Abstract
Advancing age and many disease states are associated with declines in nicotinamide adenine dinucleotide (NAD+) levels. Preclinical studies suggest that boosting NAD+ abundance with precursor compounds, such as nicotinamide riboside or nicotinamide mononucleotide, has profound effects on physiological function in models of aging and disease. Translation of these compounds for oral supplementation in humans has been increasingly studied within the last 10 years; however, the clinical evidence that raising NAD+ concentrations can improve physiological function is unclear. The goal of this review was to synthesize the published literature on the effects of chronic oral supplementation with NAD+ precursors on healthy aging and age-related chronic diseases. We identified nicotinamide riboside, nicotinamide riboside co-administered with pterostilbene, and nicotinamide mononucleotide as the most common candidates in investigations of NAD+-boosting compounds for improving physiological function in humans. Studies have been performed in generally healthy midlife and older adults, adults with cardiometabolic disease risk factors such as overweight and obesity, and numerous patient populations. Supplementation with these compounds is safe, tolerable, and can increase the abundance of NAD+ and related metabolites in multiple tissues. Dosing regimens and study durations vary greatly across interventions, and small sample sizes limit data interpretation of physiological outcomes. Limitations are identified and future research directions are suggested to further our understanding of the potential efficacy of NAD+-boosting compounds for improving physiological function and extending human health span.
Keywords: Clinical trials, Nicotinamide riboside, Nutrition, Physiology, Successful aging
Aging is the primary risk factor for a host of chronic disorders, including cardiovascular and metabolic diseases, chronic kidney disease, Alzheimer’s disease, and related dementias, which represent many of the leading causes of death in developed and developing countries. As the number of older adults rises rapidly, the personal and societal burden of chronic diseases will subsequently grow. This heightens the need for efficacious interventions that can mitigate age-related declines in physiological function by targeting fundamental mechanisms of aging to extend healthspan and reduce morbidity in midlife and older adults.
A central molecule in cellular energy metabolism and nutrient sensing is nicotinamide adenine dinucleotide (NAD+), an essential coenzyme in redox reactions and co-substrate for nonredox-related NAD+-dependent enzymes (1). NAD+ concentrations in humans may be ~10%–80% lower with advancing age (2–8). Additionally, numerous diseases are associated with reduced NAD+ levels, including metabolic disorders, cancer, and neurodegenerative diseases (1,9). Although such declines in NAD+ levels have not been observed universally across all tissues (10), the potential benefits of restoring tissue and cellular NAD+ abundance back to young, healthy concentrations on physiological function with aging remain a prevailing question in biomedical research.
Promising preclinical findings indicate that increasing NAD+ abundance is causally linked to improvements in physiological function and select markers of healthspan. NAD+ precursors, that is, endogenous molecules involved in the synthesis or regeneration of NAD+, can improve outcomes such as glucose and insulin regulation (11), mitochondrial oxidative metabolism (12), muscle and physical functioning (13), vascular function (14), and cognitive function (15) in numerous preclinical mouse models of aging, obesity, and Alzheimer’s disease. These improvements in physiological function in animal models may be mediated in part by improvements in mitochondrial function and reductions in oxidative stress, DNA-damage pathways, and inflammation (14,16,17). These observations have spurred interest in translating NAD+-boosting strategies for human health.
Herein, we summarize the currently available results from clinical trials assessing the efficacy of NAD+ precursors for increasing NAD+ levels, promoting healthy aging, and improving age- and disease-associated declines in physiological function. Of the numerous NAD+ precursors available for study in humans, most data have been collected on supplementation with niacin (ie, nicotinamide and nicotinic acid). Niacin has long been recognized for improving lipid metabolism and blood lipid levels (18); however, more recent meta-analyses have concluded that niacin may not be an effective strategy for reducing cardiovascular or all-cause mortality (19,20), limiting the biomedical significance of this treatment. More pragmatically, niacin also is commonly associated with side effects, particularly painful flushing, which further hinders its clinical use by lowering treatment adherence. Nevertheless, niacin supplementation has been investigated for its potential neuroprotective effects and may be useful as a therapeutic strategy to combat neuropathological conditions (21). Moreover, supplementation with niacin improves muscle performance and mitochondrial biogenesis in patients with adult-onset mitochondrial myopathy (22). Thus, although niacin may not be a viable NAD+-boosting compound for treating cardiometabolic disorders, it may hold promise as a therapy in other pathophysiological settings. The following discussion will not include niacin therapies but interested readers are directed to reviews on niacin (23,24). Rather, we specifically focus this review on supplementation with nicotinamide riboside (NR); NR in combination with the polyphenol, pterostilbene; and nicotinamide mononucleotide (NMN), as these compounds make up the majority of published clinical trials to date (Figure 1). Additionally, we excluded the review of clinical trials that studied the effects of a single dose of an NAD+ precursor as we were primarily interested in the chronic effects of supplementation. Short-term administration (ie, less than 2 weeks of treatment) of NAD+-boosting compounds is not the focus of this review but will be discussed where applicable. Lastly, we discuss the limitations of the research to date and potential future directions for further investigation of oral supplementation with NAD+-boosting compounds in humans. For the purposes of this review, we will use the term “NAD+ metabolome” to refer to the interconnected small molecules that are involved in, or products of, the biosynthesis and breakdown of NAD+ (25).
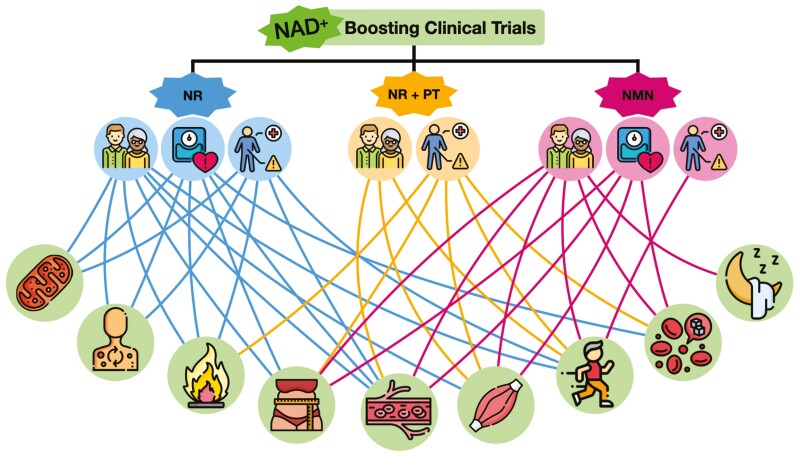
Figure 1.
Conceptual display of physiological outcomes assessed in currently published clinical trials investigating chronic supplementation of nicotinamide adenine dinucleotide (NAD+) precursors. Nicotinamide riboside (NR), NR plus pterostilbene (PT), and nicotinamide mononucleotide (NMN) are the main NAD+-boosting compounds that have been tested in clinical trials to date. Studies have been performed in healthy young, midlife, and older populations (middle, far left icon), as well as populations with risk factors such as overweight/obesity (middle, second from left icon), and patient populations (middle, third from left icon). Physiological outcomes assessed include, but are not limited to, (bottom left to right) mitochondrial function, metabolism, inflammation, body composition, vascular function, muscular strength, exercise capacity/physical function, glucose-insulin regulation, and sleep. Designed with resources from flaticon.com.
NAD+-Precursor Clinical Trials
Nicotinamide Riboside
Much of the human data on NAD+-boosting strategies for improving physiological function come from clinical trials investigating oral supplementation with NR (Figure 2). The focus of many of these trials was to assess the safety and efficacy of supplementation with NR for increasing NAD+ levels or NAD+-related metabolites in the context of healthy aging and diseased populations over intervention durations ranging from 1 to 20 weeks. Additional physiological outcomes were investigated to gain insight into the effects of chronic supplementation on cardiovascular, metabolic, motor, skeletal muscle, and adipose tissue function, with variable results. Of note, most of the trials performed to date have consisted of relatively small sample sizes (n ≤ 40), thus limiting the definitive conclusions that can be drawn regarding any treatment-induced changes in physiological function.
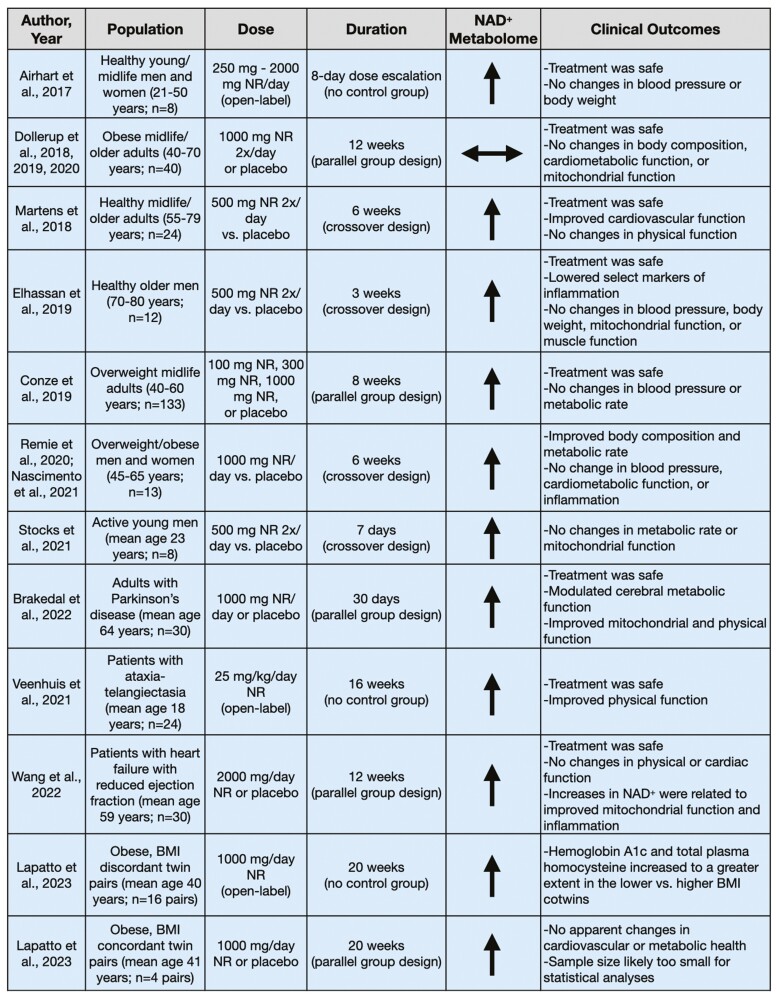
Figure 2.
