Abstract
Background
Sarcopenia diagnosis is partly based on handgrip strength (HGS) assessment. The gold-standard dynamometer for this measurement is the Jamar. The electronic Gripwise is a smaller and lighter one, and its measurements are correlated with the Jamar’s in laboratory tests. Our study aimed to confirm this correlation in aged patients.
Methods
This monocenter cross-sectional study was performed in patients of 65 years and older admitted at the University Hospital. Participants were assessed either in a seated or bedridden position, randomly allocated to begin the measurements with the Jamar or the Gripwise.
Results
Among 649 aged inpatients assessed for eligibility, 348 were included (mean age: 79 ± 9; 52% females). The intraclass correlation coefficient was 0.93 (95% confidence interval [CI] 0.92–0.94, p < .001) for the maximum value measured with both devices and 0.94 (95% CI 0.93–0.95, p < .001) for the mean values. However, there was a significant difference in detecting low values (<16 kg in women, <27 kg in men), found in 48% of patients with Jamar, and 71% with Gripwise (p < .001). Thus, we determined alternate cutoffs for diagnosing HGS low values with the Gripwise (<12 kg in women, <22 kg in men), further validated in a supplementary validation population (n = 70). The diagnostic performances of these alternative cutoffs were high (93% sensitivity and 87% specificity in women; 94% sensitivity and 96% specificity in men).
Conclusions
The correlation of the Gripwise with the Jamar was confirmed in aged inpatients. However, lower values recorded with the Gripwise require alternate cutoffs for a relevant low HGS diagnosis.
Keywords: Frailty, Inpatients, Muscle strength dynamometer, Sarcopenia
Sarcopenia affects 10% to 27% of adults aged 60 years and older and is associated with high morbimortality in older adults (1,2). According to the revised consensus of the European Working Group on Sarcopenia in Older People (EWGSOP2), the diagnosis of sarcopenia is based, at the “Assess” step, on the measurement of handgrip strength (HGS) (threshold for low HGS below 16 kg in women and 27 kg in men) or the 5 consecutive sit-to-stand chair test (threshold for low muscle strength above 15 seconds) in a clinical setting (1). The gold standard dynamometer used for HGS measurement is the Jamar (3). In the last version of the hydraulic Jamar, measurement readings were improved with a digital screen. However, this device is heavy to handle (1.5 lb). To address this issue, a standardized method for assessing HGS in a seated position called the Southampton protocol is recommended by Roberts et al. (3). The observer should uphold the base of the dynamometer on the palm of their hand as the subject holds it. Despite these instructions, Jamar may still be inappropriate when measuring HGS in very weak patients (3). Plus, an annual recalibration back to the constructor is mandatory.
Many other dynamometers have tried to compete with the Jamar. Some of them showed excellent reliability compared to Jamar, with high correlation coefficients (4–10), but reliability was lower for some others (9,11–14). Furthermore, these other dynamometers are as heavy as the Jamar (6,8–10) or cannot be connected with an app or electronic medical record (4,5,9).
The first electronic Bodygrip dynamometer developed by Gripwisetech has already been compared to the Jamar (15). This device has been further improved in the Gripwise version, including a smart wireless connected application, which records the successive values of each patient and can implement the medical record. On mechanical controls, its measurements were correlated with the Jamar. The device is smaller and lighter (0.64 lb), and its resolution is at 0.1 kgf. An application has also been developed to use the device in exergames, giving feedback on the calibrated effort required to complete each task.
The aim of our study was to confirm the correlation of the Gripwise measurements with the Jamar’s when handled by aged patients. We targeted the hospitalization setting, in which sarcopenia is frequent and to ease the recruitment of a high number of patients in a short period (16,17).
Method
This study follows the STROBE checklist for cross-sectional study reports and the CONSORT 2010 checklist.
Study Design, Setting, and Participants
This was a monocenter randomized cross-sectional study. It was performed in the following 5 units within the University Hospital: gastroenterology (50 beds), rheumatology and internal medicine (44 beds), geriatric medicine (20 beds), dermatology, and infectiology (20 beds). The study received ethical committee approval from the First West Committee for the Protection of Persons (n°2021T3-11 DM, on July 15, 2021; ID-RCB 2021-A00927-34, NCT05060874). In accordance with the current French law and after validation by the ethical committee, all patients were informed about the study, both orally and using an information letter, and their non-opposition to participate was sought.
