Abstract
The evaluation of biopsied solid organ tissue has long relied on visual examination using a microscope. Immunohistochemistry is critical in this process, labeling and detecting cell lineage markers and therapeutic targets. However, while the practice of immunohistochemistry has reshaped diagnostic pathology and facilitated improvements in cancer treatment, it has also been subject to pervasive challenges with respect to standardization and reproducibility. Efforts are ongoing to improve immunohistochemistry, but for some applications, the benefit of such initiatives could be impeded by its reliance on monospecific antibody-protein reagents and limited multiplexing capacity. This perspective surveys the relevant challenges facing traditional immunohistochemistry and describes how mass spectrometry, particularly liquid chromatography-tandem mass spectrometry, could help alleviate problems. In particular, targeted mass spectrometry assays could facilitate measurements of individual proteins or analyte panels, using internal standards for more robust quantification and improved interlaboratory reproducibility. Meanwhile, untargeted mass spectrometry, showcased to date clinically in the form of amyloid typing, is inherently multiplexed, facilitating the detection and crude quantification of 100s to 1000s of proteins in a single analysis. Further, data-independent acquisition has yet to be applied in clinical practice, but offers particular strengths that could appeal to clinical users. Finally, we discuss the guidance that is needed to facilitate broader utilization in clinical environments and achieve standardization.
Keywords: mass spectrometry, clinical proteomics, targeted proteomics, untargeted proteomics, tissue analysis, pathology, standardization, immunohistochemistry
Graphical Abstract
Highlights
-
•
Immunohistochemistry workflows are poorly standardized.
-
•
Mass spectrometry may complement existing tests for proteins in solid tissues.
-
•
Targeted workflows could help clarify borderline immunohistochemistry cases.
-
•
Untargeted assays provide relative quantification of 100s to 1000s of proteins.
-
•
Guidance is needed to help clinical labs develop robust tools for solid tissues.
In Brief
For many years, immunohistochemistry has been the primary tool in pathology for protein localization and quantification in solid tissues, but it is poorly standardized and suffers from practical limitations. The field is ripe for novel complementary technologies to help with diagnosis. This perspective focuses on the issues hampering immunohistochemistry and highlights how liquid chromatography-tandem mass spectrometry in particular is well-positioned to complement existing testing. We also discuss where guidance is needed to ensure rigor in clinical environments.
The examination of solid organ tissue is essential in the diagnosis, prognosis, and therapeutic management of cancer and other disorders. Components of this practice have existed for more than a century, including formalin fixation, paraffin embedding, and stains such as hematoxylin and eosin (1, 2, 3, 4). The introduction of immunohistochemistry (IHC) in the 1970s signaled a major shift in the field, specifically from diagnosis based primarily on morphology to a more comprehensive approach incorporating cell lineage and other protein expression information. Although IHC has broadly reshaped diagnostic criteria and advanced the practice of pathology, its rapid adoption was accompanied by a lack of standardization in workflows and reagents, hampering reproducibility (5, 6, 7, 8). Efforts are ongoing to improve standardization, particularly in the area of companion diagnostics, but these efforts may not address fundamental weaknesses of the technology, which would limit the long-term benefits of clinical tissue protein measurements in patient care. The field is thus ripe for introducing new complementary technologies for protein quantification in tissues that are able to overcome these issues, particularly for select applications. Techniques amenable to multiplexing are most desired, given the need for multianalyte panels to overcome the deficits of existing single biomarker tests in predicting responses to therapy. This perspective focuses on the challenges facing IHC and highlights how mass spectrometry, including liquid chromatography-tandem mass spectrometry (LC-MS/MS) in particular, could complement IHC to close gaps in existing testing using both targeted and untargeted acquisition approaches. We also discuss where further guidance is needed to ensure that rigorous assays are developed in clinical environments.
Challenges in Immunohistochemistry Standardization
Immunohistochemistry utilizes anti-protein antibody reagents to bind and label antigens in tissue sections for visualization by microscopy. In the preanalytical phase, a sample is procured via biopsy or surgical procedure, preserved using formaldehyde in water (a.k.a., formalin; variable concentration, pH, time, and temperature), and embedded with paraffin wax to yield a formalin-fixed paraffin-embedded (FFPE) tissue block (9). In preparation for immunostaining, one or more sections (4–10 μm thick) of the block are cut using a microtome and mounted on a glass slide. Cut sections are subjected to deparaffinization, antigen retrieval (e.g., with heat), and treatments (e.g., biotin quenching) to prevent method-related artifacts. Finally, immunostaining itself consists of multiple incubation steps separated by rinsing: incubation with primary antibody against the target of interest, incubation with a reporter-linked secondary antibody conjugated typically via biotin to an enzyme (horseradish peroxidase), and addition of a chromogenic substrate (3,3′-diaminobenzidine) to produce color deposition at the site of antigen. Counterstaining is then performed to improve the visualization of labeled markers in relation to morphology. A pathologist views the results and renders an interpretation.
The introduction of IHC in the mid-1970s as ancillary testing to histologic staining represented a major step forward in reducing ongoing, well-documented inconsistencies in microscopy-based interpretation (10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24). However, despite the enormous and continually expanding benefit of IHC to patient care, its practice has struggled to meet the standards that we normally ascribe to clinical laboratory testing. As noted by Dr Clive Taylor (former Chair of Pathology at the University of Southern California and frequent contributor to this subject), the treatment of IHC has more closely mirrored that of the differential and special staining methods that preceded it in anatomic pathology (AP):
“Not only is there much to be learned, but also there is much to be “unlearned”: the AP laboratory is handicapped by its legacy; it performs stains, not assays…” (25)
The result is that IHC still exists today as more of an art than as a well-characterized and scientific assay (26). For example, judgments of quality in IHC are typically based on whether staining patterns please the eye, resulting in different laboratories differentially tweaking the practice of the same IHC test at different institutions (7). Proficiency testing (PT) results from external quality control organizations provide a window into the scale of irreproducibility that exists. Vyberg and Nielsen (27), for example, examined IHC performance encompassing more than 30,000 slides from 2003 to 2015 in the Nordic Immunhistochemical Quality Control international PT program and found that 20% to 30% of staining results in breast cancer and other diseases were "insufficient" for diagnostic use. Interlaboratory comparisons performed as part of clinical trials are equally sobering, showing discordance rates up to 75% when testing for treatment-related markers such as estrogen receptor (ER), progesterone receptor (PR), and human epidermal growth factor receptor 2 (HER2) (6).
Unfortunately, the measures of irreproducibility highlighted by PT and other published interlaboratory comparisons only tell part of the story, since such evaluations typically start with precut sections of the same FFPE block. More specifically, in addition to the effects of interlaboratory variability in immunohistochemical staining workflows, there exist inconsistencies in sample collection and processing (i.e., the preanalytical phases of testing). In particular, the handling conditions for invasively-obtained solid organ biopsy samples can differ substantially from patient-to-patient in ways that laboratories cannot reasonably oversee or that may be challenging to document (e.g., length of warm ischemia versus cold ischemia, (7)). The preanalytical variables in IHC are numerous, only partially investigated, and subject to conflicting findings regarding their effects on end results [reviewed in (28)]. Moreover, re-collection of a sample is typically impossible or at least highly impractical due to the invasiveness of collection (e.g., tissue collected during surgery or endoscopy). This contrasts with the testing of many biofluids, where blood draw conditions can be more strictly defined and samples can often be recollected if there is a deviation from the procedure.
The lack of standardization in IHC is particularly problematic for companion diagnostics, which are standalone tests used to assign patients to targeted cancer therapies. Such testing ultimately classifies patients into descriptive categories (e.g., responder versus non-responder) but does so based on a semi-quantitative interpretation of staining patterns. Cases of breast cancer undergo separate IHC evaluations for HER2, ER, and PR, with the status of each interpreted based on the number of individual cells highlighted through the IHC reaction, typically with additional consideration of staining intensity. In the case of HER2, IHC results, on a scale of 0, 1+, 2+, or 3+ (Fig. 1), are paired with results from in situ hybridization (ISH) to determine the appropriateness of anti-HER2 therapy (29). More nuanced tiered scoring systems, such as the histochemical score (H-score), have been developed to account for tissue heterogeneity by using an estimate of the percentage of cells that are stained at different intensities (resulting in a weighted score from 0 to 300) (30). Although such developments capture increasingly granular information from tissue samples, the use of visually-driven metrics as proxies for the quantity of biomarkers is still problematic because quantification by the human eye is imprecise, and staining appearance will vary between laboratories. The situation is especially troublesome when evaluating samples with results near decision-point cutoffs (e.g., HER2 1+ versus 2+), for which small differences in counting/scoring can affect categorization (7, 31). The effects can be dramatic and are at least partially responsible for the substantial variability in response rates to cancer immunotherapies observed in clinical trials (32, 33, 34, 35, 36). In one study, Lambein et al. (37) re-performed HER2 IHC evaluation for a set of 150 consecutive cases of invasive breast cancer that had been previously designated HER2-IHC-negative (0+ or 1+) based on IHC performed at a local laboratory. Overall concordance was modest at 86%. However, for cases previously scored as 0 at the local laboratory (n = 102), concordance was only 15%, with 78 of 102 cases recategorized as 1+ and 9 cases recategorized as 2+. In light of more recent clinical trials showing the treatment benefit of HER2-low cases with emerging therapies (e.g., trastuzumab-deruxtecan), these differences could lead to real clinical consequences (38).
Fig. 1.
Immunohistochemical (IHC) Interpretation for HER2 protein expression in Breast Cancer.A, IHC 0 (negative), no staining is observed or membrane staining that is incomplete and is faint/barely perceptible and in ≤10% of tumor cells. B, IHC 1+ (negative), incomplete membrane staining that is faint/barely perceptible and in >10% of tumor cells. C, IHC 2+ (equivocal), weak to moderate complete membrane staining observed in >10% of tumor cells. D, IHC 3+ (positive), circumferential membrane staining that is complete, intense, and in >10% of tumor cells.
Ongoing Efforts to Improve Testing
The potential for adverse effects from divergent practices in IHC was recognized by many early users. As the utilization and scope of IHC rapidly expanded, Dr Taylor pointed out the need to clearly and comprehensively define the steps and variables that could impact patient results. He and others have invoked a “total test approach to standardization” that encompasses the pre-analytical, analytical, and post-analytical phases of IHC (7, 8, 10, 25, 39, 40, 41, 42, 43, 44). However, the widespread alignment of practices with the total test philosophy has been slow, reflecting the scale and complexity of procedural factors as well as the practical challenges to modifying established IHC workflows. As of today, differences in practice between IHC users are likely to persist as a potential source of irreproducibility. However, as noted by Torlakovic et al., (45) it is not necessarily desirable to fully align procedures, instruments, and reagents, and thus “freeze” IHC method development, as this would stifle innovation, limit the use of new products, and impose significant practical restrictions. Efforts to achieve standardization of results have instead focused on the development of tools to detect problems, both in the assay and specimen, as well as to achieve a more meaningful comparison of results between institutions. Initiatives on multiple fronts have been pursued, with several highlighted below.
One major pursuit is that of more systematically designed assay controls, carefully selected for each IHC application [reviewed in (45, 46)]. Historically, IHC controls have provided only a narrow window into gauging assay performance. For example, the use of archived tissue as an external positive control sample may demonstrate the adequacy of an IHC protocol for the detection of analyte at some amount or concentration, but that level is typically undefined. It may not be clear, for example, whether the IHC results are adequate for judging the presence of much lower or higher concentrations potentially under consideration in a patient sample. This ambiguity does not exist for most other qualitative assays found in clinical laboratories (e.g., urine drug screening). In other such tests, a “positive” or “negative” result depends on the analyte concentration being measured above or below a specified numerical cutoff (with units composed of physical properties, e.g., nanograms per milliliter). In particular, the lowest amount of a substance that can be reliably detected is typically identified as the limit of detection (LOD), a concentration at which test performance can be evaluated daily. As a step in this direction, an international committee of experts in IHC has suggested the deployment of so-called “immunohistochemistry critical assay performance controls” (iCAPCs) (45, 46). The iCAPCs are a set of tissues assigned to a particular IHC test (e.g., appendix, liver, and tonsil assigned to pan-keratin). These tissues have well-characterized expression patterns for the antigen in question and, furthermore, express the antigen across a full range of diagnostically relevant levels. As such, typically at least one iCAPC contains cells or other structures with known low (but still “positive”) expression of the antigen, in turn providing a descriptive representation of an IHC assay’s LOD. Once test performance is established using iCAPCs (i.e., primary external controls), secondary controls such as other archived tissue can be employed for more routine assay monitoring. The use of iCAPCs has been shown to reduce interobserver variability in IHC, for example, in BRAFV600 E testing (47).
For more quantitative IHC applications (e.g., HER2), qualitative assay controls may be inadequate for assuring reproducibility, even if improved. To prevent errors in companion diagnostic testing, Dr Steven Bogen has instead emphasized the need to introduce traceable units of measure, international standards, and calibrators for quantitative assay monitoring (6). Proposals include on-slide calibration standards created via the deposition of a target protein (or its peptide epitope) in spots at known concentrations. One configuration enables IHC calibration traceable to an existing, widely available NIST standard (SRM 1932, a fluorescein solution). Calibration efforts have culminated with the recent announcement of the National Cancer Institute (NCI)-funded Consortium for Analytic Standardization in Immunohistochemistry, which should help enable laboratories to determine a quantitative LOD for companion diagnostic assays via a distributable set of well-characterized calibrators (5).