Clinical trials assessing oral supplementation with nicotinamide riboside (NR).
Generally healthy populations
The majority of early studies investigating dietary supplementation with NR were performed in healthy young, midlife, and older adults to gain preliminary insight into treatment safety and efficacy. Airhart et al. performed an open-label, nonrandomized trial to initially investigate the pharmacokinetics and NAD+-boosting capabilities of orally supplemented NR (26). Eight healthy men and women (aged 21–50 years) took increasing doses of NR (250 mg/d up to 2 000 mg/d) over an 8-day period. In this timeframe, NAD+ abundance in whole blood (measured by mass spectrometry) increased twofold on average and no clinically significant changes were seen in the prespecified laboratory safety endpoints (ie, serum potassium, creatine kinase, glucose, uric acid, and alanine aminotransferase). Furthermore, no changes in blood pressure, body weight, or white blood cell count were observed. There were slight reductions in hematocrit, hemoglobin, and platelet count, though these changes have not been observed in subsequent trials. This trial was not blinded nor placebo controlled, but it provided some initial evidence that supplementation with NR was safe in humans and could effectively boost NAD+ abundance.
Martens et al. performed the first randomized, double-blind, placebo-controlled, crossover design clinical trial of NR treatment in healthy midlife and older men and women (age 55–79 years) (27). Twenty-four participants were supplemented with NR (500 mg twice per day) capsules for 6 weeks to investigate its safety, tolerability, and efficacy for raising NAD+ levels and its associated metabolites, versus placebo treatment. Oral supplementation with NR was safe, well tolerated, and increased NAD+ levels (assessed by mass spectrometry) in peripheral blood mononuclear cells by ~60%. Supplementation with NR also increased nicotinic acid adenine dinucleotide (NAAD), a product of NR utilization (28), by approximately sevenfold. Additionally, the investigators gained initial insight into the effects of chronic supplementation with NR for improving cardiovascular and other physiological functions, although it is stated that these outcomes were exploratory in nature. They found that NR decreased casual (resting) systolic blood pressure and arterial stiffness (carotid–femoral pulse wave velocity), with the largest improvements seen in those with above-normal systolic blood pressure (≥120 mmHg) at baseline. However, the authors note that the effects were not statistically significant after correction for multiple comparisons. Additional outcomes, such as endothelial function, motor function, and exercise capacity, were unchanged following the intervention. Although these preliminary results from Martens et al. suggest selecting cardiovascular benefits of supplementation with NR, other trials have shown no changes in blood pressure following treatment with NR (29–31). A larger (targeted enrollment = 118 participants), appropriately powered, parallel-group design, randomized controlled trial with a 3-month treatment duration is underway to assess the therapeutic potential of NR on blood pressure and arterial stiffness in midlife and older adults (ClinicalTrials.gov Identifier: NCT03821623) (32).
Elhassan et al. performed a small, double-blind, placebo-controlled, crossover design study in 12 older men (age 70–80 years) to assess the effects of 3 weeks of supplementation with NR (500 mg twice per day), with a 3-week washout period between treatments (29). The investigators sought to determine whether NR could boost the NAD+ metabolome (assessed by mass spectrometry) and alter skeletal muscle mitochondrial bioenergetics. They found that 3 weeks of supplementation increased skeletal muscle NAAD (twofold) and products of nicotinamide methylation clearance pathways (fivefold; ie, N-methyl nicotinamide [MeNAM]; N-methyl-2-pyridone-5-carboxamide [Me2PY]; and N-methyl-4-pyridone-5-carboxamide [Me4PY]) but did not change NAD+ levels. Further, supplementation had no effect on mitochondrial bioenergetics, despite inducing downregulation of energy metabolism. Additionally, neither hand grip strength nor forearm muscle blood flow was changed after supplementation with NR, nor were cardiometabolic parameters such as blood pressure, body weight, and lipid profiles affected by the intervention. However, Elhassan et al. did observe ~50%–70% reductions in select plasma markers of inflammation, including interleukin (IL)-6, IL-5, and IL-2. Chronic inflammation is a hallmark of aging and many age-associated diseases, so the potential for supplementation with NR to modulate systemic inflammation is a promising finding. Larger studies in both sexes are warranted to examine the effects of NR on reducing tonic inflammation in midlife and older adults.
Some investigators have posited that the benefits of NAD+-boosting therapies may be more efficacious in settings of cellular stress, such as that elicited by acute exercise. This led Stocks et al. to perform a placebo-controlled, crossover design study assessing the effects of 7 days of supplementation with NR (500 mg twice per day) on whole-body substrate utilization and skeletal muscle mitochondrial function at rest and following acute steady-state exercise in 8 young men (33). NR treatment did not increase NAD+ levels in skeletal muscle (assessed by mass spectrometry) but did increase the abundance of the deaminated NAD+ precursors nicotinic acid mononucleotide and nicotinic acid riboside (approximately two- to threefold), as well as methylated nicotinamide breakdown products (Me2PY and Me4PY). However, 7 days of oral supplementation did not change whole-body metabolism or substrate utilization at rest or during exercise. Furthermore, Stocks et al. found that skeletal muscle mitochondrial function, skeletal muscle sirtuin activity, and poly(ADP-ribose) polymerase 1 (PARP1; an NAD+-consuming enzyme) protein content, measured at rest and postexercise, were unchanged following 7 days of supplementation with NR. The impact of a longer duration of supplementation with NR on NAD+-consuming enzymes is unknown.
Overweight and obese populations
Given the role of NAD+ in metabolism and promising preclinical results, there has been considerable interest in testing dietary supplementation with NR in overweight and obese adults with the goal of improving body composition and metabolic function. Thus far, 4 clinical trials have been performed assessing supplementation with NR in this population.
Dollerup et al. conducted a 12-week randomized, double-blind, placebo-controlled, parallel-group design clinical trial in 40 sedentary men (age 40–70 years) with a body mass index (BMI) >30 kg/m2 (34–36). The investigators aimed to determine the safety, tolerability, and efficacy of NR for improving insulin sensitivity and other metabolic markers, as well as pancreatic and mitochondrial function. They found that NR (1 000 mg twice per day) was safe, well tolerated, and resulted in increased urinary NR and NAD+-related catabolites (measured by mass spectrometry). However, there was no change in skeletal muscle NAD+ metabolites (measured via a florescence-based cycling enzymatic assay) and supplementation decreased nicotinamide phosphoribosyltransferase (NAMPT), an enzyme involved in the biosynthesis of NAD+ from nicotinamide. Furthermore, 12 weeks of supplementation with NR did not affect insulin sensitivity, body composition, resting energy expenditure, pancreatic function, incretin hormone secretion, or skeletal muscle mitochondrial respiration, content, or morphology. Dollerup et al.’s findings question whether oral supplementation with NR can be effective in improving metabolic health in sedentary, obese men.
Considering the need to understand the optimal dose of NR for improving health, Conze et al. (30) investigated the kinetics and dose-dependent effects of oral supplementation with NR. Overweight (BMI 25–30 kg/m2), but otherwise healthy men and women (age 40–60 years; n = 133) were randomized to placebo or 100, 300, or 1 000 mg of supplementation with NR per day for 8 weeks, which resulted in dose-dependent increases in whole-blood NAD+ levels measured by mass spectrometry (100 mg = +10%; 300 mg = +48%; 1 000 mg = +139%), which were significantly different from placebo in the 300 and 1 000 mg groups. Plasma nicotinamide also was increased in the 1 000 mg group versus placebo, and plasma and urinary levels of MeNAM and Me2PY increased in the 300 and 1 000 mg groups. However, despite increasing the abundance of NAD+, no clinical effects (blood pressure, body weight, blood chemistries, or resting energy expenditure) were observed.
A crossover design study was performed in 13 overweight or obese (BMI 27–35 kg/m2) men and women (age 45–65 years) who were supplemented with placebo or NR (1 000 mg/d) for 6 weeks each to investigate changes to metabolic phenotypes, mitochondrial function, and brown adipose tissue activity (31,37). Supplementation with NR resulted in increased markers of NAD+ synthesis in skeletal muscle (NAAD increased approximately sevenfold; MeNAM increased approximately fourfold; measured via mass spectrometry), as well as decreased fat mass (−4%), increased fat-free mass (+2%), and increased sleeping metabolic rate (+4%), the latter which was likely due to the increase in fat-free mass. However, the intervention did not affect insulin sensitivity, ambulatory blood pressure, systemic inflammation, brown adipose tissue activity, or cold-induced thermogenesis. The select, minor benefits on metabolic health seen in this study are somewhat promising but, to our knowledge, no other studies of NR have replicated these results.
