Abstract
Klebsiella aerogenes strains with reduced levels of d-amino acid dehydrogenase not only fail to use alanine as a growth substrate but also become sensitive to alanine in minimal media supplemented with glucose and ammonium. The inability of these mutant strains to catabolize the alanine provided in the medium interferes with both pathways of glutamate production. Alanine derepresses the nitrogen regulatory system (Ntr), which in turn represses glutamate dehydrogenase, one pathway of glutamate production. Alanine also inhibits the enzyme glutamine synthetase, the first enzyme in the other pathway of glutamate production. Therefore, in the presence of alanine, strains with mutations in dadA (the gene that codes for a subunit of the dehydrogenase) exhibit a glutamate auxotrophy when ammonium is the sole source of nitrogen. The alanine catabolic operon of Klebsiella aerogenes, dadAB, was cloned, and its DNA sequence was determined. The clone complemented the alanine defects of dadA strains. The operon has a high similarity to the dadAB operon of Salmonella typhimurium and the dadAX operon of Escherichia coli, each of which codes for the smaller subunit of d-amino acid dehydrogenase and the catabolic alanine racemase. Unlike the cases for E. coli and S. typhimurium, the dad operon of K. aerogenes is activated by the Ntr system, mediated in this case by the nitrogen assimilation control protein (NAC). A sequence matching the DNA consensus for NAC-binding sites is located centered at position −44 with respect to the start of transcription. The promoter of this operon also contains consensus binding sites for the catabolite activator protein and the leucine-responsive regulatory protein.
Alanine plays many important roles in the growth and physiology of enteric bacteria. It is one of the major amino acids present in proteins (33) and can be used in the biosynthesis of the amino acid valine (46) and of the vitamin biotin (16), and both the l and d stereoisomers of alanine are major constituents of the peptidoglycan layer (43). Alanine pools in Klebsiella aerogenes and Escherichia coli are higher than levels of other amino acids when cells are grown under a variety of laboratory conditions (37, 42). In E. coli, the presence of exogenous alanine causes the differential expression of many operons. This differential expression occurs both in a leucine-responsive regulatory protein (Lrp)-dependent manner (12) and independently of Lrp (28). Therefore, alanine has been implicated as a regulatory molecule that plays a role in transcriptional regulation. Catabolism of alanine yields pyruvate and ammonia; thus, alanine provides a source of carbon, nitrogen, and energy.
Alanine catabolism has been well studied in the enteric bacteria E. coli and Salmonella typhimurium (30, 38). The catabolism proceeds in a two-step process in which l-alanine is converted to d-alanine by alanine racemase, and then the d-alanine is converted to pyruvate and ammonia by the membrane-associated, heterodimeric d-amino acid dehydrogenase (38). The genes that code for the racemase (dadX in E. coli; dadB in S. typhimurium) and the smaller subunit of the dehydrogenase (dadA) have been characterized and form an operon that is regulated by exogenous alanine and carbon limitation (23, 44, 45, 47–49). Lrp has been implicated in the derepression of the dad operons of both organisms in response to alanine (21, 29), and Lobocka et al. have shown that the activation of the E. coli operon under carbon-limiting conditions is dependent on the catabolite activator protein (CAP) (23). Neither the dehydrogenase nor the racemase activity is regulated by nitrogen in either organism (30).
We observed that the addition of l-alanine to minimal medium supplemented with glucose and ammonium prevented the growth of many of the K. aerogenes strains in our collection. To understand this alanine sensitivity, we studied alanine catabolism in K. aerogenes. Here we present the characterization of the dadAB operon from K. aerogenes and discuss how strains unable to catabolize alanine develop a glutamate auxotrophy when grown in minimal medium containing glucose, ammonium, and alanine.
MATERIALS AND METHODS
Bacterial strains and plasmids.
The K. aerogenes and E. coli strains and plasmids used in this work are listed in Table 1. It should be noted that although the species K. aerogenes has been subsumed into the species K. pneumoniae, we retain the older name for historical purposes and to distinguish strain W70 from the nitrogen-fixing K. pneumoniae strain M5a1, from which it differs considerably. pCB925 is the original clone isolated containing the dadAB operon of K. aerogenes. pCB752 is a subclone of pCB925 and was constructed by digesting pCB925 with HindIII and cloning a 3.5-kb fragment into pBluescript (pBS) SK−; pCB752 complements the Dad phenotype of KC2668. The dad promoter regions from E. coli and K. aerogenes were amplified by PCR and cloned into pRJ800. Primers were designed that hybridize to the upstream, oppositely oriented open reading frame (ORF) (alape [GGGGAATTCCATAGAATCGATCGTCGCCAT]) and to the start of the coding region of dadA (alaph [GATTAAGCTTCCAGGCGCTGGCAACG]) to amplify the E. coli dad promoter. Chromosomal DNA from E. coli DH5α was used as a template for PCR, the conditions for which are described below. The resulting DNA fragment was purified and digested with EcoRI and HindIII (the restriction sites were present in the primers) and cloned into pRJ800, resulting in plasmid pCB889. The dad promoter region from K. aerogenes was cloned the same fashion (primers kalapeco [GGGGAATTCATGGAGTCAATCGTAGCCAT] and kalaphin [CCCCCAAGCTTCAATCTCCCAGTATGACG]) except that pCB752 was used as a DNA template. The resulting PCR fragment was cloned into pRJ800, resulting in pCB888. The DNA sequence of each promoter was determined to ensure that no errors were generated during the amplification by Taq DNA polymerase. Plasmid pPC36 contains nac driven by the isopropyl-β-d-thiogalactopyranoside IPTG-inducible tac promoter (13, 15).
TABLE 1.
Strains and plasmids used in this work
| Strain or plasmida | Relevant genotype or characteristics | Reference or source |
|---|---|---|
| K. aerogenes | ||
| MK9682 | gltB200 ntr-45 | 4 |
| KB958 | hutC515 nac-1 | 7 |
| MK1 | Prototroph | B. Magasanik |
| KC1422 | hutC515 dadA1 (Mu cts hP1 1) | This laboratory |
| KC2001 | gltB200 ntr-45 ntrC5::Tn5-131 | 25 |
| KC2004 | hutC515 Δ[bla]-2 nac-203::Tn5-131 | This laboratory |
| KC2569 | hutC515 dadA1 nas-3::Tn5-131 | 5 |
| KC2616 | hutC515 Δ[bla]-2 crp-91::Tn5-131 | This laboratory |
| KC2668 | hutC515 Δ[bla]-2 dadA1 | This laboratory |
| KC2725 | nac-203::Tn5-131 in KC2668 | This laboratory |
| KC2863b | gdh-3 in KC2668 | This laboratory |
| KC3345c | hutC515 nac-203::Tn5-131 | P1 (KC958) × KC2004 |
| KC3346d | hutC515 Δ[bla]-2 | P1 (KC3345) × KC2668 |
| KC3821 | KC3346/pCB888 | This work |
| KC3847 | KC3346/pPC36 | This work |
| KC3848 | KC3847/pCB888 | This work |
| KC3902 | KC2668/pCB515 | This laboratory |
| KC3914 | hutC515 (Mu cts hP1 1)/pEG5005 | This work |
| KC4080 | nas-3::Tn5-131 in KC3346 | This work |
| KC4469e | hutC515 Δ[bla]-2 crp-91::Tn5-131 | P1 (KC2616) × KC3346 |
| E. coli | ||
| W3110 | Prototroph | R. G. Matthews |
| EB3364 | nac-28 in W3110 | 32 |
| EB3846 | W3110/pPC36 | This work |
| Plasmids | ||
| pBS SK− | High-copy-number cloning plasmid, Apr | Stratagene |
| pEG5005 | Carries Mu d5005, Apr Kmr | 20 |
| pRJ800 | Promoterless lacZ fusion plasmid, Apr | 2 |
| pPC36 | Low-copy-number tacp::nac fusion, Smr Spr | S. Maloy |
| pCB515 | Low-copy-number gdhA, Smr Spr | This laboratory |
| pCB752 | K. aerogenes dadAB operon in pBS SK− | This work |
| pCB888 | K. aerogenes dadAp in pRJ800 | This work |
| pCB889 | E. coli dadAp in pRJ800 | This work |
| pCB925 | Mu d5005::dadAB | This work |
All K. aerogenes strains listed here are derived from strain W70; all E. coli strains are derived from strain K-12.
