Abstract
Introduction:
Several gastrointestinal diseases have been linked to acute pancreatitis, but the risk of acute pancreatitis in microscopic colitis (MC) has not been studied.
Methods:
We conducted a nationwide, population-based, matched cohort study in Sweden of 12,140 patients with biopsy-verified MC (diagnosed in 2003–2017), 57,806 matched reference individuals, and 12,781 siblings without MC with follow-up until 2021. Data on MC were obtained from all of Sweden’s regional pathology registers (n=28) through the ESPRESSO cohort. Data on acute pancreatitis were collected from the National Patient Register. Adjusted hazard ratios (aHRs) and 95% confidence intervals (CIs) were calculated using Cox regression.
Results:
During a mean follow-up of 9.9 years (SD=4.3), 146 MC patients and 437 reference individuals were diagnosed with acute pancreatitis (127.8 vs. 80.1 per 100,000 person-years), corresponding to an aHR of 1.57 (95%CI=1.30–1.90). Moreover, we found a positive association between MC and acute non-gallstone-related pancreatitis (aHR 1.99 (95%CI=1.57–2.51)), but not with acute gallstone-related pancreatitis (aHR 1.08 (95%CI=0.78–1.49)). Comparing patients with MC to their unaffected siblings yielded an aHR of 1.28 (95%CI=0.92–1.78).
The risk of acute pancreatitis remained elevated also for MC patients with a follow-up exceeding 10 years (aHR 1.75 (95%CI=1.14–2.67)).
Conclusion:
This nationwide study of more than 12,000 patients with MC demonstrated an increased risk of acute pancreatitis after MC. Hence, clinicians should have a low threshold for evaluation of acute pancreatitis in patients with MC. Also, these patients should receive advice and care aimed at reducing the risk of acute pancreatitis.
Keywords: microscopic colitis, epidemiology, pancreatitis
INTRODUCTION
Microscopic colitis (MC) is a recently recognized inflammatory condition of the large intestine(1). The disease is usually divided into lymphocytic colitis (LC) or collagenous colitis (CC), depending on the histopathological presentation of a colonic biopsy. The most prominent symptom in MC is watery, non-bloody diarrhea. However, other symptoms, such as abdominal pain, weight loss, fatigue, and arthralgia, have also been reported(2). In a previous study we found a substantial increase in the incidence of MC in Sweden during the past decades(3) with incidence rates (IRs) approaching those of inflammatory bowel disease(4, 5). Acute pancreatitis is an acute inflammation of the pancreatic gland that clinically presents with acute onset of upper abdominal pain, elevated pancreatic enzymes in blood or urine and/or radiological signs of pancreatic inflammation. Risk factors for acute pancreatitis include alcohol abuse(6) and gallstone disease(7). Associations with other gastrointestinal diseases, such as celiac disease(8) and inflammatory bowel disease (IBD)(9, 10), have been described in the literature. However, in a large proportion of acute pancreatitis cases, no underlying risk factor can be determined. The severity of acute pancreatitis and the associated symptoms vary. Some patients require only conservative treatment whereas other patients may need intensive care. The mortality ranges between 3%(11) and 30%(12) depending on the severity of disease.
To our knowledge, no published work exists on the association between MC and acute pancreatitis. There are several reasons to hypothesize that an association between the two disorders is present. First, MC shares inflammatory characteristics with acute pancreatitis(13, 14). Second, many patients with MC are treated with budesonide, a steroid(15), which has been linked to onset of acute pancreatitis. Third, there is a known association between celiac disease and IBD and acute pancreatitis (of note, both celiac disease(16) and IBD(17) have been associated to MC). As patients with IBD (and likely also patients with MC) risk delayed or missed diagnosis of acute pancreatitis due to shared symptoms(18), added knowledge on the association between MC and acute pancreatitis may lower the threshold for evaluation of acute pancreatitis in MC. Moreover, a detected association between the two disease entities may also aid in generating new hypotheses about shared pathogenic mechanisms. Hence, this study aims to explore the association between MC and acute pancreatitis in a nationwide, population-based cohort.
METHODS
Setting
This study is based on biopsy data derived from Swedish regional pathology registers. All Swedish citizens have a unique personal identity number(19) (PIN) through which we linked data from Swedish healthcare registers. Moreover, health care in Sweden is largely tax-funded, with a policy of providing all Swedish citizens equal access to health care.
Identification of patients with MC
The data used in this study were collected as part of the ESPRESSO study(20), which includes data on all gastrointestinal biopsies in Sweden from 1965 to 2017. The ESPRESSO study contains biopsy data on 2.1 million unique individuals. Classification of biopsies in Sweden is done according to the Systematized Nomenclature of Medicine (SNOMED) system. Based on the histopathological characteristics of a biopsy, the SNOMED system classifies each biopsy by a specific code. We retrieved data on all biopsies coded M40600 for CC and M47170 for LC (Figure 1). Previously, we have examined the validity of these codes, finding a positive predictive value (PPV) of 95% for MC(21). Of 215 randomly selected patients with a SNOMED code indicative of MC, 207 (96%) had diarrhea(21).
Figure 1.
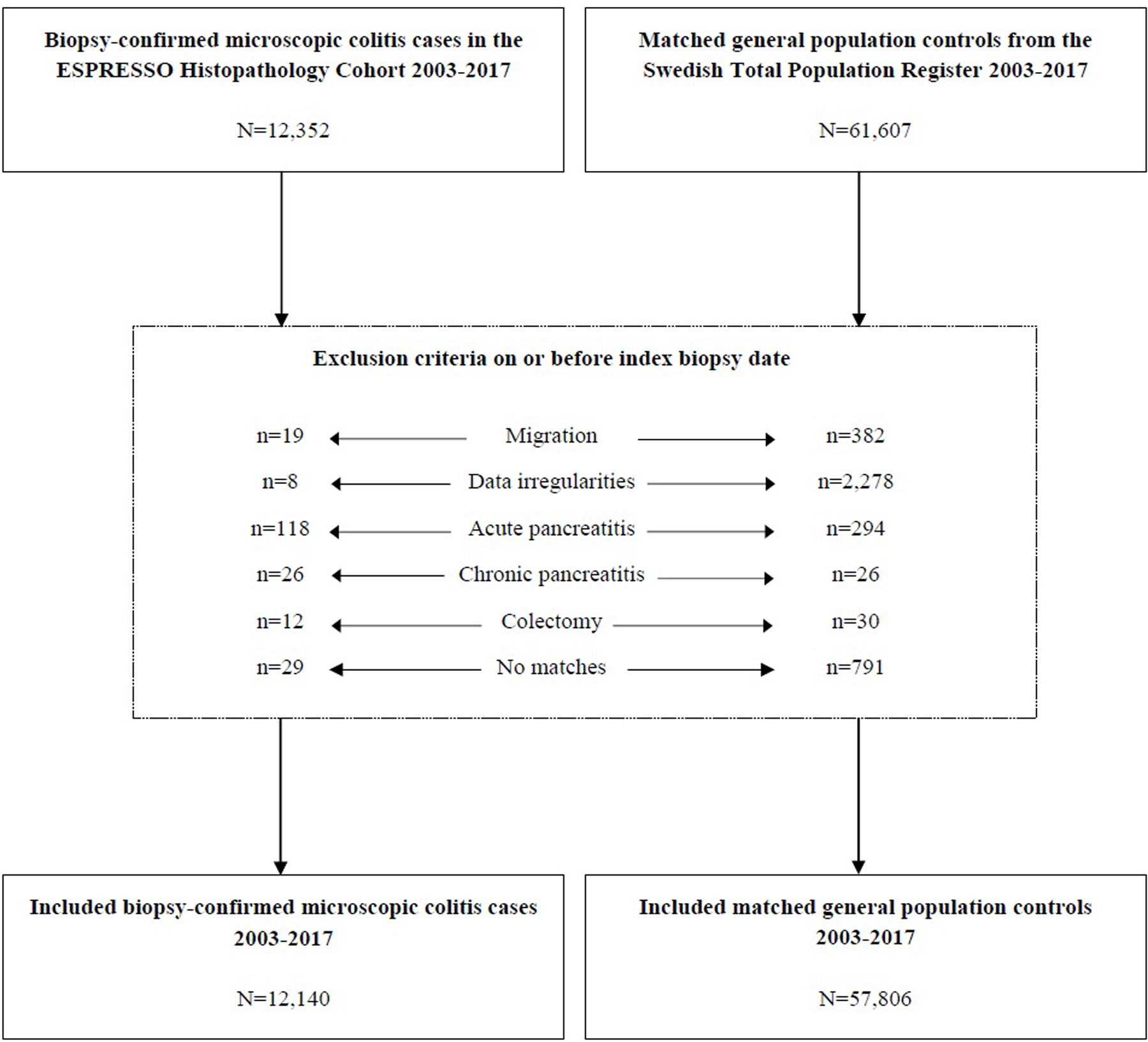
Flowchart of inclusion of patients with biopsy-confirmed microscopic colitis in the ESPRESSO Histopathology Cohort and matched general population controls from the Swedish Total Population Register 2003–2017.