Many of the abovementioned efforts, including iCAPCs and slide-based calibrators, may help standardize the analytical phase of IHC, but they cannot assess sample quality. Additional strategies are needed to ensure that pre-analytical handling and processing are appropriate and have not compromised the immunoreactivity of the protein of interest in the sample (48)). Ideally, protein epitope integrity would be evaluated by using the same protein somewhere else in the tissue. However, the likelihood that the same protein is present at similar concentrations from sample to sample is very low. Further, the detection of the protein elsewhere in the tissue does not guarantee it is detectable in the area of interest. Rather than the protein of interest, it could also be possible to use other proteins that are present in relatively constant amounts in specific cell types. For example, more than 30 years ago, Battifora described vimentin as a general marker of sample processing quality (49), based on its ubiquity and vulnerability to fixation conditions. Others have since continued to advocate for the use of housekeeping or structural proteins to verify tissue quality. For example, Taylor has cited the potential of so-called quantitative internal reference standards, which consist of reference analytes in FFPE tissue (e.g., desmin, caldesmon) for which the effects of fixation and antigen retrieval have been measured, quantitatively (using techniques other than IHC, perhaps at the individual cell level), and compared with those of key test analytes (7, 50). Once the properties of a reference analyte are established, it can be evaluated by IHC alongside the test analyte in patient samples (in a separate IHC reaction). Similarly, Neumeister et al. (48) described a tissue quality index, reliant on a panel of proteins with empirically defined relationships to delays in fixation (cold ischemic time) based on quantitative immunofluorescence studies. The authors demonstrated, for example, that measurements of cytokeratin, extracellular signal-regulated kinase 1/2 (ERK1/2), and phosphorylated heat shock protein 27 (pHSP-27) can be applied to breast to tissue to evaluate for adverse delays in processing that could result in false negative ER expression.
Besides the standardization of analytical and pre-analytical processes in IHC, calibrated image analyses could also serve to enhance reproducibility, particularly in the area of the interpretation of staining patterns [reviewed in (7, 30, 51, 52, 53, 54)]. The development of computer-based interpretation algorithms closely follows the trend to digitalize anatomic pathology workflows in general, which would facilitate remote pathology consultations, create permanent storage records, and help in teaching medical students and resident pathologists. Whole slide imaging, in particular, has provided a wealth of data for the development of artificial intelligence (AI) tools, including machine learning algorithms (52). With respect to diagnostic image analysis, computer-aided approaches improve accuracy via a reduction in visual and cognitive biases (30, 55). AI may become most impactful when it pushes visual interpretation beyond what might be considered reasonable for the human eye. For example, the evaluation of heterogeneity of proliferation markers in cancers is particularly challenging, but digital image analysis has, for example, improved diagnosis and prognosis when used to assess Ki67 expression in gastroenteropancreatic neuroendocrine neoplasms, compared to manual procedures (56).
Is This the Best Path Forward?
The abovementioned strategies are encouraging for the future of IHC standardization, addressing concerns related to preanalytical, analytical, and post-analytical workflows. However, there are still a few issues that might prompt the development of complementary strategies in parallel, as the efforts described earlier continue to unfold.
One question to consider is whether there is really sufficient precedent to guarantee that a test like IHC could be broadly standardized. The belief that standardization of IHC is achievable partially hinges on the similarities between IHC and the enzyme-linked immunosorbent assay (ELISA) (7, 8, 57). Dr Taylor described ELISA as a quantitative “gold standard” for measuring proteins in serum and a model for IHC, noting:
“[An] enhanced IHC assay would serve accurately to quantify analytes (proteins) in tissue sections, analogous to the use of the ELISA method in the clinical laboratory. ELISA employs similar principles and essentially the same reagents as are used in IHC but is subject to much more rigorous control at all levels” (7).
Dr Bogen similarly points to the clinical laboratory and its aspirational models of performance, citing reported error rates of <1% in clinical chemistry, immunology, and hematology (6). However, it must be recognized that immunoassays are frequently not reproducible between manufacturers and assay platforms. This is true even for ELISAs that are FDA-approved/cleared and coupled with well-characterized reference calibration materials. An example of this phenomenon is the thyroglobulin immunoassay, for which traceability to reference material/calibrators has long existed and yet harmonization has never been achieved among commercial systems (58, 59). Further, while clinical immunoassays appear less prone to error in PT compared to IHC, this is partially due to the fact that only identical ELISA platforms are compared head-to-head in PT assessments (which were really designed to demonstrate that each clinical laboratory performs FDA-approved/cleared devices properly). Dr Mogens Vyberg highlights the inequity in juxtaposing error rates of IHC and another clinical testing in a 2019 editorial response to Bogen, writing that:
“[Regarding differences in error rates]…the reasons are complex, because the above findings [for IHC] reflect not a single assay, but very many different assays for different analytes; even for the same analyte, different IHC laboratories may use 1 of 20 or more different antibodies in attempting to stain for, for example, keratin.” (60)
Ultimately, both IHC and ELISA are techniques dependent on monospecific anti-protein antibodies, a factor that could prevent either from achieving harmonization. The remarkable failure of standardization of antibody reagents in research supports this assertion. These tools helped fuel a reproducibility crisis due to the challenges of cross-reactivity and batch-to-batch variability (61). While one might hope that antibody reagents somehow perform better in clinical environments, there are no specific reasons to believe this is the case.
Another question to consider is how limited multiplexing capacity may prevent IHC from achieving all of its potential. IHC is normally chromogenic and, in its standard form, can only be multiplexed x2 (i.e., red and black). Performing additional analyses for each case in order to provide quality measures (e.g., an internal quality control) can be laborious and tissue consumptive. Alternatively, IHC by fluorescence (i.e., immunofluorescence) can be multiplexed more easily than conventional IHC, but the improvement is often modest and carries other practical disadvantages [reviewed in (62, 63, 64, 65)]. Commercial systems such as the Phenocycler (Akoya Biosciences) and other cyclic immunofluorescence approaches add many more channels (purportedly up 100 or more analytes) without the loss of spatial information, but they are expensive and require proprietary analyzers and reagents. The higher number of analytes is accompanied by time-consuming cycles of antibody staining, imaging, and fluorophore bleaching, potentially lasting days (66). Whether these new approaches could practically help assess tissue quality is not yet clear.
Mass Spectrometry as a Complementary Technique
Because laboratories are faced with heterogeneous and constantly changing choices of antibodies for IHC, which could hinder reproducibility within and across testing sites, it may ultimately be sensible to move on from technologies that primarily rely on antibodies for protein detection. An ideal complement to IHC in clinical tissue protein detection would address the abovementioned concerns, enable the measurement of proteins via orthogonal principles, and better facilitate highly multiplexed analyses. Mass spectrometric methods might meet these needs. There are two general approaches to the analysis of solid tissues: (1) mass spectrometry imaging (MSI), which combines multiplexed protein detection with spatial information (analogous to multiplexed immunofluorescence) and (2) LC-MS, which can quantify many, many analytes from solubilized tissues. Both are potentially viable in clinical workflows using targeted or untargeted acquisition methods.
From the perspective of practicing surgical pathologists, the most obvious and intuitive applications of mass spectrometry to solid tissues involve imaging. The prototypical example uses matrix-assisted laser desorption/ionization (MALDI), which raster-scans a laser beam across the surface of a matrix-coated tissue section and ionizes the underlying biomolecules for spatially registered mass analysis (by TOF or FT-ICR) at 5 to 200 μm resolution (67, 68, 69, 70, 71). The identification of intact proteins from MALDI-MS is challenging, particularly for larger proteins that are not reliably fragmented for adequate sequence coverage (72). However, database searching options and approaches to protein identification via LC-MS/MS analyses performed in parallel are becoming more widely available (68, 72). MALDI imaging is not inherently quantitative, but several approaches exist to help make the workflow more quantitative [reviewed in (70)]. Importantly, the factors impacting quantification are complex and as a result, quantification by MALDI-MS lags behind LC-MS/MS-based approaches (73).
Due to the challenges inherent in intact protein identification by MALDI-MS (63), other imaging techniques have combined MS with the principles of IHC (64, 66, 74, 75, 76). Such techniques include mass cytometry imaging, which uses monoclonal antibodies conjugated to isotopically pure rare earth metal atoms in order to encode protein epitopes for simultaneous analysis of up to ∼40 markers at cellular resolution (77). Standard imaging mass cytometry subjects a sample to laser ablation, which then vaporizes and atomizes a small area of sample (less than 5 μm2). The resulting aerosol is introduced into an inductively coupled plasma (ICP) ion source and ions are detected by TOF mass analysis (66, 77). Similar or improved spatial resolution can be achieved using the principles of time-of-flight secondary ion mass spectrometry (TOF-SIMS), which uses a primary ion (e.g., Ar+, O2+) beam for regiospecific sampling and generation of secondary ions from a test sample. Multiplexed ion beam imaging by time of flight (MIBI-TOF) is an implementation of TOF-SIMS, using the technology to image metal-tagged antibodies applied to tissue sections. In addition to rare earth metals, antibodies can also be labeled with organic molecules. For example, the Tag-Mass approach (78), a type of MALDI-IHC, uses a photocleavable peptide-based MALDI-MS method for targeted imaging of tissues. Yagnik et al. (63) developed a similar method that labels antibodies with both a fluorescent molecule (for standard immunofluorescence) and a photocleavable peptide for MALDI-MS detection. The dual labeling of targets allows standard immunofluorescence and MALDI-MSI on the same tissue sections.
The major appeal of MSI is the study of biomarkers in relation to intact tissue morphology. For example, imaging data could help determine surgical margins or determine the spatial distribution of immunomodulatory proteins in the tumor microenvironment, which could be beneficial (79, 80). Additionally, MSI methods could offer higher throughput compared to mass spectrometry techniques that are coupled to chromatographic separation. However, two drawbacks need to be recognized. First, at least some of the abovementioned MSI configurations do not actually break free from monospecific anti-protein antibody reagents and thus issues with reproducibility could still apply. Second, the capacity to achieve reasonable throughput for clinical environments is unclear. Mass cytometry, for example, requires an extended antibody incubation period (potentially overnight) and a relatively slow scanning process (MIBI-TOF scanning of a 100 μm × 100 μm field of view requires 3 min, which corresponds to 5 h for a 1 mm2 area) (76).
LC-MS/MS, the major conceptual alternative to MSI for tissue analyses, already has a significant presence in clinical laboratories for the analysis of biofluids, particularly in the areas of therapeutic drug monitoring, metabolic disease evaluation, endocrinology, and toxicology (81, 82, 83, 84, 85, 86, 87, 88, 89, 90, 91, 92, 93). Protein quantification by mass spectrometry has also taken hold (81, 94), owing to critical advantages with respect to specificity (95, 96, 97, 98, 99), sensitivity/dynamic range (96, 100, 101, 102, 103), overcoming assay interference (104, 105, 106), multiplexing (107, 108, 109), and throughput/turnaround time (103, 110). While some of these assays employ anti-peptide antibodies (for enhanced sensitivity through peptide immunoaffinity enrichment), the use of anti-protein reagents is generally avoided. Target quantification is based instead on the measurement of appropriately selected surrogate peptides that are readily generated by proteolysis. Multiplexed measurements are commonplace, a function of the instrumentation rather than the use of specialized reagents.
An important prerequisite to the successful transfer of LC-MS workflows to clinical care is compatibility with FFPE tissues. Fortunately, the use of LC-MS with solid tissue is already well established in the research space (when sampled in bulk from the whole section or from more intentionally selected areas using microdissection). In discovery proteomics, for example, LC-MS/MS analyses are routinely employed for comprehensive protein identification in FFPE tissues (see review by Gustafsson et al. (9)). A key finding in this body of work is that bottom-up proteolysis-dependent techniques, when coupled with heat-based protein extraction (heat-induced antigen retrieval (9, 111)), produce highly comparable results for FFPE and frozen tissue across 100s to 1000s of proteins at a time (112).
Targeted LC-MS/MS Applications for Solid Tissue
The simplest translation of current clinical LC-MS/MS workflows to solid tissues would emulate the existing IHC practice of measuring a single protein to a few protein targets in each specimen. The use of multiple reaction monitoring (MRM) for this, in particular, could lend substantial objectivity and precision to this process (Fig. 2). Sprung et al. (113) demonstrated the feasibility of the approach, applying MRM to analyze over 100 peptides previously identified in frozen and FFPE renal carcinoma tissue using shotgun proteomic analyses. The results demonstrated equivalent precision (generally <20% CV) between measurements involving FFPE and frozen tissues, addressing the hypothesis that protein cross-linking in FFPE tissues would add undue variability in MRM measurements. Since this point, a growing number of publications have demonstrated the potential of LC-MS/MS to quantify the targets of cancer immunotherapy in applications that normally rely on IHC-based companion diagnostics (Table 1). A common finding among these reports is that more quantitative, objective analyses improve case stratification within and between diagnostic categories, more clearly delineating individuals likely to respond to immunotherapy or to harbor specific genetic alterations.
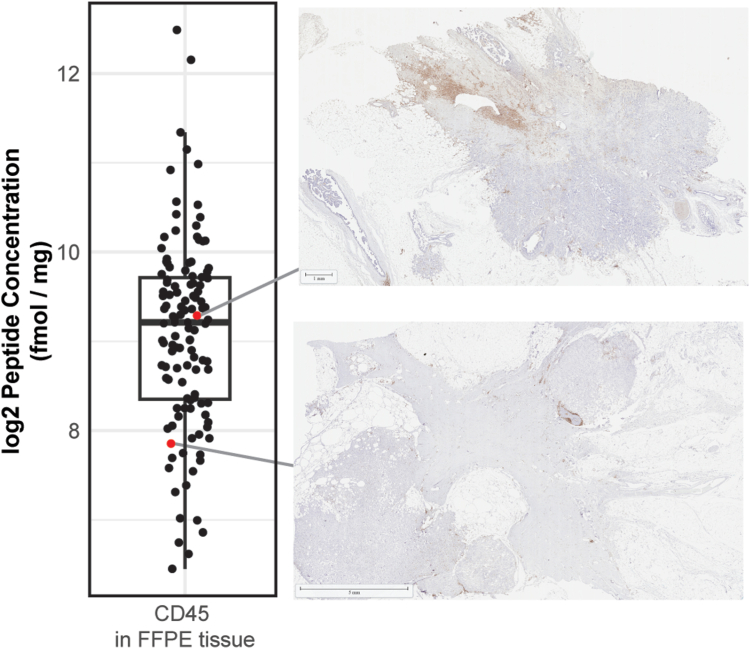
Fig. 2.
MRM-MS associated with CD45 Immunohistochemistry. MRM-MS results are shown at two different concentrations, demonstrating the correlation between visual IHC and a quantitative MRM-MS-based readout. Formalin-fixed paraffin-embedded tissues were stained with monoclonal mouse anti-human CD45 (Clone 2B11 + PD7/26, DAKO) and polyclonal anti-Rabbit IgG (Poly-HRP, Leica) before being counterstained with hematoxylin (Biocare).
Table 1.