gdh-3 in a mutation that results in an approximately threefold increase in GDH activity when cells are grown in glucose-ammonium minimal medium.
Transduction of strain KC2004 to Dad+ (growth on alanine as the sole carbon and nitrogen source), using phage P1 grown on strain KC958.
Transduction of strain KC2668 to Dad+ (growth on alanine as the sole carbon and nitrogen source), using phage P1 grown on strain KC3345.
Transduction of strain KC3346 to crp-91::Tn5-131 (tetracycline resistance), using phage P1 grown on strain KC2616.
Enzyme assays.
Cells were grown in W4 salts (6) supplemented with various carbon and nitrogen sources (0.4 and 0.2% [wt/vol], respectively) as indicated in Tables 2 to 5. Cells were grown to mid-log phase (50 Klett units), washed with 1% KCl, and concentrated 10-fold (approximately 1 mg of protein per ml). Cells were permeabilized with toluene for the d-amino acid dehydrogenase and alanine racemase assays, with 0.2% hexadecyl trimethylammonium bromide (CTAB) for the glutamine synthetase (GS) assay, and with both CTAB and 0.02% sodium deoxycholate for the glutamate dehydrogenase (GDH) assay. The d-amino acid dehydrogenase assay was performed in 100 mM phosphate buffer (pH 7.4), with 20 mM d-alanine used as the substrate. The amount of pyruvate produced in each assay was determined as described previously (49). Specific activity is reported as nanomoles of pyruvate produced/minute/milligram of protein. The alanine racemase assay was performed as described previously (48) except that to fully inactivate the K. aerogenes enzyme, the incubation at 85°C prior to the addition of d-amino oxidase was increased to 10 min. Specific activity is reported as nanomoles of d-alanine formed/minute/milligram of protein. The GDH was assayed as described previously (11) and measured the 2-ketoglutarate-dependent β-NADPH oxidation. Specific activity is reported as nanomoles of NADPH oxidized/minute/milligram of protein (with 2-ketoglutarate-independent oxidation subtracted). The GS transferase assay, which determines total (both adenylylated and unadenylylated) GS levels present, was performed as described previously (6). Specific activity is reported as nanomoles of γ-glutamyl hydroxamate formed/minute/milligram of protein. Total protein concentration was determined by the method of Lowry et al. (24).
TABLE 2.
Regulation of d-amino acid dehydrogenase and alanine racemase activities in K. aerogenes
| Strain | Relevant genotype | Growth mediuma | Sp actb
|
|
|---|---|---|---|---|
| d-Amino acid dehydrogenase | Alanine racemase | |||
| MK1 | Wild type | GN | 13 ± 3 | 22 ± 10 |
| GNala | 386 ± 162 | 428 ± 100 | ||
| Gala | 331 ± 32 | 415 ± 70 | ||
| Alac | 769 ± 62 | 615 ± 36 | ||
| KC2725 | dadA1 nac-203 | GNala | 14 ± 3 | 228 ± 30 |
| KC2001 | ntrC5 | GN | 10 ± 4 | ND |
| GNala | 240 ± 7 | ND | ||
| MK9682 | ntr-45 | GNglnd | 42 ± 6 | ND |
| GNglnalad | 629 ± 70 | ND | ||
| KC3346 | wild type | GN | 7 ± 1 | ND |
| GNala | 356 ± 59 | ND | ||
| KC3345 | nac-203 | GN | 14 ± 3 | ND |
| GNala | 206 ± 18 | ND | ||
| KC3847 | pPC36e | GN | 10 ± 4 | ND |
| GNIPTG | 52 ± 11 | ND | ||
| KC4469 | crp | GNala | 262 ± 38 | ND |
| Alac | 336 ± 98 | ND | ||
Growth medium was W4 salts supplemented with 0.4% glucose (G), 0.2% (NH4)2SO4 (N), 0.2% l-alanine (ala), 0.2% l-glutamine (gln), and 1 mM IPTG (to induce nac expression from the tac promoter [pPC36]) as indicated.
Average and standard deviation of at least three independent assays. ND, not determined.
0.4% l-alanine.
0.2% l-glutamine added to supply glutamate (MK9682 is a glutamate auxotroph).
Contains nac driven by the IPTG-inducible tac promoter.
TABLE 5.
Regulation of d-amino acid dehydrogenase activity in E. coli
| Strain | Relevant genotype | Growth mediuma | d-Amino acid dehydrogenase sp actb |
|---|---|---|---|
| W3110 | GN | 19 ± 1 | |
| GNala | 250 ± 26 | ||
| EB3364 | nac-28 | GN | 21 ± 8 |
| GNala | 325 ± 25 | ||
| EB3846 | pPC36c | GN | 20 ± 9 |
| GNIPTG | 30 ± 12 |
Growth medium was W4 salts supplemented with 0.4% glucose (G), 0.2% (NH4)2SO4 (N), 0.05% l-alanine (ala), and 1 mM IPTG (to induce nac expression from the tac promoter [pPC36]) as indicated.
Average and standard deviation of at least three independent assays.
Contains nac driven by the IPTG-inducible tac promoter.
Genetic and molecular techniques.
Generalized transduction using phage P1vir was performed as described previously (18). The use of phage Mu cts hP1 1 and plasmid pEG5005 to create bacterial gene libraries was performed as described previously (20) except that to induce lysis by phage Mu in K. aerogenes, the cells were shifted to 45°C for 3 h before harvesting. DNA digestion with restriction endonucleases, ligation of DNA fragments, DNA electrophoresis in agarose gels, and cell transformations were performed by standard protocols (1, 27). Plasmid DNA was purified by using alkaline lysis minipreps (27) or Qiaprep miniprep spin columns (Qiagen). DNA fragments were purified from agarose gels by electroelution and precipitation with ethanol (27) or by using Qiaquick gel extraction spin columns (Qiagen). DNA sequencing was performed on double-stranded DNA templates via the dideoxy method, using a Sequenase 2.0 sequencing kit (United States Biochemical), with one difference from the manufacturer’s protocol: the NaOH denaturation of templates was replaced with a heat denaturation step of 95°C for 3 min and immediate shift to ice. Additional sequence determination was performed by the University of Michigan Core Facilities. PCR was performed with Taq DNA polymerase (Gibco-BRL). Reactions were performed with 50 pmol of each primer, 0.1 to 1 μg of DNA template, 0.2 mM deoxyribonucleic triphosphates, 1.5 mM MgCl2, 0.5 U of Taq DNA polymerase, and buffer conditions specified by the manufacturer (Gibco-BRL). The PCR cycle used contained an initial 3-min denaturation step at 94°C, followed by 1 min at 56°C, 1 min at 72°C, and 1 min at 94°C for 32 cycles and a final extension step of 3 min at 72°C.