ESPRESSO, Epidemiology Strengthened by Histopathology Reports.
General population reference individuals (comparators)
Because this study was based on data from the ESPRESSO study, each exposed individual (MC) was matched to, ≤5 reference individuals from the general population. Matching was done according to age, sex, county of residence (all at time of diagnosis), and index date (i.e., reference individuals started follow-up on the date of MC diagnosis for their corresponding exposed individual). Reference individuals (with no previous biopsy indicating MC) from the general population were identified through the Total Population Register(22) (Figure 1).
Sibling comparators
Using the Multigeneration Register (part of the Total Population Register(22)), we identified siblings to our exposed population (patients with MC). Access to siblings allowed us to control for intrafamilial confounding (shared genetics and early environmental factors). We identified 12,781 full siblings without MC at index date.
Outcomes
Information on our outcome measure acute pancreatitis (main outcome), acute pancreatitis (gallstone related acute pancreatitis and acute pancreatitis not related to gallstones), was collected from the National Patient Register (NPR). This register stores diagnostic and procedural codes according to the international classification of disease (ICD) system and attained nationwide coverage on inpatient care diagnosis in 1987. Also, data on diagnoses in outpatient care have been available since 2001. The PPV of diagnoses in the NPR is generally approximately 90%(23). The accuracy of ICD codes indicating acute pancreatitis has been assessed in a 2011 validation study(24). The authors reported a PPV of 98% (including definitive and probable cases of acute pancreatitis). We defined incident acute pancreatitis as a first-time record of ICD-codes K850–859. Gallstone-related acute pancreatitis was defined as ICD-code K851 or a procedure code indicating cholecystectomy (listed in the appendix) within 90 days of a diagnosis of acute pancreatitis. Acute pancreatitis not related to gallstones was defined as all acute pancreatitis not considered to be gallstone related. For all outcomes, both primary and secondary diagnostic codes were considered.
Follow-up
Follow-up started on the day of the first biopsy, consistent with MC (for the exposed population) and on the corresponding day for the reference population. To allow access to outpatient data during the study period we commenced follow-up in 2003, using the 2-year time span from 2001–2003 as a washout period for prior diagnosis of acute pancreatitis. Because the ESPRESSO study(20) does not contain data on outcomes beyond 2021, we ended our study period on 31 December 2021. Follow-up ended on the date of acute pancreatitis diagnosis, date of death, date of emigration, or 31 December 2021, whichever occurred first. When examining our secondary outcomes, study participants were not censored if they experienced another outcome than the one under investigation. The end of follow-up also occurred for reference individuals and siblings if they were diagnosed with MC. On the same date, these individuals were reclassified as exposed.
Other covariates
Information on age, sex, county of residence, country of birth, and emigration was collected from the Total Population Register(22). To adjust for socioeconomic status, we gathered data on education level at the time of diagnostic biopsy. This information was retrieved from the longitudinal integrated database for health insurance and labor market studies (LISA)(25). Categories were defined as compulsory school (≤9 years), upper secondary school (10–12 years), and college (≥13 years). If information on the level of education was missing, study participants were placed in a missing category.
Data on celiac disease were collected from the ESPRESSO study by identifying all duodenal biopsies coded (according to the SNOMED system) as M58 (including subgroups) or the celiac disease diagnostic code D6218. A validation study using patient charts revealed that 95% of patients with a duodenal biopsy indicating villous atrophy had CD(26). IBD was identified by defining patients with ≥1 biopsy consistent with IBD and ≥1 ICD code indicating IBD. This approach has been found to have a 95% PPV for identifying IBD(4, 27, 28).
We also extracted information on dispensed budesonide (Anatomical Therapeutical Chemical (ATC) code: A07EA06) and thiopurines (ATC codes: L04AX01 and L01BB02). Moreover, there is a well-documented association between thiopurines and acute pancreatitis(29, 30). Thiopurines may also be prescribed to MC patients that cannot be weaned from steroids, which becomes a potential confounder. Beginning on 1 July 2005, the Prescribed Drug Register stores data on almost 100% of dispensed prescribed medications(31).
Exclusion criteria
Figure 1 outlines exclusions. Exclusion criteria were applied equally for all study participants. All study participants who had died, emigrated, or undergone a colectomy on or before index date were excluded. Also, everyone with a previously recorded ICD-code indicating acute or chronic pancreatitis were omitted.
Sensitivity analyses
To examine the robustness of our result, several sensitivity analyses were conducted. First, to enhance the specificity of our exposure, we conducted a sensitivity analysis where all study participants with a prior dispensation of proton pump inhibitors (PPIs (ATC-code=A02BC)), selective serotonin reuptake inhibitors (SSRIs) (ATC-code=N06AB) and non-steroidal anti-inflammatory drugs (NSAIDs (ATC-code=M01A)) prior to enrollment were excluded. Second, we added dispensed prednisolone (ATC-code=H02AB06) prior to enrollment as an exclusion criterion and dispensed prednisolone after enrollment as a censoring event to control for steroid-induced acute pancreatitis. Third and fourth, restricted cohorts were created to evaluate whether dispensed budesonide or thiopurines influenced our results. All additional analyses including data on dispensed medications from the prescribed drug register included all MC patients and matched reference individuals enrolled after or on 2006–01–01. This lag period of 6 months since the starting date for the prescribed drug register (1 July 2005) was intended to rule out prevalent therapy. Fifth, to enhance specificity of our outcome measure, we carried out an analysis where only the primary diagnostic code was considered. Sixth, in an effort to control for smoking we added adjustment for COPD (defined as an ICD code for COPD and age >40 at the time of the first diagnosis) and finally, to assess the influence of intrafamilial confounding (shared genetics and early environmental factors), we used unexposed siblings to our exposed population as comparators.
Patient and Public Involvement
No patient participated in the planning or design of this study.
Statistical analysis
We conducted a matched cohort study comparing the rate of acute pancreatitis in our exposed population (MC) with our reference population. Using a Kaplan-Meier estimator, we computed unadjusted rates for both groups and calculated absolute rate differences at 0, 10, and 15 years of follow-up. Using a Cox proportional hazard model, we also computed adjusted hazard ratios (aHRs) and 95% confidence intervals (CIs). We confirmed the proportionality assumption by running a Schoenfeld residuals test. To control for a confounding effect by the matching variables (age, sex, county of residence, and year of biopsy) we adjusted for these matching variables(32). Our main model also included adjustment for level of education (as a proxy for socioeconomic status) and comorbidities (IBD and celiac disease, diagnosed before the index date). Employing the same model, we also calculated aHRs for strata defined by sex, calendar period (2003–2010, 2011–2017), calendar period with a maximum of 3 years of follow-up (2003–2010, 2011–2017), age at MC diagnosis (<50 or ≥50 years), years of follow-up (<1, 1-<5, 5-<10, ≥10 years), country of birth (Nordic or other), education level (≤9, 10–12, ≥13 years), and IBD/celiac disease at baseline. To control for intrafamilial confounding, full siblings to patients with MC were used as comparators. This analysis was based on family-specific strata.
Statistical significance of heterogeneity was tested by introducing an interaction term - MC status and the relevant strata - into the main model. A p-value of <0.05 was considered statistically significant.
All statistical analyses were conducted using Stata/IC 14.2 for Mac (StataCorp, 4905 Lakeway Dr, College Station, TX 77845). Forest plot (Figure 2) was created using the metan package(33). To estimate the influence of unmeasured confounding we used the e-value package by Linden et al.(34)
Figure 2.
