Abstract
Atypical femoral fractures (AFFs) are rare adverse effects of bisphosphonate therapy. We report an unusual case of bilateral diaphyseal AFFs in an antiresorptive-naïve Singaporean Chinese female with Graves’ disease. She presented with complete right AFF requiring surgical fixation, and persistent left incomplete AFF for over four years. Femoral bowing, varus femoral geometry, and ethnic influence likely contributed to the AFFs’ formation. This case may provide insights into the pathogenesis of AFFs in high-risk Asian populations.
Keywords: atypical femoral fracture, bisphosphonate-naïve, antiresorptive-naïve, hyperthyroidism, Asian ethnicity
INTRODUCTION
Atypical femoral fractures (AFFs) are rare stress fractures of the femur with unique radiological and clinical features as outlined by the American Society for Bone and Mineral Research (ASBMR) 2014 Task Force report.1 They are commonly associated with long-term bisphosphonate (BP) therapy for osteoporosis but are under-recognized in antiresorptive-naïve patients. Although the overall incidence of AFF in the BP-naïve population is low at 0.3 to 0.9 per 100,000 person-years, compared to 55 to 113 per 100,000 person-years in BP users,1–3 a study on the Southeast Asian (SEA) population revealed that 47.8 % of AFFs were non-BP related.2
The risk of AFF is three to six times higher among Asians, with the highest incidence in those from the SEA region.2,3 Differences in lower limb geometry, genetic variation in bone architecture and BP metabolism have been hypothesized to account for the higher incidence.3 We report an unusual case of an antiresorptive-naive Singaporean Chinese female with hyperthyroidism who developed bilateral AFFs persisting for four years.
CASE
A 67-year-old Singaporean Chinese female presented in 2019 with a two-day history of right thigh pain after minimal trauma. She fell in a sitting position but could still get up and was able to ambulate with pain. Two days later, she experienced right lower extremity weakness and loss of weight-bearing ability. This was associated with mild, intermittent left thigh pain lasting for one year. She was initially diagnosed with antalgic gait secondary to leg length discrepancy. A bilateral full-length lower limb radiograph was performed in 2018 (Figure 1). Her pain improved and she was lost to follow-up.
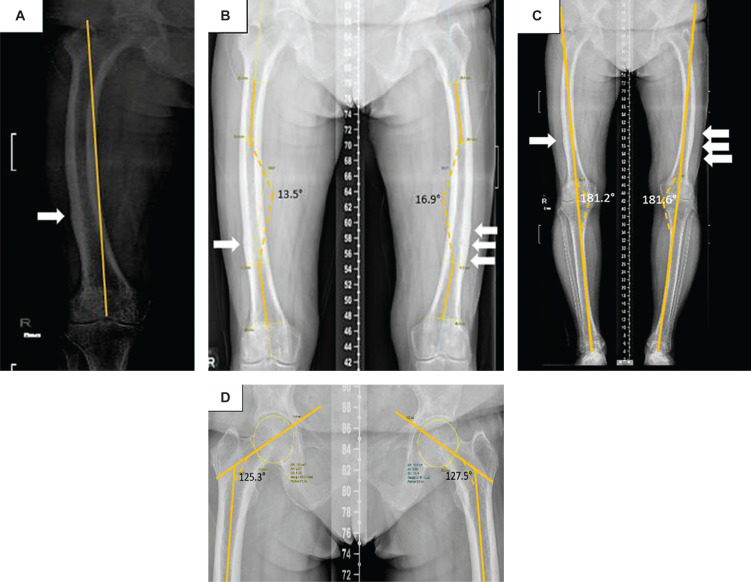
Figure 1.
Bilateral lower limb full-length radiographs taken in 2018. (A) Severe (Grade III) lateral femoral bowing, determined by drawing a reference line from the tip of the greater trochanter to the centre of the intercondylar notch and identifying its position being off the medial cortex using Park et al’s grading system for anterolateral femoral bowing.4 (B) Lateral femoral bowing angle measured by the angulation between the proximal and distal quarters of the femoral diaphysis using Yau’s method5 showing right and left lateral bowing angle of 13.5° and 16.9° respectively. (C) The standing anatomical femorotibial angle (FTA) measured 181.2° on the right and 181.6° on the left (yellow line), suggestive of a varus alignment.6 (D) Femoral neck-shaft angle (FSA) of 125.3° on right and 127.5° on left, consistent with varus hip alignment.6,7 Multiple transverse linear lucencies (white arrows) and cortical thickening at the mid to distal thirds of femoral shafts were seen.
