therapeutic strategy for DPN.
In our current work, we focused on the study on the late-stage DPN model mice as also indicated in our previously published work [27, 52]. There is currently no FDA-approved disease-modifying therapy for DPN [53], and drug combination therapy such as prostaglandin E1 plus lipoic acid combination therapy was usually used to relieve the symptoms of the disease [54]. Thus, we here failed to find suitable in vivo control group.
Mitochondrial dysfunction and oxidative stress are tightly implicated in DPN pathogenesis [55, 56], and Vin improved mitochondrial respiration dysfunction and ATP production in DPN mice. Notably, vascular dysfunction is associated with DPN pathology because metabolic insult of diabetes may directly affect nerve functions due to compromised nerve vascular supply [28]. Vin was reported as a vasodilator with its capability in increasing ATP production that is an important regulator of blood flow perfusion and velocity [57, 58]. In our work, we found that Vin effectively improved peripheral vascular dysfunctions in DPN mice. Thus, all findings have highlighted the potential of Vin in the treatment of DPN. Moreover, as Vin is currently used as a vasodilator drug, its obtained preclinical and clinical data should no doubt provide valuable references for subsequent development of anti-DPN drug based on this approved drug.
NLRP3 inflammasome is a central regulator of inflammation [10, 32, 36]. Although it is acknowledged that GPR40 and its downstream scaffold protein β-Arrestin2 are involved in inflammasome inhibition in several diseases [10, 11], our work might be the first to report the involvement of GPR40/β-Arrestin2/NLRP3 signaling in DPN-like pathology in mice. In addition, we also determined the tissue distribution selectivity for NLRP3 protein in sciatic nerve tissues over DRG tissues, which may better our understanding of the inflammation event in DPN pathology.
Interestingly, GPR40 agonists generally render no influences on GPR40 protein expression, but there are still some exceptions. For example, GPR40 can be activated by long-chain fatty acid docosahexaenoic acid (DHA) and DHA downregulated GPR40 protein expression in bone marrow stromal cells (BMSC) [59]. Here, we found that Vin as a GPR40 agonist can also stimulate GPR40 protein expression of DRG neurons and sciatic nerve cells in DPN model mice.
It was noted that MNCV and sensory sensitivity assays are potent for DPN research [32] according to the Diabetes Complications Consortium guidelines, assays of TEM, SNCV and CAMP related assays should be of high complementation to the current work.
In conclusion, as summarized in Fig. 8y, Vin as a GPR40 agonist ameliorated DPN-like pathology in DPN mice. Vin functioned as a GPR40 activator to suppress NLRP3 inflammasome activation through either β-Arrestin2 or β-Arrestin2/IκBα/NF-κB signaling, improve mitochondrial dysfunction through CaMKKβ/AMPK/SIRT1/PGC-1α signaling and alleviate oxidative stress through Nrf2 signaling in DRG and sciatic nerve tissues. Our findings have highly supported that GPR40 activation shows promise as a therapeutic strategy for DPN and highlighted the potential of Vin in the treatment of this disease.
Supplementary information
Acknowledgements
This work was supported by the National Natural Science Foundation of China (82273930), Innovative Research Team of Six Talent Peaks Project in Jiangsu Province (TD-SWYY-013), the Open Project of Chinese Materia Medica First-Class Discipline of Nanjing University of Chinese Medicine (No. 2020YLXK018) and “Qing Lan” project. The authors thank Xia-lin Zhu (Central Hospital Affiliated to Shandong First Medical University) and Xiao-ju Xu (Shanghai Ninth People’s Hospital, Shanghai JiaoTong University School of Medicine) for expert technical assistance.
Author contributions
XS, JWX, and XX designed the study. XS reviewed the paper. JWX, XX, YL, YCW, YJH, and JZY performed the animal and cell experiments. JWX and XX analyzed interpreted data. JWX and JYW wrote the paper. XS, JWX, XX, YL, YCW, YJH, JZY, and JYW are the guarantors of this work and, as such, have full access to all data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. All authors approved the paper.
