Abstract
Cognitive deficit is a common comorbidity in temporal lobe epilepsy (TLE) and is not well controlled by current therapeutics. How epileptic seizure affects cognitive performance remains largely unclear. In this study we investigated the role of subicular seizure-activated neurons in cognitive impairment in TLE. A bipolar electrode was implanted into hippocampal CA3 in male mice for kindling stimulation and EEG recording; a special promoter with enhanced synaptic activity-responsive element (E-SARE) was used to label seizure-activated neurons in the subiculum; the activity of subicular seizure-activated neurons was manipulated using chemogenetic approach; cognitive function was assessed in object location memory (OLM) and novel object recognition (NOR) tasks. We showed that chemogenetic inhibition of subicular seizure-activated neurons (mainly CaMKIIα+ glutamatergic neurons) alleviated seizure generalization and improved cognitive performance, but inhibition of seizure-activated GABAergic interneurons had no effect on seizure and cognition. For comparison, inhibition of the whole subicular CaMKIIα+ neuron impaired cognitive function in naïve mice in basal condition. Notably, chemogenetic inhibition of subicular seizure-activated neurons enhanced the recruitment of cognition-responsive c-fos+ neurons via increasing neural excitability during cognition tasks. Our results demonstrate that subicular seizure-activated neurons contribute to cognitive impairment in TLE, suggesting seizure-activated neurons as the potential therapeutic target to alleviate cognitive impairment in TLE.
Keywords: temporal lobe epilepsy, cognitive deficit, subicular seizure-activated neurons
Introduction
Epilepsy is a common neurological disorder, characterized by spontaneous and recurrent seizures, affecting 70 million people worldwide [1]. Among these, temporal lobe epilepsy (TLE) is the most common type of epilepsy that is not well controlled by available anti-seizure drugs [2, 3]. TLE is frequently accompanied by cognitive impairment [4, 5], and cognitive impairment shares many common properties with TLE in pathological changes including neuronal loss and sclerosis in the temporal lobe regions [6, 7]. Clinical data show that epilepsy patients bear 2.5 times more risk of incident dementia than people without epilepsy [8]. Similarly, epileptiform abnormalities are more frequently observed in Alzheimer’s disease patients [9]. The elderly with TLE and those with mild cognitive impairment display similar symptoms, such as language decline, memory deficits and brain atrophy [10]. Unfortunately, cognitive comorbidities often fail to be diagnosed [11], and no effective therapeutic strategy is available to improve cognitive decline in epilepsy [12]. Thus, revealing the mechanism of TLE-associated cognitive impairment is crucial to the development of precise treatment.
Neurons activated by event experience inducing persistent biophysical or biochemical changes to store and recall memories are called engram cells, and subsequent reactivation of engram cells by available cues causes memory retrieval [13]. Immediate early genes (IEG), including c-fos et al. [14], are activated in response to the neuronal activity which could regulate synaptic plasticity-related gene expression to mediate specific behavior [15]. Researchers have recently reported that memory-labeled and seizure-labeled neurons are largely overlapped in hippocampal CA1, suggesting seizure resets the memory engram to cause retrograde amnesia [16]. However, epileptic seizures arise from local foci and widely propagate to extensive brain regions [17]. How seizure-activated neurons in these brain regions participate in cognitive impairment remains largely elusive.
The subiculum is the major output of the hippocampus, which projects to the entorhinal cortex and other downstream brain regions [18]. Physiologically, the subiculum codes spatial working memory and navigation information, including place, speed, and trajectory [18–20]. More importantly, prior studies have noted the importance of the subiculum in the beginning, spreading, and generalizing process of hippocampal seizure [21–23]. Extensive evidence from specimens of patients or animal models has supported that the subiculum is highly activated in TLE [24, 25]. In addition, cognitive tasks inducing activation of c-fos expression are also found in the subiculum [26]. Recently, ultra-high field magnetic resonance imaging data have indicated that lower subicular volume is associated with high dementia risk for the senior [27]. The feature of the subiculum in the cognitive process and its key role in TLE make it a potential target for controlling epilepsy-associated cognitive impairment. However, it is obscure whether subicular c-fos induction has a causal role in coordinating circuit modifications required to encode cognition performance in epilepsy. In this study, we attempted to label the subicular seizure-activated neurons with a special promoter that carries enhanced synaptic activity-responsive element (E-SARE). By combining with designer receptors that are exclusively activated by designer drugs (DREADDs), we manipulate these neurons to unravel its role in cognitive dysfunction in TLE model. Our study found that chemogenetic inhibition of subicular seizure-activated neurons not only attenuates seizure severity but also cognitive deficits in object location memory (OLM) and novel object recognition (NOR) tasks in epilepsy by enhancing the recruitment of cognition-responsive c-fos+ neurons.
Materials and methods
Animals
Male wild-type (WT) C57BL/6 mice (25–30 g, 2–4 months old) were used in this study. They were group-maintained before surgery in cages with a 12-h light/dark cycle (lights on from 8:30 to 20:30), an ambient temperature of 22 ± 2 °C, and with ad libitum food and water. All the mice used in this study after surgery were housed individually for better recovery. For all the behavior tests, we performed by daylight (9:00–18:00). All experiments were carried out following the National Institutes of Health guidelines, and all procedures were approved by the local ethics review committee of Zhejiang Chinese Medical University. Although epilepsy affects both sexes, the susceptibility to epilepsy has shown gender differences in many previous reports [28–30]. In the present study, we used male animals uniformly in order to reduce the number of animals and minimize variability.
Virus
For calcium fluorometric recording, AAV-E-SARE-ERT2-Cre-ERT2 (serotype: AAV2/9, viral titers: 1.45 × 1013 particles/mL, Cat#S0364-9-H50, Taitool, 0.1 μL) 1:1 mixed with AAV-hSyn-DIO-GCaMP6m (serotype: AAV2/9, viral titers: 1.31 × 1013 particles/mL, Cat#S0227-2R-H50, Taitool, 0.1 μL) were microinjected to the subiculum (AP: −3.4 mm, ML: −2.0 mm, DV: −1.8 mm) of WT mice. To chemogenetically inhibit subicular seizure-activated neurons or kindling cage-activated neurons, AAV-E-SARE-ERT2-Cre-ERT2 (serotype: AAV2/9, viral titers: 1.45 × 1013 particles/mL, Cat#S0364-9-H50, Taitool, 0.1 μL) mixed with AAV-EF1a-DIO-hM4Di-mCherry (serotype: AAV2/9, viral titers: 3.46 × 1012 particles/mL, Cat#HYMBE1370, Obio, 0.1 μL) at a ratio of 1 to 1 were injected to the subiculum. To chemogenetically inhibit subicular seizure-activated GABAergic interneurons, AAV-E-SARE-ERT2-Cre-ERT2 (serotype: AAV2/9, viral titers: 1.45 × 1013 particles/mL, Cat#S0364-9-H50, Taitool, 0.1 μL) mixed with AAV-VGAT-DIO-hM4Di-mCherry (serotype: AAV2/9, viral titers: 2.81 × 1012 particles/mL, CAT#PT-0618, BrainVTA, 0.1 μL) at a ratio of 1 to 1 were injected to the subiculum. To chemogenetically inhibit whole CaMKIIα+ neurons, AAV-CaMKIIα-hM4Di-mCherry (serotype: AAV2/8, viral titers: 2.31 × 1013 particles/mL, Cat#HYMBH557, Obio, 0.2 μL) were injected. To record the calcium activity of whole subicular CaMKIIα+ neurons, AAV-CaMKIIα-GCaMP6s (serotype: AAV2/8, viral titers: 7.05 × 1012 particles/mL, Cat#S0229-8, Taitool, 0.2 μL) were injected. To synchronously record and manipulate the activity of subicular seizure-activated neurons, the virus was injected in a 1:1:1 mixture of AAV-E-SARE-ERT2-Cre-ERT2 (serotype: AAV2/9, viral titers: 1.45 × 1013 particles/mL, Cat#S0364-9-H50, Taitool, 0.07 μL), AAV-hSyn-DIO-GCaMP6m (serotype: AAV2/9, viral titers: 1.31 × 1013 particles/mL, Cat#S0227-2R-H50, Taitool, 0.07 μL) and AAV-EF1a-DIO-hM4Di-mCherry (serotype: AAV2/9, viral titers: 3.46 × 1012 particles/mL, Cat#HYMBE1370, Obio, 0.07 μL). AAV-E-SARE-ERT2-Cre-ERT2, AAV-hSyn-DIO-GCaMP6m, and AAV-CaMKIIα-GCaMP6s were available products and obtained from Shanghai Taitool Bioscience Co., LTD (Shanghai, China). AAV-EF1a-DIO-hM4Di-mCherry and AAV-CaMKIIα-hM4Di-mCherry were available products obtained from Obio Technology Co., LTD (Shanghai, China). AAV-VGAT-DIO-hM4Di-mCherry was available and obtained from BrainVTA Co., LTD (Wuhan, China).
