Abstract
SARS-CoV-2 infection causes injuries of not only the lungs but also the heart and endothelial cells in vasculature of multiple organs, and induces systemic inflammation and immune over-reactions, which makes COVID-19 a disease phenome that simultaneously affects multiple systems. Cardiovascular diseases (CVD) are intrinsic risk and causative factors for severe COVID-19 comorbidities and death. The wide-spread infection and reinfection of SARS-CoV-2 variants and the long-COVID may become a new common threat to human health and propose unprecedented impact on the risk factors, pathophysiology, and pharmacology of many diseases including CVD for a long time. COVID-19 has highlighted the urgent demand for precision medicine which needs new knowledge network to innovate disease taxonomy for more precise diagnosis, therapy, and prevention of disease. A deeper understanding of CVD in the setting of COVID-19 phenome requires a paradigm shift from the current phenotypic study that focuses on the virus or individual symptoms to phenomics of COVID-19 that addresses the inter-connectedness of clinical phenotypes, i.e., clinical phenome. Here, we summarize the CVD manifestations in the full clinical spectrum of COVID-19, and the phenome-wide association study of CVD interrelated to COVID-19. We discuss the underlying biology for CVD in the COVID-19 phenome and the concept of precision medicine with new phenomic taxonomy that addresses the overall pathophysiological responses of the body to the SARS-CoV-2 infection. We also briefly discuss the unique taxonomy of disease as Zheng-hou patterns in traditional Chinese medicine, and their potential implications in precision medicine of CVD in the post-COVID-19 era.
Keywords: COVID-19, cardiovascular disease, PheWAS, precision medicine, Traditional Chinese Medicine
Infection of severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) via the respiratory route was originally found to cause an acute infectious disease characterized by a diffused pulmonary inflammation and hypoxia similar to the SARS-CoV-caused severe acute respiratory syndrome (SARS), and thus, was initially defined as novel coronavirus pneumonia (NCP) [1, 2]. The World Health Organization (WHO) later named it coronavirus disease 2019 (COVID-19) [3], which quickly progressed as a global pandemic and has caused 766,440,796 confirmed cases and 6,932,591 deaths throughout the world as of May 25, 2023 (https://www.who.int/emergencies/diseases/novel-coronavirus-2019). It is now known that clinical spectrum of SARS-CoV-2 infection ranges across from asymptomatic, mild illness, moderate illness, or severe illness to critical illness [4]. The clinical manifestations of COVID-19 are also substantially heterogeneous with fever, cough, sore throat, malaises, acute respiratory distress syndrome (ARDS) and/or multi-organ failure [1–5]. Patients with preexisting cardiovascular disease (CVD) such as hypertension, arrhythmias, heart failure, etc. were at higher risk of infection and associated with quicker progression to severe COVID-19 illness and death [3, 5]. It has been also found that newly emerging cardiac injuries by SARS-COV-2 infection [6, 7] and various cardiovascular comorbidities and complications including myocarditis, myocardial infarction, heart failure, atrial and ventricular arrhythmias, venous thromboembolism, stroke, and microvascular disease, occurred in many acute COVID-19 patients with no preexisting CVD [8–11]. The exact effects and mechanisms of SARS-CoV-2 infection on the cardiovascular system, however, are still not very clear. Several potential mechanisms may coexist in some patients. Direct SARS-CoV-2 viral infection of cardiac myocytes and endothelial cells across vascular beds of different organs, acute cardiac stress due to respiratory failure and hypoxemia, hypercoagulability, and systemic inflammation and immune over-reactions may all be the underlying causes for the complex spectrum of clinical phenotypes [3, 12–21]. More accumulating evidence supports that the SARS-CoV-2 infection caused a multisystem disease involving injuries of many systems and organs [3, 12–21], and thus, COVID-19 should be considered not a simple phenotypic but a phenomic disease (Fig. 1) [19, 22–26]. Therefore, a deeper understanding of the risk factors and the short- and long-term cardiovascular effects of SARS-CoV-2 infection [21, 27] at a phenomic level [23–25, 28] is required for more precise diagnosis and effective treatment of CVD in the context of wide-spread infection and reinfection of SARS-CoV-2 variants (Table 1) [29, 30], the long-COVID [31], and COVID-19 vaccination [32].
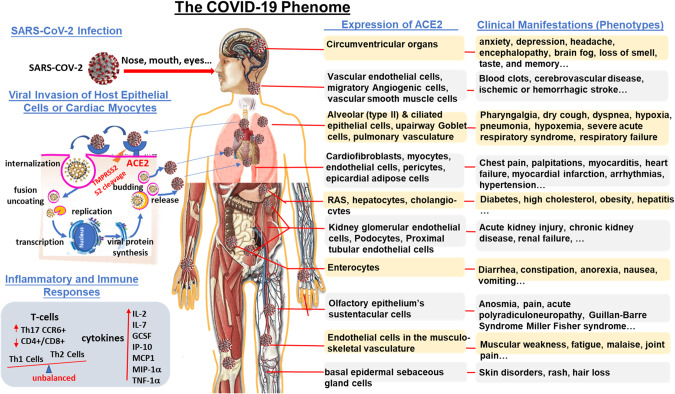
Fig. 1. The COVID-19 phenome.
The etiologic virus, severe acute respiratory syndrome-coronavirus 2 (SARS-CoV-2), infects human through the upper respiratory tract and eyes. Cell entry of SARS-CoV-2 depends on binding of the viral spike glycoprotein receptor on the surface of the virion to a transmembrane protein angiotensin converting enzyme 2 (ACE2) expressed on various host tissues throughout the body and on priming of spike protein by host cell transmembrane protease serine 2 (TMPRSS2) trim the spike glycoprotein and facilitate its binding to ACE2 and cell entry of the SARS-CoV-2 virion [75–80]. ACE2 is abundantly present in humans, not only in the epithelia of oral and nasal mucosa, nasopharynx, and lung, which provides routes of invasion for the SARS-CoV-2, but also in brain, heart, stomach, small intestine, colon, bone marrow, spleen, liver, kidney, skin, thymus and lymph nodes and in arterial and venous endothelial cells and arterial smooth muscle cells in all organs [82]. Therefore, ACE2 is the key to SARS-CoV-2 infection and provides the molecular basis for the pathogenesis of the main clinical manifestations and the crucial mechanisms of the COVID-19 phenome that affects multiple systems.
Table 1.
| WHO label | Variant name | Country first detected/date | Spike mutations | Attributes |
|---|---|---|---|---|
| Alpha | B.1.1.7 | United Kingdom/September 2020 | N501Y, D614G, P681H, 69del, 70del, 144del, E484K, S494P, A570D | ↑ transmissibility (~50%), ↑ Severity, ↑ Case fatality. Drastically reduced circulation in the EU/EEA following the emergence of Delta; No impact on susceptibility to EUA monoclonal antibody treatments; Minimal impact on neutralization by convalescent and post-vaccination sera |
| Beta |
B.1.351 B.1.351.2 B.1.351.3 |
South Africa/December 2020 | D80A, D215G, 241del, 242del, 243del, K417N, K1191N, E484K, | ↑ Transmissibility (~50%), ↓ Susceptibility to EUA monoclonal antibody treatments ↓ Neutralization to convalescent & post-vaccination sera |
| Gamma |
P.1 P.1.1 P.1.2 |
Brazil/January 2021 | L18F, T20N, P26S, D138Y, R190S, K417T, E484K, N501Y, D614G |
↓ Susceptibility to bamlanivimab/etesevimab monoclonal antibody treatments ↓ Neutralization to convalescent & post-vaccination sera |
| Delta |
B.1.617.2 AY.1 AY.2 |
India/ May 2021 | T19R, V70F, T95I, G142D, E156-, F157-, R158G, A222V, W258L, K417N, L452R, T478K, D614G, P681R, D950N |
↑ Transmissibility ↓ Susceptibility to EUA monoclonal antibody treatments ↓ Neutralization to post-vaccination sera |
| Omicron | B.1.1.529 | South Africa/November, 2021 | A67V, del69-70, T95I, del142-144, Y145D, del211, ins214EPE, T547K, D614G, H655Y, N679K, L212I, P681H, N764K, D796Y, N856K, Q954H, N969K, L981F |
↑ Transmissibility ↑ Risk of re-infection Deletion in the S gene, leading to S gene target failure (SGTF) in some PCR assays. SGTF can be used as a proxy marker to screen for Omicron. |
Herein, we summarize the manifestations of CVD in the full clinical spectrum of COVID-19, and the phenome-wide association study (PheWAS) of CVD interrelated to COVID-19. We discuss the underlying biology for CVD in the COVID-19 phenome and the concept of precision medicine with new disease taxonomy that highlights the inter-connectedness of clinical phenotypes (clinical phenome) and addresses the overall pathophysiological responses of the body to virus infections. We then provide a perspective on a unique phenomic taxonomy of disease based on the theory and clinical practice of the “treatment by differentiation of Zheng-Hou patterns (TDZH)” principles for personalized diagnosis, treatment, and prevention of disease, and discuss their potential implications in precision medicine of CVD in the post-COVID-19 era.
CVD as dominant risk factors for severe COVID-19 and death
When COVID-19 struck, preexisting CVD is a major risk factor for a severe clinical course of COVID-19 and is closely associated with adverse outcomes and death [33, 34]. According to the earliest reports on COVID-19, patients with preexisting CVD were at higher risk of SARS-CoV-2 infection and complications or mortality—up to 40% of hospitalized patients had cardiovascular or cerebrovascular disease. In a published clinical cohort study of 138 COVID-19 patients, acute cardiac injury, shock, and arrhythmia were observed in 7.2%, 8.7%, and 16.7% of patients, respectively, with poorer outcomes and higher prevalence of transfer to the intensive care unit (ICU) [33]. Furthermore, 44.4% of these patients in the ICU had cardiac arrhythmias, including malignant ventricular arrhythmias [33]. Cardiac injury is associated with severe outcome and death in patients with COVID-19. Death rates of 10.5%, 7.3%, and 6.0% for patients with cardiac disease, diabetes, and hypertension, respectively, have been reported [34]. Up to 36% of patients with SARS-CoV-2 infection were affected by severe myocardial injury with a higher prevalence than those stable cases without cardiac injury, commensurate with the elevation of cardiac troponin (cTn) [35–39]. Pathologically, interstitial mononuclear inflammatory infiltrates were observed in the heart of COVID-19 patients [40]. Therefore, in the face of COVID-19, SARS-CoV-2 infection may cause further exacerbation of CVD through host inflammatory or immune responses-induced cardiovascular injury. The blood concentrations of interleukin (IL)-2, IL-7, granulocyte-colony stimulating factor, interferon-γ inducible protein 10 (IP-10), monocyte chemoattractant protein 1, macrophage inflammatory protein 1-α, and tumor necrosis factor-α were all increased in COVID-19 patients [33, 41, 42]. Higher CCR6 Th17 cells and lower CD4/CD8 T cells, unbalanced reactions of Th1 and Th2 cells were also observed in COVID-19 patients (Fig. +++1, 2) [33, 41, 42]. Increased inflammation reactions and cytokine storm would lead to tissue swelling, ischemia-reperfusion injury, and heart failure of COVID-19 patients.
