Abstract
Objective: The apparent survival benefit of being overweight or obese in critically ill patients (the obesity paradox) remains controversial. Our aim is to report on the epidemiology and outcomes of obesity within a large heterogenous critically ill adult population.
Design: Retrospective observational cohort study.
Setting: Intensive care units (ICUs) in Australia and New Zealand.
Participants: Critically ill patients who had both height and weight recorded between 2010 and 2018.
Outcome measures: Hospital mortality in each of five body mass index (BMI) strata. Subgroups analysed included diagnostic category, gender, age, ventilation status and length of stay.
Results: Data were available for 381 855 patients, 68% of whom were overweight or obese. Increasing level of obesity was associated with lower unadjusted hospital mortality: underweight (11.9%), normal weight (7.7%), overweight (6.4%), class I obesity (5.4%), and class II obesity (5.3%). After adjustment, mortality was lowest for patients with class I obesity (adjusted odds ratio, 0.78; 95% CI, 0.74–0.82). Adverse outcomes with class II obesity were only seen in patients with cardiovascular and cardiac surgery ICU admission diagnoses, where mortality risk rose with progressively higher BMIs.
Conclusion: We describe the epidemiology of obesity within a critically ill Australian and New Zealand population and confirm that some level of obesity is associated with lower mortality, both overall and across a range of diagnostic categories and important subgroups. Further research should focus on potential confounders such as nutritional status and the appropriateness of BMI in isolation as an anthropometric measure in critically ill patients.
The apparent survival benefit conferred by being overweight or class I obesity (the obesity paradox) has attracted controversy since it was first described.1, 2, 3 At the core of this phenomenon is that a “normal” body mass index (BMI) of 18.5–24.9 kg/m2 is the ratio of weight (in kg) to height (in m2) at which mortality should be lowest, and that by extension, an ideal body weight for a given height exists.2
Obesity is often associated with a range of comorbidities that usually correlate with a reduction in life span. Therefore, the intuitive response to the inverse relationship between obesity and mortality is of scepticism, particularly, as there is no accepted physiological mechanistic model that explains how mild levels of obesity could be protective.4, 5 A number of confounding issues have been postulated for these results. These include the sole use of BMI as the definition of obesity, selection bias, treatment bias, time varying exposure, and failing to account for underlying malnutrition and socio-economic status.2, 6, 7, 8, 9, 10, 11, 12 Furthermore, the assumption that a normal BMI is the same across ethnicity, culture, time or through the life span has been challenged.13, 14, 15
While most observational studies that have described this relationship in critically ill patients have been either single centre or the analysis of patients with a single common pathophysiological process (eg, stroke, acute and chronic renal failure, cardiovascular disease, pneumonia), two recent large studies using national datasets also demonstrated a J-shaped relationship between BMI and mortality, with the nadir occurring above 30 kg/m2.16, 17, 18, 19, 20, 21, 22, 23
We sought to describe the distribution of BMI in critically ill patients within Australian and New Zealand intensive care units (ICUs), and to confirm whether the obesity paradox existed. Our hypotheses were that after adjusting for confounders, increasing levels of obesity would be associated with lower mortality, and that this relationship would vary in different pre-specified subgroups.
Methods
The Australian and New Zealand Intensive Care Society (ANZICS) Adult Patient Database (APD) — one of four clinical quality registries administered by the ANZICS Centre for Outcome and Resource Evaluation (CORE) — was used to identify all patients aged 16 years or older admitted to one of the 166 contributing ICUs who had both weight and height recorded between 2010 and 2018. The ANZICS APD encompasses 90% of all ICU admissions in Australia and New Zealand over this period.24 The BMI was calculated by dividing weight (in kilograms), by height (in metres) raised to the power of 2. Definitions of overweight and obesity were taken from the World Health Organization and were divided into underweight (BMI ≤ 18.4 kg/m2), normal (BMI 18.5–24.9 kg/m2), overweight (BMI 25.0–29.9 kg/m2), class I obesity (BMI 30.0–34.9 kg/m2), and class II obesity and above (BMI ≥ 35.0 kg/m2).25 To avoid double-counting patient outcomes, ICU readmissions during the same hospital episode and extreme BMI values that were considered unrealistic (≤ 10 kg/m2 and ≥ 60 kg/m2) were excluded.5 The study was approved by the Central Australian Human Research Ethics Committee (CAHREC 19-3339).
Data regarding baseline characteristics (age and gender), the presence of comorbidities (defined by the ANZICS APD data dictionary), and outcomes were retrieved.26 Illness severity was described using the Acute Physiology and Chronic Health Evaluation (APACHE) III scoring system and the Australian and New Zealand Risk of Death (ANZROD).27, 28, 29 ANZROD is derived from locally collected variables and components of the APACHE scoring system. It provides accurate mortality prediction for admissions to Australian and New Zealand ICUs.29
The reason for ICU admission was taken from the ANZICS modification of the APACHE III diagnostic coding system.26 Individual admission diagnoses were grouped into eight major system-based categories. A subsequent analysis was also conducted on several subgroups identified a priori, including gender, age quartiles, operative and non-operative diagnostic codes, ventilated and non-ventilated patients, emergency and elective patients, care type (high dependency unit v ICU) and, given that metabolic reserve has been given as a plausible underlying mechanistic cause for the obesity paradox, ICU length of stay in three epochs (< 4 days, 4–9 days, and ≥ 10 days).30, 31, 32, 33
In-hospital mortality was examined in all patients and in the subgroups described above. Secondary outcomes included ICU mortality and both ICU and hospital length of stay.