Her past medical history included Graves’ disease with suboptimal control for 15 years. Her main exercise was aqua aerobics. There was no personal or family history of fracture, autoimmune disease or malignancy. She attained menopause at the age of 50. She was on carbimazole and had never been on bisphosphonates, denosumab, long-term corticosteroids or proton-pump inhibitors.
On examination, her right thigh was tender and swollen. Neurovascular examination of the lower limbs was otherwise unremarkable. She was thyrotoxic but not in thyroid storm. She was overweight by Asian body mass index (BMI) criteria (BMI 24.9, height 164 cm, weight 67 kg). There was no blue sclera, hearing loss, abnormal dentition, or skeletal deformities.
Diagnostic assessment
On admission, a bilateral femoral radiograph was performed (Figure 2) which showed an acute complete right diaphyseal AFF and bilateral chronic incomplete AFFs, in accordance with the ASBMR 2014 criteria.1 A review of her old radiographs revealed the presence of bilateral cortical thickening and multiple transverse linear lucent lines at the lateral femoral cortices since 2018, suggestive of chronic stress fractures with bony remodelling (Figure 1).
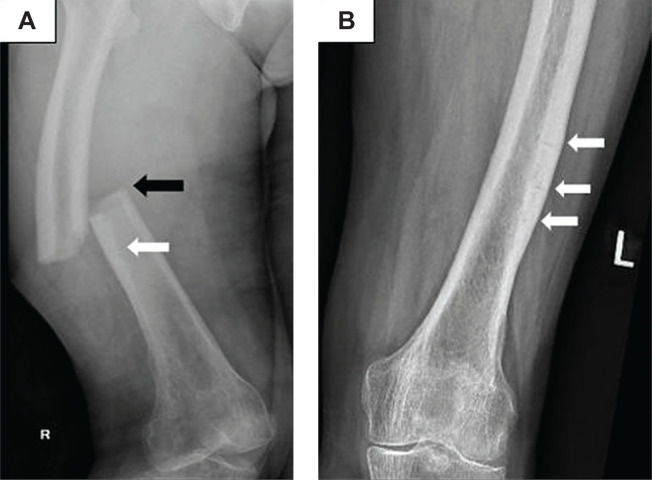
Figure 2.
Radiographs of bilateral femurs on admission in 2019. (A) A complete, non-comminuted transverse fracture of the mid right femoral shaft with medial spike (black arrow), consistent with a right complete AFF; and a faint transverse lucent line below indicative of incomplete AFF (white arrow). (B) Left incomplete AFFs (white arrows) with periosteal cortical thickening and multiple intracortical transverse fracture lines.1
There was bilateral Grade III (severe) lateral femoral bowing based on the grading system for anterolateral femoral bowing made by Park et al.,4 with a lateral femoral bowing angle of 13.5° on the right and 16.9° on the left (Figure 1A and 1B).5 The standing anatomical femorotibial angle (FTA) measured 181.2° on the right and 181.6° on the left, suggesting a varus alignment (Figure 1C).6 Varus femoral neck-shaft angles (FSA) measuring 125.3° on the right and 127.5° on the left were also seen (Figure 1D).6,7
Laboratory investigations (Table 1) showed normal serum parathyroid hormone (PTH), corrected calcium, phosphate, and creatinine levels. She was vitamin D deficient. Thyroid panel showed primary hyperthyroidism. Serum alkaline phosphatase (ALP) and fasting C-telopeptide (CTX) were elevated at 136 U/L (2.27 μkat/L) and 1.11 μg/L respectively. The bone turnover markers may be elevated due to increased bone remodeling during acute AFF healing and may also be due to hyperthyroidism.1,8 Dual-energy x-ray absorptiometry (DXA) showed osteopenia.
Table 1.