Studies reporting application of targeted MRM-Based companion diagnostics (FFPE Tissue)
| Reference | MRM targets (peptides) | IAE | FFPE tissues analyzed | Highlights |
|---|---|---|---|---|
| Hembrough 2012 (119) | EGFR (IPLENLQIIR) | No | NSCLC xenograft tumors (n = 10) NSCLC patient samples (n = 23) |
|
| Hembrough 2013 (129) | EGFR (IPLENLQIIR) HER2 (ELVSEFR) HER3 (LAEVPDLLEK) IGF-1R (GNLLINIR) |
No | Breast cancer, 2 cohorts: 1) HER2 IHC variable (n = 10) 2) HER2 IHC 3+ (n = 19) |
|
| Catenacci 2014 (118) | Met (TEFTTALQR) | No | Gastroesophageal cancer (n = 130) NSCLC |
|
| Steiner 2015 (120) | HER2, 6 peptides monitored | No | Breast cancer (n = 40) |
|
| Catenacci 2016 (117) | EGFR (IPLENLQIIR) FGFR2 (EAVTVAVK) HER2 (ELVSEFR) HER3 (LAEVPDLLEK) IGF-1R (GNLLINIR) Met (TEFTTALQR) |
No | Gastroesophageal cancer (n = 139) |
|
| Kennedy 2016 (128) | >500 peptide targets | Yes | Breast cancer (n = 3) |
|
| Nuciforo 2016 (114) | HER2 (ELVSEFR) | No | Breast cancer, invasive: HER2 0+ (n = 39) HER2 1+ (n = 49) HER2 2+ (n = 49) HER2 3+ (n = 133) |
|
| An 2017 (36) | HER2 (ELVSEFR) | No | Gastric cancer (n = 237) |
|
| Do 2020 (115) | HER2 (VLQGLPR) JAM1 (VTFLPTGITFK) |
No | Breast cancer (n = 210) |
|
| Kennedy 2021 (116) | HER2 (GLQSLPTHDPSPLQR) +22 additional targets |
Yes | Breast cancer (n = 119) |
|
Abbreviations: EGFR, epidermal growth factor receptor; HER2, human receptor tyrosine-protein kinase erbB-2; IAE, immunoaffinity enrichment; JAM1, Junctional adhesion molecule A; Met, hepatocyte growth factor receptor; NSCLC, non-small cell lung cancer.
Percentage of tumor cells in the dissected area, surface area of dissected tissue, peptide concentration, total ion current in a separate full scan acquisition, and cytokeratin 19.
In the setting of anti-HER2 therapy (traztuzumab).
The majority of studies in Table 1 focused on the quantification of HER2 in breast cancer. Nuciforo et al. (114) quantified HER2 in the set of 270 FFPE breast cancer samples that had been classified previously as HER2 0, 1+, 2+, or 3+ by IHC. As shown by these authors and in subsequent reports, HER2 quantity (measured in amol per microgram of total protein), generally correlates with IHC and amplification status by ISH, but there is a marked spread of concentrations with existing tiers and important differences with the IHC-based readout. For example, while a threshold of 740 amol/μg showed an agreement of >90% with IHC and ISH, a higher threshold (>2200 amol/μg) was associated with improvements in survival in patients treated with anti-HER2 adjuvant therapy. Do et al. (115) applied MRM-based HER2 measurement to 210 cases of invasive ductal carcinoma, investigating the capacity of quantitative HER2 results to differentiate the FISH status in HER2-equivocal cases (defined as IHC 2+, normally subject follow-up FISH testing). MRM-based HER2 quantification distinguished HER2+/FISH-negative from HER2+/FISH-positive cases with an AUROC value of >0.9, indicating that MRM-based measurement could be potentially utilized to avoid FISH in some cases. Kennedy et al. (116) applied HER2-MRM to 119 FFPE and 96 frozen breast cancer biopsy samples, highlighting the potential to identify HER2-low expression (IHC 1+ or 2) cases for which emerging targeting compounds (trastuzumab-deruxtecan) show benefit (34). In the study, using peptide-immunoaffinity enrichment (immuno-MRM), HER2 was quantified above a lower limit of quantification in all samples, improving the potential for stratification in this area.
Several studies have also focused on LC-MS/MS-based quantification of prognostic and therapy-selection markers in gastric and gastroesophageal cancers. As in breast cancer, a subset of patients who harbor HER2 amplification in gastric cancer (defined by IHC 3+ or positive FISH) are candidates for HER2-targeted therapy, based on a demonstrated benefit in the ToGA (Trastuzumab for Gastric Cancer) trial. However, substantial variations in response rates to anti-HER2 treatment have been observed in subsequent clinical trials, demanding a better approach to testing. Catenacci et al. (117) developed an MRM-based assay for quantification of HER2 in 139 cases, identifying a wide range of HER2 expression, even within specific groups. The degree of HER2 amplification was linearly correlated with MRM-based HER2 measurement. Notably, the assay was multiplexed (building off a previous assay described in (118)), enabling simultaneous measurements of other oncoproteins, including Met tyrosine kinase receptor, epidermal growth factor receptor (EGFR), and receptor tyrosine-protein kinase erbB-3 (HER3), which was relevant in several specific patient vignettes, for which co-alterations in FGR2 or HER3 readily explained the treatment course observed in each case. An et al. (36) applied MRM-based HER2 measurement to a set of 237 FFPE cases of gastric cancer, identifying a 115-fold range of HER2 concentrations in HER2+ disease. A cutoff of 1825 amol/μg was determined to predict the benefit from the addition of trastuzumab to chemotherapy.
In addition to illustrating the potential therapeutic benefit of an MRM-based readout, these early studies have helped elucidate the steps of method development, which mirror those for assays in biofluids. For example, identification of surrogate peptides can begin with recombinant protein, proteolysis, and analysis with a high-resolution orbitrap MS (100, 119). The appropriateness of candidate peptides is characterized on the basis of sequence specificity and chromatographic features (e.g., peak shape, retention time, precision of the LC-MS/MS analysis). The use of multiple peptides per target (e.g., 6 peptides monitored for HER2 in (120)) is prudent early in method development to help ensure adequate operating characteristics of the final assay (100).
From here, method development may differ substantially from biofluid testing. One of the most important ways tissues may differ is in assay calibration. As we have learned for biofluids, accurate calibration for protein biomarkers is incredibly complex and is likely to be even more complicated for solid tissues (121, 122, 123, 124). As with measuring a protein in the blood, urine, or cerebral spinal fluid, reliable quantification in a tissue matrix will require the addition of stable isotope-labeled internal standards, which will be in the form of synthetic peptides, synthetic cleavable peptides (also called winged peptides), or intact proteins. These internal standards can help control for variation in sample preparation. However, the effectiveness of a spiked internal standard will depend on when it is added and how closely the conditions to which it is exposed match that of the analyte ultimately being tested. Unlike biofluids, to which internal standards can be added at initial processing, a generalizable solution for accomplishing this is not yet clear with solid tissues. Importantly, stable isotope-labeled internal standards can also be used as internal calibrators, which has been used for apolipoprotein A-I in human serum and is planned for use in reference measurement procedures being developed by the International Federation for Clinical Chemistry (125, 126).
In addition to using internal standards as internal calibrators to convert instrument signal to protein concentration, it is also possible to use external calibrators. For example, Hembrough et al. (119) described the creation of a standard curve by serial dilution of a light peptide from EGFR in a tryptic digest from the organism Pyrococcus furiosus that contained isotope-labeled peptide so that every serial dilution had the same concentration of labeled peptide. Catenacci et al. (118) described spiking increasing amounts of the light peptide into lysate from a formalin-fixed cell line. The use of these alternative matrices as the background for external calibrators borrows from clinical measurements in biofluids (e.g., chicken serum as a calibration diluent for human serum thyroglobulin, (102, 127). More recent approaches have developed a reverse-curve approach to assay calibration, whereby the heavy isotope-labeled peptide is spiked into a matched matrix at different concentrations, keeping the unlabeled, endogenous peptide constant to serve as the internal standard (128). While this approach has the advantage of using a more favorable calibrator background matrix, this approach, and others before it rely on peptide calibrators instead of protein-based calibrators, which might be able to provide a higher order of test accuracy (122). Other approaches to external calibration could conceivably leverage slide-based calibrators, as is being explored for IHC, which would allow the calibration materials to be processed in parallel to patient samples, as is standard for current clinical LC-MS/MS assays of biofluids.
Tissue sampling and processing are two other analytical processes that will be quite different from biofluids and will greatly impact assay sensitivity and interpretation of results. Variations in sampling procedures could include whether to sample from a slide (or more than one) versus from the FFPE block. If sampling from a slide, it is possible to use laser capture microdissection (LCM) or manual microdissection. The amount of tissue, determined by the thickness of the section and the area sampled for slides and by the diameter of the punch and the depth of samples for tissue blocks, will also have a significant influence on the quality of the results. The majority of assays listed in Table 1 employed LCM of tissues on slides, which enabled some degree of control over tumor area (e.g., 12 mm2 of a 10 μm thick section (117, 118, 119, 129)), whereas others used manual procedures (scalpel microdissection of 20 μm thick area delimited by an expert pathologist (120) or manual scraping of 3x 10 μm sections (128)).
The instrument signal or calculated concentration will need to be normalized to the amount of sample used in the digestion. Given differences in sampling practices as well as the variability in tissue content from one slide to the next, different normalization strategies have been also proposed (e.g., quantity of analyte measured per total protein, per cells analyzed, per other protein biomarkers). As part of their study, Do et al. investigated five normalization factors, including tumor area (μm2), total cell count, total protein, total peptide, and light-heavy-peptide peak area ratios for 49 surrogate peptides from a range of epithelial cell-specific markers and common housekeeping proteins. From this last analysis, junctional adhesion molecule 1 (JAM1), an epithelial-specific protein was determined to be the best normalization factor (based on discrimination of HER2+/FISH-negative from HER2+/FISH-positive cases). As another example, Kennedy et al. (116), described the measurement of HER2 as a part of a 23-plex immuno-MRM-MS assay. In that study, normalization of HER2 levels to glyceraldehyde 3-phosphate dehydrogenase (GAPDH) improved concordance between immuno-MRM-MS and predicate assays for tumor classification. It is important to note that while the described normalization factors were beneficial in these individual studies, the potential limitations of each will require further consideration to not lead to misinterpretation. For example, GAPDH has been utilized as a normalization factor for gene or protein expression in other contexts (130, 131), but it is not perfect and could, for example, be affected by the variability in glycolytic capacity between tumors. Further studies (ideally larger and prospective) correlating normalized results and clinical outcomes could help clarify these issues.
After the tissue has been sampled, it must be processed and digested prior to analysis by LC-MS/MS, which must also be optimized. Kennedy et al. (128) evaluated many different combinations of protein extraction and trypsin digestion methods and identified an approach that yielded high precision across many analytes as well as excellent correlation between paired FFPE and frozen tissues.
For low-abundance proteins, it may be necessary to utilize peptide immunoaffinity enrichment to achieve better sensitivity. For clinical care, it has been difficult to measure low-abundance proteins directly in biofluids, especially using the normal to high-flow conditions that are most commonly available in those laboratories (200–1000 μl/min). Instead, peptide immunoaffinity enrichment can enhance sensitivity, using anti-peptide antibodies immobilized to a solid support (e.g., magnetic beads) to selectively isolate and enrich specific peptides from complex biological samples. This approach has been greatly beneficial, as has been shown for the high-sensitivity quantification of serum thyroglobulin (102, 127) and in other applications (see review by Neubert, et al. (94)). As noted earlier, several tissue-based assays have included peptide immunoaffinity enrichment, including a highly multiplexed analysis of breast cancer tissue (116).
Untargeted LC-MS/MS Analyses for Solid Tissues: the Example of Amyloid Typing
Although targeted analyses are most similar to the existing application of IHC, the use of untargeted analyses should also be considered. In particular, the translation of high multiplexing capacity directly to medical care is highly appealing, given the tremendous gain over IHC in deciphering the breadth of phenotypic data contained in biological samples. A potential hindrance to clinical application is that the high sensitivity and specificity of modern untargeted LC-MS/MS workflows (and, in turn, their reliability) are imparted by instrumentation atypical of clinical laboratories, namely, nanoflow chromatographs and high-resolution mass analyzers (e.g., orbitrap). However, the plausibility of leveraging these systems clinically is no longer in question, given the success of clinical LC-MS/MS-based amyloid typing, which generally employs sub-microliter-per-minute flow rates and data-dependent acquisition (DDA) run on hybrid-orbitrap mass spectrometers (132, 133, 134). Furthermore, due to continual improvements in mass spectrometry instrumentation, highly sensitive, comprehensive protein analyses are increasingly possible at higher flow rates (135, 136), which will make running these tests more robust for new users.
The chemical typing of amyloid exemplifies the characteristics of applications for which untargeted LC-MS/MS analyses may be well suited. Systemic amyloidosis is a heterogeneous group of conditions in which proteins prone to misfolding deposit in tissues and result in organ damage. The correct identification of protein contained in amyloid deposits is essential for guiding treatment. However, more than 30 endogenous proteins and exogenous substances (e.g., enfuvirtide) can contribute to amyloid formation, posing an analytical challenge (137, 138, 139, 140). Even if well-performing reagent antibodies existed against all amyloidogenic proteins, an immunohistochemical evaluation for each would exhaust all available tissue in many situations and require significant time for interpretation. Meanwhile, untargeted LC-MS/MS analyses do not require additional tissue consumption when considering additional amyloid type-specific proteins. Furthermore, a broadened proteomic examination allows for the detection of amyloidogenic substances that may not have otherwise been considered (or previously known), as well as additional markers useful for gauging the adequacy of amyloid sampling (i.e., the amyloid-associated proteins: serum amyloid P, apolipoprotein E, victronectin, apolipoprotein A4, and clusterin (141)). Of further benefit to amyloid typing specifically is that the database searching process itself does not require peptides generated from proteolytic digestion to be consistent from sample-to-sample, which is critical, since the proteins found in amyloid deposits appear truncated in unexpected ways (142).
Other similarly imposing applications to subtype tissues exist in histopathology, requiring the consideration of growing lists of protein markers for which suitable anti-protein antibody reagents may not be available. One example is the subtyping of pituitary adenomas, traditionally distinguished by pituitary hormone expression—that is, growth hormone, prolactin, adrenocorticotrophic hormone, thyroid stimulating hormone, luteinizing hormone, and follicle-stimulating hormone. In addition to these proteins, the evaluation of transcription factors (e.g., PIT-1, SF-1, T-PIT) is needed to meet the standard of care (143). In general, higher multiplexing will become increasingly important in medicine, particularly considering that a single or a handful of biomarkers are often insufficient to stratify patients in treatment categories (144). Further, multi-arm precision oncology trials require an assessment for the expression of many proteins, and IHC is a bottleneck.
