Abstract
Transcatheter aortic valve replacement (TAVR) is continually evolving, with a recent emphasis on a “minimalist” approach toward reducing procedural invasiveness, duration, and recovery time. Whereas a better understanding of the relationship between TAVR and new conduction disturbances has led to improved periprocedural management, intraprocedural rapid-pacing techniques have not evolved beyond traditional right ventricular temporary pacing. An alternative strategy utilizing the left ventricular guidewire for rapid pacing has been developed with evidence supporting its safety, effectiveness, and potential reductions in procedure time and cost. This review will outline the current best practices in left ventricular pacing for TAVR, a practical technique that embraces the minimalist approach to TAVR and may be considered for routine use. It aims to explore the current evidence and combine this with expert opinion to offer a strategy for temporary pacing that encourages efficiencies for physicians and patients without compromising periprocedural safety.
Keywords: Left ventricular pacing, Transcatheter aortic valve implantation, Transcatheter aortic valve replacement
Introduction
Transcatheter aortic valve replacement (TAVR) has evolved to become a safe, efficient procedure with an emphasis on a “minimalist” approach with regard to procedural invasiveness, duration, and recovery.1 Advancements in preprocedural planning, valve deployment technique, delivery system size, and deliverability have coincided with increasing operator comfort towards minimizing the use of procedural anesthesia, with most cases now performed under conscious sedation. Even with regard to monitoring for new conduction disease, physicians have become more skilled in determining pre- and post-TAVR risk for atrioventricular (AV) block, with risk stratification resulting in earlier discharge or earlier triage to enhanced monitoring or permanent pacemaker implantation when required.2,3 Despite these improvements, intraprocedural rapid-pacing techniques have not generally evolved beyond traditional temporary transvenous right ventricular (RV) pacing.
During TAVR, RV temporary pacing is routinely practiced via internal jugular (IJ) or femoral venous access, with placement of a transvenous temporary pacing wire within the RV. Intraprocedural rapid ventricular pacing is required for safe balloon-expandable valve deployment and, although not mandatory, is also frequently employed with self-expanding valves. Rapid pacing is necessary to reduce cardiac output in order to allow for precise valve positioning at deployment and to prevent valve embolization. However, RV pacing carries some risk of RV perforation with pericardial effusion or cardiac tamponade4 as well as vascular injury. Additionally, depending on technique, there is a risk of loss of capture and subsequent valve misplacement or embolization with RV pacing. Concerns for RV injury in the pediatric population led to a novel technique in balloon aortic valvuloplasty in which the left ventricular (LV) guidewire was used to perform rapid ventricular pacing.5 More recently, this alternative strategy for rapid pacing has been developed and proven to be safe and effective in adults undergoing balloon aortic valvuloplasty or TAVR.6, 7, 8 Furthermore, its use has been shown to reduce procedural cost and length of procedure including fluoroscopy time9,10 and it is now increasingly regarded as a reasonable alternative to RV pacing for TAVR.3,11
This review will outline current best practices in LV pacing for TAVR, a practical technique that embraces the minimalist approach to TAVR and may be considered for routine use. It aims to integrate the current evidence base with relevant expert opinion in order to offer a strategy for rapid ventricular pacing that encourages efficiencies for physicians and patients without compromising periprocedural safety.
LV Pacing Best Practice
This recommended approach to unipolar LV guidewire pacing outlines the key steps of LV guidewire pacing during the phases of the TAVR procedure; the procedure setup, LV guidewire placement, circuit grounding and connection, capture testing and valve deployment (Table 1). It is based on current evidence and author experience, noting some technical considerations and troubleshooting tips. Operators must consider suitability for LV pacing, as outlined in Figure 1.
Table 2.
Equipment list for left ventricular unipolar pacing during transcatheter aortic valve replacement
|
|
|
|
|
|
|
Abbreviations: LV, left ventricular; TAVR, transcatheter aortic valve replacement.
Figure 2.