In the longest NR clinical trial to date, Lapatto et al. investigated 5 months of oral supplementation with NR (38). Sixteen BMI-discordant twin pairs (within-pair difference in BMI ≥2.5 kg/m2; average BMI 30 kg/m2; mean age ~40 years old) were supplemented with NR (increasing from 250 to 1 000 mg/d by 1 month, 1 000 mg/d for the remaining 4 months). The investigators measured several outcomes, including mitochondrial biogenesis, satellite cell differentiation, gut microbiome composition, and cardiovascular and metabolic health. No placebo group was included in this open-label trial, so it is not possible to determine if any changes observed over the treatment period can be attributed to NR per se. It is possible, however, to assess changes in outcomes with treatment in the heavier versus leaner BMI-discordant cotwins. Multiple markers of NAD+ abundance in whole blood (measured by mass spectrometry) increased in all twins over the course of the intervention, with Me4PY increasing to a greater extent in the heavier versus leaner cotwins. Muscle mitochondrial biogenesis, satellite cell differentiation, gut microbiome composition, insulin sensitivity, and cardiovascular outcomes did not differ with treatment within the BMI-discordant twin pairs but hemoglobin A1c and total plasma homocysteine increased more in the lower compared with the higher BMI cotwins. Additionally, 4 BMI-concordant twin pairs (within-pair difference in BMI <2.5 kg/m2; average BMI 32 kg/m2; mean age ~41 years old) were assessed. Twins within a pair were randomized to receive placebo or NR (same dose and duration as BMI-discordant twin pairs). The limitations imposed by the small sample size and statistical tests utilized aside, descriptively speaking, there were no obvious effects of NR in the 4 BMI-concordant twin pairs on any outcomes.
Patient populations
Although NR has mostly been studied in generally healthy midlife and older adults, supplementation with NR may be more effective in individuals with greater baseline physiological dysfunction, such as those with neurodegeneration or cardiovascular diseases. Currently, 3 trials have investigated oral supplementation with NR in patient populations.
Brakedal et al. examined its effects in newly diagnosed men and women with Parkinson’s disease (mean age 64 years) (39). Thirty days of supplementation with NR (1 000 mg/d) elicited increases in brain NAD+ (assessed by magnetic resonance spectroscopy), and NAD+-related metabolites in the cerebrospinal fluid. However, the response was variable, with only 10/13 participants demonstrating an increase in NAD+ levels, and 9/10 exhibiting an increase ≥10%. In the participants that exhibited increased brain NAD+ after supplementation with NR, cerebral metabolism was increased in select brain regions, indicative of increased bioenergetic efficiency, which was related to modest improvements in motor function. In addition, those who took NR exhibited improved mitochondrial, lysosomal, and proteasomal function within skeletal muscle and peripheral blood mononuclear cells. Select markers of inflammation measured in serum and cerebrospinal fluid also were reduced after 30 days of supplementation; however, many of the markers were also reduced following the placebo treatment. Combined results suggest initial promise for NR as a potential neuroprotective therapy in Parkinson’s disease; however, larger trials are needed to confirm its beneficial effects in this population.
Veenhuis et al. performed an open-label trial investigating the effects of 4 months of supplementation with NR (25 mg/kg/d) in 24 children and young men and women with the neurodegenerative disorder ataxia telangiectasia (40). Preclinical models of this disorder are characterized by impaired DNA repair, oxidative stress responses, and energy metabolism, including neuronal NAD+ deficiency (41,42). NR-related plasma metabolites (measured by mass spectrometry) tended to increase (~eightfold increase in Me2PY, ~eightfold increase in Me4PY, ~32-fold increase in MeNAM, and ~threefold increase in nicotinamide) in all subjects. Additionally, ataxia rating scores improved after the 4-month treatment period, though no placebo group was available for comparison. These results highlight how NAD+-boosting strategies may be beneficial even in young populations when NAD+ abundance is compromised due to disease processes.
Further extending the range of populations in which supplementation with NR has been tested, Wang et al. explored the therapeutic potential of NR treatment in 30 patients with heart failure with reduced ejection fraction (mean age 59 years) (43). The investigators found that 12 weeks of oral supplementation with NR (500 mg/d titrated up to 2 000 mg/d by Week 3) was safe, well tolerated, and approximately doubled whole blood NAD+ levels (measured via mass spectrometry). Supplementation did not improve functional capacity (6-minute walk test) or cardiac function, but changes in NAD+ in response to NR treatment correlated with improved peripheral blood mononuclear cell mitochondrial respiration and reduced NLRP3 inflammasome gene expression. These results suggest that treatment with NR may lower systemic inflammation in this population by acting on circulating immune cells.
Overall, chronic supplementation with NR has been safely administered at doses up to 2 000 mg/d and its chronic effects have been investigated for up to 20 weeks. Across multiple tissues, supplementation with NR has been reported to increase the abundance of NAD+ and its related metabolites (measured in plasma, whole blood, peripheral blood mononuclear cells, brain, skeletal muscle, and urine). However, increases in NAD+ abundance have not been observed in all studies, including in many of these same tissues. The variability between studies likely arises from the different NR dosing regimens and treatment durations used, and from the different subject populations investigated.
Studies to date on NR are limited by small sample sizes and many lack proper control groups. Oral supplementation with NR may improve select markers of physiological function in certain cohorts of midlife and older adults and, more recently, patient populations. However, multiple randomized controlled trials have shown no effect of NR for improving common clinical outcomes such as insulin sensitivity, energy expenditure, or exercise capacity. These inconsistent findings highlight the need for larger clinical trials in populations of healthy midlife and older adults as well as those with diseases to rigorously evaluate whether there are therapeutic benefits of supplementation with NR.
Nicotinamide Riboside + Pterostilbene
An important class of mammalian NAD+-dependent enzymes are the sirtuins, which play a pivotal role in many functions essential for maintaining cellular homeostasis (44). It has been proposed that sirtuins may mediate physiological improvements evoked by supplementation with NAD+-boosting compounds; however, data on the ability of current NAD+ precursors to increase the activity of sirtuin enzymes in humans are limited. Some researchers have begun to study dietary supplements that combine NR with the polyphenol and putative SIRT-1 activator, pterostilbene (45–47), in hopes of increasing NAD+ abundance such that these NAD+-dependent enzymes can carry out their necessary functions. This section will review the small number of studies that have implemented interventions with the combination therapy of NR plus pterostilbene (Figure 3).
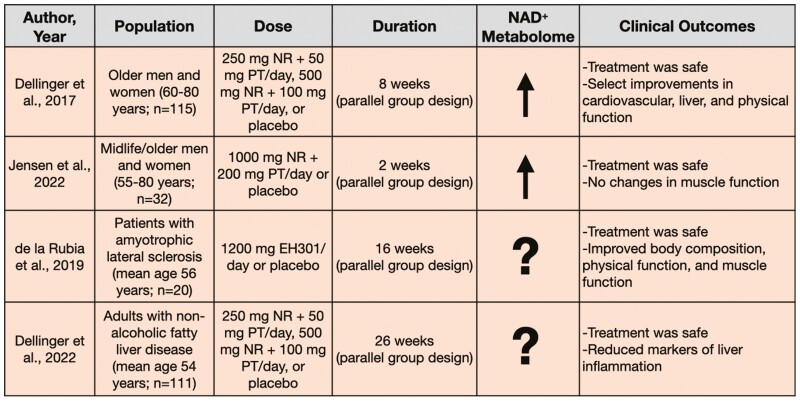
Figure 3.
Clinical trials assessing oral supplementation with nicotinamide riboside (NR) plus pterostilbene (PT).
Generally healthy populations
Dellinger et al. performed a 3-arm trial in 115 generally healthy men and women (age 60–80 years) (48). Participants were randomized to receive a low dose (250 mg NR plus 50 mg pterostilbene), high dose (500 mg NR plus 100 mg pterostilbene), or placebo treatment for 8 weeks. NR plus pterostilbene was found to be safe and increased NAD+ in whole blood (measured by mass spectrometry) in a dose-dependent fashion. That is, NR plus pterostilbene increased NAD+ by 40% in the low-dose group and 90% in the high-dose group. Additionally, despite normal baseline values, the low-dose group saw a reduction in diastolic blood pressure by 3.4 mmHg and decreased plasma alanine transaminase, suggestive of positive effects on cardiovascular and liver function, respectively. Although the high dose did not induce the same positive effects on cardiovascular and liver function as the low dose, it did improve select measures of mobility including the 6-minute walk test and the 30-second chair stand test. Lastly, the high dose also increased total and low-density lipoprotein cholesterol, which is associated with an increased risk of cardiovascular disorders. The latter observation further supports that the low dose might represent a stronger candidate for supplementation in older adults.
Jensen et al. also investigated NR plus pterostilbene for promoting muscle stem cell function in midlife and older adults with muscular injury (49). Thirty-two men and women (age 55–80 years) were randomized to daily supplementation of NR plus pterostilbene (1 000 mg NR plus 200 mg pterostilbene) or placebo. After 2 weeks of supplementation, participants were exposed to electrically induced eccentric muscle work to cause skeletal muscle injury. Skeletal muscle biopsies were obtained before injury and 2 hours, 2 days, 8 days, and 30 days after injury. Jensen et al. observed whole blood, but not skeletal muscle, NAD+ levels were increased approximately threefold after supplementation with NR plus pterostilbene (measured by mass spectrometry). However, muscle stem cell content, proliferation, cell size, and histological analyses of muscle recovery were unchanged. This study found that supplementing NR plus pterostilbene at double the amount initially investigated was safe and increased NAD+ abundance in whole blood, but was not an effective therapy for increasing skeletal muscle NAD+ or muscle recovery in response to injury.
Patient populations
Trials also have been performed to test the NR plus pterostilbene combination in patient populations. A double-blind, randomized, placebo-controlled trial assessed NR plus pterostilbene in the form of EH301 (Elysium Health) in men and women with amyotrophic lateral sclerosis (ALS; mean age 56 years) (50). Sixteen weeks of supplementation with EH301 (1 200 mg/d) led to improvements in an ALS functional rating score, forced vital capacity, and muscular strength. Furthermore, EH301 modestly decreased fat weight (~1 kg) and increased skeletal muscle weight (~0.5 kg) compared to placebo. After the 16-week intervention, subjects were given the option to continue treatment via an open-label extension to the study and were reevaluated after 1 year of supplementation. ALS functional rating scores and muscle function were maintained whereas pulmonary function slightly declined; however, no placebo group was included for comparison at the 1-year mark. Although NAD+ levels were not measured in this trial, the functional improvements observed suggest the initial promise of NR plus pterostilbene for patients with ALS.
