Abstract
Background
Older adults reporting higher energy levels have better physical function. It is not known if these associations persist among older adults reporting fatigue or if higher energy is associated with cognitive function. We examined longitudinal associations between self-reported energy, gait speed, and cognition, stratified by fatigue, in 2 613 participants (aged 74.6 ± 2.87 years) in the Health, Aging and Body Composition Study.
Methods
Self-reported energy (0–10, dichotomized at median) and fatigue (present/absent) were measured at baseline. Usual and rapid-paced gait speed (m/s), modified Mini-Mental State Examination (3MS), and Digit Symbol Substitution Test (DSST) were measured at baseline and annually over 8 years. Linear mixed effect models compared changes in gait speed, 3MS, and DSST between higher and lower energy groups within fatigue strata.
Results
At baseline, 724 participants (27%) were fatigued; 240 (33%) coreported higher energy (9% of total). The remaining 1 889 participants were fatigue-free (73%); 1 221 (65%) coreported higher energy (47% of total). Those with fatigue and higher energy had average rapid gait declines of 0.007 m/s per year (p = .04) after adjustment for demographics, comorbidities, depressive symptoms, and exercise. DSST declines were found among only fatigue-free participants (β = 0.17, p = .01). No statistically significant associations with energy were found for fatigue-free participants, or for usual gait or 3MS.
Conclusions
Asking about older adults’ energy levels as well as fatigue may identify a subgroup of older adults protected against physical and cognitive decline, even among those with fatigue.
Keywords: Mobility, Healthy aging, Physical function
Lower energy and higher fatigue are common complaints in older age (1). Energy is often conflated with fatigue in the research literature, and the constructs are treated as opposites on a single continuum (2). Although energy and fatigue may overlap, they also are distinct perceptual states. Self-reported energy is “an individual’s potential to perform mental and physical activity,” synonyms for which include vitality (2,3). Self-reported energy is associated with vitality, general health, and motivation (4–6) In contrast, fatigue is “an unpleasant physical, cognitive, and emotional symptom described as tiredness not relieved by common strategies that restore energy” (7,8). Fatigue is a frequently evaluated quality of life metric in patients with chronic illness (2–4). Importantly, each state has independent predictors (2,9–11). For example, Boolani et al. discovered that depression predicted fatigue (not energy), and body mass index (BMI) and physical activity predicted energy levels (not fatigue) (9). Moreover, neuroimaging studies suggest energy and fatigue have different neurobiological correlates (2,12). Thus, it is important to examine the effects of energy and fatigue perception on physical and cognitive function separately from each other.
Fatigue has been associated with poorer physical function, including prodromal Parkinson’s disease and mild Parkinsonian signs (13–18). Fatigue has also been associated with poorer cognitive function in clinical populations, including slower information processing speeds and memory impairment (19–22). Work done by us (17,18,23,24) and others (2,10,25) indicates higher levels of self-reported energy are cross-sectionally associated with faster gait speed but not cognition (13–18). However, the associations of energy with physical and cognitive function have not been tested longitudinally, nor separately for those with and without fatigue.
Our primary comparison was between those who reported higher versus lower energy among older adults coreporting fatigue. We investigated the gait speed decline of community-dwelling older adults from the longitudinal Health, Aging and Body Composition Study (HABC), for those with higher/lower energy within strata of fatigued and fatigue-free participants. We focused on gait speed decline at usual and rapid pace, because of previously demonstrated associations with energy and fatigue (3,9,12). We conducted a secondary analysis of declines in cognitive function among those reporting higher energy within strata of fatigued and fatigue-free participants.
Method
Health, Aging and Body Composition Study is a population-based study conducted in Memphis, Tennessee, and Pittsburgh, Pennsylvania, with active follow-up starting in 1997–1998 and ending in 2011 (26). A total of 3 075 men and women between ages 70 and 79 joined the study; 45% of the women and 33% of the men were Black; remaining participants were White (26). To be eligible to enroll, at baseline participants had to be free of difficulty walking a quarter mile or climbing 10 stairs (26). HABC was designed to evaluate changes in body composition with age, and assess differences in the onset of functional limitation and mortality by race and sex (26).
For the current analyses, we selected those with data on both self-reported energy and fatigue levels at Study Year 2 (calendar years 1998–1999); this is the first time when energy and fatigue were collected concurrently, and it represents the baseline for this analysis. Each participant with energy and fatigue status at Year 2 also had to have at least 3 measurements over the next 8 years of follow-up of rapid gait speed, usual gait speed, Teng Mini-Mental State Examination [3MS], and Digit Symbol Substitution Test [DSST]. Demographics, smoking, and the presence of chronic conditions were assessed at HABC’s Year 1 visit.
Main Exposures: Energy and Fatigue Status
Our primary exposure was participants’ self-reported energy stratified by fatigue status at Year 2. Self-reported energy at Year 2 was measured by asking participants to describe their usual energy levels in the past month on a scale of 0 to 10, where 0 is no energy and 10 is the most energy they have ever had. Energy was dichotomized at the median (≥7 was considered higher energy; Supplementary Table 1). All analyses compare the higher energy group to the referent lower energy group. Of note, the HABC study only provided information from a single question about energy, rather than multidimensional or scaled questionnaires. However, we have consistently shown in past studies that higher scores on this question about energy were associated with more physical activity (by objectively measured physical activity and self-report), higher physical performance, physical activity, and fewer depressive symptoms (18,24). These associations were independent of adjustment for fatigue, as assessed by the tiredness question used in this study (18,24). Fatigue was measured at Year 2 by asking participants over the past month whether, on average, they have been feeling unusually tired during the day (response options: yes or no). In addition, tiredness scales have been used to assess fatigue in prior studies (7,8,27,28). Single-item fatigue screening questions have also been validated for use in populations with cancer (29).