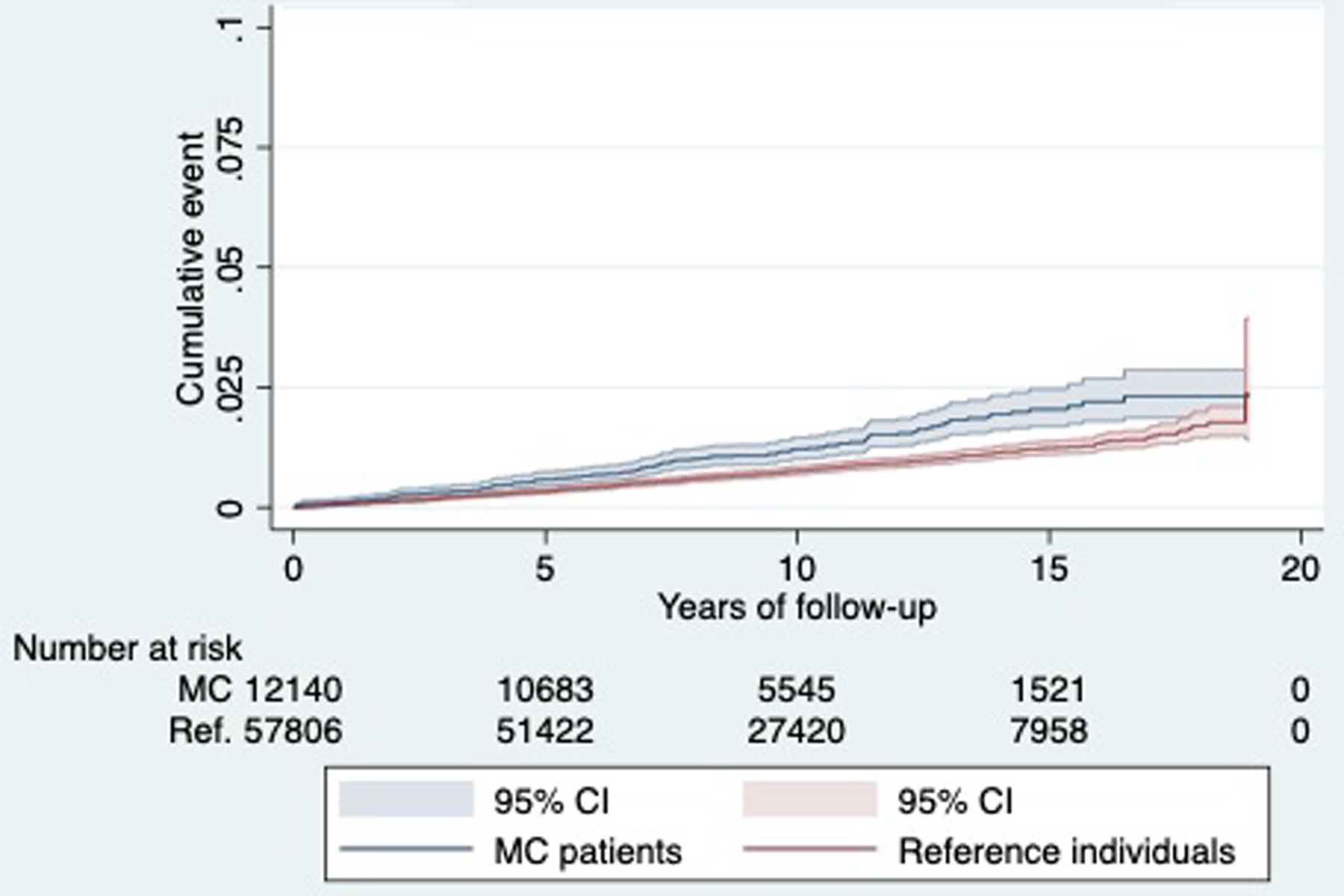
Kaplan-Meier failure estimates
Ethics
This study was approved by the Stockholm Ethics Review Board. Because the study was strictly register-based, ethical approval or informed consent was not required(35).
RESULTS
We identified 12,140 patients with MC, 57,806 matched reference individuals, and 12,781 siblings (Table 1). As expected, most (72%) patients with MC were female. Mean age at diagnosis was 60.2 years (standard deviation (SD)=16.8). LC was the more common subtype, constituting 68% of all patients with an MC diagnosis. Mean length of follow-up was 9.9 years (SD=4.3). Some 89% of our study population were born in a Nordic country, and over 30% had completed ≥13 years of schooling.
Table 1:
Summary statistics for MC patients and matched reference individuals.
| Reference | MC | CC | LC | |
|---|---|---|---|---|
| n [%] | n [%] | n [%] | n [%] | |
| Total | 57,806 [100.00] | 12,140 [100.00] | 3,905 [100.00] | 8,235 [100.00] |
| Male | 16,082 [27.8] | 3,400 [28.0] | 886 [22.7] | 2,514 [30.5] |
| Female | 41,724 [72.2] | 8,740[72.0] | 3,019 [77.3] | 5,721 [69.5] |
| AGE AT START FOLLOW UP | ||||
| Mean [SD] years | 60.2 [16.8] | 60.8 [16.9] | 64.3 [14.8] | 59.2 [17.5] |
| Median [IQR] years | 63.0 [50.0–72.6] | 63.6 [50.7–73.2] | 65.9[55.9–74.9] | 62.2 [48.1–72.3] |
| < 50 years | 14,423 [25.0] | 2,925 [24.1] | 642 [16.4] | 2,283 [27.7] |
| > = 50 years | 43,383 [75.0] | 9,215 [75.9] | 3,263[83.6] | 5,952 [72.3] |
| YEARS OF FOLLOW UP | ||||
| Mean [SD] years | 9.9 [4.3] | 9.7 [4.3] | 9.6 [4.3] | 9.8 [4.3] |
| Median [IQR] years | 9.7 [6.9–13.1] | 9.6 [6.7–12.9] | 9.5 [6.8–12.8] | 9.6 [6.7–12.9] |
| < 1 years | 1,301 [2.3] | 259 [2.1] | 107 [2.7] | 152 [1.9] |
| 1 < 5 years | 5,083 [8.8] | 1,198 [9.9] | 443 [11.3] | 755 [9.2] |
| 5 < 10 years | 24,001 [41.5] | 5,138 [42.3] | 1,597 [40.9] | 3,541 [43.0] |
| > = 10 years | 27,420 [47.4] | 5,545 [45.7] | 1,758 [45.0] | 3,787 [46.0] |
| YEAR OF START FOLLOW UP | ||||
| 2003 – 2010 | 30,115 [52.1] | 6,305 [51.9] | 2,112 [54.1] | 4,193 [50.9] |
| 2011 – 2017 | 27,691 [47.9] | 5,835 [48.1] | 1,793 [45.9] | 4,042 [49.1] |
| REASON FOR END OF FOLLOW-UP | ||||
| Emigration | 731 [1.3] | 80[0.7] | 14 [0.36] | 66 [0.8] |
| 31 Dec. 2021 | 43,667 [75.5] | 8,778 [72.3] | 2,641[67.6] | 6,137 [74.5] |
| Diagnosed with MC | 99 [0.2] | 0 | 0 | 0 |
| Diagnosed with acute pancreatitis | 437 [0.8] | 146 [1.2] | 54 [1.4] | 92 [1.1] |
| Death | 12,872 [22.3] | 3,136 [25.8] | 1,196 [30.6] | 1,940 [23.6] |
| COUNTRY OF BIRTH | ||||
| Nordic | 51,118 [88.4] | 11,392 [93.8] | 3,743 [95.8] | 7,649[92.9] |
| Other | 6,688 [11.6] | 748[6.2] | 162 [4.2] | 586 [7.1] |
| EDUCATION | ||||
| Compulsory school (< = 9 yrs) | 14,382 [24.9] | 3,072 [25.3] | 1,151 [29.5] | 1,921 [23.3] |
| Upper secondary school (10–12 yrs) | 23,778 [41.1] | 4,968 [40.9] | 1,633 [41.8] | 3,335 [40.5] |
| College or university (> = 13 yrs) | 18,143 [31.4] | 3,773 [31.1] | 1,030 [26.4] | 2,743 [33.3] |
| NA | 1,503 [2.6] | 327 [2.7] | 91 [2.3] | 236 [2.9] |
| COMORBIDITY AT DIAGNOSIS DATE | ||||
| IBD | 55 [s0.1] | 336 [2.8] | 112 [2.9] | 224 [2.7] |
| Celiac disease | 39 [0.07] | 415 [3.4] | 136[3.5] | 279 [3.4] |
| MEDICATION DURING FOLLOW-UP* | ||||
| Budesonide | 132 [0.27] | 5,169 [50.1] | 1,896 [57.7] | 3,273 [46.5] |
| Thiopurines | 5 [0.01] | 224 [2.2] | 78 [2.4] | 146 [2.1] |
Restricted cohort including study participants enrolled after or on 2006–01–01
MC and later acute pancreatitis
During follow-up, 151 patients with MC and 437 reference individuals were diagnosed with acute pancreatitis (Table 1). These figures correspond to an incidence rate (IR) of 127.8 (95%CI=109.0–149.9) per 100,000 person-years for the exposed population and 80.1 (95%CI=73.1–85.6) for the reference population (Table 2). Figure 2 depicts the crude incidence rates for the exposed population (MC) and reference individuals. Average time until acute pancreatitis were comparable for patients with MC and reference individuals, 6.2 years, and 6.5 years respectively.
