Abstract
The blood–brain barrier (BBB) impairment plays a crucial role in the pathological processes of aging-accompanied neurological diseases (AAND). Meanwhile, circadian rhythms disruption and gut microbiota dysbiosis are associated with increased morbidity of neurological diseases in the accelerated aging population. Importantly, circadian rhythms disruption and gut microbiota dysbiosis are also known to induce the generation of toxic metabolites and pro-inflammatory cytokines, resulting in disruption of BBB integrity. Collectively, this provides a new perspective for exploring the relationship among circadian rhythms, gut microbes, and the BBB in aging-accompanied neurological diseases. In this review, we focus on recent advances in the interplay between circadian rhythm disturbances and gut microbiota dysbiosis, and their potential roles in the BBB disruption that occurs in AAND. Based on existing literature, we discuss and propose potential mechanisms underlying BBB damage induced by dysregulated circadian rhythms and gut microbiota, which would serve as the basis for developing potential interventions to protect the BBB in the aging population through targeting the BBB by exploiting its links with gut microbiota and circadian rhythms for treating AAND.
Key words: Blood–brain barrier, Neurological diseases, Aging, Gut microbiota, Circadian rhythms
Graphical abstract
This review focuses on the recent advances in the interplay between circadian rhythm disturbances and gut microbiota dysbiosis, and their potential roles in the BBB disruption that occurs in aging-accompanied neurological diseases.

1. Introduction
Aging is an uncontrolled biological process that poses challenges to human health and becomes a social problem that can't be ignored1, 2, 3. Aging is regarded as a common risk factor for various human diseases4 and by reducing sensory, motor, circadian rhythms, and cognitive functions, aging affects the brain morphologically and functionally, resulting in neurological diseases5. Importantly, circadian rhythms disruption, characterized by phase shifts and reduced expression of many genes and proteins involved in circadian rhythms greatly impacts aging and longevity in many ways6,7. Disturbances in the circadian rhythms induce disorders of cognitive function, metabolism, mental function, motor control, alertness, blood–brain barrier (BBB) damage, and sleep/wake cycles8. A prospective cohort study of 72,242 participants further supported that disturbances of circadian rhythm are a risk factor for the development of common neurodegenerative and psychiatric disorders9. Interestingly, the amount and function of different microbial species fluctuate over time during aging, leading to gut microbiota dysbiosis10. The gut microbiota continuously exchanges nutrients, genetic material, and metabolites with the host throughout its life cycle which regulates the homeostasis in the host, including brain function and blood–brain barrier (BBB) integrity11. In individuals with neurological disorders, including Alzheimer's disease (AD), Parkinson's disease (PD), stroke, and multiple sclerosis (MS), circadian rhythm disturbances and gut microbiota dysbiosis are common symptoms12, 13, 14. Accumulating data suggest that either circadian rhythms or gut dysbiosis contribute to aging-accompanied neurological diseases (AAND)15. Notably, the disruption of the circadian system can alter microbiome communities and perturb host metabolism, energy homeostasis, and inflammatory pathways, and gut microbiota can regulate host circadian rhythms and metabolism as a transducer of dietary cues16. Genetic defects of a biological clock, timing or restriction of food availability, and light/dark phase changes can significantly affect microbial oscillations, leading to a reduction in microbial abundance and species17. In addition, gut microbiota-derived metabolites, including short-chain fatty acids (SCFAs) and bile acids (BA), can alter circadian rhythms18, indicating that circadian rhythms and gut microbiota can affect each other, and their interplays can induce subsequent effects.
The BBB provides nutrients to the central nervous system (CNS), maintains homeostasis, and regulates its communication with the periphery, forming a protective barrier for the CNS19. The changes and destruction of the structure and functional components of the BBB could occur naturally with the aging process20. Aging itself may worsen the disruption of different components of the BBB, thus accelerating the progression of brain damage and an ever-increasing global aging population has stimulated the exploration of the relationship between AAND and BBB, to prevent or delay the prevalence of AAND.
Therefore, there is an urgent need to further investigate the role of the BBB in AAND and the underlying mechanisms of BBB damage induced by aging-accompanied circadian rhythms disruption and dysbiosis of gut microbiota which play important roles in regulating BBB integrity21,22. Further elucidation of the interplay of gut microbiota with circadian rhythms could also shed light on the systemic regulatory mechanisms of aging and the BBB. Here, in this review, we first describe how BBB, circadian rhythms, and gut microbiota are altered during the aging process and how these alterations are exacerbated in AAND. We then discuss the effect of the interplay between circadian rhythms disruption and dysbiosis of gut microbiota on BBB integrity. We then discuss and propose potential mechanisms underlying BBB damage induced by dysregulated circadian rhythms and gut microbiota, which could serve as the basis for developing potential interventions to protect the BBB in the aging population through targeting the BBB by exploiting its links with gut microbiota and circadian rhythms for treating AAND.
2. Aging, neurological diseases, and BBB damage
2.1. Effect of aging on BBB and the underlying mechanisms
BBB consists of brain microvascular endothelial cells, astrocytes endfeet, pericytes, basement membrane, and the extracellular matrix, and BBB is a highly specialized barrier19 (Fig. 1A). Aging impairs the structure and function of BBB and aging-related physiological and pathological changes of BBB have been reviewed20 (Fig. 1B). The decrease of channel ions and surface vector expression in endothelial cells, the increase in vasoactive mediators, and the activation of astrocytes and monocytes/macrophages are the main factors for BBB hyperpermeability23. Meanwhile, oxygen and nitrogen free radicals, matrix metalloproteinase (MMP) and cyclooxygenase attack the cell membrane, degrade tight junction proteins (TJPs) between endothelial cells, and destroy the BBB integrity24, 25, 26. For instance, the effect of reactive oxygen species on BBB function has been documented in superoxide dismutase (SOD)-deficient mice, where ischemia/reperfusion induces increased endothelial permeability to macromolecules27.
Figure 1.
The aging process causes structural and functional impairments of BBB. (A) Components of the blood–brain barrier (BBB). (B) Cross-sectional structure of the healthy BBB with tight junction protein (TJPs) and adherence junction proteins (AJPs) in endothelial cells in adults; disruption of the BBB structure in the elderly, loss of TJPs and AJPs, and overactivation of transcellular pathways.
In addition, aging has been reported to be accompanied by the decreased number of vesicles for receptor-mediated endocytosis, as well as the increased number of nests for non-specific protein leakage28. Furthermore, a study from aged individuals and rodents showed that the expression or function of the glucose transporter-1 and P-glycoprotein (P-gp), a special protein in cerebral blood vessels, changes with age29, and the extent of tracer leakage in the BBB depends on the aging process30.
Aging affected post-stroke BBB recovery by profound alterations in methylation (hypermethylation and repression) in the expression of structural protein (e.g., claudin-5) and genes activat-ion (e.g., Sox9, Snai1) that is involved in endothelial to mesenchymal transformation, angiogenesis repression and epigenetic regulation, indicating that DNA methylation is critically involved in BBB repair after stroke31.
2.2. BBB impairment is a cause of aging-accompanied neurological diseases
The role of BBB injury in the occurrence and development of aging-related neurological diseases has been a special research focus for a few years32,33 and we have summarized it in Table 1. Magnetic resonance imaging -based findings indicate that the integrity damage of BBB appears in the hippocampus at the early stage of AD, and this causes synaptic and neuronal alternations and cognitive dysfunction, finally leading to more apparent AD manifestations34. ApoE4 catabolizes BBB integrity by activating MMP-9 in pericytes and endothelial cells, resulting in cognitive decline in AD patients35. Notably, BBB damage and vascular hyperpermeability are signs of AD, so targeting BBB could be a promising treatment strategy for AD therapy36.
Table 1.
Summary of aging-induced BBB damage in AD, PD, stroke and the underlying mechanisms.
| Ref. | Neurological diseases | Species | BBB changes | Possible mechanisms |
|---|---|---|---|---|
| Barisano et al., 202234 | Alzheimer's disease | Human | Loss of tight junction (TJ) or adherence junction (AJ) and increased transendothelial translocation | Associated with the loss of pericyte coverage |
| Zhang et al., 202038 | Alzheimer's disease | Human | Increase of BBB permeability | APOE4 can catabolize BBB integrity by activating the MMP9 pathway in pericytes and endothelial cells |
| Lan et al., 202239 | Parkinson's disease | Mouse | Reduce of tight junction proteins | Elevated levels of VEGFA and iNOS |
| Ruan et al., 202238 | Parkinson's disease | Mouse | Loss of endothelial integrity of the brain | Microglia-mediated activation of matrix metalloproteinase-2 and 9 |
| Kortekaas et al., 200540 | Parkinson's disease | Human | Decrease of P-gp function | PD patients carry the 1T allele of the MDR3435 gene |
| Rite et al., 200741 | Parkinson's disease | Mouse | Increase of BBB permeability | Inflammatory response and activation of astroglia |
| Candelario-Jalil et al., 202242 | Ischemic stroke | Mouse | Endothelial dysfunction | Increased MMP, oxidative stress burst, microglia activation and peripheral immune cell infiltration |
| Phillips et al., 202331 | Ischemic stroke | Mouse | Persistent BBB dysfunction with increased permeability of the paracellular barrier | DNA methylation |
| Alvarez et al., 201145 | Multiple sclerosis | Human and mouse | Reduced expression of TJ and AJ proteins | Immune cell infiltration and upregulation of pro-inflammatory cytokines |
BBB disruption also is implicated in the pathogenesis of PD37. Reduced expression of TJPs, increased vascular permeability, and increased accumulation of oligo-α-syn in activated astrocytes were found in the brain of PD mouse model38. In addition, astrocytic vascular endothelial growth factor is an essential mediator in BBB disruption in PD39. Of note, BBB dysfunction and decrease of P-gp, have been reported to be detected in PD patients40. Therefore, it is assumed that the destruction of BBB occurs ahead of dopaminergic neuron loss in the substantia nigra and contributes to the progress of PD, suggesting that protecting the integrity of BBB is a potential treatment strategy for PD41.
Age is the most important and nonmodifiable risk factor for all types of strokes. Stroke-induced BBB damage can be caused by inflammation-urged destruction including MMP increase, oxidative stress burst, microglial cell activation, and peripheral immune cell infiltration42. Aging-accompanied BBB vulnerability enhances the risk and worsens the outcome of ischemic stroke43.
MS is a chronic demyelinating neuroinflammatory disease that affects the brain and spinal cord. CNS vasculature in MS patients has shown downregulated expression of TJPs within the lesions compared to the vascular system in normal white matter44. In addition, the loss of TJPs and activation of proteolytic enzymes that occur in response to inflammation can promote degradation of the extracellular matrix and destruction of the BBB45.
Circadian rhythms disturbances and gut microbial dysbiosis are not major pathogenesis in diseases caused by mutations in autosomal dominant genes such as Huntington's disease (HD) and amyotrophic lateral sclerosis (ALS)46. HD etiology is entirely due to neurodegeneration caused by autosomal dominant HTT mutations, and BBB dysfunction is in HD47. ALS is a genetic variant of spinal cord motor neuron damage that occurs as a result of muscle weakness, atrophy, and spasticity48,49. Autopsy studies revealed impaired BBB structure in ALS patients50, and imaging studies showed early changes in BBB function51. These observations raise the possibility that the importance of BBB disruption may be overlooked in the diagnosis and treatment of AAND.
Aging and neurodegeneration-associated BBB hyperpermeability has been reviewed by Knox52 and BBB damage is a cause instead of a result of AAND44. Therefore, treatments that could enhance BBB integrity would contribute critically to delaying AAND.
3. Aging, neurological diseases, and circadian rhythms dysfunction
3.1. Effect of aging on circadian rhythms
Circadian rhythms are controlled by a set of clock genes, repressors including period circadian regulator (Per1, Per2, and Per3) and cryptochromes (Cry1 and Cry2), activators including Clock and brain and muscle ARNT-like protein-1 (Bmal1)53. Circadian rhythm disorders play an important role in many age-related health consequences. An investigation of the prospective association of circadian rhythm disorders and frailty in more than 1000 elderly showed that disturbed circadian rhythms may be an early sign or risk factor for frailty in the elderly54.
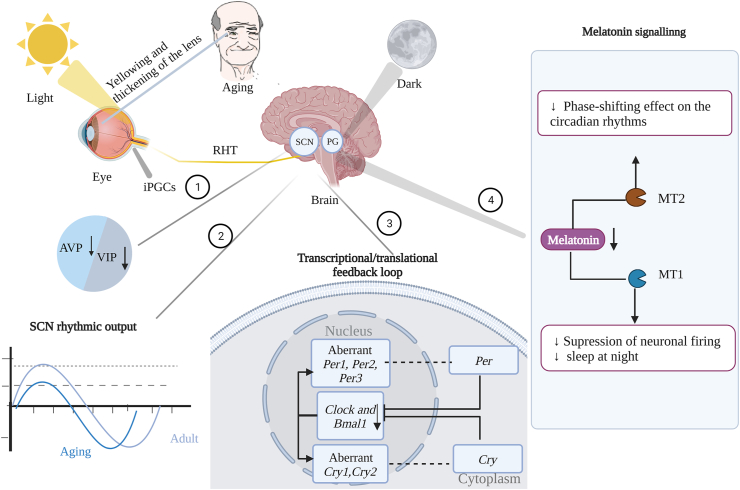
Sleep time, duration, and quality change with the increase of age, the normal circadian rhythms and sleep cycle are disrupted and older adults have less overall sleep and sleep also tends to be more fragmented55. Deterioration of circadian input pathways, the suprachiasmatic nucleus (SCN), and output pathways throughout the aging process reduce the robustness of the systemic circadian clockwork system56. Decreased neuronal numbers and reduced electrical activity were found in the SCN in the monkey model of normal aging57. Aging-related changes in the neuron of SCN lead to a decline in SCN function and disruption in the amplitude and cycle length of circadian behavior, with reduced expression of arginine vasopressin (AVP) and vasoactive intestinal polypeptide (VIP) expression, resulting in a disturbance of the circadian rhythm58. Circadian clock gene expression is also one of the changes in aging-related circadian rhythm disruption59. Rhythmic expression of the Bmal1, Clock and Per2 genes have been shown to decrease or be disrupted with age increasing60. Interestingly, the regulation of Bmal1, Clock, and expression is influenced by the redox status of the orphan nuclear receptor REV-ERB-alpha, which activity is decreased by oxidative stress61. In addition to the typical core circadian genes, a large number of clock-controlled genes in the human prefrontal cortex exhibit rhythmic variation62. One of the most common damaging effects caused by aging SCN is a significant decrease in pineal melatonin secretion and altered circadian rhythms of melatonin have a negative physiological as well as pathological impact on the elderly63. In a word, circadian clock disorder speeds up the aging progress and circadian system damage can lead to harmful health outcomes, especially for the elderly population64 (Fig. 2).
Figure 2.
Disruption of circadian rhythms in aging. Light is received by melanopsin-expressing intrinsically photosensitive retinal ganglion cells (ipRGCs) that send signals to the suprachiasmatic nucleus (SCN) via the retinal hypothalamic tract (RHT). Gradual yellowing and thickening of lenses due to aging may reduce sensitivity to light, with a concomitant reduction of arginine vasopressin (AVP) and vasoactive intestinal polypeptide (VIP) expression ①, the amplitude of SCN rhythmic output is attenuated in aging (blue line) relative to the young (grey line) ②, aberrant circadian clock genes ③, and a decline secretion of melatonin from the pineal gland (PG) ④.
3.2. Association between circadian rhythms disruption and aging-accompanied neurological diseases
Dysfunction of the sleep/wake cycle and alertness are general symptoms of neurodegenerative disease, and disruption of circadian rhythms might deteriorate the disease progress65. Disturbed sleep/wake cycle is a common and debilitating symptom of AD66. For example, significant loss of AVP and VIP-expressing neurons in the SCN has been demonstrated in AD, and SCN dysfunction and degeneration are at the root of circadian rhythms disturbances in AD67. Expression of BMAL1 and CLOCK decreased significantly in senescent cells as well as in aged rodent brain tissue68. In addition, increased neuronal activity and accumulation of pathogenic proteins in the brain are caused by disturbances in circadian rhythms and sleep. This pathological protein deposition, in turn, leads to neuronal death and promotes neurodegeneration69. Inflammation and oxidative stress, which are regulated by sleep and circadian rhythms, further promote neurodegeneration70. These results suggest that circadian rhythms disruption may play an important role in AD development.
It is estimated that 80%–90% of PD patients suffer from sleep disturbances71. Sleep/wake cycle disruption, circadian rhythms disorder of temperature and heart rate, uncontrolled regulation of clock genes, and diminished neuronal activity in the SCN have been identified in PD patients69. Of note, decreased intrinsical photosensitive retinal ganglion cells (ipRGCs) density and complexity of the plexus in PD patients resulted in decreased circadian rhythms and dislocation72. In addition, there is also evidence showing that melatonin release patterns are disrupted and that weak light entrainment signals can exacerbate the effect73. It has been reported that the circadian rhythm regulation of melatonin secretion in PD patients is decreased74 and the expression of BMAL1 was significantly lower at night in PD patients, accompanied byrenbha exercise severity and sleep quality75. In addition, taking melatonin along with bright light exposure could help improve the quality of sleep in PD patients73. Therefore, early detection of sleep disorders and improvement in sleep quality may help delay disease progression and provide long-term clinical benefits76.