This study was conducted in 2 steps: first, the main study (training population) and, following first statistical analyses showing unexpected lower HGS values when using the Gripwise, compared to the measurements obtained with the Jamar, the inclusion of a supplementary validation sample, in order to validate alternate cutoffs for low HGS when using the Gripwise. In the main study, the recruitment started on September 1, 2021 and for the second step on December 19, 2022. All inpatients aged 65 years and older were listed the day following their admission in the participating units by B.B. Patients were screened for eligibility by the investigators each business day. The exclusion criteria were being unable to communicate and/or to squeeze both hands. Thus, in case of high fatigue or pain, non-French speaking, and oral comprehension or expression impairment, patients were not included. Patients with perfusion on the back of the hand were not included considered as unable to squeeze completely their hand. The other reasons for not participating were collected too, such as refusal, infectious isolation, discharge from the hospital units before study proposal, or previous inclusion. The trained investigators, 2 geriatricians (B.B., C.V.) and 2 nurses expert in geriatrics (S.L., C.K.), offered the patients to participate in the study. After validation of the inclusion, the random allocation to each arm was registered, and the measurements were then performed.
After inclusion in the study, participants were randomly allocated to begin with the hydraulic Jamar first (arm A) or the electronic Gripwise first (arm B), both in the training and the validation studies. The randomization sequence was generated by the statistician of the study using permutated blocks of varying size and stratified by investigator, using R software (version 4.0.5, 2021, the R Foundation for Statistical Computing, Vienna, Austria).
Variables, Data Sources, and Measurement
The Jamar devices were recalibrated before the start of the study, and the Gripwise devices were new ones. The variable hand span was set at the second position for all participants, the most reliable and consistent position advocated for routine use according to Roberts et al., except for small hands set at the first position (3). Before measurements, the patient’s and investigator’s hands were disinfected with a hydroalcoholic solution and devices with alcoholic wipes.
The position of each patient during all assessments (either seated or bedridden, according to patient’s preference or health condition) and the dominant hand to start with were recorded (start with the right hand if nondetermined). When assessed in a seated position, the Southampton protocol was used: the participants were comfortably seated in a standard chair with back support and fixed arms. They were asked to rest their forearms on the arms of the chair with their wrist just over the end of the arm of the chair with the wrist in a neutral position or between 0° and 30° dorsiflexion, thumb facing upwards. The hand was positioned so that the thumb was around on one side of the handle, and the 4 fingers were around the other side (3). When assessed in a bedridden position, patients were lying on their back, arms resting on the bed, elbows bent at 90°, forearms in a vertical position, wrist in a neutral position or between 0° and 30° dorsiflexion, and fingers facing upwards.
After the subject was positioned appropriately, the participant was encouraged with incentive orders to squeeze as long and as tightly as possible or until the needle stopped rising. Three measurements were consecutively performed on each hand with the first device according to the randomization group. A 10-second gap between the 3 measurement attempts was respected to ensure a steady position instead of alternating sides. The interval of 1-minute rest between the successive measurement sets was respected according to Watanabe et al.’s recommendations (18). The Gripwise device was set for a 10-second recording measurement; its values could either be read directly on the screen of the device or on the app of a connected mobile phone.
The patient’s satisfaction was assessed enquiring whether one device was easier to use or not and, if so, which one. Sociodemographic (age, gender) and medical (weight, height, body mass index [BMI], updated Charlson Comorbidity Index [CCI], length of stay, planned/unplanned admission) characteristics were collected in the medical record. Data were registered on the Open Clinica online Case Report Form.
Sample Size
For the training study
According to the previous study that compared the former version Bodygrip dynamometer to the Jamar, the correlation between the highest HGS measurement obtained for the nondominant hand of each dynamometer was excellent within inpatients intraclass correlation coefficients (ICC: 0.95 95% confidence interval [CI] [0.9–0.97]) (15). Regarding the study aim, we considered the correlation should be excellent if the ICC would be equal or greater than 0.90. For an estimated ICC of 0.90 ± .02 with an alpha risk at 5%, the necessary number of subjects to include was computed at 348.
For the validation study
Consistent with the first analysis in the training study, the largest width of the 95% CI was 0.22. The error probability mean in diagnosis was 0.23. Considering an effect size uncertainty of 10%, the necessary number of subjects was computed at 70 to test the agreement of cutoffs on repeated observations. Both calculations were performed using the Sample size software (Version 1.0).
Statistical Analysis
Qualitative variables were described by numbers, percentages, and 95% CIs. Quantitative variables were described by means (standard deviation) or medians (interquartile range) according to their distribution.
The primary objective of the training study was to compare the maximum value reached with both dynamometers. Therefore, analysis of the primary endpoint was carried out by computing the ICC between these maxima based on the Bland and Atman concordance method.
The secondary endpoints such as the comparison of values according to patient’s position (seated/bedridden) or between the 3 measurement attempts for each tool were evaluated by a paired Student’s t test, if the distribution of the quantitative variables follows a normal distribution, otherwise by the Wilcoxon signed ranks test. For each position (seated/bedridden), the ICC between the values obtained with both devices was compared using a 2-way randomized model for simple measurements.