Outcomes: Gait Speed at Usual and Rapid Pace, Modified Mini-Mental State Examination Score, and Digit Symbol Substitution Test Score
Gait speed (meters/second) was measured each year between Years 2 and 10 (except Year 7) by instructing participants to complete a 20-m walk from a standing start, once at their usual pace, and once at a rapid pace (as fast as comfortably able) (30). Memory, orientation, visuospatial skills, fluency, and reasoning were evaluated with the 3MS, a screening test for cognitive impairment with a score range of 0–100. Normal (subclinical dementia, and dementia) status scores are >85 (80–85, <80), respectively (24,31,32). The 3MS was measured at Years 3, 5, 7, 9, and 10. Working memory, executive function, processing speed, and visuospatial skills were evaluated using the DSST, a neuropsychiatric test that involves matching symbols to numbers (33,34). DSST scores are calculated per total number of accurate symbol to number matches made in 90 seconds (35). The DSST was captured at Year 5 and Years 7–10 (30).
Covariates (Potential Confounders)
Covariates were assessed at Year 1 of HABC. Demographics include self-reported age, race (Black or White) and sex (male or female). Health characteristics evaluated were smoking status (current, past, ever, never) and BMI. Chronic disease covariates include congestive heart failure, cerebrovascular disease, coronary heart disease, incident hypertension, diabetes, peripheral arterial disease, and arthritis. These conditions were measured via self-report questionnaires, medications listed, or Health Care Finance Administration diagnosis (24). Depressive symptoms were evaluated using the Center for Epidemiologic Studies Depression (CES-D) 10-item questionnaire, which was collected annually between Years 3 and 13 (19). Physical activity was measured as self-reported walking time, calculated as minutes walking for exercise and other walking over the past week.
Statistical Analyses
Participants were stratified by fatigue status at baseline (Year 2) and all analyses were conducted separately in the fatigued group and the fatigue-free group. Within each stratum, the lower-energy group was used as the referent. In bivariate analyses, t tests or chi square tests were used to evaluate the association of each continuous or categorical covariate comparing the higher versus lower energy groups. The distribution of continuous variables was evaluated with histograms and Q–Q plots prior to using t tests and expected values (>5 in each cell) were checked for categorical variables prior to using chi square tests. Unadjusted linear mixed effects models were run to assess the average annual change in usual and rapid gait speed within fatigued and fatigue-free participants. The average annual change was used to estimate the average gait speed after 8 years of follow-up and the average gait speed decline. Next, linear mixed effects models were created, stratified by fatigue status, to evaluate the relationship of energy status with the following outcomes over 8 years: gait speed (usual and rapid) and cognitive function (via the 3MS and DSST). Each model has a random intercept (to account for repeated outcome measures within person) and slope for energy status with an unstructured covariance G matrix and restricted maximum likelihood (REML) estimation method. The Schwarz Bayesian information criteria were used during stepwise model-building procedure for each outcome to determine the covariates included in the final models. Those results were used to select covariates for inclusion in each model. An interaction term between year and energy was used to establish the group differences of the rate of change in each outcome and are the primary results of interest; year is coded as study (visit) year (2–10) as opposed to calendar time. All analyses were conducted using SAS version 9.4 (SAS Institute, Cary, NC) (36).
Results
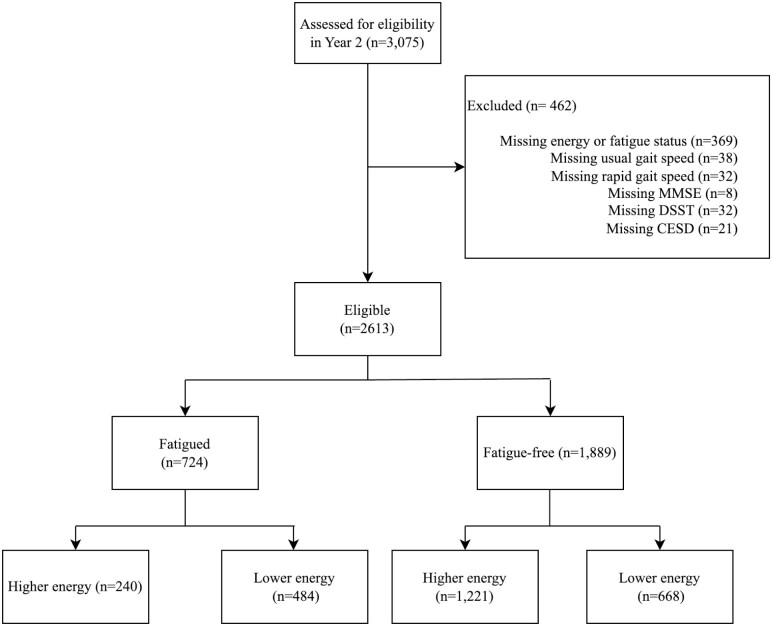
There were 2 613 participants included in this analysis (Figure 1). Of those, 724 (28.4%) self-reported fatigue at baseline and 1 889 (71.6%) did not. Those reporting fatigue were less likely to report higher energy compared to fatigue-free participants (33.2% vs 64.6%, p < .001) and more likely to have coronary heart disease (26.8% vs 19.4%, p < .001) and incident hypertension (57.3% vs 52.1%, p = .02; Supplementary Table 1).
Figure 1.
Flowchart for inclusion into the study. Participants were assessed for eligibility, stratified by fatigue status, and then further stratified into higher and lower energy groups.