Stereotactic surgery for viral injection and electrode/optic fiber implantation
These protocols were according to our previous studies [21, 31, 32]. Mice were anesthetized by intraperitoneal injection of sodium pentobarbital (50 mg/kg). When mice were deeply anesthetized, they were mounted in a stereotaxic apparatus (RWD Life Science, Shenzhen, China). During surgery, if mice showed any pain reflex, we would inject the extra 10% of the initial dose of sodium pentobarbital to keep mice in a stable painless status. Virus (0.2 μL) was injected into the subiculum (AP: −3.4 mm, ML: −2.0 mm, DV: −1.8 mm) by a 1 μL syringe (Gaoge Industrial and Trading Co. LTD, Shanghai, China) controlled by an injection pump (Micro 4, World Precision Instruments, USA) at constant speed at 40 nL/min. After viral injection, to deliver the electrical stimulation, one bipolar electrode (795500, each 0.125 mm in diameter; A.M. Systems) was embedded into the right ventral CA3 region (AP: −2.9 mm; ML: −3.2 mm; DV: −3.2 mm). One electrode or optic fiber (Inper Ltd., China) was embedded into the subiculum for electroencephalography (EEG) recording or Ca2+ activity measurement. Four additional holes are punched around the target nucleus for cranial nail fixation of dental cement. Mice were housed for 3 weeks for recovery and viral expression. After all the behavioral tests, we check the location of each fiber or electrode, as well as viral infection for further data analysis.
Hippocampal kindling
The hippocampal kindling was performed as previously [21, 33, 34]. After surgery recovery, the afterdischarge threshold (ADT) of each animal was measured (monophasic square-wave pulses, 20 Hz, 1 ms/pulse, 40 pulses) by using a constant-current stimulator (ADINSTRUMENTS, Australia). Mice received an electrical stimulation started at 40 µA with an increase of 20 µA each time (1 min interval for each time). ADT was defined as the minimal stimulation current induced 5 s afterdischarge duration (ADD). The length of ADD was defined as the duration of the electrical stimulation onset to the end of the epileptiform discharge event in EEG records. The length of generalized seizures (GS) duration (GSD) was defined as the duration of the GS onset to the end of epileptiform discharge event in EEG records. The amplitude of the continuous discharge greater than threefold of baseline was considered as the epileptiform discharge [35]. At the same time, subicular EEGs were recorded by the Powerlab software (ADINSTRUMENTS, Australia). We equally grouped all the mice in the kindling experiments based on their ADTs. On the next day, we kindled all the mice with 10 stimulations (400 µA, 20 Hz, 2 s trains, 1 ms monophasic square-wave pulses, 40 pulses, 30 min intervals between each stimulation). Seizure severity was classified according to the Racine scale [36]: (1) facial movement; (2) head nodding; (3) unilateral forelimb clonus; (4) bilateral forelimb clonus and rearing; and (5) rearing and falling. Stages 1–3 were considered to be focal seizures and stages 4–5 as GS [35]. When animals had three consecutive seizure stage 5, they were regarded as fully kindled. After that, mice were kept in stable secondary GS (sGS) after each kindling stimulation.
Labeling neurons activated following seizure
We used an AAV system containing an activity-dependent promoter, E-SARE to drive ERT2-CreERT2 expression in activated neurons by driving the downstream IEG expression [37]. For specifically labeling subicular activated neurons or GABAergic interneurons following seizure, we injected AAV-E-SARE-ERT2-Cre-ERT2 mixed with AAV-EF1a-DIO-hM4Di-mCherry or AAV-VGAT-DIO-hM4Di-mCherry. When tamoxifen is present, the E-SARE downstream Cre-recombinase-derived hM4Di-mCherry or VGAT-hM4Di-mCherry were permanently expressed on seizure-activated neurons or seizure-activated GABAergic interneurons. When mice were fully kindled, mice were intraperitoneally injected with tamoxifen (200 mg/kg; Sigma-Aldrich, dissolved in corn oil) on the next day. Tamoxifen was dissolved in the corn oil (20 mg/mL) in the water bath at 40 °C. Three hours after the tamoxifen injection, 2 s of electrical stimulation (three times, 30 min apart) was performed each day for consecutive 2 days. To minimize the non-specific neuronal labeling by environmental background noise, the mice were kept in the home cages in the quiet room for 8 h before and after tamoxifen delivery. As a control, we labeled kindling cage-activated neurons, all the experiment process (including tamoxifen injection, handling, kindling cage context et al.) were similar to the labeling of seizure-activated neurons, apart from the three kindling-induced seizures.
Chemogenetic manipulation
Chemogenetics—DREADDs are developed by engineered G-protein-coupled receptors (GPCR). DREADDs are activated by the clozapine analog, to drive the activation of GPCR signaling including Gq, Gi, Gs, Golf, and β-arrestin. Among these, Gi-DREADD was constructed by a point mutation in muscarinic acetylcholine receptor 4 (termed hM4Di) that targets to the rectifying potassium channels leading to neuronal hyperpolarization to inhibit neuronal activity [38]. To investigate the effect of chemogenetic inhibition of subicular seizure-activated neurons on seizure and associated cognitive impairment, clozapine-N-oxide (CNO, 1 mg/kg, ab141704, Abcam, USA) dissolved in the 0.9% saline was intraperitoneally injected 30 min before the kindling or training phase in cognitive tasks. The dosage chosen in chemogenetic experiments was according to previous study [39]. For the kindling, 2 s of electrical stimulation (three times, 30 min apart) was performed to investigate the seizure behavior changes after CNO treatment, and the data of seizure stage, latency to GS, and GSD were presented as the average of these three times stimulations.
Object location memory (OLM) and novel object recognition (NOR) tasks
The OLM and NOR were performed as previously [40]. Before the test, experimenters continuously handled mice for 5 days to decrease their anxiety and stress. Mice were placed into the empty experimental apparatus for 10 min exploration before the tasks. The hand-made acrylic experimental chamber with specific cues (45 cm height, 45 cm width, 45 cm length) was used in these behavioral tests. On the testing day, mice were placed in the room at least 1 h before the experiment. In the training period, mice were placed into the experimental chamber with two same objects placed on the same side from the wall at a distance of 5 cm for 10 min. Then mice were put back into their home cages for 30 min. The exploration time in the test phase was 5 min. For the OLM, one of the objects was randomly moved to a new location at the same distance from the wall. For the NOR experiments, one of the objects in the training phase was replaced by a new distinct shape and color object. All the objects used in our study had no pungnent odor. Video recording of the experiments was analyzed by ANY-MAZE system (Stoelting, USA). Exploration was considered as directing the nose at a distance less than 2 cm from the object or touching the object with nose. Mice that did not explore any object during the first minute of the test session were excluded from the analysis. The exploration time of each object was manually recorded in a blinded manner. The discrimination index (DI) was calculated by [(Tmoved – Tunmoved)/(Tmoved + Tunmoved)] × 100% or [(Tnovel − Tfamiliar))/(Tnovel + T familiar)] × 100%.
Y-maze
The Y-maze test was performed using an apparatus with three arms (30 cm length, 10 cm wide, 20 cm height) at 120° angles to form a “Y” shape. The mice were placed in the distal end of an arm and allowed to explore the maze for 8 min. The percentage of spontaneous alternations (entries into an arm that differed from the arm entered in the previous two entries) was calculated with the following formula: (alternations/(arms entries – 2)) × 100.
Fiber photometry
GCaMP is a high-affinity Ca2+ probe composed of a GFP. When intracellular Ca2+ concentration increases, which triggers the conformational change and then emits green fluorescence to reflect the Ca2+ activity of neurons [41]. After the microinjection of AAV-CaMKIIα-GCaMP6s, mixed AAV-E-SARE-ERT2-Cre-ERT2 and AAV-hSyn-DIO-GCaMP6m or mixed AAV-E-SARE-ERT2-Cre-ERT2, AAV-hSyn-DIO-GCaMP6m and AAV-EF1a-DIO-hM4Di-mCherry, one optical fiber (Inper Ltd., China) was embedded into the subiculum for neuronal activity recording. Fiber photometry of calcium signals was performed one week after surgery recovery in naïve mice or two weeks after tamoxifen-induced viral expression in kindled mice. Our study used the fiber photometry system (Nanjing Thinkertech, Nanjing, China) for all the related Ca2+ activity recording experiments [31, 32, 34]. The fiber photometry system uses a complementary metal-oxide-semiconductor surface array imaging method to collect the fluorescence brightness of each fiber in a multimode fiber bundle in real time so that multiple channels can be recorded simultaneously. The fiber photometry system used a 488 nm diode laser (target channel, light intensity 0.01–0.03 mW) and a 405 nm diode laser (reference channel, light intensity 0.01–0.03 mW) to record signal in the subiculum. The intensity of light was measured by an optical power meter (THORLABS, USA). Calcium signal fiber optic recording works as follows: the excitation light at 488 nm is reflected by a dichroic mirror, coupled to an optical transducer at the objective, and transmitted to the target brain region through an optical fiber connected to a ceramic insert. Changes in fluorescence intensity of GCaMP expressed in the target brain region can be recorded in response to the excitation light. These fluorescence changes of GFP will be collected by the photomultiplier tube, and the amplifier output is converted into a voltage signal, which is displayed and recorded as a real-time digital display after low-pass filtering in the computer. After the behavior tests, all the primary data were recorded and then imported into MATLAB software to analyze the fluorescence signal changes. All the data were corrected to avoid the effect of fluorescence attenuation during long-term exposure recording. We set and calculated the average fluorescence signal in the baseline period before the kindling or exploring onset as the value F0. The fluorescence signal changes were reflected as the (F − F0)/F0 shown as the linear graph of average fluorescence, heatmaps, and statistical plots.