Laboratory investigations during admission in 2019
| Blood investigation | Results | Reference range |
|---|---|---|
| Corrected calcium, mmol/L | 2.31 | 2.1- 2.6 |
| Phosphate, mmol/L | 1.06 | 0.65- 1.65 |
| Magnesium, mmol/L | 0.83 | 0.65- 0.95 |
| Creatinine, μmol/L | 50 | 50- 90 |
| Intact Parathyroid hormone (PTH), pmol/L | 3.39 | 1.3- 7.6 |
| 25-hydroxyvitamin D, ng/mL | 16.1 | 30- 100 |
| Alkaline phosphatase (ALP), U/L | 136 | 39- 99 |
| C-telopeptide (CTX), μg/L | 1.11 | Postmenopausal: 0.177-1.015 |
| Free Triiodothyronine (fT3), pmol/L | 6.57 | 2.5- 5.5 |
| Free Thyroxine (fT4), pmol/L | 24.33 | 10- 20 |
| Thyroid stimulating hormone (TSH), mIU/L | <0.004 | 0.4- 4.0 |
| TSH Receptor Antibody, IU/L | 2.6 | <2.0 |
| Fasting glucose, mmol/L | 6.8 | 3.9- 6.0 |
| (mg/dL) | -122.5 | (70.3- 108.1) |
| DXA scan: | ||
|
0.696 (-1.1) | T-score <-1.0 to >-2.5: Osteopenia; |
|
0.753 (-1.4) | T-score <-2.5: Osteoporosis |
|
0.731 (-2.4) | |
BMD denotes bone mineral density
Abnormal values are indicated in bold. Full blood count, renal panel and liver function tests were unremarkable.
Therapeutic intervention
A Thomas splint was applied while awaiting optimization for surgery. Oral cholecalciferol 50,000 IU once weekly and calcium carbonate 1250 mg BD were initiated. Carbimazole was increased from 15 mg to 40 mg daily, and propranolol 20 mg BD was started for thyrotoxicosis. Following normalization of the FT3 and FT4 levels, she underwent intramedullary nailing (IMN) of the right femur on the fourth hospital day. Surgical fixation of the left femur was not concurrently performed as she was asymptomatic at that time and also with the consideration of the potential difficulties of performing prophylactic nailing on her severely bowed left femur.4
Outcome and follow-up
Post-operative recovery was uncomplicated. Carbimazole was tapered once patient achieved euthyroidism, with care to avoid hypothyroidism that may suppress bone turnover and delay AFF healing.8 Serum 25-hydroxyvitamin D (34.1 ug/L; 85.11 nmol/L), ALP (97 U/L; 1.62 μkat/L), and CTX (0.96 ug/L) levels eventually normalized.
Follow-up imaging showed stable alignment of the IMN and progressive healing of the right AFF (Figure 3A). However, in 2020, she reported a recurrence of left thigh pain and a repeat left femoral radiograph showed persistence of the stress fractures. After a multidisciplinary discussion, she opted for conservative therapy for the left incomplete AFF. She continued to experience intermittent, mild left thigh pain during ambulation. In 2022, 4 years since her first consult, the latest radiographs done showed non-healing lucent lines on the left femur (Figure 3B). Magnetic resonance imaging (MRI) of the left thigh (Figures 3C and D) showed chronic periosteal thickening and sclerosis of the lateral aspects of the distal femoral shaft with areas of serous marrow atrophy, likely secondary to the reparative process from cortical stress fractures. She declined prophylactic nailing of the left femur. Calcium and cholecalciferol supplementation were continued and she was advised to avoid strenuous lower limb activity. She was offered treatment with an anabolic agent (teriparatide) in the hopes of facilitating fracture healing but this was declined due to her concern of the possible adverse effects.
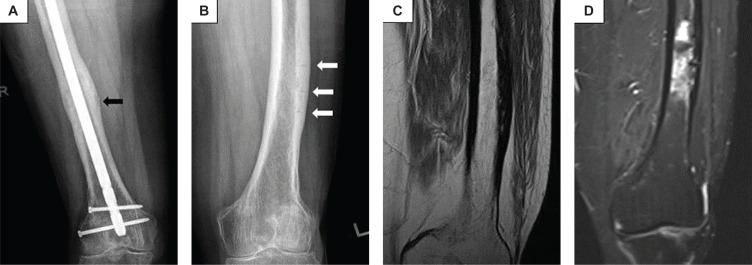
Figure 3.
Radiographs and Magnetic resonance imaging (MRI) during follow-up visits in 2020 and 2022. (A) Radiograph of right femur at 10 months (2020) after surgery showing callus formation, progressive healing of right mid-shaft AFF (black arrow) and stable alignment of the IMN. (B) Radiograph of left femur taken in 2022 showed persistence of the transverse lucent lines and thickened lateral cortex (white arrows), suggestive of persistent incomplete AFFs. (C) Turbo spin-echo proton-density weighted (TSE PD) MRI of left thigh showing chronic periosteal thickening and cortical sclerosis of the lateral aspects of the distal femoral shaft while (D) Turbo inversion recovery magnitude (TIRM) MRI shows irregular intramedullary high fluid signal of the distal left femoral shaft adjacent to the area of chronic cortical sclerosis, likely reflecting areas of bone marrow oedema secondary to reparative process from atypical cortical stress fractures.
