1. Background
The cancer patients, survivors and care-givers across the world are seeking for Traditional Systems of Medicine (TSM) treatments in respective countries, in spite of the recent advances in the conventional oncology and expect their treating doctors to know about it [1].
A meta-analysis suggested that only 33% of patients disclose the details of TSM to the primary physician; and reasons of hiding the details are lack of time and interest of primary care provider, fear of denial or disapproval by treating consultant and assumption of TSM being safe [2].
In Sweden 26% [3], 31.4% in USA [4], Europe 35.9% [5], Japan 44.6% [6], in Canada 93% [7] of cancer patients used alternative treatments during their experience of Cancer. These numbers suggest the need to look in the worldwide demand of inclusive cancer care with TSM integration.
In India widely preferred TSM, Ayurveda is being used by almost every second patient [8].
The expectations from TSM are maintenance therapies, immune enhancers, strengthening self-defense mainly; while patients expect to attenuate the tumor burden; even when following conventional western medicine guidelines. In Japan, the Kampo system of medicine and modern system are working together [9].
The complementary and alternative medicines presently include the wide range of treatments from herbs, herbal medicine, Ayurveda – the ancient Indian System of Medicine, Homeopathy, Unani, Siddha system, Acupressure, Acupuncture, Oncology massage, Yoga therapies, Kampo medicine, Naturopathy, Tibetan, Peruvian, African and list may continue [10].
2. An initiative of integrative cancer care
2.1. Team building
The core team for cancer management in our institute includes a surgeon with a specialization in cancer surgery or an onco-surgeon, a medical oncologist, and a radiation oncologist.
To make the team more robust other clinicians such as a specialist pathologist – onco-pathologist, a palliative physician, and a specialist radiologist, an interventional radiologist, nuclear medicine consultants are added to the team.
This team approach becomes more inclusive after addition of specially trained oncology nurses, a psychologist, a clinical nutritionist, a physiotherapist, an occupational therapist, speech therapist, pharmacists, clinical psychologist, geneticists and molecular biologist.
In a progressive step towards providing all cancer treatments under one roof; a physician of Ayurveda having basic orientation of cancer treatment modules, pain management physicians have been added to the team for inclusive Cancer Care at this multi-specialty tertiary cancer care center.
2.2. Challenges for integrative cancer treatments (ICT)
This was one of the very few institutes of the western India where the integrative cancer care was initiated as team work for patient centric care. To begin with the concepts were discussed from Ayurveda about the Cancer and the translational clinical interpretations of those concepts to the application. The inquisitive discussions were quite interesting when it came to selection of drugs or medicines and referencing from ancient classical texts of Ayurveda to data in modern science, biosciences and publications.
The next step was to identify the potential areas with limitations of modern cancer care in those areas. In the beginning radiation induced dermatitis and Oral Mucositis was the area identified to explore the role of Ayurveda medicines in integrative way. Despite of advancement of radiation technology in Radiotherapy from Conformal Radiotherapy (CRT) to Intensity Modulated Radiotherapy (IMRT); still Oral mucositis was the troubling side effect for the Head & Neck Squamous Cell Carcinoma (HNSCC) patients undergoing radiotherapy. At times, some patients could not complete the treatment of radiation or chemo-radiation due to the severity of side effects and could get a recurrence. After discussions with radiation oncologists, we finalized a clinical protocol with standard assessment scale.
Our medical director extended affirmation and initially we treated only few patients and clinical observations were discussed in Tumor Board of the hospital. It was then decided that we should continue the oral Ayurveda Gargle Regimen for patients of HNSCC and assess after a significant number of patients and compare with the standard of care. The grade of acute toxicity was observed reduced for most of the HNSCC patients. At the end of initial two years when the observations were assessed, the results were quite encouraging in acute as well as late radiation toxicities and were again discussed in Tumor Board. Though the data suggested that the intensity of Oral mucositis had been controlled better with Ayurveda Gargle Regimen; there were debates on if reduction in side effects of Radiotherapy can lead to reducing the effects of Radiotherapy. The tumor board then suggested to observe the same set of patients for third year. After three years the data was again presented and discussed. Later the paper was published with three years follow up [11]. Thereafter, our Medical Director suggested a Randomized Controlled Trial for the Oral Mucositis. The approval from Scientific Advisory Committee and Institutional Ethics Committee were the two major rounds of learnings and discussions on various scientific and ethical aspects of integration. The trial completed the recruitment at present. It was a challenging task to conduct a trial in a public charitable hospital, as making funds available was a big task.