The distribution of patients detected as impaired with low HGS (<16 kg in women and < 27 kg in men) was compared between the 2 devices using McNemar tests. In post hoc analyses, we determined cutoffs maximizing sensitivity and specificity for low HGS diagnosis using the Gripwise using ROC curves and Youden’s Index method, as compared to the recommended cutoffs using the Jamar. This analysis was conducted separately in men and women. To evaluate the agreement of the diagnoses obtained with the Jamar (reference) and the Gripwise, the kappa coefficient and its 95% CI were computed and we performed an asymptotic test for the simple kappa coefficient with the null hypothesis value of kappa was 0. The level of agreement was interpreted according to the value kappa, consistent with the interpretation of Cohen’s kappa: kappa between 0 and 0.20, no level of agreement; 0.21–0.39, minimal level; 0.40–0.59, weak level; 0.60–0.79, moderate level; 0.80–0.90 strong level; and above 0.90 almost perfect level of agreement (19).
The search for risk factors leading to low HGS was carried out. The studied risk factors were variables known or suspected to be associated with HGS including age, sex, BMI, CCI, chronic cancer disease, department of hospitalization, planned hospitalization, and hospital length of stay. First, a univariate analysis was computed, by comparing the group identified as having low HGS with the others, using Chi square tests or Fisher’s exact tests for qualitative variables and unpaired Student’s t tests or Mann–Whitney tests for quantitative variables, according to their distribution. All variables whose p value is less than .20 in univariate analysis were considered for inclusion in the multivariate logistic regression analysis. We used a backward procedure among qualified variables for selecting independent risk factors associated with low HGS, with the threshold of p < .10 to remain in the model. We explored collinearity among of the multivariate models by calculating the variance inflation factor, with a value more than 5 indicating collinearity.
In the validation study, the analyses were conducted separately in men and in women. The ROC curves were performed to observe the AUC and its 95% CI of Gripwise values to predict the low HGS. The sensitivity, the specificity and their 95% CI were determined with the training study cutoffs. The kappa coefficient and 95% CI were computed to evaluate the agreement of the diagnoses obtained with Jamar (reference) and Gripwise in the testing population, and we performed an asymptotic test for the simple kappa coefficient with the null hypothesis value of kappa was 0. The level of agreement was interpreted according to the value kappa, consistent with the interpretation of Cohen’s kappa, as defined above (19).
In both studies (training and validation), a p < .05 was considered statistically significant. For the secondary endpoints, the analyses were conducted on an exploratory basis. The analyses were carried out using SAS V9.4 (SAS Institute Inc., Cary, NC) and R version 3.6.2 (The R Foundation for Statistical Computing, Vienna, Austria).
Results
Participants
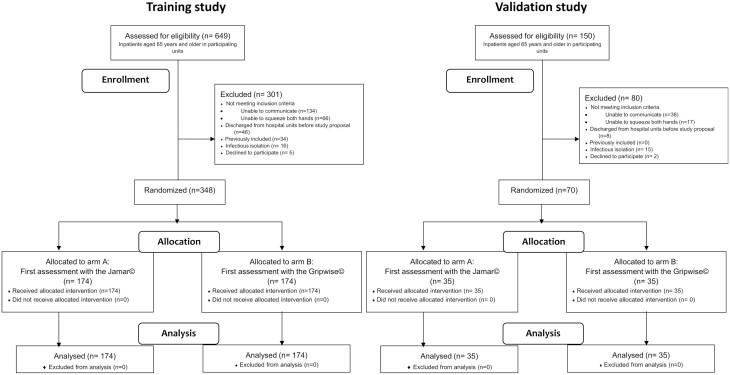
The main study’s recruitment elapsed from September 1 to November 18, 2021. Among 649 eligible inpatients aged 65 years and older in the participating units, 348 were included (Figure 1). Included patients were hospitalized in the internal medicine and rheumatology unit (n = 152), gastroenterology unit (n = 106), dermatology and infectiology unit (n = 53), and geriatric medicine unit (n = 37).
Figure 1.
Flow diagrams of HandGAges study.
The validation study recruitment elapsed from December 19 to 28, 2022. Out of 150 aged inpatients, 70 were included (Figure 1) in the same units, except the geriatric medicine one temporarily affected to COVID patients with hygiene restrictions. The characteristics of both populations are presented in Table 1. There was no missing data for the main variables of interest.
Table 1.