DISCUSSION
The exact pathogenesis of AFFs remains elusive, with several proposed mechanisms. It has been hypothesized that BPs and conditions with low bone turnover predispose to AFF formation by suppressing bone remodeling, which involves (a) osteocyte apoptosis; (b) increased production of receptor activator of nuclear factor-kappa B ligand (RANKL); (c) osteoclastic resorption of damaged tissue and (d) osteoblastic formation to replace resorbed bone.1 BPs tend to localize at sites with high bone turnover, such as stress fracture sites. They impair targeted remodeling and intracortical repair of these sites, leading to microcrack propagation and fracture.1 Suppressed bone remodeling may also weaken bone material properties, resulting in harder but more brittle bones with decreased resistance to microcrack progression.1,7
It is interesting that our patient has long-standing hyper-thyroidism, a condition usually associated with accelerated bone remodeling.8 Hyperthyroidism shortens the bone turnover cycle from seven months to three to four months, by increasing the number of osteoclast resorption sites. The ratio of bone resorption to bone formation is increased, leading to cumulative new bone loss and osteoporosis. Unfortunately, we do not have bone histomorphometry analysis of the AFF lesions. To our knowledge, this is the first reported case of persistent AFF in a BP-naïve patient and in a patient with Graves’ disease.
Reports of AFFs in patients without prior antiresorptive use or in conditions with low bone turnover suggest alternative mechanisms. The propensity for AFFs to be bilateral and at a similar location on contralateral limbs suggests a relationship between lower limb geometry and AFF formation.1,7 Patients who developed AFFs had significantly greater anterior and lateral bowing angles of the femoral diaphysis compared to controls and those with typical femoral fractures.6 Using 2D-3D X-ray scanner EOS™ imaging technology to compare femur geometry, each degree increase in lateral bowing was associated with a 46% increase in AFF risk.6,7
The mean femoral anterior radius of curvature in elderly Japanese females was reported as 104 cm compared to 120 cm in Americans. AFF patients were found to have even higher curvatures, with a mean of 59.4 cm (range 48.2 to 81.4 cm).6 A smaller radius indicates higher femoral curvature. As femur length increases, femoral bowing decreases. This may explain the higher incidence of AFF among Asians, who have shorter femurs and greater bowing compared to Caucasians.
As bowing increases, the area of greatest tensile stress is hypothesized to migrate distally, giving rise to a more distal AFF location.6,7 The average lateral bowing angle was 10.10° in the diaphyseal group and 3.33° in the subtrochanteric group, with logistic regression analysis showing increased diaphyseal fractures in lateral bowing angles greater than 5.25°.6
Although these studies used different methods in measuring femoral bowing, they all showed that anterolateral femoral bowing was associated with an increased risk for diaphyseal AFF. We used the visual grading system by Park et al., (Figure 1A) to identify severe lateral femoral bowing in our patient, as this may be a convenient and practical method for clinicians to identify at-risk femoral curvature in the clinical setting.4 We also measured the angulation between the proximal and distal quarters of the femoral diaphysis to identify an increase in lateral bowing (Figure 1B).5
Other aspects of lower limb geometry hypothesized to influence the risk of AFF are the FSA and FTA. There are suggestions that AFF patients had more varus FSA compared to the non-fractured or typical femoral fracture subjects,6,7,9 but other studies did not find such an association.6 Varus FSA of less than 128.3° yielded 69% sensitivity and 63% specificity for the development of AFF.7 FSA may influence the location of AFF, with smaller FSA in patients with subtrochanteric AFF (125.8°), compared to mid-shaft AFF (130.8°) and non-fractured controls (131.8°).9 Standing anatomical FTA of patients with diaphyseal AFF were reported to be significantly larger (varus alignment, mean 183.3°) compared to those with subtrochanteric AFF (172.8°), typical femoral fracture and Japanese population cohort (177.6°).6
Femoral bowing, varus FSA and FTA alignment in our patient may alter the mechanical axis of the lower limb, leading to concentration of tensile stress in the anterolateral cortex, stress damage, and microcrack formation.6,7 Over time, this abnormal repetitive loading may go beyond the body’s capacity to repair by targeted remodeling, leading to stress fractures.1 This was supported by biomechanical analysis using quantitative computed tomography/finite element (CT/FE), demonstrating that AFFs occur precisely where maximum tensile stress appears and the location of AFF is determined by individual stress distribution, strongly influenced by femoral bowing and weakly correlated with FSA.9 Future studies should be done to validate the use of these measurements and grading system to better identify individuals at higher risk of AFF.