Immunohistochemistry
For immunohistochemistry, mice were deeply anesthetized, perfused with saline, followed by cold 4% paraformaldehyde (PFA). Notably, in c-fos staining experiments, animals were sacrificed for 1.5 h after three GS or behavior test sessions (OLM or NOR). Brains were fixed in 4% PFA at least 6 h but not exceeding 24 h at 4 °C. After that, we changed the solution into 30% sucrose and stored it at 4 °C to full dehydration. The coronal slices at the thickness of 30 µm were obtained by the frozen slicer (ThermoFisher, NX70, USA). After all the preparation, brain slices were first incubated in the PBS solution containing 0.3% Triton X-100 and 5% normal donkey serum for 2 h at room temperature. Secondly, primary antibodies including mouse anti-c-fos (ab208942, Abcam, USA), rabbit anti-CaMKIIα (ab552476, Abcam, USA), and rabbit anti-GABA (A2052, Sigma, USA) were added and incubated at 4 °C overnight. All the above primary antibodies were diluted at the ratio of 1 to 500 in our study. Thirdly, on the second day, brain slices were washed with PBS three times and then incubated by the secondary antibody donkey anti-mouse Alexa Fluor 488 (ab150105, Abcam, USA), donkey anti-rabbit Alexa Fluor 488 (ab150073, Abcam, USA) and donkey anti-rabbit Alexa Fluor 647 (ab150075, Abcam, USA). All the above secondary antibodies were diluted at the ratio of 1 to 500 in our study. The fluorescence signals were detected by a fluorescence microscope (Leica DM6B, Germany). We imaged nine consecutive coronal sections based on setting up the electrode or fiber position as the fifth section. The immunofluorescence representative images represent the average of one mouse. The c-fos+, CaMKIIα+, GABA+, and Virus+ neurons were collected and counted by the researcher blind to the group allocation.
Statistical analysis
All the experiments were performed in a blinded way. Seizure stage data are expressed as the median with 95% CI and other data are expressed as the mean ± SEM. Data analysis was performed blind to the experimenters using Prism (GraphPad Software, Version 8.3) and SPSS (IBM, Version 22.0). Normally distributed data were tested by one-way analysis of variance followed by post hoc Turkey test analysis for multiple comparisons or post hoc Dunnett test analysis for the comparison to the control group, and paired or unpaired Student’s t test for two-group comparisons. Non-normally distributed data were tested by the Kruskal-Wallis test or Mann-Whitney test. Correlation among the c-fos+ number was evaluated using Pearson’s correlation analysis. A two-tailed P < 0.05 was considered statistically significant. Detailed statistic parameters are provided in Supplementary Table S1 with the paper.
Results
Functional validation of seizure-activated neurons in the subiculum
In order to label the seizure-activated neurons, we injected AAV-E-SARE-ERT2-Cre-ERT2 mixed with AAV-EF1a-DIO-hM4Di-mCherry into the subiculum. E-SARE is a synthetic promoter, short for the enhanced synaptic activity-responsive element which induces IEG transcription and translation in neurons [37]. When tamoxifen is present, the E-SARE downstream Cre-recombinase-derived hM4Di-mCherry was permanently expressed on seizure-activated neurons (Fig. 1a). Here, we implanted the bipolar electrode into hippocampal CA3 for kindling stimulation and EEG recording. The stable hippocampal kindling-induced TLE model was constructed by daily 10 stimulations until all animals were fully kindled. When animals were fully kindled, mice were kept in a stable sGS after each kindling stimulation. On the next day, tamoxifen was intraperitoneally injected into mice to drive the virus to label subicular seizure-activated neurons by three times kindling stimulations for consecutive 2 days (Fig. 1a, b). The kindling model used here is a canonical animal model to study TLE, which is induced by repetitive brief electrical stimulation of the hippocampus or amygdala [42, 43], resembling clinical focal seizure with sGS.
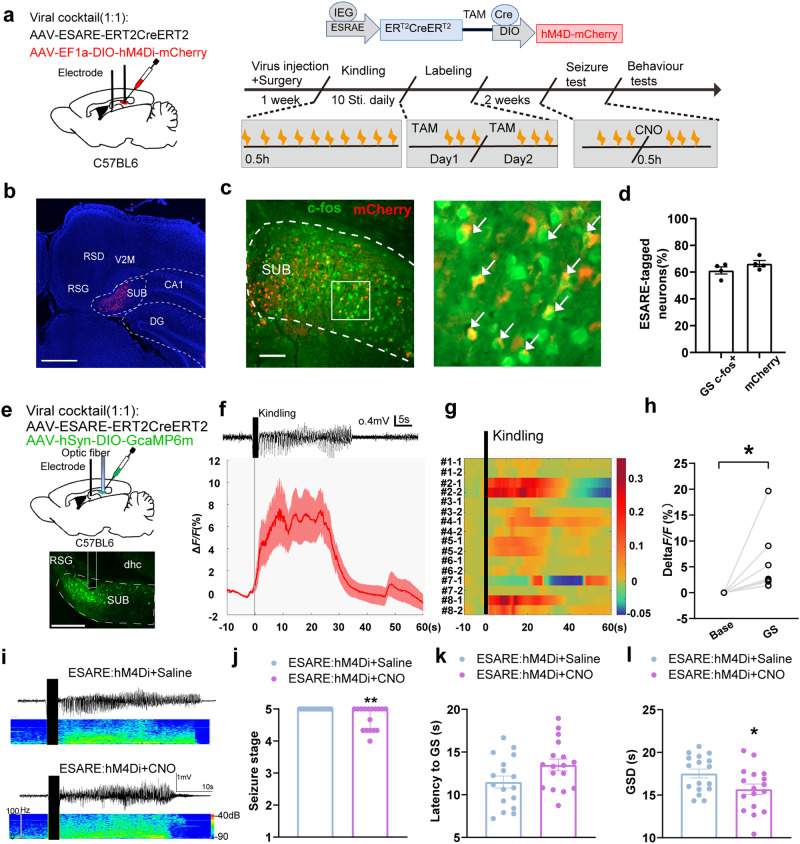
Fig. 1. Chemogenetic inhibition of subicular seizure-activated neurons alleviates seizure generalization.
a The diagram of the experimental procedures for the labeling and manipulating of subicular seizure-activated neurons in the hippocampal kindling model. b Representative image of viral expression, scale bar, 500 µm. SUB subiculum, DG dentate gyrus, RSD retrosplenial dysgranular cortex, RSG retrosplenial granular cortex, V2M secondary visual cortex, c Representative and enlarged images show seizure-labeled mCherry+ neurons with c-fos staining in the subiculum. Scale bar, 100 µm. d Percentage of seizure-labeled mCherry neurons co-stained with c-fos, and percentage of c-fos overlapped with seizure-labeled mCherry. (n = 4 mice). e Recording configuration and the representative image for fluorometric monitoring of Ca2+ activity of subicular seizure-activated neurons during GS. Scale bar, 400 μm. f Representative EEG and mean fluorescence values of Ca2+ activity of subicular seizure-activated neurons during GSs. g Heatmaps illustration of calcium activity (two representative GSs from each mouse, n = 8 mice). The number of each trial from eight mice is indicated in the figure. h Quantification of the average ΔF/F0 during base and GS. Paired T-test. *P < 0.05 compared with Base. i Typical EEGs and power spectrogram recorded from the subiculum during GS. The solid black vertical bar indicates kindling stimulation. Effects of chemogenetic inhibition of subicular seizure-activated neurons on seizure stage (j), and latency to GS (k), and GSD (l). *P < 0.05, **P < 0.01 compared with E-SARE-hM4D group+saline group. (n = 17 for E-SARE-hM4D + saline group, n = 17 for E-SARE-hM4D + CNO group). For (h), Mann Whitney test. For (i, j), unpaired T-test. For (h), data are expressed as the median with 95% CI and other data are presented as mean ± SEM.
We first used the immunohistochemistry assays by staining the c-fos+ following GS to validate the specificity and efficiency of this technique and the data demonstrated that ~65% of mCherry labeled neurons were immunopositive for seizure-activated c-fos+ (specificity) and ~61.2% of neurons immunopositive for c-fos+ were co-labeled with mCherry (efficiency) (Fig. 1c, d).
Next, we labeled the subicular seizure-activated neurons with the Cre recombinase-dependent expression of GCaMP6m to record the neuronal activity during the seizure (Fig. 1e). The average fluorescence changes of Ca2+ activity of subicular seizure-activated neurons increased immediately after the kindling stimulation, and decreased to the baseline level after seizure (Fig. 1f). The Ca2+ activity of each mouse shown in the heatmaps illustration indicated the consistent phenomenon (Fig. 1g). There were some variabilities in Fig. 1g because photometry signal reflects the fluorescence changes depending on the amount of viral expression, the optic fiber location to the viral expression, and the intensity of each seizure event. While, each mouse showed a similar increase in amplitude, suggesting the repeatability of this data. Compared to baseline, the calcium activity of subicular seizure-activated neurons during GS dominantly increased (Fig. 1h). These data further suggest that subicular seizure-activated neurons were indeed involved in seizure events.