(a-d) Grounding strategies for unipolar left ventricular pacing—the anode. This demonstrates the 3 possible anode options; (a) Subcutaneous needle, (b) intravascular wire, and (c) grounding pad. The red alligator clip should be placed at the tip of the grounding pad cable (red arrow). (d) When using the grounding pad, we recommend positioning on the lateral chest wall as shown. This is in proximity to the heart but does not obscure fluoroscopic or echocardiographic views.
Figure 3.
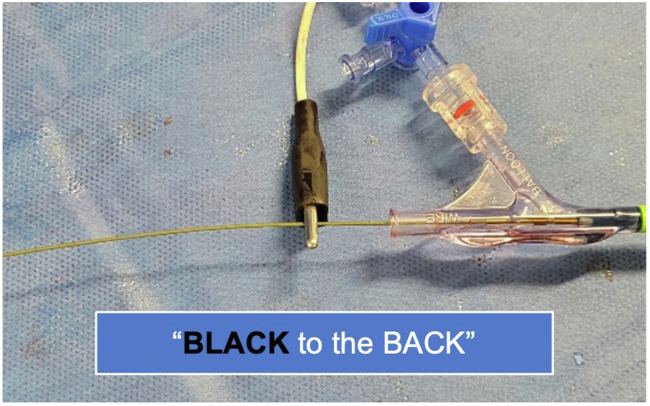
Circuit connection—the cathode: To complete the circuit, the black alligator clip should be placed on the left ventricular guidewire (cathode) distal to the valve delivery catheter as shown.
Figure 4.
Setup for capture check. Capture check should be done with valve system positioned with leading nose-cone in ascending aorta and left ventricular guidewire at the apex.
Figure 5.
Hemodynamics during rapid left ventricular pacing. Example of hemodynamic tracings during capture check.
Table 1.
A step-by-step guide to unipolar LV guidewire pacing
|
|
|
|
|
|
|
|
Abbreviations: LV, left ventricular; RV, right ventricular; TAVR, transcatheter aortic valve replacement; VVI, ventricular pacing and ventricular sensing.
Figure 1.
A proposed algorithm for temporary pacing in TAVR.
Abbreviations: HAVB/CHB, high-degree atrioventricular block/complete heart block; IJV, internal jugular vein; LV, left ventricular; LVOT, left ventricular outflow tract; PPM, pacemaker; RBBB, right bundle branch block; RV, right ventricular; TAVR, transcatheter aortic valve replacement; TPW, temporary pacing wire.
Tips and Tricks
This section covers some special considerations and troubleshooting tips when employing an LV pacing strategy.
The Threshold Check
Threshold checks should be performed using constant current and not constant voltage—this will depend on the configuration of the pacing generator box. Most contemporary external pacemaker generators deliver constant current which will be set in mA and not volts. The threshold check is recommended to be ∼50% of the threshold planned to be used during deployment,12 which is usually 25mA—hence a check at 12mA is sufficient. A threshold check using a very low current may result in poor capture when utilizing LV pacing. This is a notable difference when compared to RV pacing and may lead to unnecessary LV wire manipulation or placement of an RV pacing lead. Impedance, in accordance with Ohm’s law (voltage [V] = current [I] x impedance [Z]), can vary depending on physiological and pharmacologic factors (e.g., dehydration, body mass index, cardiac failure/myocardial scar, and antiarrhythmic drug use). We recommend this safety margin when performing capture check as the pacing wire is not fixed to the myocardium, it is easy to do and there are catastrophic consequences of loss of capture during deployment.
Sensed vs. Asynchronous Ventricular Pacing
Asynchronous pacing can be done but may increase the risk of an R-on-T or mask a premature ventricular complex. Hence, using the ventricular sensing and ventricular pacing mode is reasonable. That being said, some operators advocate for using asynchronous ventricular pacing mode for simplicity. It should be noted that if one chooses to pace in asynchronous ventricular pacing mode, the Capture Check should be done at deployment-range heart rates in order to outcompete intrinsic conduction and clearly demonstrate capture and attention given in the event ventricular tachycardia is induced. It can be difficult to appreciate capture at first because of the large electrocardiogram (EKG) spikes from LV pacing. New operators should observe a sustained hemodynamic change with loss of pulsatility and pressure lowering after 2-3 seconds, ignoring the electrical artifact on telemetry monitoring. Capture is improved by starting at a lower rate and performing ramp pacing to entrain the ventricle and mitigate the impact of the effective refractory period limiting capture.