Studies have shown that part of stroke survivors suffer from sleep/wake cycle disorders77. Sleep/wake cycle disorders are not only a risk factor for the prevalence of stroke, but also worsen the detrimental effects of stroke, thus hindering the rehabilitation of stroke patients, influencing the outcome of stroke, and increasing the recurrence rate78.
Circadian rhythms play a key role in innate and adaptive immunity, and circadian rhythms disorders are increasingly recognized as a factor in autoimmune diseases79. A study of experimental autoimmune encephalomyelitis (an animal model of MS) suggests that BMAL1 is associated with the accumulation and activation of immune cells and that genetic variability in the Clock gene may be associated with MS risk79. Since circadian rhythms play a critical role in health, and chronic disruptions to the clock are accompanied by adverse health consequences59, active interventions for circadian rhythm deregulation in neurological diseases are required although the importance of circadian rhythms is often overlooked in aging populations.
4. Aging, neurological diseases, and gut microbiota dysbiosis
4.1. Aging-accompanied gut microbiota dysbiosis
The gut microbiota establishes reciprocal relationships with the host and consists of diverse and dynamic microbiota, including bacteria, archaea, viruses, fungi, and protozoa80. Highly diverse and stable microbiota play an important role in maintaining overall human health81. For example, gut microbiota is crucial for regulating aspects of innate and adaptive immune responses, as well as anti-aging, and maintaining the body's dynamic balance82,83. In addition, gut microbial metabolites SCFAs, serotonin (5-HT), dopamine and 5-amino valeric acid can regulate neurotransmission and promote the maturation of CNS cells84. The microbiota–gut–brain axis mediates bidirectional communication between the gut microbiota and the CNS and is essential for maintaining gastrointestinal and CNS homeostasis as well as microbial homeostasis in humans and animals85.
Of note, significant changes in the bacterial flora of the fecal microbiome were reported in the elderly compared to infants and young adults86. For example, the species diversity of Bacteroides increased slightly, while the number of Bifidobacteria which are used as probiotics decreased. Prevotaceae is commonly found in the stomach of people who maintain a low animal fat and high carbohydrate diet and is lost in centenarians87. Notably, the abundance of total bacteria and Bacillus simulans in mice feces showed diet-dependent diurnal fluctuations88 and the number and proportion of Bifidobacterium and Clostridium difficile in intestinal flora decreased with age89 (Fig. 3). It has been reported that aging promotes detrimental effects on the gut microbiome, metabolism, neurovascular integrity, cognition, and mental health90. In addition, aging-related variations in gut microbiota reveal the disruption of the gut barrier, invasion of microbes into blood, and production of pro-inflammatory cytokines91. Changes in the gut microbiota are associated with morbidity and mortality in the elderly population92 (Fig. 3).
Figure 3.
Schematic showing changes in gut microbiota during aging. The increase in harmful microbiota in the gut of older adults results in an imbalance of microbiota homeostasis ①, a decrease in short-chain fatty acids (SCFAs) ②, and disruption of the gut barrier ③ compared to younger adults. In the blood of the elderly, gut microbiota invasive, ④ pro-inflammatory factors interleukin-6 (IL-6), IL-17, IL-23, and interferon-γ (IFN-γ) increased, anti-inflammatory factor IL-10 decreased ⑤.
4.2. Roles of gut microbiota dysbiosis in aging-accompanied neurological diseases
An imbalance in the gut microbiota is associated with the development and progression of neurological disorders10,93. For example, bacterial infection triggered by gut microbial dysbiosis promotes the onset of chronic inflammatory responses in the CNS of vulnerable groups to induce synaptic degeneration and amyloidosis, driving AD development94. In a Drosophila AD model, enterobacteria infection worsens the progress of AD by stimulating immune hemocyte recruitment to the brain, thereby provoking neurodegeneration95. In addition, there is a positive association between the increased abundance of Enterobacteriaceae and the severity of certain PD symptoms in the majority of PD patients with gastrointestinal problems96 and PD patients exhibit different gut microbiota-related metabolic profiles relative to healthy individuals, including the level of β-glucuronic acid tryptophan as well as SCFAs concentrations97. Gut microbiota is also involved in ischemic stroke98. For example, analysis of the cecum flora of mice after ischemic stroke revealed an increase in the relative abundance of Peptococcus and a decrease in the proportion of Prevotella. When effector T cells are transported from the gut to the brain, they localize to the pia mater and enhance ischemic neuroinflammation by secreting interleukin-1799. Additionally, antibiotic-induced gut dysbiosis reduces infarct volume in a mouse model of stroke, an effect that is dependent on gut bacteria98,99. Gut microbiota dysbiosis is characterized by less microbial diversity (fewer bacterial phyla) and upregulation of pro-inflammatory species (actinomycetes, bifidobacterial, and streptococci) in the gut of MS patients, and disordered microbial diversity resulted in significant downregulation of beneficial SCFA, suggesting that gut microbial population and its metabolites changes are associated with the onset and progression of MS100.
Direct signal transduction of SCFAs is decreased with age, and the ability to regulate neuroplasticity, epigenetics, and gene expression is lost101; however, nutritional intervention with prebiotics, synbiotics, or their metabolites such as SCFAs may help restore aging-related decreased composition and function of gut microbes, enhance gut barrier integrity and reduce chronic inflammation102. In addition, sodium butyrate has been shown to alleviate the degeneration of dopaminergic neurons and ameliorate locomotor impairment in PD model103, prevent 1-methyl-4-phenylpyridinium (MPP+)-induced neurotoxicity104, and induce microglial apoptosis accompanied by a robust decrease in LPS-induced pro-inflammatory responses, implicating sodium butyrate in the direct neuroprotection of dopaminergic neurons105.
5. Circadian rhythms dysfunction, BBB disruption, and aging effect
5.1. Roles of circadian rhythms in BBB integrity
The circadian clock affects the permeability of the BBB and endocytosis across the BBB is upregulated during sleep106. Permeability at the BBB is dynamically regulated by circadian rhythms and sleep. An endogenous circadian rhythm in the BBB controls transporter function, regulating permeability across the BBB107. Permeability of the drosophila BBB to xenobiotics is higher at night and the permeability rhythm is driven by the circadian regulation of efflux and depends on a molecular clock in the perineurial glia of the BBB108. In circadian clock mutants, the oscillatory state of heterogeneous material efflux from the BBB is disrupted in mice. In human microvascular endothelial cell lines, the molecular clock was found to drive intracellular magnesium cycling through transcriptional regulation of magnesium transporter TRPM7, which in turn appears to cycle the outflow of xenobiotics, suggesting that the circadian clock is capable of regulating BBB efflux109.
5.2. Circadian rhythms disruption leads to BBB damage, aging effect
Sleep is important for immune regulation at central and peripheral levels and sleep disorders are a risk factor for developing neurodegenerative diseases110, and the regulation of sleep by circadian rhythms appear to be vital in maintaining the integrity of BBB22. Sleep deprivation (SD) induces a systemic hypo-inflammation characterized by the release of several molecules such as cytokines, chemokines, and acute phase proteins; all of which may promote changes in BBB cellular components, especially in brain endothelial cells111. For example, sleep restriction leads to increased activity of several pro-inflammatory mediators, including C-reactive protein, interleukin-1β (IL-1β), IL-6, IL-17, interferon-γ (IFN-γ), and tumor necrosis factor-α (TNF-α)22,112 (Fig. 4). In addition, SD promotes pericyte detachment from the capillary wall, and the loss of pericyte–endothelial cell interactions occurs concurrently with a decrease in TJPs between epithelia and increased BBB permeability with exogenous tracers22. It is well known that sleep and its disorders affect the function of the basic organs and systems of the body. BBB dysfunction caused by sleep disorder plays an important role in neurodegenerative and autoimmune diseases of the CNS113. For example, increased sleep fragmentation (SF) in mice with experimental autoimmune encephalomyelitis (EAE) promotes leukocyte infiltration across the blood-spinal cord barrier and impairs immune regulation, thereby worsening EAE114. Of note, ablation of Bmal1 resulted in pericyte dysfunction, BBB hyperpermeability, and marked astrogliosis115. A noteworthy study showed that Bmal1 deficiency leads to transcription downregulation of platelet-derived growth factor receptor beta (Pdgfrβ), and exhibited age-dependent loss of pericyte coverage followed by BBB hyperpermeability115. Interestingly, TJPs were found to be regulated by circadian rhythms at both the mRNA and protein levels. For example, time-dependent oscillations of occludin mRNA and tissue barrier function were lost in circadian-disordered mice (clock−/−)116. In addition, sleep restriction not only reduced endothelial and induced nitric oxide synthase, endothelin 1, and glucose transporter protein expression in BBB, but also reduced brain uptake of 2-deoxyglucose and the expression of several TJPs117. Furthermore, the downregulation of glucose transporter-1 in brain microvasculature, enriched with sleep disturbance and decreased glucose transport across the BBB, also contributes to BBB disruption117.
Figure 4.
Schematic diagram of the interplay between circadian rhythms and gut microbiota. Circadian rhythms disruption can alter the microbial abundance and composition, and gut microbiota dysbiosis can in turn disrupt the circadian rhythms of the host. ①Gut microbiota dysbiosis reduces the production of metabolites, including short-chain fatty acids (SCFAs) (propionate, butyrate, acetate) and bile acids, affecting circadian rhythms; ②the SCN controls the secretion of endocrine glandular hormones, which play a role in circadian rhythms and affect the gut microbiota, in turn, which can influence the secretion of these hormones through feedback mechanisms; ③ the neural pathway of the vagus nerve is the most direct way for the gut microbiota to communicate with the brain, and gut microbiota dysbiosis is accompanied by a decrease in vagal function; ④gut microbiota dysbiosis and circadian rhythm disorders are associated with decreased synthesis and inactivation of neurotransmitters; ⑤increased release of LPS and defects in clock genes lead to a sustained increase in pro-inflammatory cytokines.
There exist age-related differences in the induction of BBB dysfunction by SD or SF. For example, SF disrupted the BBB integrity in aged mice, but not in young mice, suggesting that the aged BBB is more sensitive to SF than adults118. Melatonin, which could regulate circadian rhythms and sleep homeostasis119, showed a declining level in aged rats and humans. We have previously reported that supplement with melatonin alleviated LPS-disrupted BBB integrity by activating AMP-activated protein kinase in old mice120. Moreover, melatonin has also been shown to alleviate BBB breakdown in diabetes-induced AD by inhibiting the loss of TJPs121. Therefore, restoration of sleep has the potential to enhance BBB integrity122.
6. Gut microbiota dysbiosis, BBB disruption, and aging effect
6.1. Role of gut microbiota in the BBB integrity
Gut microbiota has been demonstrated to be critically involved in BBB integrity123. For example, germ-free (GF) adult mouse brain showed disorganized TJPs and increased BBB permeability with low expression of occludin and claudin-5. However, the conventionalization of GF adult mice through transplant of the fecal microbiota from pathogen-free adult mice or monocolonization of GF mice with either Clostridium tyrobutyricum or Bacteroides thetaiotaomicron that produced SCFAs restored the function of the BBB and claudin-5 level was elevated21. Meanwhile, SCFAs have also been shown to support BBB integrity as beneficial metabolizers124. In addition, strains producing sodium butyrate or acetate/propionate modulate BBB permeability in GF animals by fermenting dietary carbohydrates21. Of note, low-dose penicillin exposure early in life had lasting changes in the gut microbiota and upregulated occludin and claudin-5 expression in mice125.
6.2. Association between gut microbiota dysbiosis and BBB disruption in neurological diseases, an aging effect
Gut microbes and metabolites can modulate BBB integrity and brain health11. Gut microbiota has been proposed as a potential regulator of the integrity of the BBB, and its regulatory effect begins with the early period of intrauterine life and propagates throughout life126. Aging decreases the levels of microbial, accompanied by a reduced ability to protect BBB124. Gut microbial disturbances result in decreased P-gp, increased BBB permeability, and neuronal death due to the accumulation of harmful blood-borne chemicals127. For example, dysregulation of the gut microbiota triggers the release of inflammatory cytokines that can activate endothelial cells and actively traverse the brain endothelial cell layer, causing damage to BBB integrity128. Microbiome-produced metabolites, such as SCFAs, can cross the BBB and affect brain function129. SCFAs increased TJPs spikes, alleviated LPS-induced TJPs mismatches, improved BBB integrity, and modulated mitochondrial network dynamics130, which is a target for the anti-aging effects of chlorpropamide131.
Microbiome–BBB interactions have been reported to influence disease progression. For example, the gut microbiome plays an important role in BBB disruption in an animal model of hypertensive cerebral small vessel disease132,133. Moreover, stroke leads to dysbiosis of gut bacteria that affects post-stroke outcomes and gut microbiota is causally associated with a poststroke cognitive impairment through LPS and butyrate134. For example, cerebral ischemic stroke caused gut microbiota dysbiosis, increased intestinal permeability, disrupted the gut barrier, and triggered gut microbiota translocation135. The Puerariae Lobatae Radix and Chuanxiong Rhizoma combination was an effective treatment for cerebral ischemic stroke that relieved the gut microbiota dysbiosis and brain-gut barrier disruption135. The decrease in beneficial intestinal microbiota due to aging and the decrease in the production of sodium butyrate may increase BBB permeability and promote postoperative cognitive dysfunction in the elderly136. In addition, dysbiosis of the gut microbiota promotes the release of toxic metabolites and pro-inflammatory cytokines that disrupt the intestinal epithelial barrier and promote BBB dysfunction, which is believed to be the basis for the development of AD126. The gut microbiome also contains a lot of viruses that are critically involved in disease development and recovery by regulating host and host gut bacterial metabolism137. In addition, zonulin-dependent alterations in intestinal permeability and gut microbiota dysregulation are accompanied by changes in BBB integrity and behavioral changes that are partially alleviated by microbiota depletion138. Excessive intestinal inflammation caused by gut microbiota dysbiosis increases BBB permeability, triggering various common chronic diseases and leading to brain aging139. Regulating and supplementing the intestinal microbiota, as well as targeting immune cells and inflammatory mediators, are required to protect BBB integrity140.
7. Interplays between circadian rhythms and gut microbiota
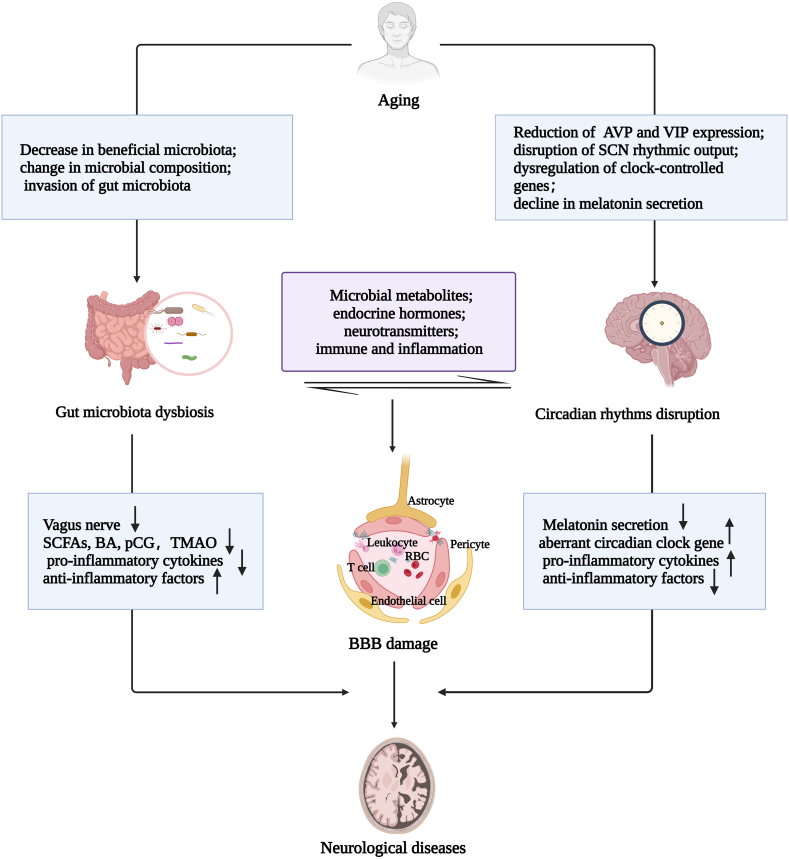
Circadian rhythms and gut microbiota are inextricably linked. Circadian rhythms disruption and gut microbiota dysbiosis occur during aging (Fig. 4). Moreover, emerging evidence shows that the interplays of circadian rhythm disturbances and gut dysbiosis disrupt BBB integrity and accelerate AAND141.
7.1. Gut microbiome is one of the key factors that is involved in maintaining host circadian rhythms
Microbial oscillators are generally promoted by plant-based, low-fat diets, and most are abolished by low-fiber, high-sugar, high-fat diets with low fiber content. Genetic, environmental, dietary, and other host factors such as sex and gut immunity determine the composition and behavior of microbial oscillators. Interaction between host and microbe affects the circadian period, with microbes affecting interactions among light- and food-entrainable circadian processes in response to environmental perturbations142. A significant difference in composition, localization, and function of the gut microbiome has been reported between day and night, depending on the feeding cycle of the host143. Taxonomic analysis of mouse fecal microbiota reveals that hourly-scale fluctuations in microbiota composition and function that follow a 24-h rhythm can lead to strong oscillations143. Mice without microorganisms (germ-free, GF) exhibit higher amplitude circadian rhythms in the light/dark cycle and longer circadian periods in constant darkness. Microbial transplantation with fecal contents of conventionally reared mice normalized circadian rhythms in GF mice, suggesting that the simultaneous activity of gut microbes modulates the host's circadian network142. Furthermore, microbiota-derived SCFAs can communicate from the microbiome to various parts within the host tissues and regulate the homeostasis of mammals144. Of note, microbiota could modulate substance uptake and storage through interleukin 3 regulated-circadian rhythms transcription factors, providing new insights into the regulation of host metabolism by gut microbiota145.