Statistical analysis
Data were analysed with STATA version 15.1 (StataCorp, Texas, USA). All data were initially assessed for normality. Group comparisons were performed using χ2 tests, Student t test for normally distributed data, and Kruskall–Wallis for non-normally distributed data and comparisons across multiple groups. Results are reported as n (%), mean (standard deviation [SD]) or median (interquartile range [IQR]) respectively. Mixed effects multivariable logistic regression was used to adjust for baseline severity of illness using ANZROD methodology, gender, year of admission, diagnostic category, region, age and hospital type, clustered by site, and site treated as a random effect. Results are presented as adjusted odds ratio (aOR) with 95% confidence interval (CI), and discrimination of the multivariable models was assessed using area under the receiver operator characteristic (AUROC). Curves were subsequently fitted with restricted cubic splines with three knots after a multivariate logistic regression adjusted for risk of death (ANZROD), hospital type, admission year, gender, region and diagnostic category, except where the covariate contained the subgroup of interest, in which case the covariate was omitted from the regression model. A BMI of 22.0 kg/m2 (being the middle of the normal range) was used as the reference for all curves. Given the size of the dataset and the multiple analyses undertaken, a two-sided P value of 0.01 was used to indicate statistical significance.
Results
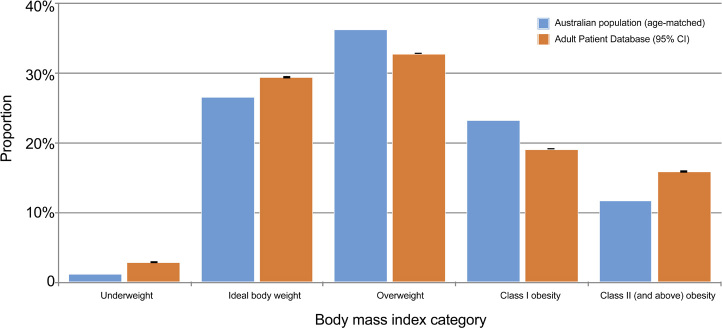
Within the study period, there were 1323071 admissions to adult Australian ICUs reported to the ANZICS APD. After exclusions (online Appendix, supplementary figure 1; available at cicm. org.au/Resources/Publications/Journal), the study dataset comprised 381 855 patients (median age, 64.5 years; IQR, 51.1–74.2 years; 59% male) from all jurisdictions within Australia and New Zealand, 68% of whom were classified as overweight or obese (Table 1). When compared with an age-matched distribution of BMI for the Australian population, patients with a BMI greater than 35 kg/m2 (class II obesity and above) were over-represented in ICUs (Figure 1), and the proportion of admissions with a BMI greater than 35 kg/m2 had increased over the sampling period (online Appendix, supplementary figure 2).5 The median APACHE III score was 48 (IQR, 36–63), and the mean risk of in-hospital mortality, as measured by ANZROD, was 7.1% (SD, 14.7%) (Table 1).
Table 1.
Characteristics of the study dataset
| Variable | All (n = 381 855) | Underweight (< 18.4 kg/m2) (n = 10 986) | Ideal body weight (18.5–24.9 kg/m2) (n = 112 064) | Overweight (25.0–29.9 kg/m2) (n = 125 194) | Class I obesity (30.0–34.9 kg/m2) (n = 73 028) | Class II and above (> 35 kg/m2) (n = 60 583) | P |
|---|---|---|---|---|---|---|---|
| Age (years), median (IQR) | 64.5 (51.1–74.2) | 60.0 (38.5–74) | 64 (46.7–75.8) | 66.6 (54.1–75.5) | 65.4 (54.3–73.7) | 60.7 (49.2–69.5) | < 0.001 |
| Male⁎ | 226 583 (59.4%) | 4278 (38.9%) | 63 067 (56.3%) | 83 215 (66.5%) | 46 327 (63.5%) | 29 696 (49.0%) | < 0.001 |
| BMI, median (IQR) | 27.5 (23.9–32.0) | 17.3 (16.3–18.0) | 22.8 (21.2–23.9) | 27.3 (26.1–28.5) | 32.0 (30.9–33.3) | 39.4 (36.8–44.0) | N/A |
| Australia | 346 774 (90.8%) | 10 149 (92.4%) | 101 403 (90.5%) | 113 860 (90.9%) | 66 244 (90.7%) | 55 118 (91.0%) | N/A |
| New Zealand | 35 081 (9.2%) | 837 (7.6%) | 10 661 (9.5%) | 11 334 (9.1%) | 6784 (9.3%) | 5465 (9.0%) | N/A |
| Hospital classification | |||||||
| Tertiary | 189 763 (49.7%) | 5599 (51.0%) | 59 440 (53.0%) | 63 044 (50.4%) | 35 358 (48.4%) | 26 322 (43.5%) | |