Then, we investigated whether selective modulation of subicular seizure-activated neurons would affect seizure. We inhibited the subicular seizure-activated neurons by intraperitoneal injection of CNO and found that CNO lowered the seizure stage (Fig. 1j), shortened the GSD (Fig. 1l), and showed a tendency to increase the latency to GS (Fig. 1k). These data suggest that subicular seizure-activated neurons are sensitive to seizure generalization. To evaluate whether the number of seizure-activated neurons affects the anti-seizure effect, we performed a correlation analysis between the decreased percentage of GSD and the number of seizure-activated neurons modulated by chemogenetics, and we found that the more activated c-fos+ neurons were inhibited, the more pronounced epilepsy protection was produced (Fig. S1). As a comparison, CNO itself showed no effect on epileptic seizures in sham-kindled mice (Fig. S2). To further confirm the specificity of subicular seizure-activated neurons on seizure-modulating effect, we labeled the kindling cage-activated neurons by injecting AAV-E-SARE-ERT2-Cre-ERT2 mixed with AAV-EF1a-DIO-hM4Di-mCherry into the subiculum (Fig. S3a, b). We found CNO did not affect the seizure stage, latency to GS, and GSD in the subicular kindling cage-labeled mice (Fig. S3c–f). Taken together, these data illustrate that subicular seizure-activated neurons contribute to seizure modulation in TLE, and inhibition of these neurons alleviates the severity of GS.
Inhibition of seizure-activated neurons in the subiculum rescues OLM and NOR deficit in epileptic mice
Spatial memory decline and episodic memory deficits are the two common cognitive comorbidities seen in TLE [44–46]. Next, we investigated whether inhibition of subicular seizure-activated neurons has any effect on TLE-associated cognitive deficits. Firstly, we used the OLM to assess spatial memory (Fig. 2a). Compared with the naïve non-kindled mice, the total exploration time of unmoved and moved objects was decreased in the kindled mice with labeling subicular seizure-activated neurons, and there was no difference between saline and CNO group in kindled mice with labeling subicular seizure-activated neurons (Fig. 2b). As shown in Fig. 2c, compared with non-kindled mice, the kindled mice treated with saline showed a decreased preference to explore the moved object, while CNO administration could rescue that (Fig. 2c). In addition, CNO did not affect the performance of the OLM task in kindling cage-labeled kindled mice (Fig. S3g, h). These data demonstrate that inhibition of subicular seizure-activated neurons improves OLM performance in kindled epileptic mice.
Fig. 2. Inhibition of subicular seizure-activated neurons rescues cognitive deficit in epileptic mice.
a Schematic of the protocol of object location memory (OLM) task. Effects of inhibition of subicular seizure-activated neurons on total exploration time (b) and discrimination index (c) for the moved object. One-way ANOVA followed by Turkey’s post hoc test. ***P < 0.001 compared with WT non-kindled group, ##P < 0.01 compared with kindled E-SARE-hM4D group injected with saline. (n = 9 mice for WT non-kindled mice, n = 10 mice for kindled E-SARE-hM4D + saline group, n = 10 mice for kindled E-SARE-hM4D + CNO group). d Schematic of the protocol of novel object recognition (NOR) task. Effects of inhibition of subicular seizure-activated neurons on total exploration time (e) and discrimination index (f) for the novel object. One-way ANOVA followed by Turkey’s post hoc test. *P < 0.05, **P < 0.01, ***P < 0.001 compared with WT non-kindled group, #P < 0.05 compared with kindled E-SARE-hM4D group injected with saline. (n = 9 mice for each group). All data are presented as mean ± SEM.
Then, NOR was used to validate the episodic memory (Fig. 2d–f). Similarly, the exploration total time of all the kindled subicular seizure-activated neurons mice (saline or CNO group) was decreased, and there was no difference in the total exploration time for exploring familiar object and novel object in kindled mice (Fig. 2e). Compared with non-kindled mice, kindled mice injected with saline had the reduced discrimination index, while kindled mice treated with CNO showed a higher preference to explore the novel object compared with mice injected with saline (Fig. 2f). As a comparison, we found CNO did not affect the performance in NOR task in kindling cage-labeled kindled mice (Fig. S3i, j). These data suggest that inhibition of subicular seizure-activated neurons improves NOR performance in kindled epileptic mice.
Next, Y-maze was used to assess the working memory which is highly attributed to the function of the prefrontal cortex [47, 48]. Chemogenetic inhibition of subicular seizure-activated neurons did not affect the spontaneous alternation in the Y-maze (Fig. S4a). Meanwhile, chemogenetic inhibition of subicular kindling cage-activated neurons did not affect the working memory (Fig. S4b). Taken together, these results indicate that inhibition of subicular seizure-activated neurons could selectively ameliorate the cognitive deficit in NOR and OLM of epileptic mice.
Inhibition of whole subicular CaMKIIα+ neuron impairs cognitive function in naïve mice
As glutamatergic and GABAergic neurons are the two main neurons in the subiculum [38], we also identified the cell type of labeled neurons. The immunohistochemistry data indicated that the 10% of subicular seizure-activated neurons are overlapped with GABA+ neurons (Fig. 3b). While, 82.6% of subicular seizure-activated neurons are CaMKIIα+ (Fig. 3a), which has been reported to be important for seizure generalization and drug resistance in TLE [2, 49]. Therefore, we next investigated the contribution of subicular whole CaMKIIα+ neurons in cognition. First of all, we recorded the neuronal activity of subicular CaMKIIα+ neurons during cognition tasks in naïve mice by in vivo CaMKIIα-GCaMP6m-based Ca2+ activity recording (Fig. 3c). Interestingly, naïve mice showed a significantly higher Ca2+ activity to the moved object than the unmoved object during OLM (Fig. 3d–f). Similarly, the activity of subicular CaMKIIα neurons was highly active when the naïve mice explored the novel object in the NOR test session (Fig. 3g–i), suggesting mice with normal cognitive function could discriminate two distinct objects. Next, we assessed the effect of inhibition of subicular CaMKIIα+ neurons on cognitive function in the naïve mice without kindling (Fig. 3j, k). Compared to the saline group, chemogenetic inhibition of subicular whole CaMKIIα+ neurons by CNO impaired the OLM (Fig. 3l, m). In the NOR task, CNO decreased the time spent exploring objects leaving the discrimination index unaffected (Fig. 3n, o). These results demonstrate that subicular CaMKIIα+ neurons are important to cognitive function, inhibition of whole subicular CaMKIIα+ neuron impairs the cognitive function in non-kindled naïve mice.
Fig. 3. Chemogenetic inhibition of whole subicular CaMKIIα+ neuron impairs cognitive function in naïve mice.
Representative images and percentage of seizure-activated neurons (red) with CaMKIIα (a) or GABA (b) staining in the subiculum. Scale bar, 100 µm (n = 4 mice). Data are represented as mean ± SEM. c Recording configuration (c1) and the representative image (c2) for fluorometric monitoring of Ca2+ activity of subicular CaMKIIα+ neurons in the naïve mice, scale bar, 500 μm. Mean fluorescence values, corresponding fluorescence heatmaps, and quantification of average ΔF/F0 of each mouse of Ca2+ activity of subicular CaMKIIα+ neurons during OLM (d–f), and NOR (g–i) test session (3 representative trails from each mouse, n = 4 mice). Paired T-test. *P < 0.05 compared with unmoved object or familiar object. j The diagram of the viral injection and representative image for CaMKIIα-hM4Di-mCherry expression in the subiculum, scale bar, 500 μm. k Schematic of the protocol of OLM and NOR task. Effects of inhibition of whole CaMKIIα+ neurons in the subiculum on total exploration time (l) and discrimination index (m) of OLM test. Unpaired T test. *P < 0.05 compared with CaMKIIα-hM4Di + saline group (n = 8 for CaMKIIα-hM4Di + saline group, n = 11 for CaMKIIα-hM4Di + CNO group). Effects of inhibition of whole CaMKIIα+ neurons in the subiculum on total exploration time (n) and discrimination index (o) of NOR test session. Unpaired T-test. *P < 0.05 compared with CaMKIIα-hM4Di + saline group (n = 10 for CaMKIIα-hM4Di + saline group, n = 8 for CaMKIIα-hM4Di + CNO group). All data are presented as mean ± SEM.
Regarding the contribution of interneurons to TLE [50, 51], we injected AAV-E-SARE-ERT2-Cre-ERT2 mixed with AAV-VGAT-DIO-hM4Di-mCherry into the subiculum to label the seizure-activated GABAergic interneurons in the subiculum (Fig. S5a, b). As a comparison, chemogenetic inhibition of seizure-activated GABAergic interneurons showed no effect on epileptic seizure (Fig. S5d–g). In addition, CNO also did not affect cognitive performance in both the OLM and NOR (Fig. S5h–k). All the above data suggest that chemogenetic inhibition of subicular seizure-activated neurons, mainly CaMKIIα+ glutamatergic neurons, reduces seizure generalization and improves performance in episodic and spatial memory tasks of hippocampal-kindled mice.