To summarize these factors more generally, untargeted LC-MS/MS analyses provide benefits in applications for which: many proteins must be examined, there is value in a broader examination of proteomic features, and/or proteins may be truncated or otherwise modified post-translationally in ways we cannot predict. In future clinical practice, solid tissue applications in which untargeted LC-MS/MS methods are useful will be determined by these factors, as well as by the ease with which a targeted method could eventually be developed. For example, certain situations that are analogous in some respects to amyloid typing may not suffer from the effects of protein truncation and thus be more amenable to the identification of surrogate peptides for the proteins of interest. Meanwhile, robust and broadly encompassing proteomic data could prove especially valuable in practice when dealing with other ongoing sophisticated challenges in anatomic pathology, particularly if effectively integrated with genomic information (145).
The Appeal of Data-Independent Acquisition in Clinical Testing
As untargeted LC-MS/MS analyses see additional clinical applications, one question to consider is whether DDA is the best we can do. In DDA, the instrument acquires fragmentation spectra for peptide precursors that were detected in wide mass-range “survey” scans (146). While the approach is capable of detecting thousands of proteins, it has several drawbacks. One is that an under-sampling of peptides occurs when evaluating complex mixtures (147), since the number of eluting peptides will exceed, by definition, the number of precursor ions for which fragmentation spectra can be acquired (148, 149). Furthermore, differences in chromatography from injection-to-injection influence which peptides are selected for fragmentation, resulting in poor reproducibility in identifications at the peptide level, even across sequential injections of the same sample. Meanwhile, since the highest abundance precursor ions are selected for analysis, low abundance ions are variably ignored, leading to reduced sensitivity of the analysis, especially for low abundance proteins. In one study, Tabb and colleagues (149) analyzed 3 sample mixtures in 144 LC-MS/MS experiments performed across eight instruments (Thermo LTQ and Orbitrap) to demonstrate that peptide detection across pairs of technical replicates only overlapped by 35% to 60%. Protein detection was more reliable, but still underwhelming (70–80% overlap). Quantification also suffers, in part due to a reliance on the integration of MS1 chromatographic peak areas or spectral counting (150). Due to chromatographic interference from overlapping peptides with the same precursor ion m/z, the former approach is typically not a viable option for the quantification of peptide content in complex mixtures. In contrast, a spectral count is the total number of spectra identified for a protein, which can correlate more reliably with protein abundance in some complicated sample types (151, 152). The measure has undergone multiple iterations to account for specific challenges, as reviewed elsewhere (151, 152). For example, one form used in amyloid typing is the normalized spectral abundance factor (NSAF) (141). The NSAF takes into account the conundrum that longer proteins will produce more detectable peptides (potentially conflating the quantitative interpretation). To facilitate comparison between samples, the measure is further normalized by all spectral counts within a sample per the following formula (153):
where NSAFk is the NSAF for a specific protein with SpC spectral counts and L length, and N is the total number of proteins identified in the sample. Despite iterative improvements to the spectral counting principle, comparing SCs between two proteins may not accurately reflect their relative abundances in some cases (a topic beyond the scope of this perspective) (152). This may not be problematic for applications focused on the identification of the most abundant (or nearly most abundant) protein in a sample (e.g., amyloid typing), but could prove limiting in other applications, particularly if greater precision is required or the quantity of a low abundance target is the goal of the assay.
The alternative untargeted acquisition method to DDA is data-independent acquisition (DIA). DIA, like DDA, employs a full m/z range survey scan, but in place of computer-driven precursor ion selection for MS/MS, the instrument accumulates all precursor ions for fragmentation across fixed sets of m/z isolation windows that, together, span the entire m/z range. Fragmentation spectra are mixed as a result (and therefore of higher complexity), but now contain data corresponding to all eluting peptides. Eliminating the stochastic precursor ion selection biased toward higher abundance peptides can improve consistency in the analysis and facilitate data capture for low abundance targets. Multiple studies suggest the approach improves consistency in protein/peptide identifications as well as quantitative reproducibility (150).
Studies of DIA applied to solid organ tissues have highlighted the benefits of the approach [Table 2, (4, 154, 155, 156, 157, 158, 159, 160, 161)]. For example, Hou and colleagues used DIA to identify putative biomarkers in esophageal carcinoma (155). In that study, 116 of 120 differentially regulated protein biomarkers identified by DIA were confirmed by targeted multiple reaction monitoring-mass spectrometry (MRM), indicating that quantification by DIA can come close to what is considered the current gold standard for mass spectrometry-based targeted protein quantification (162). Additionally, the authors were able to leverage the peptide transition information from the DIA experiments in the development of their MRM method for verification studies. In other studies by Weke (157) and Keme (158), the application of DIA identified many more proteins compared to previous studies that applied DDA to similar tissues.
Table 2.
Reported applications of DIA to solid tissues in cancer proteomics
| Reference | Solid tissues evaluateda | Protein analysis results | Highlight(s) |
|---|---|---|---|
| Hou 2015 (155) | Paired tissues: Esophageal squamous cell carcinoma tissue and adjacent normal tissue (n = 10, frozen tissues only) | 1758 proteins quantified; 467 proteins differentially expressed | High correlation between quantitative information derived from DIA (SWATH) and co-performed MRM experiments |
| Gao 2017 (160) | Paired tissues: Hepatocellular carcinoma (HBV-associated) and adjacent normal liver tissue (discovery set n = 14, verification set n = 6, frozen tissues only) | 4216 unique proteins quantified; 338 differentially expressed | Aspects of metabolic reprogramming identified; DIA (SWATH) data consistent with previous western-blot/IHC-validated results |
| Schwarzfischer 2017 (156) | Burkitt lymphoma: 5 frozen, 5 FFPE DLBCL: 6 frozen, 9 FFPE |
Frozen: 2938 proteins quantified, 1125 differentially regulated FFPE: 1442 proteins quantified, 404 differentially regulated |
Quantitative results reproducible between frozen and FFPE tissues; corroborating metabolomic studies were performed, demonstrating high secretion of pyruvic acid in BL compared to DLBCL |
| Keam 2018 (158) | Paired tissues: Prostate cancer (PCa), before and 14 days after radiation therapy (n = 8 patietns, comprising 2 with frozen and FFPE tissue, 1 frozen only, 5 FFPE only) | >5000 proteins quantified, 49 differentially expressed | Deeper proteomic analyses obtained for prostatic tissue by DIA compared to previously reported MS pipeline applied to PCa (159) |
| Zhu 2019 (4) | Multiple prostate cancer cohorts (frozen and FFPE tissues); multiple diffuse large B-cell lymphoma cohorts (n = 41 patients in first cohort, n = 52 patients in second cohort, 113 FFPE samples) | 3030 proteins quantified in one PCa cohort, 4144 proteins in second cohort | Panel of protein biomarker candidates identified for PCa diagnosis; myeloperoxidase identified as prognostic marker in DLBCL |
| Janacova 2020 (161) | Endometrial cancer (n = 34, all FFPE): 15 – previous tamoxifen treatment (ET) 19 – no previous tamoxifen (EN) 11 – adjacent myometrium |
904 proteins quantified; Differential expression of calcyphosin (CAPS) and stathmin confirmed (STMN1) | CAPS and STMN1 also appeared useful as prognostic indicators |
| Marchione 2020 (159) | 18 tissue types evaluated using HYPERsol protocol + separate analyses of 32 archival samples of malignant peripheral nerve sheath tumor (MPNST), melanoma, and synovial sarcoma | 4000–5000 proteins/sample | DIA-based analyses applied to distinguish histomorphologic mimics |
| Weke 2022 (157) | Glioblastoma (GBM) FFPE microdissections (n = 5) | >1700 proteins identified; >1400 quantified | Leveraged multiple sample processing approaches; Protein identifications increased compared to DDA; High quantitative precision for GBM markers: GFAP, FN1, VIM, and MBP |
Abbreviations: BL, Burkitt’s lymphoma; DLBCL, diffuse large B-cell lymphoma; HYPERSol, high-yield protein extraction and recovery by direct solubilization; MPNST, malignant peripheral nerve sheath tumor; MRM, multiple reaction monitoring; PCa, prostate cancer; SWATH, sequential window acquisition of all theoretical spectral.
High-yield protein extraction and recovery by direct solubilization.
Samples other than solid tissues are excluded from this table.
Whether these reported advantages of DIA in research will directly benefit patient testing remains to be seen. Furthermore, the notion of better reproducibility of DIA is not without debate or potential caveats (163), and the specific limitations of DIA should be considered. These include higher complexity of the data processing since spectra are mixed and must be deconvoluted. DIA may also be a lower throughput than DDA since the generation of spectral libraries may be needed (154). Effective use of this technology will require successful collaboration between end users in clinical laboratories and computer scientists. For example, an understanding of false discovery rates is beyond the scope of most practicing pathologists. Nonetheless, better reproducibility at the peptide level, if achievable, is appealing clinically for several reasons. Raw data that are more amenable to replication across injections can better facilitate the development of robust quality assurance measures mirroring those already in practice for targeted methods run on triple quadrupole mass spectrometers (e.g., transition ion ratios). Better reproducibility at the peptide level [e.g., >90% overlap in peptide lists between technical replicates (154)] should also benefit the identification of mutations or polymorphisms in patient samples wherein data corresponding to specified peptides will not be missing. Finally, more comprehensive and reproducible data will be more amenable to reexamination as new biomarkers are discovered. Also notable is that open-source options are becoming more prevalent for data processing and should be eventually adaptable into pipelines appropriate for use in clinical environments, given that this has already been demonstrated when handling shotgun proteomics data [e.g., Crux Pipeline (141)].
Guidance is Needed for Reliability and Standardization
The limited clinical application of mass spectrometry in solid tissues to date reflects a number of factors, including potential costs but also a range of requirements specific to the use of the technology in patient care. Many prerequisites, including those relating to test quality, are defined in government regulations, whereas others may be institution-specific. In the United States, the Centers for Medicare and Medicaid Services regulates all clinical laboratory testing, via the Clinical Laboratory Improvements Amendments of 1988 (CLIA). The specific rules governing clinical laboratories can be found in Code of Federal Regulations, Title 42, part 493 (42 CFR 493, “Laboratory Requirements”). While many of these regulations have general implications for operating a clinical lab, successful adherence to others (e.g., proficiency testing, method performance verification, and quality control) must be accounted for on an assay-by-assay basis.
Key areas that must be addressed include method validation and performance verification. Generally speaking, assays using mass spectrometry-based platforms are considered high-complexity and, with few exceptions, are not FDA-cleared or approved. In addition, almost all mass spectrometry-based assays are categorized as laboratory developed tests (LDTs), meaning in vitro diagnostic tests that are designed, manufactured, and used within a single laboratory. As a result, despite the overall improvements in analytical sensitivity and specificity, these tests still have the potential for variability between laboratories. The lack of regulatory clearance or approval means that these tests are subject to additional requirements during method validation and verification of performance characteristics. According to regulation § 493.1253, developers of such assays must (at a minimum) establish assay accuracy, precision, analytical specificity, reportable range, and reference intervals. How these performance characteristics are established or judged is not detailed by government regulations, and thus is still subject to the discretion of a laboratory medical director. Accreditation organizations, such as the College of American Pathologists (CAP), set certain baseline standards. For example, CAP checklists include brief specifications of an evaluation of test accuracy and the suitability of samples used during verification. However, this guidance is limited and recommendations for method development and validation must be found elsewhere.
One source of guidance in test design and validation is the Clinical and Laboratory Standards Institute (CLSI), a non-profit organization serving device manufacturers and those clinical laboratories that are developing and running LDTs. CLSI creates standards of practice across areas of laboratory medicine based on expert consensus, with the intent of improving testing quality, safety, and efficiency. The standards cover many aspects of method development, validation, performance verification, and post-implementation monitoring. Some guidelines address mass spectrometry, but are not all-encompassing, and do not specifically address analyses involving solid tissues. These guidelines include, C50 (“Mass Spectrometry in the Clinical Laboratory: General Principles”), C62 (“Liquid Chromatography-Mass Spectrometry Methods”, recently updated in 2022), and C64 (“Quantitative Measurement of Proteins and Peptides by Mass Spectrometry”). As detailed by Lynch in 2016 (164), the impetus for development of these documents is the widening user-base and a need for standardization, as well as to fill in gaps not addressed by other guidance documents (e.g., the FDA’s Guidance for industry: Bioanalytical method validation). Document C62, in particular, offers guidance with respect to LC-MS methods, focused on quantitative analyses performed on tandem mass spectrometers (with a special emphasis on triple quadrupole mass spectrometers). Assay elements included in this guidance document include calibration, validation, quality assurance, quality control, among others. Document C64 built upon C62, focused instead on the quantitative measurement of proteins, recognizing that protein and peptide analyses involve unique challenges and additional workflows. One other key recognition in the document is that although some proteins may exist as a single specified molecular composition, most will exist as a complex family of related proteoforms, differing in length, amino acid sequence, and post-translational modifications. As acknowledged in C64, this reality may stifle standardization and harmonization since two assays (just like different immunoassays) may not actually measure the exact same target or set of targets, making the selection of the analyte (i.e., proteotypic peptide) an important step.
The transition to measuring proteins in tissues represents another step forward where consensus and guidance will be needed. Of course, many of the challenges affecting IHC will undoubtedly also need to be addressed for mass spectrometry-based approaches. Basic questions to consider include:
-
•
What is an appropriate form of calibration?
-
•
Can the appropriate matrix be used for analytical validation?
-
•
What are suitable quality control materials?
-
•
What are the most appropriate (or minimum) validation studies?
-
•
How should proficiency testing be performed?
-
•
What are appropriate quality assurance measures?