Disruption of microbiome rhythms affects circadian fluctuations in host physiology and disease susceptibility146. Disturbances in microbiome rhythms may at least partially contribute to an increased risk of obesity and metabolic syndrome associated with insufficient sleep and circadian misalignment147. Changes in motor activity rhythm cycles and canonical clock gene expression were found in high-fat diet (HFD)-induced gut microbiota dysregulated mice148. Circadian rhythm disturbance that is caused by untimely nutrient exposure and overeating increases the risk of disease149. Dietary intervention with prebiotics including β-glucan and inulin attenuates the effects of HFD-induced circadian asynchrony while increasing the richness of gut microbial species150. In addition, mice fed with heat-killed Lactobacillus became active in the dark and showed increased non-rapid eye movement-sleep during light151. Probiotic and prebiotic supplementation showed beneficial effects on sleep and fecal metabolite-related alterations152. For example, prebiotic-rich diets modulate pre- and post-stress sleep/wake cycles and induce stress protection in circadian physiology and gut microbiota in rats153, suggesting that probiotics can improve circadian clock and sleep. In a mice model of SD-induced microbiota disorders, probiotic and prebiotic supplementation was found to reverse microbiota disorder by suppressing oxidative stress and inflammation response154,155. Furthermore, twelve-week probiotics treatment had a beneficial effect on insulin metabolism markers and triglycerides, and contributed to improving cognitive and metabolic disorders in AD patients156. Of note, clinical trials revealed that administration of probiotics (for 12 weeks) or synbiotics (for 4 weeks) could significantly improve motor functions in PD patients157. Thus, to better understand the role of intestinal flora in mediating aging-accompanied changes of the circadian rhythm would provide a novel insight into circadian-related insomnia and benefit the treatment of circadian rhythm disturbance and circadian rhythm-related insomnia158.
7.2. Effect of circadian rhythms on the gut microbiota
Mice and human studies show that gut microbiota exhibits circadian oscillations of the compositional and functional distributions at specific times of the day depending on feeding rhythms143. The function of gut microbiota rhythmicity is highly dependent on the host circadian clock159. Disruption of the circadian system can alter microbiome communities and perturb host metabolism160 and lifestyle stressors such as altered sleep and eating patterns that may disturb the host circadian system also influence the gut microbiome147.
Altered microbiota rhythmicity is associated with other Clock network mutations, including both positive (such as Bmal1 knockout) and negative (for example, Per1 and Per2 double mutant) loop components160. Mice deficient in key circadian rhythm genes, including Bmal1 or Per1/2, have disrupted microbial rhythmicity and composition. In addition, Clock gene mutant mice also exhibit changes in microbial richness and diversity in the stool microbiota. The host circadian machinery influences microbial oscillations and composition; however, reciprocally, microbiota modulates host circadian rhythms. Furthermore, a comparison between GF and specific pathogen-free mice confirmed that microbiota induces significant diurnal host circadian rhythms in liver hepatocytes161. Interestingly, time-restricted feeding can restore cellular circadian rhythms in Cry1/2-deficient and liver-specific Bmal1 and Rev-erba/b-deficient mice162. Regular light/dark cycle-regulated Per2 gene expression is critical for circadian oscillations, and gut microbiota homeostasis. Per2 knockout altered circadian oscillations in the large intestine, improved gut microbiota diversity, and increased the abundance of Clostridium, leading to a decrease in circulating SCFAs163. In Bmal1SCNfl/– mice, rhythmic loss of microbial function was shown164. Loss of bacterial taxa and their products due to a desynchronization of the intestinal clock may lead to metabolic abnormalities in shift work164.
Insufficient sleep and circadian rhythm disorders lead to an altered gut microbial diversity, which may be followed by changes in microbial structure and function165. For example, poor sleep quality is linked to a reduced abundance of Verrucomicrobia and Lentisphaerae and suggests that there may be a connection between SD, gut microbiome, and cognitive accessibility in the healthy elderly166. In addition, SD can cause specific changes in the intestinal microbiome, with Lachnospiraceae and Ruminococcaceae increasing and Lactobacillaceae decreasing, which promote intestinal permeability, inflammation in adipose tissue, and insulin sensitivity in mice167. Moreover, short-term SD has mild effects on gut microbiome, with Firmicutes: Bacteroidetes ratio and Coriobacteriaceae and Erysipelotrichaceae being upregulated, while Tenericutes are downregulated168.
Damage of circadian and feeding rhythms increases intestinal permeability and alters intestinal barrier function and intestinal microbiota composition169. For example, under time-restricted HFD conditions, light/dark cycle information transmitted by ipRGCs is critical for the daily oscillations of gut microbes. In addition, paradoxical light exposure, such as dim light at night can alter the composition, relative abundance, and daily oscillations of the gut microbiota170. Interestingly, mice exposed to dim light at night had a significant difference in microbial diversity compared to mice exposed to standard light/dark photoperiod171. Furthermore, studies have shown that photoperiod disruption results in the down-regulation of genes that promote beneficial host immune responses, while genes related to the synthesis and transport of endotoxin LPS are up-regulated17. Of note, dietary prebiotic intervention (β-glucan and inulin) could ameliorate circadian desynchrony induced by shifted light/dark cycle in mice by modulating circadian gene expression15.
Distinct clustering in terms of beta-diversity was observed when compared with the mice under normal light/dark cycle. For example, diurnal oscillation of intestinal microbiota can be influenced by diet composition and feeding rhythms and play a key role in regulating circadian liver transcriptome and maintaining host circadian rhythms172. In addition, apple polyphenol extract modulates bile acid metabolism and gut microbiota by regulating circadian rhythms in daytime-restricted HFD-feeding male mice173.
7.3. Possible mechanism underlying BBB damage induced by circadian rhythms and gut microbiota
7.3.1. Effects of gut microbial metabolites on BBB
Gut microbiota is critical for the normal barrier function during pre- and postnatal periods as BBB permeability was increased in GF mice21. Gut microbial metabolites could also regulate BBB permeability and modulate microglia homeostasis, neuroinflammation, and cognitive function174. One of the key microbial metabolites connecting altered gut microbial composition with brain disorder is SCFA (mainly butyrate, propionate, and acetate) which is produced by anaerobic bacterial fermentation of complex plant-based polysaccharides15. Unconjugated BA have also been shown to both increase circadian genes and change the expression of biological clock genes in the mouse ileum, colon, and liver175.
Butyrate and propionate promote BBB integrity and protect the cerebrovascular system from damage21,176. Moreover, colonizing GF mice with butyric acid-producing C. tyrobutyricum or acetic and propionic acid-producing Bacteroides or oral administration of sodium butyrate restored the BBB permeability177. The administration of sodium butyrate increased occludin expression in the frontal cortex and hippocampus and increased brain histone deacetylation, which was also detected in C. tyrobutyricum inoculated GF mice178. A physiologically relevant dose of propionic acid showed the modulatory effect being independent of TJPs expression in an in vitro model of human BBB. Instead, propionate enhanced the integrity of BBB by mitigating oxidative and pro-inflammatory pathways and reducing the expression of low-density lipoprotein receptor-related protein, a specific efflux transporter178.
The beneficial effect of SCFAs on disrupted BBB is mainly based on the downregulation in paracellular permeability by restoration of junctional complex proteins via affecting their transcription, intercellular localization, or proteolytic degradation179. In an in vitro BBB model, propionate inhibits pathways associated with non-specific microbial infection through a CD14-dependent mechanism, suppresses lipoprotein receptor-related protein-1 expression, and protects BBB from oxidative stress through NRF2 signaling176. Protective effects of propionate on the BBB include mitigation against deleterious inflammatory and oxidative stimuli via the erythroid 2-like 2 signaling176.
The gut microbes metabolize tyrosine and phenylalanine to p-cresol, which is then metabolized by host enzymes to p-cresol glucuronide (pCG). pCG can act as an antagonist of toll-like receptor 4 (TLR4) and can antagonize the effects of LPS and mice that are exposed to pCG show reduced BBB permeability180. Metabolism of microbially produced methylamines associated with vascular disease has been shown to correlate with BBB integrity. The methylamine trimethylamine N-oxide (TMAO) enhances BBB integrity and protects it from inflammation178, whereas the TMAO precursor trimethylamine impaired BBB function and disrupted TJPs integrity. Chronic exposure to TMAO can protect mice from inflammation-induced cognitive impairment, inhibiting astrocyte and microglial activity in a brain181.
SCFAs, particularly butyric acid, may regulate the production of brain-derived neurotrophic factor (BDNF), signal the brain via the vagus and induce biosynthesis of neurotransmitters in the CNS. In addition, metabolites may also have negative neuromodulatory activities: p-cresol treatment has been shown to modulate oxytocinergic and opioidergic systems, dopamine turnover, and receptor activity in specific brain regions in mice or rats178.
Aside from the SCFAs, BA metabolism may be a potential link between circadian rhythms and gut microbiota182. Secondary BA deoxycholic acid and ursodeoxycholic acid may modulate BBB integrity. For example, injection of deoxycholic acid in rats increased BBB permeability demonstrated by colorimetric assay and albumin immunoreactivity183. Increased deoxycholic acid by gut microbiota is sufficient to induce the production of neurotransmitter 5-HT in enterochromaffin cells184. Increased circulating bacterially produced BA may increase the BBB permeability by redistributing the localization of occludin, ZO-1, and ZO-2 and inducing the phosphorylation of occludin via a Rac1-dependent mechanism183. On the contrary, in an in vitro model mimicking the effect of severe hyperbilirubinemia on BBB, ursodeoxycholic acid partially restored the barrier integrity by protecting the endothelial cells from apoptosis185.
BA signaling to the brain encompasses both direct and indirect pathways, that is, circulating BA can cross the BBB to act directly in the brain via their receptors or increase the release of fibroblast growth factor 19 to regulate neuronal activity103. BA replacement therapies, such as ursodeoxycholic acid, have been used to change internal BA and microbiota composition, and polyphenolic compounds, have been shown to significantly alter microbiota composition associated with BA metabolism and immune function186.
7.3.2. Effect of neurotransmitters on BBB
The gut microbiome can produce a variety of mammalian neurotransmitters, such as dopamine (Bacillus, Escherichia, Lactobacillus, Lactococcus, and Streptococcus), 5-HT (Escherichia, Enterococcus, Lactobacillus, and Streptococcus), acetylcholine (Lactobacillus and Bacillus), and gamma-aminobutyric acid (GABA; Bifidobacterium and Lactobacillus), all of which affect host health and maintain homeostasis187,188. Bacteria have been shown to produce and/or deplete a variety of mammalian neurotransmitters, including DA, 5-HT, or GABA. Accumulating evidence in animals suggests that bacterial manipulation of these neurotransmitters may have an impact on host physiology, and preliminary human studies suggest that microbiota-based interventions can also alter neurotransmitter levels189. Furthermore, SCFAs participate in the synthesis of several neurotransmitters and their receptors, including dopamine, 5-HT, and GABA103.
More than 50% of DA in the human body is synthesized in the gut190. DA and its receptors are widely distributed in the gut190. It has been shown that Bacteroides uniform through fecal bacteria transplantation can increase DAT/dopamine binding efficiency, while Prevotella copri is inversely associated with DAT binding103. Lactobacillus plantarum PS128 has been reported to alleviate striatal DA reduction in MPTP-induced PD models103 and L. plantarum DR7 can reduce DA metabolism-related enzymes, β-hydroxylase and tyrosine hydroxylase103. Additionally, Clostridium coccoides and Clostridium septum have been shown to have β-glucuronidase enzymatic activity, which can convert peripheral DA into its active form103. The complete degradation of DA by Clostridium tetani and Bacillus cereus further verifies that neurotransmitters of the host are modulated by gut microbiota.
DA imbalance is associated with AAND, including PD, which is often accompanied by abnormal dopamine levels-induced circadian rhythm disturbance. The daily rhythmicity of DA can be disrupted by decoupling between the interlocking loops of the clock circuits or by quasi-periodic clock behavior caused by misalignment with the light/dark cycles191. The neurotoxin 6-hydroxydopamine has been shown to alter motor activity and clock gene expression in the rat dorsal striatum and rat forebrain192. We have recently shown that D1 receptor-mediated endogenous tissue plasminogen activator increase induces BBB damage after ischemic stroke193. In addition, it has been reported that LPS-damaged BBB could be repaired by cabergoline, a specific dopamine D2 receptor agonist194 and phenylethylamine, a full dopamine receptor agonist produced by Morganella morganii strains, is capable of crossing the BBB195.
The gastrointestinal tract contains most of the body's 5-HT. Around 90% of 5-HT is synthesized in the periphery of human body, mainly by enterochromaffin cells190, which takes up tryptophan from the diet to synthesize 5-HT190. Indigenous spore-forming bacteria (predominantly Clostridia, can promote the biosynthesis of 5-HT in colonic enterochromophils by increasing the expression of tryptophan hydroxylase 1, the rate-limiting enzyme. The presence of Clostridium ramosum promotes 5-HT secretion, furthermore, several gut metabolites have been identified as the signaling molecules to trigger 5-HT synthesis184. For example, SCFAs are important determinants of enteric 5-HT production via promoting tryptophan hydroxylase 1 transcription196.
Tryptamine is found in low concentrations in the brain, produced by selected bacterial strains of the Clostridium sporogenes and Ruminococcus gnavus species, and acts through epithelial serotonin receptor, increasing colonic secretion and gut transit time without affecting the colonic 5-HT excretion178. Tyramine, deoxycholic acid, and 4-aminobenzoic acid were found to stimulate 5-HT synthesis both in vitro and in vivo. Moreover, microbiota-associated metabolites stimulate enterochromaffin cells to release 5-HT178.
Host-microbiota interactions play critical roles in the regulation of essential 5-HT-related biological processes184. For example, primary SP from mouse and human microbiota promotes 5-HT biosynthesis which supplies 5-HT to the mucosa, lumen, and circulating platelets184. This increased 5-HT production, which is taken up and distributed by platelets, may work as a hormone-like regulatory signal that could influence membrane permeability in the host organs and tissues in the brain. Transiently increased permeability of the BBB allows for plasma 5-HT to enter the CNS and be distributed by the volume transmission197. In addition, tryptophan (Trp) is the only precursor of 5-HT, a key monoamine neurotransmitter involved in the regulation of central neurotransmission and intestinal physiological functions. Growing evidence showed that changes in the composition of the gut microbiota affect the gut-brain axis by regulating Trp metabolism198. For example, Morinda officinalis oligosaccharides increases tryptophan hydroxylase levels in the gut microbiota to increase 5-HT levels in the brain199. In particular, the levels of Trp-derived 3-hydroxytryptamine pathway metabolites were significantly lower in stool samples from AD patients and direct supplementation of Trp by dietary intervention upregulated 5-HT levels in the hippocampus and frontal cortex, preventing memory loss in aged animals200.
L. plantarum IS-10506 and the probiotic mixture of Bifidobacterium bifidum BGN4 and Bifidobacterium longum could upregulate the expression of BDNF, accompanied by a 5-HT increase in rat brain. Meanwhile, IS-10506 treatment can increase 5-HT transporter expression in the hippocampus201. In addition, treatment of aged senescence-accelerated mice P8 (SAMP8) mice with ProBiotic-4, a probiotic preparation composed of Bifidobacterium lactis, Lactobacillus casei, B. bifidum, and Lactobacillus acidophilus, significantly decreased Firmicutes/Bacteroidetes (F:B) ratio, Proteobacteria, Pseudomonas (genus), and Lachnospiraceae NK4A136 group increased the levels of claudin-1, occludin, and ZO-1202.
Plasma 5-HT levels in PD patients are found to be decreased and serotoninergic intervention has been reported to reduce Aβ-42 levels and the risk of cognitive decline in PD203. Therefore, the effects of gut microbiota in modulating neurotransmitters both in the periphery and central nervous system can help optimize available methods to prevent or restore dopaminergic deficits in PD103.
Gut microbiota could also regulate GABA production, an inhibitory neurotransmitter which is involved in various physiological functions and it was found that the levels of GABA in the gut coincide with that in the CNS204. Strandwitz et al.205 isolate a variety of GABA-producing bacteria, of which Bacteroides generated a lot of GABA. Transcriptome-based analysis showed that GABA-producing pathways are expressed by Bacteroides, Parabacteroides, and Escherichia. In addition, human intestinally derived Lactobacillus brevis and Bifidobacterium dentium can also produce GABA efficiently206. Interestingly, gut microbiota-derived GABA shows neuroprotective effects in Clostridium elegans, highlighting the role of bacterially-produced GABA in keeping brain homeostasis205.
7.3.3. Effects of endocrine hormones on the BBB
The microbiota–gut–brain axis and circadian rhythms often interact through the action of hormones, and the SCN directly or indirectly controls many endocrine glands. Hormones secreted from these glands act in circadian rhythms and influence the gut microbiota, which in turn can influence the secretion of these hormones through feedback mechanisms207. Glucocorticoids appear to act as feedback messengers to modulate the links among circadian rhythms, the endocrine system, and the gut microbiota208. Moreover, previous studies have found that glucocorticoid can regulate circadian rhythms209, and glucocorticoid therapy can alter the composition of the intestinal microbiota210.