Pacing in the Dilated Left Ventricle
The uncommon combination of severe aortic stenosis and a dilated left ventricle may degrade capture due to leak of electrical current. This may occur when the distance from the end of the insulated wire (i.e., the valve system nose cone) to where the wire contacts the LV apex is longer. In the scenario when this anatomy is noted and the capture check fails, the operator should consider RV pacing wire insertion.
Loss of Capture
While rare, reasons for loss of capture include:
-
•
Incorrect or insecure alligator clip positioning (inverted polarity, i.e., red to the back of the wire leads to significantly higher thresholds in a unipolar configuration).
-
•
Loss of grounding—for example, grounding needle is pulled out of the skin.
-
•
Loss of apical position of LV wire (e.g., If postdilating, the balloon shaft is not as stiff as the delivery system and may change guidewire contact with the LV apex).
-
•
Pacing delivery during the refractory period.
-
•
Oversensing can inhibit pacing and theoretically can occur from noise in the system.
Discussion
This review outlines the current best practices for utilizing the LV guidewire for rapid ventricular pacing during TAVR. In the framework of minimalist TAVR, this strategy provides a method for effective rapid ventricular pacing that can be routinely performed in almost all cases, while improving safety and decreasing procedure time and expense.
Conventional RV pacing techniques are somewhat inconsistent depending on operator preference, preprocedure EKG, and valve type. For example, procedure times may be longer if IJ venous access is desired in cases deemed higher risk for atrioventricular block, namely, baseline right bundle branch block (RBBB).13 Where some operators may prefer IJ venous access for more secure RV lead placement, others may prefer to persist with femoral placement of the pacing wire to ensure shorter procedure times—and only consider IJ pacing wire placement if there is confirmed conduction disturbance. Procedural time has been shown to be shortened by 7-13 minutes when RV pacing is not utilized9,14,15 and total cost per-patient at 30 days is significantly reduced, driven by materials and cath lab time spared.14 These combined benefits are important for cath lab efficiencies, budgets, patient access, and productivity as TAVR volumes continue to rise.
In the infrequent scenario when a retained pacemaker wire is needed, the operator can proceed with venous access and placement as required while continuing on-demand LV pacing. Alternatively, a reasonable strategy in those deemed high-risk would be upfront IJ venous access with temporary pacing wire placement, with consideration for a pacing wire with an active fixation lead, in this small subgroup and otherwise continuing an LV pacing strategy (Figure 1). With high-risk features such as baseline RBBB, complete heart block can be induced on LV guidewire placement before an LV pacing circuit is established and an upfront RV pacing strategy may be safest. For those RV pacing from the femoral vein and then inserting another pacing wire from the IJ vein when it is retained, risks for venous and RV injury would conceptually be doubled. We support a consistent approach to rapid pacing to ensure best practice for patient outcomes, cost-effectiveness, and workflow efficiency.
A reasonable concern in LV pacing is its reliability in maintaining capture through rapid pacing at valve deployment as well as in the postdeployment period where back-up pacing may be needed. These steps involve utilization of the LV guidewire for valve delivery, positioning, deployment, and withdrawal; careful wire control is required with the potential for wire displacement. Loss of capture occurs in 1% of cases using RV pacing, with a deflated or partially inflated balloon at the pacer tip resulting in 0.5% loss of capture rate compared to 4% when the balloon is fully inflated.16 The EASY TAVI14 randomized controlled trial compared safety and procedural efficiency of conventional RV pacing methods to LV pacing. 303 patients were randomized 1:1 and the trial demonstrated shorter procedural and fluoroscopy times with similar procedural safety and efficacy, suggesting no difference in loss of capture resulting in valve embolization. Another study by Hokken et al.9 compared an LV pacing (n = 488, 46% self-expanding valves) or no pacing (n = 139, all self-expanding valves) strategy in patients without high-risk EKG features to those with high-risk features who had RV pacing (n = 45, 51% self-expanding valves). There was no difference in valve embolization rate across all groups with only one patient requiring bailout RV pacemaker for poor LV capture (0.2%). Savvoulidis et al.15 examined 1226 consecutive patients, 2/3 of which had LV pacing and the remainder RV pacing. They found no differences in embolization due to loss of capture between groups. Loss of capture is a concern regardless of pacing modality, but current evidence dismisses the notion that LV pacing significantly worsens procedural success.