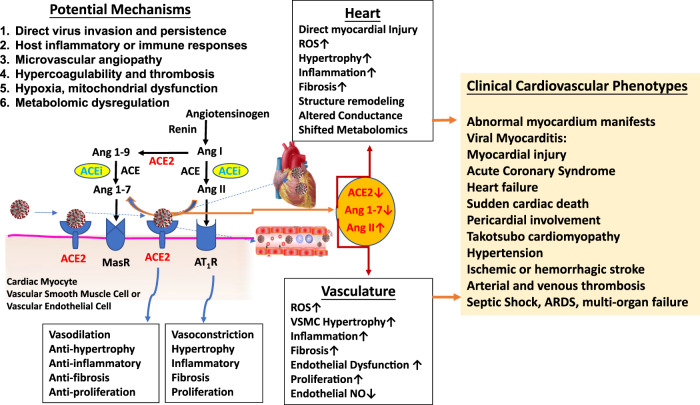
Fig. 2. Cardiovascular manifestations and potential underlying mechanisms in the COVID-19 phenome.
ACE2 is highly expressed in cardiac myocytes and vascular endothelial cells of many organs [82–84] and is a dominant mechanism for negative regulation of the renin-angiotensin system (RAS) by metabolizing angiotensin II (Ang II) into the beneficial peptide hormone angiotensin 1–7 (Ang 1–7) that controls vasoconstriction and blood pressure [80, 83, 84]. Direct binding of SARS-CoV-2 to ACE2 receptor and indirect proteolytic processing and shedding down regulate ACE2 and activate the RAS axis which in turn causes an increase of Ang II and vascular endothelial injury and higher blood pressure, and partly drives the systemic and heterogenic manifestations of COVID-19 phenome [85, 86]. The involvement of the circulatory system as the interrelated clinical manifestations of CVD in the COVID-19 phenome may include at least six potential mechanisms: 1) direct injury by virus invasion and persistence, 2) systematic host immune responses and inflammation (cytokine storm), 3) microvascular endothelial damage and angiopathy, 4) hypercoagulability and thrombosis, 5) hypoxia, and mitochondrial dysfunction, and 6) metabolomic dysregulation.
In addition, severe hypoxia from ARDS caused by acute lung injury due to the SARS-CoV-2 infection may impair myocardial oxygen demand-supply relationship and result in mitochondrial dysfunction and myocardial injury from increased oxygen demand. Except for altered myocardial demand-supply ratio, increased coronary blood flow due to systemic inflammation and increased shear stress may lead to plaque rupture and result in in acute myocardial infarction [43]. Prothrombotic milieu with exaggerated systemic inflammation may further aggravate the risk. Cerebrovascular disease and ischemic stroke associated with COVID-19 were reviewed and it seems that the SARS-Cov-2 infection induced higher ischemic stroke than influenza-A infection [44, 45]. In addition to ARDS, patients with severe COVID-19 can often develop cytokine storm, resulting in arteriovenous thrombosis, and acute multi-organ injuries including heart and kidney [46]. Preexisting CVD in the setting of COVID-19 may contribute to early clinical adverse outcomes and SARS-CoV-2 infection may have longer implications for overall cardiovascular health [34]. Therefore, prolonged clinical follow-up should be given to severe COVID-19 cases with preexisting CVD after recovery from acute COVID-19.
It should be pointed out that the majority of current findings to date on the risk factors, however, are from hospital-based studies that may not represent the broader at-risk population [47, 48]. And evidence on these risk factors is fragmented with studies focusing only on single clinical outcomes rather than covering the whole clinical spectrum and the progression of COVID-19 from initial diagnosis with positive PCR test through hospitalization, ICU admission, and recovery or death. To differentiate the risk factors associated with asymptomatic or mild cases from other risk factors associated with hospitalization and death we need to examine all the risk factors across all severity levels of COVID-19. To improve predictive ability, other than the traditional epidemiology, many novel approaches including big data, biomarker panels, genome-wide association study (GWAS), phenomics and PheWAS can be used [19, 49–54].
CVD as consequences of COVID-19
Cardiac abnormalities, ranging from infra-clinic elevations of myocardial necrosis biomarkers to acute myocarditis and cardiac dysfunction, have been reported during the acute COVID-19 phase [4, 35, 55]. The most common complications include arrhythmia (atrial fibrillation, ventricular tachyarrhythmia, and ventricular fibrillation), cardiac injury, fulminant myocarditis, heart failure, pulmonary embolism, and disseminated intravascular coagulation. Due to the effects of the virus infection on the respiratory and cardiovascular systems, the systemic inflammation and immune responses, the proarrhythmic pharmacotherapies of COVID-19 and other drug interactions, and the associated autonomic imbalance that enhances arrhythmogenicity, more patients with arrhythmias including the lethal long Q-T have been reported [3, 9, 56]. COVID-19 patients with cardiac injury were with higher prevalence amongst patients requiring ICU admission. The biomarker levels of myocardial injury (cTn and N-terminal pro-B-type natriuretic peptide), coagulation (D-dimer), and kidney function (serum creatinine) were significantly higher in patients requiring ICU admission than in those not treated in the ICU [3, 46, 55, 57, 58]. Myocardial injury, as assessed by serum analysis of lactate dehydrogenase (LDH), cTnI, creatine kinase-myocardial band (CK-MB), and myoglobin, was associated with severe illness and death of COVID-19 [35]. The prevalence of thrombo-inflammatory syndrome and subsequent cardiovascular dysfunction prompted the implementation of antithrombotic therapy and strategies targeting major pro-inflammatory cytokines involved in COVID-19 cytokine storm. Mechanistically, SARS-CoV-2, following proteolytic cleavage of its spike protein by a serine protease, binds to the transmembrane ACE2 to enter macrophages, perivascular pericytes, endothelial cells, and cardiomyocytes. This may lead to endothelial dysfunction, microvascular dysfunction, and causes thrombosis or instability of atherosclerotic plaques, thus leads to the occurrence of acute coronary syndrome (ACS), and myocardial dysfunction and damage, including myocardial infarction. The mechanisms by which the SARS-Cov-2 damages cardiomyocytes also include that the virus damages endothelial cells and causes thrombosis or damages the stability of atherosclerotic plaques, thus leading to the occurrence of ACS (Fig. 2) [43]. Initial immune and inflammatory responses induce a severe cytokine storm [interleukin (IL)-6, IL-7, IL-22, IL-17, etc.] during the rapid progression phase of COVID-19. Therefore, it was suggested that early evaluation and continued monitoring of cTnI, NT-proBNP, LDH, myoglobin, and D-dimer after hospitalization may predict and identify COVID-19 patients with severe complications such as cardiac injury [43].
COVID-19-associated myocarditis differs from COVID-19 respiratory failure by an early shock state [59]. Two distinct etiologies of primary acute heart failure exist in surprisingly equal incidence in patients with COVID-19: viral myocarditis and Takotsubo cardiomyopathy [60]. COVID myocarditis, Takotsubo cardiomyopathy (or broken-heart syndrome) [61–63], and severe COVID-19 can be clinically indistinguishable. All can present with dyspnea and evidence of cardiac injury, although in myocarditis and Takotsubo cardiomyopathy, this is due to primary cardiac dysfunction as compared to respiratory failure in severe COVID-19. Evidence of cardiac injury and heart failure in the form of ECG changes (atrial fibrillation, long Q-T, ventricular tachyarrhythmia, and ventricular fibrillation) or elevated serum high-sensitivity troponin and cardiac MRI findings in patients with COVID-19 should prompt consideration of concurrent myocarditis. However, not all heart failure from COVID-19 is from direct viral infection; some patients develop Takotsubo cardiomyopathy [63, 64]. Severe myocarditis associated with COVID-19 should be distinguished from septic cardiomyopathy following SARS-CoV-2 infection, which is associated with severe infection and induces Takotsubo cardiomyopathy. It has increasingly been recognized in clinical practice, accounting for up to 2% of ACS presentations. In fact, the clinical presentation can be indistinguishable from a myocardial infarction. It should first be distinguished from ACS, because myocarditis and Takotsubo cardiomyopathy have been included in the category of myocardial infarction in the absence of obstructive coronary artery disease (MINOCA) [65].
The development of new guidelines for effective treatment strategies requires concerted efforts to refine our understanding of the mechanisms underlying CVD and large-scale clinical trials to reduce the burden of COVID-19 hospitalization and mortality [66].
Cardiovascular complications after recovery from COVID-19
While most people with COVID-19 get better within a few days to a few weeks after infection, some people who have been infected or reinfected with the SARS-CoV-2 can experience long-term effects from their infection with signs, symptoms, and conditions that continue or develop after acute COVID-19 infection, known as “long-COVID” [31]. Each time a person is infected or reinfected with SARS-CoV-2, they have a risk of developing long-COVID. Although COVID-19 has been spreading rapidly as a global pandemic for over 3 years, the long-term outcomes for the patients who recovered from this disease are still unclear. A few studies have investigated cardiovascular outcomes in the post-acute phase of the COVID-19; [9, 67, 68] however, most were limited to hospitalized individuals who represent the minority of patients with COVID-19, and all had a short duration of monitoring and follow-up for cardiovascular outcomes [27, 69]. Fulminant myocarditis was observed in a convalescent patient one week after respiratory symptoms were resolved [70]. Sudden cardiac death (SCD) was found in many non-hospitalized patients with mild symptoms who were found dead at their home during self-quarantine in the epicenter of COVID-19 in Italy [70]. The insidious cardiovascular injury might be a potential trigger of SCD. Acute COVID-19 infection is characterized by mononuclear cell reactivity and panendothelialitis, contributing to a high incidence of thrombosis in large and small blood vessels, both arterial and venous. The concern is that background inflammation and thrombosis may persist and evolve silently, and manifest later in an insidious manner. There may be chronic sequelae even after an apparent “complete recovery” and hospital discharge. Myocardial injury might result in ventricular fibrosis which may lead to cardiac arrhythmias and SCD. Thus, cardiovascular events may exist for a long period of time after resolution of the acute COVID-19. These cardiovascular risk factors are particularly important because they may lead to further cardiovascular injury and death.