There is some precedent for answering these questions: (1) Existing amyloid typing pipelines and (2) targeted quantitative assays developed in CLIA-regulated environments. With respect to untargeted analyses, in their 2013 perspective, Theis and colleagues (142) defined what steps in the workflow could potentially contribute to variability in the processing of samples for amyloid typing. Unsurprisingly, many of the factors that they identified overlap with those that affect immunohistochemistry. E.g., just as with IHC, LC-MS/MS could be affected by specimen processing features (e.g., fixation technique), sample preparation factors (e.g., antigen retrieval), and tumor heterogeneity. Other steps are specific to LC-MS/MS: choice/setup of collection technique (e.g., LCM), chromatography, etc. To meet CLIA requirements, Theis describes addressing the six criteria required for all LDTs: Accuracy, analytical precision, analytical sensitivity, analytical specificity, reference range, and reportable range. However, it is clear that the experiments performed to address the six criteria will not be the same for all tissue assays. In addition, the interpretation of untargeted measurements aimed at tissue subtyping is very different from targeted assays aimed at quantifying a small number of proteins. Further, while one benefit of untargeted proteomics is the ability to discover new amyloidogenic proteins, it is less clear how confirmation of those discoveries should be performed in a CLIA laboratory, particularly in the absence of well-characterized standards (including for amyloid). The field would greatly benefit from more general consensus on what experiments should be performed to validate untargeted LC-MS/MS assays in tissues, although the previous examples in the literature may be a helpful starting point to answer the questions listed above (140, 141, 142).
Since targeted quantitative LC-MS/MS assays in tissues are more analogous to what is normally done in clinical laboratories, standard approaches to validation may be more appropriate with less ambiguous interpretation and subjectivity. Most targeted assays described to date have included some form of validation, although there are many elements of robustness that still need to be assessed. Method comparisons have generally been performed against IHC (or other traditional immunoassays), putting laboratories in the position of asserting assay acceptability compared with a potentially inferior gold-standard technology. Effects of tissue heterogeneity are likely to be an ongoing area of study; normalization will be particularly important in this regard. As described above, multiple strategies have been applied to the normalization of HER2 in tissue samples. While some of these approaches produce encouraging findings in early studies, the peculiarities of each and the potential for misinterpretation will have to be investigated. The challenge of normalization is already a major problem in proteomics more generally and is described elsewhere (131, 165).
Where Do We Go From Here?
At present, although MS may be a viable alternative to IHC in many respects, it still suffers from significant concerns, many of which are relevant to IHC. This raises the question of, “how do we move forward?” We envision 3 steps in the development of best practices in clinical tissue mass spectrometry.
Development of Programs to Share Clinical Cases Between Institutions
As noted earlier, tissue analyses almost universally lack standard materials. Furthermore, for LC-MS/MS analyses of proteins in general, there are typically no relevant PT materials available. This leaves laboratories in the precarious position of trying to verify the quality of their own assays. The latter might include retesting previous cases, which can demonstrate consistent performance over time, but does not speak to the accuracy of the analytical method or the strategies used in data processing (i.e., it is possible to be wrong twice if nothing about an assay has changed). For quantitative assays, we might borrow from alternative assessment programs for LC-MS/MS assays of proteins in biofluids, which were designed to help promote harmonization. For example, clinical laboratories share samples biannually to assess the comparability of thyroglobulin testing across laboratories (127). The multiplexed nature of tissue MS results will make similar programs particularly important, since interpretation is more complicated.
Improve Raw Data Accessibility
Development of best practices in data analysis and interpretation will require data sharing in order to directly compare data-processing pipelines. Data sharing has become increasingly robust in the research space, particularly through the efforts of the National Cancer Institute’s Clinical Proteomic Tumor Analysis Consortium (CPTAC). For example, the proteomic data commons (https://proteomic.datacommons.cancer.gov/pdc/) provides the raw data resulting from the accumulation of knowledge with respect to cancer biology, which has significant secondary benefits. These raw data, which meet certain quality characteristics, allow users to test analysis pipelines. Raw data from clinical laboratories will also improve the transparency of the data analysis process, because processed data should correlate with disease processes across institutions, just like quantitative data in biofluid assays.
Include Anatomic Pathologists in Assay Development and Data Interpretation
As clinical mass spectrometry becomes increasingly sophisticated, there may be a desire to shift the entire operation to the hands of technical experts (e.g., analytical chemists) or centralize testing in reference laboratories away from health care facilities. However, there is a significant potential for risk in the misinterpretation of proteomic data if results are not carefully interpreted in light of a patient’s clinical history and other laboratory testing results. There are also cases in which correct interpretation requires advanced or more obscure medical knowledge and a formal interface between anatomic pathologists and proteomic data can be essential. For example, Figure 3 illustrates a clinical case in which amyloid typing failed to identify the amyloid type (i.e., the protein responsible for the amyloidosis), until the search database was modified to include an exogenous substance of clinical relevance to the patient.
Fig. 3.
An illustrated pitfall in amyloid typing. Database searching was performed without and with the inclusion of an exogenous amyloidogenic substance, enfuvirtide (amino acid sequence YTSLIHSLIEESQNQQEKNEQELLELDKWASLWNWF). The data are from a clinical sample evaluated using the workflow described in reference (141). Protein abundance is expressed in the form of NSAF for the 5 most abundant amyloid-associated proteins in the sample in addition to enfuvirtide. Apo-AI, Apolipoprotein A-I; Apo-E, Apolipoprotein E; ApoA-IV, Apolipoprotein A-IV; DB, database; IGKC, Immunoglobulin kappa constant; SAP, Serum amyloid P-component.
Conclusion
As a clinical diagnostic platform, IHC has deficiencies and we propose that solutions incorporating mass spectrometry, or more specifically, LC-MS/MS, have many advantages that will help advance diagnosis, prognosis, and therapeutic management based on tissue biopsies. Successful implementation over the long term will require careful attention to factors related to validation and quality monitoring. Because these methods differ fundamentally from other applications of LC-MS/MS in clinical laboratories, thoughtful approaches are needed for calibration, validation, and quality assurance and we look forward to new advances in this exciting direction for clinical proteomics.
Conflict of interest
Dr Mandy Paulovich, M.D., Ph.D., is the founder of Precision Assays. The rest of the authors have nothing to disclose.
Acknowledgments
The authors thank Dr Geoff Baird for providing thoughts on the future of mass spectrometry in the solid tissue space and issues surrounding multiplexing. Dr Kelly Smith performed tissue sampling for the case shown in Figure 3.
Funding and additional information
This work was partially funded by the National Institutes of Health (R01CA235575, U01CA214114, U19AG065156, P30 CA015704). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Author contributions
W. S. P., A. N. H., M. R. K., J. R. W., and A. G. P writing–original draft. M. R. K and J. J. K. resources.
Contributor Information
Andrew N. Hoofnagle, Email: ahoof@uw.edu.
Amanda G. Paulovich, Email: apaulovi@fredhutch.org.
References
- 1.Blow N. Tissue preparation: tissue issues. Nature. 2007;448:959–963. doi: 10.1038/448959a. [DOI] [PubMed] [Google Scholar]
- 2.Titford M. The long history of hematoxylin. Biotech. Histochem. 2005;80:73–78. doi: 10.1080/10520290500138372. [DOI] [PubMed] [Google Scholar]
- 3.Race G.J., Tillery G.W., Dysert P.A., 2nd A history of pathology and laboratory medicine at Baylor University Medical Center. Proc. (Bayl. Univ. Med. Cent.) 2004;17:42–55. doi: 10.1080/08998280.2004.11927956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhu Y., Weiss T., Zhang Q., Sun R., Wang B., Yi X., et al. High-throughput proteomic analysis of FFPE tissue samples facilitates tumor stratification. Mol. Oncol. 2019;13:2305–2328. doi: 10.1002/1878-0261.12570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bogen S.A., Dabbs D.J., Miller K.D., Nielsen S., Parry S.C., Szabolcs M.J., et al. A consortium for analytic standardization in immunohistochemistry. Arch. Pathol. Lab. Med. 2022;147:584–590. doi: 10.5858/arpa.2022-0031-RA. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bogen S.A. A Root cause analysis into the high error rate in clinical immunohistochemistry. Appl. Immunohistochem. Mol. Morphol. 2019;27:329–338. doi: 10.1097/PAI.0000000000000750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Taylor C.R. Quantitative in situ proteomics; a proposed pathway for quantification of immunohistochemistry at the light-microscopic level. Cell Tissue Res. 2015;360:109–120. doi: 10.1007/s00441-014-2089-0. [DOI] [PubMed] [Google Scholar]
- 8.Taylor C.R. An exaltation of experts: concerted efforts in the standardization of immunohistochemistry. Hum. Pathol. 1994;25:2–11. doi: 10.1016/0046-8177(94)90164-3. [DOI] [PubMed] [Google Scholar]
- 9.Gustafsson O.J., Arentz G., Hoffmann P. Proteomic developments in the analysis of formalin-fixed tissue. Biochim. Biophys. Acta. 2015;1854:559–580. doi: 10.1016/j.bbapap.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 10.Taylor C.R. The total test approach to standardization of immunohistochemistry. Arch. Pathol. Lab. Med. 2000;124:945–951. doi: 10.5858/2000-124-0945-TTTATS. [DOI] [PubMed] [Google Scholar]
- 11.Ooms E.C., Anderson W.A., Alons C.L., Boon M.E., Veldhuizen R.W. Analysis of the performance of pathologists in the grading of bladder tumors. Hum. Pathol. 1983;14:140–143. doi: 10.1016/s0046-8177(83)80242-1. [DOI] [PubMed] [Google Scholar]
- 12.Thomas G.D., Dixon M.F., Smeeton N.C., Williams N.S. Observer variation in the histological grading of rectal carcinoma. J. Clin. Pathol. 1983;36:385–391. doi: 10.1136/jcp.36.4.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Beck J.S. Observer variability in reporting of breast lesions. J. Clin. Pathol. 1985;38:1358–1365. doi: 10.1136/jcp.38.12.1358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dixon M.F., Brown L.J., Gilmour H.M., Price A.B., Smeeton N.C., Talbot I.C., et al. Observer variation in the assessment of dysplasia in ulcerative colitis. Histopathology. 1988;13:385–397. doi: 10.1111/j.1365-2559.1988.tb02055.x. [DOI] [PubMed] [Google Scholar]
- 15.Reid B.J., Haggitt R.C., Rubin C.E., Roth G., Surawicz C.M., Van Belle G., et al. Observer variation in the diagnosis of dysplasia in Barrett's esophagus. Hum. Pathol. 1988;19:166–178. doi: 10.1016/s0046-8177(88)80344-7. [DOI] [PubMed] [Google Scholar]
- 16.Melville D.M., Jass J.R., Morson B.C., Pollock D.J., Richman P.I., Shepherd N.A., et al. Observer study of the grading of dysplasia in ulcerative colitis: comparison with clinical outcome. Hum. Pathol. 1989;20:1008–1014. doi: 10.1016/0046-8177(89)90273-6. [DOI] [PubMed] [Google Scholar]
- 17.Stel H.V., Vroom T.M., Blok P., van Heerde P., Meijer C.J. Therapy-relevant discrepancies between diagnoses of institutional pathologists and experienced hematopathologists in the diagnosis of malignant lymphoma. Pathol. Res. Pract. 1989;184:242–247. doi: 10.1016/S0344-0338(89)80126-8. [DOI] [PubMed] [Google Scholar]
- 18.van Ginneken A.M., van der Lei J. Understanding differential diagnostic disagreement in pathology. Proc. Annu. Symp. Comput. Appl. Med. Care. 1991:99–103. [PMC free article] [PubMed] [Google Scholar]
- 19.Kay E.W., Barry Walsh C.J., Whelan D., O'Grady A., Leader M.B. Inter-observer variation of p53 immunohistochemistry--an assessment of a practical problem and comparison with other studies. Br. J. Biomed. Sci. 1996;53:101–107. [PubMed] [Google Scholar]
- 20.Riber C., Tønnesen H., Aru A., Bjerregaard B. Observer variation in the assessment of the histopathologic diagnosis of acute appendicitis. Scand. J. Gastroenterol. 1999;34:46–49. doi: 10.1080/00365529950172826. [DOI] [PubMed] [Google Scholar]
- 21.Hirokawa M., Carney J.A., Goellner J.R., DeLellis R.A., Heffess C.S., Katoh R., et al. Observer variation of encapsulated follicular lesions of the thyroid gland. Am. J. Surg. Pathol. 2002;26:1508–1514. doi: 10.1097/00000478-200211000-00014. [DOI] [PubMed] [Google Scholar]