Competing interests
The authors declare no competing interests.
Footnotes
These authors contributed equally: Jia-wen Xu, Xu Xu
Contributor Information
Jia-ying Wang, Email: wangjy@njucm.edu.cn.
Xu Shen, Email: xshen@njucm.edu.cn.
Supplementary information
The online version contains supplementary material available at 10.1038/s41401-023-01135-1.
References
- 1.Liu XS, Fan B, Szalad A, Jia L, Wang L, Wang X, et al. MicroRNA-146a mimics reduce the peripheral neuropathy in type 2 diabetic mice. Diabetes. 2017;66:3111–21. doi: 10.2337/db16-1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yang Y, Ostbye T, Tan SB, Abdul Salam ZH, Ong BC, Yang KS. Risk factors for lower extremity amputation among patients with diabetes in Singapore. J Diabetes Complications. 2011;25:382–6. doi: 10.1016/j.jdiacomp.2011.08.002. [DOI] [PubMed] [Google Scholar]
- 3.Feldman EL, Nave KA, Jensen TS, Bennett DLH. New horizons in diabetic neuropathy: mechanisms, bioenergetics, and pain. Neuron. 2017;93:1296–313. doi: 10.1016/j.neuron.2017.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pop-Busui R, Boulton AJ, Feldman EL, Bril V, Freeman R, Malik RA, et al. Diabetic neuropathy: a position statement by the American Diabetes Association. Diabetes Care. 2017;40:136–54. doi: 10.2337/dc16-2042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Roy Chowdhury SK, Smith DR, Saleh A, Schapansky J, Marquez A, Gomes S, et al. Impaired adenosine monophosphate-activated protein kinase signalling in dorsal root ganglia neurons is linked to mitochondrial dysfunction and peripheral neuropathy in diabetes. Brain. 2012;135:1751–66. doi: 10.1093/brain/aws097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Choi J, Chandrasekaran K, Inoue T, Muragundla A, Russell JW. PGC-1alpha regulation of mitochondrial degeneration in experimental diabetic neuropathy. Neurobiol Dis. 2014;64:118–30. doi: 10.1016/j.nbd.2014.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chandrasekaran K, Anjaneyulu M, Choi J, Kumar P, Salimian M, Ho CY, et al. Role of mitochondria in diabetic peripheral neuropathy: influencing the NAD+-dependent SIRT1-PGC-1alpha-TFAM pathway. Int Rev Neurobiol. 2019;145:177–209. doi: 10.1016/bs.irn.2019.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Karki P, Kurihara T, Nakamachi T, Watanabe J, Asada T, Oyoshi T, et al. Attenuation of inflammatory and neuropathic pain behaviors in mice through activation of free fatty acid receptor GPR40. Mol Pain. 2015;11:6. doi: 10.1186/s12990-015-0003-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ma D, Tao B, Warashina S, Kotani S, Lu L, Kaplamadzhiev DB, et al. Expression of free fatty acid receptor GPR40 in the central nervous system of adult monkeys. Neurosci Res. 2007;58:394–401. doi: 10.1016/j.neures.2007.05.001. [DOI] [PubMed] [Google Scholar]
- 10.Yan Y, Jiang W, Spinetti T, Tardivel A, Castillo R, Bourquin C, et al. Omega-3 fatty acids prevent inflammation and metabolic disorder through inhibition of NLRP3 inflammasome activation. Immunity. 2013;38:1154–63. doi: 10.1016/j.immuni.2013.05.015. [DOI] [PubMed] [Google Scholar]
- 11.Lin C, Chao H, Li Z, Xu X, Liu Y, Bao Z, et al. Omega-3 fatty acids regulate NLRP3 inflammasome activation and prevent behavior deficits after traumatic brain injury. Exp Neurol. 2017;290:115–22. doi: 10.1016/j.expneurol.2017.01.005. [DOI] [PubMed] [Google Scholar]