Xie et al. used national healthcare databases from the US Department of Veterans Affairs to build a 153,760 COVID-19 cohort, a 5,637,647 contemporary control cohort, and a 5,859,411 historical control cohort, corresponding to 12,095,836 person-years of follow-up, to estimate risk factors and 1-year burdens of a set of pre-specified incident cardiovascular outcomes [27]. They found that, beyond the first 30-day of infection, patients with COVID-19 were at increased risks and 12-month burdens of incident CVD, including cerebrovascular disorders, dysrhythmias, inflammatory heart disease, ischemic heart disease, heart failure, thromboembolic disease and other cardiac disorders. The risks were evident regardless of age, race, sex and other cardiovascular risk factors, including obesity, hypertension, diabetes, chronic kidney disease and hyperlipidemia; they were also evident in people without any preexisting CVD before exposure to COVID-19, providing evidence that these risks manifested even in people at low risk of CVD and might be intrinsic to SARS-CoV-2 infection. These risks and burdens were evident among individuals who were not hospitalized during the acute phase of the infection and increased in a graded fashion according to the care setting during the acute phase (non-hospitalized, hospitalized, and admitted to ICU). Thus, in survivors of acute COVID-19 the risks and 1-year burden of CVD are substantial [27] and warrant more attentions. Careful follow-up of cured COVID-19 patients with or without preexisting CVD would be critical for better understanding the long-term impact of this illness and protection of these patients from future CVD [71, 72].
Angiotensin converting enzyme 2 (ACE2) in the COVID-19 phenome and CVD
SARS-CoV-2 is a new β-coronavirus belonging to the Orthocoronavirinae subfamily of the Coronaviridae family. With a 29,903 base single-stranded RNA genome SARS-CoV-2 encodes a total of 29 proteins [2]. It is now clear that cell entry of SARS-CoV-2 depends on 1) binding of the viral spike glycoprotein receptor on the surface of the virion to a transmembrane protein ACE2 expressed on various host tissues throughout the body [73], and 2) priming of spike protein by host cell transmembrane protease serine 2 (TMPRSS2) (Fig. 1) [73–76]. In the internalization process of SARS-CoV-2, TMPRSS2, furin, and cathepsins B and L trim the spike glycoprotein and facilitate its binding to ACE2 and cell entry of the SARS-CoV-2 virion [75–80]. At the same time on the cell membrane, S2 cleaves to the cell membrane, and RNA is uncoated and replicated in the cytoplasm. If the virus binds to ACE2 only, the S2 is cleaved in the lysosome by endoannexation [81]. Furthermore, ACE2 is abundantly present in humans in the epithelia of the upper airway and lung, which provides routes of invasion for the SARS-CoV-2. Therefore, ACE2 is the key to SARS-CoV-2 infection. ACE2 is also widely expressed in various human organs throughout the body (brain, oral and nasal mucosa, nasopharynx, lung, heart, stomach, small intestine, colon, bone marrow, spleen, liver, kidney, skin, thymus, and lymph nodes) and in arterial and venous endothelial cells and arterial smooth muscle cells in all organs [80, 82]. This provides the molecular basis for understanding the pathogenesis of the main clinical manifestations and the crucial mechanisms of the COVID-19 phenome that affects multiple systems (Fig. 1).
A very recent study [60] on genetic landscape of the coronavirus receptor ACE2 found that human plasma ACE2 shares a genetic basis with COVID-19, CVD and some other related diseases. Elevated ACE2 levels show a causal relationship with COVID-19 severity, hospitalization, and infection, as shown by a cis-pQTLn (protein quantitative trait loci)-based Mendelian randomization analysis. The genetic correlations between ACE2 levels and both COVID-19 and CVD risk imply that the cardiovascular complications seen in COVID-19 patients may be intrinsic to the disease and mechanistically driven by ACE2 [60].
ACE2 is highly expressed in cardiac myocytes and vascular endothelial cells of many organs [82–84]. ACE2 is a dominant mechanism for negative regulation of the renin-angiotensin system (RAS) by metabolizing angiotensin II (Ang II) into the beneficial peptide hormone angiotensin 1–7 that controls vasoconstriction and blood pressure (Fig. 2) [80, 83, 84]. The activation of the RAS axis by ACE2 downregulation due to direct binding of SARS-CoV-2 to ACE2 and indirect proteolytic processing and shedding causes an increase of Ang II and vascular endothelial injury and higher blood pressure, and partly drives the systemic and heterogenic manifestations of COVID-19 phenome (Fig. 2) [85–87]. It was reported that blood concentration of Ang II in COVID-19 patients is higher than healthy controls, and positively correlated with SARS-CoV-2 virus loads [78, 79]. Clinical evidence also showed that higher SARS-CoV-2 viral loads linked to an increase in myocardial injury-related enzymes such as hs-TnI and CK-MB [88]. Large cohort data found that plasma levels of soluble ACE2 are higher in men than in women [89] and correlated with cardiovascular outcomes and cardiometabolic and inflammatory biomarkers [87, 89, 90]. Since ACE2 level is genetically correlated with both COVID-19 and CVD [60], cardiac and vascular diseases are risk and causative factors for the COVID-19 comorbidities, severe cases and death. Clinically, COVID-19 is not only frequently exacerbated by preexisting CVD, but also directly aggravated by SARS-CoV-2 infection-induced cardiovascular complications (Fig. 2), including viral myocarditis, abnormal myocardium manifests, myocardial injury, ACS, heart failure, SCD, pericardial involvement, Takotsubo cardiomyopathy [9, 63], hypertension, cerebrovascular disease (ischemic or hemorrhagic stroke), blood clots (arterial and venous thrombosis), septic shock (ARDS, multi-organ failure) [9]. The involvement of the cardiovascular system as the interrelated clinical manifestations of CVD in the COVID-19 phenome may include the following mechanisms: 1) direct virus invasion and persistence, 2) systematic host immune responses and inflammation (cytokine storm), 3) microvascular angiopathy, 4) hypercoagulability and thrombosis, 5) hypoxia, and mitochondrial dysfunction, and 6) metabolomic dysregulation (Fig. 2).
PheWAS of CVD in the COVID-19 phenome
The novel virus-caused COVID-19 pandemic has taught us a lesson: COVID-19 is not a phenotypic disease that attacks only one or two organs or systems but a phenomic disease that affects multiple organs and systems of the whole body and thus manifests with an integrated and interconnected multiple clinical phenotypes. With the simultaneous involvement of direct SARS-CoV-2 viral infection of many cell types, including cardiac myocytes and endothelial cells across vascular beds of different organs, it induces hypoxemia, systemic inflammation and immune over-reactions, and acute multi-organ failure (Fig. 1) [3]. COVID-19 has also called the need for identifying clinical and biologic risk factors and long-term sequelae for acute infectious disease. Therefore, a full and deeper understanding of the cardiovascular phenotypes in the setting of COVID-19 at the phenome level requires a paradigm shift from the current phenotypic study that focuses on the virus itself or a few organs (lung, heart, kidney, etc.) infected by the virus to phenomics and PheWAS that highlights the inter-connectedness of clinical phenotypes (such as NCP and CVD) and addresses the overall pathophysiological responses of the body as a whole (Fig. 1) [3, 23, 49, 50, 91].
In the last decade, PheWAS has been used to replicate hundreds of known genotype–phenotype associations and has added context to genetic discoveries [23, 49, 53, 92–94]. It has been validated that PheWAS may differentiate between true pleiotropy and clinical comorbidity [54], help define disease subtypes and repurpose medications [3, 95]. PheWAS has also been proven to be useful with purpose-collected research data and may create a rich resource for more efficient and detailed genome-phenome analysis to usher in new discoveries to advance precision medicine [49, 91–98].
Using an unbiased PheWAS approach to clinical diagnoses in a large data set, Regan et al. identified novel phenotypic associations between risk alleles for severe COVID-19 infection with relevant comorbidities [19]. They found that the phenotypes associated with the rs657152 risk allele (ABO) with greater odds ratio (OR) include heart failure (OR, 1.09; 95% CI, 1.03–1.14; q = 0.046), diabetes (OR, 1.05; 95% CI, 1.02–1.07; q = 0.004) and hypercholesterolemia (OR, 1.04; 95% CI, 1.02–1.06; q = 0.004); and that the phenotypes associated with rs1819040 risk allele (KANSL1) include atrial fibrillation and flutter (OR, 1.07; 95% CI, 1.04–1.10; q = 0.0084) and pulmonary fibrosis (OR, 0.80; 95% CI, 0.71–0.89; q = 0.035) [19]. These results suggest that genetic predisposition for these cardiovascular phenotypes may have pleiotropic effects on CVD and COVID-19 related complications. Individuals carrying these genetic markers, known for their role in host antiviral immune response and inflammation, may have modified risk of CVD, as well as autoimmune and inflammatory disorders, which in turn may amplify the risk of severe COVID-19 and adverse outcomes and may have broader long-term health implications [19]. Therefore, understanding preexisting clinical diagnoses related to COVID-19 outcomes informs the need for targeted screening to support specific vulnerable populations to improve disease prevention and healthcare delivery. A PheWAS of COVID-19 prognosis across the medical phenome in 53,853 COVID-19 patients found that preexisting conditions including heart disease, renal failure, and respiratory failure were strongly associated with hospitalization; and that hematopoietic conditions, mental disorders in non-Hispanic Whites, and circulatory system and genitourinary conditions in non-Hispanic Blacks were associated with ICU admission/mortality [50].
Integrating a GWAS of 7885 COVID-19 hospitalization cases and 961,804 controls from the COVID-19 Host Genetics Initiative with mRNA expression, splicing, and protein levels (n = 18,502), Pathak et al. identified 27 genes in the inflammation and coagulation pathways associated with COVID-19 hospitalization [99]. Using phenome-wide and laboratory-wide association scans in Vanderbilt Biobank (n = 85,460) to functionally characterize the 27 genes they further identified coagulation-related clinical symptoms, immunologic, and blood-cell-related biomarkers. When these findings were replicated across trans-ethnic studies consistent effects were observed in individuals of diverse ancestral backgrounds in Vanderbilt Biobank, pan-UK Biobank, and Biobank Japan. Therefore, putative causal genes do impact COVID-19 severity and symptomology through the host inflammatory response [99].
Song et al. [52]. performed a PheWAS of 1809 phenotypes and COVID-19 disease progression in the Veterans Health Administration Million Veteran Program. They observed an increased risk of COVID-19 and its disease progression with a history of circulatory, endocrine, respiratory, urinary, and dermatologic disease groups. Patients with prevalent congestive heart failure, ischemic heart disease, hypertensive heart and/or renal disease, obesity, fluid/electrolyte/acid-base disorders, disorder of lipid metabolism, type 2 diabetes, respiratory failure/insufficiency/arrest, active bronchitis and bronchiolitis, pneumonia, urinary tract infection, renal failure, chronic ulcer of skin, and superficial cellulitis and abscess were associated with an increased risk across all COVID-19 disease stages. Mental health and sense disease groups overall were associated with a decreased risk of COVID-19 and the subsequent disease stages. When analyses were restricted to symptomatic COVID-19 cases, they found that older age, higher body mass index, males and patients with a history of respiratory, kidney, bacterial or metabolic comorbidities had greater COVID-19 severity [52].