Primer extension analysis.
RNA was isolated by the method of Xiong et al. (51). The primer extension analysis was performed as previously described (35).
Mobility shift assays.
To radiolabel the DNA fragments used in the mobility shift assays, the plasmids containing either the E. coli dad promoter (pCB889) or K. aerogenes dad promoter (pCB888) were incubated with EcoRI, purified (by ethanol precipitation), radiolabeled by using Klenow fragment and [32P]dATP, again purified, and finally digested with HindIII (27). For experiments using Lrp, pCB888 was further digested with PstI (after the DNA was purified), which resulted in a radiolabeled DNA fragment of appropriate length to use as a control. For mobility shift assays with the nitrogen assimilation control protein (NAC), the labeled DNA fragments were incubated with purified NAC or NAC dilution buffer 6 (19) and a 10-fold excess of calf thymus DNA in a total volume of 10 μl. The binding mixtures were incubated for 20 min at room temperature, and then 1 μl of type II loading buffer (27) was added. Each reaction was loaded into a 4% TE (10 mM Tris-HCl, 1 mM EDTA)–polyacrylamide gel, and the fragments were separated by electrophoresis at 4°C and 13.3 V/cm in a Hoefer electrophoresis chamber. The gel was transferred to 3MM filter paper and dried. Autoradiographs were obtained by exposing X-ray film at −70°C with an intensifying screen. Films were developed in a X-Omat developer. For the Lrp mobility shifts assays, the protocol was essentially the same as described by Ernsting et al. (17) except that more target DNA (0.1 μg) and more Lrp (8 to 200 nM) were used in each reaction. Electrophoresis and gel transfer were performed as for the NAC mobility shift assays.
Nucleotide sequence accession number.
The sequence of the K. aerogenes dadAB operon has been entered in GenBank under accession no. AF016253.
RESULTS
Alanine sensitivity of a dadA mutant of K. aerogenes.
To determine if K. aerogenes could catabolize alanine in a way analogous to that of E. coli and S. typhimurium, we assayed strain MK1 for both alanine racemase and d-amino acid dehydrogenase activity in the presence and absence of l-alanine. Both enzyme activities were present, and both were induced by the presence of alanine in the growth medium (Table 2). Somewhat surprisingly, strain KC2668, which is derived from strain MK1, failed to grow on alanine as a carbon or nitrogen source. Moreover, the presence of alanine at 0.2% (wt/vol) prevented growth of this strain in minimal medium supplied with glucose and ammonium as carbon and nitrogen sources. Thus, we were unable to measure the induction of the racemase or the dehydrogenase in KC2668. This alanine sensitivity was not observed in the nearly isogenic strain KC2725, although this strain still failed to use alanine as a carbon or nitrogen source. KC2725 had low levels of d-amino acid dehydrogenase, even in the presence of alanine. Alanine racemase levels were comparable to those in the wild type (Table 2). We therefore concluded that the failure of both KC2668 and KC2725 to grow on alanine as the sole source of carbon or nitrogen was a result of the low levels of d-amino acid dehydrogenase activity.
Both KC2668 and KC2725 were derived from strain MK53 (hutC515). In fact, nearly all of the strains we tested that were derived from MK53 exhibited the alanine-sensitive phenotype. MK53 was isolated after treatment with ethyl methanesulfonate (36), and it is therefore likely that the mutation that reduces the dehydrogenase activity occurred during this mutagenesis.
We hypothesized that the mutation that results in the loss of the dehydrogenase activity could be located in the dadA gene, which codes for the smaller subunit of the dehydrogenase. Using P1 transduction, we mapped the allele responsible for the failure to catabolize alanine. dadA should be linked approximately 1% to nas, the gene that encodes the assimilatory nitrate reductase in S. typhimurium (39), and to nar, the gene that encodes the respiratory reductase in E. coli (9). The nar and nas alleles are linked in K. pneumoniae (22). Using a nas-3::Tn5-131 dad+ strain of K. aerogenes as a donor (KC4080), we transduced KC2668 either to tetracycline resistance (encoded by transposon Tn5-131 [8]) or to the ability to grow with alanine as the nitrogen source. We then tested each of the transductants for the other phenotype. The dad allele in KC2668 was 0.8% linked to nas. The linkage of the dad allele to nas was our first evidence that the mutation that abolished d-amino acid dehydrogenase activity in KC2668 was in the K. aerogenes dadA gene.
A clone of the dadAB operon complements the alanine defects of KC2668.
To clone the gene responsible for the reduced dehydrogenase activity in KC2668, we used the in vivo cloning strategy developed by Groisman and Casadaban (20). KC3914 can grow on alanine normally, is lysogenic for phage Mu, and contains plasmid pEG5005 (20). A Mu lysate was prepared from this strain and used to transduce strain KC1422 (dadA1) to kanamycin resistance and the ability to grow with alanine as the nitrogen source. Transformation of KC1422 with the plasmid from one such transductant verified that the plasmid (pCB925) contained the information necessary to allow the dadA strain to grow with alanine as the sole nitrogen source. A 3.5-kb HindIII fragment was cloned from this plasmid into pBS SK− (Stratagene), and the resulting plasmid, pCB752, was introduced into KC2668. pCB752 allowed KC2668 to grow with alanine as the sole carbon or nitrogen source. It also abolished the toxicity of alanine observed for KC2668. This plasmid restored high levels of the dehydrogenase and increased the levels of the racemase in KC2668 (data not shown).
Determination of the DNA sequence of a majority of the 3.5-kb HindIII fragment showed that this fragment contained an operon highly similar to the dadAX operon of E. coli and the dadAB operon of S. typhimurium. The operon contains a promoter region followed by dadA and dadB, with a 9-bp spacer between the two genes. Upstream of the region is an oppositely oriented ORF similar in sequence to the ORF reported by Lobocka et al. in the analogous region from E. coli (23). This ORF was only partially sequenced. The DNA sequence of the dad operon was found to be 76% identical to that of the dadAX operon of E. coli. The deduced amino acid sequence of the alanine racemase of K. aerogenes was 79 and 77% identical to the corresponding proteins from E. coli and S. typhimurium, respectively, while the deduced amino acid sequence of the dehydrogenase of K. aerogenes was 88% identical to the protein from E. coli. Regions of high identity included the putative flavin adenine dinucleotide binding domain of the smaller subunit of the dehydrogenase and the lysine proposed to interact with pyridoxal phosphate and the glycine-rich hinge of the racemase.
