Abstract
The majority of patients with congenital adrenal hyperplasia (CAH) present with a deficiency of 21-hydroxylase or 11-beta-hydroxylase, which account for 90% and 7% of cases, respectively. However, CAH due to 17α-hydroxylase deficiency (17OHD) is an extremely rare form of CAH (<1% of all CAH cases) that leads to a deficiency of cortisol and sex steroids, along with features of aldosterone excess. This is a case of a 51-year-old single female who was referred to us for the evaluation of new-onset hypertension and hypokalaemia of one-year duration. She was born out of a second-degree consanguineous marriage and reared as a female. She was diagnosed to have testicular feminization syndrome when she presented with a history of primary amenorrhea, absence of secondary sexual characteristics, and bilateral labial swellings at pubertal age. Subsequently, she underwent gonadectomy at the age of 16. Due to the presence of hypertension, metabolic alkalosis and bilaterally enlarged adrenals on CT scan, 46, XY disorders of sexual development (DSD) was considered. A karyotype confirmed the presence of 46, XY chromosomal sex, and genetic analysis revealed a mutation in the CYP17A1 gene, thus confirming the diagnosis of 17α-hydroxylase deficiency.
Keywords: disorders of sexual development (DSD), congenital adrenal hyperplasia, rare cases, hypertension, 46, XY DSD, 17α-hydroxylase deficiency
INTRODUCTION
Disorders of sexual development (DSD) refer to a diverse group of congenital disorders that result in a discrepancy between an individual’s sex chromosomes, gonads, and/or the anatomic sex. The 46, XY DSD could be due to a defect in gonadal development or androgen biosynthesis, an error in steroidogenesis as that found in congenital adrenal hyperplasia (CAH), or due to a defect in androgen action.
Congenital adrenal hyperplasia occurs due to enzyme defects involved in adrenal and gonadal steroid biosynthesis. Most CAH cases result from a deficiency of 21-hydroxylase or 11-beta-hydroxylase, accounting for 90% and 7% of cases, respectively.1,2 Deficiency of 21-hydroxylase and 11-beta-hydroxylase affects only the adrenal steroidogenesis, whereas those with a mutation in 17α-hydroxylase/17,20-lyase (CYP17A1) and 3-β-hydroxysteroid dehydrogenase type 2 (HSD3B2) have an additional impairment in gonadal steroid biosynthesis. Consequently, 21-hydroxylase and 11-beta-hydroxylase cause DSD exclusively in 46, XX individuals. Congenital adrenal hyperplasia due to a mutation in STAR protein, cholesterol side chain cleavage (CYP11A1), and CYP17A1 all cause DSD exclusively in 46, XY individuals. Moreover, deficiencies in HSD3B2 and P450 oxidoreductase cause DSD in both sexes.
Congenital adrenal hyperplasia caused by 17α-hydroxylase deficiency (17OHD) is extremely rare, occurring in approximately 1 in 50,000 individuals.3 The classical description of 17OHD is that of a phenotypic female (46, XX or underandrogenized 46, XY) who presents at puberty with primary amenorrhea and lack of secondary sexual characteristics, with low-renin hypertension, and hypokalaemic metabolic alkalosis. Here, we report a case of 46, XY DSD due to 17OHD, whose diagnosis was confirmed by CYP17A1 genotyping. It is important to diagnose this rare condition because persistent hypokalemia in these individuals can lead to life-threatening cardiac arrhythmias, entailing careful monitoring and treatment in the acute phase. In the long-term, there is a risk of osteoporosis if they have not been started on gonadal steroid replacement from the time of puberty. Additionally, they are at a greater risk of developing gonadoblastoma and an invasive mixed germ cell tumor.