| Metropolitan | 25 983 (6.8%) | 1020 (9.3%) | 7480 (6.7%) | 7760 (6.2%) | 4597 (6.3%) | 5126 (8.5%) | < 0.001 |
| Regional/rural | 47 663 (12.5%) | 1976 (18.0%) | 14 283 (12.8%) | 14 290 (11.4%) | 8591 (11.8%) | 8523 (14.1%) | |
| Private | 118 446 (31.0%) | 2391 (21.8%) | 30 861 (27.5%) | 40 100 (32.0%) | 24 482 (33.5%) | 20 612 (34.0%) | |
| Emergency admission | 184 007 (48.2%) | 7114 (65.1%) | 60 330 (54.1%) | 57 908 (46.4%) | 31 210 (42.9%) | 27 445 (45.5%) | < 0.001 |
| Hours preceding ICU (h), median (IQR) | 11.0 (5.2–29.4) | 10.4 (4.5–35.0) | 10.7 (4.7–29.7) | 12.1 (5.4–30.1) | 12.0 (5.7–29.8) | 9.2 (5.2–26.7) | < 0.001 |
| HDU† | 294 776 (77.2%) | 8186 (75.0%) | 87 369 (78.5%) | 98 549 (79.2%) | 56 663 (77.9%) | 44 011 (72.9%) | < 0.001 |
| ICU† | 84 986 (22.4%) | 2725 (25.0%) | 23 970 (21.5%) | 25 896 (20.8%) | 16 039 (22.0%) | 16 356 (27.1%) | < 0.001 |
| Chronic disease† | |||||||
| Respiratory | 23 291 (6.1%) | 1365 (12.4%) | 6437 (5.8%) | 6212 (5.0%) | 3984 (5.5%) | 5293 (8.7%) | < 0.001 |
| Cardiovascular | 31 052 (8.1%) | 668 (6.1%) | 8054 (7.2%) | 10 667 (8.5%) | 6562 (9.0%) | 5101 (8.4%) | < 0.001 |
| Liver | 5975 (1.6%) | 272 (2.5%) | 1972 (1.8%) | 1834 (1.5%) | 1065 (1.5%) | 832 (1.4%) | < 0.001 |
| Renal | 10 124 (2.7%) | 313 (2.9%) | 3014 (2.7%) | 2997 (2.4%) | 2025 (2.8%) | 1775 (2.9%) | < 0.001 |
| Lymphoma | 3332 (0.9%) | 149 (1.4%) | 1280 (1.1%) | 1084 (0.9%) | 508 (0.7%) | 311 (0.5%) | < 0.001 |
| Metastatic disease | 15 066 (4.0%) | 567 (5.2%) | 5514 (4.9%) | 5035 (4.0%) | 2547 (3.5%) | 1403 (2.3%) | < 0.001 |
| Leukaemia | 4001 (1.1%) | 123 (1.1%) | 1449 (1.3%) | 1364 (1.1%) | 653 (0.9%) | 412 (0.7%) | < 0.001 |
| Immunosuppressed‡ | 24 021 (6.3%) | 1199 (10.9%) | 8591 (7.7%) | 7604 (6.1%) | 3882 (5.3%) | 2745 (4.5%) | < 0.001 |
| Two or more chronic diseases | 15 994 (4.2%) | 636 (5.8%) | 4847 (4.3%) | 4842 (3.9%) | 2955 (4.1%) | 2714 (4.5%) | < 0.001 |
| APACHE III score, mean (SD) | 51.8 ± 23.6 | 55.5 ± 24.9 | 53.1 ± 24.1 | 52.5 ± 23.3 | 51.1 ± 22.8 | 48.0 ± 23.9 | |
| APACHE III score, median (IQR) | 48 (36–63) | 52 (38–68) | 49 (36–65) | 49 (37–63) | 47 (36–61) | 44 (31–59) | < 0.001 |
| Less age score,§ median (IQR) | 36 (26–50) | 42 (30–57) | 38 (27–52) | 36 (27–50) | 36 (26–49) | 34 (24–48 | < 0.001 |
| APACHE III RoD (%), mean (SD) | 15.3% ± 20.0% | 13.1% ± 19.0% | 11.8% ± 18.4% | 10.8% ± 17.5% | 10.3% ± 17.1% | < 0.001 | |
| APACHE III RoD (%), median (IQR) | 4.3% (1.6–12.5%) | 7.3% (2.7–19.4%) | 5.1% (1.9–14.8%) | 4.2% (1.6–12.2%) | 3.8% (1.4–10.7%) | 3.3% (1.3–10.2%) | |
| ANZROD (%), mean (SD) | 7.1% ± 14.7% | 10.0% ± 16.6% | 7.9% ± 15.4% | 7.0% ± 14.7% | 6.3% ± 13.8% | 6.1% ± 13.6% | < 0.001 |
| ANZROD (%), median (IQR) | 1.4% (0.5–5.4%) | 2.8% (0.8–10.1%) | 1.7% (0.6–6.6%) | 1.4% (0.5–5.2%) | 1.2% (0.4–4.4%) | 1.0% (0.4–4.3%) | |
| Mechanical ventilation | 163 568 (42.8%) | 4000 (36.4%) | 47 391 (42.3%) | 57 028 (45.6%) | 32 689 (44.8%) | 22 460 (37.1%) | < 0.001 |
ANZROD = Australia and New Zealand Risk of Death; APACHE = Acute Physiology and Chronic Health Evaluation; HDU = high dependency unit; ICU = intensive care unit; IQR = interquartile range; RoD = risk of death; SD = standard deviation.
n = 381 775 (43 were indeterminate and 37 had unknown gender).
Australian and New Zealand Intensive Care Society (ANZICS) modification of the APACHE III diagnosis list (ANZICS Adult Patient Database data dictionary).
Combination of immunosuppressed, immunosuppressive therapy, immune suppressing disease, acquired immune deficiency syndrome (AIDS) from the ANZICS modified APACHE III chronic disease definitions.
APACHE III score with age component removed.
Figure 1.