The predicted promoter region of the dadAB operon of K. aerogenes was very similar to the promoter of the E. coli operon (23). Sequence comparison of the two promoters (Fig. 1) revealed an exact match in the promoter region from positions −7 to −38 (positions 198 to 167 in Fig. 1). This promoter most resembles a sigma-70-dependent promoter. The promoter also contains several putative binding sites for different activators. There is a close match to the CAP-cyclic AMP (cAMP) recognition sequence located centered at approximately −60 (position 146 in Fig. 1) with respect to the start of transcription. In addition, there are several putative Lrp binding sequences. Also of interest is a putative binding site for NAC. The regions containing the putative binding sites of these transcriptional regulators have a high level of sequence identity compared to the promoter of the E. coli dad operon, with the exception of the binding site for NAC, which is not present in the E. coli promoter.
FIG. 1.
Comparison of the regulatory regions of dadAB from K. aerogenes and dadAX from E. coli. The lower DNA sequence is from dadAX as reported by Lobocka et al. (23). The start of transcription and the proposed −10 and −35 promoter regions are in boldface. Solid underlines indicate putative CAP-cAMP binding sites, ΔΔΔΔΔ indicates the NAC binding site (the asterisk indicates the C-to-T change that most likely is the reason NAC fails to bind to the dad promoter from E. coli), and the broken lines indicate putative Lrp binding sites.
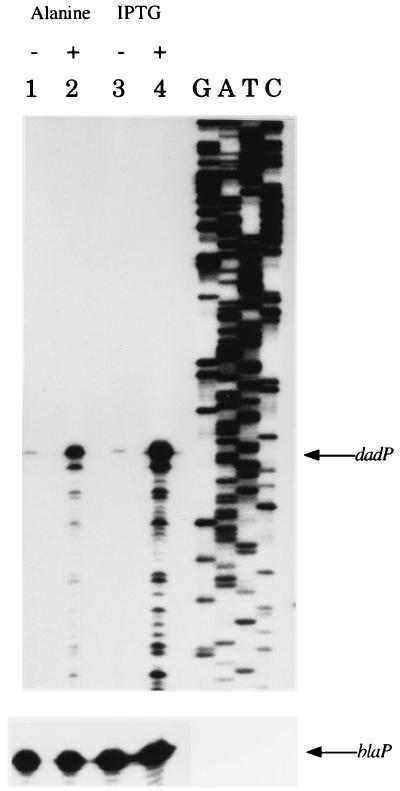
The sequence identity of the promoter led us to predict that transcription of the K. aerogenes operon would initiate from the same nucleotide identified in E. coli. We used primer extension analysis to map the 5′ end of the mRNA transcript generated from this promoter to test this prediction (Fig. 2). Total RNA was obtained from KC3821 (dadA+, pCB888) grown in glucose-ammonium minimal medium with or without 0.2% l-alanine. We identified one major product that corresponds with the previously identified start of transcription of the E. coli dadAX operon. The amount of extended product was increased by the presence of alanine (Fig. 2, lanes 1 and 2). This result verifies that the predicted sigma-70-dependent promoter is indeed the promoter of this operon and that transcription is induced by alanine from this promoter.
FIG. 2.
Mapping the transcriptional start site of dadAB by primer extension. The sequence lanes have been oppositely labeled such that the coding strand sequence is denoted, although the actual reactions were performed with the primer extension primer (RJ800EXT) and is thus the noncoding strand. Below is a control extension reaction of the β-lactamase gene (blaP) also present on the plasmid to ensure equal loading. RJ800EXT hybridizes to plasmid pRJ800 downstream of the multiple cloning site; thus, the extended products shown are from the plasmid-borne promoter (pCB888) only. Total RNA was isolated from the strains grown in glucose-ammonium minimal medium supplemented with ampicillin (100 μg/ml) for plasmid maintenance and with alanine or IPTG as indicated. Lanes: 1, KC3821, no addition; 2, KC3821, supplemented with 0.2% l-alanine; 3, KC3848, no addition; 4, KC3848, supplemented with 1 mM IPTG.
Glutamate relieves the alanine sensitivity of dadA strains.
We found three nutritional supplements that allowed strain KC2668 (dadA1) to grow in glucose-ammonium minimal medium in the presence of alanine. First, the addition of glutamate (0.5% [wt/vol]) to the medium allowed growth in the presence of alanine. Second, the addition of amino acids that could be catabolized to yield glutamate (arginine, asparagine, aspartate, glutamine, histidine, and proline) allowed growth, but those that could not be catabolized to glutamate (cysteine, glycine, lysine, methionine, phenylalanine, serine, threonine, and tryptophan) failed to allow growth. Finally, the addition of the branch-chained amino acids (leucine, isoleucine, and valine) allowed growth, most likely by reducing the amount of alanine transported into the cell.
We also identified several mutant strains that failed to grow with alanine as the sole source of carbon or nitrogen (presumably dadA1) that were not sensitive to alanine. Each of these strains has higher levels of GDH than does MK1 (Table 3). Strains that had higher levels of GDH expression, because they possessed either multiple copies of the gdhA gene (KC3902, pCB515) or a mutation that increased the expression of gdhA (KC2863, gdh-3), grew in the presence of alanine, as did a strain that lacked a functional NAC (KC2725, nac-203). NAC has been shown to be a repressor of gdhA (40).
TABLE 3.
Regulation of GDH activity in the presence of alanine
| Strain | Relevant genotype | Growth mediuma | GDH sp actb |
|---|---|---|---|
| MK1 | Wild type | GNgln | 310 ± 25 |
| GN | 227 ± 15 | ||
| Ggln | 47 ± 6 | ||
| GNala | 47 ± 3 | ||
| KC2668 | dadA1 | GNgln | 417 ± 11 |
| GN gln ala | 332 ± 8 | ||
| GN ala pro | 51 ± 5 | ||
| Ggln | 27 ± 5 | ||
| KC3902 | dadA1, pCB515c | GN | 1,233 ± 232 |
| GNala | 185 ± 48 | ||
| KC2725 | dadA1 nac-203 | GN | 410 ± 49 |
| GNala | 365 ± 43 | ||
| KC2863d | dadA1 gdh-3 | GN | 1,244 ± 268 |
| GNala | 874 ± 102 |
Growth medium was W4 salts supplemented with 0.4% glucose (G), 0.2% (NH4)2SO4 (N), 0.2% l-alanine (ala), 0.2% proline (pro), and 0.04% l-glutamine (gln) as indicated.
Average and standard deviation of at least three independent assays.
pCB515 contains gdhA in multicopy (pSC101 origin of replication, four to five copies per cell [14]).
gdh-3 in a mutation that results in an approximate threefold increase in GDH activity when cells are grown in glucose-ammonium minimal medium.
Nitrogen regulation of the dadAB operon.
Initial studies of the nitrogen regulation of the dadAB operon were complicated by the effect of alanine on the nitrogen regulatory (Ntr) system of K. aerogenes (26, 31). Using GS as a reporter for Ntr derepression, we found that the presence of 0.2% l-alanine in the growth media activated GS expression two- to sixfold (Table 4). l-Alanine has been shown to inhibit the GS from both E. coli (50) and K. aerogenes (41), and we found that GS activity is inhibited by d-alanine as well (data not shown). Alanine at 40 mM can inhibit nearly 90% of GS activity. The inhibition of GS by alanine would result in a starvation for glutamine and thus derepression of the Ntr system. This derepression would lead to the higher levels of GS (Table 4) and the relief of the glutamine starvation caused by alanine.