Notably, long-term gut flora dysbiosis results in overactivation of the hypothalamic–pituitary–adrenal axis (HPA) and the neuroimmune system, as well as altered neurotransmitter levels, followed by dysfunctional signaling, inflammation, and neuronal death211. Gut microbial disorders can lead to an increase in LPS, which crosses the intestinal epithelial barrier and activates the HPA, where the adrenocorticotropin-releasing hormone is released, leading to increased BBB permeability140. In addition, corticosterone treatment reduces TJPs expression in brain endothelial cells for in vitro BBB212 and RU486, glucocorticoids receptor antagonist, could inhibit the effects of dexamethasone on functional recovery, indicating that barrier recovery was mediated by glucocorticoid receptor signaling213. Interestingly, glucocorticoids are also involved in regulating immune responses and demonstrate rhythmic alterations in animal models of MS79. Moreover, orexins could initiate and maintain wakefulness, and loss of orexin-producing neurons causes narcolepsy, and modulation of orexin and its effects on sleep appear to modulate Aβ pathology in the brain214.
7.3.4. Effects of vagus regulation on BBB
The intestine and the brain are connected by millions of nerve cells, and vagus, the 10th cranial nerve that transmits bidirectional regulatory signals between the brain and internal organs, is an essential part of the gut–brain connection215.
In addition to endocrine and immune signaling pathways, neural pathways involving the vagus is likely to be the most direct route for the gut microbiota to communicate with the brain. Stimulation of the vagus reduces LPS stimulation-induced co-localization of neutrophils and endothelial cell adhesion molecule (ICAM)-1, as well as downregulates gene expression of inflammatory mediators (IL-6, C–X–C motif chemokine ligand 1, CXCL-1) and attenuates inflammatory responses in brain regions216. In addition, the neuroprotective effect of a series of noninvasive vagus nerve stimulation (nVNS) stimulation is spatially associated with the protection of BBB integrity from ischemic stroke-induced injury and reduction in infarct size in rats217.
It is of note that Paenalcaligenes hominins travel into the brain via the blood and vagus nerve218. Vagotomy can prevent the accumulation and infiltration of extracellular vesicles into the hippocampus and inhibit the oral gavage of Paenalcaligenes hominis-induced downregulation of BDNF expression in the hippocampus218.
7.3.5. Effects of immune and inflammation on BBB
Chronic circadian rhythm disturbances have been shown to deteriorate stroke outcomes in a specific direction and schedule by initiating inflammatory responses in the brain219. In addition, dual silencing of CRY1 and CRY2 proteins produced a sustained upregulation in pro-inflammatory cytokines IL-6 and TNF-α and upregulated expression of inducible nitric oxide synthase (iNOS) in fibroblasts, demonstrating a direct link between molecular clock disruption and inflammatory response increase220. Circadian rhythm disorder regulates the composition of intestinal microbiota221, which plays a key role in regulating host immunity. For example, dysbiosis of intestinal flora leads to chemokine secretion and subsequently to recruitment of neutrophils, monocytes, dendritic cells, and macrophages222. Neutrophils produce ROS, MMP3, lipid carrier protein-2, and neutrophil extracellular traps to degrade TJPs and disrupt BBB structure223,224. Moreover, microbiota transfer from intestinal clock-deficient mice into GF mice altered intestinal gene expression and suppressed immune cell recruitment225 and BaWeiBaiDuSan, a herbal formula, has been shown to alleviate polymicrobial sepsis-induced liver injury in mice by modulating macrophage anti-inflammatory activity and increasing the gut microbiota Lactobacillus johnsonii226. Notably, age-related changes in the composition or function of the intestinal microbiota lead to a decrease in intestinal epithelial barrier function of the elderly, driving systemic chronic inflammation227 and contributing to aging-related diseases228.
The altered gut microbial composition can lead to disruption of the intestinal epithelial barrier, which may permit microbiota-derived factors to enter the circulation126. Circulating LPS and high-mobility group box 1 (HMGB1) act as pathogen-associated molecular patterns and danger-associated molecular patterns, respectively, thereby “alarming” the immune system and promoting systemic inflammation. In addition, CNS-produced type I interferons bind to metabolites that are derived from dietary Trp by intestinal microbiota to activate transcription factor aryl hydrocarbon receptor signaling in astrocytes and inhibit CNS inflammation229. SCFAs have been shown to have an integral role in promoting microglia maturation and normal function230.
It has been shown that secoisolariciresinol diglucoside, the main lignan in flaxseed, can prevent systemic inflammatory response-induced BBB permeability by directly inhibiting BBB interactions with inflammatory cells and reducing the inflammatory state of leukocytes231. Interestingly, trans-10-hydroxy-2-decenoic acid, the exclusive lipid component of royal jelly, can alleviate LPS-induced BBB dysfunction, therefore it may be a potential candidate for the treatment of BBB damage-related diseases232. In addition, dietary saturated fatty acid was identified as a key regulator in the gut, altering T helper 1 and Th17/Treg cell homeostasis in autoimmune neuroinflammation233. Dendrobium officinale polysaccharide (DOP) has received attention as it may alleviate AD-related cognitive impairment via regulating microglial activation. DOP treatment appears to reshape the perturbation of gut microbiota caused by circadian rhythm disruption, including the up-regulated abundance of Akkermansia and Alistipes, and the down-regulated abundance of Clostridia. DOP seems to restore histopathological changes, reduce inflammatory cell infiltration, and strengthen mucosal integrity. Furthermore, DOP could reverse the levels of metabolites related to cognitive function improvement92.
7.4. Molecular mechanisms underlying aging, circadian rhythms, gut microbiota, and the BBB (Fig. 5)
Figure 5.
Molecular mechanisms underlying aging, circadian rhythms, gut microbiota, and the BBB. ①Binding of LPS and high-mobility group box 1 (HMGB1) to toll-like receptor 4 (TLR4) signaling in the myeloid differentiation factor 88 (MyD88 -dependent pathway induces activation of NF-κB, leading to the release of pro-inflammatory factors that contribute to BBB destruction; ②increased HDAC expression and histone hypoacetylation lead to BBB damage. SCFAs contribute to the integrity of the BBB by inhibiting HDAC to promote the expression of genes such as NRF2 and increasing nuclear factor kappa B (NF-κB) acetylation and inhibiting its transcriptional activity. ③Activation of the TLR-MyD88 signaling pathway by LPS produces IL-22 to further trigger STAT3 phosphorylation, activated STAT3 inhibits the expression of the biological clock transcriptional repressor REV-ERBα, which leads to an increase in the amplitude of nuclear factor interleukin 3 regulatory factor (NFIL3) expression.
TLR expression and activation occur in response to microbial cues and can be regulated by circadian rhythms, leading to significant functional differences in microbial responses at different times of the day162,234. Both LPS and HMGB1 can bind to TLR 4 on multiple types of immune cells in the blood. LPS stimulates TLR4 receptors by interacting with CD4 and MD-14 proteins, triggering an inflammatory response235. The downstream pathways of TLR4 activation include the myeloid differentiation factor 88 (MyD88)-dependent and the MyD88-independent pathways. TLR4 signaling in the MyD88-dependent pathway induces the activation of nuclear factor kappa B (NF-κB), leading to the release of proinflammatory cytokines and several TLR agonists can activate NF-κB236. For example, FLZ, a novel squamosamide derivative, reverses PD-related microbiota activation and restores BBB struc-ture via TLR4/MyD88/NF-κB pathway to protect PD models237.
Of note, systemic administration of TMAO prevented LPS-induced impairment of BBB function in mice181. The host-gut co-metabolite p-cresol glucosinolate improved BBB integrity in vivo and prevented LPS-induced BBB permeabilization in vitro by acting as a TLR4 antagonist238. The TLR4 agonist LTA increases circulating levels of cytokine and cytokine mRNA expression and is also involved in the transcriptional downregulation of TJPs in the brain239. NI 0101, an anti-TLR4 antibody, can potentially block TLR4 ligands and inhibit cytokines release240. Agonistic anti-TLR4 mAb significantly decreases NF-κB activation and the production of IL-6, IL-1β, and TNF-α241. TAK-242, a small-molecule-specific inhibitor of TLR4, disrupts the interaction of TLR4 with its adaptor molecules, thereby inhibiting its downstream signaling events242. Therefore, the TLR4 is a potential therapeutic target to block systemic inflammation, thereby preventing BBB dysfunction and alleviating AAND.
SCFAs can pass the cell membrane and inhibit intracellular HDAC. SCFAs-stimulated acetylation of histone and non-histone proteins via inhibition of classes I and IIb histone deacetylases, and crosstalk of these signaling pathways with transcriptional factors NF-κB and NRF2 as mainstream mechanisms179. NRF2 translocates to the nucleus and binds to the adjacent regions of the Il-1β and Il-6 genes, suppressing the expression of inflammatory factors243. For example, microbiota-derived SCFAs program circadian rhythms in the small intestine via HDACs244. In addition, 3,4-dihydroxyphenylpropionic acid reduces circadian changes in ischemia/reperfusion injury by inhibiting macrophage pro-inflammatory activation through inhibition of HDAC activity245. BBB damage induced by increased HDAC expression and histone hypoacetylation play a key role in the recovery after stroke246. In stroke, valproic acid treatment could increase histone acetylation and restore BBB integrity247. Inhibition of HDACs induces hyperacetylation of NF-κB/p65, leading to regulation of p65 binding to IκB and downregulation of NF-κB transcriptional activity248. Considering that small molecular weights of SCFAs can easily enter the brain, SCFAs could be a promising class of HDACIs for PD therapy. Until now, the therapeutic potential of sodium butyrate as HDACIs has been reported in several studies103.
Gut microbiota has been shown to regulate the amplitude of circadian rhythms of the transcription factor NFIL3. The regulation of NFIL3 was specific for the signals of gram-negative bacteria such as LPS and flagellin145. The microbiota-induced NFIL3 increase is involved in decreased expression of REV-ERBα protein145 and the effect is mediated through Myd88 signaling in CD11c+ dendritic cells. Briefly, flagellin and LPS are recognized by TLRs on dendritic cells, and IL-23 is recruited to activated group 3 innate lymphoid cells (ILC3s) which could phosphorylate signal transducer and activator of transcription 3 (STAT3) signal via IL-22 in 2 intestinal epithelial cells (IECs)16. Phosphorylated STAT3 could decrease of REV-ERBα protein expression and REV-ERBα can mediate the circadian rhythms of NFIL316.
Altered intestinal microbial composition and an abnormal increase in the ratio of thick-walled bacteria anaplasma induced HMGB1 release from the intestine into circulation via exosomes249,250. NF-κB activation increases glial cell activation and the expression of ICAM-1, IL-6, IL-8, and monocyte chemoattractant protein 1251, which contribute to BBB disruption252; whereas inhibition of NF-κB activity could restore low BBB permeability and upregulate the expression of TJP in rat brain microvascular endothelial cells253. Circadian rhythms has been shown to be involved in influencing microglia immune activity, as Clock is a positive regulator of NF-κB-mediated transcription254. In another mouse model-based study, DOP was found to improve intestinal barrier dysfunction by upregulating TJPs expression through inhibition of NF-κB activation and neuroinflammation and to modulate the gut microbiota to provide neuroprotection against cognitive impairment255.
8. Conclusions
As the pace of human population aging continues to accelerate, aging as a risk factor for neurodegenerative diseases has received increasing attention from society. During aging, alterations in circadian rhythms, gut microbiota dysbiosis, and disruption of BBB integrity can trigger or accelerate age-accompanied neurological diseases. In particular, BBB damage plays a crucial role in the pathological process of age-accompanied neurological diseases. Accumulating evidence supports the potentially important role of dysregulated interplays between gut microbiota and circadian rhythms in promoting BBB disruption and neurological disease pathogenesis. While progress is being made, it is critical to better understand the mechanisms underlying age-related neurological disorders accompanied by BBB disruption and/or interactions between gut microbiota and circadian rhythms. Further research in this direction will bring the translational potential for future clinical applications in promoting healthy aging and treating age-related neurodegeneration. In addition, interventions based on the interplays between circadian rhythms and gut microbiota, such as a healthy and regular diet, adequate sleep duration, and melatonin, may improve some symptoms of aging-related neurological disorders. Regulating circadian rhythms and gut microbiota could restore the structure and function of the BBB and improve neurovascular microenvironment homeostasis, providing potential therapeutic strategies for addressing AAND (Fig. 6).
Figure 6.
Summary of interplays among circadian rhythms, gut microbiota, and BBB in aging-accompanied neurological diseases.
Acknowledgments
This work was supported by Jiaxing Plan of Science and Technology (2022AY30028), China. This work was also supported by the National Natural Science Foundation of China (81870973, 81671145).
Footnotes
Peer review under the responsibility of Chinese Pharmaceutical Association and Institute of Materia Medica, Chinese Academy of Medical Sciences.
Contributor Information
Yanping Wang, Email: ypwang93@163.com.
Xinchun Jin, Email: xinchunjin@gmail.com.
Wei Wang, Email: wangwei@ccmu.edu.cn.
Author contributions
Yanping Wang: writing-original draft, writing-review & editing; Weihong Du: writing-review & editing, figure making; Xiaoyan Hu: writing-review & editing; Xin Yu: writing & reference collection; Chun Guo: review & editing; Xinchun Jin: writing-review & editing, conceptualization, supervision; Wei Wang: review & editing, conceptualization, supervision.
Conflicts of interest
The authors declare no conflicts of interest.