Dellinger et al. have since expanded on their original investigation in healthy adults to assess NR plus pterostilbene in 111 adults with nonalcoholic fatty liver disease (mean age 54 years) (51). Their previously employed low- and high-dosing regimens (low: 250 mg NR plus 50 mg pterostilbene; high: 500 mg NR plus 100 mg pterostilbene) were found to be safe and well tolerated for 26 weeks of treatment, though the primary outcome of hepatic fat fraction was not changed by the intervention. In the subset of subjects who were classified as protocol compliant (>80% compliance), select markers of liver damage and inflammation were reduced by the low dose of NR plus pterostilbene relative to placebo supplementation. Insulin resistance and C-reactive protein were improved from baseline in the low-dose group but were not different from the placebo group. Of note, the 2 trials by Dellinger et al. suggest greater clinical efficacy with the lower dose of NR plus pterostilbene (48,51).
In summary, limited data exist on the effects of NR plus pterostilbene, but currently completed trials suggest that this combination therapy may improve select markers of cardiovascular, liver, pulmonary, and muscular function in healthy and diseased populations. Though NR plus pterostilbene may boost NAD+ in a dose-dependent manner, the optimal dosing paradigm to elicit beneficial clinical outcomes is less clear, with lower doses potentially possessing greater clinical efficacy. Of note, no trials have directly compared this combined treatment to supplementation with either NR or pterostilbene in isolation, so it is unclear whether NR plus pterostilbene is truly beneficial in combination or whether the efficacy is driven primarily by 1 or the other major bioactive component.
Nicotinamide Mononucleotide
Numerous preclinical trials have been performed with another NAD+ precursor, NMN, and some have shown promising beneficial effects on physiological outcomes (14,52–54). At first, due to the initial high costs of manufacturing this compound at concentrations necessary for human consumption, translation of NMN into the clinical space lagged compared to other NAD+-boosting compounds such as NR. More recently, NMN has become more available and less expensive; as a result, several clinical trials have recently been completed investigating the efficacy of oral supplementation with NMN in humans, which are outlined subsequently (Figure 4). Of note, at the end of 2022, the Food and Drug Administration announced that NMN can no longer be sold as a dietary supplement in the United States due to its authorization as an investigational new drug. Although clinical trials on the compound can continue to move forward, this announcement may severely limit the availability of NMN products to the general public.
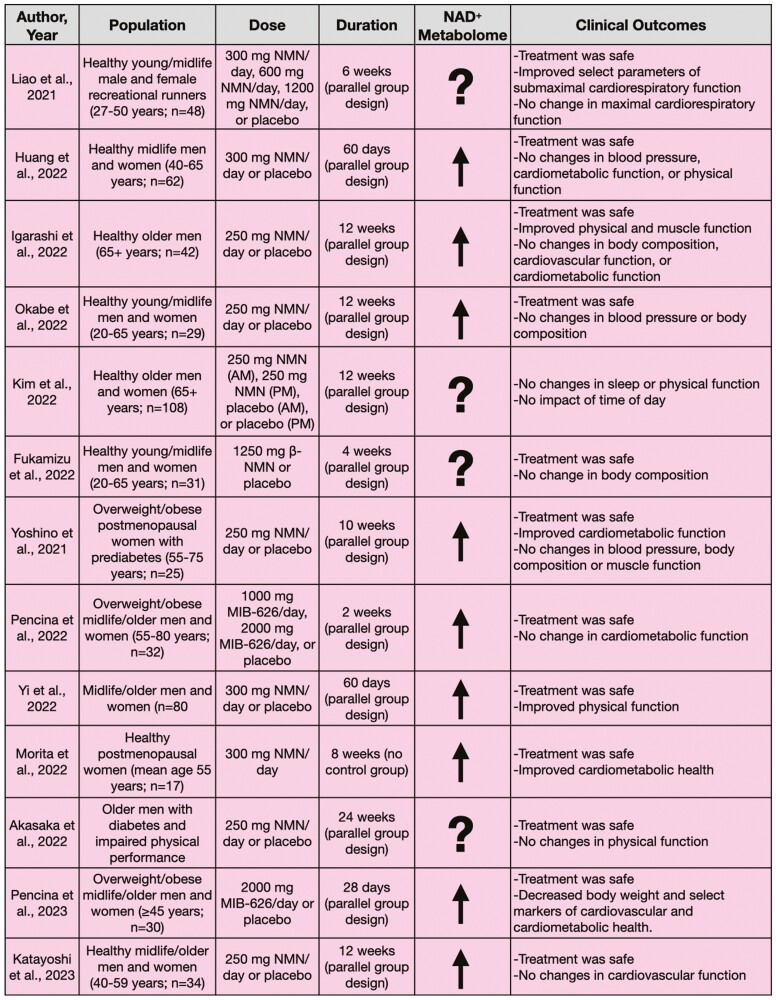
Figure 4.
Clinical trials investigating oral supplementation with nicotinamide mononucleotide (NMN).
Generally healthy populations
Fukamizu et al. recently found that 4 weeks of oral supplementation with NMN (1 250 mg/d) was safe and well tolerated in 31 healthy men and women of varied ages (20–65 years) (55). Indeed, no serious adverse events were reported and hematological tests (eg, white blood cell count), clinical biochemical tests (eg, cholesterol), urinalysis, body composition, and vital signs were unchanged over the 4-week treatment period. However, metabolomic analyses of blood or urine samples were not performed to confirm the efficacy of supplementation for increasing NAD+ or NAD+-related metabolite abundance, limiting the interpretation and potential significance of these results. This study provided initial evidence supporting the safety of NMN, but more rigorous pharmacokinetics studies are necessary to understand the NAD+-boosting potential of orally supplemented NMN.
Liao et al. studied healthy male and female recreational runners (age 27–50 years) in a double-blind, randomized controlled trial assessing the effects of different doses of NMN on aerobic capacity (56). Participants were randomized into 1 of 4 groups (12 participants/group): low dose (300 mg/d NMN), medium dose (600 mg/d NMN), high dose (1 200 mg/d NMN), or placebo control; standardized exercise training was performed in each group across the 6-week intervention. Changes to the NAD+ metabolome were not assessed in this study. Submaximal exercise performance was improved in the medium- and high-dose groups compared to the control group. However, maximal aerobic exercise capacity was unchanged in all groups. These results from Liao et al. suggest NMN treatment was safe when supplemented at up to 1 200 mg/d but efficacious for aerobic performance only at higher (600 and 1 200 mg/d) doses. Whether supplementation with NMN can induce similar or greater beneficial effects on cardiopulmonary exercise performance in midlife and older adults (age 50+ years) is unknown.
In a multicenter, double-blind, placebo-controlled, parallel-group design clinical trial, Huang investigated the effects of 60 days of supplementation with NMN (300 mg/d) in 62 healthy men and women (age 40–65 years) (57). NAD+ levels in the serum (assessed by a colorimetric quantification kit) increased (~10%) after the intervention but functional outcomes, including the 6-minute walk test, blood pressure, insulin sensitivity, and well-being, were unchanged. Overall, this study supports the efficacy of NMN for increasing serum NAD+ abundance in healthy adults, though no clinical benefits were observed.
Another multicenter, randomized controlled trial by Yi et al. assessed the effects of daily oral supplementation with 300, 600, and 900 mg of NMN or placebo for 60 days in 80 midlife/older adults (age 40–65 years) (58). Blood NAD+ concentrations (assessed by a colorimetric quantification kit) increased in the 300 mg (approximately threefold), 600 mg (approximately sixfold), and 900 mg (approximately fivefold) groups but were unchanged in the placebo group. Participants in the 600 mg and 900 mg groups walked ~1.5-fold further during the 6-minute walk test after the intervention. Insulin sensitivity was not affected by supplementation with NMN. Blood-based analysis of biological age (Aging.Ai 3.0 calculator) was unchanged in the NMN-treated groups, but increased significantly in the placebo group after 60 days. This trial showed positive effects of supplementation with NMN on physical function in healthy midlife/older adults and observed no significant differences in treatment efficacy between 600 and 900 mg/d NMN.
Morita et al. performed a single-arm, 8-week intervention trial in 17 healthy postmenopausal women (age 50–80 years) and observed the clinical effects of supplementation with NMN at 300 mg/d (59). Blood nicotinamide abundance increased by approximately fourfold but blood NAD+ levels decreased by ~20% after supplementation (measured by mass spectrometry) compared to baseline. This was accompanied by declines in hemoglobin A1c (a measure of chronic blood glucose concentrations) and elevated high-density lipoprotein cholesterol. No significant changes in body fat percentage, basal metabolic rate, blood pressure, grip strength, or leukemic cell sirtuin 1 mRNA expression were observed. These results should be interpreted with caution as no placebo control group was studied for comparison.