TABLE 4.
Derepression of GS by alanine in K. aerogenes MK1
| Growth mediuma | GS sp actb |
|---|---|
| GNgln | 149 ± 44 |
| GN | 476 ± 135 |
| GNala | 947 ± 98 |
| Ggln | |
| 0.2% | 1,452 ± 37 |
| 0.04% | 2,130 ± 233 |
Growth medium was W4 salts supplemented with 0.4% glucose (G), 0.2% (NH4)2SO4 (N), 0.2% l-alanine (ala), and l-glutamine (gln) as indicated.
Detergent-treated cells were assayed for the total amount of GS present in the transferase assay (6). Results are the average and standard deviation of at least three independent assays.
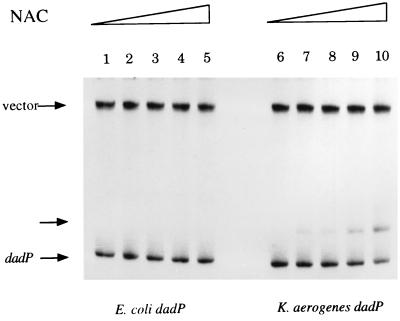
To avoid these complexities, we used a series of Ntr mutants to study the regulation of the dadAB operon (Table 2). A strain that cannot derepress the Ntr system (KC2001, ntrC5) was unable to induce the dehydrogenase activity fully. A strain that has constitutive derepression of the Ntr system (MK9682, ntr-45) had increased expression of the dehydrogenase in the absence of alanine. Many genes and operons that exhibit Ntr-dependent regulation also require the NAC protein for this regulation (3). A strain that lacks NAC (KC3345, nac-203) failed to derepress the dehydrogenase fully, much like the Ntr-deficient mutant (Table 2). In addition, a strain that contains an IPTG-inducible nac (KC3847, pPC36) had IPTG-dependent derepression of the dehydrogenase. Purified NAC bound to the promoter of the K. aerogenes dad operon in gel mobility shift assays (Fig. 3, lanes 6 to 10). We can therefore conclude that the induction of dad is Ntr dependent and that this dependency is mediated through NAC.
FIG. 3.
Interaction of the dad promoters from E. coli and K. aerogenes with NAC. pCB888, which contains the dad promoter from K. aerogenes, and pCB889, which contains the dad promoter from E. coli, were digested and radiolabeled as described in Materials and Methods. Each was then incubated with buffer 6 (19) or increasing amounts of purified NAC (0, 16.5, 22, 33, and 66 nM) for 20 min. The bound and unbound fragments were then separated by electrophoresis on a 4% TE–polyacrylamide gel run for 2 h at 13.3 V/cm at 4°C. The gel was dried, and the DNA was visualized by exposure to X-ray film. Unbound vector and dad promoter bands are indicated by labeled arrows; the unlabeled arrow indicates the NAC-dadAp complex.
The consensus sequence for NAC binding in NAC-activatable promoters is ATA-N9-TAT (3). Such a site is present in the dad promoter, centered at position −44 with respect to the start of transcription (Fig. 1). This sequence is not present in the promoter of the E. coli operon. An E. coli strain that lacks a functional NAC (EB3364, nac-28) showed no loss of the dehydrogenase induction by alanine, and a strain (EB3846, pPC36) with an IPTG-inducible nac did not exhibit any IPTG-dependent induction of dad (Table 5). NAC did not bind to the promoter of the E. coli dad operon (Fig. 3, lanes 1 to 5). Therefore, NAC is not involved in the regulation of the E. coli dad operon.
To verify that the promoter activated by NAC was the same promoter induced by alanine, we performed primer extension analysis using strain KC3848, in which nac can be induced by IPTG. Primer extension analysis using RNA isolated from this strain grown in the presence of 1 mM IPTG yielded a product identical to the product observed by alanine induction (Fig. 2, lanes 2 and 4). Therefore, NAC activates the predicted sigma-70-dependent promoter and most likely does so from a site centered at −44.
Other regulators of the dadAB operon.
Alanine racemase and d-amino acid dehydrogenase are both slightly repressed by glucose (Table 2, strain MK1; cf. Ala with GNala). It is likely that the slight activation seen in the absence of glucose is due to CAP-cAMP binding to the CAP-cAMP binding site located centered at −60 with respect to the start of transcription (Fig. 1), since a crp mutant (KC4469 [Table 2]) fails to repress the dehydrogenase activity in the presence of glucose. This activation by CAP-cAMP is likely to be occurring in a manner equivalent to that described for the operon from E. coli (23).
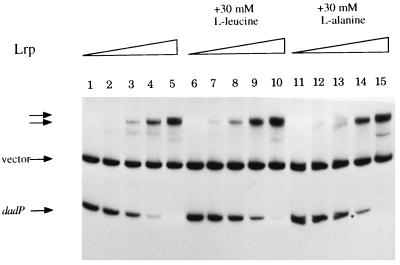
Lrp has been implicated in the regulation of the dad operons of E. coli and S. typhimurium (21, 29). The proposed Lrp binding sites of E. coli have high sequence identity with the corresponding regions in the promoter of K. aerogenes (Fig. 1). To test whether Lrp could bind in this promoter region, we performed gel mobility shift assays using Lrp purified from E. coli (Fig. 4). Incubation with Lrp yielded several retarded species, suggesting that more than one Lrp dimer bound to these fragments. The addition of 30 mM leucine or 30 mM alanine to the binding reaction reduced the amount of free target shifted (Fig. 4; cf. lane 4 with lanes 9 and 14) but did not abolish the ability of Lrp to shift the target to the lowest-mobility product.
FIG. 4.
Interaction of Lrp with the promoter region of dadAB from K. aerogenes. pCB888 was digested and radiolabeled as described in Materials and Methods; 0.1 μg of DNA was incubated with increasing amounts of Lrp (lanes 1, 6, and 11, no Lrp; lanes 2, 7, and 12, 8 nM; lanes 3, 8, and 13, 23 nM; lanes 4, 9, and 14, 68 nM; lanes 5, 10, 15, 200 nM) and incubated for 20 min at room temperature; 30 mM l-leucine (lanes 6 to 10) or 30 mM l-alanine (lanes 11 to 15) was added prior to the addition of Lrp. Separation of the bound and unbound fragments by electrophoresis and visualization of the DNA fragments by autoradiography are described in Materials and Methods. The vector and unbound dad promoter bands are indicated by labeled arrows; the two unlabeled arrows indicate the Lrp-dadAp complexes. The unlabeled band that appears in lanes 5, 10, and 15 is most likely the result of an Lrp-vector DNA complex due to the high levels of Lrp present.