Conversely, LV pacing appears to have several notable advantages: it spares RV instrumentation and central venous access and their associated vascular injury risks; it reduces fluoroscopy and overall procedural time devoted to both access and lead placement; it overcomes recent pandemic-related supply-chain shortages where transvenous pacing wires have been unavailable or supply has been limited; and it lowers overall procedural cost related to equipment use and cath lab time.
Cardiac tamponade associated with TAVR is uncommon but life-threatening and can relate to RV perforation, LV guidewire injury (both from valve deployment and pacing), and annular rupture during valve intervention. A thin-walled, crescent-shaped structure, the RV is smaller in mass than the LV and has a free wall that is 3-5mm thinner,17 making it more at risk for perforation. There is little data on cardiac tamponade due to RV pacing lead placement. In two trials comparing LV and RV pacing techniques, tamponade was caused by an RV lead in 1.3% of cases, whereas no LV wire-related tamponade was seen.14,15 One study comparing full, partial, and no inflation of a balloon-tipped pacing wire for RV pacing demonstrated a 1.4% RV perforation rate in the deflated group, with no perforations in the partial and full inflation groups but significantly higher rate of capture loss.16 Overall, there appears to be a trade-off between maintaining capture and preventing RV injury when performing RV pacing. LV pacing may be a safer and more reliable technique and future studies are needed for a more definitive evaluation.
Despite this, care must be taken with the stiff LV guidewire and operators should balance the importance of safe wire position with achieving pacing capture. Classically, the most common 0.035” LV guidewires used include the preshaped SAFARI2 (Boston Scientific, Massachusetts, USA) and CONFIDA (Medtronic, Minnesota, USA) wires, as well as the unshaped Lunderquist (Cook Medical Inc., Indiana, USA), Amplatz Extra Stiff (Cook Medical, Inc., Indiana, USA), and Amplatz Super Stiff (Boston Scientific, Massachusetts, USA) wires. With different wire properties and shape, their conductivity and contact with the LV is varied with one study suggesting slightly lower thresholds achieved with the CONFIDA wire compared to the SAFARI2 wire.18 This finding may not have significant clinical relevance as all the aforementioned wires appear to work well. EASY TAVI showed safety across 4 wires with over 80% use of Amplatz Extra Stiff and Amplatz Super Stiff wires and less than 5% of CONFIDA wire use.14
Future Directions
Despite more recent evidence of high reliability, earlier concern for LV injury, loss of capture, and high thresholds from unipolar pacing has led to the development of dedicated LV-pacing wire systems. The Wattson temporary pacing guidewire (Teleflex, Inc., Minnesota, USA) is an 0.035” guidewire with an insulated stainless steel core and multiple exposed distal electrodes to facilitate consistent capture. It has bipolar pacing capabilities with early experience demonstrating procedural success with reliable pacing and no loss of capture.19 The Electroducer Sleeve (Electroducer, Grenoble, France) is a designed electroconductive sleeve device that can act as both sheath and enhanced electrical conductor to allow direct wire pacing, whether it be on the LV guidewire for TAVR or on the coronary guidewire for urgent transcoronary pacing in percutaneous coronary intervention. Results of a pilot study of 60 patients who underwent TAVR (n = 39) or percutaneous coronary intervention (n = 21) demonstrated its safety and hemodynamic effectiveness.20 SavvyWire (Opsens, Quebec, Canada) is a preshaped, sensor-guided LV guidewire that is designed for valve delivery, hemodynamic measurements (e.g., simultaneous LV and aortic pressures), and LV pacing. It has 2 sizes (small and X-small) and a feasibility study (NCT05082337) has completed with pending recruitment for a pivotal safety and efficacy trial (NCT05492383) titled SAvvyWire EFficacy and SafEty in Transcatheter Aortic Valve Implantation Procedures (SAFE-TAVI). Solo Pace (Solo Pace Inc, California, USA) is a first to market purpose built complete pacing kit for TAVR that is under development.21 It encompasses all modes of TAVR pacing including RV pacing (if desired), as well as LV unipolar pacing with a dedicated preshaped guidewire insulated to optimize pacing thresholds with or without the delivery system in place as an insulator. Furthermore, it includes a grounding pad for reliable routine grounding that is easily built into the workflow of the procedure. The system will be validated in a clinical trial to support approval of a TAVR pacing kit. There is an included table-mounted pace generator that is controllable by the operator from the sterile field with intuitive assistive technology that decreases user workload. There is experience with the use of grounding pads as a grounding anode instead of utilizing more established techniques involving a needle in the skin6 or an intravascular wire.22 The Booker Back defibrillator pad (TZ Medical, Oregon, USA) comes with a 9-ft cable with an exposed pin that can be clamped by the alligator clip directly and demonstrates good function as a ground, having been used successfully in over 300 TAVR cases with acceptable thresholds and no loss of capture (D. Daniels MD, personal communication, January 23, 2023).
Regardless of pacing strategy, operators must consider the postprocedural management of a patient with new conduction disturbance. The overall incidence of pacemaker implantation can be estimated at around 6.7%3 and varies between valve types. Balloon-expandable valves, the most widely used currently, result in pacemaker insertion rates of 4-24% in those treated with the SAPIEN 3 (Edwards Lifesciences, California, USA)10,23, 24, 25 and 5.8% in the newer-generation SAPIEN 3 Ultra (Edwards Lifesciences).23 Self-expanding valves have historically delivered higher pacemaker risk with rates with a pacemaker incidence of 15-27% in those treated with CoreValve Evolut R valves10,26 with the later-to-market self-expanding valves ACURATE Neo (Boston Scientific) and Portico (Abbott Structural Heart, Minnesota, USA)27,28 achieving lower rates. The development of the cusp-overlapping projection technique has allowed for a higher implantation depth more consistently, with significantly lower pacemaker rates seen in self-expanding valves.29
With identification of high-risk features that are anatomical (LV outflow tract calcification, membranous septum length), electrical (pre-existing conduction disease, notably RBBB), and procedural (valve type, balloon valvuloplasty, depth of implant), operators have become better at identifying patients more at risk for retention of a temporary pacing wire and/or permanent pacemaker. A patient-specific strategy whereby high-risk patients have upfront IJ venous pacing wire placement is a reasonable approach. However, current evidence and clinical experience suggest that an LV pacing strategy does not compromise even those with high-risk features. High-quality randomized trials are needed not only evaluating safety and efficacy of LV pacing but also evaluating the workflow and cost-effectiveness of LV pacing in TAVR. Pending results from these important upcoming trials, LV pacing may become the preferred rapid pacing strategy, in line with previous advancements in achieving a “minimalist” approach to TAVR.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Disclosure Statement
Dr Walsh is a consultant and proctor for Edwards Lifesciences. Dr Woods is has equity in Solo Pace Inc., and InHeart. He declares intellectual property with Attune Medical, is a consultant for Abbott, is on the education board at Atricure, received travel funds from Abbott and Atricure, has research funds from Biosense Webster. Dr Daniels is founder and CEO of Solo Pace, Inc.
Footnotes
Supplemental data for this article can be accessed on the publisher’s website.