References
- 1.The Lancet Diabetes E. Opening the door to treating ageing as a disease. Lancet Diabetes Endocrinol. 2018;6:587. doi: 10.1016/S2213-8587(18)30214-6. [DOI] [PubMed] [Google Scholar]
- 2.Childs B.G., Durik M., Baker D.J., van Deursen J.M. Cellular senescence in aging and age-related disease: from mechanisms to therapy. Nat Med. 2015;21:1424–1435. doi: 10.1038/nm.4000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Flatt T. A new definition of aging? Front Genet. 2012;3:148. doi: 10.3389/fgene.2012.00148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kirkwood T.B. Understanding the odd science of aging. Cell. 2005;120:437–447. doi: 10.1016/j.cell.2005.01.027. [DOI] [PubMed] [Google Scholar]
- 5.Satoh A., Imai S.I., Guarente L. The brain, sirtuins, and ageing. Nat Rev Neurosci. 2017;18:362–374. doi: 10.1038/nrn.2017.42. [DOI] [PubMed] [Google Scholar]
- 6.Kondratova A.A., Kondratov R.V. The circadian clock and pathology of the ageing brain. Nat Rev Neurosci. 2012;13:325–335. doi: 10.1038/nrn3208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kondratov R.V. A role of the circadian system and circadian proteins in aging. Ageing Res Rev. 2007;6:12–27. doi: 10.1016/j.arr.2007.02.003. [DOI] [PubMed] [Google Scholar]
- 8.Bass J., Lazar M.A. Circadian time signatures of fitness and disease. Science. 2016;354:994–999. doi: 10.1126/science.aah4965. [DOI] [PubMed] [Google Scholar]
- 9.Chen S.J., Deng Y.T., Li Y.Z., Zhang Y.R., Zhang W., Chen S.D., et al. Association of circadian rhythms with brain disorder incidents: a prospective cohort study of 72,242 participants. Transl Psychiatry. 2022;12:514. doi: 10.1038/s41398-022-02278-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Caracciolo B., Xu W., Collins S., Fratiglioni L. Cognitive decline, dietary factors and gut-brain interactions. Mech Ageing Dev. 2014;136–137:59–69. doi: 10.1016/j.mad.2013.11.011. [DOI] [PubMed] [Google Scholar]
- 11.Parker A., Fonseca S., Carding S.R. Gut microbes and metabolites as modulators of blood–brain barrier integrity and brain health. Gut Microb. 2020;11:135–157. doi: 10.1080/19490976.2019.1638722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Navarro-Lopez V., Mendez-Miralles M.A., Vela-Yebra R., Fries-Ramos A., Sanchez-Pellicer P., Ruzafa-Costas B., et al. Gut microbiota as a potential predictive biomarker in relapsing-remitting multiple sclerosis. Genes. 2022;13:930. doi: 10.3390/genes13050930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wiefels M.D., Furar E., Eshraghi R.S., Mittal J., Memis I., Moosa M., et al. Targeting gut dysbiosis and microbiome metabolites for the development of therapeutic modalities for neurological disorders. Curr Neuropharmacol. 2024;1:123–139. doi: 10.2174/1570159X20666221003085508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xu J., Du W., Zhao Y., Lim K., Lu L., Zhang C., et al. Mitochondria targeting drugs for neurodegenerative diseases-design, mechanism and application. Acta Pharm Sin B. 2022;12:2778–2789. doi: 10.1016/j.apsb.2022.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cheng W.Y., Ho Y.S., Chang R.C. Linking circadian rhythms to microbiome–gut–brain axis in aging-associated neurodegenerative diseases. Ageing Res Rev. 2022;78 doi: 10.1016/j.arr.2022.101620. [DOI] [PubMed] [Google Scholar]
- 16.Choi H., Rao M.C., Chang E.B. Gut microbiota as a transducer of dietary cues to regulate host circadian rhythms and metabolism. Nat Rev Gastroenterol Hepatol. 2021;18:679–689. doi: 10.1038/s41575-021-00452-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Deaver J.A., Eum S.Y., Toborek M. Circadian disruption changes gut microbiome taxa and functional gene composition. Front Microbiol. 2018;9:737. doi: 10.3389/fmicb.2018.00737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.van de Wouw M., Boehme M., Lyte J.M., Wiley N., Strain C., O'Sullivan O., et al. Short-chain fatty acids: microbial metabolites that alleviate stress-induced brain–gut axis alterations. J Physiol. 2018;596:4923–4944. doi: 10.1113/JP276431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hu X., Geng P., Zhao X., Wang Q., Liu C., Guo C., et al. The NG2-glia is a potential target to maintain the integrity of neurovascular unit after acute ischemic stroke. Neurobiol Dis. 2023;180 doi: 10.1016/j.nbd.2023.106076. [DOI] [PubMed] [Google Scholar]
- 20.Wang Y., Du W., Sun Y., Zhang J., Ma C., Jin X. CRTC1 is a potential target to delay aging-induced cognitive deficit by protecting the integrity of the blood–brain barrier via inhibiting inflammation. J Cerebr Blood Flow Metabol. 2023;43:1042–1059. doi: 10.1177/0271678X231169133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Braniste V., Al-Asmakh M., Kowal C., Anuar F., Abbaspour A., Toth M., et al. The gut microbiota influences blood–brain barrier permeability in mice. Sci Transl Med. 2014;6:263ra158. doi: 10.1126/scitranslmed.3009759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Medina-Flores F., Hurtado-Alvarado G., Contis-Montes de Oca A., Lopez-Cervantes S.P., Konigsberg M., Deli M.A., et al. Sleep loss disrupts pericyte–brain endothelial cell interactions impairing blood–brain barrier function. Brain Behav Immun. 2020;89:118–132. doi: 10.1016/j.bbi.2020.05.077. [DOI] [PubMed] [Google Scholar]
- 23.Kurz C., Walker L., Rauchmann B.S., Perneczky R. Dysfunction of the blood–brain barrier in Alzheimer's disease: evidence from human studies. Neuropathol Appl Neurobiol. 2022;48 doi: 10.1111/nan.12782. [DOI] [PubMed] [Google Scholar]
- 24.Jin X., Liu J., Liu K.J., Rosenberg G.A., Yang Y., Liu W. Normobaric hyperoxia combined with minocycline provides greater neuroprotection than either alone in transient focal cerebral ischemia. Exp Neurol. 2013;240:9–16. doi: 10.1016/j.expneurol.2012.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu J., Weaver J., Jin X., Zhang Y., Xu J., Liu K.J., et al. Nitric oxide interacts with caveolin-1 to facilitate autophagy–lysosome-mediated claudin-5 degradation in oxygen–glucose deprivation-treated endothelial cells. Mol Neurobiol. 2016;53:5935–5947. doi: 10.1007/s12035-015-9504-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang X., Liu Y., Sun Y., Liu W., Jin X. Blood brain barrier breakdown was found in non-infarcted area after 2-h MCAO. J Neurol Sci. 2016;363:63–68. doi: 10.1016/j.jns.2016.02.035. [DOI] [PubMed] [Google Scholar]
- 27.Chrissobolis S., Faraci F.M. The role of oxidative stress and NADPH oxidase in cerebrovascular disease. Trends Mol Med. 2008;14:495–502. doi: 10.1016/j.molmed.2008.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yang A.C., Stevens M.Y., Chen M.B., Lee D.P., Stähli D., Gate D., et al. Physiological blood–brain transport is impaired with age by a shift in transcytosis. Nature. 2020;583:425–430. doi: 10.1038/s41586-020-2453-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bartels A.L., Kortekaas R., Bart J., Willemsen A.T., de Klerk O.L., de Vries J.J., et al. Blood–brain barrier P-glycoprotein function decreases in specific brain regions with aging: a possible role in progressive neurodegeneration. Neurobiol Aging. 2009;30:1818–1824. doi: 10.1016/j.neurobiolaging.2008.02.002. [DOI] [PubMed] [Google Scholar]
- 30.Montagne A., Barnes S.R., Sweeney M.D., Halliday M.R., Sagare A.P., Zhao Z., et al. Blood–brain barrier breakdown in the aging human hippocampus. Neuron. 2015;85:296–302. doi: 10.1016/j.neuron.2014.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Phillips C.M., Stamatovic S.M., Keep R.F., Andjelkovic A.V. Epigenetics and stroke: role of DNA methylation and effect of aging on blood–brain barrier recovery. Fluids Barriers CNS. 2023;20:14. doi: 10.1186/s12987-023-00414-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Erdő F., Denes L., de Lange E. Age-associated physiological and pathological changes at the blood–brain barrier: a review. J Cerebr Blood Flow Metabol. 2017;37:4–24. doi: 10.1177/0271678X16679420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rosenberg G.A. Neurological diseases in relation to the blood–brain barrier. J Cerebr Blood Flow Metabol. 2012;32:1139–1151. doi: 10.1038/jcbfm.2011.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Barisano G., Montagne A., Kisler K., Schneider J.A., Wardlaw J.M., Zlokovic B.V. Blood–brain barrier link to human cognitive impairment and Alzheimer's disease. Nat Cardiovasc Res. 2022;1:108–115. doi: 10.1038/s44161-021-00014-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang Q., Xie C. Apolipoprotein E drives early blood–brain barrier damage in Alzheimer's disease. Neurosci Bull. 2021;37:281–283. doi: 10.1007/s12264-020-00596-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Montagne A., Nation D.A., Sagare A.P., Barisano G., Sweeney M.D., Chakhoyan A., et al. APOE4 leads to blood–brain barrier dysfunction predicting cognitive decline. Nature. 2020;581:71–76. doi: 10.1038/s41586-020-2247-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Poewe W., Seppi K., Tanner C.M., Halliday G.M., Brundin P., Volkmann J., et al. Parkinson disease. Nat Rev Dis Prim. 2017;3 doi: 10.1038/nrdp.2017.13. [DOI] [PubMed] [Google Scholar]
- 38.Ruan Z., Zhang D., Huang R., Sun W., Hou L., Zhao J., et al. Microglial activation damages dopaminergic neurons through MMP-2/-9-mediated increase of blood–brain barrier permeability in a Parkinson's disease mouse model. Int J Mol Sci. 2022;23:2793. doi: 10.3390/ijms23052793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lan G., Wang P., Chan R.B., Liu Z., Yu Z., Liu X., et al. Astrocytic VEGFA: an essential mediator in blood–brain-barrier disruption in Parkinson's disease. Glia. 2022;70:337–353. doi: 10.1002/glia.24109. [DOI] [PubMed] [Google Scholar]
- 40.Kortekaas R., Leenders K.L., van Oostrom J.C., Vaalburg W., Bart J., Willemsen A.T., et al. Blood–brain barrier dysfunction in parkinsonian midbrain in vivo. Ann Neurol. 2005;57:176–179. doi: 10.1002/ana.20369. [DOI] [PubMed] [Google Scholar]
- 41.Rite I., Machado A., Cano J., Venero J.L. Blood–brain barrier disruption induces in vivo degeneration of nigral dopaminergic neurons. J Neurochem. 2007;101:1567–1582. doi: 10.1111/j.1471-4159.2007.04567.x. [DOI] [PubMed] [Google Scholar]
- 42.Candelario-Jalil E., Dijkhuizen R.M., Magnus T. Neuroinflammation, stroke, blood–brain barrier dysfunction, and imaging modalities. Stroke. 2022;53:1473–1486. doi: 10.1161/STROKEAHA.122.036946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mohawk J.A., Green C.B., Takahashi J.S. Central and peripheral circadian clocks in mammals. Annu Rev Neurosci. 2012;35:445–462. doi: 10.1146/annurev-neuro-060909-153128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cai W., Zhang K., Li P., Zhu L., Xu J., Yang B., et al. Dysfunction of the neurovascular unit in ischemic stroke and neurodegenerative diseases: an aging effect. Ageing Res Rev. 2017;34:77–87. doi: 10.1016/j.arr.2016.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Alvarez J.I., Cayrol R., Prat A. Disruption of central nervous system barriers in multiple sclerosis. Biochim Biophys Acta. 2011;1812:252–264. doi: 10.1016/j.bbadis.2010.06.017. [DOI] [PubMed] [Google Scholar]
- 46.Sweeney M.D., Zhao Z., Montagne A., Nelson A.R., Zlokovic B.V. Blood–brain barrier: from physiology to disease and back. Physiol Rev. 2019;99:21–78. doi: 10.1152/physrev.00050.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Drouin-Ouellet J., Sawiak S.J., Cisbani G., Lagacé M., Kuan W.L., Saint-Pierre M., et al. Cerebrovascular and blood–brain barrier impairments in Huntington's disease: potential implications for its pathophysiology. Ann Neurol. 2015;78:160–177. doi: 10.1002/ana.24406. [DOI] [PubMed] [Google Scholar]
- 48.Hardiman O., Al-Chalabi A., Chio A., Corr E.M., Logroscino G., Robberecht W., et al. Amyotrophic lateral sclerosis. Nat Rev Dis Prim. 2017;3 doi: 10.1038/nrdp.2017.71. [DOI] [PubMed] [Google Scholar]
- 49.Zhou Y., Tang J., Lan J., Zhang Y., Wang H., Chen Q., et al. Honokiol alleviated neurodegeneration by reducing oxidative stress and improving mitochondrial function in mutant SOD1 cellular and mouse models of amyotrophic lateral sclerosis. Acta Pharm Sin B. 2023;13:577–597. doi: 10.1016/j.apsb.2022.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Garbuzova-Davis S., Hernandez-Ontiveros D.G., Rodrigues M.C., Haller E., Frisina-Deyo A., Mirtyl S., et al. Impaired blood–brain/spinal cord barrier in ALS patients. Brain Res. 2012;1469:114–128. doi: 10.1016/j.brainres.2012.05.056. [DOI] [PubMed] [Google Scholar]
- 51.Garbuzova-Davis S., Sanberg P.R. Blood–CNS barrier impairment in ALS patients versus an animal model. Front Cell Neurosci. 2014;8:21. doi: 10.3389/fncel.2014.00021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Knox E.G., Aburto M.R., Clarke G., Cryan J.F., O'Driscoll C.M. The blood–brain barrier in aging and neurodegeneration. Mol Psychiatr. 2022;27:2659–2673. doi: 10.1038/s41380-022-01511-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhang R., Lahens N.F., Ballance H.I., Hughes M.E., Hogenesch J.B. A circadian gene expression atlas in mammals: implications for biology and medicine. Proc Natl Acad Sci U S A. 2014;111:16219–16224. doi: 10.1073/pnas.1408886111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cai R., Gao L., Gao C., Yu L., Zheng X., Bennett D., et al. Circadian disturbances and frailty risk in older adults: a prospective cohort study. Res Sq. 2023 doi: 10.21203/rs.3.rs-2648399/v1. Available from. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Froy O. Circadian aspects of energy metabolism and aging. Ageing Res Rev. 2013;12:931–940. doi: 10.1016/j.arr.2013.09.002. [DOI] [PubMed] [Google Scholar]
- 56.Welz P.S., Benitah S.A. Molecular connections between circadian clocks and aging. J Mol Biol. 2020;432:3661–3679. doi: 10.1016/j.jmb.2019.12.036. [DOI] [PubMed] [Google Scholar]
- 57.Eghlidi D.H., Luna S.L., Brown D.I., Garyfallou V.T., Kohama S.G., Urbanski H.F. Gene expression profiling of the SCN in young and old rhesus macaques. J Mol Endocrinol. 2018;61:57–67. doi: 10.1530/JME-18-0062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Maejima T., Tsuno Y., Miyazaki S., Tsuneoka Y., Hasegawa E., Islam M.T., et al. GABA from vasopressin neurons regulates the time at which suprachiasmatic nucleus molecular clocks enable circadian behavior. Proc Natl Acad Sci U S A. 2021;118 doi: 10.1073/pnas.2010168118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hood S., Amir S. The aging clock: circadian rhythms and later life. J Clin Invest. 2017;127:437–446. doi: 10.1172/JCI90328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chang H.C., Guarente L. SIRT1 mediates central circadian control in the SCN by a mechanism that decays with aging. Cell. 2013;153:1448–1460. doi: 10.1016/j.cell.2013.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Preitner N., Damiola F., Lopez-Molina L., Zakany J., Duboule D., Albrecht U., et al. The orphan nuclear receptor REV-ERBalpha controls circadian transcription within the positive limb of the mammalian circadian oscillator. Cell. 2002;110:251–260. doi: 10.1016/s0092-8674(02)00825-5. [DOI] [PubMed] [Google Scholar]
- 62.Chen C.Y., Logan R.W., Ma T., Lewis D.A., Tseng G.C., Sibille E., et al. Effects of aging on circadian patterns of gene expression in the human prefrontal cortex. Proc Natl Acad Sci U S A. 2016;113:206–211. doi: 10.1073/pnas.1508249112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Verma A.K., Singh S., Rizvi S.I. Aging, circadian disruption and neurodegeneration: interesting interplay. Exp Gerontol. 2023;172 doi: 10.1016/j.exger.2022.112076. [DOI] [PubMed] [Google Scholar]
- 64.Sellix M.T., Evans J.A., Leise T.L., Castanon-Cervantes O., Hill D.D., DeLisser P., et al. Aging differentially affects the re-entrainment response of central and peripheral circadian oscillators. J Neurosci. 2012;32:16193–16202. doi: 10.1523/JNEUROSCI.3559-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Videnovic A., Lazar A.S., Barker R.A., Overeem S. 'The clocks that time us'—circadian rhythms in neurodegenerative disorders. Nat Rev Neurol. 2014;10:683–693. doi: 10.1038/nrneurol.2014.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Petit D., Gagnon J.F., Fantini M.L., Ferini-Strambi L., Montplaisir J. Sleep and quantitative EEG in neurodegenerative disorders. J Psychosom Res. 2004;56:487–496. doi: 10.1016/j.jpsychores.2004.02.001. [DOI] [PubMed] [Google Scholar]
- 67.Farajnia S., Michel S., Deboer T., vanderLeest H.T., Houben T., Rohling J.H., et al. Evidence for neuronal desynchrony in the aged suprachiasmatic nucleus clock. J Neurosci. 2012;32:5891–5899. doi: 10.1523/JNEUROSCI.0469-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wyse C.A., Coogan A.N. Impact of aging on diurnal expression patterns of CLOCK and BMAL1 in the mouse brain. Brain Res. 2010;1337:21–31. doi: 10.1016/j.brainres.2010.03.113. [DOI] [PubMed] [Google Scholar]
- 69.Abbott S.M., Videnovic A. Chronic sleep disturbance and neural injury: links to neurodegenerative disease. Nat Sci Sleep. 2016;8:55–61. doi: 10.2147/NSS.S78947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Scheiermann C., Kunisaki Y., Frenette P.S. Circadian control of the immune system. Nat Rev Immunol. 2013;13:190–198. doi: 10.1038/nri3386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Arnulf I., Leu S., Oudiette D. Abnormal sleep and sleepiness in Parkinson's disease. Curr Opin Neurol. 2008;21:472–477. doi: 10.1097/WCO.0b013e328305044d. [DOI] [PubMed] [Google Scholar]