Of those who reported fatigue, 240 (33.2%) also reported higher energy, corresponding to 9.2% of the total sample (Table 1). Among the fatigued participants, those simultaneously reporting higher energy as compared to those reporting lower energy were more likely to be Black (45.8% vs 36.8%, p = .02), had less postsecondary education (36.8% vs 41.1%, p = .02), lower 3MS scores (89.0 vs 90.6, p = .01), and fewer depressive symptoms (5.8 vs 7.1, p < .01; Table 1). Differences in both usual gait speed (1.09 vs 1.06 m/s, p = .07) and rapid gait speed (1.46 vs 1.42 m/s, p = .11) at baseline were not statistically significant by energy group amongst fatigued participants (Table 1). There was no statistically significant difference in DSST scores by energy group amongst fatigued participants (33.1 vs 25.5, p = .05).
Table 1.
Sample Characteristics in Fatigued and Fatigue-Free Participants by Energy Status in the Health, Aging, and Body Composition Study at Baseline, Total N = 2 613
| Fatigue (n = 724) | Fatigue-Free (n = 1 889) | |||||
|---|---|---|---|---|---|---|
| Mean ± SD or n (column %) | ||||||
| Higher Energy | Lower Energy | p Value | Higher Energy | Lower Energy | p Value | |
| Energy level | 240 (33.2) | 484 (66.8) | 1 221 (64.7) | 668 (35.3) | ||
| Demographics and baseline health status | ||||||
| Age, y | 74.7 ± 2.9 | 74.9 ± 2.9 | .52 | 74.5 ± 2.8 | 74.7 ± 2.9 | .12 |
| Black | 110 (45.8) | 178 (36.8) | .02 | 475 (38.9) | 233 (34.9) | .08 |
| Female | 154 (64.2) | 306 (63.2) | .80 | 555 (45.5) | 319 (47.8) | .34 |
| Postsecondary education | 88 (36.8) | 199 (41.1) | .02 | 580 (47.6) | 306 (46.0) | .15 |
| Diabetes | 32 (13.3) | 79 (16.3) | .29 | 153 (12.5) | 105 (15.7) | .05 |
| Congestive heart failure | 8 (3.4) | 21 (4.4) | .49 | 20 (1.7) | 19 (2.9) | .08 |
| Cerebrovascular disease | 18 (7.5) | 47 (10.0) | .29 | 69 (5.7) | 65 (9.8) | <.01 |
| Peripheral arterial disease | 13 (5.6) | 31 (6.7) | .56 | 42 (3.5) | 44 (6.8) | <.01 |
| Coronary heart disease | 57 (24.0) | 135 (28.4) | .21 | 204 (16.9) | 160 (24.5) | <.01 |
| Incident hypertension | 130 (54.2) | 285 (58.9) | .23 | 588 (48.2) | 395 (59.2) | <.01 |
| Cancer | 37 (15.5) | 83 (17.2) | .56 | 198 (16.3) | 133 (19.9) | .05 |
| Current smoker | 103 (42.9) | 199 (41.1) | .78 | 587 (48.2) | 326 (48.8) | .22 |
| 400 m walk time, m/s | 333.1 ± 69.2 | 339.0 ± 61.8 | .86 | 311.1 ± 51.1 | 328.8 ± 57.6 | <.01 |
| Baseline values of outcome variables | ||||||
| Usual gait speed, m/s | 1.09 ± 0.20 | 1.06 ± 0.21 | .07 | 1.19 ± 0.21 | 1.12 ± 0.21 | <.01 |
| Rapid gait speed, m/s | 1.46 ± 0.31 | 1.42 ± 0.33 | .11 | 1.62 ± 0.32 | 1.52 ± 0.33 | <.01 |
| 3MS*, 0–100 | 89.0 ± 8.2 | 90.6 ± 7.5 | .01 | 90.9 ± 7.6 | 91.1 ± 7.1 | .05 |
| CES-D†, 0–60 | 5.8 ± 5.2 | 7.1 ± 7.0 | <.01 | 3.4 ± 4.0 | 4.8 ± 5.1 | <.01 |
| DSST‡ | 33.1 ± 16.5 | 35.5 ± 13.5 | .05 | 37.3 ± 14.4 | 37.0 ± 14.2 | .64 |
Notes: t Tests were used for continuous variables, and chi-square tests for categorical variables.
*Teng Mini-Mental State Examination.
†Center for Epidemiologic Studies Depression scale.
‡Digit Symbol Substitution Test.
Among the fatigue-free group, 1 221 (64.6%) also reported higher energy, corresponding to 46.7% of the total sample (Table 1). Compared to those with lower energy, those with higher energy were less likely to have cerebrovascular disease (5.7% vs 9.8%, p < .01), peripheral arterial disease (3.5% vs 6.8%, p < .01), coronary heart disease (16.9% vs 24.5%, p < .01), and incident hypertension (48.2% vs 59.2%, p < .01; Table 1). The fatigue-free participants with higher energy also had fewer depression symptoms and faster gait speed as compared to those reporting lower energy (Table 1). Differences in baseline 3MS (90.9 vs 91.1, p = .05) or DSST (37.3 vs 37.0, p = .64) were not statistically significant (Table 1).