Additional factors such as Vitamin D deficiency has been associated with the development of AFF.1 Serum 25-hydroxyvitamin D concentrations of less than 16 ng/ mL increased the risk of subtrochanteric AFF in 1 series (Odds ratio 3.2).10 Vitamin D deficiency leads to reduced calcium absorption and impaired bone mineralization. The resultant osteomalacia may predispose to femoral bowing and insufficiency fractures.11 The patient was vitamin D deficient when she presented with right complete AFF, but there was no associated secondary hyperparathyroidism, hypocalcaemia, or hypophosphatemia. Her chest radiograph did not show signs of childhood rickets and she is of normal adult height. Despite repleting her vitamin D and normalization of serum ALP, the left incomplete AFFs persisted for years.
Evidence of genetic influence on AFF was first reported in 3 sisters with AFFs and long-term BP therapy. Whole exome sequencing showed p.Asp188Tyr mutation in the GGPS1 gene in the mevalonate pathway critical to osteoclast function, which is inhibited by BP.7 Twenty-one rare genetic variants and polygenicity were subsequently reported.12 AFFs were also found to be associated with 7 monogenetic bone disorders, such as osteogenesis imperfecta, pycnodysostosis, and hypophosphatasia.12 The patient did not have any clinical features of these conditions; her serum ALP level a few years prior to her AFF was normal. Future studies should look into genetic factors that may play a role in predisposing risk of AFF in the Asian and Singaporean populations. These factors may be associated with bone metabolism and its interaction in the setting of BP exposure.
Previous studies in BP users have reported that only 5% to 18% of non-surgically managed incomplete AFFs showed radiological regression at an average of 11 months to 5.3 years, more frequently when BPs are discontinued.13 The delayed healing was thought to be due to the long skeletal half-life of BPs and its suppression of intracortical bone remodelling. Our patient was not exposed to BP, but she has lower limb geometries that predispose to increased tensile load on the lateral femoral cortex and she is of high-risk ethnicity, supporting that AFFs are stress fractures that developed from recurrent abnormal loading on the lateral cortex and/or due to inherent factors such as genetic predisposition.
AFFs are associated with increased morbidity, with delayed fracture healing in 26% of cases and a low rate of spontaneous healing among the conservatively managed incomplete AFFs.1 Symptomatic patients with cortical lucency are at increased risk of progressing to complete fracture and prophylactic nail fixation is recommended.1,13 However, some patients may opt for non-surgical management, consisting of limitation of weight-bearing activities, adequate calcium and vitamin D replacement, and cessation of antiresorptive medication (where applicable). Anabolic agents, such as teriparatide administration for 1 to 24 months, have been used to accelerate fracture healing in AFFs, although high-quality data on its use is lacking.1,7 If there is no improvement after 2 to 3 months of conservative management, prophylactic nail fixation should strongly be considered.1
CONCLUSION
In summary, we have presented a case of persistent bilateral AFFs in a BP-naïve patient of Singaporean Chinese ethnicity. It highlights the fact that AFFs can be chronic, and the presenting symptoms can be mild and intermittent; subtle early radiographic changes can be easily missed until a precipitant such as low trauma or the initiation of BP therapy leads to a complete fracture. This case may serve to provide a better understanding of the pathogenesis of AFFs, especially the role of mechanical and inherent factors in high-risk ethnic groups. With the rising prevalence of osteoporosis and antiresorptive use, further research is needed to better understand the exact pathogenesis of AFFs and the role of femoral geometry, genetic and clinical risk factors in identifying individuals at high-risk for AFFs. Further studies are also needed in understanding the optimal treatment regimen to prevent future fracture in the high-risk AFF individuals.
Ethical Considerations
Patient consent was obtained before submission of the manuscript.
Statement of Authorship
The authors certified fulfillment of ICMJE authorship criteria.