- 72.Ortuño-Lizarán I., Esquiva G., Beach T.G., Serrano G.E., Adler C.H., Lax P., et al. Degeneration of human photosensitive retinal ganglion cells may explain sleep and circadian rhythms disorders in Parkinson's disease. Acta Neuropathol Commun. 2018;6:90. doi: 10.1186/s40478-018-0596-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Willis G.L., Kelly A.M., Kennedy G.A. Compromised circadian function in Parkinson's disease: enucleation augments disease severity in the unilateral model. Behav Brain Res. 2008;193:37–47. doi: 10.1016/j.bbr.2008.04.017. [DOI] [PubMed] [Google Scholar]
- 74.Liu Y., Niu L., Liu X., Cheng C., Le W. Recent progress in non-motor features of Parkinson's disease with a focus on circadian rhythm dysregulation. Neurosci Bull. 2021;37:1010–1024. doi: 10.1007/s12264-021-00711-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Cai Y., Liu S., Sothern R.B., Xu S., Chan P. Expression of clock genes Per1 and Bmal1 in total leukocytes in health and Parkinson's disease. Eur J Neurol. 2010;17:550–554. doi: 10.1111/j.1468-1331.2009.02848.x. [DOI] [PubMed] [Google Scholar]
- 76.Liu X., Yu H., Wang Y., Li S., Cheng C., Al-Nusaif M., et al. Altered motor performance, sleep EEG, and Parkinson's disease pathology induced by chronic sleep deprivation in Lrrk2(G2019S) mice. Neurosci Bull. 2022;38:1170–1182. doi: 10.1007/s12264-022-00881-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Pajediene E., Pajeda A., Urnieziute G., Paulekas E., Liesiene V., Bileviciute-Ljungar I., et al. Subjective and objective features of sleep disorders in patients with acute ischemic or haemorrhagic stroke: it is not only sleep apnoea which is important. Med Hypotheses. 2020;136 doi: 10.1016/j.mehy.2019.109512. [DOI] [PubMed] [Google Scholar]
- 78.Duss S.B., Seiler A., Schmidt M.H., Pace M., Adamantidis A., Muri R.M., et al. The role of sleep in recovery following ischemic stroke: a review of human and animal data. Neurobiol Sleep Circadian Rhythms. 2017;2:94–105. doi: 10.1016/j.nbscr.2016.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Downton P., Jo Early, Gibbs J.E. Circadian rhythms in adaptive immunity. Immunology. 2020;161:268–277. doi: 10.1111/imm.13167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Qin J., Li R., Raes J., Arumugam M., Burgdorf K.S., Manichanh C., et al. A human gut microbial gene catalogue established by metagenomic sequencing. Nature. 2010;464:59–65. doi: 10.1038/nature08821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Spielman L.J., Gibson D.L., Klegeris A. Unhealthy gut, unhealthy brain: the role of the intestinal microbiota in neurodegenerative diseases. Neurochem Int. 2018;120:149–163. doi: 10.1016/j.neuint.2018.08.005. [DOI] [PubMed] [Google Scholar]
- 82.Belkaid Y., Hand T.W. Role of the microbiota in immunity and inflammation. Cell. 2014;157:121–141. doi: 10.1016/j.cell.2014.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Bäckhed F., Ding H., Wang T., Hooper L.V., Koh G.Y., Nagy A., et al. The gut microbiota as an environmental factor that regulates fat storage. Proc Natl Acad Sci U S A. 2004;101:15718–15723. doi: 10.1073/pnas.0407076101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Park J., Kim C.H. Regulation of common neurological disorders by gut microbial metabolites. Exp Mol Med. 2021;53:1821–1833. doi: 10.1038/s12276-021-00703-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Morais L.H., Schreiber HLt, Mazmanian S.K. The gut microbiota–brain axis in behaviour and brain disorders. Nat Rev Microbiol. 2021;19:241–255. doi: 10.1038/s41579-020-00460-0. [DOI] [PubMed] [Google Scholar]
- 86.Odamaki T., Kato K., Sugahara H., Hashikura N., Takahashi S., Xiao J.Z., et al. Age-related changes in gut microbiota composition from newborn to centenarian: a cross-sectional study. BMC Microbiol. 2016;16:90. doi: 10.1186/s12866-016-0708-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Xu C., Zhu H., Qiu P. Aging progression of human gut microbiota. BMC Microbiol. 2019;19:236. doi: 10.1186/s12866-019-1616-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Song D., Yang C.S., Zhang X., Wang Y. The relationship between host circadian rhythms and intestinal microbiota: a new cue to improve health by tea polyphenols. Crit Rev Food Sci Nutr. 2021;61:139–148. doi: 10.1080/10408398.2020.1719473. [DOI] [PubMed] [Google Scholar]
- 89.Anand R., Song Y., Garg S., Girotra M., Sinha A., Sivaraman A., et al. Effect of aging on the composition of fecal microbiota in donors for FMT and its impact on clinical outcomes. Dig Dis Sci. 2017;62:1002–1008. doi: 10.1007/s10620-017-4449-6. [DOI] [PubMed] [Google Scholar]
- 90.Hoffman J.D., Parikh I., Green S.J., Chlipala G., Mohney R.P., Keaton M., et al. Age drives distortion of brain metabolic, vascular and cognitive functions, and the gut microbiome. Front Aging Neurosci. 2017;9:298. doi: 10.3389/fnagi.2017.00298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Biagi E., Nylund L., Candela M., Ostan R., Bucci L., Pini E., et al. Through ageing, and beyond: gut microbiota and inflammatory status in seniors and centenarians. PLoS One. 2010;5 doi: 10.1371/journal.pone.0010667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.GBD 2017 Causes of Death Collaborators Global, regional, and national age-sex-specific mortality for 282 causes of death in 195 countries and territories, 1980-2017: a systematic analysis for the global burden of disease study 2017. Lancet (London, England) 2018;392:1736–1788. doi: 10.1016/S0140-6736(18)32203-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Sorboni S.G., Moghaddam H.S., Jafarzadeh-Esfehani R., Soleimanpour S. A comprehensive review on the role of the gut microbiome in human neurological disorders. Clin Microbiol Rev. 2022;35 doi: 10.1128/CMR.00338-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kumar D.K., Choi S.H., Washicosky K.J., Eimer W.A., Tucker S., Ghofrani J., et al. Amyloid-β peptide protects against microbial infection in mouse and worm models of Alzheimer's disease. Sci Transl Med. 2016;8:340ra72. doi: 10.1126/scitranslmed.aaf1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Wu S.C., Cao Z.S., Chang K.M., Juang J.L. Intestinal microbial dysbiosis aggravates the progression of Alzheimer's disease in Drosophila. Nat Commun. 2017;8:24. doi: 10.1038/s41467-017-00040-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Chen H., Zhao E.J., Zhang W., Lu Y., Liu R., Huang X., et al. Meta-analyses on prevalence of selected Parkinson's nonmotor symptoms before and after diagnosis. Transl Neurodegener. 2015;4:1. doi: 10.1186/2047-9158-4-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Unger M.M., Spiegel J., Dillmann K.U., Grundmann D., Philippeit H., Bürmann J., et al. Short chain fatty acids and gut microbiota differ between patients with Parkinson's disease and age-matched controls. Parkinsonism Relat Disorders. 2016;32:66–72. doi: 10.1016/j.parkreldis.2016.08.019. [DOI] [PubMed] [Google Scholar]
- 98.Houlden A., Goldrick M., Brough D., Vizi E.S., Lénárt N., Martinecz B., et al. Brain injury induces specific changes in the caecal microbiota of mice via altered autonomic activity and mucoprotein production. Brain Behav Immun. 2016;57:10–20. doi: 10.1016/j.bbi.2016.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Benakis C., Brea D., Caballero S., Faraco G., Moore J., Murphy M., et al. Commensal microbiota affects ischemic stroke outcome by regulating intestinal γδ T cells. Nat Med. 2016;22:516–523. doi: 10.1038/nm.4068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Shahi S.K., Freedman S.N., Mangalam A.K. Gut microbiome in multiple sclerosis: the players involved and the roles they play. Gut Microb. 2017;8:607–615. doi: 10.1080/19490976.2017.1349041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Schroeder B.O., Bäckhed F. Signals from the gut microbiota to distant organs in physiology and disease. Nat Med. 2016;22:1079–1089. doi: 10.1038/nm.4185. [DOI] [PubMed] [Google Scholar]
- 102.Bosco N., Noti M. The aging gut microbiome and its impact on host immunity. Gene Immun. 2021;22:289–303. doi: 10.1038/s41435-021-00126-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Bi M., Feng L., He J., Liu C., Wang Y., Jiang H., et al. Emerging insights between gut microbiome dysbiosis and Parkinson's disease: pathogenic and clinical relevance. Ageing Res Rev. 2022;82 doi: 10.1016/j.arr.2022.101759. [DOI] [PubMed] [Google Scholar]
- 104.Kidd S.K., Schneider J.S. Protection of dopaminergic cells from MPP+-mediated toxicity by histone deacetylase inhibition. Brain Res. 2010;1354:172–178. doi: 10.1016/j.brainres.2010.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Chen P.S., Wang C.C., Bortner C.D., Peng G.S., Wu X., Pang H., et al. Valproic acid and other histone deacetylase inhibitors induce microglial apoptosis and attenuate lipopolysaccharide-induced dopaminergic neurotoxicity. Neuroscience. 2007;149:203–212. doi: 10.1016/j.neuroscience.2007.06.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Artiushin G., Zhang S.L., Tricoire H., Sehgal A. Endocytosis at the Drosophila blood–brain barrier as a function for sleep. Elife. 2018;7 doi: 10.7554/eLife.43326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Cuddapah V.A., Zhang S.L., Sehgal A. Regulation of the blood–brain barrier by circadian rhythms and sleep. Trends Neurosci. 2019;42:500–510. doi: 10.1016/j.tins.2019.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Zhang S.L., Yue Z., Arnold D.M., Artiushin G., Sehgal A. A circadian clock in the blood–brain barrier regulates xenobiotic efflux. Cell. 2018;173:130–139. doi: 10.1016/j.cell.2018.02.017. e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Zhang S.L., Lahens N.F., Yue Z., Arnold D.M., Pakstis P.P., Schwarz J.E., et al. A circadian clock regulates efflux by the blood–brain barrier in mice and human cells. Nat Commun. 2021;12:617. doi: 10.1038/s41467-020-20795-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Srinivasan M., Walker C. Circadian clock, glucocorticoids and NF-kappaB signaling in neuroinflammation-implicating glucocorticoid induced leucine Zipper as a molecular link. ASN Neuro. 2022;14 doi: 10.1177/17590914221120190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Hurtado-Alvarado G., Domínguez-Salazar E., Pavon L., Velázquez-Moctezuma J., Gómez-González B. Blood–brain barrier disruption induced by chronic sleep loss: low-grade inflammation may be the link. J Immunol Res. 2016;2016 doi: 10.1155/2016/4576012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Hurtado-Alvarado G., Becerril-Villanueva E., Contis-Montes de Oca A., Domínguez-Salazar E., Salinas-Jazmín N., Pérez-Tapia S.M., et al. The yin/yang of inflammatory status: blood–brain barrier regulation during sleep. Brain Behav Immun. 2018;69:154–166. doi: 10.1016/j.bbi.2017.11.009. [DOI] [PubMed] [Google Scholar]
- 113.Pan W., Kastin A.J. The blood–brain barrier: regulatory roles in wakefulness and sleep. Neuroscientist. 2017;23:124–136. doi: 10.1177/1073858416639005. [DOI] [PubMed] [Google Scholar]
- 114.He J., Hsuchou H., He Y., Kastin A.J., Mishra P.K., Fang J., et al. Leukocyte infiltration across the blood-spinal cord barrier is modulated by sleep fragmentation in mice with experimental autoimmune encephalomyelitis. Fluids Barriers CNS. 2014;11:27. doi: 10.1186/2045-8118-11-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Nakazato R., Kawabe K., Yamada D., Ikeno S., Mieda M., Shimba S., et al. Disruption of bmal1 impairs blood–brain barrier integrity via pericyte dysfunction. J Neurosci. 2017;37:10052–10062. doi: 10.1523/JNEUROSCI.3639-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Kyoko O.O., Kono H., Ishimaru K., Miyake K., Kubota T., Ogawa H., et al. Expressions of tight junction proteins Occludin and Claudin-1 are under the circadian control in the mouse large intestine: implications in intestinal permeability and susceptibility to colitis. PLoS One. 2014;9 doi: 10.1371/journal.pone.0098016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.He J., Hsuchou H., He Y., Kastin A.J., Wang Y., Pan W. Sleep restriction impairs blood–brain barrier function. J Neurosci. 2014;34:14697–14706. doi: 10.1523/JNEUROSCI.2111-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Opp M.R., George A., Ringgold K.M., Hansen K.M., Bullock K.M., Banks W.A. Sleep fragmentation and sepsis differentially impact blood–brain barrier integrity and transport of tumor necrosis factor-α in aging. Brain Behav Immun. 2015;50:259–265. doi: 10.1016/j.bbi.2015.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Borjigin J., Zhang L.S., Calinescu A.A. Circadian regulation of pineal gland rhythmicity. Mol Cell Endocrinol. 2012;349:13–19. doi: 10.1016/j.mce.2011.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Wang X., Xue G.X., Liu W.C., Shu H., Wang M., Sun Y., et al. Melatonin alleviates lipopolysaccharide-compromised integrity of blood–brain barrier through activating AMP-activated protein kinase in old mice. Aging Cell. 2017;16:414–421. doi: 10.1111/acel.12572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Song J., Whitcomb D.J., Kim B.C. The role of melatonin in the onset and progression of type 3 diabetes. Mol Brain. 2017;10:35. doi: 10.1186/s13041-017-0315-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Xie L., Kang H., Xu Q., Chen M.J., Liao Y., Thiyagarajan M., et al. Sleep drives metabolite clearance from the adult brain. Science. 2013;342:373–377. doi: 10.1126/science.1241224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Bien-Ly N., Watts R.J. The blood–brain barrier's gut check. Sci Transl Med. 2014;6:263fs46. doi: 10.1126/scitranslmed.aaa2543. [DOI] [PubMed] [Google Scholar]
- 124.Hu L., Zhu S., Peng X., Li K., Peng W., Zhong Y., et al. High salt elicits brain inflammation and cognitive dysfunction, accompanied by alternations in the gut microbiota and decreased SCFA production. J Alzheimers Dis. 2020;77:629–640. doi: 10.3233/JAD-200035. [DOI] [PubMed] [Google Scholar]
- 125.Leclercq S., Mian F.M., Stanisz A.M., Bindels L.B., Cambier E., Ben-Amram H., et al. Low-dose penicillin in early life induces long-term changes in murine gut microbiota, brain cytokines and behavior. Nat Commun. 2017;8 doi: 10.1038/ncomms15062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Liu S., Gao J., Liu K., Zhang H.L. Microbiota–gut–brain axis and Alzheimer's disease: implications of the blood–brain barrier as an intervention target. Mech Ageing Dev. 2021;199 doi: 10.1016/j.mad.2021.111560. [DOI] [PubMed] [Google Scholar]
- 127.Sampson T.R., Debelius J.W., Thron T., Janssen S., Shastri G.G., Ilhan Z.E., et al. Gut microbiota regulate motor deficits and neuroinflammation in a model of Parkinson's disease. Cell. 2016;167 doi: 10.1016/j.cell.2016.11.018. 1469-80.e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Masuda H., Mori M., Uchida T., Uzawa A., Ohtani R., Kuwabara S. Soluble CD40 ligand contributes to blood–brain barrier breakdown and central nervous system inflammation in multiple sclerosis and neuromyelitis optica spectrum disorder. J Neuroimmunol. 2017;305:102–107. doi: 10.1016/j.jneuroim.2017.01.024. [DOI] [PubMed] [Google Scholar]
- 129.Logsdon A.F., Erickson M.A., Rhea E.M., Salameh T.S., Banks W.A. Gut reactions: how the blood–brain barrier connects the microbiome and the brain. Exp Biol Med. 2018;243:159–165. doi: 10.1177/1535370217743766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Knox E.G., Aburto M.R., Tessier C., Nagpal J., Clarke G., O'Driscoll C.M., et al. Microbial-derived metabolites induce actin cytoskeletal rearrangement and protect blood–brain barrier function. iScience. 2022;25 doi: 10.1016/j.isci.2022.105648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Mao Z., Liu W., Huang Y., Sun T., Bao K., Feng J., et al. Anti-aging effects of chlorpropamide depend on mitochondrial complex-II and the production of mitochondrial reactive oxygen species. Acta Pharm Sin B. 2022;12:665–677. doi: 10.1016/j.apsb.2021.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Nelson J.W., Phillips S.C., Ganesh B.P., Petrosino J.F., Durgan D.J., Bryan R.M. The gut microbiome contributes to blood–brain barrier disruption in spontaneously hypertensive stroke prone rats. FASEB J. 2021;35 doi: 10.1096/fj.202001117R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Wu X.N., Zhou Q., Huang Y.D., Xie X., Li Z., Wu Y., et al. Structure-based discovery of orally efficient inhibitors via unique interactions with H-pocket of PDE8 for the treatment of vascular dementia. Acta Pharm Sin B. 2022;12:3103–3112. doi: 10.1016/j.apsb.2022.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Wang H., Zhang M., Li J., Liang J., Yang M., Xia G., et al. Gut microbiota is causally associated with poststroke cognitive impairment through lipopolysaccharide and butyrate. J Neuroinflammation. 2022;19:76. doi: 10.1186/s12974-022-02435-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Chen R., Wu P., Cai Z., Fang Y., Zhou H., Lasanajak Y., et al. Puerariae Lobatae Radix with chuanxiong rhizoma for treatment of cerebral ischemic stroke by remodeling gut microbiota to regulate the brain–gut barriers. J Nutr Biochem. 2019;65:101–114. doi: 10.1016/j.jnutbio.2018.12.004. [DOI] [PubMed] [Google Scholar]