Four randomized controlled trials have assessed supplementation with NMN across 12-week interventions. A broad range of physiological outcomes was assessed but NMN had varying effectiveness. Igarashi et al. found that supplementation with NMN (250 mg/d) in 42 healthy older men (age 65+ years) increased NAD+ levels by sixfold in whole blood (measured by mass spectrometry) (60). NR (~1.4-fold increase), NMN (~1.7-fold increase), nicotinic acid mononucleotide (~23-fold increase), and nicotinic acid riboside (~sevenfold increase) also were increased compared to placebo treatment. Select improvements in motor function, such as increased gait speed and grip strength, were observed, but skeletal muscle mass did not change. Additional outcomes such as fat mass, lipids, insulin sensitivity, glucose tolerance, cognitive function, blood pressure, and endothelial function were unchanged after the intervention. Okabe et al. found that 12 weeks of NMN treatment (250 mg/d) in 29 healthy men and women (age 20–65 years) were safe and increased NAD+ levels in whole blood by ~75% (assessed via mass spectrometry) (61). However, similar to the findings of Igarashi et al., no changes to blood panels, blood pressure, or body composition were observed. Kim et al. investigated the circadian implications of NMN treatment (250 mg/d) through a comparison of 12 weeks of morning versus afternoon supplementation with NMN or placebo in 108 healthy older men and women (age 65+ years) (62). They found that sleep quality, fatigue, and physical performance were not different from placebo. Lastly, Katayoshi et al. assessed the cardiovascular effects of 12 weeks of oral supplementation of NMN (250 mg/d) in 34 healthy midlife/older adults (age 40–59 years) (63). Serum nicotinamide levels (measured by mass spectrometry) were increased by ~60% but NAD+ abundance was below detection limits at all time points. No significant changes in blood pressure or arterial stiffness were observed. The varied outcomes assessed in these studies limit comparison between trials, but together, the results suggest limited effectiveness of 12 weeks of supplementation of NMN in generally healthy adults.
Overweight and obese populations
Three studies have been performed assessing the efficacy of NMN treatment in overweight and obese cohorts. Yoshino et al. performed a 10-week trial in 25 overweight and obese postmenopausal women with prediabetes (age 55–75 years; BMI 25.0–44.9 kg/m2) who were randomized to receive either supplementation with NMN (250 mg/d) or placebo (64). NMN was found to be safe, increased NAD+ content (~50%) in peripheral blood mononuclear cells (measured via mass spectrometry), and increased levels of the muscle NMN metabolites MeNAM, Me2PY, and Me4PY, which the authors suggested may indicate greater NAD+ turnover in skeletal muscle. Furthermore, muscle insulin sensitivity was greater after NMN treatment. However, Yoshino et al. reported that other important clinical outcomes such as body composition, blood pressure, plasma glucose, lipids, and muscle strength and fatigability were unchanged following the intervention.
Additionally, Pencina et al. performed a short-term, 2-week, placebo-controlled study in 32 overweight and obese men and women (age 55–80 years, BMI 28–40 kg/m2) and found that MIB-626, a microcrystalline polymorph of β-NMN oral formulation (1 000 vs 2 000 mg/d), was safe and increased whole blood NAD+ levels (measured by mass spectrometry) in a dose-dependent manner (~100% increase in 1 000 mg/d group; ~200% increase in 2 000 mg/d group) (65). Metabolic markers such as glucose and cholesterol were unchanged.
The same investigators also performed a 4-week, placebo-controlled study in 30 overweight or obese adults (age ≥45 years; mean BMI 29.2 kg/m2) assessing the safety and physiological effects of oral supplementation with 2 000 mg MIB-626 (66). Follow-up blood sampling also was performed 28 days after the completion of supplementation. Whole blood NAD+ (measured by mass spectrometry) increased by ~150%, and NAD+-related metabolites such as Me2PY, nicotinamide, and 1-methylnicotinamide also were higher after supplementation, but all declined to baseline levels after 28 days without treatment. Body weight, total cholesterol, low-density lipoprotein cholesterol, and diastolic blood pressure decreased in the treatment group compared to placebo. In contrast, muscle performance (eg, strength, fatiguability, power), physical function (eg, aerobic capacity), muscle bioenergetics (eg, creatine phosphate depletion and recovery), fasting glucose, and insulin sensitivity were unchanged. Combined, these studies suggest supplementation with NMN is safe and can increase NAD+ abundance in overweight and obese adults. Select clinical improvements also were observed, though how between-study differences in treatment duration and participant sex affected these findings requires further interrogation.
Patient populations
Akasaka et al. assessed the safety and efficacy of NMN treatment in a population of diabetic, physically impaired older men (grip strength <26 kg or walking speed <1.0 m/s) (67). Fourteen participants (mean age 81 years) were supplemented with NMN (250 mg/d) or placebo for 24 weeks. Supplementation with NMN was safe and tolerable, but no changes in grip strength or walking speed were observed between groups. NAD+ levels were not measured. The lack of improvement in physical function in a population with impaired baseline function suggests that this outcome may not be responsive to supplementation with NMN.
Together, supplementation with NMN appears to be safe for up to 24 weeks and, when measured, tends to boost at least some components of the NAD+ metabolome. However, the efficacy of NMN for improving physiological function is less clear. Whereas some studies have seen beneficial effects of NMN on muscle insulin sensitivity and submaximal exercise performance, several trials have reported no effects of NMN on cardiovascular, metabolic, motor, cognitive, and sleep outcomes. Additional trials are needed to understand the optimal dose, outcomes, and populations in which to harness supplementation with NMN for improving physiological function. Studies to date have mostly focused on healthy adults, including those that are physically active, or adults with cardiovascular disease risk factors. Therefore, studying different patient populations will be necessary to understand the effects of NMN treatment in those with greater baseline physiological dysfunction.
Limitations
Although not universally observed, several clinical studies have shown that NAD+ precursors can boost NAD+ abundance and levels of NAD+-related metabolites in several different tissues. Interventions have raised NAD+ levels in midlife and older adults by ~10%–100% (27,30,39,43,48,49,57), with some trials seeing even greater improvements (60,65). Many of these initial investigations also have reported on the safety and tolerability of supplementation across varied doses and durations with no indications of serious adverse effects. However, there are important limitations to consider regarding the investigation of NAD+ precursor supplementation in humans.
Oral Bioavailability of NAD+ Precursors
Although some studies have quantified an increase in NAD+ abundance after treatment with NR or NMN, many clinical trials to date do not provide evidence that oral supplementation of these NAD+ precursors raised tissue NAD+ levels. It is important to note that the exact metabolic fates of orally administered NR and NMN in humans are complex and incompletely understood. Moreover, interindividual differences in intestinal transport and metabolism of these compounds likely play a role in the absorption of oral NR or NMN and may be involved in the variability of metabolites quantified between studies (68). Different pathways have been identified in attempts to understand the oral bioavailability of NR, such as the conversion of NR to NAD+ via NAAD (28). Due to its hydrophilicity, NR is expected to have a low passive permeability across the human intestinal mucosa (26). Indeed, there is evidence to suggest a role of the gut microbiome in the degradation of oral NR to nicotinic acid, which may boost in vivo NAD+ via microbiota-dependent deamidation pathways (69,70). Additionally, the ability of NR to transport freely into cells versus the need for NMN to be dephosphorylated to NR or utilize a transporter for cell entry is controversial (71,72). Answers to these questions are crucial to our understanding of the regulation of intestinal and systemic NAD+ metabolism. Overall, limited data exist on the pharmacokinetics of orally supplemented NAD+ precursors in humans. As such, definitive studies on the pharmacokinetics of orally dosed NR and NMN are needed.
Quantification of NAD+ Abundance
In general, the lack of standardization of sample collection procedures and analytical methods limits our ability to compare NAD+ assay results among clinical trials. NAD+ and its related metabolites are highly unstable. As such, neutralizing samples and appropriate storage with internal standards are necessary for clinical trials during which the degradation of samples over the course of the intervention is a critical issue. Interlaboratory differences in internal standards and sensitivity of assays also weaken our understanding of the effects of orally dosed NAD+ precursors on NAD+ abundance among studies. An additional weakness is that many extraction solvents used to maintain NAD+ degrade NADH due to differences in pH-dependent stability of the 2 metabolites, thus limiting quantification to only part of the NAD+ metabolome. Moreover, the selection of the proper extraction solvent is critical to prevent the degradation of NAD+ into other metabolites. Mass spectrometry and nuclear magnetic resonance spectroscopy are the most common and accurate methods for measuring NAD+ levels (73). Although much progress has been made to improve the quantification of NAD+ across different tissues, currently available techniques are expensive, require technical expertise, and still may exhibit inherent sensitivity issues. Thus, further optimization and standardization of these methods are necessary to establish greater confidence in NAD+ quantification within and between studies. NAD+ data from clinical trials to date should be interpreted with caution given the limitations and variability of NAD+ quantification methods utilized across studies (73).
Of note, clinical trials that measure changes to NAD+ abundance after precursor supplementation typically use assays that quantify steady-state levels of NAD+ and its related metabolites. However, if supplementation with NAD+ precursors is increasing NAD+ synthesis, this also will be accompanied by increased NAD+ consumption. Thus, assessing only steady-state NAD+ levels provides an incomplete picture of the efficacy of orally dosed NAD+ precursors for boosting in vivo NAD+ bioavailability. Isotope tracer studies are necessary to determine how NAD+ precursors are modulating NAD+ biosynthesis flux.