DISCUSSION
In this work, we have shown that K. aerogenes expresses both alanine racemase and d-amino acid dehydrogenase activities and that the dadAB operon is regulated by the Ntr system through NAC. A dadA mutant not only fails to use alanine as a carbon or nitrogen source but becomes sensitive to the presence of the amino acid in glucose-ammonium minimal medium. A clone containing the dadAB operon of K. aerogenes complements these growth defects and codes for the genes for the smaller subunit of the dehydrogenase (dadA) and the racemase (dadB).
The most surprising behavior of KC2668 was its sensitivity to the presence of alanine in glucose-ammonium minimal medium. It is apparent that dadA mutants of E. coli and S. typhimurium do not exhibit this sensitivity, and therefore the K. aerogenes sensitivity to alanine could be explained by a difference in the physiology of these organisms. We believe the inability of K. aerogenes dadA mutants to grow on glucose-ammonium minimal medium supplemented with 0.2% l-alanine is due to a glutamate auxotrophy established through alanine’s derepression of the Ntr system. K. aerogenes assimilates ammonia two ways, depending on its availability. When ammonium levels are high, GDH (ammonia + 2 ketoglutarate→glutamate) is derepressed and is responsible for meeting the glutamate demand of the cell. Under these conditions, only a low level of GS (ammonia + glutamate→glutamine) is needed to supply the cell with glutamine. When ammonium levels are low, GDH is fully repressed and GS levels are greatly enhanced. The elevated levels of GS allow for an increased ability of the cell to scavenge ammonia and make glutamine. However, since GDH is fully repressed under nitrogen limitation, most of the glutamine produced by GS is converted back to glutamate by glutamate synthase (glutamine + 2-ketoglutarate→2 glutamate). Thus, in nitrogen-limiting conditions, GS levels are elevated to provide the cell not only with glutamine but with glutamate as well. Both l- and d-alanine can inhibit GS in vitro. This interaction of alanine with GS could explain the derepression of Ntr in the wild-type strain even though ammonia levels are high: inhibition of GS by alanine would lead to a drop in glutamine pools, which would in turn derepress Ntr, as has been seen with other inhibitors of GS such as methionine sulfoximine (10). Derepression of the Ntr system by alanine would affect both pathways of glutamate synthesis. First, Ntr derepression would lead to greater expression of the glnA ntrBC (glnALG) operon, and so GS levels would be increased until there was enough GS to overcome the alanine inhibition and restore the glutamine pool to an appropriate level. Second, gdhA would be repressed by the Ntr system (via NAC), and so the presence of alanine would ultimately result in repressed levels of GDH similar to that of nitrogen-limited conditions. Therefore, in the presence of alanine, K. aerogenes must assimilate all of its nitrogen through GS and glutamate synthase. Under these conditions, the alanine is being catabolized by the high levels of the racemase and the dehydrogenase. However, if the strain is unable to catabolize alanine, as with KC2668, the accumulation of alanine would be even greater and the amounts of GS needed to overcome the inhibition would be greater still. It is our belief that in the presence of 0.2% l-alanine, a dadA mutant is unable to make enough GS to provide all of the glutamine and glutamate necessary for growth. Conditions that overcome the alanine sensitivity in dadA strains all yield alternative sources of glutamate, either by catabolism of other substrates such as glutamine or proline or by raising the amount of GDH present in the cell. These increased amounts of GDH provide additional glutamate from the high levels of ammonia available. These results also predict that in the presence of alanine, KC2668 contains enough GS to satisfy the demand for glutamine needed for biosynthesis and growth but not enough GS to provide for all of the nitrogen assimilation needed by the cell.
The alanine sensitivity of K. aerogenes dadA strains can therefore be attributed to the role that NAC plays in the repression of gdhA. Neither E. coli or S. typhimurium represses GDH when nitrogen is limited and so would not exhibit the sensitivity to alanine accumulation.
Alanine is present in intracellular levels as high as 2 mM during growth in minimal medium (42). This amount of alanine might be enough to inhibit the activity of GS, and so it is possible that even in the absence of exogenous alanine, the amino acid has an in vivo effect on GS. This might partially explain the slight elevation of GS levels in cells grown in glucose-ammonium minimal medium lacking exogenous glutamine (Table 4). However, GS is a highly regulated enzyme, and we provide no direct evidence that normal pool levels of alanine have any effect on the enzyme’s activity.
The dadAB operon of K. aerogenes is activated by the Ntr system, and this activation is achieved by NAC. In contrast, E. coli has no reported nitrogen regulation of its dad operon. The apparent binding site in the K. aerogenes promoter matches the consensus sequence for sites able to activate transcription. ATA-N9-TAT (3). We can therefore include dadAB in the NAC regulon. The binding site in the dad promoter matches the derived consensus sequence but differs from the other characterized NAC-activated promoters in its position relative to the start of transcription. The other characterized NAC-activated operons all have a NAC binding site centered at −64 with respect to transcription, while the site in the dad operon is located at −44. While this is the first natural site found to be located at a position other than −64, there is evidence that NAC should be able to function normally from a site located at −44. Pomposiello and Bender (35) showed that a site centered at −42 activates transcription of an artificial promoter construct, although less than the same site centered at −64. The fact that we see only a twofold decrease in the dehydrogenase levels in a nac mutant is consistent with the idea that NAC activation is weaker from a site at −44 than from a site at −64. However, induction of nac by artificial means increased the dehydrogenase levels as much as fivefold.
The dehydrogenase activity is slightly repressed by the presence of glucose in the growth medium. The derepression of the dehydrogenase in the absence of glucose is presumably due to CAP-cAMP, as a binding site was identified in the promoter region of the operon and a crp mutant of K. aerogenes failed to derepress the dehydrogenase activity in the absence of glucose. Catabolite repression and the roles of cAMP and CAP in K. aerogenes are basically identical to the system as it is defined in E. coli (34). Two CAP-cAMP binding sites were identified in the E. coli promoter, and it is possible that only one of these binding sites, the upstream site centered at −60 with respect to the start of transcription, is active in K. aerogenes. The other CAP binding site in E. coli dadAX overlaps the −10 region of the promoter, and a role for CAP in activation from this position is unclear. The corresponding site from K. aerogenes diverges significantly and has only 6 of 14 nucleotides from the CAP-binding consensus sequence. We predict that this site would not be active in K. aerogenes.
Lrp has been proposed to be a repressor of the dadAX operon of E. coli (29). We have shown that purified Lrp from E. coli binds in the promoter region of the K. aerogenes dadAB but provide no evidence for the protein’s role in the regulation of the operon. It had been shown previously that the presence of leucine or alanine in the binding reaction abolished the binding of Lrp to the dad promoter of E. coli, but our results were different: while leucine and alanine apparently reduced the affinity of Lrp for the K. aerogenes dad promoter, they did not abolish it. Whether the observed dissimilarity is due to a difference between the two organisms, the reaction conditions of the assay, or the protein used (purified wild-type or histidine-tagged protein) is unclear.
In conclusion, it is clear that the catabolism of alanine is important in developing a metabolic balance in the cell. The inability to catabolize the amino acid can affect growth by interfering with several regulatory systems of the cell, most notably Ntr. Thus, even an important metabolite such as alanine, necessary for protein and cell wall synthesis as well as in metabolism, must be maintained at a balanced level; otherwise, it can become toxic.
ACKNOWLEDGMENTS
This work was supported by Public Health Service grant GM 47156 from the National Institutes of Health to R.A.B.