Next challenge was to explore possibilities to prescribe Ayurveda medicines per-oral, with ongoing chemotherapy drugs. There was again a series of brainstorming discussions, referring data; then for a new task to plan Ayurveda treatment for Chemotherapy Induced Nausea and Vomiting (CINV). These were the opportunities for integrative care.
The discussions were continued to explore more areas where Ayurveda can contribute. While discussing with onco-surgeons it was decided to explore possible benefit of Ayurveda treatment in patients of HNSCC those who were not eligible for Chemotherapy though indicated due to medical reasons like chronic kidney disease. The brainstorming continued with hemato-oncologists treating blood cancers where the Chemotherapy planning might have differed due to derailed liver profile. Ayurveda medicines indicated for liver diseases were planned and gradual correction in liver profile was observed. In some thyroid malignancies after surgery radioactive iodine is the treatment of choice; which leads to severe gastritis. The addition of Ayurveda medicines was helpful and so continued-add-on therapy.
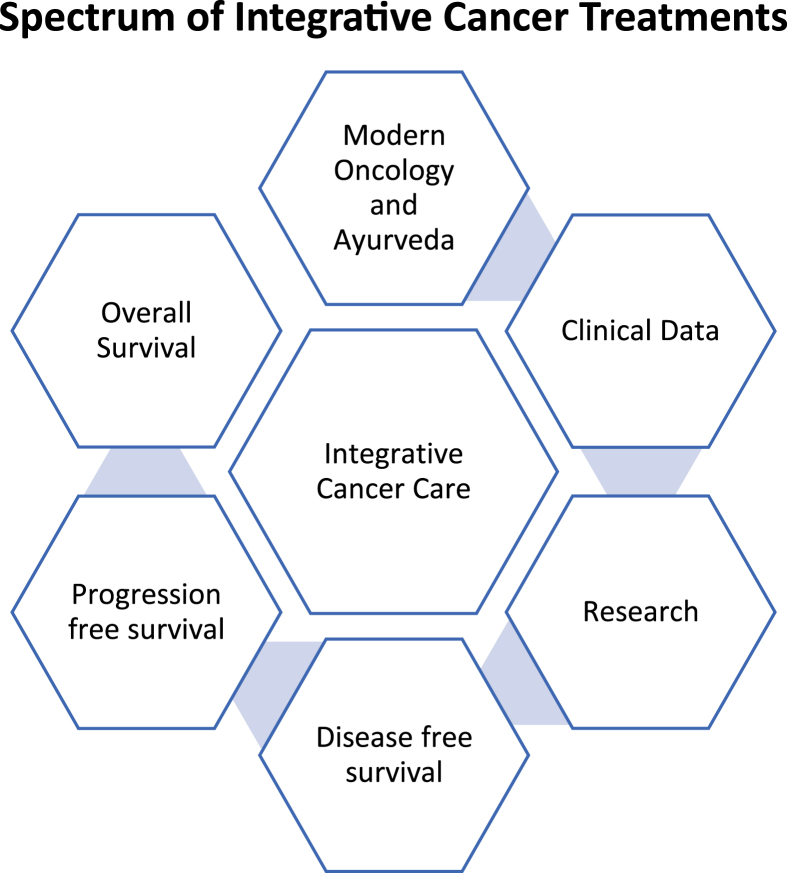
2.3. Spectrum of integrative cancer treatments
The role of integrative treatment has been categorized in three groups as per the intended goal; viz. Disease-Free Survival (DFS), Progression Free Survival (PFS), Overall Survival (OS).
A step ahead from supportive care; the role of Ayurveda was also explored for treating borderline Ovarian Cancer, as stand-alone treatment, post standard of care for PFS. The overall summary as per types of cancer, where integrated treatment was prescribed has been listed in Table 1.
Table 1.