CASE
A 51-year-old single female was referred for evaluation of new-onset hypertension accompanied by hypokalemia with documented bilateral adrenal hyperplasia. She was born out of a second-degree consanguineous marriage and reared as a female. Family history was unremarkable. She had a history of primary amenorrhea, absence of secondary sexual characteristics, and bilateral labial swelling at pubertal age and was mistakenly diagnosed to have testicular feminization syndrome as her karyotype was 46, XY. The patient did not have any gender dysphoria and identified socially as a female. Initially, she was normotensive. The patient and her family members consented to gonadectomy, penile reduction and feminine genitoplasty for this case. Subsequently, when she was 16 years old, she underwent bilateral gonadectomy where her bilateral labioscrotal gonads were removed, and her phallus was surgically reduced.
Given that the patient presented with hypertension, hypokalaemia, metabolic alkalosis, and bilaterally enlarged adrenals on abdominal imaging, the diagnosis of 17OHD was considered. Anthropometry demonstrated eunuchoid body proportion [height 154 cm, weight 51 kg, BMI 21.6 kg/m2, and upper segment: lower segment ratio 0.94, arm span 175 cm]. Blood pressure was 160/100 mm Hg. Both axillary and pubic hair were absent, and the breast was Tanner stage 1. Genital examination revealed female external genitalia with single urethral opening in the perineum (post-genitoplasty).
Investigations
Serum sodium was 135 mEq/L [normal range (NR) 135-145 mEq/L] and potassium was 2 mEq/L [NR: 3.5-4.5 mEq/L]. Hormonal investigations revealed an 8 am basal cortisol of 3.6 mcg/dl [NR: 4.3-22.4 μg/dL, assay sensitivity (AS) 0.20-75 μg/dL, CV <7%]; plasma adrenocorticotropic hormone (ACTH) 137 pg/ml [NR 0-46 pg/mL, AS: 9 pg/ mL, CV<9.6%]; follicle-stimulating hormone 95.24 mIU/ mL [NR:1.4-18.1 mIU/mL, AS:0.3 – 200 mIU/mL, CV<4%] and luteinizing hormone 25.74 mIU/mL [NR:1.5-9.3 mIU/ mL, AS:0.07 – 200 mIU/mL, CV<3.8%]; serum testosterone <7 ng/dL [NR:14-76 ng/dL, AS:10 – 1500 ng/dL, CV <7.6%]. Bone mineral density was assessed using dual energy X-ray absorptiometry [Hologic Discovery Wi DXA machine] which showed a lumbar spine and femoral neck T score of minus 3.8 and minus 4 respectively.
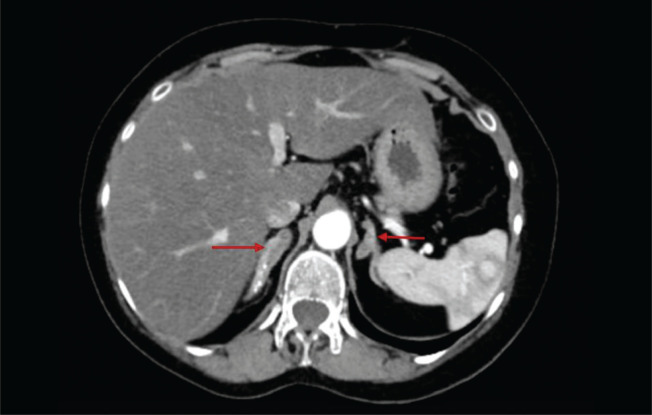
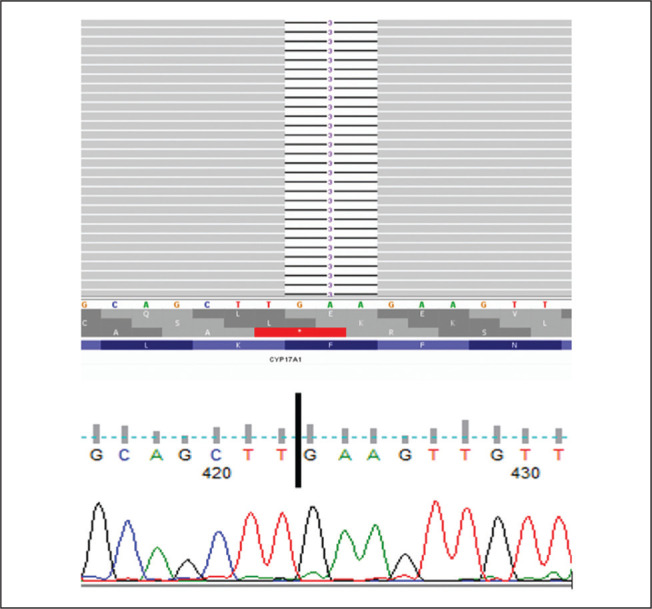