We are grateful to R. Matthews for helpful discussions and for supplying purified E. coli Lrp.
REFERENCES
- 1.Ausubel F M, Brent R, Kingston R E, Moore D D, Seidman J G, Smith J A, Struhl K, editors. Current protocols in molecular biology. New York, N.Y: Greene Publishing Associates and Wiley-Interscience; 1991. [Google Scholar]
- 2.Ball C A, Osuna R, Ferguson K C, Johnson R C. Dramatic changes in Fis levels upon nutrient upshift in Escherichia coli. J Bacteriol. 1992;174:8043–8056. doi: 10.1128/jb.174.24.8043-8056.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bender R A. The role of the NAC protein in nitrogen regulation of Klebsiella aerogenes. Mol Microbiol. 1991;5:2575–2580. doi: 10.1111/j.1365-2958.1991.tb01965.x. [DOI] [PubMed] [Google Scholar]
- 4.Bender R A, Macaluso A, Magasanik B. Glutamate dehydrogenase: genetic mapping and isolation of regulatory mutants of Klebsiella aerogenes. J Bacteriol. 1976;127:141–148. doi: 10.1128/jb.127.1.141-148.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bender R A, Friedrich B. Regulation of assimilatory nitrate reductase formation in Klebsiella aerogenes W70. J Bacteriol. 1990;172:7256–7259. doi: 10.1128/jb.172.12.7256-7259.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bender R A, Janssen K A, Resnick A D, Blumenberg M B, Foor F, Magasanik B. Biochemical parameters of glutamine synthetase from Klebsiella aerogenes. J Bacteriol. 1977;129:1001–1009. doi: 10.1128/jb.129.2.1001-1009.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bender R A, Snyder P M, Bueno R, Quinto M, Magasanik B. Nitrogen regulation system of Klebsiella aerogenes: the nac gene. J Bacteriol. 1983;156:444–446. doi: 10.1128/jb.156.1.444-446.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Berg D E, Egner C, Hirschel B J, Howard J, Johnsrud L, Jorgensen R A, Tlsty T D. Insertion, excision, and inversion of Tn5. Cold Spring Harbor Symp Quant Biol. 1980;45:115–123. doi: 10.1101/sqb.1981.045.01.020. [DOI] [PubMed] [Google Scholar]
- 9.Berlyn M K B, Low K B, Rudd K E, Singer M. Linkage map of Escherichia coli K-12, edition 9. In: Neidhardt F C, Curtiss III R, Ingraham J L, Lin E C C, Low K B, Magasanik B, Reznikoff W S, Riley M, Schaechter M, Umbarger H E, editors. Escherichia coli and Salmonella: cellular and molecular biology. Washington, D.C: American Society for Microbiology; 1996. pp. 1715–1902. [Google Scholar]
- 10.Brenchley J E. Effect of methionine sulfoximine and methionine sulfane on glutamate synthesis in Klebsiella aerogenes. J Bacteriol. 1973;114:666–673. doi: 10.1128/jb.114.2.666-673.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brenchley J E, Magasanik B. Mutants of Klebsiella aerogenes lacking glutamate dehydrogenase. J Bacteriol. 1974;117:544–550. doi: 10.1128/jb.117.2.544-550.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Calvo J M, Matthews R G. The leucine-responsive regulatory protein, a global regulator of metabolism in Escherichia coli. Microbiol Rev. 1994;58:466–490. doi: 10.1128/mr.58.3.466-490.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chow W, Berg D E. Tn5tac1, a derivative of transposon Tn5 that generates conditional mutations. Proc Natl Acad Sci USA. 1988;85:6468–6472. doi: 10.1073/pnas.85.17.6468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Churchward G, Belin D, Nagamine Y. A pSC101-derived plasmid which shows no sequence homology to other commonly used cloning vectors. Gene. 1984;31:165–171. doi: 10.1016/0378-1119(84)90207-5. [DOI] [PubMed] [Google Scholar]
- 15.DeBoer H A, Comstock L J, Vasser M. The tac promoter: a functional hybrid derived from the trp and lac promoters. Proc Natl Acad Sci USA. 1983;80:21–25. doi: 10.1073/pnas.80.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Eisenberg M A, Star C. Synthesis of 7-oxo-8-aminopelargonic acid, a biotin vitamer, in cell-free extracts of Escherichia coli biotin auxotrophs. J Bacteriol. 1968;96:1291–1297. doi: 10.1128/jb.96.4.1291-1297.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ernsting B R, Denninger J W, Blumenthal R M, Matthews R G. Regulation of the gltBDF operon of Escherichia coli: how is a leucine-insensitive operon regulated by the leucine-responsive regulatory protein? J Bacteriol. 1993;175:7160–7169. doi: 10.1128/jb.175.22.7160-7169.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Goldberg R B, Bender R A, Streicher S L. Direct selection for P1-sensitive mutants of enteric bacteria. J Bacteriol. 1974;118:810–814. doi: 10.1128/jb.118.3.810-814.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Goss T J, Bender R A. The nitrogen assimilation control protein, NAC, is a DNA binding transcription activator in Klebsiella aerogenes. J Bacteriol. 1995;177:3546–3555. doi: 10.1128/jb.177.12.3546-3555.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Groisman E A, Casadaban M J. Mini-Mu bacteriophage with plasmid replicons for in vivo cloning and lac gene fusing. J Bacteriol. 1986;168:357–364. doi: 10.1128/jb.168.1.357-364.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hecht K, Zhang S, Klopotowski T, Ames G F. d-Histidine utilization in Salmonella typhimurium is controlled by the leucine-responsive regulatory protein (Lrp) J Bacteriol. 1996;178:327–331. doi: 10.1128/jb.178.2.327-331.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lin J T, Goldman B S, Stewart V. Structures of genes nasA and nasB, encoding assimilatory nitrate and nitrite reductases in Klebsiella pneumoniae M5a1. J Bacteriol. 1993;175:2370–2378. doi: 10.1128/jb.175.8.2370-2378.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lobocka M, Hennig J, Wild J, Klopotowski T. Organization and expression of the Escherichia coli K-12 dad operon encoding the smaller subunit of d-amino acid dehydrogenase and the catabolic alanine racemase. J Bacteriol. 1994;176:1500–1510. doi: 10.1128/jb.176.5.1500-1510.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lowry O H, Rosebrough N J, Farr A L, Randall R J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 25.Macaluso A, Best E A, Bender R A. Role of the nac gene product in the nitrogen regulation of some NTR-regulated operons of Klebsiella aerogenes. J Bacteriol. 1990;172:7249–7255. doi: 10.1128/jb.172.12.7249-7255.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Magasanik B. Regulation of nitrogen utilization. In: Neidhardt F C, Curtiss III R, Ingraham J L, Lin E C C, Low K B, Magasanik B, Reznikoff W S, Riley M, Schaechter M, Umbarger H E, editors. Escherichia coli and Salmonella: cellular and molecular biology. Washington, D.C: American Society for Microbiology; 1996. pp. 1344–1356. [Google Scholar]
- 27.Maniatis T, Fritsch E F, Sambrook J. Molecular cloning: a laboratory manual. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1982. [Google Scholar]
- 28.Martin C. The clp (CS31A) operon is negatively controlled by Lrp, ClpB, and l-alanine at the transcriptional level. Mol Microbiol. 1996;21:281–292. doi: 10.1046/j.1365-2958.1996.00651.x. [DOI] [PubMed] [Google Scholar]
- 29.Mathew E, Zhi J, Freundlich M. Lrp is a direct repressor of the dad operon in Escherichia coli. J Bacteriol. 1996;178:7234–7240. doi: 10.1128/jb.178.24.7234-7240.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McFall, E., and E. B. Newman. Amino acids as carbon sources, p. 358–379. In F. C. Neidhardt, R. Curtiss III, J. L. Ingraham, E. C. C. Lin, K. B. Low, B. Magasanik, W. S. Reznikoff, M. Riley, M. Schaechter, and H. E. Umbarger (ed.), Escherichia coli and Salmonella: cellular and molecular biology. American Society for Microbiology, Washington, D.C.