Inhibition of subicular seizure-activated neurons enhances recruitment of the cognition-activated c-fos+ neurons
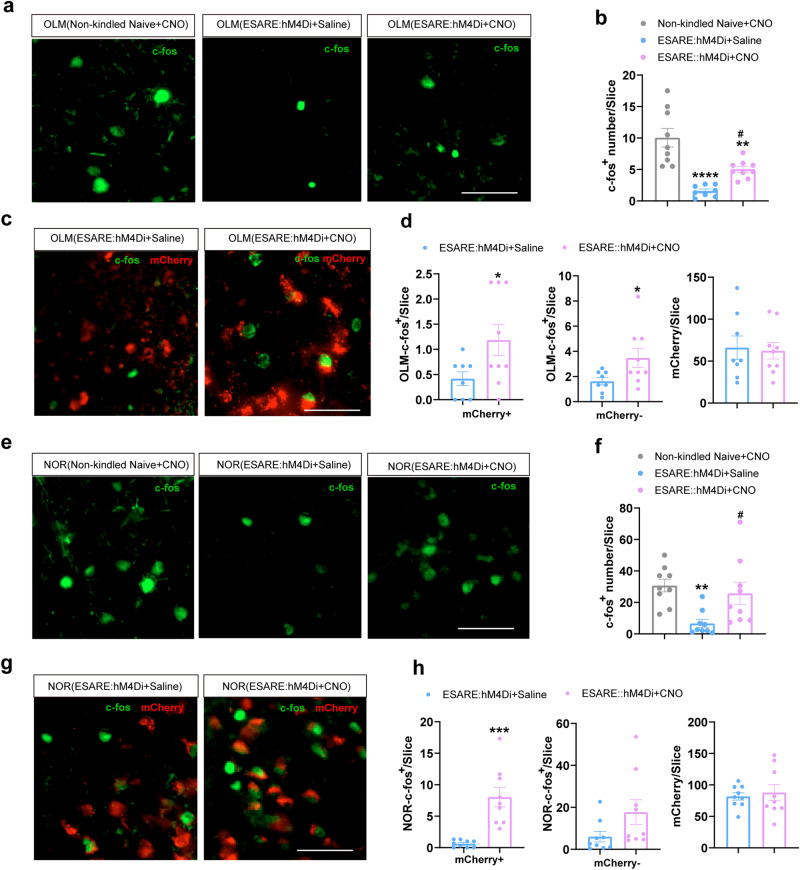
Next, we asked the reason why inhibition of subicular seizure-activated neurons showed a protective effect on cognition in TLE. To answer this question, we quantified the cognition-task activated c-fos+ neurons by immunohistochemistry. Compared with the non-kindled naïve mice, the kindled mice showed a decreased number of OLM-activated c-fos+ neurons (Fig. 4a, b), and NOR-activated c-fos+ neurons (Fig. 4e, f). The number of OLM- and NOR-activated c-fos+ showed little overlap with the seizure-labeled mCherry in the subiculum. We found that the most of cognition-activated c-fos+ neurons were CaMKIIα+ (Fig. S6a–d). Importantly, inhibition of subicular seizure-activated neurons by CNO increased the number of OLM-activated and NOR-activated c-fos+ neurons in kindled mice (Fig. 4b, f). Interestingly, the increased OLM-activated and NOR-activated c-fos+ neurons included both seizure-labeled mCherry+ neurons and mCherry- neurons (Fig. 4d–h). These above results indicate that seizure-activated neurons might affect the activation of cognition tasks-responsive neurons, a part of which are seizure activated mCherry+ neurons itself, and inhibition of subicular seizure-activated neurons increases the activation of cognition-responsive neurons.
Fig. 4. Inhibition of subicular seizure-activated neurons enhances recruitment of cognition task-responsive c-fos+ neurons.
a Representative image of OLM-activated c-fos+ in the subiculum of naïve non-kindled mice, kindled mice in E-SARE-hM4D injected with saline or CNO group. Scale bar, 50 μm. b Quantification of the number of OLM-activated c-fos+ neurons. One-way ANOVA followed by Turkey’s post hoc test. (n = 9 for non-kindled naïve group, n = 8 for kindled E-SARE-hM4D + saline group, n = 9 for kindled E-SARE-hM4D + CNO group). **P < 0.01 and ****P < 0.0001 compared with non-kindled naïve + CNO group, #P < 0.05 compared with kindled E-SARE-hM4D + saline group. c Representative images show seizure-labeled mCherry neurons with OLM-activated c-fos+ in the subiculum of kindled E-SARE-hM4D mice in saline and CNO group. Scale bar, 50 μm. d Statistical data for the number of mCherry-overlapped OLM-activated c-fos+ (left), mCherry negative OLM-activated c-fos+ (middle), and labeled virus (right). (n = 8 for kindled E-SARE-hM4D + saline group, n = 9 for kindled E-SARE-hM4D + CNO group). Unpaired T test. *P < 0.05 compared with kindled E-SARE-hM4D + saline group. e Representative images show NOR-activated c-fos staining in the subiculum of naïve non-kindled mice and kindled E-SARE-hM4D mice in the saline and CNO group. Scale bar, 100 μm. f Quantification of the number of NOR-activated c-fos+ neurons. One-way ANOVA followed by Turkey’s post hoc test. (n = 9 for each group). **P < 0.01 compared with non-kindled naïve + CNO group, #P < 0.05 compared with kindled E-SARE-hM4D + saline group. g Representative images of seizure-labeled mCherry neurons with NOR-activated c-fos+ staining in the subiculum of the naïve non-kindled mice and kindled E-SARE-hM4D mice in saline and CNO group. Scale bar, 50 μm. h Statistical data for the number of mCherry-overlapped NOR-activated c-fos+ (left), mCherry negative NOR-activated c-fos+ (middle), and labeled virus (right). (n = 9 for each group). Unpaired T test. ***P < 0.01 compared with kindled E-SARE-hM4D + saline group. All data are presented as mean ± SEM.
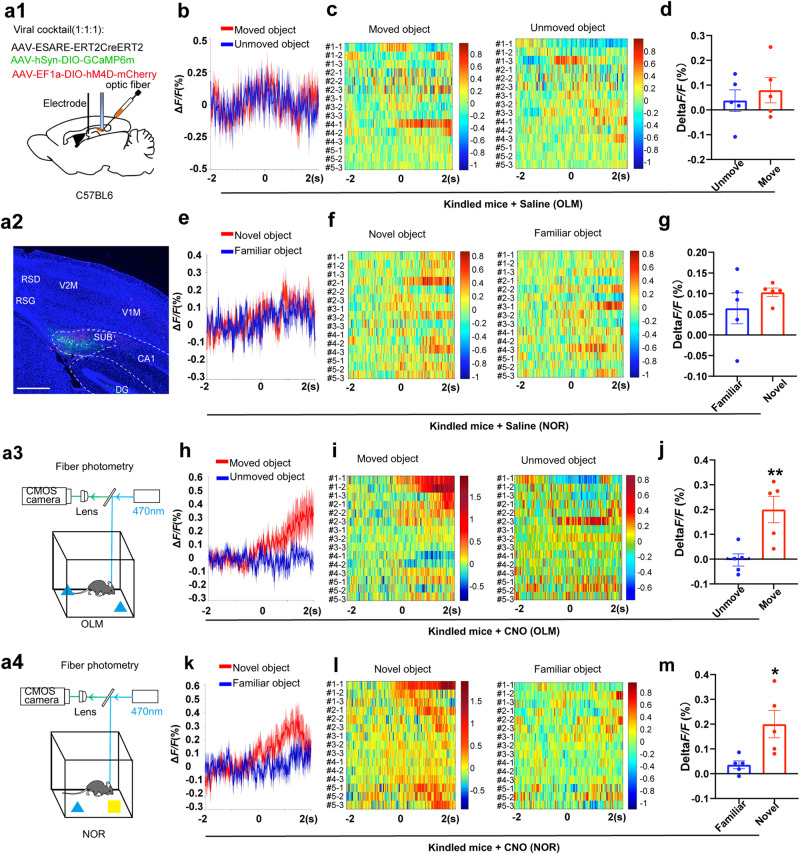
Next, to record and manipulate the seizure-activated neurons during cognitive tasks, we injected AAV-E-SARE-ERT2-Cre-ERT2 mixed with AAV-hSyn-DIO-GCaMP6m and AAV-EF1a-DIO-hM4Di-mCherry into the subiculum (Fig. 5a). We found that the kindled mice demonstrated a similar fluorescence change in mean ΔF/F during exploring the unmoved object and moved object in OLM (Fig. 5b–d). Likewise, there was no difference in mean ΔF/F during exploring the familiar object and novel object in NOR (Fig. 5e–g). These data indicate that the kindled mice could not discriminate the distinct object in the OLM and NOR tests. Interestingly, CNO treatment increased the Ca2+ activity of seizure-activated neurons when kindled mice explored the moved object in OLM (Fig. 5h–j) and the novel object in NOR (Fig. 5k–m), which might be due to the enhancement of task-activated c-fos+ neurons. Taken together, the above data indicate that subicular seizure-activated neurons fail to be activated properly in OLM and NOR behavior tasks in epileptic mice, and inhibition of subicular seizure-activated neurons could increase the recruitment of cognition task-activated neurons.