Distribution of obesity in Australian and New Zealand intensive care units versus age-matched Australian population
Unadjusted hospital mortality (Table 2) demonstrated that increasing levels of obesity were associated with lower mortality. There was a similar pattern for ICU mortality, and the ICU length of stay was shortest for the most obese patients (Table 2).
Table 2.
Study outcomes
| Variable | All (n = 381 855) | Underweight (< 18.4 kg/m2) (n = 10 986) | Ideal body weight (18.5–24.9 kg/m2) (n = 112 064) | Overweight (25.0–29.9 kg/m2) (n = 125 194) | Class I obesity (30.0–34.9 kg/m2) (n = 73 028) | Class II and above (> 35 kg/m2) (n = 60 583) | P |
|---|---|---|---|---|---|---|---|
| Primary outcome | |||||||
| Hospital mortality | 24 999 (6.6%) |
1304 (11.9%) |
8604 (7.7%) |
7949 (6.4%) |
3937 (5.4%) |
3205 (5.3%) |
< 0.001 |
| Secondary outcomes | |||||||
| ICU | 15 956 | 719 | 5294 | 5195 | 2593 | 2155 | < 0.001 |
| mortality | (4.2%) | (6.6%) | (4.7%) | (4.2%) | (3.6%) | (3.6%) | |
| ICU LOS (days), median (IQR) | 1.7 (0.9–3.2) | 1.9 (0.9–3.7) | 1.8 (0.9–3.4) | 1.8 (0.9–3.1) | 1.7 (0.9–3.1) | 1.5 (0.9–3.0) | < 0.001 |
| Hospital LOS (days), median (IQR) | 8.3 (4.9–14.9) | 9.7 (5.1–18.6) | 8.7 (5.0–15.9) | 8.4 (5.2–14.8) | 8.2 (5.0–14.2) | 7.4 (4.0–13.7) | < 0.001 |
ICU = intensive care unit; IQR = interquartile range; LOS = length of stay.
Unadjusted mortality for each diagnostic category by BMI category (online Appendix, supplementary table 1) demonstrated a similar association for all diagnostic categories, with the exception of cardiac surgery and admissions with a cardiovascular diagnosis. For these diagnostic categories, there was a marked increase in unadjusted mortality for patients with a BMI greater than 35 kg/m2 (class II obesity and above), such that patients with class I obesity had the lowest mortality.
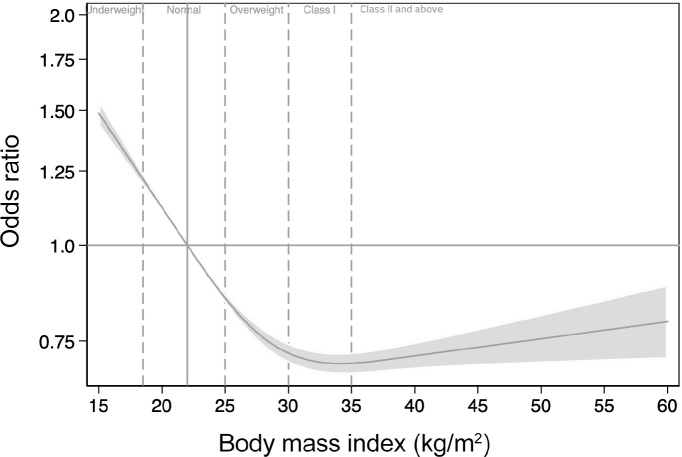
After adjustment for baseline illness severity, hospital type, region, diagnostic category and year of admission, the BMI classification at which adjusted mortality was lowest was class I obesity (aOR, 0.78; 95% CI, 0.74–0.82; P < 0.001) (online Appendix, supplementary table 2), and the association between BMI and mortality described the obesity paradox (Figure 2).
Figure 2.
Mortality adjusted for illness severity, hospital type, admission year, gender, region, and diagnostic category fitted with restricted cubic splines with three knots against body mass index
Evidence of the obesity paradox appeared in all of the subgroups defined a priori, with mortality highest in underweight patients and a fall in mortality up to class I obesity (online Appendix, supplementary figures 3–10). However, there were differences in the odds ratio of mortality at higher levels of obesity across subgroups. For patients with an admission diagnostic category of respiratory disease and sepsis, the adjusted mortality was no different to the unadjusted one and continued to demonstrate that higher levels of obesity were associated with lower mortality (online Appendix, supplementary figure 3, panels C and F), although the confidence intervals above 35.0 kg/m2 are broad. Similarly, for patients with an ICU length of stay greater than 240 hours, there was also an apparent reduction in mortality at a BMI greater than 35 kg/m2 (class II obesity and above) (online Appendix, supplementary figure 9, panel C).
In contrast, for the diagnostic categories of cardiac surgery, cardiovascular disease and trauma, there was a clear nadir in mortality at class I obesity and an increase in mortality at higher levels of obesity (online Appendix, supplementary figure 3, panels A, B and G). This phenomenon was also observed in males, patients aged over 65 years, post-operative admissions, ventilated patients, elective admissions, and patients with an ICU length of stay of less than 96 hours (online Appendix, supplementary figure 4, panel B; supplementary figure 5, panels C and D; supplementary figure 6, panel A; supplementary figure 7, panel A; supplementary figure 8, panel A; and supplementary figure 9, panel A, respectively). The similarity in the relationship between mortality and BMI for the categories of post-operative and elective admission is due to the high proportion of overlap between these groups (97% of elective admissions are operative).