The Spectrum of Clinical Data of Integrative Ayurveda Treatments at our Tertiary Cancer Hospital.
| Sr. No. | Site of Cancer | Sub-site of Cancer | Type | Maintenance/Recurrent/Metastatic | ICT intended for DFS/PFS/OS | Minimizing side effects |
|---|---|---|---|---|---|---|
| 1 | Oral Cavity | Tongue | Squamous Cell Carcinoma (SCC) | All | All | Radiation Toxicity |
| Base of Tongue | Squamous Cell Carcinoma (SCC) | All | All | Radiation Toxicity | ||
| Buccal Mucosa | Squamous Cell Carcinoma (SCC) | All | All | Radiation Toxicity | ||
| GBS/Alveolus | Squamous Cell Carcinoma (SCC) | All | All | Radiation Toxicity | ||
| Lip | Squamous Cell Carcinoma (SCC) | All | DFS | Radiation Toxicity | ||
| Maxilla | Squamous Cell Carcinoma (SCC) | Recurrent | PFS | Radiation Toxicity | ||
| Soft Palate | Squamous Cell Carcinoma (SCC) | All | DFS | Radiation Toxicity | ||
| 2 | Oropharynx | Tonsils | Human Papilloma Virus (HPV) | Maintenance | DFS | Chemotherapy Toxicity |
| Non-HPV | Maintenance | DFS | Radiation Toxicity | |||
| Larynx/Vocal Cord | Squamous Cell Carcinoma (SCC) | Recurrent | PFS | Radiation Toxicity | ||
| 3 | Nasopharynx | Nasopharynx | Papillary | Recurrent | PFS | Chemotherapy Toxicity |
| Squamous Cell Carcinoma (SCC) | Recurrent | PFS/OS | Radiation Toxicity | |||
| 4 | Thyroid | Recurrent | PFS/OS | Radio-Iodine Toxicity | ||
| 5 | Mediastinal | Thymoma | Metastatic | OS | Chemotherapy Toxicity | |
| SCLC (Small Cell Lung Cancer) | Metastatic | OS | Chemotherapy Toxicity | |||
| NSCLC (Non-small cell Lung Cancer) | Metastatic | OS | Radiation Toxicity | |||
| 6 | Upper GI (Gastro-intestinal) | Esophagus | Adenocarcinoma | Recurrent | PFS/OS | Chemotherapy Toxicity |
| Squamous Cell Carcinoma (SCC) | Recurrent | PFS/OS | Radiation Toxicity | |||
| Stomach | Adenocarcinoma | Recurrent | PFS | Chemotherapy Toxicity | ||
| Signet Cell | Metastatic | OS | Chemotherapy Toxicity | |||
| Gastrointestinal stromal tumor | Metastatic | OS | Chemotherapy Toxicity | |||
| 7 | Hepato-billiary | Common Bile Duct | Metastatic | OS | Chemotherapy Toxicity | |
| Gall Bladder | Maintenance/Metastatic | PFS/OS | Chemotherapy Toxicity | |||
| Pancreas | Maintenance/Metastatic | PFS/OS | Chemotherapy Toxicity | |||
| Peri-ampullary | Metastatic | OS | Chemotherapy Toxicity | |||
| Hepatocellular Carcinoma | Metastatic | OS | ||||
| 8 | Lower GI (Gastro-intestinal) | Cecum | Maintenance/Metastatic | PFS/OS | Chemotherapy Toxicity | |
| Colon | Maintenance/Metastatic | All | Chemotherapy Toxicity | |||
| Sigmoid | Maintenance/Metastatic | PFS/OS | ||||
| Rectum | Squamous Cell Carcinoma (SCC) | Maintenance/Metastatic | PFS/OS | Radiation Toxicity | ||
| Adenocarcinoma | Maintenance/Metastatic | PFS/OS | Chemotherapy Toxicity | |||
| 9 | Peritoneum | Primary Peritoneal cancer | Recurrent | PFS/OS | Chemotherapy Toxicity | |
| Pseudomyxoma peritonei | Recurrent | PFS/OS | ||||
| 10 | Gynaecology Cancers | Breast | Hormone Positive | Maintenance/Metastatic | PFS/OS | Chemotherapy Toxicity |
| Triple Negative | Maintenance/Metastatic | PFS/OS | ||||
| Ovary | Adenocarcinoma | All | All | |||
| Papillary | Recurrent | PFS | ||||
| Clear cell | Maintenance/Recurrent | PFS | ||||
| Cervix | Squamous Cell Carcinoma (SCC) | Maintenance/Metastatic | PFS/OS | Radiation Toxicity | ||
| Endometrium | All | Radiation Toxicity | ||||
| 11 | Sarcoma | Ewing's | Metastatic | OS | Chemotherapy Toxicity | |
| Leiomyosarcoma | Metastatic | OS | ||||
| Osteogenic | Metastatic | OS | ||||
| Gastrointestinal stromal tumor | Metastatic | OS | ||||
| Dermatofibrosarcoma | Metastatic | OS | ||||
| Synovial | Maintenance | DFS/PFS | ||||
| 12 | Uro-genital Cancers | Renal | Renal Cell carcinoma | Metastatic | ||
| Adrenal | Maintenance/Metastatic | |||||
| Prostate | Hormone refractory | Recurrent/Metastatic | PFS/OS | |||
| Castration resistant | Recurrent/Metastatic | PFS/OS | ||||
| Urinary Bladder | Metastatic | OS | ||||
| 13 | Liquid Tumors | Hodgkin's Lymphoma | Maintenance | PFS/OS | Chemotherapy Toxicity | |
| Acute Myeloid Leukemia | Maintenance/Relapse | PFS/OS | Chemotherapy Toxicity | |||
| Chronic Myeloid Leukemia | Maintenance/Relapse | PFS/OS | ||||