Fig. 5. Inhibition of subicular seizure-activated neurons enhances neural activity during cognition tasks.
a Recording configuration (a1), representative image (a2), and recording diagram for fluorometric monitoring of Ca2+ activity of subicular seizure-activated neurons in OLM (a3) and NOR (a4), scale bar, 500 μm. The calcium activity of subicular seizure-activated neurons on mean fluorescence values (b), corresponding fluorescence heatmaps (c), and quantification of average ΔF/F0 of each mouse (d) during OLM test in kindled mice injected with saline (three representative trails from each mouse, n = 5 mice, quantification of average ΔF/F0 data represents the average of three trails from each mouse). The calcium activity of subicular seizure-activated neurons on mean fluorescence values (e), corresponding fluorescence heatmaps (f), and quantification of average ΔF/F0 of each mouse (g) during NOR test in kindled mice injected with saline (three representative trails from each mouse, n = 5 mice, quantification of average ΔF/F0 data represents the average of three trails from each mouse). The calcium activity of subicular seizure-activated neurons on mean fluorescence values (h), corresponding fluorescence heatmaps (i), and quantification of average ΔF/F0 of each mouse (j) during OLM test after chemogenetic inhibition (three representative trails from each mouse, n = 5 mice, quantification of average ΔF/F0 data represents the average of three trails from each mouse). For (h), paired T test. **P < 0.01 compared with the unmoved object. The calcium activity of subicular seizure-activated neurons on mean fluorescence values (k), corresponding fluorescence heatmaps (l), and quantification of average ΔF/F0 of each mouse (m) during NOR test after chemogenetic inhibition (three representative trails from each mouse, n = 5 mice, quantification of average ΔF/F0 data represents the average of three trails from each mouse). Paired T test, *P < 0.05 compared with a familiar object. All data are presented as mean ± SEM.
Discussion
Serving as the “output” of the hippocampal complex, the subiculum plays a vital role in both cognition and TLE, in that the subiculum could be a potential candidate brain area to treat TLE-associated cognitive impairment. In this study, we specifically labeled the seizure-activated neurons in the subiculum and sought to inhibit these neurons by chemogenetic manipulation to investigate the effect on TLE-associated cognitive deficits. We reported for the first time that subicular seizure-activated neurons contribute to cognitive impairment in epilepsy, and inhibition of subicular seizure-activated neurons reduces the epilepsy-associated cognitive deficits possibly owing to the enhancement of the number of cognition-responsive ensembles.
Understanding the mechanism of seizure-associated cognitive impairment is important for the development of precise treatment. Several reports have shown that cognitive deficits are one of the common comorbidities of epilepsy, and there are ~25% of patients suffer from attention and memory problems [4, 11, 52]. Unfortunately, cognitive comorbidities are often neglected and undiagnosed in epilepsy [11], and there is no available treatment to restore cognitive decline in epilepsy [12]. Classic treatment mainly focuses on the “excitation-inhibition” theory, including activating inhibitory GABAergic neurons or inhibiting excitatory neurons, which often leads to unnegligible side effects [53]. For example, it has been reported that chemogenetic inhibition of granule cells by expressing CaMKIIα-hM4Di in hippocampal dentate gyrus disrupted cognition in naïve mice [12]. Consistent with their conclusions, our study found a similar phenomenon that inhibition of whole subicular CaMKIIα+ neurons induced cognitive decline in naïve non-kindled mice. In our study, we investigated the effect of inhibition of subicular seizure-activated neurons on seizure severity at first. As previous studies (including our group) reported that the anti-seizure effect of CNO was transient and reversible, lasting only several hours [39, 54–58]. The next day we performed cognitive-behavioral tests on all epileptic animals in randomized groups (Saline vs CNO). CNO treatment was injected acutely to test whether inhibiting seizure-activated neurons would restore cognitive deficit. We found that chemogenetic inhibition of subicular seizure-activated neurons could restore cognitive performance in both NOR and OLM tests. These results show cognitive improvement that may result from the inhibition of these seizure-activated neurons, but not alleviation of seizures.
Interestingly, chemogenetic inhibition of seizure-activated neurons GABAergic interneurons showed no effect on epileptic seizure and cognitive behavior. Two possible reasons may account for the abovementioned conclusion. (1) Subicular GABAergic neurons show a high degree of heterogeneity. In our previous study, we found that activation of subicular GABAergic neurons retarded early sGS acquisition. However, subicular parvalbumin (PV)-expressing GABAergic neurons and somatostatin (SST)-expressing GABAergic neurons showed opposite effect in seizure when the tested animals were fully kindled. Activation of SST-expressing GABAergic neurons showed an anti-seizure effect, however, activation of PV-expressing GABAergic neurons deteriorated seizure via depolarized signaling [21]. In this study, we labeled the subicular seizure-activated GABAergic neurons that were probably both PV-expressing and SST-expressing GABAergic neurons. Therefore, no effect to be observed would be foreseeable. (2) The limited number of GABAergic neurons are labeled. Here, we used a seizure-dependent manner to label subicular seizure-activated GABAergic interneurons that might be less than the number of the total GABAergic neurons, which may limit the overall effect on seizure. Notably, our present study shows that the subicular seizure-activated neurons, mainly targeting the subicular CaMKIIα+ glutamatergic neurons, were effective to alleviate both seizure and cognitive decline in epilepsy. This might be an important target for precise medication for epilepsy-associated cognitive deficits. Further, it is necessary to elucidate the function of subicular seizure-activated neurons in different TLE animal models such as the kainic acid model and pilocarpine model in the behavioral outcomes in both seizure and cognition [59].
Intact circuits guarantee correct information processing of learning and memory [60]. Repeated hippocampal seizures cause brain-wide reorganization of circuits and synchronization of hippocampal activity [17], which might lead to the fact that cognition engrams cells could not be activated during task performance. Then, we revealed how subicular seizure-activated neurons affect cognition. GCaMP6-based Ca2+ activity recording data indicated that subicular principal neurons exhibited higher response during exploring the moved object in OLM and novel object in NOR in the naïve non-kindled mice. However, we found that the kindled mice displayed similar neuronal activity no matter what object they were exploring in the behavior test. This suggested that epileptic seizure might indeed affect the functional activation of subicular neurons during cognitive task performance. Interestingly, we found a very small population of cognition-activated c-fos+ neurons in kindled mice. The number of OLM- and NOR-activated c-fos+ showed little overlap with the seizure-labeled mCherry in the subiculum. This might be due to the following two possibilities: (1) neurons responding to seizure and cognition are different populations in the subiculum, (2) seizure saturates the long-term potentiation in engram cells, limiting their further re-activation during cognitive tasks. Furthermore, inhibition of subicular s seizure-activated neurons could reverse this situation, as indicated by the increased number of OLM-activated and NOR-activated c-fos+ neurons and increased neuronal activity of subicular neurons during cognitive tasks in fiber photometry recording.
In conclusion, our results illustrated the subicular mechanism of epilepsy-associated cognitive dysfunction and supported subicular seizure-activated neurons as the potential target to alleviate cognitive impairment in the TLE. However, our results indicated that the specificity and efficiency of this E-SARE labeling technique were limited [37, 61], which may influence the interpretation of the results. As the half-life of tamoxifen (several hours) is much longer than the duration of the labeled ictal event (several minutes) [62, 63], it is inevitable that DREADDs expression can be not only in seizure-activated neurons in the subiculum but also some other events-activated neurons, such as interictal state-activated neurons [64]. In the future, drugs that can control the time window of the expression of Cre-recombinase more precisely may be able to solve this dilemma [65, 66].
Supplementary information
Acknowledgements
This project was supported by grants from the National Key R&D Program of China (2021ZD0202803 and 2020YFA0803902), the National Natural Science Foundation of China (82022071), and the Natural Science Foundation of Zhejiang Province (LD22H310003).
Author contributions
ZC, LY, and YW, contributed to conception and design of the study. LY, QZ, XQW, XYQ, FF, NXL, YYZ, QYZ, MDZ, YW, FW, CLX, and YPR contributed to acquisition and analysis of data. LY, YW, and ZC contributed to drafting the manuscript and figures.
Competing interests
The authors declare no competing interests.
Footnotes
These authors contributed equally: Lin Yang, Qi Zhang, Xue-qing Wu
Contributor Information
Yi Wang, Email: wang-yi@zju.edu.cn.
Zhong Chen, Email: chenzhong@zju.edu.cn.
Supplementary information
The online version contains supplementary material available at 10.1038/s41401-023-01129-z.