Average annual decline in both usual and rapid gait speed was similar for both fatigued and fatigue-free participants in unadjusted linear mixed effects models (Table 2). Overall, among fatigued participants, there was a 26% decline in usual gait speed and a 20% decline in rapid gait speed over the 8-year study period (Table 2). Fatigued participants coreporting higher energy had lesser average declines in usual and rapid gait speed during follow-up compared to those with lower energy levels (24% vs 27% and 18% vs 21%, respectively; Table 2). Among fatigue-free participants, there was a 23% decline in usual gait speed and a 19% decline in rapid gait speed during the 8-year study period (Table 3). Fatigue-free participants coreporting higher energy had lesser average declines in usual and rapid gait speed during follow-up compared to those with lower energy levels (24% vs 25% and 17% vs 20%, respectively; Table 3).
Table 2.
Results of Unadjusted Linear Mixed Effects Models Reporting the Annual Average Change and Estimated Average Decline Over Eight Years in Rapid and Usual Gait Speed Among Fatigued Participants, by Energy Status, N = 724
| Fatigued Participants | ||||||
|---|---|---|---|---|---|---|
| All Fatigued | Higher Energy | Lower Energy | ||||
| Average Annual Gait Speed Change (β (p Value)) |
Estimated Average Gait Speed Decline | Average Annual Gait Speed Change (β (p Value)) |
Estimated Average Gait Speed Decline | Average Annual Gait Speed Change (β (p Value)) |
Estimated Average Gait Speed Decline | |
| Usual gait speed | −0.035 (<.001) | 26.2% | −0.033 (<.001) | 24.2% | −0.036 (<.001) | 27.2% |
| Rapid gait speed | −0.035 (<.001) | 19.6% | −0.032 (<.001) | 17.5% | −0.038 (<.001) | 21.4% |
Note: Results are unadjusted, and the betas and estimated averages are for change in gait speed over the course of the study.
Table 3.
Results of Unadjusted Linear Mixed Effects Models Reporting the Annual Average Change and Estimated Average Decline Over Eight Years in Rapid and Usual Gait Speed Among Fatigue-Free Participants, by Energy Status, N = 1 889
| Fatigue-Free Participants | ||||||
|---|---|---|---|---|---|---|
| All Fatigue-Free | Higher Energy | Lower Energy | ||||
| Average Annual Gait Speed Change (β (p Value)) |
Estimated Average Gait Speed Decline | Average Annual Gait Speed Change (β (p Value)) |
Estimated Average Gait Speed Decline | Average Annual Gait Speed Change (β (p Value)) |
Estimated Average Gait Speed Decline | |
| Usual gait speed | −0.034 (<.001) | 23.3% | −0.034 (<.001) | 23.5% | −0.035 (<.001) | 25.0% |
| Rapid gait speed | −0.038 (<.001) | 19.2% | −0.035 (<.001) | 17.3% | −0.037 (<.001) | 19.5% |
Note: Results are unadjusted, and the betas and estimated averages are for change in gait speed over the course of the study.
Results were similar in fully adjusted models (Table 4). The annual rate of change for rapid gait speed but not usual gait speed was larger in the higher versus lower energy group, indicating less decline, for fatigued participants (β = 0.007, p = .03; Table 4). No difference was discovered for the annual rate of change in DSST by energy group among fatigued participants (β = 0.08, p = .5) or for 3MS among fatigued participants (β = 0.05, p = .6). Fatigue-free participants reporting higher energy had borderline significant larger average annual rapid gait speed declines (β = 0.004, p = .05) in Models 1 and 2, but not after full adjustment in Model 3 (β = 0.003, p = .18; Table 4). The annual rate of change for DSST was larger in the higher versus lower energy group for the fatigue-free group (β = 0.17, p = .01), but not for the 3MS (β = 0.08, p = .2).
Table 4.
Linear Mixed Effect Models Reporting Longitudinal Associations Between Self-Reported Energy and Annual Change in Usual and Rapid Gait Speed in Fatigued and Fatigue-Free Participants in the Health, Aging and Body Composition Study, Total N = 2 613
| Fatigued Participants | Fatigue-Free Participants | |||||||
|---|---|---|---|---|---|---|---|---|
| Outcome | Usual Gait Speed | Rapid Gait Speed | Usual Gait Speed | Rapid Gait Speed | ||||
| β | p Value | β | p Value | β | p Value | β | p Value | |
| Model 1: Demographics* | ||||||||
| Higher energy | −0.009 | .32 | −0.02 | .15 | 0.02 | .0004 | 0.01 | .08 |
| Year | −0.04 | <.0001 | −0.04 | <.0001 | −0.04 | <.0001 | −0.04 | <.0001 |
| Higher energy × year | 0.004 | .11 | 0.007 | .03 | 0.002 | .10 | 0.004 | .05 |
| Model 2: Model 1 + comorbidities† | ||||||||
|---|---|---|---|---|---|---|---|---|
| Higher energy | −0.01 | .29 | −0.02 | .15 | 0.02 | .001 | 0.01 | .13 |
| Year | −0.04 | <.0001 | −0.04 | <.0001 | −0.04 | <.0001 | −0.04 | <.0001 |
| Higher energy × year | 0.004 | .10 | 0.007 | .04 | 0.002 | .11 | 0.004 | .05 |
| Model 3: Model 2 + depressive symptoms and exercise‡ | ||||||||
|---|---|---|---|---|---|---|---|---|
| Higher energy | −0.006 | .54 | −0.01 | .24 | 0.02 | <.01 | 0.01 | .07 |
| Year | −0.04 | <.0001 | −0.04 | <.0001 | −0.04 | <.0001 | −0.04 | <.0001 |
| Higher energy × year | 0.002 | .29 | 0.007 | .04 | 0.002 | .14 | 0.003 | .18 |
Notes: Low energy is the referent, and baseline gait speed was adjusted for in all models.
*Adjusted for race, sex, age, education.