| Acute Lymphoblastic Leukemia | Maintenance/Relapse | PFS/OS | Chemotherapy Toxicity | |||
| Chronic Lymphocytic Leukemia | Maintenance/Relapse | PFS/OS | ||||
| Non-Hodgkin's Lymphoma | Maintenance/Relapse | PFS/OS | Chemotherapy Toxicity | |||
| Multuiple Myeloma | Metastatic | OS | Chemotherapy Toxicity | |||
| 14 | Neuro Endocrine Tumors | Recurrent | PFS/OS | Chemotherapy Toxicity |
DFS - Disease Free Survival; PFS - Progression Free Survival; OS - Overall Survival.
The intent of Integrative Cancer Treatments at our hospital has been discussed in Table 1. Present discussion paper is based on the clinical observations and experiences till date from this center; some leads from which may come to the aid of setting up basic goals for integrative treatments in upcoming cancer centres with interdisciplinary units. The intended goals of DFS, PFS, OS discussed in this paper, may get revised / modified in near future with gathering of clinical data from multiple integrative cancer units; based on continued periodic assessments in maintenance, receurrence &/ or seconadry prevention strategies. The essesntial check-points for integrative cancer treatments are the systematic inclusion of the patients, understanding scope & limitations of every system of medicine involved in patient care, deciding the intent with duaration of integrative treatments and dose-dependent herbal / herbo-mineral interventions. The system for close monitoring of drug procurement, herb selection as per text references and meticulous dispensing in therapeutic dosage as per posology were the key factors of Integrative Ayurveda Cancer Therapy. This process was keenly observed to avoid so called heavy metal contamination in herbal drugs.
There were questions raised towards the use of Ayurveda treatments and the possible renal and hepatic toxicity; but it did not confirm on whether the data collected was from the prescriptions by qualified Ayurveda physicians or from OTC herbs being sold under the label of Ayurveda. In our institution, the data has been maintained along with the detailed reports of renal function tests and liver function tests at periodic interval for every patient being treated with integrative approach.
In spite of first-hand clinical data, published case report, case series, case control studies, randomized trials; the funding remains one of the challenges for conducting more integrative clinical studies with robust study design. It would have been contributory help if Government funding agencies or some industry support these studies from Ayurveda & Integrative Cancer Care.
The patients who were willing to undergo Ayurveda cancer treatments during or after their modern cancer treatments; had been offered Ayurveda treatments either add-on with modern treatments or after standard of care treatment for that respective disease. The phase of treatment has been tabulated in Table 1.
3. Discussion
3.1. Standpoint
The ancient and traditional systems of medicines like Ayurveda mainly have clinical experience-based interpretation of diseases including diagnostics [12] as well as prognostication [13], in the languages those were well versed during the era when the doctrines were written.
In spite of all efforts in evidence-based medicine; the response to the treatment still varies [14], which directly affects the survival of the patients.
The specific causality of partial response or no response remains unclear [15] despite of series of clinical trials, research; and this leads to the apprehension in patients and their relatives leading to use Add-on Traditional Systems of Medicines (TSM), like Ayurveda, Siddha, Unani etc for the diseases like Cancer. Most of the times patients are willing to undergo TSM [16] with the Physician of respective systems of medicine. The lack of availability of Physicians of such traditional system of medicines or unawareness about the availability among patients as well as primary service providers in healthcare system; make patients search and opt for over the counter (OTC) products [17]. The OTC products at times mention tall claims, which misguides patients and by the time they return to Oncology experts, the upstaging of cancer might already has occurred.