Table 2:
Incidence rates (acute pancreatitis) for MC patients diagnosed in Sweden 2003–2017 and for matched reference individuals
| Reference | MC | CC | LC | |
|---|---|---|---|---|
| N Total | 57,806 | 12,140 | 3,905 | 8,235 |
| N events | 437 | 151 | 55 | 96 |
| Incidence proportion [%] | 0.8 | 1.2 | 1.4 | 1.2 |
| Person years | 569,367 | 118,160 | 37,589 | 80,571 |
| Incidence rate/100,000 pyears [95% CI] | 80.1 [73.1–85.6] | 127.8 [109.0–149.9] | 146.3 [112.3–190.6] | 119.1 [97.5–145.5] |
| SEX | ||||
| Males | 98.2 [83.7–115.3] | 158.7 [120.3–209.4] | 174.9 [103.6–295.2] | 153.2 [110.5–212.4] |
| Females | 73.4 [65.7–82.1] | 116.6 [95.9–141.7] | 138.6 [102.1–188.2] | 105.1 [81.6–135.4] |
| AGE AT START FOLLOW UP | ||||
| < 50 years | 46.4 [36.9–58.3] | 79.9 [54.4–117.3] | 110.8 [55.4–221.6] | 71.1 [44.8–112.8] |
| ≥ 50 years | 93.2 [84.3–103.0] | 146.0 [122.5–174.0] | 154.8 [116.3–206.0] | 141.2 [113.1–176.3] |
| YEARS OF FOLLOW UP | ||||
| < 1 | 64.0 [46.2–88.8] | 108.3 [62.9–186.6] | 104.0 [39.0–277.0] | 110.4 [57.4–212.1] |
| 1 < 5 | 68.5 [58.3–80.6] | 119.2 [91.3–155.7] | 146.2 [95.3–224.2] | 106.7 [75.9–150.1] |
| 5 < 10 | 87.9 [75.8–102.0] | 126.3 [96.3–165.8] | 136.4 [85.9–216.4] | 121.6 [86.9–170.2] |
| > = 10 | 100.6 [82.7–122.4] | 162.4 [114.8–229.6] | 194.2 [110.3–342.0] | 147.8 [95.4–229.2] |
| START OF FOLLOW UP | ||||
| 2003 – 2010 | 81.6 [72.8–91.5] | 116.7 [94.6–144.0] | 161.7 [118.2–221.4] | 95.2 [71.8–126.3] |
| 2011 – 2017 | 77.4 [66.4–90.4] | 146.7 [114.8–187.4] | 118.7 [72.7–193.8] | 159.2 [119.9–211.2] |
| START OF FOLLOW UP (with max 3 years of follow-up) |
||||
| 2003 – 2010 | 67.2 [52.0–87.0] | 76.9 [45.5–129.8] | 82.8 [34.5–198.9] | 73.9 [38.5–142.1] |
| 2011 – 2017 | 67.5 [51.7–88.2] | 135.3 [89.9–203.7] | 77.0 [28.9–205.2] | 161.0 [102.7–252.5] |
| COUNTRY OF BIRTH | ||||
| Nordic | 78.1 [70.7–86.2] | 121.0 [63.0–232.6] | 150.1 [115.0–196.0] | 117.7 [95.5–145.1] |
| Other | 95.8 [74.7–122.8] | 128.2 [108.8–151.2] | 61.9 [8.7–439.1] | 137.5 [68.7–274.9] |
| EDUCATION | ||||
| Compulsory school (< = 9 yrs) | 108.8 [92.6–127.8] | 178.1 [135.0–235.0] | 192.3 [124.1–298.1] | 169.7 [118.7–242.7] |
| Upper secondary school (10–12 yrs) | 77.0 [66.6–89.0] | 122.9 [95.6–158.0] | 159.4 [108.6–234.2] | 105.0 [75.4–146.3] |
| College or university (> = 13 yrs) | 63.8 [53.1–76.5] | 83.1 [58.8–117.5] | 75.9 [37.9–151.7] | 85.8 [57.5–128.0] |
| NA | 64.4 [33.5–123.7] | 410.2 [205.1–820.2] | 294.6 [41.5–2091.2] | 434.6 [207.2–911.5] |
| COMORBIDITY | ||||
| with IBD | NA [NA-NA] * | 115.5 [43.3–307.7] | 86.1 [12.1–611.0] | 130.4 [42.0–404.2] |
| without IBD | 80.1 [73.1–87.8] | 128.2 [109.0–150.7] | 148.2 [113.5–193.6] | 118.8 [97.0–145.6] |
| with celiac disease | 602.1 [150.6–2407.4] | 116.1 [79.2–348.4] | 69.1 [9.7–490.0] | 216.9 [97.5–482.8] |
| without celiac disease | 79.8 [72.8–87.5] | 126.4 [107.3–148.8] | 149.4 [114.4–195.1] | 115.7 [94.1–142.2] |
No events
Siblings without MC had an IR of 85.3 (95%CI=71.0–102.5) per 100,000 person-years compared to 112.3 (95%CI=89.8–140.4) in their siblings with MC.
Our Cox proportional hazards model adjusted for the matching variables (age at start of follow-up, sex, county, calendar year), education level, and comorbidities at baseline (IBD and celiac disease). Compared to the reference population, MC patients were 1.6 times more likely to be diagnosed with acute pancreatitis during follow-up (aHR=1.57, 95%CI=1.30–1.90) (Table 3). Stratifying by sex, the aHR was 1.50 (95%CI=1.07–2.10) for males and 1.62 (95%CI=1.29–2.03) for females. Examining CC and LC separately, the aHR was 1.67 (95% CI=1.22–2.28) for CC and 1.53 (95%CI=1.21–1.94) for LC (Table 3).
Table 3: Acute pancreatitis hazard ratios for MC patients diagnosed in Sweden 2003–2017 compared to matched reference individuals.