Supplementary Material
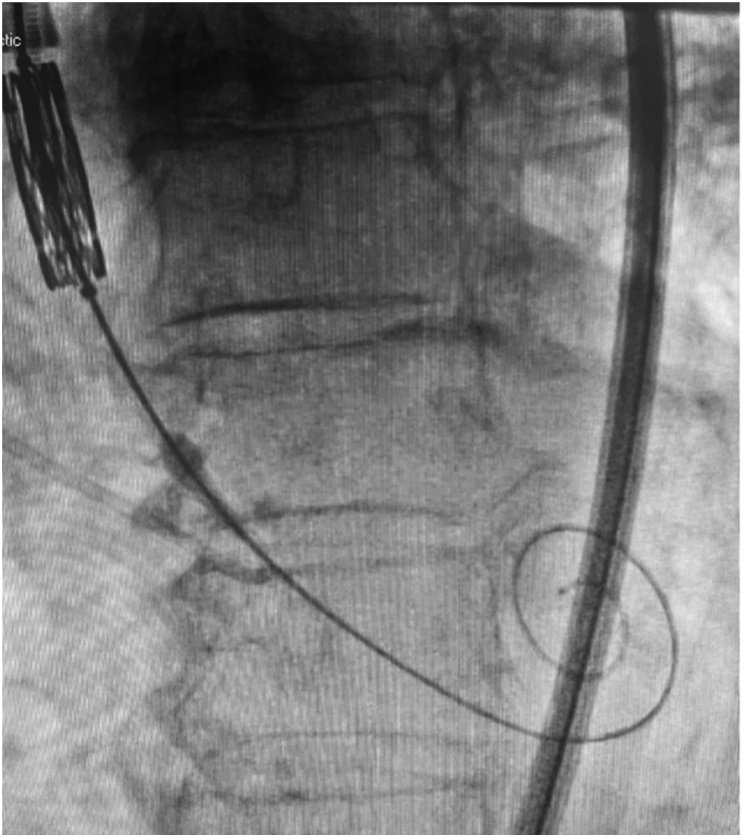
Desired fluoroscopic appearance of LV guidewire. Demonstrates compressed appearance in systole suggesting “snug” position in the LV apex. This position is best confirmed on echocardiography, where possible.
References
- 1.Wood D.A., Lauck S.B., Cairns J.A., et al. The vancouver 3M (multidisciplinary, multimodality, but minimalist) clinical pathway facilitates safe next-day discharge home at low-, medium-, and high-volume transfemoral transcatheter aortic valve replacement centers: the 3M TAVR study. JACC Cardiovasc Interv. 2019;12(5):459–469. doi: 10.1016/j.jcin.2018.12.020. [DOI] [PubMed] [Google Scholar]
- 2.Sammour Y., Krishnaswamy A., Kumar A., et al. Incidence, predictors, and implications of permanent pacemaker requirement after transcatheter aortic valve replacement. JACC Cardiovasc Interv. 2021;14(2):115–134. doi: 10.1016/j.jcin.2020.09.063. [DOI] [PubMed] [Google Scholar]
- 3.Rodes-Cabau J., Ellenbogen K.A., Krahn A.D., et al. Management of conduction disturbances associated with transcatheter aortic valve replacement: JACC scientific expert panel. J Am Coll Cardiol. 2019;74(8):1086–1106. doi: 10.1016/j.jacc.2019.07.014. [DOI] [PubMed] [Google Scholar]
- 4.Tanaka T., Sugiura A., Kavsur R., et al. Leaflet-to-annulus index and residual tricuspid regurgitation following tricuspid transcatheter edge-to-edge repair. EuroIntervention. 2022;18:e169–e178. doi: 10.4244/EIJ-D-21-00862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Karagoz T., Aypar E., Erdogan I., Sahin M., Ozer S., Celiker A. Congenital aortic stenosis: a novel technique for ventricular pacing during valvuloplasty. Catheter Cardiovasc Interv. 2008;72(4):527–530. doi: 10.1002/ccd.21695. [DOI] [PubMed] [Google Scholar]
- 6.Faurie B., Abdellaoui M., Wautot F., et al. Rapid pacing using the left ventricular guidewire: reviving an old technique to simplify BAV and TAVI procedures. Catheter Cardiovasc Interv. 2016;88(6):988–993. doi: 10.1002/ccd.26666. [DOI] [PubMed] [Google Scholar]
- 7.Hilling-Smith R., Cockburn J., Dooley M., et al. Rapid pacing using the 0.035-in. Retrograde left ventricular support wire in 208 cases of transcatheter aortic valve implantation and balloon aortic valvuloplasty. Catheter Cardiovasc Interv. 2017;89(4):783–786. doi: 10.1002/ccd.26720. [DOI] [PubMed] [Google Scholar]
- 8.Kleczynski P., Dziewierz A., Socha S., et al. Direct rapid left ventricular wire pacing during balloon aortic valvuloplasty. J Clin Med. 2020;9(4):1017. doi: 10.3390/jcm9041017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hokken T.W., de Ronde M., Wolff Q., et al. Insights in a restricted temporary pacemaker strategy in a lean transcatheter aortic valve implantation program. Catheter Cardiovasc Interv. 2022;99(4):1197–1205. doi: 10.1002/ccd.30026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ben-Shoshan J., Konigstein M., Zahler D., et al. Comparison of the Edwards SAPIEN S3 versus medtronic Evolut-R devices for transcatheter aortic valve implantation. Am J Cardiol. 2017;119(2):302–307. doi: 10.1016/j.amjcard.2016.09.030. [DOI] [PubMed] [Google Scholar]