Characteristics of the Study Populations
| Baseline Characteristics | Training Population (n = 348) | Validation Population (n = 70) |
|---|---|---|
| Age (y), mean ± SD | 79 ± 9 | 78 ± 9 |
| Sex, n (%) | ||
| Female | 181 (52) | 30 (43) |
| Male | 167 (48) | 40 (57) |
| Body mass index (kg/m²), mean ± SD | 26 ± 6 | 28 ± 6 |
| Updated Charlson Comorbidity Index, n (%) | ||
| 0 | 70 (20) | 20 (29) |
| 1–2 | 154 (44) | 29 (41) |
| 3–4 | 73 (21) | 12 (17) |
| ≥5 | 51 (15) | 9 (13) |
| Chronic cancer disease, n (%) | 104 (30) | 19 (27) |
| Department, n (%) | ||
| Geriatrics | 36 (10) | 0 (0) |
| Internal medicine | 140 (40) | 41 (59) |
| Dermatology, infectious disease | 53 (15) | 10 (14) |
| Rheumatology | 13 (4) | 0 (0) |
| Gastroenterology | 106 (30) | 19 (27) |
| Planned hospitalization, n (%) | ||
| Yes | 98 (28) | 17 (24) |
| No | 250 (72) | 53 (76) |
| Position, n (%) | ||
| Sitting down | 171 (49) | 41 (59) |
| Bedridden | 177 (51) | 29 (41) |
| Dominant hand, n (%) | ||
| Right | 295 (85) | 59 (84) |
| Left | 19 (5) | 4 (6) |
| Not determined | 34 (10) | 7 (10) |
Comparison of Measurements Between Both Devices
Measurements assessed with the Gripwise were significantly lower than those assessed with the Jamar for both maximal and mean values of the 3 measurements (Table 2). These lower values with the Gripwise were observed regardless of the dominant/nondominant hand, the order of the measurements, and the position (seated/bedridden). There was a significant decrease in HGS values from the first to the third measure for each device. However, this was not clinically relevant because the maximum decrease between the first and third measurements was less than 1 kg (Supplementary Table 1).
Table 2.
Comparison Between the Jamar and the Gripwise Measurements and Intraclass Correlation Coefficient (ICC)
| Jamar | Gripwise | p Value* | ICC (95% CI) | rho | p Value† | |
|---|---|---|---|---|---|---|
| All patients (n = 348) | ||||||
| Overall maximum values, median (IQR) | 19 (14; 28) | 16 (11; 24) | <.001 | 0.93 (0.92; 0.94) | 0.93 | <.001 |
| Overall means of values, median (IQR) | 16 (12; 25) | 13 (9; 20) | <.001 | 0.94 (0.93; 0.95) | 0.94 | <.001 |
| Dominant hand (n = 314) | ||||||
| Maximum values, median (IQR) | 18 (13; 26) | 15 (10; 22) | <.001 | 0.92 (0.91; 0.93) | 0.92 | <.001 |
| Means of values, median (IQR) | 17 (12; 24) | 14 (10; 21) | <.001 | 0.92 (0.91; 0.94) | 0.92 | <.001 |
| No dominant hand (n = 314) | ||||||
| Maximum values, median (IQR) | 18 (12; 26) | 14 (9; 22) | <.001 | 0.94 (0.92; 0.95) | 0.94 | <.001 |
| Means of values, median (IQR) | 16 (12; 24) | 13 (9; 20) | <.001 | 0.93 (0.92; 0.94) | 0.94 | <.001 |
| First measure (n = 348) | ||||||
| Maximum values, median (IQR) | 18 (14; 27) | 15 (10; 23) | <.001 | 0.92 (0.90; 0.93) | 0.91 | <.001 |
| Second measure (n = 348) | ||||||
| Maximum values, median (IQR) | 18 (14; 27) | 15 (11; 23) | <.001 | 0.93 (0.91; 0.94) | 0.93 | <.001 |
| Third measure (n = 348) | ||||||
| Maximum values, median (IQR) | 18 (13; 26) | 14 (10; 22) | <.001 | 0.92 (0.90; 0.93) | 0.92 | <.001 |
| According to position | ||||||
| Seated (n = 171) | ||||||
| Maximum values, median (IQR) | 18 (14; 28) | 15 (11; 22) | <.001 | 0.94 (0.92; 0.95) | 0.94 | <.001 |
| Means of values, median (IQR) | 15 (11; 24) | 12 (8; 19) | <.001 | 0.95 (0.93; 0.96) | 0.96 | <.001 |
| Bedridden (n = 177) | ||||||
| Maximum values, median (IQR) | 20 (15; 29) | 16 (12; 25) | <.001 | 0.93 (0.91; 0.94) | 0.93 | <.001 |
| Means of values, median (IQR) | 17 (13; 26) | 14 (10; 21) | <.001 | 0.93 (0.91; 0.95) | 0.93 | <.001 |
Notes: ICC (95% CI) = intraclass correlation coefficients and 95% confidence interval.