Adjusted for age, sex, county, calendar period, level of education, IBD and celiac disease
| MC | CC | LC | |
|---|---|---|---|
| HR [95% CI] | HR [95% CI] | HR [95% CI] | |
| Total | 1.57 [1.30–1.90] | 1.67 [1.22–2.28] | 1.53 [1.21–1.94] |
| SEX | |||
| Males | 1.50 [1.07–2.10] | 1.69 [0.89–3.21] | 1.43 [0.97–2.13] |
| Females | 1.62 [1.29–2.03] | 1.66 [1.16–2.37] | 1.60 [1.19–2.16] |
| AGE AT START FOLLOW UP | |||
| < 50 years | 1.58 [0.98–2.55] | 1.65 [0.75–3.63] | 1.56 [0.85–2.84] |
| ≥ 50 years | 1.57 [1.28–1.94] | 1.69 [1.21–2.38] | 1.52 [1.17–1.97] |
| YEARS OF FOLLOW UP | |||
| < 1 | 1.82 [0.96–3.43] | 1.42 [0.47–4.33] | 2.07 [0.95–4.50] |
| 1 < 5 | 1.71 [1.24–2.36] | 1.78 [1.07–2.97] | 1.68 [1.12–2.54] |
| 5 < 10 | 1.33 [0.96–1.85] | 1.39 [0.80–2.42] | 1.31 [0.87–1.96] |
| > = 10 | 1.75 [1.14–2.67] | 2.38 [1.17–4.82] | 1.49 [0.88–2.54] |
| START OF FOLLOW UP | |||
| 2003 – 2010 | 1.38 [1.08–1.77] | 1.74 [1.20–2.51] | 1.18 [0.85–1.66] |
| 2011 – 2017 | 1.92 [1.43–2.57] | 1.53 [0.86–42.73] | 2.08 [1.48–2.93] |
| START OF FOLLOW UP (with max 3 years of follow-up) |
|||
| 2003 – 2010 | 1.04 [0.56–1.92] | 0.83 [0.29–2.39] | 1.19 [0.55–2.56] |
| 2011 – 2017 | 1.94 [1.18–3.20] | 1.04 [0.35– 3.07] | 2.44 [1.38–4.33] |
| COUNTRY OF BIRTH | |||
| Nordic | 1.62 [1.33–1.98] | 1.85 [1.34–2.55] | 1.52 [1.18–1.96] |
| Other | 2.32 [0.50–10.8] | NA [NA-NA]** | 3.54 [0.61–20.3] |
| EDUCATION | |||
| Compulsory school (< = 9 yrs) | 1.66 [1.19–2.30] | 1.65 [0.98–2.77] | 1.68 [1.10–2.56] |
| Upper secondary school (10–12 yrs) | 1.64 [1.23–2.21] | 2.07 [1.31–3.26] | 1.42 [0.97–2.09] |
| College or university (> = 13 yrs) | 1.20 [0.79–1.80] | 0.92 [0.39–2.13] | 1.29 [0.80–2.07] |
| NA | 5.71 [1.99–16.6] | 3.34 [0.33–33.6] | 10.62 [2.69–41.9] |
| COMORBIDITY | |||
| with IBD* | NA [NA-NA] | NA [NA-NA]* | NA [NA-NA]* |
| without IBD | 1.57 [1.30–1.90] | 1.65 [1.21–2.26] | 1.53 [1.21–1.94] |
| with celiac disease* | NA [NA-NA] | NA [NA-NA]* | NA [NA-NA]* |
| without celiac disease | 1.59 [1.32–1.93] | 1.70 [1.25–2.32] | 1.55 [1.22–1.96] |
No events
Only event in reference individuals
Defining age categories as below or above age 50, we found similar aHRs for both groups (Table 3). Examining the association between MC and acute pancreatitis across different calendar periods (2003–2010, 2011–2017), we found a higher aHR (1.92, 95%CI=1.43–2.57) during the past decade compared to the aHR of the earlier calendar period (2003–2010: 1.38, 95%CI=1.08–1.77)). In a separate analysis we restricted follow-up to 3 years to make the different calendar periods more comparable. Again, the same pattern emerged with a higher aHR (1.94, 95%CI=1.18–3.20)) during the most recent calendar period, 2011–2017. The aHR for the earlier calendar period was 1.04 (95%CI=0.56–1.92). The aHR was highest during the first year of follow-up (1.82, 95%CI=0.96–3.43) when stratifying on years of follow-up. However, MC patients followed for ≥10 years had an almost identical aHR (1.75, 95%CI=1.14–2.67). (Table 3). Excluding all study participants with a follow up less than one year did not materially change our point estimate, aHR (1.56, 95%CI=1.28–1.90)) remained unchanged. Stratifying by educational attainment returned similar aHRs across levels (Table 3).
Secondary outcomes
Classifying acute pancreatitis as gallstone related or gallstone unrelated, aHRs differed with a significant increase for gallstone unrelated acute pancreatitis (n(MC)=106, n(reference population)=250), (aHR=1.99 (95%CI=1.57–2.51)); no association was detected for gallstone related acute pancreatitis (n(MC)=45), n(reference population)=206) (aHR=1.08 (95%CI=0.78–1.49). Table 4 outlines IRs and aHRs for our secondary outcomes.
Table 4:
INCIDENCE RATES AND ADJUSTED HAZARD RATIOS (ADJUSTED FOR AGE, SEX, COUNTY, CALENDAR PERIOD, LEVEL OF EDUCATION, IBD AND CELIAC DISEASE) FOR SECONDARY OUTCOMES FOR MC PATIENTS DIAGNOSED IN SWEDEN 2003–2017 AND FOR THEIR MATCHED REFERENCE INDIVIDUALS.
| ACUTE GALLSTONE RELATED PANCREATITIS | ||||
|---|---|---|---|---|
| Reference | MC | CC | LC | |
| N TOTAL | 57,806 | 12,140 | 3,905 | 8,235 |
| N EVENTS | 206 | 45 | 17 | 28 |
| INCIDENCE PROPORTION [%] | 0.4 | 0.4 | 0.4 | 0.3 |
| PERSON YEARS | 570,323 | 118,617 | 37,738 | 80.881 |
| INCIDENCE RATE/100,000 PYEARS [95% CI] | 36.1 [31.5–41.4] | 37.9 [28.3–50.8] | 45.0 [28.0–72.5] | 34.6 [23.9–50.1] |
| ADJUSTED HAZARD RATIO [95% CI] | 1.0 [ref] | 1.08 [0.78–1.49] | 1.09 [0.64–1.85] | 1.08 [0.71–1.63] |
|
| ||||
| ACUTE NON-GALLSTONE RELATED PANCREATITIS | ||||
| Reference | MC | CC | LC | |
| N TOTAL | 57,806 | 12,140 | 3,905 | 8,235 |
| N EVENTS | 250 | 106 | 38 | 68 |
| INCIDENCE PROPORTION [%] | 0.4 | 0.9 | 1.0 | 0.8 |
| PERSON YEARS | 570,378 | 118,357 | 37,672 | 80,685 |
| INCIDENCE RATE/100,000 PYEARS [95% CI] | 43.8 [38.7–49.6] | 89.6 [74.0–108.3] | 100.9 [73.4–138.6] | 84.3 [66.4–106.9] |
| ADJUSTED HAZARD RATIO [95%CI) | 1.0 [ref] | 1.99 [1.57–2.51] | 2.21 [1.49–3.26] | 1.88 [1.40–2.52] |
Sensitivity analysis
To assess the robustness of our observed association, several sensitivity analyses were performed. First, we excluded all study subjects with a prior dispensation of PPIs, NSAIDs or SSRIs. These added exclusions left our estimate virtually unchanged, aHR 1.58 (95%CI=1.28–1.95). Second, omitting all study participants with a prior dispensation of prednisolone and adding dispensation of prednisolone as a censoring event yielded a somewhat lower aHR of 1.33 (95%CI=1.02–1.72). Third, stratifying by use or non-use of budesonide, we found an aHR of 1.84 (95%CI=1.37–2.48) for budesonide users compared to their matched reference individuals. Corresponding figures for MC patients not treated with budesonide were 1.31 (95%CI=0.95–1.80), p for heterogeneity=0.16. Fourth, we performed a similar analysis examining whether the association between MC and acute pancreatitis differed in patients treated with thiopurines. In total, 224 MC patients had been treated with thiopurines. In this restricted cohort five MC patients and one reference individual later developed acute pancreatitis. These figures corresponded to an aHR of 42.1 (95%CI=3.44–514.4). Naturally, the aHR (1.53, 95%CI=1.24–1.90) for MC patients not treated with thiopurines resembled our main result. Fifth, we investigated the association between MC and acute pancreatitis using only the primary diagnostic code. This restriction, however, had no substantial effect on our our point estimate, aHR 1.59 (95%CI=1.30–1.96). Sixth, to control for intrafamilial confounding we used unaffected siblings as comparators, estimating an aHR of 1.28 (95%CI=0.92–1.78). Finally, in an effort to control for smoking we added adjustment for chronic obstructive pulmonary disease. This additional adjustment, however, did not materially change our results. The aHR for acute pancreatitis in this model was 1.57 (95%CI=1.30–1.90). For non-gallstone acute pancreatitis, the aHR was 1.97 (95%CI=1.56–2.49) when adding adjustment for COPD. Moreover, to estimate the influence of unmeasured confounding, we calculated the e-value for the association between MC and acute pancreatitis finding that the strength of an unmeasured confounder (e.g., smoking) would have to be 2.5-fold to both MC and acute pancreatitis to reduce our observed aHR of 1.57 to 1. To shift the lower limit of the CI below 1, the strength of the association between the unmeasured confounder and the exposure and outcome would have to be 1.93-fold.