- 22.Lloyd R.V., Erickson L.A., Casey M.B., Lam K.Y., Lohse C.M., Asa S.L., et al. Observer variation in the diagnosis of follicular variant of papillary thyroid carcinoma. Am. J. Surg. Pathol. 2004;28:1336–1340. doi: 10.1097/01.pas.0000135519.34847.f6. [DOI] [PubMed] [Google Scholar]
- 23.Douglas-Jones A., Shah V., Morgan J., Dallimore N., Rashid M. Observer variability in the histopathological reporting of core biopsies of papillary breast lesions is reduced by the use of immunohistochemistry for CK5/6, calponin and p63. Histopathology. 2005;47:202–208. doi: 10.1111/j.1365-2559.2005.02208.x. [DOI] [PubMed] [Google Scholar]
- 24.Van Den Brekel M.W.M., Lodder W.L., Stel H.V., Bloemena E., Leemans C.R., Van Der Waal I. Observer variation in the histopathologic assessment of extranodal tumor spread in lymph node metastases in the neck. Head Neck. 2012;34:840–845. doi: 10.1002/hed.21823. [DOI] [PubMed] [Google Scholar]
- 25.Taylor C.R. Immunohistochemistry: growing pains, from a stain to an assay. Appl. Immunohistochem. Mol. Morphol. 2019;27:325–326. doi: 10.1097/PAI.0000000000000770. [DOI] [PubMed] [Google Scholar]
- 26.Torlakovic E.E., Nielsen S., Vyberg M., Taylor C.R. Getting controls under control: the time is now for immunohistochemistry. J. Clin. Pathol. 2015;68:879–882. doi: 10.1136/jclinpath-2014-202705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vyberg M., Nielsen S. Proficiency testing in immunohistochemistry--experiences from nordic immunohistochemical quality control (NordiQC) Virchows Arch. 2016;468:19–29. doi: 10.1007/s00428-015-1829-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Engel K.B., Moore H.M. Effects of preanalytical variables on the detection of proteins by immunohistochemistry in formalin-fixed, paraffin-embedded tissue. Arch. Pathol. Lab. Med. 2011;135:537–543. doi: 10.5858/2010-0702-RAIR.1. [DOI] [PubMed] [Google Scholar]
- 29.Wolff A.C., Hammond M.E.H., Allison K.H., Harvey B.E., Mangu P.B., Bartlett J.M.S., et al. Human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists Clinical Practice Guideline Focused Update. Arch. Pathol. Lab. Med. 2018;142:1364–1382. doi: 10.5858/arpa.2018-0902-SA. [DOI] [PubMed] [Google Scholar]
- 30.Aeffner F., Wilson K., Martin N.T., Black J.C., Hendriks C.L.L., Bolon B., et al. The gold standard paradox in digital image analysis: manual versus automated scoring as ground truth. Arch. Pathol. Lab. Med. 2017;141:1267–1275. doi: 10.5858/arpa.2016-0386-RA. [DOI] [PubMed] [Google Scholar]
- 31.Welsh A.W., Moeder C.B., Kumar S., Gershkovich P., Alarid E.T., Harigopal M., et al. Standardization of estrogen receptor measurement in breast cancer suggests false-negative results are a function of threshold intensity rather than percentage of positive cells. J. Clin. Oncol. 2011;29:2978–2984. doi: 10.1200/JCO.2010.32.9706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Garrison L.P., Jr., Babigumira J.B., Masaquel A., Wang B.C., Lalla D., Brammer M. The lifetime economic burden of inaccurate HER2 testing: estimating the costs of false-positive and false-negative HER2 test results in US patients with early-stage breast cancer. Value Health. 2015;18:541–546. doi: 10.1016/j.jval.2015.01.012. [DOI] [PubMed] [Google Scholar]
- 33.Jacobs T.W., Gown A.M., Yaziji H., Barnes M.J., Schnitt S.J. HER-2/neu protein expression in breast cancer evaluated by immunohistochemistry. A study of interlaboratory agreement. Am. J. Clin. Pathol. 2000;113:251–258. doi: 10.1309/980M-E24R-V19K-595D. [DOI] [PubMed] [Google Scholar]
- 34.Modi S., Park H., Murthy R.K., Iwata H., Tamura K., Tsurutani J., et al. Antitumor activity and safety of trastuzumab deruxtecan in patients with HER2-low-expressing advanced breast cancer: results from a phase ib study. J. Clin. Oncol. 2020;38:1887–1896. doi: 10.1200/JCO.19.02318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sheffield B.S., Garratt J., Kalloger S.E., Li-Chang H.H., Torlakovic E.E., Gilks C.B., et al. HER2/neu testing in gastric cancer by immunohistochemistry: assessment of interlaboratory variation. Arch. Pathol. Lab. Med. 2014;138:1495–1502. doi: 10.5858/arpa.2013-0604-OA. [DOI] [PubMed] [Google Scholar]
- 36.An E., Ock C.Y., Kim T.Y., Lee K.H., Han S.W., Im S.A., et al. Quantitative proteomic analysis of HER2 expression in the selection of gastric cancer patients for trastuzumab treatment. Ann. Oncol. 2017;28:110–115. doi: 10.1093/annonc/mdw442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lambein K., Van Bockstal M., Vandemaele L., Geenen S., Rottiers I., Nuyts A., et al. Distinguishing score 0 from score 1+ in HER2 immunohistochemistry-negative breast cancer: clinical and pathobiological relevance. Am. J. Clin. Pathol. 2013;140:561–566. doi: 10.1309/AJCP4A7KTAYHZSOE. [DOI] [PubMed] [Google Scholar]
- 38.Modi S., Jacot W., Yamashita T., Sohn J., Vidal M., Tokunaga E., et al. Trastuzumab deruxtecan in previously treated HER2-low advanced breast cancer. N. Engl. J. Med. 2022;387:9–20. doi: 10.1056/NEJMoa2203690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Miller R.T. Avoiding pitfalls in diagnostic immunohistochemistry-important technical aspects that every pathologist should know. Semin. Diagn. Pathol. 2019;36:312–335. doi: 10.1053/j.semdp.2019.05.002. [DOI] [PubMed] [Google Scholar]
- 40.Gown A.M. Diagnostic immunohistochemistry: what can go wrong and how to prevent it. Arch. Pathol. Lab. Med. 2016;140:893–898. doi: 10.5858/arpa.2016-0119-RA. [DOI] [PubMed] [Google Scholar]
- 41.Yaziji H., Barry T. Diagnostic Immunohistochemistry: what can go wrong? Adv. Anat. Pathol. 2006;13:238–246. doi: 10.1097/01.pap.0000213041.39070.2f. [DOI] [PubMed] [Google Scholar]
- 42.Hofman F.M., Taylor C.R. Immunohistochemistry. Curr. Protoc. Immunol. 2013 doi: 10.1002/0471142735.im2104s103. [DOI] [PubMed] [Google Scholar]
- 43.Moskaluk C.A. Standardization of clinical immunohistochemistry: why, how, and by whom? Am. J. Clin. Pathol. 2002;118:669–671. doi: 10.1309/KM95-6LVL-UNLB-R3RH. [DOI] [PubMed] [Google Scholar]
- 44.Cheung C.C., Taylor C.R., Torlakovic E.E. An audit of failed immunohistochemical slides in a clinical laboratory: the role of on-slide controls. Appl. Immunohistochem. Mol. Morphol. 2017;25:308–312. doi: 10.1097/PAI.0000000000000305. [DOI] [PubMed] [Google Scholar]
- 45.Torlakovic E.E., Nielsen S., Francis G., Garratt J., Gilks B., Goldsmith J.D., et al. Standardization of positive controls in diagnostic immunohistochemistry: recommendations from the international ad hoc expert committee. Appl. Immunohistochem. Mol. Morphol. 2015;23:1–18. doi: 10.1097/PAI.0000000000000163. [DOI] [PubMed] [Google Scholar]
- 46.Torlakovic E.E., Francis G., Garratt J., Gilks B., Hyjek E., Ibrahim M., et al. Standardization of negative controls in diagnostic immunohistochemistry: recommendations from the international ad hoc expert panel. Appl. Immunohistochem. Mol. Morphol. 2014;22:241–252. doi: 10.1097/PAI.0000000000000069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gale N.S., Kalloger S.E., Cai E., Abozina A., Derakhshan F., Hickey T., et al. Immunohistochemistry critical assay performance controls (ICAPC) reduce interobserver variability in the interpretation of BRAFV600E immunohistochemistry. Appl. Immunohistochem. Mol. Morphol. 2020;28:422–427. doi: 10.1097/PAI.0000000000000784. [DOI] [PubMed] [Google Scholar]
- 48.Neumeister V.M., Parisi F., England A.M., Siddiqui S., Anagnostou V., Zarrella E., et al. A tissue quality index: an intrinsic control for measurement of effects of preanalytical variables on FFPE tissue. Lab. Invest. 2014;94:467–474. doi: 10.1038/labinvest.2014.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Battifora H. Assessment of antigen damage in immunohistochemistry. The vimentin internal control. Am. J. Clin. Pathol. 1991;96:669–671. doi: 10.1093/ajcp/96.5.669. [DOI] [PubMed] [Google Scholar]
- 50.Taylor C.R. Quantifiable internal reference standards for immunohistochemistry: the measurement of quantity by weight. Appl. Immunohistochem. Mol. Morphol. 2006;14:253–259. doi: 10.1097/00129039-200609000-00001. [DOI] [PubMed] [Google Scholar]
- 51.Lin E., Fuda F., Luu H.S., Cox A.M., Fang F., Feng J., et al. Digital pathology and artificial intelligence as the next chapter in diagnostic hematopathology. Semin. Diagn. Pathol. 2023;40:88–94. doi: 10.1053/j.semdp.2023.02.001. [DOI] [PubMed] [Google Scholar]
- 52.Niazi M.K.K., Parwani A.V., Gurcan M.N. Digital pathology and artificial intelligence. Lancet Oncol. 2019;20:e253–e261. doi: 10.1016/S1470-2045(19)30154-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Aeffner F., Zarella M.D., Buchbinder N., Bui M.M., Goodman M.R., Hartman D.J., et al. Introduction to digital image analysis in whole-slide imaging: a white paper from the digital pathology association. J. Pathol. Inform. 2019;10:9. doi: 10.4103/jpi.jpi_82_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Joshi S., Watkins J., Gazinska P., Brown J.P., Gillett C.E., Grigoriadis A., et al. Digital imaging in the immunohistochemical evaluation of the proliferation markers Ki67, MCM2 and Geminin, in early breast cancer, and their putative prognostic value. BMC Cancer. 2015;15:546. doi: 10.1186/s12885-015-1531-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lykkegaard Andersen N., Brugmann A., Lelkaitis G., Nielsen S., Friis Lippert M., Vyberg M. Virtual double staining: a digital approach to immunohistochemical quantification of estrogen receptor protein in breast carcinoma specimens. Appl. Immunohistochem. Mol. Morphol. 2018;26:620–626. doi: 10.1097/PAI.0000000000000502. [DOI] [PubMed] [Google Scholar]
- 56.Zhang M., Tan C., Wang X., Ding X., Zhang B., Yang Z., et al. Digital image analysis of Ki67 heterogeneity improves the diagnosis and prognosis of gastroenteropancreatic neuroendocrine neoplasms. Mod. Pathol. 2023;36 doi: 10.1016/j.modpat.2022.100017. [DOI] [PubMed] [Google Scholar]
- 57.Taylor C.R., Levenson R.M. Quantification of immunohistochemistry--issues concerning methods, utility and semiquantitative assessment II. Histopathology. 2006;49:411–424. doi: 10.1111/j.1365-2559.2006.02513.x. [DOI] [PubMed] [Google Scholar]
- 58.Giovanella L., Feldt-Rasmussen U., Verburg F.A., Grebe S.K., Plebani M., Clark P.M. Thyroglobulin measurement by highly sensitive assays: focus on laboratory challenges. Clin. Chem. Lab. Med. 2015;53:1301–1314. doi: 10.1515/cclm-2014-0813. [DOI] [PubMed] [Google Scholar]
- 59.Spencer C., Petrovic I., Fatemi S. Current thyroglobulin autoantibody (TgAb) assays often fail to detect interfering TgAb that can result in the reporting of falsely low/undetectable serum Tg IMA values for patients with differentiated thyroid cancer. J. Clin. Endocrinol. Metab. 2011;96:1283–1291. doi: 10.1210/jc.2010-2762. [DOI] [PubMed] [Google Scholar]
- 60.Vyberg M. A commentary: quality assurance in immunohistochemistry. Appl. Immunohistochem. Mol. Morphol. 2019;27:327–328. doi: 10.1097/PAI.0000000000000771. [DOI] [PubMed] [Google Scholar]
- 61.Baker M. Reproducibility crisis: blame it on the antibodies. Nature. 2015;521:274–276. doi: 10.1038/521274a. [DOI] [PubMed] [Google Scholar]
- 62.Tan W.C.C., Nerurkar S.N., Cai H.Y., Ng H.H.M., Wu D., Wee Y.T.F., et al. Overview of multiplex immunohistochemistry/immunofluorescence techniques in the era of cancer immunotherapy. Cancer Commun. (Lond.) 2020;40:135–153. doi: 10.1002/cac2.12023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yagnik G., Liu Z., Rothschild K.J., Lim M.J. Highly multiplexed immunohistochemical MALDI-MS imaging of biomarkers in tissues. J. Am. Soc. Mass Spectrom. 2021;32:977–988. doi: 10.1021/jasms.0c00473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Marsh-Wakefield F., Ferguson A.L., Liu K., Santhakumar C., McCaughan G., Palendira U. Approaches to spatially resolving the tumour immune microenvironment of hepatocellular carcinoma. Ther. Adv. Med. Oncol. 2022;14 doi: 10.1177/17588359221113270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.van Dam S., Baars M.J.D., Vercoulen Y. Multiplex tissue imaging: spatial revelations in the tumor microenvironment. Cancers (Basel) 2022;14:3170. doi: 10.3390/cancers14133170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Giesen C., Wang H.A., Schapiro D., Zivanovic N., Jacobs A., Hattendorf B., et al. Highly multiplexed imaging of tumor tissues with subcellular resolution by mass cytometry. Nat. Methods. 2014;11:417–422. doi: 10.1038/nmeth.2869. [DOI] [PubMed] [Google Scholar]
- 67.Norris J.L., Caprioli R.M. Analysis of tissue specimens by matrix-assisted laser desorption/ionization imaging mass spectrometry in biological and clinical research. Chem. Rev. 2013;113:2309–2342. doi: 10.1021/cr3004295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Aichler M., Walch A. MALDI imaging mass spectrometry: current frontiers and perspectives in pathology research and practice. Lab. Invest. 2015;95:422–431. doi: 10.1038/labinvest.2014.156. [DOI] [PubMed] [Google Scholar]