The anxiety of patients about the side effects or adverse effects of the modern oncology treatments, chemotherapy and radiotherapy in particular; keep them looking for TSM as safer option [18].
Our team believed that, though Ayurveda is considered as a safer option most of the times, patients may not get a proper prescription in therapeutic dosage without the Ayurveda Physician with Oncology exposure.
Overall, cancer is a time sensitive disease and even delay in diagnosis can change the prognosis [19]. Therefore, for providing upfront clinical consultation to willing patients, inclusion of an Ayurveda Physician with experience in Oncology has been done.
The advantage of the inclusion of an TSM/Ayurveda Physician in the interdisciplinary team keeps all diagnostic and treatment data together along with the follow up data as well as surveillance data [20].
We experienced a lesser chance of losing the proper track of treatment and Ayurveda can be added for such patients at appropriate stages, being discussed in this paper.
There are certain known side effects of Chemotherapy [21] and Radiotherapy [22] which affect the quality of life in patients undergoing cancer treatment; efforts to minimize the same have been initiated at our center by inclusion of Ayurveda medicines with primary care.
In continued patient centric efforts, the study conducted in this hospital revealed the statistically significant reduction in Radiation Induced Oral Mucositis during radiotherapy for HNSCC with an Ayurveda Gargle regimen and with the added advantage of lesser recurrence after follow up of three years [11].
In cancers like Ovarian Cancers, which are known for high recurrence rate; limited affordable options are available for maintenance treatment [23]. The level IV evidence on significant Progression Free Survival (PFS) when prescribed an Ayurveda Maintenance Therapy [24] in Recurrent Ovarian Cancers has opened up discussion on exploring the role of Ayurveda hand in hand with modern cancer care for expanding the horizons of cancer care.
The scope for integrative approach remains unexplored in treatment of aggressive cancers like Pancreas cancer, carcinoma Gall bladder, Osteosarcoma, Melanoma and leukemia in early as well as advanced stages. Effect of these integrative approaches need to be studies while following such patients long term for overall survival (OS); like we have long term observational studies in breast cancer patients. Triple negative breast cancer is another area where the role of herbs from TSM has been explored [25].
Our Integrative Cancer Unit has added a valuable support in patients who are not eligible for standard chemotherapy though indicated; due to personal, social as well as medical reasons like derailed renal functions [26] or deranged liver functions [27]. This will eventually surface more and more areas to identify for comprehensive treatments.
3.2. Oblique point
The cancer treatment in today's world is unanimously based on data after Randomized Controlled Trials (RCTs) [28].
TSM lacks not only clinical evidences but also robust testing of clinical data [29] and does not comment on generalization of outcome. The uniformity in diagnosis and treatment selection are other challenges with TSM. Free exchange of knowledge, understanding global terminologies and open interaction form the basis of any integrative platform [30].
Out of pocket expenses in cancer are burden in high income countries; for a middle-income country like India and other low-income countries, the government has floated many health schemes at state and national levels [31]. Modern cancer care has been included in all these healthcare schemes with uniform guidelines; whereas TSM is still at fringe and struggling to generate uniform baseline data [32].
3.3. Crossover point
In our institute the discussions happen among in Multi-disciplinary Tumor Boards about planning and execution of studies like radiation induced Oral Mucositis in HNSCC and other potential areas.
The initiative of generating level IV evidences can be a stepping stone towards level I evidence for TSM based integrative cancer care.
The existing FDA approved Ayurveda formulations can be tested for re-purposing [33] in treatment of various side effects of Chemotherapy and Radiotherapy; in adjuvant settings; in supportive care; in palliative care in cancer patients.
Pillars of integrative Cancer Treatment at Deenath Mangeshkar Hospital.
3.4. Debate point
There is need for both Randomized Controlled Trials (RCT) as well as Real World Data (RWD) to provide the totality of evidence towards definitive answers for treatment decisions [34]. The RCTs and RWDs collectively can answer questions related to access, adherence, tolerance, short-term and long-term safety of interventions in patients’ care.
There cannot be two types of medical systems; mainstream and alternative. No medicine can be alternative as far as its rigorously tested for safety and efficacy [35]; the parameters of testing differ from RCT to RWD.