Discussion
In this nationwide, matched cohort study of >12,000 patients with MC we found a 1.6-fold increased risk of later acute pancreatitis (95%CI=1.30–1.90). Stratifying on disease duration, we found the highest aHRs for MC patients followed for <1 year and those followed for >10 years. The elevated aHR during the first year of follow-up is likely, partly, explained by surveillance bias. However, when excluding all MC patients diagnosed with acute pancreatitis within 1 year of MC diagnosis, the aHR was still significantly elevated.
Regarding our secondary outcomes, we found an increased risk of acute pancreatitis unrelated to gallstones (aHR=1.99, 95%CI=1.57–2.51), whereas no association was detected for gallstone-related acute pancreatitis (aHR=1.08, 95%CI=0.78–1.49). Our sensitivity analyses strengthened the robustness of our main result, indicating that our observed association, to some extent, is independent of the effects of dispensed medications (e.g., PPIs, SSRIs, NSAIDs, prednisolone, thiopurines) and intrafamilial confounding.
As far as we know, no studies on the association between MC and acute pancreatitis exist. However, there are reports on the relationship between IBD(9, 10) and acute pancreatitis and celiac disease(8) and acute pancreatitis. A nationwide study from Denmark(9) examining the risk of acute pancreatitis in IBD found increased standardized incidence ratios (SIRs) for Crohn’s disease (SIR=4.3, 95% CI=2.9–6.1)) and ulcerative colitis (SIR=2.1, 95% CI=1.6–2.8)), whereas a Swedish matched cohort study investigating the risk of pancreatitis in patients with celiac disease(8) found an HR of 2.85 (95% CI=2.53–3.21). There is also a known association between autoimmune pancreatitis (AIP) type2 and IBD; some 15% of patients with AIP type2 are estimated to have a concomitant diagnosis of IBD(36). A 2019 study(37), outlining holistic prevention strategies for pancreatitis stressed the importance of smoking cessation, healthy foods as well as a cautious use of endoscopic retrograde cholangiopancreatography (ERCP) and drugs known to induce acute pancreatitis. Hence, awareness of our observed association may benefit patients with MC.
Several plausible mechanisms may be involved in the observed association between MC and acute pancreatitis. First, although the heterogeneity between aHRs for MC patients treated and untreated with budesonide did not attain statistical significance, we noted a tendency towards a higher risk for acute pancreatitis in MC patients on budesonide. This trend may suggest that acute pancreatitis in MC patients is related to disease intensity and/or is iatrogenically caused. The Swedish environmental classification of pharmaceuticals (FASS) lists pancreatitis as a side effect of budesonide, and the risk of steroid-induced acute pancreatitis is well documented(38). To an extent dispensed thiopurines(29) also contributed to our findings. MC patients treated with thiopurines were more prone to develop acute pancreatitis (aHR=42.1 (95%CI=3.44–514.4)) (we urge caution in interpreting this result as it was based on few events). Nevertheless, our result remained essentially unchanged when excluding MC patients with a record of thiopurine treatment. The association with thiopurine, however, corroborates the known association with acute pancreatitis(29).
Second, the inflammatory activity in MC and acute pancreatitis are both characterized by cytokines involved in the Th1/Th17 pathways (13, 14) and there is evidence that IL-6, which is upregulated in active CC(13) also correlates to severity of acute pancreatitis(39). In addition, there are findings from animal-studies indicating that elevated levels of IL-6 increase the susceptibility to acute pancreatitis(40). Third, smoking is associated to both MC(41) and acute pancreatitis(42) and may explain part of our finding. Finally, we found no association between MC and gallstone-related disease, further strengthening the notion that MC and pancreatitis might share environmental or genetic risk factors. This finding is likely explained by the fact that a gallstone wedged in the papilla Vateri suffices to trigger acute pancreatitis regardless of concomitant MC.
One strength of this study is the nationwide coverage, which minimizes selection bias and makes for a sizeable cohort, allowing for precise calculations of relative risks across various strata. Another strength is that the Swedish PIN enabled us to capture our study population’s outcomes and exposures of interest. Moreover, our exposure information (biopsy verified MC) was assessed in a validation study, finding a PPV of 95%(43). The accuracy of an ICD code for acute pancreatitis in Sweden has also been examined. Based on a random selection of patients with a primary or secondary diagnosis of acute pancreatitis under inpatient care, the authors reported a PPV of 98%(24). Access to the Prescribed Drug Register permitted us to perform several sensitivity analyses incorporating information on dispensed medication.
We acknowledge some limitations. The registers used for this study lack information on important lifestyle factors such as body mass index (BMI), alcohol and smoking. However, because MC is associated with lean BMI(44) and acute pancreatitis is associated with higher BMI(45), added data on BMI would likely have strengthened our observed association. Moreover, a strong link has been found between alcohol use and pancreatitis(6). However, studies focusing on the relationship between MC and alcohol intake have reported contradictory results ranging from a positive correlation(46) to no association(47, 48). Notably, one of the studies (48) showing no association was performed on a Swedish cohort. Thus, whether alcohol use plays an integral part in the causal pathway is difficult to determine based on à priori knowledge. Smoking is more prevalent among patients with MC(41) and is therefore an important confounder. Our attempt to control for smoking did not have a meaningful impact on our main result. Nor did it notably change our aHR for non-gallstone-related acute pancreatitis, which is strongly linked to smoking(49). However, it should be noted that only a minority of smokers develop COPD and/or receive an ICD-code for the disease. Nevertheless, our calculated e-value suggests our observed association, to some degree, is independent of unmeasured confounding. In addition, as our data only contained information on incident MC, we were unable to contrast the risk of acute pancreatitis in patients with active disease to that of the patients who had been brought to clinical remission.
Furthermore, surveillance bias may partly explain the observed association, as patients with MC generally are followed by a gastroenterologist providing MC patients better access to gastroenterological evaluation and testing for amylase in the event of an episode with acute abdominal pain. Thus, the probability of identifying acute pancreatitis with a less severe presentation increases. Our study was conducted based on Swedish data. We caution that because of genetic dissimilarities and differing exposures to alcohol, tobacco, and other substances, as well as occupational and other factors, our findings cannot unquestionably be extrapolated to other countries.
In conclusion, we found an increased probability of MC patients developing acute pancreatitis. This elevated probability remained after >10 years of follow-up. Our findings suggest clinicians should have a low threshold for evaluating for acute pancreatitis in patients with MC. Our finding of a significantly increased probability of acute pancreatitis unrelated to gallstones motivates further studies on the biologic mechanisms involved in the observed association. Also, our finding stresses the importance of patients with MC being subject to advice and care aimed at avoiding development of acute pancreatitis.
Supplementary Material
Figure 3:
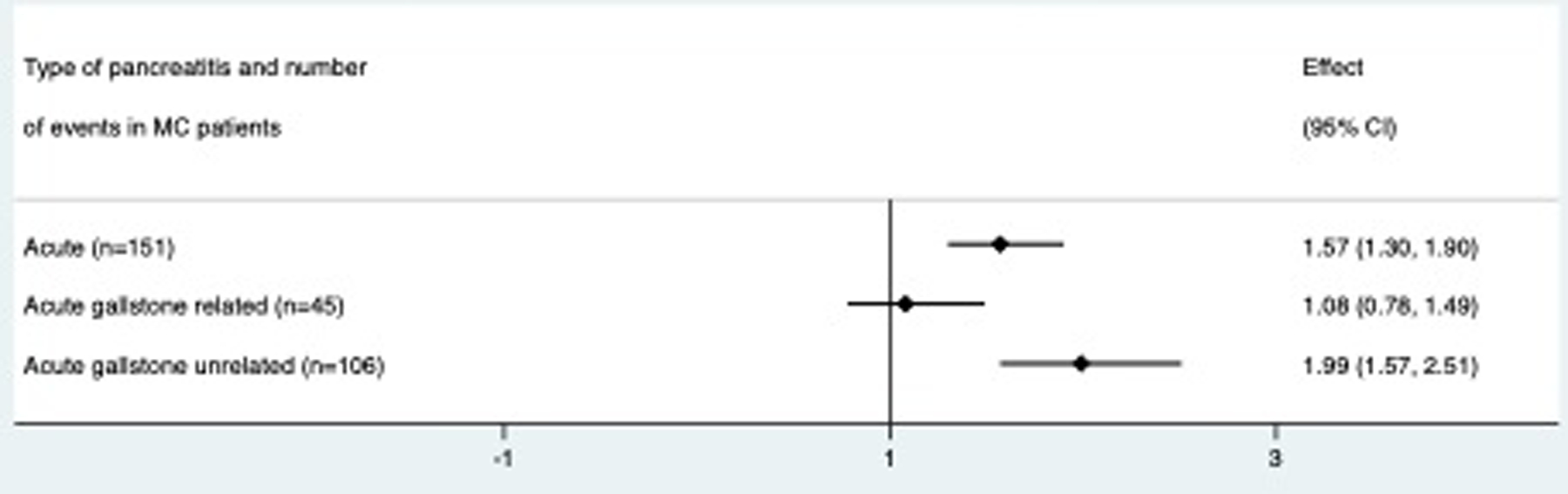
Forest plot of main and secondary outcomes
Study highlights:
What is known:
The association between microscopic colitis and acute pancreatitis has not yet been examined.