- 11.Lilly S.M., Deshmukh A.J., Epstein A.E., et al. 2020 ACC expert consensus decision pathway on management of conduction disturbances in patients undergoing transcatheter aortic valve replacement: a report of the American College of Cardiology Solution Set Oversight Committee. J Am Coll Cardiol. 2020;76(20):2391–2411. doi: 10.1016/j.jacc.2020.08.050. [DOI] [PubMed] [Google Scholar]
- 12.Mulpuru S.K., Madhavan M., McLeod C.J., Cha Y.M., Friedman P.A. Cardiac pacemakers: function, troubleshooting, and management: part 1 of a 2-part series. J Am Coll Cardiol. 2017;69(2):189–210. doi: 10.1016/j.jacc.2016.10.061. [DOI] [PubMed] [Google Scholar]
- 13.Auffret V., Webb J.G., Eltchaninoff H., et al. Clinical impact of baseline right bundle branch block in patients undergoing transcatheter aortic valve replacement. JACC Cardiovasc Interv. 2017;10(15):1564–1574. doi: 10.1016/j.jcin.2017.05.030. [DOI] [PubMed] [Google Scholar]
- 14.Faurie B., Souteyrand G., Staat P., et al. Left ventricular rapid pacing via the valve delivery guidewire in transcatheter aortic valve replacement. JACC Cardiovasc Interv. 2019;12(24):2449–2459. doi: 10.1016/j.jcin.2019.09.029. [DOI] [PubMed] [Google Scholar]
- 15.Savvoulidis P., Mechery A., Lawton E., et al. Comparison of left ventricular with right ventricular rapid pacing on tamponade during TAVI. Int J Cardiol. 2022;360:46–52. doi: 10.1016/j.ijcard.2022.05.035. [DOI] [PubMed] [Google Scholar]
- 16.Tanaka M., Yanagisawa R., Yashima F., et al. A novel technique to avoid perforation of the right ventricle by the temporary pacing lead during transcatheter aortic valve implantation. Cardiovasc Interv Ther. 2021;36(3):347–354. doi: 10.1007/s12928-020-00676-0. [DOI] [PubMed] [Google Scholar]
- 17.Sanz J., Sanchez-Quintana D., Bossone E., Bogaard H.J., Naeije R. Anatomy, function, and dysfunction of the right ventricle: JACC state-of-the-art review. J Am Coll Cardiol. 2019;73(12):1463–1482. doi: 10.1016/j.jacc.2018.12.076. [DOI] [PubMed] [Google Scholar]
- 18.Tamura Y., Tamura Y., Konami Y., et al. Comparison of left ventricular pacing performance among pre-shaped guidewires designed for transfemoral-approach transcatheter aortic valve implantation. Heart Vessels. 2022;37(3):460–466. doi: 10.1007/s00380-021-01938-4. [DOI] [PubMed] [Google Scholar]
- 19.Hensey M., Sathananthan J., Alkhodair A., et al. Single-center prospective study examining use of the Wattson temporary pacing guidewire for transcatheter aortic valve replacement. Catheter Cardiovasc Interv. 2020;96(4):968–971. doi: 10.1002/ccd.28832. [DOI] [PubMed] [Google Scholar]
- 20.Wintzer-Wehekind J., Lefevre T., Benamer H., et al. A direct wire pacing device for transcatheter heart valve and coronary interventions: a first-in-human, multicentre study of the Electroducer Sleeve. EuroIntervention. 2023;18:1150–1555. doi: 10.4244/EIJ-D-22-00662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Daniels D. Left ventricle pacing Algorithm during TAVR: the "Solo pace" system. 2022. https://www.tctmd.com/slide/left-ventricle-pacing-algorithm-during-tavr-solo-pace-system Presented at Transcatheter Valve Therapeutics 2022.