Several observational large cohort studies including either patients with atrial fibrillation or heart failure or healthy children and adults have demonstrated that circulating ACE2 antigen levels are higher in men than women [87, 89, 90], increase with age, and correlate with cardiovascular outcomes in COVID-19 [87, 90]. A genome-wide analysis of soluble ACE2 in plasma of 3442 heart failure patients identified three genome-wide significant protein quantitative trait loci (pQTL): a cispQTL on chromosome X (located near the cognate ACE2 gene) and two trans-loci on chromosomes 12 and 21 encompassing the genes encoding transcription factors HNF1A, and ERG, respectively.
Yang et al. conducted a genome-wide association meta-analysis of plasma ACE2 levels in a cohort of over 28,000 individuals of the SCALLOP Consortium [60]. They summarized the cross sectional epidemiologic correlates of circulating ACE2 and estimated relevant genetic correlations with cardiometabolic phenotypes, COVID-19, and other human complex traits and diseases. They detected that plasma ACE2 was genetically correlated with vascular diseases, severe COVID-19, and a wide range of human complex diseases and medications. An X-chromosome cis-pQTL-based Mendelian randomization analysis suggested a causal effect of elevated ACE2 levels on COVID-19 severity (odds ratio (OR), 1.63; 95% CI, 1.10–2.42; P = 0.01), hospitalization (OR, 1.52; 95% CI, 1.05–2.21; P = 0.03), and infection (OR, 1.60; 95% CI, 1.08 to 2.37; P = 0.02). The causal evidence for ACE2 suggests inhibition of circulating ACE2 may be a promising pharmacological approach for treating COVID-19 and its comorbidities including NCP and CVD. Transcription factors such as HNF1A and HNF4A play essential roles in ACE2 regulation and could provide alternative paths to pharmacological modulation of ACE2 plasma levels [60]. In addition to ARDS, patients with severe COVID-19 can often develop acute myocardial injury and cytokine storm, resulting in arteriovenous thrombosis, and multi-organ injuries including heart and kidney [46].
Millions of patients around the world are on treatment with ACE-Is and ARBs for hypertension, heart failure, coronary artery disease, or kidney disease. The understandings of ACE2 as the key to SARS-CoV-2 infection [43, 73–75] and the clinical poor outcomes of patients with hypertension and other cardiovascular comorbidities among COVID-19 patients [100–102] had promoted concerns that angiotensin-converting enzyme inhibitors (ACEIs) and AT1 angiotensin receptor blockers (ARBs) would increase susceptibility of patients who are using the drugs for the treatment of hypertension to SARS CoV-2 and the likelihood of severe COVID-19 illness [3, 103] Speculation about worse outcomes among patients on ACEIs and ARBs during the COVID-19 pandemic has caused widespread anxiety among patients and their care providers. On the other hand, the harms of indiscriminate withdrawal of these medications on cardiovascular outcomes are well documented [86, 104]; There is also widespread speculation about the potential benefits of ACE-Is and ARBs, based on biological plausibility arguments and animal data and small clinical studies on patients with other viral respiratory infections [86, 104]. Careful targeting of the RAS axis may be needed in these COVID-19 patients to optimize their clinical outcomes, including the use of ACEIs and ARBs.
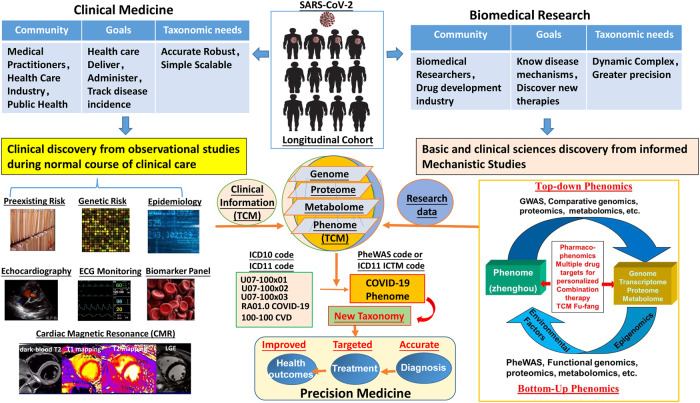
From the PheWAS point of view, therefore, COVID-19 has highlighted the demand to innovate the current taxonomy of human disease based on the emerging concept of precision medicine, which was put forward by an ad hoc committee at the USA National Research Council in 2011 [105]. In medical practice, taxonomy of human disease often refers to the International Classification of Diseases (ICD), a system established more than 100 years ago that WHO uses to track disease incidence, physicians use as a basis for standardized diagnoses, and the health-care industry uses to determine reimbursement for care [106, 107]. In the current ICD, definition and classifications of diseases are based on phenotypic changes in an anatomic system or organ or at even a single molecule [106, 107]. Precision medicine is based on new knowledge network for biomedical research and a new taxonomy of disease for more precise diagnosis, therapy, prevention, and identification of clinical and biologic risk factors and long-term sequelae for new acute infectious disease like COVID-19. Phenomics provides a major opportunity to accelerate an equitable realization of the promise of precision medicine that is based on new knowledge network for biomedical research and a new taxonomy of disease. A paradigm shift to PheWAS of COVID-19 phenome, therefore, is essential for more precise diagnosis and effective therapeutics with reduced mortality of CVD and improved overall outcomes in this time of COVID-19 with world-wide spread of infection and vaccination of SARS-CoV-2 and its variants. The major challenges to make such a paradigm shift include how to 1) define a clinical phenome, 2) integrate multi-omics big data from longitudinal cohorts with detailed interrelated phenotypes in the phenome at the systematic level, 3) establish a new disease knowledge network for creation of a more accurate disease definition and classification system, and 4) ultimately establish new taxonomy of disease and achieve the goal of improving the accuracy of clinical diagnosis and treatment [23, 49, 91].
In this aspect, the traditional Chinese medicine (TCM), which uses a unique phenomic taxonomy of disease for diagnosis and therapeutics based on the theory of TDZH”, may offer alternative theoretical and clinical guidance for more precise diagnosis and effective treatment of the COVID-19 phenome [3, 108–114] that is consistent with the concept of precision medicine [49, 91, 105].
TCM has a well-documented history of nearly 3000 years for battling infectious diseases in the scale of either endemic, epidemic, or pandemic [115–117] and has developed a theoretical system of prevention and treatment of “pestilence” (refers to fatal epidemic disease, termed “Wen” or “Yi”) over the years [115–117]. During the 2002–2003 SARS epidemic in China, for example, mounted convincing evidence has proven the high efficacy of TCM in the prevention and treatment of the SARS-CoV infection, including shorter hospitalization and disease course, marked improvement of symptoms from dyspnea and malaise, reduced side effects caused by conventional therapeutics and steroid treatments, and reduced fatality [118–123]. When the newly developed fatal infectious disease first struck in China in the end of 2019, there was no clear diagnosis and many thought it was a new outbreak of SARS. Although the sequence and structure of the pathogen virus SARS-CoV-2 were then quickly identified [2, 76, 124, 125] and the disease was defined as NCP [1, 2, 126], there were no approved specific anti-viral drugs or vaccine available for NCP but only a few options for symptomatic relief. Since SARS-CoV-2 showed similarities to SARS in many aspects, including the virus genes, etiology, epidemiology, pathological processes, and clinical manifestations, TCM was quickly incorporated into COVID-19 treatment guidelines as a mandatory strategy for medical interventions [127]. As of March 23, 2020, a total of 74,187 diagnosed patients, accounting for 91.5% of the infected population in China, were treated with TCM [128]. A plethora of publications have provided strong evidence that TCM effectively alleviated the symptoms of patients with COVID-19, delayed or stopped the disease’s progression from mild to severe or critical illness, and reduced severe and critical cases and all-cause death, which played an indispensable role in the management of the disease and contributed significantly to controlling the spread of COVID-19 in China in a short time [128–137]. More details about the rationales and outcomes of TCM in the treatment of COVID-19 can be found in several excellent review articles [131, 138–142].
TCM uses a unique theoretical and clinical system of disease taxonomy different from Western medicine to approach COVID-19. In TCM, Zheng-Hou is used to define disease taxonomy. “Zheng” is a summary of a series of interrelated clinical manifestations (symptoms and signs, especially tongue and pulse manifestations) caused by the pathogenesis and etiology at a certain pathological stage, and “Hou” is a summary of dynamically interrelated clinical manifestations affected by environmental factors. Differentiation of Zheng-Hou means to comprehensively analyze and integrate the sum of all pathogenesis and the whole dynamically interrelated clinical symptoms and signs (phenotypes) affected by pathological factors collected using the “four diagnostic methods” (looking, listening, questioning, and feeling the pulse) so as to identify the etiology, nature, time and location of a disease, and the relations between pathogens and patients, thereby to determine what Zheng-Hou the disease belongs to. The specific corresponding therapy decision is then made according to the outcome of differentiating Zheng-Hou. In summary, TDZH means treatment based on diagnosis of Zheng-Hou via holistic analysis of the overall sum of a series dynamically interrelated clinical phenotypes, i.e., the clinical phenome, caused by the pathogenesis under certain environmental conditions. Therefore, Zheng-Hou is treated with a combination of multiple herbs, called Fu-Fang or Fangji, to address the problems identified in the whole phenome of Zheng-Hou, usually by strengthening defense mechanisms and at the same time eliminating pathogenic factors through integrated and combined modes of actions to harmonize multiple on-targets with off-targets [111, 112].
Accordingly, the SARS-CoV-2 infection-induced disease in Wuhan was then defined as “Han-Shi-Du Yi” [140], which can be further differentiated by five TCM Zheng-Hou in the progression stages closely corresponding to the clinical spectrum of COVID-19 [4, 128, 140]. Each Zheng-Hou is treated with a corresponding TCM Fangji [128, 142].
The fundamental difference between TCM and the Western medicine on the COVID-19 is in the definition of the disease taxonomy. While the Western medicine defines COVID-19 as a disease solely based on the symptoms or signs of respiratory system (eg, cough, sore throat, malaise, CT imaging of pneumonia, etc.) [4], TCM defines COVID-19 as a disease with the patient’s overall holistic clinical manifestations. Therefore, in Western medicine CVD is considered either a risk factor or a complication of COVID-19 and is treated separately; but in TCM, CVD is considered an intrinsic component of the Zheng-Hou in Han-Shi-Du Yi and is treated together with all other interrelated problems as a whole in the Zheng-Hou.
For example, while the individuals who tested positive for SARS-CoV-2 infection but had no symptoms consistent with COVID-19 (no fever, cough, sore throat, or malaise) are classified into the category of asymptomatic and needed no treatment by Western medicine, these individuals were found to have languid, abdominal fullness and distention, thick and greasy tongue coating and could be diagnosed as “Han-Shi” or “Pi-Wei Han-Shi Qi-Zhi” by TCM and needed treatment with either Huoxiang Zhengqi dropping pills or Lianhua Qingwen Capsules [142, 143].