- 12.Fandy TE, Abdallah I, Khayat M, Colby DA, Hassan HE. In vitro characterization of transport and metabolism of the alkaloids: vincamine, vinpocetine and eburnamonine. Cancer Chemother Pharmacol. 2016;77:259–67. doi: 10.1007/s00280-015-2924-3. [DOI] [PubMed] [Google Scholar]
- 13.Du T, Yang L, Xu X, Shi X, Xu X, Lu J, et al. Vincamine as a GPR40 agonist improves glucose homeostasis in type 2 diabetic mice. J Endocrinol. 2019;240:195–214. doi: 10.1530/JOE-18-0432. [DOI] [PubMed] [Google Scholar]
- 14.Adeghate J, Nurulain S, Tekes K, Feher E, Kalasz H, Adeghate E. Novel biological therapies for the treatment of diabetic foot ulcers. Expert Opin Biol Ther. 2017;17:979–87. doi: 10.1080/14712598.2017.1333596. [DOI] [PubMed] [Google Scholar]
- 15.De Gregorio C, Contador D, Diaz D, Carcamo C, Santapau D, Lobos-Gonzalez L, et al. Human adipose-derived mesenchymal stem cell-conditioned medium ameliorates polyneuropathy and foot ulceration in diabetic BKS db/db mice. Stem Cell Res Ther. 2020;11:168. doi: 10.1186/s13287-020-01680-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Muller KA, Ryals JM, Feldman EL, Wright DE. Abnormal muscle spindle innervation and large-fiber neuropathy in diabetic mice. Diabetes. 2008;57:1693–701. doi: 10.2337/db08-0022. [DOI] [PubMed] [Google Scholar]
- 17.Kan M, Guo G, Singh B, Singh V, Zochodne DW. Glucagon-like peptide 1, insulin, sensory neurons, and diabetic neuropathy. J Neuropathol Exp Neurol. 2012;71:494–510. doi: 10.1097/NEN.0b013e3182580673. [DOI] [PubMed] [Google Scholar]
- 18.Yerra VG, Kalvala AK, Kumar A. Isoliquiritigenin reduces oxidative damage and alleviates mitochondrial impairment by SIRT1 activation in experimental diabetic neuropathy. J Nutr Biochem. 2017;47:41–52. doi: 10.1016/j.jnutbio.2017.05.001. [DOI] [PubMed] [Google Scholar]
- 19.Latham JR, Pathirathna S, Jagodic MM, Choe WJ, Levin ME, Nelson MT, et al. Selective T-type calcium channel blockade alleviates hyperalgesia in ob/ob mice. Diabetes. 2009;58:2656–65. doi: 10.2337/db08-1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yerra VG, Areti A, Kumar A. Adenosine Monophosphate-activated protein kinase abates hyperglycaemia-induced neuronal injury in experimental models of diabetic neuropathy: effects on mitochondrial biogenesis, autophagy and neuroinflammation. Mol Neurobiol. 2017;54:2301–12. doi: 10.1007/s12035-016-9824-3. [DOI] [PubMed] [Google Scholar]
- 21.Saleh A, Sabbir MG, Aghanoori MR, Smith DR, Roy Chowdhury SK, Tessler L, et al. Muscarinic toxin 7 signals via Ca2+/calmodulin-dependent protein kinase kinase beta to augment mitochondrial function and prevent neurodegeneration. Mol Neurobiol. 2020;57:2521–38. doi: 10.1007/s12035-020-01900-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Calcutt NA, Smith DR, Frizzi K, Sabbir MG, Chowdhury SK, Mixcoatl-Zecuatl T, et al. Selective antagonism of muscarinic receptors is neuroprotective in peripheral neuropathy. J Clin Invest. 2017;127:608–22. doi: 10.1172/JCI88321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fujita T, Matsuoka T, Honda T, Kabashima K, Hirata T, Narumiya S. A GPR40 agonist GW9508 suppresses CCL5, CCL17, and CXCL10 induction in keratinocytes and attenuates cutaneous immune inflammation. J Investig Dermatol. 2011;131:1660–7. doi: 10.1038/jid.2011.123. [DOI] [PubMed] [Google Scholar]