†Additionally adjusted for baseline diabetes and hypertension.
‡Additionally adjusted for height, and baseline cardiovascular disease, arthritis, peripheral arterial disease, depressive symptoms, and minutes walked per week.
In sensitivity analyses for primary outcomes using 8 as a cutoff for higher energy, 114 participants reported both fatigue and higher energy; among fatigued participants, the interaction of higher energy and year was significantly associated with usual gait speed decline (β = 0.006, p = .03) but not rapid gait speed decline (β = 0.005, p = .21) in models adjusting for demographics and chronic conditions (Supplementary Table 3).
Discussion
In this sample of community-dwelling older adults, participants with higher energy showed less decline in rapid gait speed than those reporting lower energy during the following 8 years, with strongest effects seen in the fatigued group. Our work further distinguishes the link with energy on gait speed decline separately from fatigue. Self-report of higher energy levels in older age, even in the presence of fatigue, may be a useful clinical indicator of less mobility decline. Conversely, self-report of lower energy, even in the absence of fatigue, may alert geriatricians to impending mobility decline.
Contrary to our hypotheses, associations of higher energy with usual gait speed were not statistically significant. It has been widely reported that usual gait speed declines with increasing age and begins to decline more rapidly in those age 80 and older (37–39). This rapid decline around age 80 is also associated with an increase in the energetic cost of walking (38,40,41). The average age of participants in this sample was 75 at baseline and 8 years of follow-up were included; perhaps as the energetic cost of walking increases at older ages among these participants, baseline energy levels would come to play a more prominent role in usual gait speed decline. In much of the existing literature, only the association of fatigue with gait speed has been evaluated, despite increasing evidence that energy is also predictive of gait speed (25). Our results add to knowledge in this field and are also among the first to assess energy in relation to both usual and rapid gait speed. Higher energy levels may reflect an underlying robustness in physical function that is more evident for challenging conditions; the rapid gait speed condition asks participants to push themselves out of their comfort zones in a way that the usual gait speed condition does not (42).
Higher self-reported energy may reflect underlying physiological and energy regulation processes that may affect both physical and cognitive function (43–45). Recent evidence suggests that self-reported energy may reflect changes in bioenergetic processes and dopamine availability occurring with older age, and/or in the prodromal stages of Parkinson’s disease (16,39,45,46). Future studies should assess whether older adults with higher energy have more favorable bioenergetic profiles than older adults with lower energy, and whether this differs based on fatigue.
We noted a relatively high prevalence of groups reporting discordant levels of energy and fatigue status (41% of the sample). Although misclassification bias cannot be ruled out, the high level of discrepancy indicates that answers to questions pertaining to energy and fatigue may involve different biological processes, further supporting the notion that energy and fatigue are not entirely overlapping constructs.
Higher energy was not associated with decline in cognitive scores among fatigued participants. These null results align with previous cross-sectional findings in this cohort using the same measure of energy (23,24). However, we found a positive association with DSST among the fatigue-free, perhaps driven by the motor component of DSST (23,24). Contrary to our hypothesis, there was no longitudinal effect of energy on memory in either the fatigued or fatigue-free group. These null results for memory align with previous cross-sectional results in this cohort using the same measure of self-reported energy, which did not find associations between higher energy and memory (24). Prior studies on fatigue and cognition were primarily in clinical populations (24); these studies did not distinguish between fatigue and energy and their results may not generalize well to healthier older adults (47). This work on self-reported energy in older adults without overt neurologic diagnoses is a step toward identifying and addressing the longitudinal impact of higher energy on clinically relevant measures of gait and cognitive function.
Limitations and Strengths
Only one measurement of energy and fatigue status was included, and changes in energy or fatigue were not assessed. Fatigue was captured via a standalone question; feeling “unusually tired” may not represent other dimensions of fatigue captured in broader fatigue scales. Similarly, a single-item measure of energy may not fully capture multifaceted aspects of energy perception. What constitutes higher energy for a given participant may not generalize to all participants. Interpretations of higher energy may vary both within and between participants; thus, self-reported energy was dichotomized at the median to help account for this variability and to directly build upon past work using this energy measure (both as a continuous and dichotomized variable) in this cohort (24). Sensitivity analyses suggest that associations with gait speed are sensitive to the cut point for higher energy; statistically significant results were not replicated for rapid gait speed in 114 participants who reported both fatigue and higher energy. There may be a survival bias due to the selection criteria requiring that outcomes be captured at least three times during follow-up; those who were less healthy or who died may be less likely to have been included. Although prior literature has shown that depression may affect gait speed (48,49), adjusting for depressive symptoms in this analysis did not dilute the effect size or strength of the association between higher energy and rapid gait speed among fatigued participants. Among fatigue-free participants, the annual rate of change in rapid gait speed was no longer statistically significant after additional adjustment. It may be the case that among fatigue-free participants, changes in rapid gait speed may be driven more by chronic conditions or exercise rather than self-reported energy itself. However, among fatigued participants, the association between higher energy and the rate of change in rapid gait speed is robust to adjustment for multidimensional confounding variables.
Strengths of this study included longitudinal assessment of clinically relevant metrics across mobility, memory, and cognition, domains critical for promoting health and well-being in older adults (50–52). The sample itself was well characterized, with excellent participant retention and generalizability to community-dwelling older adults in the United States (26). Energy and fatigue were captured as distinct perceptual states, which may more accurately reflect the neurobiology underlying each construct (2). The energy measure used has been shown to correlate with objective metrics of energy expenditure (23), and results for gait speed reproduce prior findings in this cohort (18,24). Previous research has demonstrated the association between fatigue and reduced gait speed (53,54); this study contributes a novel assessment of the association between energy and gait speed in the presence of fatigue, thereby highlighting the distinct clinical relevance of energy to physical function. Our results underscore the importance of developing more comprehensive assessments to evaluate individual’s feelings of energy. Overall, we aim to approach this topic with caution and encourage further investigation to ascertain the authenticity and reproducibility of these findings.