- 22.Scarsini R., Kotronias R.A., De Maria G.L., et al. Routine left ventricular pacing for patients undergoing transcatheter aortic valve replacement. Struct Heart. 2019;3(6):478–482. [Google Scholar]
- 23.Rheude T., Pellegrini C., Lutz J., et al. Transcatheter aortic valve replacement with balloon-expandable valves: comparison of SAPIEN 3 Ultra versus SAPIEN 3. JACC Cardiovasc Interv. 2020;13(22):2631–2638. doi: 10.1016/j.jcin.2020.07.013. [DOI] [PubMed] [Google Scholar]
- 24.Vahanian A., Urena M., Walther T., et al. Thirty-day outcomes in patients at intermediate risk for surgery from the SAPIEN 3 European approval trial. EuroIntervention. 2016;12(2):e235–e243. doi: 10.4244/EIJV12I2A37. [DOI] [PubMed] [Google Scholar]
- 25.Fadahunsi O.O., Olowoyeye A., Ukaigwe A., et al. Incidence, predictors, and outcomes of permanent pacemaker implantation following transcatheter aortic valve replacement: analysis from the U.S. Society of Thoracic Surgeons/American College of Cardiology TVT Registry. JACC Cardiovasc Interv. 2016;9(21):2189–2199. doi: 10.1016/j.jcin.2016.07.026. [DOI] [PubMed] [Google Scholar]
- 26.Sorajja P., Kodali S., Reardon M.J., et al. Outcomes for the commercial use of self-expanding prostheses in transcatheter aortic valve replacement: a report from the STS/ACC TVT registry. JACC Cardiovasc Interv. 2017;10(20):2090–2098. doi: 10.1016/j.jcin.2017.07.027. [DOI] [PubMed] [Google Scholar]
- 27.Toggweiler S., Nissen H., Mogensen B., et al. Very low pacemaker rate following ACURATE neo transcatheter heart valve implantation. EuroIntervention. 2017;13(11):1273–1280. doi: 10.4244/EIJ-D-17-00252. [DOI] [PubMed] [Google Scholar]
- 28.Millan-Iturbe O., De Backer O., Bieliauskas G., et al. Transcatheter aortic valve implantation with the self-expanding portico valve system in an all-comers population: procedural and clinical outcomes. EuroIntervention. 2018;14(6):621–628. doi: 10.4244/EIJ-D-18-00488. [DOI] [PubMed] [Google Scholar]
- 29.Pascual I., Hernandez-Vaquero D., Alperi A., et al. Permanent pacemaker reduction using cusp-overlapping projection in TAVR: a propensity score analysis. JACC Cardiovasc Interv. 2022;15(2):150–161. doi: 10.1016/j.jcin.2021.10.002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Desired fluoroscopic appearance of LV guidewire. Demonstrates compressed appearance in systole suggesting “snug” position in the LV apex. This position is best confirmed on echocardiography, where possible.