Finally, for patients admitted under the diagnostic categories of neurology, sepsis or “other diagnosis”, there was an apparent plateau in mortality above class I obesity (online Appendix, supplementary figure 3 panels F, G and H) as well as for the remaining subgroup comparisons of female gender, non-operative categories, emergency admissions, and patients with an ICU length of stay of 96–120 hours (online Appendix, supplementary figure 4, panel A; supplementary figure 6, panel B; supplementary figure 8, panel B; and supplementary figure 9, panel B, respectively)
Discussion
This large retrospective study examining the epidemiology of obesity in Australian and New Zealand ICUs confirms that patients with a BMI greater than 35 kg/m2 are over-represented relative to the Australian population. Furthermore, our results confirm that increasing levels of obesity were associated with lower unadjusted mortality (Table 2). After adjustment for a range of confounders, mortality was lowest for patients with a BMI between 30.0 and 34.9 kg/m2 (class I obesity) (Figure 2).
Subgroup analysis undertaken to explore contributors to the paradox confirm that the phenomenon is consistent across a range of categories, including diagnostic category, gender, age quartiles, operative category, ventilation status, elective status, and ICU length of stay.
It is not clear, however, what contributes to the different patterns of mortality at higher BMI categories, with substantial variation in mortality seen in different subgroups at higher levels of obesity. For some subgroups, the relationship is U-shaped, with an increase in mortality as BMI increases above 35 kg/m2. In contrast, there are several subgroups in which mortality continues to fall as BMI increases. For the remaining subgroups, there is a plateau in mortality above class I obesity. However, for this latter group, the odds ratios never approach 1. It is difficult to isolate any single common theme among these apparently disparate subgroups which provides a physiological rationale for these different patterns of in-hospital mortality.
Age appears to have a complex effect on the relationship between BMI and mortality. Our data indicate lower mortality with increasing level of obesity for patients aged less than 50 years, while the association is more complex with increasing age such that mortality increases in each successive age quartile as the BMI increases. Given the over-representation of older patients in ICUs generally, this may be responsible for skewing the results slightly. Nevertheless, it remains the case that, in critical illness, some obesity is protective for all, but that at older ages, morbid obesity is associated with an increased probability of death and lends support to the contention that the BMI at which mortality is lowest is not stable across the age spectrum.34
Our results suggest that, in critical illness, the BMI at which mortality is lowest is higher than that which has traditionally been taken to be the ideal body weight. There is evidence in the literature of this rightward shift in both healthy and diseased states.14,35 Afzal and colleagues,14 in comparing the mortality outcomes of thee large registry studies, in which each cohort was recruited during successive decades in Denmark, report that the BMI at which mortality is lowest has increased with successive generations.14 Similarly, Thomson and colleagues,15 in reporting outcomes from the Women’s Health Initiative, demonstrate that the BMI at which mortality is lowest occurs for patients who are overweight. More recently, Gribsholt et al35 found that the adjusted 30-day mortality of more than 90 000 Danish hospitalisations was also lowest for patients who are overweight. Of more relevance to critical care, the mortality for critically ill patients has been reported to be lowest for patients who are either overweight or have class I obesity in heterogenous cohorts; for patients with specific pathophysiological processes, such as sepsis, pneumonia and acute respiratory distress syndrome; and for ventilated patients.33,36, 37, 38, 39 Our results support these findings, both overall and in specific subgroups, in a much larger cohort.
Possible physiological explanations for the obesity paradox have been advanced and include:
-
■
the endocrine functions of adipose tissue, resulting in higher levels of anti-inflammatory mediators, including leptin, adinopectin and soluble tumour necrosis factor receptor 2;
-
■
the presence of high density lipoproteins, which bind bacterial lipopolysaccharides;
-
■
the upregulation of the renin-angiotensin system, producing protective haemodynamic effects;
-
■
possible ischaemic pre-conditioning as a result of obstructive sleep apnoea; and
-
■
the nutritional, metabolic and energy reserve provided by adipose tissue.32, 33
Non-physiological methodological explanations have also been postulated, including selection bias, collider bias, reverse causation, and failing to account for confounders such as chronic disease, sarcopenia, malnutrition, and smoking status.40, 41 The observation in these data that patients who have class II obesity or more had less acute physiological derangement at admission suggests that some degree of selection bias may be occurring. However, given the strength of the effect seen here and that the BMI category at which mortality is lowest remains class I obesity after adjustment for illness severity, it seems unlikely that selection bias alone is completely responsible.
It is possible that BMI is a poor proxy for adiposity and body shape and the paradox is not so much a paradox as the poor use of an inappropriate and outdated anthropometric measure. While it is intuitively attractive to assume that an individual has an optimal or ideal body weight for a given height, it does not necessarily follow that this weight is the same for all individuals, that it is the same through their life cycle, or that it is the same in health as it is in sickness.
Strengths and limitations
The major strength of this paper is the large, multicentre database from which the data are drawn, incorporating data from 166 ICUs representing the majority of Australian and New Zealand ICUs, which provides strong external validity. The breadth of the data also meant that the same comparisons could be made across a range of diagnostic categories, offering a significant advantage over the large number of single pathophysiological process or single centre studies that exist.