Conclusion
Our findings indicate that higher energy levels are associated with lesser gait speed decline, especially for those with fatigue, when participants are asked to exert themselves. Future research should explore the potential physiologic mechanisms through which self-reported energy may reflect decreased bioenergetic capacity. Lower self-reported energy may be an early warning sign for functional aging, including decreasing cognitive function. As such, self-reported energy may be a candidate for inclusion in a risk stratification algorithm for mobility impairment. Improved understanding of the physiology of self-reported energy may lead to novel treatments that directly target energy-specific biology and innovative approaches for maintaining energy and mobility in older age.
Supplementary Material
Contributor Information
Rebecca Ehrenkranz, Department of Epidemiology, University of Pittsburgh School of Public Health, Pittsburgh, Pennsylvania, USA.
Xiaonan Zhu, Department of Epidemiology, University of Pittsburgh School of Public Health, Pittsburgh, Pennsylvania, USA.
Nancy W Glynn, Department of Epidemiology, University of Pittsburgh School of Public Health, Pittsburgh, Pennsylvania, USA.
Marnie Bertolet, Department of Epidemiology, University of Pittsburgh School of Public Health, Pittsburgh, Pennsylvania, USA.
Sarah B Berman, Department of Neurology, University of Pittsburgh School of Medicine, Pittsburgh, Pennsylvania, USA.
James B Hengenius, Department of Epidemiology, University of Pittsburgh School of Public Health, Pittsburgh, Pennsylvania, USA.
Caterina Rosano, Department of Epidemiology, University of Pittsburgh School of Public Health, Pittsburgh, Pennsylvania, USA.
Lewis A Lipsitz, (Medical Sciences Section).
Funding
This work was supported by the following funding sources: National Institute on Aging (NIA) Contracts N01-AG-6-2101, N01-AG-6-2103, and N01-AG-6-2106; NIA grant R01-AG028050; and NINR grant R01-NR012459. This research was funded in part by the Intramural Research Program of the NIH, National Institute on Aging. National Institute on Aging Kirschstein Institutional National Research Service Award (T32-AG055381) was awarded to the University of Pittsburgh (PIs: Drs. Mary Ganguli and C.R.) [R.E.].
Conflict of Interest
None.
Data Availability
The data used in this study is publicly available through Health ABC: https://healthabc.nia.nih.gov/.
Ethics Statement
Health ABC was approved through the Institutional Review Boards of the University of Pittsburgh (approval #960212) and the University of Tennesee, Knoxville (approval #5531).
References
- 1. Zengarini E, Ruggiero C, Pérez-Zepeda MU, et al. Fatigue: relevance and implications in the aging population. Exp Gerontol. 2015;70:78–83. 10.1016/j.exger.2015.07.011 [DOI] [PubMed] [Google Scholar]
- 2. Loy BD, Cameron MH, O’Connor PJ.. Perceived fatigue and energy are independent unipolar states: supporting evidence. Med Hypotheses. 2018;113:46–51. 10.1016/j.mehy.2018.02.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lerdal A. A concept analysis of energy. Its meaning in the lives of three individuals with chronic illness. Scand J Caring Sci. 1998;12(1):3–10. 10.1080/02839319850163075 [DOI] [PubMed] [Google Scholar]
- 4. Düzel E, Bunzeck N, Guitart-Masip M, Düzel S.. NOvelty-related Motivation of Anticipation and exploration by Dopamine (NOMAD): implications for healthy aging. Neurosci Biobehav Rev. 2010;34(5):660–669. 10.1016/j.neubiorev.2009.08.006 [DOI] [PubMed] [Google Scholar]
- 5. Penninx BWJH, Guralnik JM, Bandeen-Roche K, et al. The protective effect of emotional vitality on adverse health outcomes in disabled older women. J Am Geriatr Soc. 2000;48(11):1359–1366. 10.1111/J.1532-5415.2000.TB02622.X [DOI] [PubMed] [Google Scholar]
- 6. Rosenbusch H, Wanders F, Pit IL.. The semantic scale network: an online tool to detect semantic overlap of psychological scales and prevent scale redundancies. Psychol Methods. 2020;25(3):380–392. 10.1037/met0000244 [DOI] [PubMed] [Google Scholar]
- 7. Mota DDCF, Pimenta CAM.. Self-report instruments for fatigue assessment: a systematic review. Res Theory Nurs Pract. 2006;20(1):49–78. 10.1891/rtnp.20.1.49 [DOI] [PubMed] [Google Scholar]
- 8. Neuberger GB. Measures of fatigue: the Fatigue Questionnaire, Fatigue Severity scale, Multidimensional Assessment of Fatigue scale, and Short Form-36 Vitality (Energy/Fatigue) subscale of the Short Form Health Survey. Arthritis Rheum. 2003;49(S5):S175–S183. 10.1002/art.11405 [DOI] [Google Scholar]
- 9. Boolani A, Manierre M.. An exploratory multivariate study examining correlates of trait mental and physical fatigue and energy. Fatigue: Biomedicine, Health & Behavior. 2019;7(1):29–40. 10.1080/21641846.2019.1573790 [DOI] [Google Scholar]
- 10. Manierre M, Jansen E, Boolani A.. Sleep quality and sex modify the relationships between trait energy and fatigue on state energy and fatigue. PLoS One. 2020;15(1):e0227511. 10.1371/journal.pone.0227511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. van Solinge H, Vanajan A, Henkens K.. Does phased retirement increase vitality in older workers? Findings from a 3-year follow-up panel study. J Aging Soc Policy. Published online 2022;35:221–240. 10.1080/08959420.2022.2029270 [DOI] [PubMed] [Google Scholar]