References
- 1.Trinka E, Kwan P, Lee B, Dash A. Epilepsy in Asia: disease burden, management barriers, and challenges. Epilepsia. 2019;60:7–21. doi: 10.1111/epi.14458. [DOI] [PubMed] [Google Scholar]
- 2.Xu C, Wang Y, Zhang S, Nao J, Liu Y, Wang Y, et al. Subicular pyramidal neurons gate drug resistance in temporal lobe epilepsy. Ann Neurol. 2019;86:626–40. doi: 10.1002/ana.25554. [DOI] [PubMed] [Google Scholar]
- 3.Engel J., Jr Introduction to temporal lobe epilepsy. Epilepsy Res. 1996;26:141–50. doi: 10.1016/S0920-1211(96)00043-5. [DOI] [PubMed] [Google Scholar]
- 4.Phuong TH, Houot M, Méré M, Denos M, Samson S, Dupont S. Cognitive impairment in temporal lobe epilepsy: contributions of lesion, localization and lateralization. J Neurol. 2021;268:1443–52. doi: 10.1007/s00415-020-10307-6. [DOI] [PubMed] [Google Scholar]
- 5.Caciagli L, Paquola C, He X, Vollmar C, Centeno M, Wandschneider B, et al. Disorganization of language and working memory systems in frontal versus temporal lobe epilepsy. Brain. 2022;146:935–53. doi: 10.1093/brain/awac150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Grote A, Heiland DH, Taube J, Helmstaedter C, Ravi VM, Will P, et al. ‘Hippocampal innate inflammatory gliosis only’ in pharmacoresistant temporal lobe epilepsy. Brain. 2022;146:549–60. doi: 10.1093/brain/awac293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Godrich D, Martin ER, Schellenberg G, Pericak-Vance MA, Cuccaro M, Scott WK, et al. Neuropathological lesions and their contribution to dementia and cognitive impairment in a heterogeneous clinical population. Alzheimers Dement. 2022;18:2403–12. doi: 10.1002/alz.12516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schnier C, Duncan S, Wilkinson T. A nationwide, retrospective, data-linkage, cohort study of epilepsy and incident dementia. Neurology. 2020;95:e1686–e1693. doi: 10.1212/WNL.0000000000010358. [DOI] [PubMed] [Google Scholar]
- 9.Lam AD, Sarkis RA. Association of epileptiform abnormalities and seizures in Alzheimer disease. Neurology. 2020;95:e2259–e2270. doi: 10.1212/WNL.0000000000010612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kaestner E, Reyes A, Chen A, Rao J, Macari AC, Choi JY, et al. Atrophy and cognitive profiles in older adults with temporal lobe epilepsy are similar to mild cognitive impairment. Brain. 2021;144:236–50. doi: 10.1093/brain/awaa397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nickels KC, Zaccariello MJ, Hamiwka LD, Wirrell EC. Cognitive and neurodevelopmental comorbidities in paediatric epilepsy. Nat Rev Neurol. 2016;12:465–76. doi: 10.1038/nrneurol.2016.98. [DOI] [PubMed] [Google Scholar]
- 12.Kahn JB, Port RG, Yue C, Takano H, Coulter DA. Circuit-based interventions in the dentate gyrus rescue epilepsy-associated cognitive dysfunction. Brain. 2019;142:2705–21. doi: 10.1093/brain/awz209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Josselyn SA, Tonegawa S. Memory engrams: recalling the past and imagining the future. Science. 2020;367:1–14. doi: 10.1126/science.aaw4325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jaworski J, Kalita K, Knapska E. c-Fos and neuronal plasticity: the aftermath of Kaczmarek’s theory. Acta Neurobiol Exp (Wars) 2018;78:287–96. doi: 10.21307/ane-2018-027. [DOI] [PubMed] [Google Scholar]
- 15.Yap EL, Greenberg ME. Activity-regulated transcription: bridging the gap between neural activity and behavior. Neuron. 2018;100:330–48. doi: 10.1016/j.neuron.2018.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Naik AA, Sun H, Williams CL, Weller DS, Julius Zhu J, Kapur J. Mechanism of seizure-induced retrograde amnesia. Prog Neurobiol. 2021;200:101984. doi: 10.1016/j.pneurobio.2020.101984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Choy M, Dadgar-Kiani E, Cron GO, Duffy BA, Schmid F, Edelman BJ, et al. Repeated hippocampal seizures lead to brain-wide reorganization of circuits and seizure propagation pathways. Neuron. 2022;110:221–36. doi: 10.1016/j.neuron.2021.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cembrowski MS, Phillips MG, DiLisio SF, Shields BC, Winnubst J, Chandrashekar J, et al. Dissociable structural and functional hippocampal outputs via distinct subiculum cell classes. Cell. 2018;173:1280–92. doi: 10.1016/j.cell.2018.03.031. [DOI] [PubMed] [Google Scholar]
- 19.Kitanishi T, Umaba R, Mizuseki K. Robust information routing by dorsal subiculum neurons. Sci Adv. 2021;7:1–19. doi: 10.1126/sciadv.abf1913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Torromino G, Autore L, Khalil V, Mastrorilli V, Griguoli M, Pignataro A, et al. Offline ventral subiculum-ventral striatum serial communication is required for spatial memory consolidation. Nat Commun. 2019;10:5721. doi: 10.1038/s41467-019-13703-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang Y, Xu C, Xu Z, Ji C, Liang J, Wang Y, et al. Depolarized GABAergic signaling in subicular microcircuits mediates generalized seizure in temporal lobe epilepsy. Neuron. 2017;95:92–105.e105. doi: 10.1016/j.neuron.2017.06.004. [DOI] [PubMed] [Google Scholar]
- 22.Vreugdenhil M, Hoogland G, van Veelen CW, Wadman WJ. Persistent sodium current in subicular neurons isolated from patients with temporal lobe epilepsy. Eur J Neurosci. 2004;19:2769–78. doi: 10.1111/j.1460-9568.2004.03400.x. [DOI] [PubMed] [Google Scholar]
- 23.Zhong K, Wu DC, Jin MM, Xu ZH, Wang Y, Hou WW, et al. Wide therapeutic time-window of low-frequency stimulation at the subiculum for temporal lobe epilepsy treatment in rats. Neurobiol Dis. 2012;48:20–6. doi: 10.1016/j.nbd.2012.05.011. [DOI] [PubMed] [Google Scholar]
- 24.Knopp A, Kivi A, Wozny C, Heinemann U, Behr J. Cellular and network properties of the subiculum in the pilocarpine model of temporal lobe epilepsy. J Comp Neurol. 2005;483:476–88. doi: 10.1002/cne.20460. [DOI] [PubMed] [Google Scholar]
- 25.Fujita S, Toyoda I, Thamattoor AK, Buckmaster PS. Preictal activity of subicular, CA1, and dentate gyrus principal neurons in the dorsal hippocampus before spontaneous seizures in a rat model of temporal lobe epilepsy. J Neurosci. 2014;34:16671–87. doi: 10.1523/JNEUROSCI.0584-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kinnavane L, Amin E, Olarte-Sánchez CM, Aggleton JP. Detecting and discriminating novel objects: the impact of perirhinal cortex disconnection on hippocampal activity patterns. Hippocampus. 2016;26:1393–413. doi: 10.1002/hipo.22615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kagerer SM, Schroeder C, van Bergen JMG, Schreiner SJ, Meyer R, Steininger SC, et al. Low subicular volume as an indicator of dementia-risk susceptibility in old age. Front Aging Neurosci. 2022;14:811146. doi: 10.3389/fnagi.2022.811146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Reddy DS. Brain structural and neuroendocrine basis of sex differences in epilepsy. Handb Clin Neurol. 2020;175:223–33. doi: 10.1016/B978-0-444-64123-6.00016-3. [DOI] [PubMed] [Google Scholar]
- 29.Hophing L, Kyriakopoulos P, Bui E. Sex and gender differences in epilepsy. Int Rev Neurobiol. 2022;164:235–76. doi: 10.1016/bs.irn.2022.06.012. [DOI] [PubMed] [Google Scholar]
- 30.Savic I. Sex differences in human epilepsy. Exp Neurol. 2014;259:38–43. doi: 10.1016/j.expneurol.2014.04.009. [DOI] [PubMed] [Google Scholar]
- 31.Chen B, Xu C, Wang Y, Lin W, Wang Y, Chen L, et al. A disinhibitory nigra-parafascicular pathway amplifies seizure in temporal lobe epilepsy. Nat Commun. 2020;11:923. doi: 10.1038/s41467-020-14648-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xu L, Lin W, Zheng Y, Chen J, Fang Z, Tan N, et al. An H2R-dependent medial septum histaminergic circuit mediates feeding behavior. Curr Biol. 2022;32:1937–48. doi: 10.1016/j.cub.2022.03.010. [DOI] [PubMed] [Google Scholar]
- 33.Wang Y, Wang Y, Xu C, Wang S, Tan N, Chen C, et al. Direct septum-hippocampus cholinergic circuit attenuates seizure through driving somatostatin inhibition. Biol Psychiatry. 2020;87:843–56. doi: 10.1016/j.biopsych.2019.11.014. [DOI] [PubMed] [Google Scholar]