Computed tomography of the abdomen (Figure 1) showed bilaterally enlarged adrenals and absent Müllerian structures, thus reinforcing the karyotype confirmation of 46,XY DSD. For molecular testing, the patient’s consent for a CYP17A1 gene analysis was obtained and the blood sample sent to the Molecular Endocrinology Laboratory at Christian Medical College, Vellore, India. DNA extraction was carried out on whole blood samples using the Qiagen Gentra Kit method. All the coding exons and splice site regions of the CYP17A1 gene were amplified using in-house designed primers. Next-generation sequencing with the Ion torrent PGM was performed using previously published protocols.4 The NGS-based approach achieved an average base coverage depth of 370x, with 99.89% of the target sequenced at 20x, covering the complete coding and splice site regions of the CYP17A1 gene. The patient was found to be homozygous positive for a reported CYP17A1: c.160_162delTTC mutation, resulting in deletion of phenylalanine at codon 54 (p. Phe54del) in exon one, as shown in Figure 2. This mutation has been described in earlier reports from both Japan and New Zealand in patients with similar phenotypes and both 46,XY and 46,XX karyotypes.5-7 Structural and functional studies of the mutant protein with cos-1 cells lines have shown that this deletion alters the protein folding, thus reducing the activity of 17α-hydroxylase (<37%) and 17,20 lyase (<8%) compared to the wildtype protein resulting in partial combined 17α-hydroxylase/17,20 lyase deficiency.7 Based on the guidelines of the American College of Human Genetics released in 2015, the identified mutation is classified as pathogenic.
Figure 1.
Contrast-enhanced computed tomography of the abdomen showing bilateral adrenal hyperplasia (red arrows).
Figure 2.
The NGS reads were aligned to hg19 reference genome and viewed on Integrative Genomics Viewer (IGV.2.9.4) as seen in the top image. The gray reads show homozygous deletion of three base pairs (GAA) at codon 54 in exon1 CYP17A1(NM_000102.4):c.160_162del (p.Phe54del). The below image shows the sanger chromatogram of CYP17A1:P.Phe54del corroborating the NGS results with homozygous deletion of three base pairs (GAA) at codon 54.
Therapeutic intervention and outcome
She was started on a once-daily dose of oral prednisolone (2.5 mg) and spironolactone (50 mg). Additionally, she received a single intravenous infusion of zoledronic acid (4 mg) once yearly dose along with oral calcium (1000 mg daily) for the management of osteoporosis due to long-standing gonadal hormone deficiency. On follow-up, her serum potassium normalized, and her blood pressure was under control.