Conclusions and perspectives on future directions
With the unprecedented COVID-19 pandemic entering its 4th year, we have gradually learnt that this SARS-CoV-2 infection-induced infectious disease is not a phenotypic disease primarily attacks the lung but a phenomic disease that simultaneously affects both respiratory and extra-respiratory systems throughout the body. Viral invasion via ACE2 causes direct injuries of various cells including cardiac myocytes and endothelial cells in vascular beds of multiple organs and induces systemic inflammation and immune over-response, cytokine storm, or hypercoagulability and thrombosis, which can cause systemic complications and are the main causes for the transition from mild to severe/critical illness or even death in COVID-19 patients.
Intrinsic to the COVID-19 phenome, the cardiovascular impact of SARS-CoV-2 infection is prominent and closely associated with COVID-19 disease severity and mortality. The cardiovascular manifestations can be acute and persist into convalescence and possibly beyond. Regardless of the severity of the initial infection up to 1/3 of those diagnosed with COVID-19 may continue health-related problems including CVD. Evaluation of post-acute COVID-19 syndrome (long-COVID-19) and recommendations for long-term surveillance, monitoring, and return to exercise or sports participation remain areas in need of further study [144–146]. The potential for long-term evolution of long-COVID-19 into chronic myocardial disease and other cardiovascular complications and their significant implications still await further definition and evaluation [146]. Therefore, interdisciplinary management and prolonged clinical follow-up should be given to all COVID-19 cases with or without preexisting CVD after recovery from acute infection.
The COVID-19 pandemic has opened many doors and created a platform for innovation and creativities and has highlighted the urgency for precision medicine to move faster and further. With the challenge from the wide-spread infection of SARS-CoV-2 and variants, COVID-19 has also called for internationally interoperable longitudinal cohorts at the phenome scale to identify clinical and biologic risk factors and long-term sequelae for acute infectious diseases. PheWAS on the TCM Zheng-hou and Fang-ji, which has been clinically proven effective in the treatment of COVID-19, including the new mutated variants, may provide new systematical insights into the pathogenic mechanisms of SARS-CoV-2 infection and therapeutic targets for COVID-19 phenome. The translation of the knowledge of phenomics, PheWAS, pharmacophenomics and pharmacogenomics into clinical application will pave a new path to building new knowledge network for creation of new taxonomy of human disease and lead to refined diagnoses, more rational treatment, and prevention of disease (Fig. 3).
Fig. 3. PheWAS and precision medicine of CVD in the COVID-19 phenome.
With the wide-spread infection of the SARS-CoV-2 and its variants COVID-19 is a phenomic disease that simultaneously affects multiple systems involving many organs including heart and vasculatures. The cardiovascular manifestations can be acute and persist as a long-COVID. Therefore, interdisciplinary management of preexisting, genetic, and epidemiologic risks for CVD and prolonged clinical monitoring (biomarker panels, ECG, echocardiography, CMR, etc.) and follow-up should be given to all COVID-19 cases during and after recovery from acute infection. A close collaboration of medical practitioners, health care industry and public health officials and professions, biomedical researchers and drug research and development industry is required to establish internationally interoperable longitudinal phenome (including TCM) cohorts for clinical and biomedical research. PheWAS of CVD and COVID-19 phenome may help to innovate the current taxonomy of human disease, i.e., to change the taxonomy and classification of disease from a phenotypic definition to a phenome-based definition, and to advance precision medicine for more accurate diagnosis and greater precision therapy of CVD and for better health outcomes in the era of post COVID-19 pandemic.
Acknowledgements
This work was supported by research grants from the Science and Technology strategic cooperation Programs of Luzhou Municipal People’s Government and Southwest Medical University (NO. 2017LZXNYD-P01 and 2019 LZXNYD-P01DUAN to Dr. Duan).
Author contributions
All the authors contributed substantially to all aspects of the article.
Competing interests
The authors declare no competing interests.
Footnotes
These authors contributed equally: Qian Cao, Xin Du, Xiao-yan Jiang.
Contributor Information
Lingyu Linda Ye, Email: lye@swmu.edu.cn.
Dayue Darrel Duan, Email: dduan@swmu.edu.cn.
References
- 1.Zhu N, Zhang D, Wang W, Li X, Yang B, Song J, et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382:727–33. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhou P, Yang XL, Wang XG, Hu B, Zhang L, Zhang W, et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579:270–3. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhang XL, Li ZM, Ye JT, Lu J, Ye LL, Zhang CX, et al. Pharmacological and cardiovascular perspectives on the treatment of COVID-19 with chloroquine derivatives. Acta Pharmacol Sin. 2020;41:1377–86. doi: 10.1038/s41401-020-00519-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gluckman TJ, Bhave NM, Allen LA, Chung EH, Spatz ES, Ammirati E, et al. 2022 ACC expert consensus decision pathway on cardiovascular sequelae of COVID-19 in adults: myocarditis and other myocardial involvement, post-acute sequelae of SARS-CoV-2 infection, and return to play: a report of the American College of Cardiology Solution Set Oversight Committee. J Am Coll Cardiol. 2022;79:1717–56. doi: 10.1016/j.jacc.2022.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Welty FK, Rajai N, Amangurbanova M. Comprehensive review of cardiovascular complications of coronavirus disease 2019 and beneficial treatments. Cardiol Rev. 2022;30:145–57. doi: 10.1097/CRD.0000000000000422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ho HT, Peischard S, Strutz-Seebohm N, Klingel K, Seebohm G. Myocardial damage by SARS-CoV-2: emerging mechanisms and therapies. Viruses. 2021;13:1880–903. doi: 10.3390/v13091880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Maiese A, Frati P, Del Duca F, Santoro P, Manetti AC, La Russa R, et al. Myocardial pathology in COVID-19-associated cardiac injury: a systematic review. Diagnostics. 2021;11:1647–61. doi: 10.3390/diagnostics11091647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cooke JP, Connor JH, Jain A. Acute and chronic cardiovascular manifestations of COVID-19: role for endotheliopathy. Methodist Debakey Cardiovasc J. 2021;17:53–62. doi: 10.14797/mdcvj.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Arevalos V, Ortega-Paz L, Rodriguez-Arias JJ, Calvo Lopez M, Castrillo-Golvano L, Salazar-Rodriguez A, et al. Acute and chronic effects of COVID-19 on the cardiovascular system. J Cardiovasc Dev Dis. 2021;8:128–47. doi: 10.3390/jcdd8100128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Crudo VL, Ahmed AI, Cowan EL, Shah DJ, Al-Mallah MH, Malahfji M. Acute and subclinical myocardial injury in COVID-19. Methodist Debakey Cardiovasc J. 2021;17:22–30. doi: 10.14797/mdcvj.1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jaiswal V, Sarfraz Z, Sarfraz A, Mukherjee D, Batra N, Hitawala G, et al. COVID-19 infection and myocarditis: a state-of-the-art systematic review. J Prim Care Community Health. 2021;12:21501327211056800. doi: 10.1177/21501327211056800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen YM, Zheng Y, Yu Y, Wang Y, Huang Q, Qian F, et al. Blood molecular markers associated with COVID-19 immunopathology and multi-organ damage. EMBO J. 2020;39:e105896. doi: 10.15252/embj.2020105896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lawler NG, Gray N, Kimhofer T, Boughton B, Gay M, Yang R, et al. Systemic perturbations in amine and kynurenine metabolism associated with acute SARS-CoV-2 infection and inflammatory cytokine responses. J Proteome Res. 2021;20:2796–811. doi: 10.1021/acs.jproteome.1c00052. [DOI] [PubMed] [Google Scholar]
- 14.Liu R, Pan J, Zhang C, Sun X. Cardiovascular complications of COVID-19 vaccines. Front Cardiovasc Med. 2022;9:840929. doi: 10.3389/fcvm.2022.840929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mohammadi A, Balan I, Yadav S, Matos WF, Kharawala A, Gaddam M, et al. Post-COVID-19 pulmonary fibrosis. Cureus. 2022;14:e22770. doi: 10.7759/cureus.22770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Papadopoulou A, Musa H, Sivaganesan M, McCoy D, Deloukas P, Marouli E. COVID-19 susceptibility variants associate with blood clots, thrombophlebitis and circulatory diseases. PLoS One. 2021;16:e0256988. doi: 10.1371/journal.pone.0256988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Parise RS, Ramesh S, Govindarajulu M, Ajoolabady A, Moore T, Dhanasekaran M. COVID-19-induced cardiovascular damage differs from other prevalent viruses. Cardiol Plus. 2021;6:231–45. doi: 10.4103/2470-7511.334401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rajpal S, Kahwash R, Tong MS, Paschke K, Satoskar AA, Foreman B, et al. Fulminant myocarditis following SARS-CoV-2 infection: JACC patient care pathways. JACC Case Rep. 2022;4:567–75. doi: 10.1016/j.jaccas.2022.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Regan JA, Abdulrahim JW, Bihlmeyer NA, Haynes C, Kwee LC, Patel MR, et al. Phenome-wide association study of severe COVID-19 genetic risk variants. J Am Heart Assoc. 2022;11:e024004. doi: 10.1161/JAHA.121.024004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sakr Y, Giovini M, Leone M, Pizzilli G, Kortgen A, Bauer M, et al. Pulmonary embolism in patients with coronavirus disease-2019 (COVID-19) pneumonia: a narrative review. Ann Intensive Care. 2020;10:124. doi: 10.1186/s13613-020-00741-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Siripanthong B, Asatryan B, Hanff TC, Chatha SR, Khanji MY, Ricci F, et al. The pathogenesis and long-term consequences of COVID-19 cardiac injury. JACC Basic Transl Sci. 2022;7:294–308. doi: 10.1016/j.jacbts.2021.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhou D, Gamazon ER. Integrative transcriptomic, evolutionary, and causal inference framework for region-level analysis: Application to COVID-19. NPJ Genom Med. 2022;7:24. doi: 10.1038/s41525-022-00296-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang YP, Zhang YY, Duan DD. From genome-wide association study to phenome-wide association study: new paradigms in obesity research. Prog Mol Biol Transl Sci. 2016;140:185–231. doi: 10.1016/bs.pmbts.2016.02.003. [DOI] [PubMed] [Google Scholar]