- 136.Wen Q., Ding Q., Wang J., Yin Y., Xu S., Ju Y., et al. Cortistatin-14 exerts neuroprotective effect against microglial activation, blood–brain barrier disruption, and cognitive impairment in sepsis-associated encephalopathy. J Immunol Res. 2022;2022 doi: 10.1155/2022/3334145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Chelluboina B., Kieft K., Breister A., Anantharaman K., Vemuganti R. Gut virome dysbiosis following focal cerebral ischemia in mice. J Cerebr Blood Flow Metabol. 2022;42:1597–1602. doi: 10.1177/0271678X221107702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Miranda-Ribera A., Serena G., Liu J., Fasano A., Kingsbury M.A., Fiorentino M.R. The Zonulin-transgenic mouse displays behavioral alterations ameliorated via depletion of the gut microbiota. Tissue Barriers. 2022;10 doi: 10.1080/21688370.2021.2000299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Mou Y., Du Y., Zhou L., Yue J., Hu X., Liu Y., et al. Gut microbiota interact with the brain through systemic chronic inflammation: implications on neuroinflammation, neurodegeneration, and aging. Front Immunol. 2022;13 doi: 10.3389/fimmu.2022.796288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Tang W., Zhu H., Feng Y., Guo R., Wan D. The impact of gut microbiota disorders on the blood–brain barrier. Infect Drug Resist. 2020;13:3351–3363. doi: 10.2147/IDR.S254403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Senatorov V.V., Jr., Friedman A.R., Milikovsky D.Z., Ofer J., Saar-Ashkenazy R., Charbash A., et al. Blood–brain barrier dysfunction in aging induces hyperactivation of TGFbeta signaling and chronic yet reversible neural dysfunction. Sci Transl Med. 2019;11 doi: 10.1126/scitranslmed.aaw8283. [DOI] [PubMed] [Google Scholar]
- 142.Leone V.A., Onishi K.G., Kennedy M., Riggle J.P., Pierre J.F., Maneval A.C., et al. Atypical behavioral and thermoregulatory circadian rhythms in mice lacking a microbiome. Sci Rep. 2022;12 doi: 10.1038/s41598-022-18291-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Thaiss C.A., Zeevi D., Levy M., Zilberman-Schapira G., Suez J., Tengeler A.C., et al. Transkingdom control of microbiota diurnal oscillations promotes metabolic homeostasis. Cell. 2014;159:514–529. doi: 10.1016/j.cell.2014.09.048. [DOI] [PubMed] [Google Scholar]
- 144.Tahara Y., Yamazaki M., Sukigara H., Motohashi H., Sasaki H., Miyakawa H., et al. Gut microbiota-derived short chain fatty acids induce circadian clock entrainment in mouse peripheral tissue. Sci Rep. 2018;8:1395. doi: 10.1038/s41598-018-19836-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Wang Y., Kuang Z., Yu X., Ruhn K.A., Kubo M., Hooper L.V. The intestinal microbiota regulates body composition through NFIL3 and the circadian clock. Science. 2017;357:912–916. doi: 10.1126/science.aan0677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Kohsaka A., Laposky A.D., Ramsey K.M., Estrada C., Joshu C., Kobayashi Y., et al. High-fat diet disrupts behavioral and molecular circadian rhythms in mice. Cell Metabol. 2007;6:414–421. doi: 10.1016/j.cmet.2007.09.006. [DOI] [PubMed] [Google Scholar]
- 147.Parkar S.G., Kalsbeek A., Cheeseman J.F. Potential role for the gut microbiota in modulating host circadian rhythms and metabolic health. Microorganisms. 2019;7:41. doi: 10.3390/microorganisms7020041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Honma K., Hikosaka M., Mochizuki K., Goda T. Loss of circadian rhythm of circulating insulin concentration induced by high-fat diet intake is associated with disrupted rhythmic expression of circadian clock genes in the liver. Metabolism. 2016;65:482–491. doi: 10.1016/j.metabol.2015.12.003. [DOI] [PubMed] [Google Scholar]
- 149.Martchenko S.E., Prescott D., Martchenko A., Sweeney M.E., Philpott D.J., Brubaker P.L. Diurnal changes in the murine small intestine are disrupted by obesogenic western diet feeding and microbial dysbiosis. Sci Rep. 2021;11 doi: 10.1038/s41598-021-98986-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Cheng W.Y., Lam K.L., Li X., Kong A.P., Cheung P.C. Circadian disruption-induced metabolic syndrome in mice is ameliorated by oat β-glucan mediated by gut microbiota. Carbohydr Polym. 2021;267 doi: 10.1016/j.carbpol.2021.118216. [DOI] [PubMed] [Google Scholar]
- 151.Miyazaki K., Itoh N., Yamamoto S., Higo-Yamamoto S., Nakakita Y., Kaneda H., et al. Dietary heat-killed Lactobacillus brevis SBC8803 promotes voluntary wheel-running and affects sleep rhythms in mice. Life Sci. 2014;111:47–52. doi: 10.1016/j.lfs.2014.07.009. [DOI] [PubMed] [Google Scholar]
- 152.Nishida K., Sawada D., Kuwano Y., Tanaka H., Rokutan K. Health benefits of lactobacillus gasseri CP2305 tablets in young adults exposed to chronic stress: a randomized, double-blind, placebo-controlled study. Nutrients. 2019;11:1859. doi: 10.3390/nu11081859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Thompson R.S., Roller R., Mika A., Greenwood B.N., Knight R., Chichlowski M., et al. Dietary prebiotics and bioactive milk fractions improve NREM sleep, enhance REM sleep rebound and attenuate the stress-induced decrease in diurnal temperature and gut microbial alpha diversity. Front Behav Neurosci. 2016;10:240. doi: 10.3389/fnbeh.2016.00240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Gao T., Wang Z., Cao J., Dong Y., Chen Y. Melatonin attenuates microbiota dysbiosis of jejunum in short-term sleep deprived mice. J Microbiol. 2020;58:588–597. doi: 10.1007/s12275-020-0094-4. [DOI] [PubMed] [Google Scholar]
- 155.Park Y.S., Kim S.H., Park J.W., Kho Y., Seok P.R., Shin J.H., et al. Melatonin in the colon modulates intestinal microbiota in response to stress and sleep deprivation. Int Res. 2020;18:325–336. doi: 10.5217/ir.2019.00093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Akbari E., Asemi Z., Daneshvar Kakhaki R., Bahmani F., Kouchaki E., Tamtaji O.R., et al. Effect of probiotic supplementation on cognitive function and metabolic status in Alzheimer's disease: a randomized, double-blind and controlled trial. Front Aging Neurosci. 2016;8:256. doi: 10.3389/fnagi.2016.00256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Abbruscato T.J., Davis T.P. Protein expression of brain endothelial cell E-cadherin after hypoxia/aglycemia: influence of astrocyte contact. Brain Res. 1999;842:277–286. doi: 10.1016/s0006-8993(99)01778-3. [DOI] [PubMed] [Google Scholar]
- 158.Tian Y., Yang W., Chen G., Men C., Gu Y., Song X., et al. An important link between the gut microbiota and the circadian rhythm: imply for treatments of circadian rhythm sleep disorder. Food Sci Biotechnol. 2022;31:155–164. doi: 10.1007/s10068-021-01015-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Wang H., Zhang H., Su Y. New insights into the diurnal rhythmicity of gut microbiota and its crosstalk with host circadian rhythm. Animals. 2022;12:1677. doi: 10.3390/ani12131677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Bishehsari F., Voigt R.M., Keshavarzian A. Circadian rhythms and the gut microbiota: from the metabolic syndrome to cancer. Nat Rev Endocrinol. 2020;16:731–739. doi: 10.1038/s41574-020-00427-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Leone V., Gibbons S.M., Martinez K., Hutchison A.L., Huang E.Y., Cham C.M., et al. Effects of diurnal variation of gut microbes and high-fat feeding on host circadian clock function and metabolism. Cell Host Microbe. 2015;17:681–689. doi: 10.1016/j.chom.2015.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Pearson J.A., Wong F.S., Wen L. Crosstalk between circadian rhythms and the microbiota. Immunology. 2020;161:278–290. doi: 10.1111/imm.13278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Zhen Y., Ge L., Xu Q., Hu L., Wei W., Huang J., et al. Normal light-dark and short-light cycles regulate intestinal inflammation, circulating short-chain fatty acids and gut microbiota in period 2 gene knockout mice. Front Immunol. 2022;13 doi: 10.3389/fimmu.2022.848248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Altaha B., Heddes M., Pilorz V., Niu Y., Gorbunova E., Gigl M., et al. Genetic and environmental circadian disruption induce weight gain through changes in the gut microbiome. Mol Metabol. 2022;66 doi: 10.1016/j.molmet.2022.101628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Zhang S.L., Bai L., Goel N., Bailey A., Jang C.J., Bushman F.D., et al. Human and rat gut microbiome composition is maintained following sleep restriction. Proc Natl Acad Sci U S A. 2017;114:E1564–E1571. doi: 10.1073/pnas.1620673114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Anderson J.R., Carroll I., Azcarate-Peril M.A., Rochette A.D., Heinberg L.J., Peat C., et al. A preliminary examination of gut microbiota, sleep, and cognitive flexibility in healthy older adults. Sleep Med. 2017;38:104–107. doi: 10.1016/j.sleep.2017.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Poroyko V.A., Carreras A., Khalyfa A., Khalyfa A.A., Leone V., Peris E., et al. Chronic sleep disruption alters gut microbiota, induces systemic and adipose tissue inflammation and insulin resistance in mice. Sci Rep. 2016;6 doi: 10.1038/srep35405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Benedict C., Vogel H., Jonas W., Woting A., Blaut M., Schurmann A., et al. Gut microbiota and glucometabolic alterations in response to recurrent partial sleep deprivation in normal-weight young individuals. Mol Metabol. 2016;5:1175–1186. doi: 10.1016/j.molmet.2016.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Voigt R.M., Forsyth C.B., Green S.J., Mutlu E., Engen P., Vitaterna M.H., et al. Circadian disorganization alters intestinal microbiota. PLoS One. 2014;9 doi: 10.1371/journal.pone.0097500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Lee C.C., Liang F., Lee I.C., Lu T.H., Shan Y.Y., Jeng C.F., et al. External light-dark cycle shapes gut microbiota through intrinsically photosensitive retinal ganglion cells. EMBO Rep. 2022;23 doi: 10.15252/embr.202052316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Sarmiento J., Pulgar R., Mandakovic D., Porras O., Flores C.A., Luco D., et al. Nocturnal light pollution induces weight gain in mice and reshapes the structure, functions, and interactions of their colonic microbiota. Int J Mol Sci. 2022;23:1673. doi: 10.3390/ijms23031673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Thaiss C.A., Levy M., Korem T., Dohnalová L., Shapiro H., Jaitin D.A., et al. Microbiota diurnal rhythmicity programs host transcriptome oscillations. Cell. 2016;167 doi: 10.1016/j.cell.2016.11.003. [DOI] [PubMed] [Google Scholar]
- 173.Cui Y., Yin Y., Li S., Wu Z., Xie Y., Qian Q., et al. Apple polyphenol extract modulates bile acid metabolism and gut microbiota by regulating the circadian rhythms in daytime-restricted high fat diet feeding C57BL/6 male mice. Food Funct. 2022;13:2805–2822. doi: 10.1039/d1fo04116a. [DOI] [PubMed] [Google Scholar]
- 174.Brunt V.E., LaRocca T.J., Bazzoni A.E., Sapinsley Z.J., Miyamoto-Ditmon J., Gioscia-Ryan R.A., et al. The gut microbiome-derived metabolite trimethylamine N-oxide modulates neuroinflammation and cognitive function with aging. Geroscience. 2021;43:377–394. doi: 10.1007/s11357-020-00257-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Govindarajan K., MacSharry J., Casey P.G., Shanahan F., Joyce S.A., Gahan C.G. Unconjugated bile acids influence expression of circadian genes: a potential mechanism for microbe-host crosstalk. PLoS One. 2016;11 doi: 10.1371/journal.pone.0167319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Hoyles L., Snelling T., Umlai U.K., Nicholson J.K., Carding S.R., Glen R.C., et al. Microbiome-host systems interactions: protective effects of propionate upon the blood–brain barrier. Microbiome. 2018;6:55. doi: 10.1186/s40168-018-0439-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Wen J., Ding Y., Wang L., Xiao Y. Gut microbiome improves postoperative cognitive function by decreasing permeability of the blood–brain barrier in aged mice. Brain Res Bull. 2020;164:249–256. doi: 10.1016/j.brainresbull.2020.08.017. [DOI] [PubMed] [Google Scholar]
- 178.Ahmed H., Leyrolle Q., Koistinen V., Karkkainen O., Laye S., Delzenne N., et al. Microbiota-derived metabolites as drivers of gut–brain communication. Gut Microb. 2022;14 doi: 10.1080/19490976.2022.2102878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Fock E., Parnova R. Mechanisms of blood–brain barrier protection by microbiota-derived short-chain fatty acids. Cells. 2023;12:657. doi: 10.3390/cells12040657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Stachulski A.V., Knausenberger T.B., Shah S.N., Hoyles L., McArthur S. A host-gut microbial amino acid co-metabolite, p-cresol glucuronide, promotes blood–brain barrier integrity in vivo. Tissue Barriers. 2023;11 doi: 10.1080/21688370.2022.2073175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Hoyles L., Pontifex M.G., Rodriguez-Ramiro I., Anis-Alavi M.A., Jelane K.S., Snelling T., et al. Regulation of blood–brain barrier integrity by microbiome-associated methylamines and cognition by trimethylamine N-oxide. Microbiome. 2021;9:235. doi: 10.1186/s40168-021-01181-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Wang T., Rong X., Zhao C. Circadian rhythms coordinated with gut microbiota partially account for individual differences in hepatitis B-related cirrhosis. Front Cell Infect Microbiol. 2022;12 doi: 10.3389/fcimb.2022.936815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Quinn M., McMillin M., Galindo C., Frampton G., Pae H.Y., DeMorrow S. Bile acids permeabilize the blood brain barrier after bile duct ligation in rats via Rac1-dependent mechanisms. Dig Liver Dis. 2014;46:527–534. doi: 10.1016/j.dld.2014.01.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Yano J.M., Yu K., Donaldson G.P., Shastri G.G., Ann P., Ma L., et al. Indigenous bacteria from the gut microbiota regulate host serotonin biosynthesis. Cell. 2015;161:264–276. doi: 10.1016/j.cell.2015.02.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Palmela I., Correia L., Silva R.F., Sasaki H., Kim K.S., Brites D., et al. Hydrophilic bile acids protect human blood–brain barrier endothelial cells from disruption by unconjugated bilirubin: an in vitro study. Front Neurosci. 2015;9:80. doi: 10.3389/fnins.2015.00080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Guan B., Tong J., Hao H., Yang Z., Chen K., Xu H., et al. Bile acid coordinates microbiota homeostasis and systemic immunometabolism in cardiometabolic diseases. Acta Pharm Sin B. 2022;12:2129–2149. doi: 10.1016/j.apsb.2021.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Zhang Z., Peng Q., Huo D., Jiang S., Ma C., Chang H., et al. Melatonin regulates the neurotransmitter secretion disorder induced by caffeine through the microbiota–gut–brain axis in zebrafish (Danio rerio) Front Cell Dev Biol. 2021;9 doi: 10.3389/fcell.2021.678190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Alkasir R., Li J., Li X., Jin M., Zhu B. Human gut microbiota: the links with dementia development. Protein Cell. 2017;8:90–102. doi: 10.1007/s13238-016-0338-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Strandwitz P. Neurotransmitter modulation by the gut microbiota. Brain Res. 2018;1693:128–133. doi: 10.1016/j.brainres.2018.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Chen Y., Xu J., Chen Y. Regulation of neurotransmitters by the gut microbiota and effects on cognition in neurological disorders. Nutrients. 2021;13:2099. doi: 10.3390/nu13062099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Kim R., Witelski T.P. Uncovering the dynamics of a circadian-dopamine model influenced by the light-dark cycle. Math Biosci. 2022;344 doi: 10.1016/j.mbs.2021.108764. [DOI] [PubMed] [Google Scholar]
- 192.Hood S., Cassidy P., Cossette M.P., Weigl Y., Verwey M., Robinson B., et al. Endogenous dopamine regulates the rhythm of expression of the clock protein PER2 in the rat dorsal striatum via daily activation of D2 dopamine receptors. J Neurosci. 2010;30:14046–14058. doi: 10.1523/JNEUROSCI.2128-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Wang Y., Wang X., Zhang X., Chen S., Sun Y., Liu W., et al. D1 receptor-mediated endogenous tPA upregulation contributes to blood–brain barrier injury after acute ischaemic stroke. J Cell Mol Med. 2020;24:9255–9266. doi: 10.1111/jcmm.15570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.You L., Jiang H. Cabergoline possesses a beneficial effect on blood–brain barrier (BBB) integrity against lipopolysaccharide (LPS) Bioengineered. 2021;12:8358–8369. doi: 10.1080/21655979.2021.1987066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Chen H., Nwe P.K., Yang Y., Rosen C.E., Bielecka A.A., Kuchroo M., et al. A forward chemical genetic screen reveals gut microbiota metabolites that modulate host physiology. Cell. 2019;177:1217–1231.e18. doi: 10.1016/j.cell.2019.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Reigstad C.S., Salmonson C.E., Rainey J.F., 3rd, Szurszewski J.H., Linden D.R., Sonnenburg J.L., et al. Gut microbes promote colonic serotonin production through an effect of short-chain fatty acids on enterochromaffin cells. Faseb J. 2015;29:1395–1403. doi: 10.1096/fj.14-259598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Csaszar-Nagy N., Bob P., Bokkon I. A Multidisciplinary hypothesis about serotonergic psychedelics. Is it possible that a portion of brain serotonin comes from the gut?. J Integr Neurosci. 2022;21:148. doi: 10.31083/j.jin2105148. [DOI] [PubMed] [Google Scholar]