What is new here:
We found a significantly increased risk for acute pancreatitis in patients with microscopic colitis compared to reference individuals.
Specifically, we found an increased risk for acute pancreatitis not related to gallstones.
Our findings indicate that the increased risk for acute pancreatitis may be related to disease severity or budesonide.
Grant support:
This work was supported by the Karolinska Institutet (Ludvigsson), Stockholm County Council (Ludvigsson), and the NIH (National Institutes of Health NIA R01 (AG068390; Ludvigsson).
None of the funding organizations has had any role in the design and conduct of the study, in the collection, management, and analysis of the data, or in the preparation, review, and approval of the manuscript.
Abbreviations used in this article:
- MC
Microscopic colitis
- LC
lymphocytic colitis
- CC
collagenous colitis
- CD
celiac disease
- IBD
inflammatory bowel disease
- PIN
personal identity number
- SNOMED
Systematized Nomenclature of Medicine
- PPV
positive predictive value
- NPR
National Patient Register
- aHR
adjusted hazard ratio
- CI
confidence interval
- SD
standard deviation
- IR
incidence rate
- aOR
adjusted odds ratio
- COPD
chronic obstructive pulmonary disease
- SIR
standardized incidence ratio
- AIP
autoimmune pancreatitis
- BMI
body mass index
Footnotes
Disclosures:
Dr. Ludvigsson coordinates a study on behalf of the Swedish IBD quality register (SWIBREG). That study has received funding from the Janssen corporation. Dr Ludvigsson has also received financial support from MSD to develop a paper reviewing national healthcare registers in China.
Dr Olén has been PI on projects at Karolinska Institutet financed by grants from Janssen, Pfizer, AbbVie, Takeda, and Ferring, and Karolinska Institutet has received fees for lectures and participation on advisory boards from Janssen, Ferring, Galapagos, Bristol Myer Squibb, Takeda, and Pfizer.
Dr Olén also reports grants from Pfizer, Galapagos, and Janssen in the context of national safety monitoring programs.
Supplemental data: This manuscript contains supplementary data.
Details of ethics approval: This study was approved by the Regional Ethics Committee, Stockholm, Sweden (Protocol no 2014/1287–31/4 and 2018/972–32).
Data transparency statement:
In accordance with Swedish regulations, the data from this study are not publicly available.
References
- 1.Burke KE, D’Amato M, Ng SC, Pardi DS, Ludvigsson JF, Khalili H. Microscopic colitis. Nat Rev Dis Primers 2021;7(1):39. [DOI] [PubMed] [Google Scholar]
- 2.Mellander MR, Ekbom A, Hultcrantz R, Lofberg R, Ost A, Bjork J. Microscopic colitis: a descriptive clinical cohort study of 795 patients with collagenous and lymphocytic colitis. Scand J Gastroenterol 2016;51(5):556–62. [DOI] [PubMed] [Google Scholar]
- 3.Bergman D, Clements MS, Khalili H, Agreus L, Hultcrantz R, Ludvigsson JF. A nationwide cohort study of the incidence of microscopic colitis in Sweden. Aliment Pharmacol Ther 2019;49(11):1395–400. [DOI] [PubMed] [Google Scholar]
- 4.Forss A, Clements M, Bergman D, Roelstraete B, Kaplan GG, Myrelid P, et al. A nationwide cohort study of the incidence of inflammatory bowel disease in Sweden from 1990 to 2014. Aliment Pharmacol Ther 2022;55(6):691–9. [DOI] [PubMed] [Google Scholar]
- 5.Weimers P, Ankersen DV, Lophaven S, Bonderup OK, Münch A, Løkkegaard ECL, et al. Incidence and prevalence of microscopic colitis between 2001 and 2016: A Danish nationwide cohort study. J Crohns Colitis 2020. [DOI] [PubMed]
- 6.Kleeff J, Whitcomb DC, Shimosegawa T, Esposito I, Lerch MM, Gress T, et al. Chronic pancreatitis. Nat Rev Dis Primers 2017;3:17060. [DOI] [PubMed] [Google Scholar]
- 7.Attasaranya S, Fogel EL, Lehman GA. Choledocholithiasis, ascending cholangitis, and gallstone pancreatitis. Med Clin North Am 2008;92(4):925–60, x. [DOI] [PubMed] [Google Scholar]
- 8.Sadr-Azodi O, Sanders DS, Murray JA, Ludvigsson JF. Patients with celiac disease have an increased risk for pancreatitis. Clin Gastroenterol Hepatol 2012;10(10):1136–42.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rasmussen HH, Fonager K, Sørensen HT, Pedersen L, Dahlerup JF, Steffensen FH. Risk of acute pancreatitis in patients with chronic inflammatory bowel disease. A Danish 16-year nationwide follow-up study. Scand J Gastroenterol 1999;34(2):199–201. [DOI] [PubMed] [Google Scholar]
- 10.Chen YT, Su JS, Tseng CW, Chen CC, Lin CL, Kao CH. Inflammatory bowel disease on the risk of acute pancreatitis: A population-based cohort study. J Gastroenterol Hepatol 2016;31(4):782–7. [DOI] [PubMed] [Google Scholar]
- 11.Singh VK, Bollen TL, Wu BU, Repas K, Maurer R, Yu S, et al. An assessment of the severity of interstitial pancreatitis. Clin Gastroenterol Hepatol 2011;9(12):1098–103. [DOI] [PubMed] [Google Scholar]
- 12.Petrov MS, Shanbhag S, Chakraborty M, Phillips AR, Windsor JA. Organ failure and infection of pancreatic necrosis as determinants of mortality in patients with acute pancreatitis. Gastroenterology 2010;139(3):813–20. [DOI] [PubMed] [Google Scholar]
- 13.Kumawat AK, Strid H, Tysk C, Bohr J, Hörnquist EH. Microscopic colitis patients demonstrate a mixed Th17/Tc17 and Th1/Tc1 mucosal cytokine profile. Mol Immunol 2013;55(3–4):355–64. [DOI] [PubMed] [Google Scholar]
- 14.Pietruczuk M, Dabrowska MI, Wereszczynska-Siemiatkowska U, Dabrowski A. Alteration of peripheral blood lymphocyte subsets in acute pancreatitis. World J Gastroenterol 2006;12(33):5344–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bergman D, Khalili H, Roelstraete B, Ludvigsson JF. Microscopic colitis and risk of cancer - a population-based cohort study. J Crohns Colitis 2020. [DOI] [PubMed]
- 16.Bergman D, Khalili H, Lebwohl B, Roelstraete B, Green PHR, Ludvigsson JF. Celiac disease and risk of microscopic colitis: A nationwide population-based matched cohort study. United European Gastroenterol J 2023;11(2):189–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Khalili H, Burke KE, Roelstraete B, Sachs MC, Olen O, Ludvigsson JF. Microscopic Colitis and Risk of Inflammatory Bowel Disease in a Nationwide Cohort Study. Gastroenterology 2020;158(6):1574–83 e2. [DOI] [PubMed] [Google Scholar]
- 18.Fousekis FS, Theopistos VI, Katsanos KH, Christodoulou DK. Pancreatic Involvement in Inflammatory Bowel Disease: A Review. J Clin Med Res 2018;10(10):743–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ludvigsson JF, Otterblad-Olausson P, Pettersson BU, Ekbom A. The Swedish personal identity number: possibilities and pitfalls in healthcare and medical research. European journal of epidemiology 2009;24(11):659–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ludvigsson JF, Lashkariani M. Cohort profile: ESPRESSO (Epidemiology Strengthened by histoPathology Reports in Sweden). Clin Epidemiol 2019;11:101–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Svensson M, Bergman D, Olen O, Myrelid P, Bohr J, Wickbom A, et al. Validating microscopic colitis (MC) in Swedish pathology registers. Scand J Gastroenterol 2019:1–7. [DOI] [PubMed]