The study has limitations. First, as a retrospective registry data, it is only possible to highlight associations, and no conclusion about causality can be drawn. It is also possible that biases in the admission threshold drive the observed effect, or that obese patients are dying before being admitted to the ICU. There is a heavy reliance on the accuracy of coding. Furthermore, there is no indication within the database regarding whether weight and height were measured or estimated, nor at what time point in the critical care admission this was undertaken, both of which are likely to be important.16 There was also a substantial proportion of patients excluded from the analysis dataset who had missing height or weight data. This is an important source of potential selection bias because nearly 70% of admissions were excluded on the basis of missing height and/or weight data. Second, the results from this dataset cannot be translated to a non-critically ill population. The existing public health message that the ideal ratio of weight to body height lies between 18.5 and 25 kg/m2 has evidential support, including an association with a range of chronic diseases and early death, and the over-representation of class II obesity and above in this dataset may speak to the increased burden of disease associated with obesity.4,42, 43, 44, 45 Furthermore, the distribution of obesity in this dataset differs from that of the Australian population, and no comparison was made to the general New Zealand population (who contribute about 10% of the admissions to the dataset). Third, the very low hospital mortality rate observed in this dataset may suggest that only survivors have both their weight and height recorded, which may introduce survival bias. Finally, we were unable to explore all possible confounders due to the limitations of the dataset. While it leaves unanswered the question of whether BMI is the best measure of body shape, there is sufficient evidence in these results to suggest it is not, and further research is urgently required to ascertain whether the inclusion of other measures, such as waist circumference, would be preferable.12, 17, 46
Future research needs to focus on the pathophysiological influences of obesity, the effects of different distributions of adipose tissue (peripheral v visceral), different phenotypes, and the effect of confounders that could not be measured in these data, including the influence of malnutrition, which may be important given the apparent lower mortality seen in the very obese with a longer ICU length of stay. Furthermore, priority should be given to teasing out the underlying reasons for the difference in mortality at class II obesity and above.
Conclusion
Overweight and obese critically ill patients are over-represented in the ICU relative to the distribution of obesity in the general community and this study confirms that the relationship described by the obesity paradox exists in this large heterogenous cohort.
While it has not been possible with the dataset available to confirm or refute any of the proposed mechanisms, there are indications in these data that should guide future research efforts.
Competing interests
None declared.
Acknowledgements:
The authors and the ANZICS CORE management committee thank clinicians, data collectors and researchers at the following contributing sites:
Albury Base Hospital ICU, Alfred Hospital ICU, Alice Springs Hospital ICU, Allamanda Private Hospital ICU, Armadale Health Service ICU, Ashford Community Hospital ICU, Austin Hospital ICU, Ballarat Health Services ICU, Bankstown–Lidcombe Hospital ICU, Bathurst Base Hospital ICU, Bendigo Health Care Group ICU, Blacktown Hospital ICU, Box Hill Hospital ICU, Brisbane Private Hospital ICU, Brisbane Waters Private Hospital ICU, Buderim Private Hospital ICU, Bunbury Regional Hospital ICU, Bundaberg Base Hospital ICU, Caboolture Hospital ICU, Cabrini Hospital ICU, Cairns Hospital ICU, Calvary Hospital (Canberra) ICU, Calvary Hospital (Lenah Valley) ICU, Calvary John James Hospital ICU, Calvary Mater Newcastle ICU, Calvary North Adelaide Hospital ICU, Calvary Wakefield Hospital (Adelaide) ICU, Campbelltown Hospital ICU, Canberra Hospital ICU, Central Gippsland Health Service ICU, Coffs Harbour Health Campus ICU, Concord Hospital (Sydney) ICU, Dandenong Hospital ICU, Epworth Eastern Private Hospital ICU, Epworth Freemasons Hospital ICU, Epworth Geelong ICU, Epworth Hospital (Richmond) ICU, Fairfield Hospital ICU, Figtree Private Hospital ICU, Fiona Stanley Hospital ICU, Flinders Medical Centre ICU, Flinders Private Hospital ICU, Footscray Hospital ICU, Frankston Hospital ICU, Fremantle Hospital ICU, Gold Coast Private Hospital ICU, Gold Coast University Hospital ICU, Gosford Hospital ICU, Gosford Private Hospital ICU, Goulburn Base Hospital ICU, Goulburn Valley Health ICU, Grafton Base Hospital ICU, Greenslopes Private Hospital ICU, Griffith Base Hospital