- 24.Verma A, Tsao NL, Thomann L, Ho YL, Iyengar S, Luoh SW, et al. A Phenome-Wide Association Study of genes associated with COVID-19 severity reveals shared genetics with complex diseases in the Million Veteran Program. PLoS Genet. 2022;18:e1010113. doi: 10.1371/journal.pgen.1010113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tuta-Quintero E, Martinez-Ayala C, Mantilla-Beltran G, Rueda-Rodriguez A, Pimentel J. Multisystem inflammatory syndrome and COVID-19: a scoping review. Bol Med Hosp Infant Mex. 2022;79:69–82. doi: 10.24875/BMHIM.21000073. [DOI] [PubMed] [Google Scholar]
- 26.Duan DD, Han Y, Li L, Zhao J, Wang Z. Pharmacophenomics: a new paradigm for pharmacology, toxicology, and personalized medicine. Chin J Pharmacol Toxicol. 2014;28:1–9. [Google Scholar]
- 27.Xie Y, Xu E, Bowe B, Al-Aly Z. Long-term cardiovascular outcomes of COVID-19. Nat Med. 2022;28:583–90. doi: 10.1038/s41591-022-01689-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nicholson JK. Molecular phenomic approaches to deconvolving the systemic effects of SARS-CoV-2 infection and post-acute COVID-19 syndrome. Phenomics. 2021;1:143–50. doi: 10.1007/s43657-021-00020-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Abdelsayed N, McKinney B, Carter M. SARS-CoV-2 complicated by a large hemorrhagic pericardial effusion. Cureus. 2022;14:e22282. doi: 10.7759/cureus.22282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fernandes Q, Inchakalody VP, Merhi M, Mestiri S, Taib N, Moustafa Abo El-Ella D, et al. Emerging COVID-19 variants and their impact on SARS-CoV-2 diagnosis, therapeutics and vaccines. Ann Med. 2022;54:524–40. doi: 10.1080/07853890.2022.2031274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ahmad MS, Shaik RA, Ahmad RK, Yusuf M, Khan M, Almutairi AB, et al. “LONG COVID”: an insight. Eur Rev Med Pharm Sci. 2021;25:5561–77. doi: 10.26355/eurrev_202109_26669. [DOI] [PubMed] [Google Scholar]
- 32.Shiravi AA, Ardekani A, Sheikhbahaei E, Heshmat-Ghahdarijani K. Cardiovascular complications of SARS-CoV-2 vaccines: an overview. Cardiol Ther. 2022;11:13–21. doi: 10.1007/s40119-021-00248-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323:1061–9. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xiong TY, Redwood S, Prendergast B, Chen M. Coronaviruses and the cardiovascular system: acute and long-term implications. Eur Heart J. 2020;41:1798–800. doi: 10.1093/eurheartj/ehaa231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Parohan M, Yaghoubi S, Seraji A. Cardiac injury is associated with severe outcome and death in patients with Coronavirus disease 2019 (COVID-19) infection: a systematic review and meta-analysis of observational studies. Eur Heart J Acute Cardiovasc Care. 2020;9:665–77. doi: 10.1177/2048872620937165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lala A, Johnson KW, Januzzi JL, Russak AJ, Paranjpe I, Richter F, et al. Prevalence and impact of myocardial injury in patients hospitalized With COVID-19 infection. J Am Coll Cardiol. 2020;76:533–46. doi: 10.1016/j.jacc.2020.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Alvarez-Garcia J, Lee S, Gupta A, Cagliostro M, Joshi AA, Rivas-Lasarte M, et al. Prognostic impact of prior heart failure in patients hospitalized with COVID-19. J Am Coll Cardiol. 2020;76:2334–48. doi: 10.1016/j.jacc.2020.09.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Giustino G, Pinney SP, Lala A, Reddy VY, Johnston-Cox HA, Mechanick JI, et al. Coronavirus and cardiovascular disease, myocardial injury, and arrhythmia: JACC focus seminar. J Am Coll Cardiol. 2020;76:2011–23. doi: 10.1016/j.jacc.2020.08.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yu Y, Xu D, Fu S, Zhang J, Yang X, Xu L, et al. Patients with COVID-19 in 19 ICUs in Wuhan, China: a cross-sectional study. Crit Care. 2020;24:219. doi: 10.1186/s13054-020-02939-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020;323:1239–42. doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 42.Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708–20. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Guzik TJ, Mohiddin SA, Dimarco A, Patel V, Savvatis K, Marelli-Berg FM, et al. COVID-19 and the cardiovascular system: implications for risk assessment, diagnosis, and treatment options. Cardiovasc Res. 2020;116:1666–87. doi: 10.1093/cvr/cvaa106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Saggese CE. COVID-19 and stroke: an emerging association. Cerebrovasc Dis. 2021;50:363. doi: 10.1159/000514132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Saggese CE, Del Bianco C, Di Ruzza MR, Magarelli M, Gandini R, Plocco M. COVID-19 and stroke: casual or causal role? Cerebrovasc Dis. 2020;49:341–4. doi: 10.1159/000509453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kario K, Morisawa Y, Sukonthasarn A, Turana Y, Chia YC, Park S, et al. COVID-19 and hypertension-evidence and practical management: guidance from the HOPE Asia Network. J Clin Hypertens. 2020;22:1109–19. doi: 10.1111/jch.13917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Williamson EJ, Walker AJ, Bhaskaran K, Bacon S, Bates C, Morton CE, et al. Factors associated with COVID-19-related death using OpenSAFELY. Nature. 2020;584:430–6. doi: 10.1038/s41586-020-2521-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Myers LC, Parodi SM, Escobar GJ, Liu VX. Characteristics of hospitalized adults with COVID-19 in an integrated health care system in California. JAMA. 2020;323:2195–8. doi: 10.1001/jama.2020.7202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Denny JC, Bastarache L, Roden DM. Phenome-wide association studies as a tool to advance precision medicine. Annu Rev Genom Hum Genet. 2016;17:353–73. doi: 10.1146/annurev-genom-090314-024956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Salvatore M, Gu T, Mack JA, Prabhu Sankar S, Patil S, Valley TS, et al. A Phenome-Wide Association Study (PheWAS) of COVID-19 outcomes by race using the electronic health records data in Michigan medicine. J Clin Med. 2021;10:1351. doi: 10.3390/jcm10071351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Verma A, Tsao NL, Thomann LO, Ho YL, Iyengar SK, Luoh SW, et al. A Phenome-Wide Association Study of genes associated with COVID-19 severity reveals shared genetics with complex diseases in the Million Veteran Program. PLoS Genet. 2022;18:e1010113. doi: 10.1371/journal.pgen.1010113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Song RJ, Ho YL, Schubert P, Park Y, Posner D, Lord EM, et al. Phenome-wide association of 1809 phenotypes and COVID-19 disease progression in the Veterans Health Administration Million Veteran Program. PLoS One. 2021;16:e0251651. doi: 10.1371/journal.pone.0251651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.George G, Gan S, Huang Y, Appleby P, Nar AS, Venkatesan R, et al. PheGWAS: a new dimension to visualize GWAS across multiple phenotypes. Bioinformatics. 2020;36:2500–5. doi: 10.1093/bioinformatics/btz944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pendergrass SA, Brown-Gentry K, Dudek S, Frase A, Torstenson ES, Goodloe R, et al. Phenome-wide association study (PheWAS) for detection of pleiotropy within the Population Architecture using Genomics and Epidemiology (PAGE) network. PLoS Genet. 2013;9:e1003087. doi: 10.1371/journal.pgen.1003087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fayol A, Livrozet M, Boutouyrie P, Khettab H, Betton M, Tea V, et al. Cardiac performance in patients hospitalized with COVID-19: a 6 month follow-up study. ESC Heart Fail. 2021;8:2232–9. doi: 10.1002/ehf2.13315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Manolis AS, Manolis AA, Manolis TA, Apostolopoulos EJ, Papatheou D, Melita H. COVID-19 infection and cardiac arrhythmias. Trends Cardiovasc Med. 2020;30:451–60. doi: 10.1016/j.tcm.2020.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tajbakhsh A, Gheibi Hayat SM, Taghizadeh H, Akbari A, Inabadi M, Savardashtaki A, et al. COVID-19 and cardiac injury: clinical manifestations, biomarkers, mechanisms, diagnosis, treatment, and follow up. Expert Rev Anti-Infective Ther. 2021;19:345–57. doi: 10.1080/14787210.2020.1822737. [DOI] [PubMed] [Google Scholar]
- 58.Sahranavard M, Akhavan Rezayat A, Zamiri Bidary M, Omranzadeh A, Rohani F, Hamidi Farahani R, et al. Cardiac complications in COVID-19: a systematic review and meta-analysis. Arch Iran Med. 2021;24:152–63. doi: 10.34172/aim.2021.24. [DOI] [PubMed] [Google Scholar]
- 59.Haussner W, DeRosa AP, Haussner D, Tran J, Torres-Lavoro J, Kamler J, et al. COVID-19 associated myocarditis: a systematic review. Am J Emerg Med. 2022;51:150–5. doi: 10.1016/j.ajem.2021.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yang Z, MacDonald-Dunlop E, Chen J, Zhai R, Li T, Richmond A, et al. Genetic landscape of the ACE2 coronavirus receptor. Circulation. 2022;145:1398–411. doi: 10.1161/CIRCULATIONAHA.121.057888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Eftekharzadeh P, Patel A, Sokolova E, Rodas A, Ahmed S. Takotsubo cardiomyopathy: a COVID-19 complication. Cureus. 2022;14:e22803. doi: 10.7759/cureus.22803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lee K, Rahimi O, Gupta N, Ahsan C. Complete AV block in vaccinated COVID-19 patient. Case Rep Cardiol. 2022;2022:9371818. doi: 10.1155/2022/9371818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Singh T, Khan H, Gamble DT, Scally C, Newby DE, Dawson D. Takotsubo syndrome: pathophysiology, emerging concepts, and clinical implications. Circulation. 2022;145:1002–19. doi: 10.1161/CIRCULATIONAHA.121.055854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gomez JMD, Nair G, Nanavaty P, Rao A, Marinescu K, Suboc T. COVID-19-associated takotsubo cardiomyopathy. BMJ Case Rep. 2020;13:e236811. doi: 10.1136/bcr-2020-236811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tamis-Holland JE, Jneid H, Reynolds HR, Agewall S, Brilakis ES, Brown TM, et al. Contemporary diagnosis and management of patients with myocardial infarction in the absence of obstructive coronary artery disease: a scientific statement from the American Heart Association. Circulation. 2019;139:e891–e908. doi: 10.1161/CIR.0000000000000670. [DOI] [PubMed] [Google Scholar]
- 66.Chammas J, Delaney D, Chabaytah N, Abdulkarim S, Schwertani A. COVID-19 and the cardiovascular system: insights into effects and treatments. Can J Physiol Pharmacol. 2021;99:1119–27. doi: 10.1139/cjpp-2021-0093. [DOI] [PubMed] [Google Scholar]