- 69.Zhu X., Xu T., Peng C., Wu S. Advances in MALDI mass spectrometry imaging single cell and tissues. Front. Chem. 2021;9 doi: 10.3389/fchem.2021.782432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Unsihuay D., Mesa Sanchez D., Laskin J. Quantitative mass spectrometry imaging of biological systems. Annu. Rev. Phys. Chem. 2021;72:307–329. doi: 10.1146/annurev-physchem-061020-053416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Rompp A., Spengler B. Mass spectrometry imaging with high resolution in mass and space. Histochem. Cell Biol. 2013;139:759–783. doi: 10.1007/s00418-013-1097-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ryan D.J., Spraggins J.M., Caprioli R.M. Protein identification strategies in MALDI imaging mass spectrometry: a brief review. Curr. Opin. Chem. Biol. 2019;48:64–72. doi: 10.1016/j.cbpa.2018.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Buchberger A.R., Delaney K., Johnson J., Li L. Mass spectrometry imaging: a review of emerging advancements and future insights. Anal. Chem. 2018;90:240–265. doi: 10.1021/acs.analchem.7b04733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Rimm D.L. Next-gen immunohistochemistry. Nat. Methods. 2014;11:381–383. doi: 10.1038/nmeth.2896. [DOI] [PubMed] [Google Scholar]
- 75.Angelo M., Bendall S.C., Finck R., Hale M.B., Hitzman C., Borowsky A.D., et al. Multiplexed ion beam imaging of human breast tumors. Nat. Med. 2014;20:436–442. doi: 10.1038/nm.3488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Keren L., Bosse M., Thompson S., Risom T., Vijayaragavan K., McCaffrey E., et al. MIBI-TOF: a multiplexed imaging platform relates cellular phenotypes and tissue structure. Sci. Adv. 2019;5 doi: 10.1126/sciadv.aax5851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Baharlou H., Canete N.P., Cunningham A.L., Harman A.N., Patrick E. Mass cytometry imaging for the study of human diseases-applications and data analysis strategies. Front. Immunol. 2019;10:2657. doi: 10.3389/fimmu.2019.02657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lemaire R., Stauber J., Wisztorski M., Van Camp C., Desmons A., Deschamps M., et al. Tag-mass: specific molecular imaging of transcriptome and proteome by mass spectrometry based on photocleavable tag. J. Proteome Res. 2007;6:2057–2067. doi: 10.1021/pr0700044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Tsujikawa T., Mitsuda J., Ogi H., Miyagawa-Hayashino A., Konishi E., Itoh K., et al. Prognostic significance of spatial immune profiles in human solid cancers. Cancer Sci. 2020;111:3426–3434. doi: 10.1111/cas.14591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wu Y., Cheng Y., Wang X., Fan J., Gao Q. Spatial omics: navigating to the golden era of cancer research. Clin. Transl. Med. 2022;12:e696. doi: 10.1002/ctm2.696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Seger C., Salzmann L. After another decade: LC-MS/MS became routine in clinical diagnostics. Clin. Biochem. 2020;82:2–11. doi: 10.1016/j.clinbiochem.2020.03.004. [DOI] [PubMed] [Google Scholar]
- 82.Maurer H.H. Mass spectrometry for research and application in therapeutic drug monitoring or clinical and forensic toxicology. Ther. Drug Monit. 2018;40:389–393. doi: 10.1097/FTD.0000000000000525. [DOI] [PubMed] [Google Scholar]
- 83.French D. Advances in clinical mass spectrometry. Adv. Clin. Chem. 2017;79:153–198. doi: 10.1016/bs.acc.2016.09.003. [DOI] [PubMed] [Google Scholar]
- 84.Leung K.S., Fong B.M. LC-MS/MS in the routine clinical laboratory: has its time come? Anal. Bioanal. Chem. 2014;406:2289–2301. doi: 10.1007/s00216-013-7542-5. [DOI] [PubMed] [Google Scholar]
- 85.la Marca G. Mass spectrometry in clinical chemistry: the case of newborn screening. J. Pharm. Biomed. Anal. 2014;101:174–182. doi: 10.1016/j.jpba.2014.03.047. [DOI] [PubMed] [Google Scholar]
- 86.Wu A.H., French D. Implementation of liquid chromatography/mass spectrometry into the clinical laboratory. Clin. Chim. Acta. 2013;420:4–10. doi: 10.1016/j.cca.2012.10.026. [DOI] [PubMed] [Google Scholar]
- 87.van den Ouweland J.M., Kema I.P. The role of liquid chromatography-tandem mass spectrometry in the clinical laboratory. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2012;883-884:18–32. doi: 10.1016/j.jchromb.2011.11.044. [DOI] [PubMed] [Google Scholar]
- 88.Seger C. Usage and limitations of liquid chromatography-tandem mass spectrometry (LC-MS/MS) in clinical routine laboratories. Wien. Med. Wochenschr. 2012;162:499–504. doi: 10.1007/s10354-012-0147-3. [DOI] [PubMed] [Google Scholar]
- 89.Grebe S.K., Singh R.J. LC-MS/MS in the clinical laboratory - where to from here? Clin. Biochem. Rev. 2011;32:5–31. [PMC free article] [PubMed] [Google Scholar]
- 90.Shushan B. A review of clinical diagnostic applications of liquid chromatography-tandem mass spectrometry. Mass Spectrom. Rev. 2010;29:930–944. doi: 10.1002/mas.20295. [DOI] [PubMed] [Google Scholar]
- 91.Vogeser M., Seger C. A decade of HPLC-MS/MS in the routine clinical laboratory--goals for further developments. Clin. Biochem. 2008;41:649–662. doi: 10.1016/j.clinbiochem.2008.02.017. [DOI] [PubMed] [Google Scholar]
- 92.French D., Drees J., Stone J.A., Holmes D.T., van der Gugten J.G. Comparison of four clinically validated testosterone LC-MS/MS assays: harmonization is an attainable goal. Clin. Mass Spectrom. 2019;11:12–20. doi: 10.1016/j.clinms.2018.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Wudy S.A., Schuler G., Sanchez-Guijo A., Hartmann M.F. The art of measuring steroids: principles and practice of current hormonal steroid analysis. J. Steroid Biochem. Mol. Biol. 2018;179:88–103. doi: 10.1016/j.jsbmb.2017.09.003. [DOI] [PubMed] [Google Scholar]
- 94.Neubert H., Shuford C.M., Olah T.V., Garofolo F., Schultz G.A., Jones B.R., et al. Protein biomarker quantification by immunoaffinity liquid chromatography-tandem mass spectrometry: current state and future vision. Clin. Chem. 2020;66:282–301. doi: 10.1093/clinchem/hvz022. [DOI] [PubMed] [Google Scholar]
- 95.Owusu B.Y., Pflaum H., Garner R., Foulon N., Laha T.J., Hoofnagle A.N. Development and validation of a novel LC-MS/MS assay for C-peptide in human serum. J. Mass Spectrom. Adv. Clin. Lab. 2021;19:1–6. doi: 10.1016/j.jmsacl.2020.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Mills J.R., Kohlhagen M.C., Dasari S., Vanderboom P.M., Kyle R.A., Katzmann J.A., et al. Comprehensive assessment of M-proteins using nanobody enrichment coupled to MALDI-TOF mass spectrometry. Clin. Chem. 2016;62:1334–1344. doi: 10.1373/clinchem.2015.253740. [DOI] [PubMed] [Google Scholar]
- 97.Ladwig P.M., Barnidge D.R., Snyder M.R., Katzmann J.A., Murray D.L. Quantification of serum IgG subclasses by use of subclass-specific tryptic peptides and liquid chromatography--tandem mass spectrometry. Clin. Chem. 2014;60:1080–1088. doi: 10.1373/clinchem.2014.222208. [DOI] [PubMed] [Google Scholar]
- 98.Picken M.M. New insights into systemic amyloidosis: the importance of diagnosis of specific type. Curr. Opin. Nephrol. Hypertens. 2007;16:196–203. doi: 10.1097/MNH.0b013e3280bdc0db. [DOI] [PubMed] [Google Scholar]
- 99.Jeppsson J.O., Kobold U., Barr J., Finke A., Hoelzel W., Hoshino T., et al. Approved IFCC reference method for the measurement of HbA1c in human blood. Clin. Chem. Lab. Med. 2002;40:78–89. doi: 10.1515/CCLM.2002.016. [DOI] [PubMed] [Google Scholar]
- 100.Phipps W.S., Greene D.N., Pflaum H., Laha T.J., Dickerson J.A., Irvine J., et al. Small volume retinol binding protein measurement by liquid chromatography-tandem mass spectrometry. Clin. Biochem. 2022;99:111–117. doi: 10.1016/j.clinbiochem.2021.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Derman B.A., Stefka A.T., Jiang K., McIver A., Kubicki T., Jasielec J.K., et al. Measurable residual disease assessed by mass spectrometry in peripheral blood in multiple myeloma in a phase II trial of carfilzomib, lenalidomide, dexamethasone and autologous stem cell transplantation. Blood Cancer J. 2021;11:19. doi: 10.1038/s41408-021-00418-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Shuford C.M., Johnson J.S., Thompson J.W., Holland P.L., Hoofnagle A.N., Grant R.P. More sensitivity is always better: measuring sub-clinical levels of serum thyroglobulin on a microLC-MS/MS system. Clin. Mass Spectrom. 2020;15:29–35. doi: 10.1016/j.clinms.2020.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Bystrom C.E., Salameh W., Reitz R., Clarke N.J. Plasma renin activity by LC-MS/MS: development of a prototypical clinical assay reveals a subpopulation of human plasma samples with substantial peptidase activity. Clin. Chem. 2010;56:1561–1569. doi: 10.1373/clinchem.2010.146449. [DOI] [PubMed] [Google Scholar]
- 104.Leinenbach A., Pannee J., Dulffer T., Huber A., Bittner T., Andreasson U., et al. Mass spectrometry-based candidate reference measurement procedure for quantification of amyloid-beta in cerebrospinal fluid. Clin. Chem. 2014;60:987–994. doi: 10.1373/clinchem.2013.220392. [DOI] [PubMed] [Google Scholar]
- 105.Clarke N.J., Zhang Y., Reitz R.E. A novel mass spectrometry-based assay for the accurate measurement of thyroglobulin from patient samples containing antithyroglobulin autoantibodies. J. Investig. Med. 2012;60:1157–1163. doi: 10.2310/JIM.0b013e318276deb4. [DOI] [PubMed] [Google Scholar]
- 106.Bystrom C.E., Sheng S., Clarke N.J. Narrow mass extraction of time-of-flight data for quantitative analysis of proteins: determination of insulin-like growth factor-1. Anal. Chem. 2011;83:9005–9010. doi: 10.1021/ac201800g. [DOI] [PubMed] [Google Scholar]
- 107.Jin Z., Collier T.S., Dai D.L.Y., Chen V., Hollander Z., Ng R.T., et al. Development and validation of apolipoprotein AI-associated lipoprotein proteome panel for the prediction of cholesterol efflux capacity and coronary artery disease. Clin. Chem. 2019;65:282–290. doi: 10.1373/clinchem.2018.291922. [DOI] [PubMed] [Google Scholar]
- 108.Collier T.S., Jin Z., Topbas C., Bystrom C. Rapid affinity enrichment of human apolipoprotein A-I associated lipoproteins for proteome analysis. J. Proteome Res. 2018;17:1183–1193. doi: 10.1021/acs.jproteome.7b00816. [DOI] [PubMed] [Google Scholar]
- 109.Li X.J., Hayward C., Fong P.Y., Dominguez M., Hunsucker S.W., Lee L.W., et al. A blood-based proteomic classifier for the molecular characterization of pulmonary nodules. Sci. Transl. Med. 2013;5 doi: 10.1126/scitranslmed.3007013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Jin M., Cataland S., Bissell M., Wu H.M. A rapid test for the diagnosis of thrombotic thrombocytopenic purpura using surface enhanced laser desorption/ionization time-of-flight (SELDI-TOF)-mass spectrometry. J. Thromb. Haemost. 2006;4:333–338. doi: 10.1111/j.1538-7836.2006.01758.x. [DOI] [PubMed] [Google Scholar]
- 111.Shi S.R., Key M.E., Kalra K.L. Antigen retrieval in formalin-fixed, paraffin-embedded tissues: an enhancement method for immunohistochemical staining based on microwave oven heating of tissue sections. J. Histochem. Cytochem. 1991;39:741–748. doi: 10.1177/39.6.1709656. [DOI] [PubMed] [Google Scholar]
- 112.Sprung R.W., Jr., Brock J.W., Tanksley J.P., Li M., Washington M.K., Slebos R.J., et al. Equivalence of protein inventories obtained from formalin-fixed paraffin-embedded and frozen tissue in multidimensional liquid chromatography-tandem mass spectrometry shotgun proteomic analysis. Mol. Cell. Proteomics. 2009;8:1988–1998. doi: 10.1074/mcp.M800518-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Sprung R.W., Martinez M.A., Carpenter K.L., Ham A.J., Washington M.K., Arteaga C.L., et al. Precision of multiple reaction monitoring mass spectrometry analysis of formalin-fixed, paraffin-embedded tissue. J. Proteome Res. 2012;11:3498–3505. doi: 10.1021/pr300130t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Nuciforo P., Thyparambil S., Aura C., Garrido-Castro A., Vilaro M., Peg V., et al. High HER2 protein levels correlate with increased survival in breast cancer patients treated with anti-HER2 therapy. Mol. Oncol. 2016;10:138–147. doi: 10.1016/j.molonc.2015.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Do M., Kim H., Yeo I., Lee J., Park I.A., Ryu H.S., et al. Clinical application of multiple reaction monitoring-mass spectrometry to human epidermal growth factor receptor 2 measurements as a potential diagnostic tool for breast cancer therapy. Clin. Chem. 2020;66:1339–1348. doi: 10.1093/clinchem/hvaa178. [DOI] [PubMed] [Google Scholar]
- 116.Kennedy J.J., Whiteaker J.R., Kennedy L.C., Bosch D.E., Lerch M.L., Schoenherr R.M., et al. Quantification of human epidermal growth factor receptor 2 by immunopeptide enrichment and targeted mass spectrometry in formalin-fixed paraffin-embedded and frozen breast cancer tissues. Clin. Chem. 2021;67:1008–1018. doi: 10.1093/clinchem/hvab047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Catenacci D.V.T., Liao W.L., Zhao L., Whitcomb E., Henderson L., O'Day E., et al. Mass-spectrometry-based quantitation of Her2 in gastroesophageal tumor tissue: comparison to IHC and FISH. Gastric Cancer. 2016;19:1066–1079. doi: 10.1007/s10120-015-0566-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Catenacci D.V., Liao W.L., Thyparambil S., Henderson L., Xu P., Zhao L., et al. Absolute quantitation of Met using mass spectrometry for clinical application: assay precision, stability, and correlation with MET gene amplification in FFPE tumor tissue. PLoS One. 2014;9 doi: 10.1371/journal.pone.0100586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Hembrough T., Thyparambil S., Liao W.L., Darfler M.M., Abdo J., Bengali K.M., et al. Selected reaction monitoring (SRM) analysis of epidermal growth factor receptor (EGFR) in formalin fixed tumor tissue. Clin. Proteomics. 2012;9:5. doi: 10.1186/1559-0275-9-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Steiner C., Tille J.C., Lamerz J., Kux van Geijtenbeek S., McKee T.A., Venturi M., et al. Quantification of HER2 by targeted mass spectrometry in formalin-fixed paraffin-embedded (FFPE) breast cancer tissues. Mol. Cell. Proteomics. 2015;14:2786–2799. doi: 10.1074/mcp.O115.049049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Steiner C., Ducret A., Tille J.C., Thomas M., McKee T.A., Rubbia-Brandt L., et al. Applications of mass spectrometry for quantitative protein analysis in formalin-fixed paraffin-embedded tissues. Proteomics. 2014;14:441–451. doi: 10.1002/pmic.201300311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Shuford C.M., Walters J.J., Holland P.M., Sreenivasan U., Askari N., Ray K., et al. Absolute protein quantification by mass spectrometry: not as simple as advertised. Anal. Chem. 2017;89:7406–7415. doi: 10.1021/acs.analchem.7b00858. [DOI] [PubMed] [Google Scholar]