- 34.Fei F, Wang X, Xu C, Shi J, Gong Y, Cheng H, et al. Discrete subicular circuits control generalization of hippocampal seizures. Nat Commun. 2022;13:5010. doi: 10.1038/s41467-022-32742-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sato M, Racine RJ, McIntyre DC. Kindling: basic mechanisms and clinical validity. Electroencephalogr Clin Neurophysiol. 1990;76:459–72. doi: 10.1016/0013-4694(90)90099-6. [DOI] [PubMed] [Google Scholar]
- 36.Racine RJ. Modification of seizure activity by electrical stimulation. II. Motor seizure. Electroencephalogr Clin Neurophysiol. 1972;32:281–94. doi: 10.1016/0013-4694(72)90177-0. [DOI] [PubMed] [Google Scholar]
- 37.Kawashima T, Kitamura K, Suzuki K, Nonaka M, Kamijo S, Takemoto-Kimura S, et al. Functional labeling of neurons and their projections using the synthetic activity-dependent promoter E-SARE. Nat Methods. 2013;10:889–95. doi: 10.1038/nmeth.2559. [DOI] [PubMed] [Google Scholar]
- 38.Urban DJ, Roth BL. DREADDs (designer receptors exclusively activated by designer drugs): chemogenetic tools with therapeutic utility. Annu Rev Pharmacol Toxicol. 2015;55:399–417. doi: 10.1146/annurev-pharmtox-010814-124803. [DOI] [PubMed] [Google Scholar]
- 39.Wang Y, Liang J, Chen L, Shen Y, Zhao J, Xu C, et al. Pharmaco-genetic therapeutics targeting parvalbumin neurons attenuate temporal lobe epilepsy. Neurobiol Dis. 2018;117:149–60. doi: 10.1016/j.nbd.2018.06.006. [DOI] [PubMed] [Google Scholar]
- 40.Sun Y, Jin S, Lin X, Chen L, Qiao X, Jiang L, et al. CA1-projecting subiculum neurons facilitate object-place learning. Nat Neurosci. 2019;22:1857–70. doi: 10.1038/s41593-019-0496-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nakai J, Ohkura M, Imoto K. A high signal-to-noise Ca2+ probe composed of a single green fluorescent protein. Nat Biotechnol. 2001;19:137–41. doi: 10.1038/84397. [DOI] [PubMed] [Google Scholar]
- 42.Goddard GV. Development of epileptic seizures through brain stimulation at low intensity. Nature. 1967;214:1020–1. doi: 10.1038/2141020a0. [DOI] [PubMed] [Google Scholar]
- 43.Morimoto K, Fahnestock M, Racine RJ. Kindling and status epilepticus models of epilepsy: rewiring the brain. Prog Neurobiol. 2004;73:1–60. doi: 10.1016/j.pneurobio.2004.03.009. [DOI] [PubMed] [Google Scholar]
- 44.Cánovas R, León I, Serrano P, Roldán MD, Cimadevilla JM. Spatial navigation impairment in patients with refractory temporal lobe epilepsy: evidence from a new virtual reality-based task. Epilepsy Behav. 2011;22:364–9. doi: 10.1016/j.yebeh.2011.07.021. [DOI] [PubMed] [Google Scholar]
- 45.Bakhtiari A, Bjørke AB, Larsson PG, Olsen KB, Nævra MCJ, Taubøll E, et al. Episodic memory dysfunction and effective connectivity in adult patients with newly diagnosed nonlesional temporal lobe epilepsy. Front Neurol. 2022;13:774532. doi: 10.3389/fneur.2022.774532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lalani SJ, Reyes A, Kaestner E, Stark SM, Stark CEL, Lee D, et al. Impaired behavioral pattern separation in refractory temporal lobe epilepsy and mild cognitive impairment. J Int Neuropsychol Soc. 2021;28:550–62. doi: 10.1017/S1355617721000734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Khodadadi M, Zare M, Rezaei M, Bakhtiarzadeh F, Barkley V, Shojaei A, et al. Effect of low frequency stimulation of olfactory bulb on seizure severity, learning, and memory in kindled rats. Epilepsy Res. 2022;188:107055. doi: 10.1016/j.eplepsyres.2022.107055. [DOI] [PubMed] [Google Scholar]
- 48.Papadogiannis A, Dimitrov E. A possible mechanism for development of working memory impairment in male mice subjected to inflammatory pain. Neuroscience. 2022;503:17–27. doi: 10.1016/j.neuroscience.2022.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Attili SM, Moradi K, Wheeler DW, Ascoli GA. Quantification of neuron types in the rodent hippocampal formation by data mining and numerical optimization. Eur J Neurosci. 2022;55:1724–41. doi: 10.1111/ejn.15639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cossart R, Dinocourt C, Hirsch JC, Merchan-Perez A, De Felipe J, Ben-Ari Y, et al. Dendritic but not somatic GABAergic inhibition is decreased in experimental epilepsy. Nat Neurosci. 2001;4:52–62. doi: 10.1038/82900. [DOI] [PubMed] [Google Scholar]
- 51.Cossart R, Bernard C, Ben-Ari Y. Multiple facets of GABAergic neurons and synapses: multiple fates of GABA signalling in epilepsies. Trends Neurosci. 2005;28:108–15. doi: 10.1016/j.tins.2004.11.011. [DOI] [PubMed] [Google Scholar]
- 52.Kanner AM, Bicchi MM. Antiseizure medications for adults with epilepsy: a review. JAMA. 2022;327:1269–81. doi: 10.1001/jama.2022.3880. [DOI] [PubMed] [Google Scholar]
- 53.Wang Y, Chen Z. An update for epilepsy research and antiepileptic drug development: toward precise circuit therapy. Pharmacol Ther. 2019;201:77–93. doi: 10.1016/j.pharmthera.2019.05.010. [DOI] [PubMed] [Google Scholar]
- 54.Miyakawa N, Nagai Y, Hori Y, Mimura K, Orihara A, Oyama K, et al. Chemogenetic attenuation of cortical seizures in nonhuman primates. Nat Commun. 2023;14:971. doi: 10.1038/s41467-023-36642-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chen LY, Liang J, Fei F, Ruan YP, Cheng HM, Wang Y, et al. Pharmaco-genetic inhibition of pyramidal neurons retards hippocampal kindling-induced epileptogenesis. CNS Neurosci Ther. 2020;26:1111–20. doi: 10.1111/cns.13434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lieb A, Weston M, Kullmann DM. Designer receptor technology for the treatment of epilepsy. EBioMedicine. 2019;43:641–9. doi: 10.1016/j.ebiom.2019.04.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhou QG, Nemes AD, Lee D, Ro EJ, Zhang J, Nowacki AS, et al. Chemogenetic silencing of hippocampal neurons suppresses epileptic neural circuits. J Clin Invest. 2019;129:310–23. doi: 10.1172/JCI95731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cǎlin A, Stancu M, Zagrean AM, Jefferys JGR, Ilie AS, Akerman CJ. Chemogenetic recruitment of specific interneurons suppresses seizure activity. Front Cell Neurosci. 2018;12:293. doi: 10.3389/fncel.2018.00293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Löscher W. Critical review of current animal models of seizures and epilepsy used in the discovery and development of new antiepileptic drugs. Seizure. 2011;20:359–68. doi: 10.1016/j.seizure.2011.01.003. [DOI] [PubMed] [Google Scholar]
- 60.Robertson EM. Memory leaks: information shared across memory systems. Trends Cogn Sci. 2022;26:544–54. doi: 10.1016/j.tics.2022.03.010. [DOI] [PubMed] [Google Scholar]
- 61.Kingsbury L, Huang S, Raam T, Ye LS, Wei D, Hu RK, et al. Cortical representations of conspecific sex shape social behavior. Neuron. 2020;107:941–53. doi: 10.1016/j.neuron.2020.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Fuchs WS, Leary WP, van der Meer MJ, Gay S, Witschital K, von Nieciecki A. Pharmacokinetics and bioavailability of tamoxifen in postmenopausal healthy women. Arzneimittelforschung. 1996;46:418–22. [PubMed] [Google Scholar]
- 63.Li C, Lim SC, Kim J, Choi JS. Effects of myricetin, an anticancer compound, on the bioavailability and pharmacokinetics of tamoxifen and its main metabolite, 4-hydroxytamoxifen, in rats. Eur J Drug Metab Pharmacokinet. 2011;36:175–82. doi: 10.1007/s13318-011-0036-y. [DOI] [PubMed] [Google Scholar]
- 64.Lai N, Cheng H, Li Z, Wang X, Ruan Y, Qi Y, et al. Interictal-period-activated neuronal ensemble in piriform cortex retards further seizure development. Cell Rep. 2022;41:111798. doi: 10.1016/j.celrep.2022.111798. [DOI] [PubMed] [Google Scholar]
- 65.Wang C, Liu H, Li K, Wu ZZ, Wu C, Yu JY, et al. Tactile modulation of memory and anxiety requires dentate granule cells along the dorsoventral axis. Nat Commun. 2020;11:6045. doi: 10.1038/s41467-020-19874-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gulmez Karaca K, Kupke J, Brito DVC, Zeuch B, Thome C, Weichenhan D, et al. Neuronal ensemble-specific DNA methylation strengthens engram stability. Nat Commun. 2020;11:639. doi: 10.1038/s41467-020-14498-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.