DISCUSSION
Congenital adrenal hyperplasia due to 17OHD is rare, first described by Biglieri et al.8 This enzyme is encoded by the CYP17A1 gene, located on chromosome 10q24.3. Individuals with this form account for about 1% of all CAH cases.9 A defect in the CYP17A1 gene results in two different types of enzyme deficiency: (1) combined 17α-hydroxylase/17,20-lyase deficiency and (2) isolated 17,20-lyase deficiency. The combined form of 17OHD is the most common and is characterized by the impaired synthesis of cortisol and gonadal steroids. Depending on the residual enzyme activity (>1% and <1%), it is classified as a combined severe and combined partial form of 17OHD, respectively. Affected 46,XY individuals due to combined severe form of 17OHD classically present with a female phenotype accompanied by primary amenorrhea, absence of secondary sexual characteristics (absent or sparse axillary and pubic hair), female external genitalia with a blind vaginal pouch and variable degrees of hypertension usually detected in adolescence. The gonads (testes) may be located in the intra-abdominal or inguinal region or in the labioscrotal folds. Combined partial form of 17OHD in 46,XY individuals presents with ambiguous genitalia or severe hypospadias with or without hypertension. The normal production of anti-müllerian hormone (AMH) from the testes leads to the regression of müllerian structures (uterus, fallopian tubes and upper one third of the vagina). Affected 46,XX females usually have normal female external and internal genitalia, but the ovaries cannot secrete estrogen during puberty resulting in absent breast development with hypergonadotropic hypogonadism, absent axillary and pubic hair due to lack of adrenal and ovarian androgens. In both 46,XX and 46,XY affected individuals, 17OHD results in impaired synthesis of cortisol and sex steroids with a consequent increase of ACTH and accumulation of steroid precursors, which are diverted into the mineralocorticoid synthesis pathway resulting in the decrease of 17-hydroxypregnenolone, 17-hydroxyprogesterone, 11-deoxycortisol, cortisol, DHEA, androstenedione, estrogen/ testosterone and consequently excess production of DOC, corticosterone, and 18-hydroxycorticosterone respectively. High levels of DOC, due to its mineralocorticoid effect, induce sodium and fluid retention associated with hypertension and hypokalaemia. Despite having a low level of cortisol, patients with 17OHD rarely develop adrenal crisis due to elevated levels of corticosterone, which contributes some glucocorticoid activity, thus preventing an adrenal crisis. Most 17OHD patients have low aldosterone levels caused by elevated levels of DOC, resulting in suppression of the renin-angiotensin system. The isolated 17,20-lyase deficiency is very rare and is characterized by low androgen production leading to genital ambiguity in males, normal production of glucocorticoids and mineralocorticoids.10
The differential diagnosis of 46, XY DSD with palpable gonads includes:
Defect in gonadal development - partial gonadal dysgenesis.
Disorder in androgen synthesis-Leydig cell hypoplasia due to LHCGR mutation, deficiency of STAR, CYP11A1, 3HSDB2, 17OHD, POR, 17βHSD3, 5αreductase type 2.
Disorder in androgen action - partial androgen insensitivity syndrome.
A thorough clinical examination is crucial in the evaluation of patients with DSD. As our patient presented with female-type external genitalia, primary amenorrhea, and palpable gonads at puberty along with hypokalemic hypertension and bilateral adrenal hyperplasia, the diagnosis of CAH due to 17α-hydroxylase deficiency (combined severe) was considered since none of the above-mentioned conditions except 17OHD will present with hypokalemia and hypertension in an individual with 46,XY DSD. Laboratory investigations are essential to establish the diagnosis in these settings, which may not be available in many centers; hence, the need to perform these tests in other facilities. We were unable to perform DOC, aldosterone, and renin levels which could reinforce the diagnosis. Nevertheless, as previously described, clinical and available biochemical findings supported the diagnosis of CAH due to 17OHD. The genetic diagnosis in this patient revealed the presence of a deleterious CYP17A1 mutation. Genetic testing is critical for confirmatory diagnosis of 17α-hydroxylase deficiency due to the presence of varying clinical and biochemical features. Several point mutations, large deletions, and duplications in the CYP17A1 gene have been reported earlier in the homozygous and compound heterozygous states.11-14
The decision about the sex of rearing in DSD is complicated and challenging, especially for those with a 46, XY karyotype. Traditionally, a female phenotype of the external genitalia with palpable gonads results in female sex rearing as it was thought that learning, or “nurture,” has a more significant influence on gender identity than biology or “nature.”15 Similarly, our patient was raised and identified as a female as the external genitalia was female with palpable gonads; consequently, feminizing genitoplasty was performed in the past. Another critical issue in the treatment of 46,XY DSD is the appropriate time of gonadectomy. Although data are limited, some reports have shown an increased risk of Sertoli and Leydig cell tumors in phenotypic girls with 17OHD16 and other forms of 46, XY DSD with undescended testis.17 Additionally, there have been no reported cases of biological fertility in individuals with 46,XY DSD due to 17OHD. From a comprehensive perspective, the decision to perform gonadectomy and feminizing genitoplasty was appropriate in this case. However, current guidelines recommend that the decision of gonadectomy should be individualized. Once the sex of the rearing is decided conclusively, the incongruent gonadal tissue can be removed after age 14 years after obtaining informed consent from parents and the concerned individual unless there are compelling indications like suspicion of malignancy.18
Most CAH cases, including 17OHD, are inherited in an autosomal recessive form. Hence, there is a 25% probability that other siblings of the index case will have CAH and a 50% probability that each will be an asymptomatic carrier. Genetic testing of other familial members is essential in the recognition of the disease for individualized management as it will aid in sex assignment and estimate the viability of fertility.