- 31.Merrick M J, Edwards R A. Nitrogen control in bacteria. Microbiol Rev. 1995;59:604–622. doi: 10.1128/mr.59.4.604-622.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Muse, W. B., III, and R. A. Bender. The nac (nitrogen assimilation control) gene from Escherichia coli. Submitted for publication. [DOI] [PMC free article] [PubMed]
- 33.Neidhardt F C, Umbarger H E. Chemical composition of Escherichia coli. In: Neidhardt F C, Curtiss III R, Ingraham J L, Lin E C C, Low K B, Magasanik B, Reznikoff W S, Riley M, Schaechter M, Umbarger H E, editors. Escherichia coli and Salmonella: cellular and molecular biology. Washington, D.C: American Society for Microbiology; 1996. pp. 13–16. [Google Scholar]
- 34.Osuna R, Bender R A. The catabolite activator protein in Klebsiella aerogenes and the gene encoding it. J Bacteriol. 1991;173:6626–6631. doi: 10.1128/jb.173.20.6626-6631.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pomposiello P J, Bender R A. Activation of the Escherichia coli lacZ promoter by the Klebsiella aerogenes nitrogen assimilation control protein (NAC), a LysR family transcription factor. J Bacteriol. 1995;177:4820–4824. doi: 10.1128/jb.177.16.4820-4824.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Prival M J, Magasanik B. Resistance to catabolite repression of histidase and proline oxidase during nitrogen-limited growth of Klebsiella aerogenes. J Biol Chem. 1971;246:6288–6296. [PubMed] [Google Scholar]
- 37.Quay S C, Dick T E, Oxender D L. Role of transport systems in amino acid metabolism: leucine toxicity and the branched-chain amino acid transport systems. J Bacteriol. 1977;129:1257–1264. doi: 10.1128/jb.129.3.1257-1265.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Reitzer L J. Ammonia assimilation and the biosynthesis of glutamine, glutamate, aspartate, asparagine, l-alanine, and d-alanine. In: Neidhardt F C, Curtiss III R, Ingraham J L, Lin E C C, Low K B, Magasanik B, Reznikoff W S, Riley M, Schaechter M, Umbarger H E, editors. Escherichia coli and Salmonella: cellular and molecular biology. Washington, D.C: American Society for Microbiology; 1996. pp. 391–407. [Google Scholar]
- 39.Sanderson K E, Hessel A, Liu S, Rudd K E. The genetic map of Salmonella typhimurium, edition VIII. In: Neidhardt F C, Curtiss III R, Ingraham J L, Lin E C C, Low K B, Magasanik B, Reznikoff W S, Riley M, Schaechter M, Umbarger H E, editors. Escherichia coli and Salmonella: cellular and molecular biology. Washington, D.C: American Society for Microbiology; 1996. pp. 1903–1999. [Google Scholar]
- 40.Schwacha A, Bender R A. The product of the Klebsiella aerogenes nac (nitrogen assimilation control) gene is sufficient for activation of the hut operons and repression of the gdh operon. J Bacteriol. 1993;175:2116–2124. doi: 10.1128/jb.175.7.2116-2124.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Streicher S L, Bender R A, Magasanik B. Genetic control of glutamine synthetase in Klebsiella aerogenes. J Bacteriol. 1975;121:320–331. doi: 10.1128/jb.121.1.320-331.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tempest D W, Meers J L, Brown C M. Influence of environment on the content and composition of microbial free amino acid pools. J Gen Microbiol. 1970;64:171–185. doi: 10.1099/00221287-64-2-171. [DOI] [PubMed] [Google Scholar]
- 43.Van Heijenoort J. Murein synthesis. In: Neidhardt F C, Curtiss III R, Ingraham J L, Lin E C C, Low K B, Magasanik B, Reznikoff W S, Riley M, Schaechter M, Umbarger H E, editors. Escherichia coli and Salmonella: cellular and molecular biology. Washington, D.C: American Society for Microbiology; 1996. pp. 1025–1034. [Google Scholar]
- 44.Wasserman S A, Walsh C T, Botstein D. Two alanine racemase genes in Salmonella typhimurium that differ in structure and function. J Bacteriol. 1983;153:1439–1450. doi: 10.1128/jb.153.3.1439-1450.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wasserman S A, Daub E, Grisafi P, Botstein D, Walsh C T. Catabolic alanine racemase from Salmonella typhimurium: DNA sequence, enzyme purification and characterization. Biochemistry. 1984;23:5182–5187. doi: 10.1021/bi00317a015. [DOI] [PubMed] [Google Scholar]
- 46.Whalen W A, Berg C M. Analysis of an avtA::Mu d1(Ap lac): metabolic role of transaminase C. J Bacteriol. 1982;150:739–746. doi: 10.1128/jb.150.2.739-746.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wild J, Klopotowski T. d-Amino acid dehydrogenase of Escherichia coli K12: positive selection of mutants defective in enzyme activity and localization of the structural gene. Mol Gen Genet. 1981;181:373–378. doi: 10.1007/BF00425614. [DOI] [PubMed] [Google Scholar]
- 48.Wild J, Hennig J, Lobocka M, Walczak W, Klopotowski T. Identification of the dadX gene coding for the predominant isozyme of alanine racemase in Escherichia coli K-12. Mol Gen Genet. 1985;198:215–322. doi: 10.1007/BF00383013. [DOI] [PubMed] [Google Scholar]
- 49.Wild J, Walczak W, Krajewska-grynkiewicz K, Klopotowski T. d-amino acid dehydrogenase: the enzyme of the first step of d-histidine and d-methionine racemization in Salmonella typhimurium. Mol Gen Genet. 1974;128:131–146. doi: 10.1007/BF02654486. [DOI] [PubMed] [Google Scholar]
- 50.Woolfolk C A, Stadtman E R. Regulation of glutamine synthetase III: cumulative feedback inhibition of glutamine synthetase from Escherichia coli. Arch Biochem Biophys. 1967;118:736–755. doi: 10.1016/0003-9861(67)90412-2. [DOI] [PubMed] [Google Scholar]
- 51.Xiong X, De la Cruz N, Reznikoff W S. Downstream deletion analysis of the lac promoter. J Bacteriol. 1991;173:4570–4577. doi: 10.1128/jb.173.15.4570-4577.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]