- 24.Umar S, Li J, Hannabass K, Vaillancourt M, Cunningham CM, Moazeni S, et al. Free fatty acid receptor G-protein-coupled receptor 40 mediates lipid emulsion-induced cardioprotection. Anesthesiology. 2018;129:154–62. doi: 10.1097/ALN.0000000000002195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu C, Cheng ZY, Xia QP, Hu YH, Wang C, He L. GPR40 receptor agonist TAK-875 improves cognitive deficits and reduces beta-amyloid production in APPswe/PS1dE9 mice. Psychopharmacology (Berl) 2021;238:2133–46. doi: 10.1007/s00213-021-05837-4. [DOI] [PubMed] [Google Scholar]
- 26.Xu X, Xu X, Hao Y, Zhu X, Lu J, Ouyang X, et al. Antispasmodic drug drofenine as an inhibitor of Kv2.1 channel ameliorates peripheral neuropathy in diabetic mice. iScience. 2020;23:101617. doi: 10.1016/j.isci.2020.101617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhu X, Chen Y, Xu X, Xu X, Lu Y, Huang X, et al. SP6616 as a Kv2.1 inhibitor efficiently ameliorates peripheral neuropathy in diabetic mice. EBioMedicine. 2020;61:103061. doi: 10.1016/j.ebiom.2020.103061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jin HY, Joung SJ, Park JH, Baek HS, Park TS. The effect of alpha-lipoic acid on symptoms and skin blood flow in diabetic neuropathy. Diabet Med. 2007;24:1034–8. doi: 10.1111/j.1464-5491.2007.02179.x. [DOI] [PubMed] [Google Scholar]
- 29.Fayed AH. Brain trace element concentration of rats treated with the plant alkaloid, vincamine. Biol Trace Elem Res. 2010;136:314–9. doi: 10.1007/s12011-009-8550-3. [DOI] [PubMed] [Google Scholar]
- 30.Al-Rashed S, Baker A, Ahmad SS, Syed A, Bahkali AH, Elgorban AM, et al. Vincamine, a safe natural alkaloid, represents a novel anticancer agent. Bioorg Chem. 2021;107:104626. doi: 10.1016/j.bioorg.2021.104626. [DOI] [PubMed] [Google Scholar]
- 31.Zhang WJ, Luo C, Huang C, Liu SC, Luo HL. Microencapsulated neural stem cells inhibit sciatic nerve injury-induced pain by reducing P2 x 4 receptor expression. Front Cell Dev Biol. 2021;9:656780. doi: 10.3389/fcell.2021.656780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sun Q, Wang C, Yan B, Shi X, Shi Y, Qu L, et al. Jinmaitong ameliorates diabetic peripheral neuropathy through suppressing TXNIP/NLRP3 inflammasome activation in the streptozotocin-induced diabetic rat model. Diabetes Metab Syndr Obes. 2019;12:2145–55. doi: 10.2147/DMSO.S223842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Feng Y, Ge Y, Wu M, Xie Y, Wang M, Chen Y, et al. Long noncoding RNAs regulate inflammation in diabetic peripheral neuropathy by acting as ceRNAs targeting miR-146a-5p. Diabetes Metab Syndr Obes. 2020;13:413–22. doi: 10.2147/DMSO.S242789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tang HY, Jiang AJ, Ma JL, Wang FJ, Shen GM. Understanding the signaling pathways related to the mechanism and treatment of diabetic peripheral neuropathy. Endocrinology. 2019;160:2119–27. doi: 10.1210/en.2019-00311. [DOI] [PubMed] [Google Scholar]