- 198.Gao K., Pi Y., Mu C.L., Farzi A., Liu Z., Zhu W.Y. Increasing carbohydrate availability in the hindgut promotes hypothalamic neurotransmitter synthesis: aromatic amino acids linking the microbiota–brain axis. J Neurochem. 2019;149:641–659. doi: 10.1111/jnc.14709. [DOI] [PubMed] [Google Scholar]
- 199.Zhang Z.W., Gao C.S., Zhang H., Yang J., Wang Y.P., Pan L.B., et al. Morinda officinalis oligosaccharides increase serotonin in the brain and ameliorate depression via promoting 5-hydroxytryptophan production in the gut microbiota. Acta Pharm Sin B. 2022;12:3298–3312. doi: 10.1016/j.apsb.2022.02.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Musumeci G., Castrogiovanni P., Castorina S., Imbesi R., Szychlinska M.A., Scuderi S., et al. Changes in serotonin (5-HT) and brain-derived neurotrophic factor (BDFN) expression in frontal cortex and hippocampus of aged rat treated with high tryptophan diet. Brain Res Bull. 2015;119:12–18. doi: 10.1016/j.brainresbull.2015.09.010. [DOI] [PubMed] [Google Scholar]
- 201.Ranuh R., Athiyyah A.F., Darma A., Risky V.P., Riawan W., Surono I.S., et al. Effect of the probiotic Lactobacillus plantarum IS-10506 on BDNF and 5HT stimulation: role of intestinal microbiota on the gut–brain axis. Iran J Microbiol. 2019;11:145–150. [PMC free article] [PubMed] [Google Scholar]
- 202.Yang X., Yu D., Xue L., Li H., Du J. Probiotics modulate the microbiota–gut–brain axis and improve memory deficits in aged SAMP8 mice. Acta Pharm Sin B. 2020;10:475–487. doi: 10.1016/j.apsb.2019.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Kotagal V., Spino C., Bohnen N.I., Koeppe R., Albin R.L. Serotonin, beta-amyloid, and cognition in Parkinson disease. Ann Neurol. 2018;83:994–1002. doi: 10.1002/ana.25236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Hashim H.M., Makpol S. A review of the preclinical and clinical studies on the role of the gut microbiome in aging and neurodegenerative diseases and its modulation. Front Cell Neurosci. 2022;16 doi: 10.3389/fncel.2022.1007166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Strandwitz P., Kim K.H., Terekhova D., Liu J.K., Sharma A., Levering J., et al. GABA-modulating bacteria of the human gut microbiota. Nat Microbiol. 2019;4:396–403. doi: 10.1038/s41564-018-0307-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Barrett E., Ross R.P., O'Toole P.W., Fitzgerald G.F., Stanton C. gamma-Aminobutyric acid production by culturable bacteria from the human intestine. J Appl Microbiol. 2012;113:411–417. doi: 10.1111/j.1365-2672.2012.05344.x. [DOI] [PubMed] [Google Scholar]
- 207.Huo R., Zeng B., Zeng L., Cheng K., Li B., Luo Y., et al. Microbiota modulate anxiety-like behavior and endocrine abnormalities in hypothalamic-pituitary-adrenal axis. Front Cell Infect Microbiol. 2017;7:489. doi: 10.3389/fcimb.2017.00489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Agusti A., Moya-Pérez A., Campillo I., Montserrat-de la Paz S., Cerrudo V., Perez-Villalba A., et al. Bifidobacterium pseudocatenulatum CECT 7765 ameliorates neuroendocrine alterations associated with an exaggerated stress response and anhedonia in obese mice. Mol Neurobiol. 2018;55:5337–5352. doi: 10.1007/s12035-017-0768-z. [DOI] [PubMed] [Google Scholar]
- 209.Sollars P.J., Weiser M.J., Kudwa A.E., Bramley J.R., Ogilvie M.D., Spencer R.L., et al. Altered entrainment to the day/night cycle attenuates the daily rise in circulating corticosterone in the mouse. PLoS One. 2014;9 doi: 10.1371/journal.pone.0111944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Guo M., Wang H., Xu S., Zhuang Y., An J., Su C., et al. Alteration in gut microbiota is associated with dysregulation of cytokines and glucocorticoid therapy in systemic lupus erythematosus. Gut Microb. 2020;11:1758–1773. doi: 10.1080/19490976.2020.1768644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Anand N., Gorantla V.R., Chidambaram S.B. The role of gut dysbiosis in the pathophysiology of neuropsychiatric disorders. Cells. 2022;12:54. doi: 10.3390/cells12010054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Na S.Y., Janakiraman M., Leliavski A., Krishnamoorthy G. High-salt diet suppresses autoimmune demyelination by regulating the blood–brain barrier permeability. Proc Natl Acad Sci U S A. 2021;118 doi: 10.1073/pnas.2025944118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Hue C.D., Cho F.S., Cao S., Dale Bass C.R., Meaney D.F., Morrison B., 3rd Dexamethasone potentiates in vitro blood–brain barrier recovery after primary blast injury by glucocorticoid receptor-mediated upregulation of ZO-1 tight junction protein. J Cerebr Blood Flow Metabol. 2015;35:1191–1198. doi: 10.1038/jcbfm.2015.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Roh J.H., Jiang H., Finn M.B., Stewart F.R., Mahan T.E., Cirrito J.R., et al. Potential role of orexin and sleep modulation in the pathogenesis of Alzheimer's disease. J Exp Med. 2014;211:2487–2496. doi: 10.1084/jem.20141788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Bonaz B., Bazin T., Pellissier S. The Vagus nerve at the interface of the microbiota–gut–brain axis. Front Neurosci. 2018;12:49. doi: 10.3389/fnins.2018.00049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Forsythe P., Bienenstock J., Kunze W.A. Vagal pathways for microbiome–brain–gut axis communication. Adv Exp Med Biol. 2014;817:115–133. doi: 10.1007/978-1-4939-0897-4_5. [DOI] [PubMed] [Google Scholar]
- 217.Yang Y., Yang L.Y., Orban L., Cuylear D., Thompson J., Simon B., et al. Non-invasive vagus nerve stimulation reduces blood–brain barrier disruption in a rat model of ischemic stroke. Brain Stimul. 2018;11:689–698. doi: 10.1016/j.brs.2018.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Lee K.E., Kim J.K., Han S.K., Lee D.Y., Lee H.J., Yim S.V., et al. The extracellular vesicle of gut microbial Paenalcaligenes hominis is a risk factor for vagus nerve-mediated cognitive impairment. Microbiome. 2020;8:107. doi: 10.1186/s40168-020-00881-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Ramsey A.M., Stowie A., Castanon-Cervantes O., Davidson A.J. Environmental circadian disruption increases stroke severity and dysregulates immune response. J Biol Rhythm. 2020;35:368–376. doi: 10.1177/0748730420929450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Narasimamurthy R., Hatori M., Nayak S.K., Liu F., Panda S., Verma I.M. Circadian clock protein cryptochrome regulates the expression of proinflammatory cytokines. Proc Natl Acad Sci U S A. 2012;109:12662–12667. doi: 10.1073/pnas.1209965109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Meng D., Yang M., Hu L., Liu T., Zhang H., Sun X., et al. Rifaximin protects against circadian rhythm disruption-induced cognitive impairment through preventing gut barrier damage and neuroinflammation. J Neurochem. 2022;163:406–418. doi: 10.1111/jnc.15701. [DOI] [PubMed] [Google Scholar]
- 222.Kronsteiner B., Bassaganya-Riera J., Philipson C., Viladomiu M., Carbo A., Abedi V., et al. Systems-wide analyses of mucosal immune responses to Helicobacter pylori at the interface between pathogenicity and symbiosis. Gut Microb. 2016;7:3–21. doi: 10.1080/19490976.2015.1116673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Krizbai I.A., Bauer H., Bresgen N., Eckl P.M., Farkas A., Szatmári E., et al. Effect of oxidative stress on the junctional proteins of cultured cerebral endothelial cells. Cell Mol Neurobiol. 2005;25:129–139. doi: 10.1007/s10571-004-1378-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Zhou Z., Li K., Chu Y., Li C., Zhang T., Liu P., et al. ROS-removing nano-medicine for navigating inflammatory microenvironment to enhance anti-epileptic therapy. Acta Pharm Sin B. 2023;13:1246–1261. doi: 10.1016/j.apsb.2022.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Heddes M., Altaha B., Niu Y., Reitmeier S., Kleigrewe K., Haller D., et al. The intestinal clock drives the microbiome to maintain gastrointestinal homeostasis. Nat Commun. 2022;13:6068. doi: 10.1038/s41467-022-33609-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Fan X., Mai C., Zuo L., Huang J., Xie C., Jiang Z., et al. Herbal formula BaWeiBaiDuSan alleviates polymicrobial sepsis-induced liver injury via increasing the gut microbiota Lactobacillus johnsonii and regulating macrophage anti-inflammatory activity in mice. Acta Pharm Sin B. 2023;13:1164–1179. doi: 10.1016/j.apsb.2022.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Parker A., Romano S., Ansorge R., Aboelnour A., Le Gall G., Savva G.M., et al. Fecal microbiota transfer between young and aged mice reverses hallmarks of the aging gut, eye, and brain. Microbiome. 2022;10:68. doi: 10.1186/s40168-022-01243-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Martel J., Chang S.H., Ko Y.F., Hwang T.L., Young J.D., Ojcius D.M. Gut barrier disruption and chronic disease. Trends Endocrinol Metabol. 2022;33:247–265. doi: 10.1016/j.tem.2022.01.002. [DOI] [PubMed] [Google Scholar]
- 229.Rothhammer V., Mascanfroni I.D., Bunse L., Takenaka M.C., Kenison J.E., Mayo L., et al. Type I interferons and microbial metabolites of tryptophan modulate astrocyte activity and central nervous system inflammation via the aryl hydrocarbon receptor. Nat Med. 2016;22:586–597. doi: 10.1038/nm.4106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Erny D., Hrabe de Angelis A.L., Jaitin D., Wieghofer P., Staszewski O., David E., et al. Host microbiota constantly control maturation and function of microglia in the CNS. Nat Neurosci. 2015;18:965–977. doi: 10.1038/nn.4030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Rom S., Zuluaga-Ramirez V., Reichenbach N.L., Erickson M.A., Winfield M., Gajghate S., et al. Secoisolariciresinol diglucoside is a blood–brain barrier protective and anti-inflammatory agent: implications for neuroinflammation. J Neuroinflammation. 2018;15:25. doi: 10.1186/s12974-018-1065-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.You M., Miao Z., Pan Y., Hu F. Trans-10-hydroxy-2-decenoic acid alleviates LPS-induced blood–brain barrier dysfunction by activating the AMPK/PI3K/AKT pathway. Eur J Pharmacol. 2019;865 doi: 10.1016/j.ejphar.2019.172736. [DOI] [PubMed] [Google Scholar]
- 233.Haghikia A., Jörg S., Duscha A., Berg J., Manzel A., Waschbisch A., et al. Dietary fatty acids directly impact central nervous system autoimmunity via the small intestine. Immunity. 2015;43:817–829. doi: 10.1016/j.immuni.2015.09.007. [DOI] [PubMed] [Google Scholar]
- 234.Sun Z., Liu Q., Lv Z., Li J., Xu X., Sun H., et al. Targeting macrophagic SHP2 for ameliorating osteoarthritis via TLR signaling. Acta Pharm Sin B. 2022;12:3073–3084. doi: 10.1016/j.apsb.2022.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Zhao Y., Jaber V., Lukiw W.J. Secretory products of the human GI tract microbiome and their potential impact on Alzheimer's disease (AD): detection of lipopolysaccharide (LPS) in AD hippocampus. Front Cell Infect Microbiol. 2017;7:318. doi: 10.3389/fcimb.2017.00318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Shihab P.K., Al-Roub A., Al-Ghanim M., Al-Mass A., Behbehani K., Ahmad R. TLR2 and AP-1/NF-kappaB are involved in the regulation of MMP-9 elicited by heat killed Listeria monocytogenes in human monocytic THP-1 cells. J Inflamm. 2015;12:32. doi: 10.1186/s12950-015-0077-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Zhao Z., Li F., Ning J., Peng R., Shang J., Liu H., et al. Novel compound FLZ alleviates rotenone-induced PD mouse model by suppressing TLR4/MyD88/NF-κB pathway through microbiota–gut–brain axis. Acta Pharm Sin B. 2021;11:2859–2879. doi: 10.1016/j.apsb.2021.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Stachulski A.V., Knausenberger T.B., Shah S.N., Hoyles L, McArthur S. A host-gut microbial amino acid co-metabolite, p-cresol glucuronide, promotes blood-brain barrier integrity in vivo. Tissue Barriers. 2023;11:2073175. doi: 10.1080/21688370.2022.2073175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Mayerhofer R., Fröhlich E.E., Reichmann F., Farzi A., Kogelnik N., Fröhlich E., et al. Diverse action of lipoteichoic acid and lipopolysaccharide on neuroinflammation, blood–brain barrier disruption, and anxiety in mice. Brain Behav Immun. 2017;60:174–187. doi: 10.1016/j.bbi.2016.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Monnet E., Lapeyre G., Poelgeest E.V., Jacqmin P., Graaf K., Reijers J., et al. Evidence of NI-0101 pharmacological activity, an anti-TLR4 antibody, in a randomized phase I dose escalation study in healthy volunteers receiving LPS. Clin Pharmacol Ther. 2017;101:200–208. doi: 10.1002/cpt.522. [DOI] [PubMed] [Google Scholar]
- 241.Tsukamoto H., Kubota K., Shichiku A., Maekawa M., Mano N., Yagita H., et al. An agonistic anti-Toll-like receptor 4 monoclonal antibody as an effective adjuvant for cancer immunotherapy. Immunology. 2019;158:136–149. doi: 10.1111/imm.13095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Matsunaga N., Tsuchimori N., Matsumoto T., Ii M. TAK-242 (resatorvid), a small-molecule inhibitor of Toll-like receptor (TLR) 4 signaling, binds selectively to TLR4 and interferes with interactions between TLR4 and its adaptor molecules. Mol Pharmacol. 2011;79:34–41. doi: 10.1124/mol.110.068064. [DOI] [PubMed] [Google Scholar]
- 243.Liao L., Dang W., Lin T., Yu J., Liu T., Li W., et al. A potent PGK1 antagonist reveals PGK1 regulates the production of IL-1β and IL-6. Acta Pharm Sin B. 2022;12:4180–4192. doi: 10.1016/j.apsb.2022.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Mehra A., Baker C.L., Loros J.J., Dunlap J.C. Post-translational modifications in circadian rhythms. Trends Biochem Sci. 2009;34:483–490. doi: 10.1016/j.tibs.2009.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Li R., Xie L., Li L., Chen X., Yao T., Tian Y., et al. The gut microbial metabolite, 3,4-dihydroxyphenylpropionic acid, alleviates hepatic ischemia/reperfusion injury via mitigation of macrophage pro-inflammatory activity in mice. Acta Pharm Sin B. 2022;12:182–196. doi: 10.1016/j.apsb.2021.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Stamatovic S.M., Phillips C.M., Martinez-Revollar G., Keep R.F., Andjelkovic A.V. Involvement of epigenetic mechanisms and non-coding RNAs in blood–brain barrier and neurovascular unit injury and recovery after stroke. Front Neurosci. 2019;13:864. doi: 10.3389/fnins.2019.00864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Wang Z., Leng Y., Tsai L.K., Leeds P., Chuang D.M. Valproic acid attenuates blood–brain barrier disruption in a rat model of transient focal cerebral ischemia: the roles of HDAC and MMP-9 inhibition. J Cerebr Blood Flow Metabol. 2011;31:52–57. doi: 10.1038/jcbfm.2010.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Chen S., Ye J., Chen X., Shi J., Wu W., Lin W., et al. Valproic acid attenuates traumatic spinal cord injury-induced inflammation via STAT1 and NF-κB pathway dependent of HDAC3. J Neuroinflammation. 2018;15:150. doi: 10.1186/s12974-018-1193-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Chen Y., Sun H., Bai Y., Zhi F. Gut dysbiosis-derived exosomes trigger hepatic steatosis by transiting HMGB1 from intestinal to liver in mice. Biochem Biophys Res Commun. 2019;509:767–772. doi: 10.1016/j.bbrc.2018.12.180. [DOI] [PubMed] [Google Scholar]
- 250.Yuan S., Liu Z., Xu Z., Liu J., Zhang J. High mobility group box 1 (HMGB1): a pivotal regulator of hematopoietic malignancies. J Hematol Oncol. 2020;13:91. doi: 10.1186/s13045-020-00920-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Li W., Cai H., Ren L., Yang Y., Yang H., Liu J., et al. Sphingosine kinase 1 promotes growth of glioblastoma by increasing inflammation mediated by the NF-κB/IL-6/STAT3 and JNK/PTX3 pathways. Acta Pharm Sin B. 2022;12:4390–4406. doi: 10.1016/j.apsb.2022.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Al-Obaidi M.M.J., Desa M.N.M. Mechanisms of blood brain barrier disruption by different types of bacteria, and bacterial-host interactions facilitate the bacterial pathogen invading the brain. Cell Mol Neurobiol. 2018;38:1349–1368. doi: 10.1007/s10571-018-0609-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Zhu H., Dai R., Zhou Y., Fu H., Meng Q. TLR2 ligand Pam3CSK4 regulates MMP-2/9 expression by MAPK/NF-κB signaling pathways in primary brain microvascular endothelial cells. Neurochem Res. 2018;43:1897–1904. doi: 10.1007/s11064-018-2607-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Spengler M.L., Kuropatwinski K.K., Comas M., Gasparian A.V., Fedtsova N., Gleiberman A.S., et al. Core circadian protein CLOCK is a positive regulator of NF-κB-mediated transcription. Proc Natl Acad Sci U S A. 2012;109:E2457–E2465. doi: 10.1073/pnas.1206274109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Sun Y., Zeng X., Liu Y., Zhan S., Wu Z., Zheng X., et al. Dendrobium officinale polysaccharide attenuates cognitive impairment in circadian rhythm disruption mice model by modulating gut microbiota. Int J Biol Macromol. 2022;217:677–688. doi: 10.1016/j.ijbiomac.2022.07.090. [DOI] [PubMed] [Google Scholar]