- 123.Huynh H.H., Kuch K., Orquillas A., Forrest K., Barahona-Carrillo L., Keene D., et al. Metrologically traceable quantification of 3 apolipoprotein E isoforms in cerebrospinal fluid. Clin. Chem. 2023;69:734–745. doi: 10.1093/clinchem/hvad056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Ruhaak L.R., Romijn F., Begcevic Brkovic I., Kuklenyik Z., Dittrich J., Ceglarek U., et al. Development of an LC-MRM-MS-based candidate reference measurement procedure for standardization of serum apolipoprotein (a) tests. Clin. Chem. 2023;69:251–261. doi: 10.1093/clinchem/hvac204. [DOI] [PubMed] [Google Scholar]
- 125.Cobbaert C.M., Althaus H., Begcevic Brkovic I., Ceglarek U., Coassin S., Delatour V., et al. Towards an SI-traceable reference measurement system for seven serum apolipoproteins using bottom-up quantitative proteomics: conceptual approach enabled by cross-disciplinary/cross-sector collaboration. Clin. Chem. 2021;67:478–489. doi: 10.1093/clinchem/hvaa239. [DOI] [PubMed] [Google Scholar]
- 126.Shi J., Zheng Y.Z., Sin D.D., DeMarco M.L. A streamlined method for quantification of apolipoprotein A1 in human plasma by LC-MS/MS. Clin. Chem. 2018;64:1782–1784. doi: 10.1373/clinchem.2018.293530. [DOI] [PubMed] [Google Scholar]
- 127.Shi J.Y., Phipps W.S., Owusu B.Y., Henderson C.M., Laha T.J., Becker J.O., et al. A distributable LC-MS/MS method for the measurement of serum thyroglobulin. J. Mass Spectrom. Adv. Clin. Lab. 2022;26:28–33. doi: 10.1016/j.jmsacl.2022.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Kennedy J.J., Whiteaker J.R., Schoenherr R.M., Yan P., Allison K., Shipley M., et al. Optimized protocol for quantitative multiple reaction monitoring-based proteomic analysis of formalin-fixed, paraffin-embedded tissues. J. Proteome Res. 2016;15:2717–2728. doi: 10.1021/acs.jproteome.6b00245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Hembrough T., Thyparambil S., Liao W.L., Darfler M.M., Abdo J., Bengali K.M., et al. Application of selected reaction monitoring for multiplex quantification of clinically validated biomarkers in formalin-fixed, paraffin-embedded tumor tissue. J. Mol. Diagn. 2013;15:454–465. doi: 10.1016/j.jmoldx.2013.03.002. [DOI] [PubMed] [Google Scholar]
- 130.Vermani L., Kumar R., Senthil Kumar N. GAPDH and PUM1: optimal housekeeping genes for quantitative polymerase chain reaction-based analysis of cancer stem cells and epithelial-mesenchymal transition gene expression in rectal tumors. Cureus. 2020;12 doi: 10.7759/cureus.12020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Wisniewski J.R., Mann M. A proteomics approach to the protein normalization problem: selection of unvarying proteins for MS-based proteomics and western blotting. J. Proteome Res. 2016;15:2321–2326. doi: 10.1021/acs.jproteome.6b00403. [DOI] [PubMed] [Google Scholar]
- 132.Picken M.M. Diagnosis of amyloid beyond Congo red. Curr. Opin. Nephrol. Hypertens. 2021;30:303–309. doi: 10.1097/MNH.0000000000000695. [DOI] [PubMed] [Google Scholar]
- 133.Hill M.M., Dasari S., Mollee P., Merlini G., Costello C.E., Hazenberg B.P.C., et al. The clinical impact of proteomics in amyloid typing. Mayo Clin. Proc. 2021;96:1122–1127. doi: 10.1016/j.mayocp.2020.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Dogan A. Amyloidosis: insights from proteomics. Annu. Rev. Pathol. 2017;12:277–304. doi: 10.1146/annurev-pathol-052016-100200. [DOI] [PubMed] [Google Scholar]
- 135.Bian Y., Zheng R., Bayer F.P., Wong C., Chang Y.C., Meng C., et al. Robust, reproducible and quantitative analysis of thousands of proteomes by micro-flow LC-MS/MS. Nat. Commun. 2020;11:157. doi: 10.1038/s41467-019-13973-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Bian Y., Gao C., Kuster B. On the potential of micro-flow LC-MS/MS in proteomics. Expert Rev. Proteomics. 2022;19:153–164. doi: 10.1080/14789450.2022.2134780. [DOI] [PubMed] [Google Scholar]
- 137.D'Souza A., Theis J.D., Vrana J.A., Dogan A. Pharmaceutical amyloidosis associated with subcutaneous insulin and enfuvirtide administration. Amyloid. 2014;21:71–75. doi: 10.3109/13506129.2013.876984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Gonzalez Suarez M.L., Zhang P., Nasr S.H., Sathick I.J., Kittanamongkolchai W., Kurtin P.J., et al. The sensitivity and specificity of the routine kidney biopsy immunofluorescence panel are inferior to diagnosing renal immunoglobulin-derived amyloidosis by mass spectrometry. Kidney Int. 2019;96:1005–1009. doi: 10.1016/j.kint.2019.05.027. [DOI] [PubMed] [Google Scholar]
- 139.Mollee P., Boros S., Loo D., Ruelcke J.E., Lakis V.A., Cao K.L., et al. Implementation and evaluation of amyloidosis subtyping by laser-capture microdissection and tandem mass spectrometry. Clin. Proteomics. 2016;13:30. doi: 10.1186/s12014-016-9133-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Gilbertson J.A., Theis J.D., Vrana J.A., Lachmann H., Wechalekar A., Whelan C., et al. A comparison of immunohistochemistry and mass spectrometry for determining the amyloid fibril protein from formalin-fixed biopsy tissue. J. Clin. Pathol. 2015;68:314–317. doi: 10.1136/jclinpath-2014-202722. [DOI] [PubMed] [Google Scholar]
- 141.Phipps W.S., Smith K.D., Yang H.Y., Henderson C.M., Pflaum H., Lerch M.L., et al. Tandem mass spectrometry-based amyloid typing using manual microdissection and open-source data processing. Am. J. Clin. Pathol. 2022;157:748–757. doi: 10.1093/ajcp/aqab185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Theis J.D., Dasari S., Vrana J.A., Kurtin P.J., Dogan A. Shotgun-proteomics-based clinical testing for diagnosis and classification of amyloidosis. J. Mass Spectrom. 2013;48:1067–1077. doi: 10.1002/jms.3264. [DOI] [PubMed] [Google Scholar]
- 143.Mete O., Lopes M.B. Overview of the 2017 WHO classification of pituitary tumors. Endocr. Pathol. 2017;28:228–243. doi: 10.1007/s12022-017-9498-z. [DOI] [PubMed] [Google Scholar]
- 144.Rizk E.M., Gartrell R.D., Barker L.W., Esancy C.L., Finkel G.G., Bordbar D.D., et al. Prognostic and predictive immunohistochemistry-based biomarkers in cancer and immunotherapy. Hematol. Oncol. Clin. North Am. 2019;33:291–299. doi: 10.1016/j.hoc.2018.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Rodriguez H., Zenklusen J.C., Staudt L.M., Doroshow J.H., Lowy D.R. The next horizon in precision oncology: proteogenomics to inform cancer diagnosis and treatment. Cell. 2021;184:1661–1670. doi: 10.1016/j.cell.2021.02.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Hu A., Noble W.S., Wolf-Yadlin A. Technical advances in proteomics: new developments in data-independent acquisition. F1000Res. 2016 doi: 10.12688/f1000research.7042.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Gillet L.C., Navarro P., Tate S., Rost H., Selevsek N., Reiter L., et al. Targeted data extraction of the MS/MS spectra generated by data-independent acquisition: a new concept for consistent and accurate proteome analysis. Mol. Cell. Proteomics. 2012;11 doi: 10.1074/mcp.O111.016717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Bruderer R., Bernhardt O.M., Gandhi T., Miladinovic S.M., Cheng L.Y., Messner S., et al. Extending the limits of quantitative proteome profiling with data-independent acquisition and application to acetaminophen-treated three-dimensional liver microtissues. Mol. Cell. Proteomics. 2015;14:1400–1410. doi: 10.1074/mcp.M114.044305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Tabb D.L., Vega-Montoto L., Rudnick P.A., Variyath A.M., Ham A.J., Bunk D.M., et al. Repeatability and reproducibility in proteomic identifications by liquid chromatography-tandem mass spectrometry. J. Proteome Res. 2010;9:761–776. doi: 10.1021/pr9006365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Barkovits K., Pacharra S., Pfeiffer K., Steinbach S., Eisenacher M., Marcus K., et al. Reproducibility, specificity and accuracy of relative quantification using spectral library-based data-independent acquisition. Mol. Cell. Proteomics. 2020;19:181–197. doi: 10.1074/mcp.RA119.001714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Anand S., Samuel M., Ang C.S., Keerthikumar S., Mathivanan S. Label-based and label-free strategies for protein quantitation. Methods Mol. Biol. 2017;1549:31–43. doi: 10.1007/978-1-4939-6740-7_4. [DOI] [PubMed] [Google Scholar]
- 152.Lundgren D.H., Hwang S.I., Wu L., Han D.K. Role of spectral counting in quantitative proteomics. Expert Rev. Proteomics. 2010;7:39–53. doi: 10.1586/epr.09.69. [DOI] [PubMed] [Google Scholar]
- 153.Zybailov B., Mosley A.L., Sardiu M.E., Coleman M.K., Florens L., Washburn M.P. Statistical analysis of membrane proteome expression changes in Saccharomyces cerevisiae. J. Proteome Res. 2006;5:2339–2347. doi: 10.1021/pr060161n. [DOI] [PubMed] [Google Scholar]
- 154.Krasny L., Huang P.H. Data-independent acquisition mass spectrometry (DIA-MS) for proteomic applications in oncology. Mol. Omics. 2021;17:29–42. doi: 10.1039/d0mo00072h. [DOI] [PubMed] [Google Scholar]
- 155.Hou G., Lou X., Sun Y., Xu S., Zi J., Wang Q., et al. Biomarker discovery and verification of esophageal squamous cell carcinoma using integration of SWATH/MRM. J. Proteome Res. 2015;14:3793–3803. doi: 10.1021/acs.jproteome.5b00438. [DOI] [PubMed] [Google Scholar]
- 156.Schwarzfischer P., Reinders J., Dettmer K., Kleo K., Dimitrova L., Hummel M., et al. Comprehensive metaboproteomics of burkitt's and diffuse large B-cell lymphoma cell lines and primary tumor tissues reveals distinct differences in pyruvate content and metabolism. J. Proteome Res. 2017;16:1105–1120. doi: 10.1021/acs.jproteome.6b00164. [DOI] [PubMed] [Google Scholar]
- 157.Weke K., Kote S., Faktor J., Al Shboul S., Uwugiaren N., Brennan P.M., et al. DIA-MS proteome analysis of formalin-fixed paraffin-embedded glioblastoma tissues. Anal. Chim. Acta. 2022;1204 doi: 10.1016/j.aca.2022.339695. [DOI] [PubMed] [Google Scholar]
- 158.Keam S.P., Gulati T., Gamell C., Caramia F., Huang C., Schittenhelm R.B., et al. Exploring the oncoproteomic response of human prostate cancer to therapeutic radiation using data-independent acquisition (DIA) mass spectrometry. Prostate. 2018;78:563–575. doi: 10.1002/pros.23500. [DOI] [PubMed] [Google Scholar]
- 159.Marchione D.M., Ilieva I., Devins K., Sharpe D., Pappin D.J., Garcia B.A., et al. HYPERsol: high-quality data from archival FFPE tissue for clinical proteomics. J. Proteome Res. 2020;19:973–983. doi: 10.1021/acs.jproteome.9b00686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Gao Y., Wang X., Sang Z., Li Z., Liu F., Mao J., et al. Quantitative proteomics by SWATH-MS reveals sophisticated metabolic reprogramming in hepatocellular carcinoma tissues. Sci. Rep. 2017;7 doi: 10.1038/srep45913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Janacova L., Faktor J., Capkova L., Paralova V., Pospisilova A., Podhorec J., et al. SWATH-MS analysis of FFPE tissues identifies stathmin as a potential marker of endometrial cancer in patients exposed to tamoxifen. J. Proteome Res. 2020;19:2617–2630. doi: 10.1021/acs.jproteome.0c00064. [DOI] [PubMed] [Google Scholar]
- 162.Thomas S.N., Zhang H. Targeted proteomic assays for the verification of global proteomics insights. Expert Rev. Proteomics. 2016;13:897–899. doi: 10.1080/14789450.2016.1229601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Fernandez-Costa C., Martinez-Bartolome S., McClatchy D.B., Saviola A.J., Yu N.K., Yates J.R., 3rd Impact of the identification strategy on the reproducibility of the DDA and DIA results. J. Proteome Res. 2020;19:3153–3161. doi: 10.1021/acs.jproteome.0c00153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Lynch K.L. Clsi C62-A: a new standard for clinical mass spectrometry. Clin. Chem. 2016;62:24–29. doi: 10.1373/clinchem.2015.238626. [DOI] [PubMed] [Google Scholar]
- 165.Gorr T.A., Vogel J. Western blotting revisited: critical perusal of underappreciated technical issues. Proteomics Clin. Appl. 2015;9:396–405. doi: 10.1002/prca.201400118. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Development of best practices in data analysis and interpretation will require data sharing in order to directly compare data-processing pipelines. Data sharing has become increasingly robust in the research space, particularly through the efforts of the National Cancer Institute’s Clinical Proteomic Tumor Analysis Consortium (CPTAC). For example, the proteomic data commons (https://proteomic.datacommons.cancer.gov/pdc/) provides the raw data resulting from the accumulation of knowledge with respect to cancer biology, which has significant secondary benefits. These raw data, which meet certain quality characteristics, allow users to test analysis pipelines. Raw data from clinical laboratories will also improve the transparency of the data analysis process, because processed data should correlate with disease processes across institutions, just like quantitative data in biofluid assays.