* p Value obtained by paired Student’s t test or by Wilcoxon signed rank test according the distribution.
†Correlation tests.
The Bland–Altman plot is shown in Supplementary Figure 1. The ICC was 0.93 (95% CI [0.92–0.94], p < .001) for the maximum value and 0.94 (95% CI [0.93–0.95], p < .001) for the mean values. The ICC values for the dominant/nondominant hand, order of measurements, and seated/bedridden position were each ≥0.92. The ICC values according to patients’ comorbidities are shown in Supplementary Table 2.
There was a significant difference in detecting low values (<16 kg in women, <27 kg in men) between both devices: found in 169 (48%) patients with the Jamar, and 247 (71%) with the Gripwise (p < .001). In women, a cutoff <12 kg used with the Gripwise was associated with an 80%-sensitivity (95% CI [71%–88%]) and an 89%-specificity (95% CI [82%–95%]) for low HGS diagnosis. In men, a cutoff <22 kg used with the Gripwise was associated with an 89%-sensitivity (95% CI [83%–96%]) and a 94%-specificity (95% CI [90%–99%]) for low HGS diagnosis. Using these new cutoffs, low HGS was detected in 160 (46%) participants using Gripwise. Agreements between both devices when using alternate cutoffs are shown in Table 3. The level of agreement was moderate in women (kappa = 0.68, 95% CI [0.57–0.79]) and strong in men (kappa = 0.83, 95% CI [0.75–0.92]).
Table 3.
Agreement Between Recommended Cutoffs for Low HGS Using the Jamar and Adapted Cutoffs Using the Gripwise in the Training Population
| Women (n = 181) | Men (n = 167) | Total (n = 348) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Using Gripwise | |||||||||
| Low HGS† | Normal HGS | kappa [95% CI] | Low HGS‡ | Normal HGS | kappa [95% CI] | Low HGS†,‡ | Normal HGS | ||
| Using Jamar | Low HGS* | 74 (88%) | 19 (20%) | 0.68 [0.57–0.79] | 69 (91%) | 7 (8%) | 0.83 [0.75–0.92] | 143 (89%) | 26 (14%) |
| Normal HGS | 10 (12%) | 78 (80%) | 7 (9%) | 84 (92%) | 17 (11%) | 162 (86%) | |||
Notes: HGS = handgrip strength; 95% CI = 95% confidence interval.
*Low HGS using Jamar: <16 kg in women and <27 kg in men.
†Low HGS using Gripwise in women: <12 kg.
‡Low HGS using Gripwise in men: <22 kg.
Twenty-two percent of participants found the Gripwise easier to use than the Jamar, 29% the Jamar easier to use than the Gripwise, 41% found them equal, and 9% had no opinion.
Factors Associated With Low Handgrip Strength
In univariate analysis, low HGS values were significantly associated with older age, CCI, department of hospitalization, and planned status of the admission (Supplementary Table 3). In the multivariate model, the older subgroup (aOR = 3.8, 95% CI [2.3–6.1] for participants aged ≥80 years, as compared to younger ones, p < .001), the CCI (aOR = 2.2, 95% CI [1.1–4.7] for scores between 3 and 4 compared to a score of 0, p = .031), and the hospitalization department were significantly and independently associated with a risk of low HGS values.
Validation Study
We further included 70 participants in the validation study. In women (n = 30), a cutoff <12 kg with the Gripwise was associated with a 93%-sensitivity (95% CI: [68%–100%]) and an 87%-specificity (95% CI [60%–98%]) for low HGS diagnosis. In men (n = 40), a cutoff <22 kg using the Gripwise was associated with a 94%-sensitivity (95% CI [71%–100%]) and a 96%-specificity (95% CI [78%–100%]) for low HGS diagnosis. Using these new cutoffs for the Gripwise measurements, low HGS was detected in 47% participants using Gripwise. Agreements between both devices when using alternate cutoffs are shown in Supplementary Table 4. The level of agreement in the validation population was strong whatever the gender (kappa = 0.80, 95% CI [0.59–1.00] in women, and kappa = 0.90, 95% CI [0.76–1.00] in men).
Discussion
In a large population of older inpatients, we found that HGS measurements recorded with the Gripwise were highly correlated to those collected with the Jamar, regardless of the dominant/nondominant hand, order of measurements, or seated/bedridden position of patients. The HGS measurements with the Gripwise were generally lower than those measured with the Jamar, resulting in overdiagnosis of low HGS with the Gripwise. Nevertheless, the use of alternative cutoffs for the Gripwise seems promising to accurately diagnose low HGS.