- 12. Loy BD, O’Connor PJ.. The effect of histamine on changes in mental energy and fatigue after a single bout of exercise. Physiol Behav. 2016;153:7–18. 10.1016/j.physbeh.2015.10.016 [DOI] [PubMed] [Google Scholar]
- 13. Rutkowski NA, Sabri E, Yang C.. Post-stroke fatigue: a factor associated with inability to return to work in patients. PLoS One. 2021;16(8):e0255538. 10.1371/journal.pone.0255538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Babulal GM, Chen L, Doherty JM, Murphy SA, Johnson AM, Roe CM.. Longitudinal changes in anger, anxiety, and fatigue are associated with cerebrospinal fluid biomarkers of Alzheimer’s disease. J Alzheimers Dis. Published online March 2, 2022;87:141–148. 10.3233/jad-215708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Chan T, Wanigatunga AA, Terracciano A, et al. Traits and treadmills: association between personality and perceived fatigability in well-functioning community-dwelling older adults. Psychol Aging. 2021;36(6):710–717. 10.1037/pag0000631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Chahine LM, Zhu X, Ehrenkranz R, Chen H, Glynn NW, Rosano C.. Changes in self-reported energy levels in prodromal Parkinson’s disease. Mov Disord. 2021;36(5):1276–1277. 10.1002/mds.28535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Tian Q, Ehrenkranz R, Rosso AL, et al. Mild Parkinsonian signs, energy decline, and striatal volume in community-dwelling older adults. J Gerontol A Biol Sci Med Sci. 2021;77:800–806. 10.1093/gerona/glab150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sprague BN, Zhu X, Ehrenkranz RC, et al. Declining energy predicts incident mobility disability and mortality risk in healthy older adults. J Am Geriatr Soc. Published online July 23, 2021;69:3134–3141. 10.1111/jgs.17372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wearden A, Appleby L.. Cognitive performance and complaints of cognitive impairment in chronic fatigue syndrome (CFS). Psychol Med. 1997;27(1):81–90. 10.1017/s0033291796004035 [DOI] [PubMed] [Google Scholar]
- 20. Cameron MH, Peterson V, Boudreau EA, et al. Fatigue is associated with poor sleep in people with multiple sclerosis and cognitive impairment. Mult Scler Int. 2014;2014:1–5. 10.1155/2014/872732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Johansson B, Rönnbäck L.. Mental fatigue and cognitive impairment after an almost neurological recovered stroke. ISRN Psychiatry. 2012;2012:1–7. 10.5402/2012/686425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Graber M, Garnier L, Duloquin G, et al. Association between fatigue and cognitive impairment at 6 months in patients with ischemic stroke treated with acute revascularization therapy. Front Neurol. 2019;10(AUG):931. 10.3389/fneur.2019.00931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Tian Q, Glynn NW, Ehrenkranz RC, Sprague BN, Rosso AL, Rosano C.. Perception of energy and objective measures of physical activity in older adults. J Am Geriatr Soc. Published online June 8, 2020:68(8):1876–1877. 10.1111/jgs.16577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ehrenkranz R, Rosso AL, Sprague BN, et al. Functional correlates of self-reported energy levels in the Health, Aging and Body Composition Study. Aging Clin Exp Res. Published online March 10, 2021:33(10):2787–2795. 10.1007/s40520-021-01788-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kowalski KL, Boolani A, Christie AD.. State and trait fatigue and energy predictors of postural control and gait. Motor Control. 2021;25(3):519–536. 10.1123/mc.2020-0106 [DOI] [PubMed] [Google Scholar]
- 26. Introducing the Health ABC Study: the dynamics of health, aging, and body composition. https://healthabc.nia.nih.gov/
- 27. de Vries J, Michielsen HJ, van Heck GL.. Assessment of fatigue among working people: a comparison of six questionnaires. Occup Environ Med. 2003;60(suppl 1):i10–i15. 10.1136/OEM.60.SUPPL_1.I10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Jackson C. The Chalder Fatigue Scale (CFQ 11). Occup Med (Lond). 2015;65(1):86–86. 10.1093/occmed/kqu168 [DOI] [PubMed] [Google Scholar]
- 29. Strasser F, Müller-Käser I, Dietrich D.. Evaluating cognitive, emotional, and physical fatigue domains in daily practice by single-item questions in patients with advanced cancer: a cross-sectional pragmatic study. J Pain Symptom Manage. 2009;38(4):505–514. 10.1016/j.jpainsymman.2008.12.009 [DOI] [PubMed] [Google Scholar]
- 30. Datasets/Documentation | Health ABC. Accessed November 21, 2019. https://healthabc.nia.nih.gov/datasets-documentation
- 31. McDowell I, Kristjansson B, Hill GB, Hébert R.. Community screening for dementia: the Mini Mental State Exam (MMSE) and Modified Mini-Mental State Exam (3MS) compared. J Clin Epidemiol. 1997;50(4):377–383. 10.1016/s0895-4356(97)00060-7 [DOI] [PubMed] [Google Scholar]
- 32. Sabe L, Jason L, Juejati M, Leiguarda R, Starkstein S.. Sensitivity and specificity of the Mini-Mental State Exam in the diagnosis of dementia. Behav Neurol. 1993;6(4):207–210. 10.3233/BEN-1993-6405 [DOI] [PubMed] [Google Scholar]