- 35.Suryavanshi SV, Kulkarni YA. NF-kappabeta: A potential target in the management of vascular complications of diabetes. Front Pharmacol. 2017;8:798. doi: 10.3389/fphar.2017.00798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cheng YC, Chu LW, Chen JY, Hsieh SL, Chang YC, Dai ZK, et al. Loganin attenuates high glucose-induced schwann cells pyroptosis by inhibiting ROS generation and NLRP3 inflammasome activation. Cells. 2020;9:1948-65. [DOI] [PMC free article] [PubMed]
- 37.Luo Q, Feng Y, Xie Y, Shao Y, Wu M, Deng X, et al. Nanoparticle-microRNA-146a-5p polyplexes ameliorate diabetic peripheral neuropathy by modulating inflammation and apoptosis. Nanomedicine. 2019;17:188–97. doi: 10.1016/j.nano.2019.01.007. [DOI] [PubMed] [Google Scholar]
- 38.Chandrasekaran K, Anjaneyulu M, Inoue T, Choi J, Sagi AR, Chen C, et al. Mitochondrial transcription factor A regulation of mitochondrial degeneration in experimental diabetic neuropathy. Am J Physiol Endocrinol Metab. 2015;309:e132–41. doi: 10.1152/ajpendo.00620.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cai X, Bao L, Ren J, Li Y, Zhang Z. Grape seed procyanidin B2 protects podocytes from high glucose-induced mitochondrial dysfunction and apoptosis via the AMPK-SIRT1-PGC-1alpha axis in vitro. Food Funct. 2016;7:805–15. doi: 10.1039/C5FO01062D. [DOI] [PubMed] [Google Scholar]
- 40.Xu W, Yan J, Ocak U, Lenahan C, Shao A, Tang J, et al. Melanocortin 1 receptor attenuates early brain injury following subarachnoid hemorrhage by controlling mitochondrial metabolism via AMPK/SIRT1/PGC-1alpha pathway in rats. Theranostics. 2021;11:522–39. doi: 10.7150/thno.49426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chandrasekaran K, Salimian M, Konduru SR, Choi J, Kumar P, Long A, et al. Overexpression of Sirtuin 1 protein in neurons prevents and reverses experimental diabetic neuropathy. Brain. 2019;142:3737–52. doi: 10.1093/brain/awz324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Eom JW, Lee JM, Koh JY, Kim YH. AMP-activated protein kinase contributes to zinc-induced neuronal death via activation by LKB1 and induction of Bim in mouse cortical cultures. Mol Brain. 2016;9:14. doi: 10.1186/s13041-016-0194-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hardie DG, Ross FA, Hawley SA. AMPK: a nutrient and energy sensor that maintains energy homeostasis. Nat Rev Mol Cell Biol. 2012;13:251–62. doi: 10.1038/nrm3311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Carling D, Sanders MJ, Woods A. The regulation of AMP-activated protein kinase by upstream kinases. Int J Obes (Lond) 2008;32:S55–9. doi: 10.1038/ijo.2008.124. [DOI] [PubMed] [Google Scholar]
- 45.Ji J, Xue TF, Guo XD, Yang J, Guo RB, Wang J, et al. Antagonizing peroxisome proliferator-activated receptor gamma facilitates M1-to-M2 shift of microglia by enhancing autophagy via the LKB1-AMPK signaling pathway. Aging Cell. 2018;17:e12774. doi: 10.1111/acel.12774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Liu JL, Mao Z, Gallick GE, Yung WK. AMPK/TSC2/mTOR-signaling intermediates are not necessary for LKB1-mediated nuclear retention of PTEN tumor suppressor. Neuro Oncol. 