We found a high correlation between Gripwise and Jamar measurements, as some other devices did before. However, most previous studies included young and/or healthy participants (4–8,10,13,14,20). In few studies that included unhealthy, older patients (9,11,12), correlation levels were lower, and only one device (Sammons Preston) had an ICC > 0.90 compared to the Jamar (9). Thus, the high correlation found in our study with targeted aged inpatients is of great interest for its use in older population. It is of note that ICC was slightly lower in patients with dementia, as compared to patients with other comorbidities. Thus, measurements obtained using the Gripwise in this specific population should thus be cautiously interpreted.
It is likely that the lower values obtained with Gripwise are related to the different shape of the handles, inducing differences in finger muscle activation. Indeed, the curved shape of the Jamar enables patients to squeeze harder by adding the thumb index clamp, which would be less involved with the straight shape of the Gripwise. Therefore, using the actual version of Gripwise dynamometer requires alternate cutoffs. The alternate cutoffs computed in the present study reported a high sensitivity and specificity, even excellent in men. The weaker concordance between the Gripwise and the Jamar in women could be due to the size of their hands, the registered contact surface pressure being different than in men. As women had lower values of HGS as compared to men, this lower concordance between dynamometers in women could also be explained by the fact that the Jamar previously showed lower performances in persons with low HGS (3).
Considering different methods to screen sarcopenia, HGS is more strongly associated with prognosis than chair stand test in inpatients (21). However, measuring HGS in inpatients is challenging, as they cannot all be transferred to a seated position. In our study, the Southampton protocol could not be used in half of the participants. There is a need for a validated dynamometer to be used either in bedridden or seated position. Here, we provide a standardized method to assess HGS reaching similar ICC in both seated/bedridden positions. It could have been of great interest to assess HGS in both positions in participants who were able to maintain the seated position, and to assess correlation between the measurements obtained in both positions. However, this was not planned in the initial protocol and each participant was assessed in only one position. Moreover, a recent study found that measuring HGS in a setting or a standing position leads to similar results regarding low HGS diagnosis (22).
In our study, older age, comorbidity, and being hospitalized in some departments were associated with low HGS. Thus, these factors could indirectly reflect both the multimorbidity burden and nutritional status of the patients. Indeed, nutritional status, physical activity level, cognitive status, or depressive disorders were previously reported as risk factors for sarcopenia (23).
The main strength of the present study is the inclusion of a large sample of patients. Most of the previously published studies comparing the Jamar to other dynamometers included less than 100 (4–7,9–14) or 200 participants (8,15). The only study that included more participants (n = 486) found a poor correlation between the Vigorimeter and Jamar (20). A second strength is that we focused on older patients, who were only investigated in 3 other studies (9,11,12), although they are at higher risk of sarcopenia, on which multicomponent interventions could have an impact (17,24). Finally, we externally validated alternative cutoffs for Gripwise. We also must acknowledge some limitations. First, this was a monocenter study that included mainly Caucasian older inpatients, which cannot assure the generalizability of our findings. Consequently, our results need to be confirmed in different settings, including community-dwelling older adults, but also younger adults and adults of different ethnicities, as HGS widely differs according to ethnicity and geographic localization (25). Second, our protocol of HGS assessment slightly differed from the Southampton protocol, as we did not alternate hands after each measurement, but consecutively performed the 3 measurements with the first hand, and then with the second one. However, this is likely that this did not lead to significant bias, as the ICC computed using first to third measurements, nondominant or dominant hand were all high. Furthermore, the randomization made that each participant could start with one or the other dynamometer. Third, it would have been of great interest to study if HGS was associated with body composition, in order to assess the performance of Gripwise not only for sarcopenia screening but also for sarcopenia diagnosis. However, this was not the main objective of our study, and thus muscle mass assessment was not initially planned in the study design. Finally, users may now be familiar with the cutoffs used in current guidelines, which could be a barrier to the implementation of this new dynamometer requiring alternate thresholds.
Conclusion
The Gripwise demonstrated highly correlated performances to the Jamar in HGS measurements in older inpatients. However, its use could lead to overdiagnosing low HGS and it is therefore necessary to validate other cutoffs in different populations, particularly in community-dwelling older adults, as well as for the diagnosis of sarcopenia by assessing the association with low muscle mass. Given the small size and weight of the Gripwise dynamometer, it is a very promising tool for prospectively tracing the evolution of the HGS in patients with chronic illnesses follow-up. This would allow to early detect the occurrence of sarcopenia and implement physical exercise and nutritional support to reverse it.