Future Directions
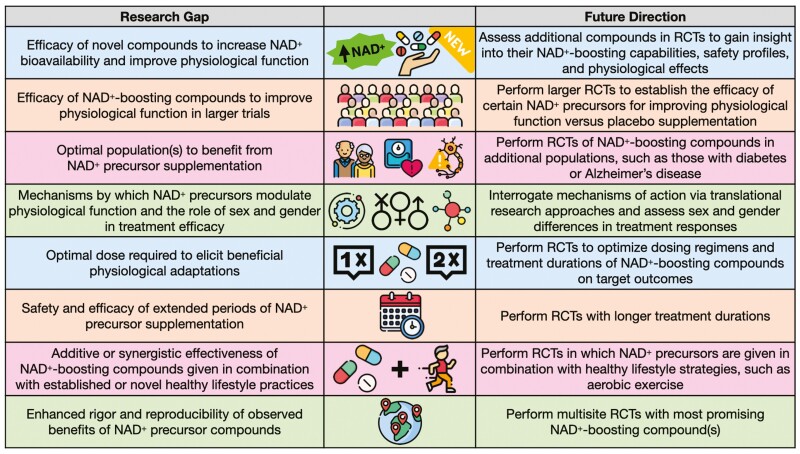
The study of NAD+ precursors for enhancing human physiological function is still in its infancy. As such, knowledge gaps exist that create opportunities for future research directions in this field (Figure 5). Additional NAD+ boosters, beyond those discussed in this review, are presently in various stages of development and may provide additional avenues for enhancing physiological function beyond the precursors currently available for human consumption. These novel NAD+-enhancing agents are harnessing different chemical structures related to NR (eg, dihydronicotinamide riboside (74)) or combining multiple compounds (75,76), including nicotinamide plus d-ribose (77), to explore novel approaches for restoring NAD+ abundance with advancing age and disease. However, it is still unclear whether supplementation with NAD+-increasing compounds can enhance physiological function and improve key clinical outcomes. Meaningful interpretation of the results of published studies is limited by small sample sizes, variations in dosing regimens, wide-ranging treatment durations, and heterogeneous populations. Therefore, future research into NAD+-boosting compounds must involve larger randomized controlled trials that are properly powered to detect effects on physiological outcomes of interest. These trials will need to be performed in both healthy populations and patients with chronic diseases to better determine the groups for whom supplementation with NAD+ precursors is most efficacious. Currently, most trials have either studied only 1 sex or have included small samples of men and women without assessing sex-specific effects of supplementation. Thus, studies that seek to determine whether sex and gender differences exist in treatment efficacy also will help to elucidate the populations who might benefit most from NAD+ precursor supplementation.
Figure 5.
Current research gaps in the field and suggested future directions. NAD+ = nicotinamide adenine dinucleotide; RCT = randomized controlled trial. Designed with resources from flaticon.com.
Differences in efficacy between preclinical and clinical findings on the promise of NAD+ boosters may be due to differences in dosing concentrations and treatment durations. That is, many preclinical studies have given animals higher concentrations of NAD+-boosting compounds, for longer relative durations, than what has been supplemented in human trials (78–80). Furthermore, preclinical studies have not found an optimal intervention duration, often supplementing mice with NAD+ precursors for anywhere between 2 and 20 weeks (14,53,81,82).
Thus, future investigations must work to identify the optimal treatment paradigm for NAD+ boosters to be supplemented to populations of interest to achieve the desired outcomes. This includes identifying appropriate dose concentrations and extending supplementation periods to further characterize the long-term safety and efficacy of treatment. However, it should be recognized that many human trials to date have not shown clinically significant health benefits, so treatment with NAD+ boosters may not be efficacious even in larger trials of longer durations.
Clinical trials to date have primarily investigated NAD+ precursors as monotherapies, so the potential synergistic effects of NAD+ boosters with other healthy lifestyle strategies, such as aerobic exercise or heat therapy (eg, regular use of sauna), are unclear. Future research assessing the combined effectiveness of NAD+-increasing supplements with conventional exercise paradigms, as well as novel interventions (83,84), will add to our understanding of the optimal use of these compounds.
Once target populations and effective regimens are better understood, multisite clinical trials are necessary to enhance the rigor and reproducibility of findings from single-site studies. Numerous trials are currently underway to enhance our understanding of NAD+-boosting therapies for novel populations and extended treatment durations. These trials will help guide the field in our efforts to better characterize the context in which NAD+ precursors are most beneficial.
Conclusion
Promising preclinical findings and safe translation of compounds for human consumption have paved the way for the investigation of oral supplementation with NAD+ precursors as a possible strategy to improve physiological function. NR, NR plus pterostilbene, and NMN are 3 commonly utilized NAD+ precursors that have been investigated. When supplemented, these compounds have been shown to be safe, well tolerated, and may boost the NAD+ metabolome. However, only limited data from select studies have found clinically relevant improvements to physiological function after treatment with these NAD+-boosting supplements. Some studies have reported improvements in cardiovascular, metabolic, and physical function, primarily in groups with chronic disorders or those with reduced baseline function, though these data come from small sample sizes and need to be confirmed. Larger trials in the future must seek to identify the optimal dose, duration, populations, and outcomes in which NAD+ precursors demonstrate the greatest promise for improving human health.
Contributor Information
Kaitlin A Freeberg, Department of Integrative Physiology, University of Colorado Boulder, Boulder, Colorado, USA.
CeAnn C Udovich, Department of Integrative Physiology, University of Colorado Boulder, Boulder, Colorado, USA.
Christopher R Martens, Department of Kinesiology and Applied Physiology, University of Delaware, Newark, Delaware, USA.
Douglas R Seals, Department of Integrative Physiology, University of Colorado Boulder, Boulder, Colorado, USA.
Daniel H Craighead, Department of Integrative Physiology, University of Colorado Boulder, Boulder, Colorado, USA.
Funding
This work was supported by the National Institutes of Health (F31HL154782 to K.A.F., K01AG054731 to C.R.M., R01AG061514 to D.R.S., and K01HL153326 to D.H.C.).
Conflict of Interest
The authors have a material transfer agreement with the Chromadex External Research Program to receive the compound nicotinamide riboside for study purposes. The authors declare no commercial or financial relationships that could be viewed as conflicts of interest.
Author Contributions
K.A.F., D.R.S., and D.H.C. conceptualized the manuscript. K.A.F., C.C.U., C.R.M., and D.H.C. curated the data. K.A.F. prepared figures. K.A.F. and D.H.C. drafted the manuscript. K.A.F., C.C.U., C.R.M., D.R.S., and D.H.C. edited and revised the manuscript. All authors approved the final version of manuscript.
References
- 1. Covarrubias AJ, Perrone R, Grozio A, Verdin E.. NAD+ metabolism and its roles in cellular processes during ageing. Nat Rev Mol Cell Biol. 2021;22(2):119–141. doi: 10.1038/s41580-020-00313-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Guest J, Grant R, Mori TA, Croft KD.. Changes in oxidative damage: inflammation and [NAD(H)] with age in cerebrospinal fluid. PLoS One. 2014;9(1):e85335. doi: 10.1371/journal.pone.0085335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Zhu X-H, Lu M, Lee B-Y, Ugurbil K, Chen W.. In vivo NAD assay reveals the intracellular NAD contents and redox state in healthy human brain and their age dependences. Proc Natl Acad Sci U S A. 2015;112(9):2876–2881. doi: 10.1073/pnas.1417921112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bagga P, Hariharan H, Wilson NE, et al. Single-voxel 1 H MR spectroscopy of cerebral nicotinamide adenine dinucleotide (NAD+) in humans at 7T using a 32-channel volume coil. Magn Reson Med. 2020;83(3):806–814. doi: 10.1002/mrm.27971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Zhou C-C, Yang X, Hua X, et al. Hepatic NAD(+) deficiency as a therapeutic target for non-alcoholic fatty liver disease in ageing. Br J Pharmacol. 2016;173(15):2352–2368. doi: 10.1111/bph.13513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Clement J, Wong M, Poljak A, Sachdev P, Braidy N.. The plasma NAD+ metabolome is dysregulated in “normal” aging. Rejuvenation Res. 2019;22(2):121–130. doi: 10.1089/rej.2018.2077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Janssens GE, Grevendonk L, Perez RZ, et al. Healthy aging and muscle function are positively associated with NAD+ abundance in humans. Nat Aging. 2022;2(3):254–263. doi: 10.1038/s43587-022-00174-3 [DOI] [PubMed] [Google Scholar]
- 8. Vreones M, Mustapic M, Moaddel R, et al. Oral nicotinamide riboside raises NAD+ and lowers biomarkers of neurodegenerative pathology in plasma extracellular vesicles enriched for neuronal origin. Aging Cell. 2023;22(1):e13754. doi: 10.1111/acel.13754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Katsyuba E, Romani M, Hofer D, Auwerx J.. NAD+ homeostasis in health and disease. Nat Metab. 2020;2(1):9–31. doi: 10.1038/s42255-019-0161-5 [DOI] [PubMed] [Google Scholar]
- 10. Peluso A, Damgaard MV, Mori MAS, Treebak JT.. Age-dependent decline of NAD+-universal truth or confounded consensus? Nutrients. 2021;14(1):101101. doi: 10.3390/nu14010101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Yoshino J, Mills KF, Yoon MJ, Imai S.. Nicotinamide mononucleotide, a key NAD(+) intermediate, treats the pathophysiology of diet- and age-induced diabetes in mice. Cell Metab. 2011;14(4):528–536. doi: 10.1016/j.cmet.2011.08.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gomes AP, Price NL, Ling AJY, et al. Declining NAD(+) induces a pseudohypoxic state disrupting nuclear-mitochondrial communication during aging. Cell. 2013;155(7):1624–1638. doi: 10.1016/j.cell.2013.11.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Frederick DW, Loro E, Liu L, et al. Loss of NAD homeostasis leads to progressive and reversible degeneration of skeletal muscle. Cell Metab. 2016;24(2):269–282. doi: 10.1016/j.cmet.2016.07.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. de Picciotto NE, Gano LB, Johnson LC, et al. Nicotinamide mononucleotide supplementation reverses vascular dysfunction and oxidative stress with aging in mice. Aging Cell. 2016;15(3):522–530. doi: 10.1111/acel.12461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wang X, Hu X, Yang Y, Takata T, Sakurai T.. Nicotinamide mononucleotide protects against β-amyloid oligomer-induced cognitive impairment and neuronal death. Brain Res. 2016;1643:1–9. doi: 10.1016/j.brainres.2016.04.060 [DOI] [PubMed] [Google Scholar]