- 22.Ludvigsson JF, Almqvist C, Bonamy AK, Ljung R, Michaelsson K, Neovius M, et al. Registers of the Swedish total population and their use in medical research. Eur J Epidemiol 2016;31(2):125–36. [DOI] [PubMed] [Google Scholar]
- 23.Ludvigsson JF, Andersson E, Ekbom A, Feychting M, Kim JL, Reuterwall C, et al. External review and validation of the Swedish national inpatient register. BMC Public Health 2011;11:450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Razavi D, Ljung R, Lu Y, Andrén-Sandberg A, Lindblad M. Reliability of acute pancreatitis diagnosis coding in a National Patient Register: a validation study in Sweden. Pancreatology 2011;11(5):525–32. [DOI] [PubMed] [Google Scholar]
- 25.Ludvigsson JF, Svedberg P, Olen O, Bruze G, Neovius M. The longitudinal integrated database for health insurance and labour market studies (LISA) and its use in medical research. Eur J Epidemiol 2019;34(4):423–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ludvigsson JF, Brandt L, Montgomery SM, Granath F, Ekbom A. Validation study of villous atrophy and small intestinal inflammation in Swedish biopsy registers. BMC Gastroenterol 2009;9:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nguyen LH, Örtqvist AK, Cao Y, Simon TG, Roelstraete B, Song M, et al. Antibiotic use and the development of inflammatory bowel disease: a national case-control study in Sweden. Lancet Gastroenterol Hepatol 2020;5(11):986–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mouratidou N, Malmborg P, Järås J, Sigurdsson V, Sandström O, Fagerberg UL, et al. Identification of Childhood-Onset Inflammatory Bowel Disease in Swedish Healthcare Registers: A Validation Study. Clin Epidemiol 2022;14:591–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ramos LR, Sachar DB, DiMaio CJ, Colombel JF, Torres J. Inflammatory Bowel Disease and Pancreatitis: A Review. J Crohns Colitis 2016;10(1):95–104. [DOI] [PubMed] [Google Scholar]
- 30.Wintzell V, Svanström H, Olén O, Melbye M, Ludvigsson JF, Pasternak B. Association between use of azathioprine and risk of acute pancreatitis in children with inflammatory bowel disease: a Swedish-Danish nationwide cohort study. Lancet Child Adolesc Health 2019;3(3):158–65. [DOI] [PubMed] [Google Scholar]
- 31.Wettermark B, Hammar N, Fored CM, Leimanis A, Otterblad Olausson P, Bergman U, et al. The new Swedish Prescribed Drug Register--opportunities for pharmacoepidemiological research and experience from the first six months. Pharmacoepidemiol Drug Saf 2007;16(7):726–35. [DOI] [PubMed] [Google Scholar]
- 32.Sjolander A, Greenland S. Ignoring the matching variables in cohort studies - when is it valid and why? Stat Med 2013;32(27):4696–708. [DOI] [PubMed] [Google Scholar]
- 33.Harris R, Bradburn M, Deeks J, Harbord R, Altman D, Sterne J. metan: fixed- and random-effects meta-analysis. Stata Journal 2008;8(1):3–28. [Google Scholar]
- 34.Linden AMM, VanderWeele TJ. Conducting sensitivity analysis for unmeasured confounding in observational studies using E-values: the evalue package. Stata J 2020;20(1):162–75. [Google Scholar]
- 35.Ludvigsson JF, Haberg SE, Knudsen GP, Lafolie P, Zoega H, Sarkkola C, et al. Ethical aspects of registry-based research in the Nordic countries. Clin Epidemiol 2015;7:491–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kamisawa T, Chari ST, Giday SA, Kim MH, Chung JB, Lee KT, et al. Clinical profile of autoimmune pancreatitis and its histological subtypes: an international multicenter survey. Pancreas 2011;40(6):809–14. [DOI] [PubMed] [Google Scholar]
- 37.Petrov MS, Yadav D. Global epidemiology and holistic prevention of pancreatitis. Nat Rev Gastroenterol Hepatol 2019;16(3):175–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sadr-Azodi O, Mattsson F, Bexlius TS, Lindblad M, Lagergren J, Ljung R. Association of oral glucocorticoid use with an increased risk of acute pancreatitis: a population-based nested case-control study. JAMA Intern Med 2013;173(6):444–9. [DOI] [PubMed] [Google Scholar]
- 39.Jain S, Midha S, Mahapatra SJ, Gupta S, Sharma MK, Nayak B, et al. Interleukin-6 significantly improves predictive value of systemic inflammatory response syndrome for predicting severe acute pancreatitis. Pancreatology 2018;18(5):500–6. [DOI] [PubMed] [Google Scholar]
- 40.Shimada M, Andoh A, Hata K, Tasaki K, Araki Y, Fujiyama Y, et al. IL-6 secretion by human pancreatic periacinar myofibroblasts in response to inflammatory mediators. J Immunol 2002;168(2):861–8. [DOI] [PubMed] [Google Scholar]
- 41.Jaruvongvanich V, Poonsombudlert K, Ungprasert P. Smoking and Risk of Microscopic Colitis: A Systematic Review and Meta-analysis. Inflamm Bowel Dis 2019;25(4):672–8. [DOI] [PubMed] [Google Scholar]
- 42.Alsamarrai A, Das SL, Windsor JA, Petrov MS. Factors that affect risk for pancreatic disease in the general population: a systematic review and meta-analysis of prospective cohort studies. Clin Gastroenterol Hepatol 2014;12(10):1635–44 e5; quiz e103. [DOI] [PubMed] [Google Scholar]
- 43.Svensson M, Bergman D, Olen O, Myrelid P, Bohr J, Wickbom A, et al. Validating microscopic colitis (MC) in Swedish pathology registers. Scand J Gastroenterol 2018;53(12):1469–75. [DOI] [PubMed] [Google Scholar]
- 44.Liu PH, Burke KE, Ananthakrishnan AN, Lochhead P, Olen O, Ludvigsson JF, et al. Obesity and Weight Gain Since Early Adulthood Are Associated With a Lower Risk of Microscopic Colitis. Clin Gastroenterol Hepatol 2019;17(12):2523–32.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhu GL, Chen SH, Fan XD, Fan JC, Men XL, Zhang YM, et al. [A prospective cohort study on BMI levels and risk of acute pancreatitis]. Zhonghua Liu Xing Bing Xue Za Zhi 2021;42(12):2131–7. [DOI] [PubMed] [Google Scholar]
- 46.Larsson JK, Sonestedt E, Ohlsson B, Manjer J, Sjöberg K. The association between the intake of specific dietary components and lifestyle factors and microscopic colitis. Eur J Clin Nutr 2016;70(11):1309–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yen EF, Pokhrel B, Du H, Nwe S, Bianchi L, Witt B, et al. Current and past cigarette smoking significantly increase risk for microscopic colitis. Inflamm Bowel Dis 2012;18(10):1835–41. [DOI] [PubMed] [Google Scholar]
- 48.Roth B, Gustafsson RJ, Jeppsson B, Manjer J, Ohlsson B. Smoking- and alcohol habits in relation to the clinical picture of women with microscopic colitis compared to controls. BMC Womens Health 2014;14:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sadr-Azodi O, Andrén-Sandberg Å, Orsini N, Wolk A. Cigarette smoking, smoking cessation and acute pancreatitis: a prospective population-based study. Gut 2012;61(2):262–7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