Supplementary Material
Acknowledgments
The authors are grateful to Pr. Achille Aouba, Pr. Christian Marcelli, Dr. Benoit Dupont, Pr. Marie-Astrid Piquet, Pr. Anne Dompmartin, Pr. Renaud Verdon, and Dr. Pablo Descatoire for having given authorization for recruitment in their hospital units. Antoine Morin, datamanager, for having designed the database. Ricardo Moura, chief executive of Gripewisetech, for letting us use the devices and accepting this challenge.
Contributor Information
Cédric Villain, Department of Geriatric Medicine, Centre Hospitalier Universitaire de Caen Normandie, Normandie Univ., UNICAEN, INSERM U1075, COMETE, Caen, France.
Soazig Lebaube, Department of Geriatric Medicine, Centre Hospitalier Universitaire de Caen Normandie, Caen, France.
Corinne Kremer, Department of Geriatric Medicine, Centre Hospitalier Universitaire de Caen Normandie, Caen, France.
Chantal Chavoix, Normandie Univ., UNICAEN, INSERM U1075, COMETE, Caen, France.
François Fournel, Clinical Research Department, Centre Hospitalier Universitaire de Caen Normandie, Caen, France.
Anaïs R Briant, Biostatistical Unit of Research Department, Centre Hospitalier Universitaire de Caen Normandie, Caen, France.
Bérengère Beauplet, Department of Geriatric Medicine, Centre Hospitalier Universitaire de Caen Normandie, Normandie Univ., UNICAEN, INSERM U1086, ANTICIPE, Caen, France; Normandy Interregional Oncogeriatric Coordination Unit, Caen, France.
Lewis A Lipsitz, (Medical Sciences Section).
Funding
This work was supported by a grant from the “Unité de Coordination en OncoGériatrie InterRégionale de Normandie” (coordination unit in Geriatric Oncology of Normandy, France), funded by the French National Cancer Institute (INCa).
Conflict of Interest
None.
Author Contributions
C.V., S.L., C.K., C.C., F.F., A.R.B., and B.B. designed the study. C.V., S.L., C.K., and B.B. included the participants. A.R.B. performed the statistical analyses. C.V. and B.B. wrote the first draft of the manuscript, which has been critically reviewed and validated by all coauthors.
References
- 1. Cruz-Jentoft AJ, Bahat G, Bauer J, et al. ; Writing Group for the European Working Group on Sarcopenia in Older People 2 (EWGSOP2), and the Extended Group for EWGSOP2. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing. 2019;48(1):16–31. 10.1093/ageing/afy169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Petermann-Rocha F, Balntzi V, Gray SR, et al. Global prevalence of sarcopenia and severe sarcopenia: a systematic review and meta-analysis. J Cachexia Sarcopenia Muscle. 2022;13(1):86–99. 10.1002/jcsm.12783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Roberts HC, Denison HJ, Martin HJ, et al. A review of the measurement of grip strength in clinical and epidemiological studies: towards a standardised approach. Age Ageing. 2011;40(4):423–429. 10.1093/ageing/afr051 [DOI] [PubMed] [Google Scholar]
- 4. Mathiowetz V. Comparison of Rolyan and Jamar dynamometers for measuring grip strength. Occup Ther Int. 2002;9(3):201–209. 10.1002/oti.165 [DOI] [PubMed] [Google Scholar]
- 5. Bellace JV, Healy D, Besser MP, Byron T, Hohman L.. Validity of the Dexter Evaluation System’s Jamar dynamometer attachment for assessment of hand grip strength in a normal population. J Hand Ther. 2000;13(1):46–51. 10.1016/s0894-1130(00)80052-6 [DOI] [PubMed] [Google Scholar]
- 6. Mathiowetz V, Vizenor L, Melander D.. Comparison of baseline instruments to the Jamar dynamometer and the B&L engineering pinch gauge. Occup Ther J Res. 2000;20(3):147–162. 10.1177/153944920002000301 [DOI] [Google Scholar]
- 7. Shechtman O, Davenport R, Malcolm M, Nabavi D.. Reliability and validity of the BTE-Primus grip tool. J Hand Ther. 2003;16(1):36–42. 10.1016/s0894-1130(03)80022-4 [DOI] [PubMed] [Google Scholar]
- 8. Shechtman O, Gestewitz L, Kimble C.. Reliability and validity of the DynEx dynamometer. J Hand Ther. 2005;18(3):339–347. 10.1197/j.jht.2005.04.002 [DOI] [PubMed] [Google Scholar]
- 9. Guerra RS, Amaral TF.. Comparison of hand dynamometers in elderly people. J Nutr Health Aging. 2009;13(10):907–912. 10.1007/s12603-009-0250-3 [DOI] [PubMed] [Google Scholar]