CONCLUSION
A 46,XY DSD due to 17OHD may be missed during adolescence. The diagnosis of 17OHD should be considered in a 46,XY DSD presenting with atypical female external genitalia, and absence of secondary sexual characters irrespective of the presence of hypertension, as the presentation of the disorder may vary and hypertension may present later in life. Genetic testing is necessary to confirm the diagnosis of 17OHD. Treatment with glucocorticoids reduces the excess mineralocorticoid precursors and usually normalizes hypokalaemia and hypertension.
Ethical Considerations
Patient consent was obtained before submission of the manuscript.
Statement of Authorship
All authors certified fulfillment of ICMJE authorship criteria.
Author Disclosure
The authors declared no conflict of interest.
Funding Source
None.
References
- 1.Merke DP, Bornstein SR. Congenital adrenal hyperplasia. Lancet. 2005;365(9477):2125–36. PMID: . 10.1016/S0140-6736(05)66736-0. [DOI] [PubMed] [Google Scholar]
- 2.Krone N, Arlt W. Genetics of congenital adrenal hyperplasia. Best Pract Res Clin Endocrinol Metab. 2009;23(2):181–92. PMID: . PMCID: . 10.1016/j.beem.2008.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Costa-Santos M, Kater CE, Auchus RJ; Brazilian Congenital Adrenal Hyperplasia Multicenter Study Group. Two prevalent CYP17 mutations and genotype-phenotype correlations in 24 Brazilian patients with 17-hydroxylase deficiency. J Clin Endocrinol Metab. 2004;89(1):49–60. PMID: . 10.1210/jc.2003-031021. [DOI] [PubMed] [Google Scholar]
- 4.Chapla A, Mruthyunjaya MD, Asha HS, et al. Maturity onset diabetes of the young in India - A distinctive mutation pattern identified through targeted next-generation sequencing. Clin Endocrinol (Oxf). 2015;82(4):533–42. PMID: . 10.1111/cen.12541. [DOI] [PubMed] [Google Scholar]
- 5.Mikami Y, Takai Y, Obata-Yasuoka M, et al. Diagnosis of female 17α-hydroxylase deficiency after gonadectomy: A case report. J Med Case Rep. 2019;13(1):235. PMID: . PMCID: . 10.1186/s13256-019-2166-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Croxson M, Ogilvie CM, Milsom S, Lewis J, Davidson J, Rumsby G. Delayed puberty from partial 17-alpha hydroxylase enzyme deficiency. N Z Med J. 2012;125(1355):71–4. PMID: . [PubMed] [Google Scholar]
- 7.Yanase T, Kagimoto M, Suzuki S, Hashiba K, Simpson ER, Waterman MR. Deletion of a phenylalanine in the N-terminal region of human cytochrome P-450(17 alpha) results in partial combined 17 alpha-hydroxylase/17,20-lyase deficiency. J Biol Chem. 1989;264(30): 18076–82. Erratum in: J Biol Chem 1989;264(35):21433. PMID: . [PubMed] [Google Scholar]