ICU, Hervey Bay Hospital ICU, Hollywood Private Hospital ICU, Holy Spirit Northside Hospital ICU, Hornsby Ku-ring-gai Hospital ICU, Hurstville Private Hospital ICU, Ipswich Hospital ICU, John Fawkner Hospital ICU, John Flynn Private Hospital ICU, John Hunter Hospital ICU, Joondalup Health Campus ICU, Kareena Private Hospital ICU, Knox Private Hospital ICU, Latrobe Regional Hospital ICU, Launceston General Hospital ICU, Lismore Base Hospital ICU, Liverpool Hospital ICU, Logan Hospital ICU, Lyell McEwin Hospital ICU, Mackay Base Hospital ICU, Macquarie University Private Hospital ICU, Manning Rural Referral Hospital ICU, Maroondah Hospital ICU, Mater Adults Hospital (Brisbane) ICU, Mater Health Services North Queensland ICU, Mater Private Hospital (Brisbane) ICU, Mater Private Hospital (Sydney) ICU, Melbourne Private Hospital ICU, Mildura Base Hospital ICU, Modbury Public Hospital ICU, Monash Medical Centre-Clayton Campus ICU, Mount Hospital ICU, Mount Isa Hospital ICU, Nambour General Hospital ICU, National Capital Private Hospital ICU, Nepean Hospital ICU, Newcastle Private Hospital ICU, Noosa Hospital ICU, North Shore Private Hospital ICU, North West Regional Hospital (Burnie) ICU, Northeast Health Wangaratta ICU, Northern Beaches Hospital ICU, Norwest Private Hospital ICU, Orange Base Hospital ICU, Peninsula Private Hospital ICU, Peter MacCallum Cancer Institute ICU, Pindara Private Hospital ICU, Port Macquarie Base Hospital ICU, Prince of Wales Hospital (Sydney) ICU, Prince of Wales Private Hospital (Sydney) ICU, Princess Alexandra Hospital ICU, Queen Elizabeth II Jubilee Hospital ICU, Redcliffe Hospital ICU, Repatriation General Hospital (Adelaide) ICU, Robina Hospital ICU, Rockhampton Hospital ICU, Rockingham General Hospital ICU, Royal Adelaide Hospital ICU, Royal Brisbane and Women’s Hospital ICU, Royal Darwin Hospital ICU, Royal Hobart Hospital ICU, Royal Melbourne Hospital ICU, Royal North Shore Hospital ICU, Royal Perth Hospital ICU, Royal Prince Alfred Hospital ICU, Shoalhaven Hospital ICU, Sir Charles Gairdner Hospital ICU, South West Healthcare (Warrnambool) ICU, St Andrew’s Hospital (Adelaide) ICU, St Andrew’s Hospital Toowoomba ICU, St Andrew’s War Memorial Hospital ICU, St George Hospital (Sydney) C, St George Hospital (Sydney) ICU, St George Hospital (Sydney) ICU, St George Private Hospital (Sydney) ICU, St John Of God Health Care (Subiaco) ICU, St John Of God Hospital (Ballarat) ICU, St John of God Hospital (Bendigo) ICU, St John Of God Hospital (Geelong) ICU, St John Of God Hospital (Murdoch) ICU, St John of God Midland Public and Private ICU, St Vincent’s Hospital (Melbourne) ICU, St Vincent’s Hospital (Sydney) ICU, St Vincent’s Hospital (Toowoomba) ICU, St Vincent’s Private Hospital (Sydney) ICU, St Vincent’s Private Hospital Fitzroy ICU, Sunnybank Hospital ICU, Sunshine Hospital ICU, Sutherland Hospital and Community Health Services ICU, Sydney Adventist Hospital ICU, Sydney Southwest Private Hospital ICU, Tamworth Base Hospital ICU, The Memorial Hospital (Adelaide) ICU, The Northern Hospital ICU, The Prince Charles Hospital ICU, The Queen Elizabeth (Adelaide) ICU, The Townsville Hospital ICU, The Valley Private Hospital ICU, The Wesley Hospital ICU, Toowoomba Hospital ICU, Tweed Heads District Hospital ICU, University Hospital Geelong ICU, Wagga Wagga Base Hospital and District Health ICU, Warringal Private Hospital ICU, Western District Health Service (Hamilton) ICU, Western Hospital (SA) ICU, Westmead Hospital ICU, Westmead Private Hospital ICU, Wimmera Health Care Group (Horsham) ICU, Wollongong Hospital ICU, Wollongong Private Hospital ICU, Wyong Hospital ICU.
Supplementary Information

References
- 1.Kopple J.D., Zhu X., Lew N.L., Lowrie E.G. Body weight-for-height relationships predict mortality in maintenance hemodialysis patients. Kidney Int. 1999;56:1136–1148. doi: 10.1046/j.1523-1755.1999.00615.x. [DOI] [PubMed] [Google Scholar]
- 2.Dixon J.B., Egger G.J., Finkelstein E.A., et al. “Obesity paradox” misunderstands the biology of optimal weight throughout the life cycle. Int J Obes (Lond) 2015;39:82–84. doi: 10.1038/ijo.2014.59. [DOI] [PubMed] [Google Scholar]
- 3.Schetz M., De Jong A., Deane A.M., et al. Obesity in the critically ill: a narrative review. Intensive Care Med. 2019;45:757–769. doi: 10.1007/s00134-019-05594-1. [DOI] [PubMed] [Google Scholar]
- 4.Obesity Collaborators G.B.D., Afshin A., Forouzanfar M.H., Reitsma M.B., et al. Health effects of overweight and obesity in 195 countries over 25 years. N Engl J Med. 2017;377:13–27. doi: 10.1056/NEJMoa1614362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Australian Institute of Health and Welfare. Australia’s health 2018 [Australia’s health series No. 16; Cat. No. AUS 221]. Canberra: AIHW, 2018. https://www.aihw.gov.au/reports/australias-health/australias-health-2018/contents/indicators-of-australias-health/selected-potentially-preventable-hospitalisations (viewed Mar 2019).