- 10. Bohannon RW. Parallel comparison of grip strength measures obtained with a MicroFET 4 and a Jamar dynamometer. Percept Mot Skills. 2005;100(3 Pt 1):795–798. 10.2466/pms.100.3.795-798 [DOI] [PubMed] [Google Scholar]
- 11. Sipers WMWH, Verdijk LB, Sipers SJE, Schols JMGA, van Loon LJC.. The Martin Vigorimeter represents a reliable and more practical tool than the Jamar dynamometer to assess handgrip strength in the geriatric patient. J Am Med Dir Assoc. 2016;17(5):466.e1–466.e7. 10.1016/j.jamda.2016.02.026 [DOI] [PubMed] [Google Scholar]
- 12. Vermeulen J, Neyens JCL, Spreeuwenberg MD, van Rossum E, Hewson DJ, de Witte LP.. Measuring grip strength in older adults: comparing the grip-ball with the Jamar dynamometer. J Geriatr Phys Ther. 2015;38(3):148–153. 10.1519/JPT.0000000000000034 [DOI] [PubMed] [Google Scholar]
- 13. Svantesson U, Nordé M, Svensson S, Brodin E.. A comparative study of the Jamar and the Grippit; for measuring handgrip strength in clinical practice. Isokinet Exerc Sci. 2009;17(2):85–91. 10.3233/IES-2009-0338 [DOI] [Google Scholar]
- 14. Hamilton GF, McDonald C, Chenier TC.. Measurement of grip strength: validity and reliability of the sphygmomanometer and Jamar grip dynamometer. J Orthop Sports Phys Ther. 1992;16(5):215–219. 10.2519/jospt.1992.16.5.215 [DOI] [PubMed] [Google Scholar]
- 15. Guerra RS, Amaral TF, Sousa AS, Fonseca I, Pichel F, Restivo MT.. Comparison of Jamar and bodygrip dynamometers for handgrip strength measurement. J Strength Cond Res. 2017;31(7):1931–1940. 10.1519/JSC.0000000000001666 [DOI] [PubMed] [Google Scholar]
- 16. Gariballa S, Alessa A.. Sarcopenia: prevalence and prognostic significance in hospitalized patients. Clin Nutr. 2013;32(5):772–776. 10.1016/j.clnu.2013.01.010 [DOI] [PubMed] [Google Scholar]
- 17. Papadopoulou SK, Tsintavis P, Potsaki P, Papandreou D.. Differences in the prevalence of sarcopenia in community-dwelling, nursing home and hospitalized individuals. A systematic review and meta-analysis. J Nutr Health Aging. 2020;24(1):83–90. 10.1007/s12603-019-1267-x [DOI] [PubMed] [Google Scholar]
- 18. Watanabe T, Owashi K, Kanauchi Y, Mura N, Takahara M, Ogino T.. The short-term reliability of grip strength measurement and the effects of posture and grip span. J Hand Surg. 2005;30(3):603–609. 10.1016/j.jhsa.2004.12.007 [DOI] [PubMed] [Google Scholar]
- 19. McHugh ML. Interrater reliability: the kappa statistic. Biochem Med. 2012;22(3):276–282. 10.11613/BM.2012.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Fike ML, Rousseau E.. Measurement of adult hand strength: a comparison of two instruments. Occup Ther J Res. 1982;2(1):43–49. 10.1177/153944928200200105 [DOI] [Google Scholar]
- 21. Verstraeten LMG, de Haan NJ, Verbeet E, van Wijngaarden JP, Meskers CGM, Maier AB.. Handgrip strength rather than chair stand test should be used to diagnose sarcopenia in geriatric rehabilitation inpatients: REStORing health of acutely unwell adulTs (RESORT). Age Ageing. 2022;51(11):afac242. 10.1093/ageing/afac242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Chen X, Liu G, Li S, et al. Handgrip measurement method affects asymmetry but not weakness identification in community-dwelling older adults. J Am Med Dir Assoc. 2023;24(3):284–291.e3. 10.1016/j.jamda.2022.10.013 [DOI] [PubMed] [Google Scholar]
- 23. Kitamura A, Seino S, Abe T, et al. Sarcopenia: prevalence, associated factors, and the risk of mortality and disability in Japanese older adults. J Cachexia Sarcopenia Muscle. 2021;12(1):30–38. 10.1002/jcsm.12651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Welch C, Majid Z, Greig C, Gladman J, Masud T, Jackson T.. Interventions to ameliorate reductions in muscle quantity and function in hospitalised older adults: a systematic review towards acute sarcopenia treatment. Age Ageing. 2021;50(2):394–404. 10.1093/ageing/afaa209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Woo J, Arai H, Ng TP, et al. Ethnic and geographic variations in muscle mass, muscle strength and physical performance measures. Eur Geriatr Med. 2014;5(3):155–164. 10.1016/j.eurger.2014.04.003 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.