The inclination for TSM with evidences to modern medicine has initiated a collaborative research and evidence generation for Ayurveda & Homeopathy; by India and United States of America [36].
3.5. Swift point
The initiative by our hospital of providing an opportunity for the willing patients to get an upfront consultation by the Ayurveda physician well-versed with Oncology principles has been contributory to the inclusive treatment planning in neo-adjuvant, adjuvant, maintenance, palliative stages as well as to diligent decision making for patients and minimize the undocumented treatments in cancer.
The practical comprehensive care will be an open-ended healthcare system with integration of TSM like Ayurveda and modern medicines with regular dialogue among the cancer treating physicians of either systems in the interest of patients, where the collective experience will generate the evidence to develop a network of integrative cancer care units in tertiary cancer hospitals.
Conflict of interest
None.
Source of funding
None.
Acknowledgement
1. Department of Onco-surgery & Department of Nuclear Medicine.
2. Thanks to the editorial team of the journal for inviting us for writing a discussion paper on documented experiences about the evolving concept of integrative cancer care.
Footnotes
Peer review under responsibility of Transdisciplinary University, Bangalore.
References
- 1.Milton D. Alternative and complementary therapies: integration into cancer care. AAOHN J. 1998;46(9):454–461. quiz 462-3. PMID: 9923206. [PubMed] [Google Scholar]
- 2.Foley H, Steel A, Cramer H, Wardle J, Adams J. Disclosure of complementary medicine use to medical providers: a systematic review and meta-analysis. Sci Rep. 2019 Feb 7;9(1):1573. 10.1038/s41598-018-38279-8. PMID: 30733573; PMCID: PMC6367405. [DOI] [PMC free article] [PubMed]
- 3.Wode K, Henriksson R, Sharp L, Stoltenberg A, Hök Nordberg J. Cancer patients' use of complementary and alternative medicine in Sweden: a cross-sectional study. BMC Complement Altern Med. 2019 Mar 13;19(1):62. 10.1186/s12906-019-2452-5. PMID: 30866916; PMCID: PMC6417272. [DOI] [PMC free article] [PubMed]
- 4.Ernst E. The prevalence of complementary/Alternative medicine in cancer. Cancer. 1998;83:777–782. doi: 10.1002/(SICI)1097-0142(19980815)83:4<777::AID-CNCR22>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 5.Molassiotis A, Fernández-Ortega P, Pud D, Ozden G, Scott JA, Panteli V, Margulies A, Browall M, Magri M, Selvekerova S, Madsen E, Milovics L, Bruyns I, Gudmundsdottir G, Hummerston S, Ahmad AM, Platin N, Kearney N, Patiraki E. Use of complementary and alternative medicine in cancer patients: a European survey. Ann Oncol. 2005 Apr;16(4):655-63. 10.1093/annonc/mdi110. Epub 2005 Feb 2. PMID: 15699021. [DOI] [PubMed]
- 6.Hyodo I, Amano N, Eguchi K, Narabayashi M, Imanishi J, Hirai M, Nakano T, Takashima S. Nationwide survey on complementary and alternative medicine in cancer patients in Japan. J Clin Oncol. 2005 Apr 20;23(12):2645-54. 10.1200/JCO.2005.04.126. Epub 2005 Feb 22. PMID: 15728227. [DOI] [PubMed]
- 7.Truant T.L., Balneaves L.G., Fitch M.I. Integrating complementary and alternative medicine into cancer care: Canadian oncology nurses' perspectives. Asia Pac J Oncol Nurs. 2015;2(4):205–214. doi: 10.4103/2347-5625.167233. PMID: 27981116; PMCID: PMC5123512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pandey L., Pasricha R., Joseph D., Ahuja R., Yanthan Y., Garg P.K., et al. Use of complementary and alternative medicine among patients with cancer in a sub-Himalayan state in India: an exploratory study. J Ayurveda Integr Med. 2021;12(1):126–130. doi: 10.1016/j.jaim.2021.01.001. Epub 2021 Feb 23. PMID: 33637425; PMCID: PMC8039360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yamakawa J., Motoo Y., Moriya J., Ogawa M., Uenishi H., Akazawa S., et al. Role of Kampo medicine in integrative cancer therapy. Evid Based Complement Alternat Med. 2013;2013 doi: 10.1155/2013/570848. Epub 2013 Aug 22. PMID: 23997796; PMCID: PMC3723058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.https://www.who.int/traditional-complementary-integrative-medicine/WhoGlobalReportOnTraditionalAndComplementaryMedicine2019.pdf.