- 16. Hou Y, Wei Y, Lautrup S, et al. NAD+ supplementation reduces neuroinflammation and cell senescence in a transgenic mouse model of Alzheimer’s disease via cGAS-STING. Proc Natl Acad Sci U S A. 2021;118(37):e2011226118. doi: 10.1073/pnas.2011226118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Zhang H, Ryu D, Wu Y, et al. NAD+ repletion improves mitochondrial and stem cell function and enhances life span in mice. Science. 2016;352(6292):1436–1443. doi: 10.1126/science.aaf2693 [DOI] [PubMed] [Google Scholar]
- 18. Villines TC, Kim AS, Gore RS, Taylor AJ.. Niacin: the evidence, clinical use, and future directions. Curr Atheroscler Rep. 2012;14(1):49–59. doi: 10.1007/s11883-011-0212-1 [DOI] [PubMed] [Google Scholar]
- 19. D’Andrea E, Hey SP, Ramirez CL, Kesselheim AS.. Assessment of the role of niacin in managing cardiovascular disease outcomes: a systematic review and meta-analysis. JAMA Netw Open. 2019;2(4):e192224. doi: 10.1001/jamanetworkopen.2019.2224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Schandelmaier S, Briel M, Saccilotto R, et al. Niacin for primary and secondary prevention of cardiovascular events. Cochrane Database Syst Rev. 2017;6(6):CD009744. doi: 10.1002/14651858.CD009744.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gasperi V, Sibilano M, Savini I, Catani MV.. Niacin in the central nervous system: an update of biological aspects and clinical applications. Int J Mol Sci . 2019;20(4):974. doi: 10.3390/ijms20040974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Pirinen E, Auranen M, Khan NA, et al. Niacin cures systemic NAD+ deficiency and improves muscle performance in adult-onset mitochondrial myopathy. Cell Metab. 2020;31(6):1078–1090. doi: 10.1016/j.cmet.2020.04.008 [DOI] [PubMed] [Google Scholar]
- 23. Penberthy WT, Kirkland JB.. Niacin. In: Marriott BP, Birt DF, Stallings VA, Yates AA, eds. Present Knowledge in Nutrition. Elsevier; 2020:209–224. doi: 10.1016/B978-0-323-66162-1.00012-3 [DOI] [Google Scholar]
- 24. Kirkland JB, Meyer-Ficca ML.. Niacin. Adv Food Nutr Res. 2018;83:83–149. doi: 10.1016/bs.afnr.2017.11.003 [DOI] [PubMed] [Google Scholar]
- 25. Harrigan GG, Goodacre R.. Metabolic Profiling. Springer Nature; 2003. [Google Scholar]
- 26. Airhart SE, Shireman LM, Risler LJ, et al. An open-label, non-randomized study of the pharmacokinetics of the nutritional supplement nicotinamide riboside (NR) and its effects on blood NAD+ levels in healthy volunteers. PLoS One. 2017;12(12):e0186459. doi: 10.1371/journal.pone.0186459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Martens CR, Denman BA, Mazzo MR, et al. Chronic nicotinamide riboside supplementation is well-tolerated and elevates NAD+ in healthy middle-aged and older adults. Nat Commun. 2018;9(1):1286. doi: 10.1038/s41467-018-03421-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Trammell SAJ, Schmidt MS, Weidemann BJ, et al. Nicotinamide riboside is uniquely and orally bioavailable in mice and humans. Nat Commun. 2016;7(1):12948. doi: 10.1038/ncomms12948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Elhassan YS, Kluckova K, Fletcher RS, et al. Nicotinamide riboside augments the aged human skeletal muscle NAD+ metabolome and induces transcriptomic and anti-inflammatory signatures. Cell Rep. 2019;28(7):1717–1728.e6. doi: 10.1016/j.celrep.2019.07.043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Conze D, Brenner C, Kruger CL.. Safety and metabolism of long-term administration of NIAGEN (nicotinamide riboside chloride) in a randomized, double-blind, placebo-controlled clinical trial of healthy overweight adults. Sci Rep. 2019;9(1):9772. doi: 10.1038/s41598-019-46120-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Remie CME, Roumans KHM, Moonen MPB, et al. Nicotinamide riboside supplementation alters body composition and skeletal muscle acetylcarnitine concentrations in healthy obese humans. Am J Clin Nutr. 2020;112(2):413–426. doi: 10.1093/ajcn/nqaa072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Freeberg KA, Craighead DH, Martens CR, You Z, Chonchol M, Seals DR.. Nicotinamide riboside supplementation for treating elevated systolic blood pressure and arterial stiffness in midlife and older adults. Front Cardiovasc Med. 2022;9:881703. doi: 10.3389/fcvm.2022.881703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Stocks B, Ashcroft SP, Joanisse S, et al. Nicotinamide riboside supplementation does not alter whole-body or skeletal muscle metabolic responses to a single bout of endurance exercise. J Physiol. 2021;599(5):1513–1531. doi: 10.1113/JP280825 [DOI] [PubMed] [Google Scholar]
- 34. Dollerup OL, Christensen B, Svart M, et al. A randomized placebo-controlled clinical trial of nicotinamide riboside in obese men: safety, insulin-sensitivity, and lipid-mobilizing effects. Am J Clin Nutr. 2018;108(2):343–353. doi: 10.1093/ajcn/nqy132 [DOI] [PubMed] [Google Scholar]
- 35. Dollerup OL, Trammell SAJ, Hartmann B, et al. Effects of nicotinamide riboside on endocrine pancreatic function and incretin hormones in nondiabetic men with obesity. J Clin Endocrinol Metab. 2019;104(11):5703–5714. doi: 10.1210/jc.2019-01081 [DOI] [PubMed] [Google Scholar]
- 36. Dollerup OL, Chubanava S, Agerholm M, et al. Nicotinamide riboside does not alter mitochondrial respiration, content or morphology in skeletal muscle from obese and insulin-resistant men. J Physiol. 2020;598(4):731–754. doi: 10.1113/JP278752 [DOI] [PubMed] [Google Scholar]
- 37. Nascimento EBM, Moonen MPB, Remie CME, et al. Nicotinamide riboside enhances in vitro beta-adrenergic brown adipose tissue activity in humans. J Clin Endocrinol Metab. 2021;106(5):1437–1447. doi: 10.1210/clinem/dgaa960 [DOI] [PubMed] [Google Scholar]
- 38. Lapatto HAK, Kuusela M, Heikkinen A, et al. Nicotinamide riboside improves muscle mitochondrial biogenesis, satellite cell differentiation, and gut microbiota in a twin study. Sci Adv. 2023;9(2):eadd5163. doi: 10.1126/sciadv.add5163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Brakedal B, Dölle C, Riemer F, et al. The NADPARK study: a randomized phase I trial of nicotinamide riboside supplementation in Parkinson’s disease. Cell Metab. 2022;34(3):396–407.e6. doi: 10.1016/j.cmet.2022.02.001 [DOI] [PubMed] [Google Scholar]
- 40. Veenhuis SJG, van Os NJH, Janssen AJWM, et al. Nicotinamide riboside improves ataxia scores and immunoglobulin levels in ataxia telangiectasia. Mov Disord. 2021;36(12):2951–2957. doi: 10.1002/mds.28788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Fang EF, Kassahun H, Croteau DL, et al. NAD+ replenishment improves lifespan and healthspan in ataxia telangiectasia models via mitophagy and DNA repair. Cell Metab. 2016;24(4):566–581. doi: 10.1016/j.cmet.2016.09.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Stern N, Hochman A, Zemach N, et al. Accumulation of DNA damage and reduced levels of nicotine adenine dinucleotide in the brains of Atm-deficient mice. J Biol Chem. 2002;277(1):602–608. doi: 10.1074/jbc.M106798200 [DOI] [PubMed] [Google Scholar]
- 43. Wang DD, Airhart SE, Zhou B, et al. Safety and tolerability of nicotinamide riboside in heart failure with reduced ejection fraction. JACC: Basic Transl Sci. 2022;7(12):1183–1196. doi: 10.1016/j.jacbts.2022.06.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Imai S, Guarente L.. NAD+ and sirtuins in aging and disease. Trends Cell Biol. 2014;24(8):464–471. doi: 10.1016/j.tcb.2014.04.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Zhang L, Zheng J, Tie X, et al. Pterostilbene and its nicotinate derivative ameliorated vascular endothelial senescence and elicited endothelium-dependent relaxations via activation of sirtuin 1. Can J Physiol Pharmacol. 2021;99(9):900–909. doi: 10.1139/cjpp-2020-0583 [DOI] [PubMed] [Google Scholar]
- 46. Guo J, Wang J, Guo R, Shao H, Guo L.. Pterostilbene protects the optic nerves and retina in a murine model of experimental autoimmune encephalomyelitis via activation of SIRT1 signaling. Neuroscience. 2022;487:35–46. doi: 10.1016/j.neuroscience.2022.01.016 [DOI] [PubMed] [Google Scholar]
- 47. Ma C, Xiang J, Huang G, et al. Pterostilbene alleviates cholestasis by promoting SIRT1 activity in hepatocytes and macrophages. Front Pharmacol. 2021;12:785403. doi: 10.3389/fphar.2021.785403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Dellinger RW, Santos SR, Morris M, et al. Repeat dose NRPT (nicotinamide riboside and pterostilbene) increases NAD+ levels in humans safely and sustainably: a randomized, double-blind, placebo-controlled study. NPJ Aging Mech Dis. 2017;3(1):17. doi: 10.1038/s41514-017-0016-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Jensen JB, Dollerup OL, Møller AB, et al. A randomized placebo-controlled trial of nicotinamide riboside+pterostilbene supplementation in experimental muscle injury in elderly subjects. JCI Insight. 2022;19:e158314. doi: 10.1172/jci.insight.158314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. de la Rubia JE, Drehmer E, Platero JL, et al. Efficacy and tolerability of EH301 for amyotrophic lateral sclerosis: a randomized, double-blind, placebo-controlled human pilot study. Amyotroph Lateral Scler Frontotemporal Degener. 2019;20(1–2):115–122. doi: 10.1080/21678421.2018.1536152 [DOI] [PubMed] [Google Scholar]