CRediT Author Statement
KSC: Conceptualization, Validation, Resources, Data Curation, Writing – original draft preparation, Writing – review and editing, Visualization, Project administration; LMD: Validation, Resources, Data Curation; LRC: Validation, Data Curation, Writing – review and editing, Visualization; LUG: Conceptualization, Validation, Writing – review and editing, Supervision.
Author Disclosure
The authors declared no conflict of interest.
Funding Source
None.
References
- 1.Shane E, Burr D, Abrahamsen B, et al. Atypical subtrochanteric and diaphyseal femoral fractures: Second report of a task force of the American Society for Bone and Mineral Research. J Bone Miner Res. 2014;29(1):1–23. PMID: . 10.1002/jbmr.1998. [DOI] [PubMed] [Google Scholar]
- 2.Gani LU, Anthony NF, Dacay LM, Tan PT, Chong LR, King TFJ. Characteristics of bisphosphonate and non-bisphosphonate related atypical femoral fracture in a South East Asian population - Secondary analysis. Bone. 2022;162:116455. PMID: . 10.1016/j.bone.2022.116455. [DOI] [PubMed] [Google Scholar]
- 3.Nguyen HH, Lakhani A, Shore-Lorenti C, et al. Asian ethnicity is associated with atypical femur fractures in an Australian population study. Bone. 2020;135:115319. PMID: . 10.1016/j.bone.2020.115319. [DOI] [PubMed] [Google Scholar]
- 4.Park YC, Song HK, Zheng XL, Yang KH. Intramedullary nailing for atypical femoral fracture with excessive anterolateral bowing. J Bone Joint Surg. 2017;99(9):726–35. PMID: . 10.2106/JBJS.16.00760. [DOI] [PubMed] [Google Scholar]
- 5.Yau W, Chiu K, Tang W, Ng T. Coronal bowing of the femur and tibia in Chinese: Its incidence and effects on total knee arthroplasty planning. J Orthop Surg (Hong Kong). 2007;15(1):32–6. PMID: . 10.1177/230949900701500108. [DOI] [PubMed] [Google Scholar]
- 6.Haider IT, Schneider PS, Edwards WB. The role of lower-limb geometry in the pathophysiology of atypical femoral fracture. Curr Osteoporos Rep. 2019;17(5):281–90. PMID: . 10.1007/s11914-019-00525-x. [DOI] [PubMed] [Google Scholar]
- 7.Starr J, Tay YKD, Shane E. Current understanding of epidemiology, pathophysiology, and management of atypical femur fractures. Curr Osteoporos Rep. 2018;16(4):519–29. PMID: . PMCID: . 10.1007/s11914-018-0464-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gorka J, Taylor-Gjevre RM, Arnason T. Metabolic and clinical consequences of hyperthyroidism on bone density. Int J Endocrinol. 2013;2013:638727. PMID: . PMCID: . 10.1155/2013/638727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Oh Y, Fujita K, Wakabayashi Y, Kurosa Y, Okawa A. Location of atypical femoral fracture can be determined by tensile stress distribution influenced by femoral bowing and neck-shaft angle: A CT-based nonlinear finite element analysis model for the assessment of femoral shaft loading stress. Injury. 2017;48(12):2736–43. PMID: . 10.1016/j.injury.2017.09.023. [DOI] [PubMed] [Google Scholar]
- 10.Girgis CM, Sher D, Seibel MJ. Atypical femoral fractures and bisphosphonate use. N Engl J Med. 2010;362(19):1848–9. PMID: . 10.1056/NEJMc0910389. [DOI] [PubMed] [Google Scholar]
- 11.Uday S, Högler W. Spot the silent sufferers: A call for clinical diagnostic criteria for solar and nutritional osteomalacia. J Steroid Biochem Mol Biol. 2019;188:141–6. PMID: . 10.1016/j.jsbmb.2019.01.004. [DOI] [PubMed] [Google Scholar]
- 12.Nguyen HH, van de Laarschot DM, Verkerk AJ, Milat F, Zillikens MC, Ebeling PR. Genetic risk factors for atypical femoral fractures (AFFs): A systematic review. JBMR Plus. 2018;2(1):1–11. PMID: . PMCID: . 10.1002/jbm4.10024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Png MA, Mohan PC, Koh JSB, Howe CY, Howe TS. Natural history of incomplete atypical femoral fractures in patients after a prolonged and variable course of bisphosphonate therapy - A long-term radiological follow-up. Osteoporos Int. 2019;30(12):2417–28. PMID: . 10.1007/s00198-019-05067-7. [DOI] [PubMed] [Google Scholar]