2011;13:184–94. doi: 10.1093/neuonc/noq163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tumurkhuu G, Shimada K, Dagvadorj J, Crother TR, Zhang W, Luthringer D, et al. Ogg1-dependent DNA repair regulates NLRP3 inflammasome and prevents atherosclerosis. Circ Res. 2016;119:e76–90. doi: 10.1161/CIRCRESAHA.116.308362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Liu X, Chhipa RR, Nakano I, Dasgupta B. The AMPK inhibitor compound C is a potent AMPK-independent antiglioma agent. Mol Cancer Ther. 2014;13:596–605. doi: 10.1158/1535-7163.MCT-13-0579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dang R, Wang M, Li X, Wang H, Liu L, Wu Q, et al. Edaravone ameliorates depressive and anxiety-like behaviors via Sirt1/Nrf2/HO-1/Gpx4 pathway. J Neuroinflammation. 2022;19:41. doi: 10.1186/s12974-022-02400-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dewanjee S, Das S, Das AK, Bhattacharjee N, Dihingia A, Dua TK, et al. Molecular mechanism of diabetic neuropathy and its pharmacotherapeutic targets. Eur J Pharmacol. 2018;833:472–523. doi: 10.1016/j.ejphar.2018.06.034. [DOI] [PubMed] [Google Scholar]
- 51.Singh R, Kishore L, Kaur N. Diabetic peripheral neuropathy: current perspective and future directions. Pharmacol Res. 2014;80:21–35. doi: 10.1016/j.phrs.2013.12.005. [DOI] [PubMed] [Google Scholar]
- 52.Xu X, Wang W, Wang Z, Lv J, Xu X, Xu J, et al. DW14006 as a direct AMPKalpha activator ameliorates diabetic peripheral neuropathy in mice. Diabetes. 2020;69:1974–88. doi: 10.2337/db19-1084. [DOI] [PubMed] [Google Scholar]
- 53.Al-Bazz DY, Nelson AJ, Burgess J, Petropoulos IN, Nizza J, Marshall A, et al. Is nerve electrophysiology a robust primary endpoint in clinical trials of treatments for diabetic peripheral neuropathy? Diagnostics (Basel). 2022;12:731–45. [DOI] [PMC free article] [PubMed]
- 54.Jiang DQ, Li MX, Ma YJ, Wang Y, Wang Y. Efficacy and safety of prostaglandin E1 plus lipoic acid combination therapy versus monotherapy for patients with diabetic peripheral neuropathy. J Clin Neurosci. 2016;27:8–16. doi: 10.1016/j.jocn.2015.07.028. [DOI] [PubMed] [Google Scholar]
- 55.Sifuentes-Franco S, Pacheco-Moises FP, Rodriguez-Carrizalez AD, Miranda-Diaz AG. The role of oxidative stress, mitochondrial function, and autophagy in diabetic polyneuropathy. J Diabetes Res. 2017;2017:1673081. doi: 10.1155/2017/1673081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chowdhury SK, Zherebitskaya E, Smith DR, Akude E, Chattopadhyay S, Jolivalt CG, et al. Mitochondrial respiratory chain dysfunction in dorsal root ganglia of streptozotocin-induced diabetic rats and its correction by insulin treatment. Diabetes. 2010;59:1082–91. doi: 10.2337/db09-1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kurita T, Sakuma H, Onishi K, Ishida M, Kitagawa K, Yamanaka T, et al. Regional myocardial perfusion reserve determined using myocardial perfusion magnetic resonance imaging showed a direct correlation with coronary flow velocity reserve by Doppler flow wire. Eur Heart J. 2009;30:444–52. doi: 10.1093/eurheartj/ehn521. [DOI] [PubMed] [Google Scholar]
- 58.Ellsworth ML. Red blood cell-derived ATP as a regulator of skeletal muscle perfusion. Med Sci Sports Exerc. 2004;36:35–41. doi: 10.1249/01.MSS.0000106284.80300.B2. [DOI] [PubMed] [Google Scholar]
- 59.Kaplamadzhiev DB, Hisha H, Adachi Y, Ikehara S, Tonchev AB, Boneva NB, et al. Bone marrow-derived stromal cells can express neuronal markers by DHA/GPR40 signaling. Biosci Trends. 2010;4:119–29. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.