- 33. Jaeger J. Digit symbol substitution test. J Clin Psychopharmacol. 2018;38(5):513–519. 10.1097/JCP.0000000000000941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Chen X, Hu N, Wang Y, Gao X.. Validation of a brain-computer interface version of the digit symbol substitution test in healthy subjects. Comput Biol Med. 2020;120:103729. 10.1016/j.compbiomed.2020.103729 [DOI] [PubMed] [Google Scholar]
- 35. Rosano C, Perera S, Inzitari M, Newman AB, Longstreth WT, Studenski S.. Digit symbol substitution test and future clinical and subclinical disorders of cognition, mobility and mood in older adults. Age Ageing. 2016;45(5):687–694. 10.1093/ageing/afw116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. SAS/STAT GLIMMIX Procedure. Accessed December 9, 2021. https://support.sas.com/rnd/app/stat/procedures/glimmix.html
- 37. Waters RL, Mulroy S.. The energy expenditure of normal and pathologic gait. Gait Posture. 1999;9(3):207–231. 10.1016/s0966-6362(99)00009-0 [DOI] [PubMed] [Google Scholar]
- 38. Waters RL, Lunsford BR, Perry J, Byrd R.. Energy-speed relationship of walking: standard tables. J Orthop Res. 1988;6(2):215–222. 10.1002/jor.1100060208 [DOI] [PubMed] [Google Scholar]
- 39. Schrack JA, Simonsick EM, Chaves PHM, Ferrucci L.. The role of energetic cost in the age-related slowing of gait speed. J Am Geriatr Soc. 2012;60(10):1811–1816. 10.1111/j.1532-5415.2012.04153.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Larish DD, Martin PE, Mungiole M.. Characteristic patterns of gait in the healthy old. Ann N Y Acad Sci. 1988;515(1):18–32. 10.1111/j.1749-6632.1988.tb32960.x [DOI] [PubMed] [Google Scholar]
- 41. Schrack JA, Simonsick EM, Ferrucci L.. The relationship of the energetic cost of slow walking and peak energy expenditure to gait speed in mid-to-late life. Am J Phys Med Rehab. 2013;92(1):28–35. 10.1097/PHM.0b013e3182644165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Middleton A, Fritz SL, Lusardi M.. Walking speed: the functional vital sign. J Aging Phys Act. 2015;23(2):314–322. 10.1123/japa.2013-0236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Manini TM. Energy expenditure and aging. Ageing Res Rev. 2010;9(1):1–11. 10.1016/j.arr.2009.08.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Dharshini SAP, Taguchi YH, Gromiha MM.. Investigating the energy crisis in Alzheimer disease using transcriptome study. Sci Rep. 2019;9(1):1–13. 10.1038/s41598-019-54782-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Schrack JA, Simonsick EM, Ferrucci L.. The energetic pathway to mobility loss: an emerging new framework for longitudinal studies on aging. J Am Geriatr Soc. 2010;58(suppl 2):S329–S336. 10.1111/j.1532-5415.2010.02913.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Schrack JA, Simonsick EM, Ferrucci L, Manuscript A.. Framework for Longitudinal Studies on Aging. J Am Geriatr Soc. 2011;58(suppl 2):1–19. 10.1111/j.1532-5415.2010.02913.x.The [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Feng LR, Regan J, Shrader JA, et al. Cognitive and motor aspects of cancer-related fatigue. Cancer Med. 2019;8(13):5840–5849. 10.1002/cam4.2490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Buchner DM, Cress ME, Esselman PC, et al. Factors associated with changes in gait speed in older adults. J Gerontol A Biol Sci Med Sci. 1996;51(6):M297–M302. 10.1093/gerona/51a.6.m297 [DOI] [PubMed] [Google Scholar]
- 49. Demakakos P, Cooper R, Hamer M, de Oliveira C, Hardy R, Breeze E.. The bidirectional association between depressive symptoms and gait speed: evidence from the English Longitudinal Study of Ageing (ELSA). PLoS One. 2013;8(7):e68632. 10.1371/journal.pone.0068632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Miller ME, Magaziner J, Marsh AP, et al. ; LIFE Investigators. Gait speed and mobility disability: revisiting meaningful levels in diverse clinical populations. J Am Geriatr Soc. 2018;66(5):954–961. 10.1111/jgs.15331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Newman AB, Simonsick EM, Naydeck BL, et al. Association of long-distance corridor walk performance with mortality, cardiovascular disease, mobility limitation, and disability. JAMA. 2006;295(17):2018–2026. 10.1001/jama.295.17.2018 [DOI] [PubMed] [Google Scholar]
- 52. Buchman AS, Leurgans SE, Boyle PA, Schneider JA, Arnold SE, Bennett DA.. Combinations of motor measures more strongly predict adverse health outcomes in old age: the Rush Memory and Aging Project, a community-based cohort study. BMC Med. 2011;9:42. 10.1186/1741-7015-9-42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Hardy SE, Studenski SA.. Fatigue and function over 3 years among older adults. J Gerontol A Biol Sci Med Sci. 2008;63(12):1389–1392. 10.1093/gerona/63.12.1389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Kalron A. Association between perceived fatigue and gait parameters measured by an instrumented treadmill in people with multiple sclerosis: a cross-sectional study. J Neuroeng Rehabil. 2015;12(1):34. 10.1186/s12984-015-0028-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data used in this study is publicly available through Health ABC: https://healthabc.nia.nih.gov/.