- 51. Dellinger RW, Holmes HE, Hu-Seliger T, et al. NRPT reduces markers of hepatic inflammation in non-alcoholic fatty liver disease: a double-blind, placebo-controlled clinical trial. Hepatology. 2022:1–14. doi: 10.1002/hep.32778 [DOI] [PubMed] [Google Scholar]
- 52. Mills KF, Yoshida S, Stein LR, et al. Long-term administration of nicotinamide mononucleotide mitigates age-associated physiological decline in mice. Cell Metab. 2016;24(6):795–806. doi: 10.1016/j.cmet.2016.09.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Tarantini S, Valcarcel-Ares MN, Toth P, et al. Nicotinamide mononucleotide (NMN) supplementation rescues cerebromicrovascular endothelial function and neurovascular coupling responses and improves cognitive function in aged mice. Redox Biol. 2019;24:101192. doi: 10.1016/j.redox.2019.101192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Lee CF, Chavez JD, Garcia-Menendez L, et al. Normalization of NAD+ redox balance as a therapy for heart failure. Circulation. 2016;134(12):883–894. doi: 10.1161/CIRCULATIONAHA.116.022495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Fukamizu Y, Uchida Y, Shigekawa A, Sato T, Kosaka H, Sakurai T.. Safety evaluation of β-nicotinamide mononucleotide oral administration in healthy adult men and women. Sci Rep. 2022;12(1):14442. doi: 10.1038/s41598-022-18272-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Liao B, Zhao Y, Wang D, Zhang X, Hao X, Hu M.. Nicotinamide mononucleotide supplementation enhances aerobic capacity in amateur runners: a randomized, double-blind study. J Int Soc Sports Nutr. 2021;18(1):54. doi: 10.1186/s12970-021-00442-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Huang H. A multicentre, randomised, double blind, parallel design, placebo controlled study to evaluate the efficacy and safety of Uthever (NMN Supplement), an orally administered supplementation in middle aged and older adults. Front Aging. 2022;3:851698. doi: 10.3389/fragi.2022.851698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Yi L, Maier AB, Tao R, et al. The efficacy and safety of β-nicotinamide mononucleotide (NMN) supplementation in healthy middle-aged adults: a randomized, multicenter, double-blind, placebo-controlled, parallel-group, dose-dependent clinical trial. GeroScience. 2022;45:29–43. doi: 10.1007/s11357-022-00705-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Morita Y, Izawa H, Hirano A, Mayumi E, Isozaki S, Yonei Y.. Clinical evaluation of changes in biomarkers by oral intake of NMN. Glycative Stress Res. 2022;9(2):33–41. doi: 10.24659/gsr.9.2_33 [DOI] [Google Scholar]
- 60. Igarashi M, Nakagawa-Nagahama Y, Miura M, et al. Chronic nicotinamide mononucleotide supplementation elevates blood nicotinamide adenine dinucleotide levels and alters muscle function in healthy older men. NPJ Aging. 2022;8(1):5. doi: 10.1038/s41514-022-00084-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Okabe K, Yaku K, Uchida Y, et al. Oral Administration of nicotinamide mononucleotide is safe and efficiently increases blood nicotinamide adenine dinucleotide levels in healthy subjects. Front Nutr. 2022;9:868640. doi: 10.3389/fnut.2022.868640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Kim M, Seol J, Sato T, Fukamizu Y, Sakurai T, Okura T.. Effect of 12-week intake of nicotinamide mononucleotide on sleep quality, fatigue, and physical performance in older Japanese adults: a randomized, double-blind placebo-controlled study. Nutrients. 2022;14(4):755755. doi: 10.3390/nu14040755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Katayoshi T, Uehata S, Nakashima N, et al. Nicotinamide adenine dinucleotide metabolism and arterial stiffness after long-term nicotinamide mononucleotide supplementation: a randomized, double-blind, placebo-controlled trial. Sci Rep. 2023;13(1):2786. doi: 10.1038/s41598-023-29787-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Yoshino M, Yoshino J, Kayser BD, et al. Nicotinamide mononucleotide increases muscle insulin sensitivity in prediabetic women. Science. 2021;372(6547):1224–1229. doi: 10.1126/science.abe9985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Pencina KM, Lavu S, dos Santos M, et al. MIB-626, an oral formulation of a microcrystalline unique polymorph of β-nicotinamide mononucleotide, increases circulating nicotinamide adenine dinucleotide and its metabolome in middle-aged and older adults. J Gerontol A Biol Sci Med Sci. 2022;78:90–96. doi: 10.1093/gerona/glac049 [DOI] [PubMed] [Google Scholar]
- 66. Pencina KM, Valderrabano R, Wipper B, et al. Nicotinamide adenine dinucleotide augmentation in overweight or obese middle-aged and older adults: a physiologic study. J Clin Endocrinol Metab. 2023:dgad027. doi: 10.1210/clinem/dgad027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Akasaka H, Nakagami H, Sugimoto K, et al. Effects of nicotinamide mononucleotide on older patients with diabetes and impaired physical performance: a prospective, placebo-controlled, double-blind study. Geriatr Gerontol Int. 2023;23(1):38–43. doi: 10.1111/ggi.14513 [DOI] [PubMed] [Google Scholar]
- 68. Mehmel M, Jovanović N, Spitz U.. Nicotinamide riboside—the current state of research and therapeutic uses. Nutrients. 2020;12(6):1616. doi: 10.3390/nu12061616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Shats I, Williams JG, Liu J, et al. Bacteria boost mammalian host NAD metabolism by engaging the deamidated biosynthesis pathway. Cell Metab. 2020;31(3):564–579. doi: 10.1016/j.cmet.2020.02.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Chellappa K, McReynolds MR, Lu W, et al. NAD precursors cycle between host tissues and the gut microbiome. Cell Metab. 2022;34(12):1947–1959. doi: 10.1016/j.cmet.2022.11.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Grozio A, Mills KF, Yoshino J, et al. Slc12a8 is a nicotinamide mononucleotide transporter. Nat Metab. 2019;1(1):47–57. doi: 10.1038/s42255-018-0009-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Schmidt MS, Brenner C.. Absence of evidence that Slc12a8 encodes a nicotinamide mononucleotide transporter. Nat Metab. 2019;1(7):660–661. doi: 10.1038/s42255-019-0085-0 [DOI] [PubMed] [Google Scholar]
- 73. Emwas A-HM. The strengths and weaknesses of NMR spectroscopy and mass spectrometry with particular focus on metabolomics research. Methods Mol Biol. 2015;1277:161–193. doi: 10.1007/978-1-4939-2377-9_13 [DOI] [PubMed] [Google Scholar]
- 74. Yang Y, Mohammed FS, Zhang N, Sauve AA.. Dihydronicotinamide riboside is a potent NAD+ concentration enhancer in vitro and in vivo. J Biol Chem. 2019;294(23):9295–9307. doi: 10.1074/jbc.RA118.005772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Altay O, Arif M, Li X, et al. Combined metabolic activators accelerates recovery in mild-to-moderate COVID-19. Adv Sci. 2021;8(17):2101222. doi: 10.1002/advs.202101222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Yulug B, Altay O, Li X, et al. Combined metabolic activators improve cognitive functions in Alzheimer’s disease patients: a randomised, double-blinded, placebo-controlled phase-II trial. Transl Neurodegener. 2023;12(1):4. doi: 10.1186/s40035-023-00336-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Xue Y, Shamp T, Nagana Gowda GA, Crabtree M, Bagchi D, Raftery D.. A combination of nicotinamide and d-ribose (RiaGev) is safe and effective to increase NAD+ metabolome in healthy middle-aged adults: a randomized, triple-blind, placebo-controlled, cross-over pilot clinical trial. Nutrients. 2022;14(11):2219. doi: 10.3390/nu14112219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Sims CA, Guan Y, Mukherjee S, et al. Nicotinamide mononucleotide preserves mitochondrial function and increases survival in hemorrhagic shock. JCI Insight. 2018;3(17):120182. doi: 10.1172/jci.insight.120182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Uddin GM, Youngson NA, Sinclair DA, Morris MJ.. Head to head comparison of short-term treatment with the NAD(+) precursor nicotinamide mononucleotide (NMN) and 6 weeks of exercise in obese female mice. Front Pharmacol. 2016;7:258. doi: 10.3389/fphar.2016.00258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Gariani K, Menzies KJ, Ryu D, et al. Eliciting the mitochondrial unfolded protein response by nicotinamide adenine dinucleotide repletion reverses fatty liver disease in mice. Hepatology. 2016;63(4):1190–1204. doi: 10.1002/hep.28245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Ryu D, Zhang H, Ropelle ER, et al. NAD+ repletion improves muscle function in muscular dystrophy and counters global PARylation. Sci Transl Med. 2016;8(361):361ra139. doi: 10.1126/scitranslmed.aaf5504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Huang P, Zhou Y, Tang W, et al. Long-term treatment of Nicotinamide mononucleotide improved age-related diminished ovary reserve through enhancing the mitophagy level of granulosa cells in mice. J Nutr Biochem. 2022;101:108911. doi: 10.1016/j.jnutbio.2021.108911 [DOI] [PubMed] [Google Scholar]
- 83. Craighead DH, Heinbockel TC, Freeberg KA, et al. Time-efficient inspiratory muscle strength training lowers blood pressure and improves endothelial function, NO bioavailability, and oxidative stress in midlife/older adults with above-normal blood pressure. J Am Heart Assoc. 2021;10(13):e020980. doi: 10.1161/JAHA.121.020980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Brunt VE, Howard MJ, Francisco MA, Ely BR, Minson CT.. Passive heat therapy improves endothelial function, arterial stiffness and blood pressure in sedentary humans. J Physiol. 2016;594(18):5329–5342. doi: 10.1113/JP272453 [DOI] [PMC free article] [PubMed] [Google Scholar]