- 11.Wanjarkhedkar P., Pingley S., Shende S., Kelkar D., Parasnis A., Sambhus M., et al. An ayurveda gargle regimen in management of radiotherapy-induced oral mucositis. South Asian J Cancer. 2020 Oct;9(4):250–252. doi: 10.1055/s-0041-1726138. Epub 2021 Jun 15. PMID: 34141687; PMCID: PMC8205557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dwivedi R.B., Dubey S.D., Gujarathi R., Singh A.Khandel S.K., Rai S. In: "Jwara Nidana Adhyaya". Charak Samhita New Edition. 1st ed. Khandel S.K., Godatwar P., Deole Y.S., Basisht G., editors. CSRTSDC; 2020. p. 34. [DOI] [Google Scholar]
- 13.Khandel S.K. In: "Indriya Sthana". Charak Samhita New Edition. 1st ed. Deole Y.S., Basisht G., editors. CSRTSDC; 2020. p. 60. [DOI] [Google Scholar]
- 14.Yang R., Niepel M., Mitchison T.K., Sorger P.K. Dissecting variability in responses to cancer chemotherapy through systems pharmacology. Clin Pharmacol Ther. 2010 Jul;88(1):34–38. doi: 10.1038/clpt.2010.96. Epub 2010 Jun 2. PMID: 20520606; PMCID: PMC2941986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Alfarouk K.O., Stock C.M., Taylor S., et al. Resistance to cancer chemotherapy: failure in drug response from ADME to P-gp. Cancer Cell Int. 2015;15:71. doi: 10.1186/s12935-015-0221-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Knecht K., Kinder D., Stockert A. Biologically-based complementary and alternative medicine (CAM) use in cancer patients: the good, the bad, the misunderstood. Front Nutr. 2020 Jan 24;6:196. doi: 10.3389/fnut.2019.00196. PMID: 32039227; PMCID: PMC6992534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Adam K., Agnieszka B., Kamila B., Katarzyna K.B. Use of the herbal OTC products and dietary supplements by patients receiving chemotherapy: survey-based study. Indian J Pharm Educ Res. 2017;51(4s):s675–s678. doi: 10.5530/ijper.51.4s.98. [DOI] [Google Scholar]
- 18.Smith P.J., Clavarino A.M., Long J.E., Anstey C.M., Steadman K.J. Complementary and alternative medicine use by patients receiving curative-intent chemotherapy. Asia Pac J Clin Oncol. 2016 Sep;12(3):265–274. doi: 10.1111/ajco.12490. Epub 2016 Apr 4. PMID: 27044569. [DOI] [PubMed] [Google Scholar]
- 19.Cone E.B., Marchese M., Paciotti M., et al. Assessment of time-to-treatment initiation and survival in a cohort of patients with common cancers. JAMA Netw Open. 2020;3(12) doi: 10.1001/jamanetworkopen.2020.30072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wheeler S.B., Basch E. Translating cancer surveillance data into effective public health interventions. JAMA. 2017;317(4):365–367. doi: 10.1001/jama.2016.20326. [DOI] [PubMed] [Google Scholar]
- 21.Marx W., McCarthy A.L., Ried K., McKavanagh D., Vitetta L., Sali A., et al. The effect of a standardized ginger extract on chemotherapy-induced nausea-related quality of life in patients undergoing moderately or highly emetogenic chemotherapy: a double blind, randomized, placebo controlled trial. Nutrients. 2017 Aug 12;9(8):867. doi: 10.3390/nu9080867. PMID: 28805667; PMCID: PMC5579660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stubbe C.E., Valero M. Complementary strategies for the management of radiation therapy side effects. J Adv Pract Oncol. 2013 Jul;4(4):219–231. doi: 10.6004/jadpro.2013.4.4.3. PMID: 25032003; PMCID: PMC4093430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Walsh C.S. Latest clinical evidence of maintenance therapy in ovarian cancer. Curr Opin Obstet Gynecol. 2020;32(1):15–21. doi: 10.1097/GCO.0000000000000592. PMID: 31833941. [DOI] [PubMed] [Google Scholar]