- 67.Huang C, Huang L, Wang Y, Li X, Ren L, Gu X, et al. 6-month consequences of COVID-19 in patients discharged from hospital: a cohort study. Lancet. 2021;397:220–32. doi: 10.1016/S0140-6736(20)32656-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Carfi A, Bernabei R, Landi F, Gemelli Against C-P-ACSG. Persistent symptoms in patients after acute COVID-19. JAMA. 2020;324:603–5. doi: 10.1001/jama.2020.12603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ayoubkhani D, Khunti K, Nafilyan V, Maddox T, Humberstone B, Diamond I, et al. Post-covid syndrome in individuals admitted to hospital with covid-19: retrospective cohort study. BMJ. 2021;372:n693. doi: 10.1136/bmj.n693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Inciardi RM, Lupi L, Zaccone G, Italia L, Raffo M, Tomasoni D, et al. Cardiac involvement in a patient with coronavirus disease 2019 (COVID-19) JAMA Cardiol. 2020;5:819–24. doi: 10.1001/jamacardio.2020.1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Li M, Dong Y, Wang H, Guo W, Zhou H, Zhang Z, et al. Cardiovascular disease potentially contributes to the progression and poor prognosis of COVID-19. Nutr Metab Cardiovasc Dis. 2020;30:1061–7. doi: 10.1016/j.numecd.2020.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Khalid M, Awan S, Jatoi NN, Jatoi HN, Yasmin F, Ochani RK, et al. Cardiac manifestations of the coronavirus disease-19: a review of pathogenesis, clinical manifestations, diagnosis, and treatment. Pan Afr Med J. 2021;39:173. doi: 10.11604/pamj.2021.39.173.27802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lan J, Ge J, Yu J, Shan S, Zhou H, Fan S, et al. Structure of the SARS-CoV-2 spike receptor-binding domain bound to the ACE2 receptor. Nature. 2020;581:215–20. doi: 10.1038/s41586-020-2180-5. [DOI] [PubMed] [Google Scholar]
- 74.Jiang RD, Liu MQ, Chen Y, Shan C, Zhou YW, Shen XR, et al. Pathogenesis of SARS-CoV-2 in transgenic mice expressing human angiotensin-converting enzyme 2. Cell. 2020;182:50–8. doi: 10.1016/j.cell.2020.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hoffmann M, Kleine-Weber H, Schroeder S, Kruger N, Herrler T, Erichsen S, et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181:271–80. doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wang Q, Zhang Y, Wu L, Niu S, Song C, Zhang Z, et al. Structural and functional basis of SARS-CoV-2 entry by using human ACE2. Cell. 2020;181:894–904. doi: 10.1016/j.cell.2020.03.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wenzel UO, Kintscher U. ACE2 and SARS-CoV-2: tissue or plasma, good or bad? Am J Hypertens. 2021;34:274–7. doi: 10.1093/ajh/hpaa175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wicik Z, Eyileten C, Jakubik D, Simoes SN, Martins DC, Jr, Pavao R, et al. ACE2 interaction networks in COVID-19: a physiological framework for prediction of outcome in patients with cardiovascular risk factors. J Clin Med. 2020;9:3743–72. doi: 10.3390/jcm9113743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wehbe Z, Hammoud S, Soudani N, Zaraket H, El-Yazbi A, Eid AH. Molecular insights Into SARS COV-2 interaction with cardiovascular disease: role of RAAS and MAPK signaling. Front Pharmacol. 2020;11:836. doi: 10.3389/fphar.2020.00836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Turner AJ, Hiscox JA, Hooper NM. ACE2: from vasopeptidase to SARS virus receptor. Trends Pharmacol Sci. 2004;25:291–4. doi: 10.1016/j.tips.2004.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Jackson CB, Farzan M, Chen B, Choe H. Mechanisms of SARS-CoV-2 entry into cells. Nat Rev Mol Cell Biol. 2022;23:3–20. doi: 10.1038/s41580-021-00418-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hamming I, Timens W, Bulthuis ML, Lely AT, Navis G, van Goor H. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J Pathol. 2004;203:631–7. doi: 10.1002/path.1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Turner AJ, Nalivaeva NN. Angiotensin-converting enzyme 2 (ACE2): Two decades of revelations and re-evaluation. Peptides. 2022;151:170766. doi: 10.1016/j.peptides.2022.170766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Turner AJ, Hooper NM. The angiotensin-converting enzyme gene family: genomics and pharmacology. Trends Pharmacol Sci. 2002;23:177–83. doi: 10.1016/S0165-6147(00)01994-5. [DOI] [PubMed] [Google Scholar]
- 85.Pang XC, Zhang HX, Zhang Z, Rinkiko S, Cui YM, Zhu YZ. The two-way switch role of ACE2 in the treatment of novel coronavirus pneumonia and underlying comorbidities. Molecules. 2020;26:142–60. doi: 10.3390/molecules26010142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Gheblawi M, Wang K, Viveiros A, Nguyen Q, Zhong JC, Turner AJ, et al. Angiotensin-converting enzyme 2: SARS-CoV-2 receptor and regulator of the renin-angiotensin system: celebrating the 20th anniversary of the discovery of ACE2. Circ Res. 2020;126:1456–74. doi: 10.1161/CIRCRESAHA.120.317015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wallentin L, Lindback J, Eriksson N, Hijazi Z, Eikelboom JW, Ezekowitz MD, et al. Angiotensin-converting enzyme 2 (ACE2) levels in relation to risk factors for COVID-19 in two large cohorts of patients with atrial fibrillation. Eur Heart J. 2020;41:4037–46. doi: 10.1093/eurheartj/ehaa697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Liu Y, Yang Y, Zhang C, Huang F, Wang F, Yuan J, et al. Clinical and biochemical indexes from 2019-nCoV infected patients linked to viral loads and lung injury. Sci China Life Sci. 2020;63:364–74. doi: 10.1007/s11427-020-1643-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kornilov SA, Lucas I, Jade K, Dai CL, Lovejoy JC, Magis AT. Plasma levels of soluble ACE2are associated with sex, metabolic syndrome, and its biomarkers in a large cohort, pointing to a possible mechanism for increased severity in COVID-19. Crit Care. 2020;24:452. doi: 10.1186/s13054-020-03141-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Sward P, Edsfeldt A, Reepalu A, Jehpsson L, Rosengren BE, Karlsson MK. Age and sex differences in soluble ACE2 may give insights for COVID-19. Crit Care. 2020;24:221. doi: 10.1186/s13054-020-02942-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Denny JC, Collins FS. Precision medicine in 2030-seven ways to transform healthcare. Cell. 2021;184:1415–9. doi: 10.1016/j.cell.2021.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Nikpay M, Mohammadzadeh S. Phenome-wide screening for traits causally associated with the risk of coronary artery disease. J Hum Genet. 2020;65:371–80. doi: 10.1038/s10038-019-0716-z. [DOI] [PubMed] [Google Scholar]
- 93.Han Y, Li L, Zhang Y, Yuan H, Ye L, Zhao J, et al. Phenomics of vascular disease: the systematic approach to the combination therapy. Curr Vasc Pharmacol. 2015;13:433–40. doi: 10.2174/1570161112666141014144829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Denny JC, Ritchie MD, Basford MA, Pulley JM, Bastarache L, Brown-Gentry K, et al. PheWAS: demonstrating the feasibility of a phenome-wide scan to discover gene-disease associations. Bioinformatics. 2010;26:1205–10. doi: 10.1093/bioinformatics/btq126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Diogo D, Tian C, Franklin CS, Alanne-Kinnunen M, March M, Spencer CCA, et al. Phenome-wide association studies across large population cohorts support drug target validation. Nat Commun. 2018;9:4285. doi: 10.1038/s41467-018-06540-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Pendergrass SA, Ritchie MD. Phenome-wide association studies: leveraging comprehensive phenotypic and genotypic data for discovery. Curr Genet Med Rep. 2015;3:92–100. doi: 10.1007/s40142-015-0067-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Roden DM. Phenome-wide association studies: a new method for functional genomics in humans. J Physiol. 2017;595:4109–15. doi: 10.1113/JP273122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Barnado A, Carroll RJ, Casey C, Wheless L, Denny JC, Crofford LJ. Phenome-wide association studies uncover a novel association of increased atrial fibrillation in male patients with systemic Lupus Erythematosus. Arthritis Care Res. 2018;70:1630–6. doi: 10.1002/acr.23553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Pathak GA, Singh K, Miller-Fleming TW, Wendt FR, Ehsan N, Hou K, et al. Integrative genomic analyses identify susceptibility genes underlying COVID-19 hospitalization. Nat Commun. 2021;12:4569. doi: 10.1038/s41467-021-24824-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Li J, Wang X, Chen J, Zhang H, Deng A. Association of renin-angiotensin system inhibitors with severity or risk of death in patients with hypertension hospitalized for coronavirus disease 2019 (COVID-19) infection in Wuhan, China. JAMA Cardiol. 2020;5:825–30. doi: 10.1001/jamacardio.2020.1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Zhang P, Zhu L, Cai J, Lei F, Qin JJ, Wang Y, et al. Response by Zhang et al. to Letter Regarding Article, “Association of inpatient use of angiotensin-converting enzyme inhibitors and angiotensin II receptor blockers with mortality among patients with hypertension hospitalized with COVID-19”. Circ Res. 2020;126:e142–e3. doi: 10.1161/CIRCRESAHA.120.317242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Zhang P, Zhu L, Cai J, Lei F, Qin JJ, Xie J, et al. Association of inpatient use of angiotensin-converting enzyme inhibitors and angiotensin II receptor blockers with mortality among patients with hypertension hospitalized with COVID-19. Circ Res. 2020;126:1671–81. doi: 10.1161/CIRCRESAHA.120.317134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Watkins J. Preventing a covid-19 pandemic. BMJ. 2020;368:m810. doi: 10.1136/bmj.m810. [DOI] [PubMed] [Google Scholar]
- 104.Kuster GM, Pfister O, Burkard T, Zhou Q, Twerenbold R, Haaf P, et al. SARS-CoV2: should inhibitors of the renin-angiotensin system be withdrawn in patients with COVID-19? Eur Heart J. 2020;41:1801–3. doi: 10.1093/eurheartj/ehaa235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.National Research Council (US) Committee on A Framework for Developing a New Taxonomy of Disease In: Toward precision medicine: building a knowledge network for biomedical research and a new taxonomy of disease. (National Academies Press (US), Washington, D.C., 2011). [PubMed]