- 8.Biglieri EG, Herron MA, Brust N. 17-hydroxylation deficiency in man. J Clin Invest. 1966;45(12):1946–54. PMID: . PMCID: . 10.1172/JCI105499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Maheshwari M, Arya S, Lila AR, et al. 17α-Hydroxylase/17,20-lyase deficiency in 46, XY: Our experience and review of literature. J Endocr Soc. 2022;6(3):bvac011. PMID: . PMCID: . 10.1210/jendso/bvac011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Auchus RJ. Steroid 17-hydroxylase and 17,20-lyase deficiencies, genetic and pharmacologic. J Steroid Biochem Mol Biol. 2017;165(Pt A): 71–8. PMID: . PMCID: . 10.1016/j.jsbmb.2016.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim YM, Kang M, Choi JH, et al. A review of the literature on common CYP17A1 mutations in adults with 17-hydroxylase/17,20-lyase deficiency, a case series of such mutations among Koreans and functional characteristics of a novel mutation. Metabolism. 2014;63(1):42–9. PMID: . 10.1016/j.metabol.2013.08.015. [DOI] [PubMed] [Google Scholar]
- 12.Kim SM, Rhee JH. A case of 17 alpha-hydroxylase deficiency. Clin Exp Reprod Med. 2015;42(2):72–6. PMID: . PMCID: . 10.5653/cerm.2015.42.2.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang M, Sun S, Liu Y, et al. New, recurrent, and prevalent mutations: Clinical and molecular characterization of 26 Chinese patients with 17alpha-hydroxylase/17,20-lyase deficiency. J Steroid Biochem Mol Biol. 2015. Jun;150:11–6. PMID: . 10.1016/j.jsbmb.2015.02.007. [DOI] [PubMed] [Google Scholar]
- 14.Asirvatham AR, Balachandran K, Jerome P, Venkatesan V, Koshy T, Mahadevan S. Clinical, biochemical and genetic characteristics of children with congenital adrenal hyperplasia due to 17α-hydroxylase deficiency. J Pediatr Endocrinol Metab. 2020. Jul 13;/j/jpem.ahead-of-print/jpem-2020-0050/jpem-2020-0050.xml. PMID: . 10.1515/jpem-2020-0050. [DOI] [PubMed] [Google Scholar]
- 15.Wilson JD, Rivarola MA, Mendonca BB, et al. Advice on the management of ambiguous genitalia to a young endocrinologist from experienced clinicians. Semin Reprod Med. 2012;30(5):339–50. PMID: . 10.1055/s-0032-1324717. [DOI] [PubMed] [Google Scholar]
- 16.Jiang JF, Deng Y, Xue W, Wang YF, Tian QJ, Sun AJ. Surgical therapy of 17α-hydroxylase deficiency in 30 patients. Zhongguo Yi Xue Ke Xue Yuan Xue Bao 2016; 38(5):559–62. PMID: . 10.3881/j.issn.1000-503X.2016.05.012. [DOI] [PubMed] [Google Scholar]
- 17.Wisniewski AB, Batista RL, Costa EMF, et al. Management of 46,XY differences/disorders of sex development (DSD) throughout life. Endocr Rev. 2019;40(6):1547–72. PMID: . 10.1210/er.2019-00049. [DOI] [PubMed] [Google Scholar]
- 18.Wiesemann C, Ude-Koeller S, Sinnecker GHG, Thyen U. Ethical principles and recommendations for the medical management of differences of sex development (DSD)/intersex in children and adolescents. Eur J Pediatr. 2010;169(6):671–9. PMID: . PMCID: . 10.1007/s00431-009-1086-x. [DOI] [PMC free article] [PubMed] [Google Scholar]