- 24.Wanjarkhedkar, P., Kulkarni, P., Hingmire, S., Kelkar, D., & Bokil, K. (2022). Ayurveda Maintenance Therapy in Recurrent Ovarian Cancer. Indian Journal of Medical and Paediatric Oncology, 43(05), 434–438. 10.1055/s-0041-1740323. [DOI]
- 25.Webb M.J., Kukard C. A review of natural therapies potentially relevant in triple negative breast cancer aimed at targeting cancer Cell vulnerabilities. Integr Cancer Ther. 2020;19 doi: 10.1177/1534735420975861. PMID: 33243021; PMCID: PMC7705812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Horie S., Oya M., Nangaku M., Yasuda Y., Komatsu Y., Yanagita M., et al. Guidelines for treatment of renal injury during cancer chemotherapy 2016. Clin Exp Nephrol. 2018;22(1):210–244. doi: 10.1007/s10157-017-1448-z. PMID: 28856465; PMCID: PMC5805816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Segal I., Rassekh S.R., Bond M.C., Senger C., Schreiber R.A. Abnormal liver transaminases and conjugated hyperbilirubinemia at presentation of acute lymphoblastic leukemia. Pediatr Blood Cancer. 2010;55(3):434–439. doi: 10.1002/pbc.22549. PMID: 20658613. [DOI] [PubMed] [Google Scholar]
- 28.Tannock I.F., Amir E., Booth C.M., Niraula S., Ocana A., Seruga B., et al. Relevance of randomised controlled trials in oncology. Lancet Oncol. 2016;17(12):e560–e567. doi: 10.1016/S1470-2045(16)30572-1. PMID: 27924754. [DOI] [PubMed] [Google Scholar]
- 29.Bloom B.S., Retbi A., Dahan S., Jonsson E. Evaluation of randomized controlled trials on complementary and alternative medicine. Int J Technol Assess Health Care. 2000 Winter;16(1):13–21. doi: 10.1017/s0266462300016123. PMID: 10815350. [DOI] [PubMed] [Google Scholar]
- 30.Institute of Medicine (US) Committee on the use of complementary and alternative medicine by the American public. Complementary and alternative medicine in the United States. National Academies Press (US); Washington (DC): 2005. https://www.ncbi.nlm.nih.gov/books/NBK83807/ 7, Integration of CAM and Conventional Medicine. Available from: [PubMed] [Google Scholar]
- 31.Patwardhan B., Tillu G. Universal health coverage and AYUSH systems. J Ayurveda Integr Med. 2018;9(1):1–2. doi: 10.1016/j.jaim.2018.03.001. PMID: 29606254; PMCID: PMC5884080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pushpakom S, Iorio F, Eyers PA, Escott KJ, Hopper S, Wells A, Doig A, Guilliams T, Latimer J, McNamee C, Norris A, Sanseau P, Cavalla D, Pirmohamed M. Drug repurposing: progress, challenges and recommendations. Nat Rev Drug Discov. 2019 Jan;18(1):41-58. doi: 10.1038/nrd.2018.168. Epub 2018 Oct 12. PMID: 30310233. [DOI] [PubMed]
- 33.Kim H.S., Lee S., Kim J.H. Real-world evidence versus randomized controlled trial: clinical research based on electronic medical records. J Kor Med Sci. 2018;33(34):e213. doi: 10.3346/jkms.2018.33.e213. PMID: 30127705; PMCID: PMC6097073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Del Paggio JC, Berry JS, Hopman WM, Eisenhauer EA, Prasad V, Gyawali B, Booth CM. Evolution of the Randomized Clinical Trial in the Era of Precision Oncology. JAMA Oncol. 2021 May 1;7(5):728-734. doi: 10.1001/jamaoncol.2021.0379. PMID: 33764385; PMCID: PMC7995135. [DOI] [PMC free article] [PubMed]
- 35.Tabish S.A. Complementary and alternative healthcare: is it evidence-based? Int J Health Sci. 2008;2(1) V-IX. PMID: 21475465; PMCID: PMC3068720. [PMC free article] [PubMed] [Google Scholar]
- 36.White J.D., O'Keefe B.R., Sharma J., Javed G., Nukala V., Ganguly A., et al. India-United States dialogue on traditional medicine: toward collaborative research and generation of an evidence base. J Glob Oncol. 2018;4:1–10. doi: 10.1200/JGO.17.00099. Epub 2017 Nov 16. PMID: 30241135; PMCID: PMC6180779. [DOI] [PMC free article] [PubMed] [Google Scholar]