- 106.Chute CG, Celik C. Overview of ICD-11 architecture and structure. BMC Med Inform Decis Mak. 2022;21:378. doi: 10.1186/s12911-021-01539-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Drosler SE, Weber S, Chute CG. ICD-11 extension codes support detailed clinical abstraction and comprehensive classification. BMC Med Inform Decis Mak. 2021;21:278. doi: 10.1186/s12911-021-01635-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Li JG, Xu H. Chinese medicine in fighting against Covid-19: role and inspiration. Chin J Integr Med. 2021;27:3–6. doi: 10.1007/s11655-020-2860-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Chen X, Yin YH, Zhang MY, Liu JY, Li R, Qu YQ. Investigating the mechanism of ShuFeng JieDu capsule for the treatment of novel Coronavirus pneumonia (COVID-19) based on network pharmacology. Int J Med Sci. 2020;17:2511–30. doi: 10.7150/ijms.46378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Jin YH, Zhan QY, Peng ZY, Ren XQ, Yin XT, Cai L, et al. Chemoprophylaxis, diagnosis, treatments, and discharge management of COVID-19: An evidence-based clinical practice guideline (updated version) Mil Med Res. 2020;7:41. doi: 10.1186/s40779-020-00270-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Duan DD, Wang Z, Zhang BL, Wang YY. Fangjiomics: revealing adaptive omics pharmacological mechanisms of the myriad combination therapies to achieve personalized medicine. Acta Pharmacol Sin. 2015;36:651–3. doi: 10.1038/aps.2015.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Duan DD, Wang Z, Wang YY. New omic and network paradigms for deep understanding of therapeutic mechanisms for Fangji of traditional Chinese medicine. Acta Pharmacol Sin. 2018;39:903–5. doi: 10.1038/aps.2018.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.He DD, Zhang XK, Zhu XY, Huang FF, Wang Z, Tu JC. Network pharmacology and RNA-sequencing reveal the molecular mechanism of Xuebijing injection on COVID-19-induced cardiac dysfunction. Comput Biol Med. 2021;131:104293. doi: 10.1016/j.compbiomed.2021.104293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Yu YN, Liu J, Zhang L, Wang Z, Duan DD, Wang YY. Clinical Zheng-hou pharmacology: the missing link between pharmacogenomics and personalized medicine? Curr Vasc Pharmacol. 2015;13:423–32. doi: 10.2174/1570161112666141014144431. [DOI] [PubMed] [Google Scholar]
- 115.Curran J. The Yellow Emperor’s classic of internal medicine. BMJ. 2008;336:777. doi: 10.1136/bmj.39527.472303.4E. [DOI] [Google Scholar]
- 116.Luo H, Tang QL, Shang YX, Liang SB, Yang M, Robinson N, et al. Can Chinese medicine be used for prevention of corona virus disease 2019 (COVID-19)? A review of historical classics, research evidence and current prevention programs. Chin J Integr Med. 2020;26:243–50. doi: 10.1007/s11655-020-3192-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Qi F, Tang W. Traditional Chinese medicine for treatment of novel infectious diseases: current status and dilemma. Biosci Trends. 2021;15:201–4. doi: 10.5582/bst.2021.01263. [DOI] [PubMed] [Google Scholar]
- 118.Han Y, Geng H, Feng W, Tang X, Ou A, Lao Y, et al. A follow-up study of 69 discharged SARS patients. J Tradit Chin Med. 2003;23:214–7. [PubMed] [Google Scholar]
- 119.Tong X, Li A, Zhang Z, Duan J, Chen X, Hua C, et al. TCM treatment of infectious atypical pneumonia—a report of 16 cases. J Tradit Chin Med. 2004;24:266–9. [PubMed] [Google Scholar]
- 120.Zhang MM, Liu XM, He L. Effect of integrated traditional Chinese and Western medicine on SARS: a review of clinical evidence. World J Gastroenterol. 2004;10:3500–5. doi: 10.3748/wjg.v10.i23.3500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Liu X, Zhang M, He L, Li YP, Kang YK. Chinese herbs combined with Western medicine for severe acute respiratory syndrome (SARS) Cochrane Database Syst Rev. 2006;10:CD004882. doi: 10.1002/14651858.CD004882.pub2. [DOI] [PubMed] [Google Scholar]
- 122.Chen Y, Guo JJ, Healy DP, Zhan S. Effect of integrated traditional Chinese medicine and western medicine on the treatment of severe acute respiratory syndrome: a meta-analysis. Pharm Pract (Granada). 2007;5:1–9. doi: 10.4321/S1886-36552007000100001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Lau JT, Leung PC, Wong EL, Fong C, Cheng KF, Zhang SC, et al. The use of an herbal formula by hospital care workers during the severe acute respiratory syndrome epidemic in Hong Kong to prevent severe acute respiratory syndrome transmission, relieve influenza-related symptoms, and improve quality of life: a prospective cohort study. J Alter Complement Med. 2005;11:49–55. doi: 10.1089/acm.2005.11.49. [DOI] [PubMed] [Google Scholar]
- 124.Lu R, Zhao X, Li J, Niu P, Yang B, Wu H, et al. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet. 2020;395:565–74. doi: 10.1016/S0140-6736(20)30251-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Han P, Li L, Liu S, Wang Q, Zhang D, Xu Z, et al. Receptor binding and complex structures of human ACE2 to spike RBD from omicron and delta SARS-CoV-2. Cell. 2022;185:630–40. doi: 10.1016/j.cell.2022.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Zhou P, Yang XL, Wang XG, Hu B, Zhang L, Zhang W, et al. Addendum: a pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;588:E6. doi: 10.1038/s41586-020-2951-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Song P, Zhao L, Li X, Su J, Jiang Z, Song B, et al. Interpretation of the Traditional Chinese Medicine portion of the diagnosis and treatment protocol for corona virus disease 2019 (Trial Version 7) J Tradit Chin Med. 2020;40:497–508. doi: 10.19852/j.cnki.jtcm.2020.03.019. [DOI] [PubMed] [Google Scholar]
- 128.Li Q, Wang H, Li X, Zheng Y, Wei Y, Zhang P, et al. The role played by traditional Chinese medicine in preventing and treating COVID-19 in China. Front Med. 2020;14:681–8. doi: 10.1007/s11684-020-0801-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Chen G, Su W, Yang J, Luo D, Xia P, Jia W, et al. Chinese herbal medicine reduces mortality in patients with severe and critical Coronavirus disease 2019: a retrospective cohort study. Front Med. 2020;14:752–9. doi: 10.1007/s11684-020-0813-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Zhuang J, Dai X, Wu Q, Cai H, Fu X, Zhang W, et al. A meta-analysis for Lianhua Qingwen on the treatment of Coronavirus disease 2019 (COVID-19) Complement Ther Med. 2021;60:102754. doi: 10.1016/j.ctim.2021.102754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Zhao Z, Li Y, Zhou L, Zhou X, Xie B, Zhang W, et al. Prevention and treatment of COVID-19 using traditional Chinese medicine: a review. Phytomedicine. 2021;85:153308. doi: 10.1016/j.phymed.2020.153308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Chu L, Huang F, Zhang M, Huang B, Wang Y. Current status of traditional Chinese medicine for the treatment of COVID-19 in China. Chin Med. 2021;16:63. doi: 10.1186/s13020-021-00461-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Buabeid M, Ijaz M, Shamim S, Huang X, Murtaza G. Therapeutic uses of traditional chinese medicines against COVID-19. Infect Drug Resist. 2021;14:5017–26. doi: 10.2147/IDR.S328261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.An X, Zhang Y, Duan L, Jin, Zhao S, Zhou R, et al. The direct evidence and mechanism of traditional Chinese medicine treatment of COVID-19. Biomed Pharmacother. 2021;137:111267. doi: 10.1016/j.biopha.2021.111267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Tian J, Yan S, Wang H, Zhang Y, Zheng Y, Wu H, et al. Hanshiyi Formula, a medicine for Sars-CoV2 infection in China, reduced the proportion of mild and moderate COVID-19 patients turning to severe status: a cohort study. Pharmacol Res. 2020;161:105127. doi: 10.1016/j.phrs.2020.105127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Ai Z, Zhou S, Li W, Wang M, Wang L, Hu G, et al. “Fei Yan No. 1” as a combined treatment for COVID-19: an efficacy and potential mechanistic study. Front Pharmacol. 2020;11:581277. doi: 10.3389/fphar.2020.581277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Zhang D, Zhang B, Lv JT, Sa RN, Zhang XM, Lin ZJ. The clinical benefits of Chinese patent medicines against COVID-19 based on current evidence. Pharmacol Res. 2020;157:104882. doi: 10.1016/j.phrs.2020.104882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Zhou W, Chen Z, Sun X, Zhong N, Liu Z. Application of traditional chinese medicine and systems pharmacology in drug prevention and treatment against COVID-19. Am J Chin Med. 2021;49:1045–61. doi: 10.1142/S0192415X21500506. [DOI] [PubMed] [Google Scholar]
- 139.Zhou LP, Wang J, Xie RH, Pakhale S, Krewski D, Cameron DW, et al. The effects of traditional Chinese medicine as an auxiliary treatment for COVID-19: a systematic review and meta-analysis. J Alter Complement Med. 2021;27:225–37. doi: 10.1089/acm.2020.0310. [DOI] [PubMed] [Google Scholar]
- 140.Zheng Y, Jin, Lin J, Zhang Y, Tian J, Lian F, et al. Understanding COVID-19 in Wuhan from the perspective of cold-dampness: clinical evidences and mechanisms. Front Med. 2021;8:617659. doi: 10.3389/fmed.2021.617659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Zhao ZH, Zhou Y, Li WH, Huang QS, Tang ZH, Li H. Analysis of traditional Chinese medicine diagnosis and treatment strategies for COVID-19 based on “The Diagnosis and Treatment Program for Coronavirus Disease-2019” from Chinese authority. Am J Chin Med. 2020;48:1035–49. doi: 10.1142/S0192415X20500500. [DOI] [PubMed] [Google Scholar]
- 142.Huang K, Zhang P, Zhang Z, Youn JY, Wang C, Zhang H, et al. Traditional Chinese Medicine (TCM) in the treatment of COVID-19 and other viral infections: efficacies and mechanisms. Pharmacol Ther. 2021;225:107843. doi: 10.1016/j.pharmthera.2021.107843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Chen X, Wu Y, Chen C, Gu Y, Zhu C, Wang S, et al. Identifying potential anti-COVID-19 pharmacological components of traditional Chinese medicine Lianhuaqingwen capsule based on human exposure and ACE2 biochromatography screening. Acta Pharm Sin B. 2021;11:222–36. doi: 10.1016/j.apsb.2020.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Holmes E, Wist J, Masuda R, Lodge S, Nitschke P, Kimhofer T, et al. Incomplete systemic recovery and metabolic phenoreversion in post-acute-phase nonhospitalized COVID-19 patients: implications for assessment of post-acute COVID-19 syndrome. J Proteome Res. 2021;20:3315–29. doi: 10.1021/acs.jproteome.1c00224. [DOI] [PubMed] [Google Scholar]
- 145.Filippetti L, Pace N, Louis JS, Mandry D, Goehringer F, Rocher MS, et al. Long-lasting myocardial and skeletal muscle damage evidenced by serial CMR during the first year in COVID-19 patients from the first wave. Front Cardiovasc Med. 2022;9:831580. doi: 10.3389/fcvm.2022.831580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Szarpak L, Pruc M, Filipiak KJ, Popieluch J, Bielski A, Jaguszewski MJ, et al. Myocarditis: a complication of COVID-19 and long-COVID-19 syndrome as a serious threat in modern cardiology. Cardiol J. 2022;29:178–9. doi: 10.5603/CJ.a2021.0155. [DOI] [PMC free article] [PubMed] [Google Scholar]