- 6.Martino J.L., Stapleton R.D., Wang M., et al. Extreme obesity and outcomes in critically ill patients. Chest. 2011;140:1198–1206. doi: 10.1378/chest.10-3023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ferreira I., Stehouwer C.D. Obesity paradox or inappropriate study designs? Time for life-course epidemiology. J Hypertens. 2012;30:2271–2275. doi: 10.1097/HJH.0b013e32835b4fe0. [DOI] [PubMed] [Google Scholar]
- 8.Standl E., Erbach M., Schnell O. Defending the con side: obesity paradox does not exist. Diabetes Care. 2013;36(Suppl):S282–S286. doi: 10.2337/dcS13-2040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Robinson W.R., Furberg H., Banack H.R. Selection bias: a missing factor in the obesity paradox debate. Obesity. 2014;22:625. doi: 10.1002/oby.20666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Banack H.R., Kaufman J.S. Does selection bias explain the obesity paradox among individuals with cardiovascular disease? Ann Epidemiol. 2015;25:342–349. doi: 10.1016/j.annepidem.2015.02.008. [DOI] [PubMed] [Google Scholar]
- 11.Robinson M.K., Mogensen K.M., Casey J.D., et al. The relationship among obesity, nutritional status, and mortality in the critically ill. Crit Care Med. 2015;43:87–100. doi: 10.1097/CCM.0000000000000602. [DOI] [PubMed] [Google Scholar]
- 12.Bozorgmanesh M., Arshi B., Sheikholeslami F., et al. No obesity paradox — BMI incapable of adequately capturing the relation of obesity with all-cause mortality: an inception diabetes cohort study. Int J Endocrinol. 2014;2014 doi: 10.1155/2014/282089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Flegal K.M., Graubard B.I., Williamson D.F., Gail M.H. Excess deaths associated with underweight, overweight, and obesity. JAMA. 2005;293:1861–1867. doi: 10.1001/jama.293.15.1861. [DOI] [PubMed] [Google Scholar]
- 14.Afzal S., Tybjaerg-Hansen A., Jensen G.B., Nordestgaard B.G. Change in body mass index associated with lowest mortality in Denmark, 1976–2013. JAMA. 2016;315:1989–1996. doi: 10.1001/jama.2016.4666. [DOI] [PubMed] [Google Scholar]
- 15.Thomson C.A., Garcia D.O., Wertheim B.C., et al. Body shape, adiposity index, and mortality in postmenopausal women: findings from the Women’s Health Initiative. Obesity. 2016;24:1061–1069. doi: 10.1002/oby.21461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Toft-Petersen A.P., Wulff J., Harrison D.A., et al. Exploring the impact of using measured or estimated values for height and weight on the relationship between BMI and acute hospital mortality. J Crit Care. 2018;44:196–202. doi: 10.1016/j.jcrc.2017.11.021. [DOI] [PubMed] [Google Scholar]
- 17.Pickkers P., de Keizer N., Dusseljee J., et al. Body mass index is associated with hospital mortality in critically ill patients: an observational cohort study. Critical Care Med. 2013;41:1878–1883. doi: 10.1097/CCM.0b013e31828a2aa1. [DOI] [PubMed] [Google Scholar]
- 18.Andersen K.K., Olsen T.S. The obesity paradox in stroke: lower mortality and lower risk of readmission for recurrent stroke in obese stroke patients. Int J Stroke. 2015;10:99–104. doi: 10.1111/ijs.12016. [DOI] [PubMed] [Google Scholar]
- 19.Wohlfahrt P., Lopez-Jimenez F., Krajcoviechova A., et al. The obesity paradox and survivors of ischemic stroke. J Stroke Cerebrovasc Dis. 2015;24:1443–1450. doi: 10.1016/j.jstrokecerebrovasdis.2015.03.008. [DOI] [PubMed] [Google Scholar]
- 20.Bramley A.M., Reed C., Finelli L., et al. Relationship between body mass index and outcomes among hospitalized patients with community-acquired pneumonia. J Infect Dis. 2017;215:1873–1882. doi: 10.1093/infdis/jix241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim H., Kim J., Seo C., et al. Body mass index is inversely associated with mortality in patients with acute kidney injury undergoing continuous renal replacement therapy. Kidney Res Clin Pract. 2017;36:39–47. doi: 10.23876/j.krcp.2017.36.1.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mariscalco G., Wozniak M.J., Dawson A.G., et al. Body mass index and mortality among adults undergoing cardiac surgery: a nationwide study with a systematic review and meta-analysis. Circulation. 2017;135:850–863. doi: 10.1161/CIRCULATIONAHA.116.022840. [DOI] [PubMed] [Google Scholar]
- 23.Ziolkowski S.L., Long J., Baker J.F., et al. Chronic kidney disease and the adiposity paradox: valid or confounded? J Ren Nutr. 2019;29:521–528. doi: 10.1053/j.jrn.2018.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Australian and New Zealand Intensive Care Society, Centre for Outcome and Resource Evaluation. ANZICS CORE annual report 2017. ANZICS, 2017. https://www.anzics.com.au/wp-content/uploads/2018/10/ANZICS-CORE-Annual-Report-2017. pdf (viewed Aug 2019).
- 25.World Health Organization. Obesity and overweight [website]. http://www.who.int/mediacentre/factsheets/fs311/en/ (viewed Nov 2019).
- 26.Australian and New Zealand Intensive Care Society Centre for Outcomes and Resource Evaluation. APD data dictionary for software programmers: ANZICS CORE — Adult Patient Database version 5.8. ANZCIS CORE; Melbourne: 2019. https://www.anzics.com.au/wp-content/uploads/2018/08/ANZICS-APD-Programmers-Data-Dictionary.pdf (viewed Nov 2019) [Google Scholar]
- 27.Knaus W.A., Wagner D.P., Draper E.A., et al. The APACHE III prognostic system. Risk prediction of hospital mortality for critically ill hospitalized adults. Chest. 1991;100:1619–1636. doi: 10.1378/chest.100.6.1619. [DOI] [PubMed] [Google Scholar]
- 28.Paul E., Bailey M., Pilcher D. Risk prediction of hospital mortality for adult patients admitted to Australian and New Zealand intensive care units: development and validation of the Australian and New Zealand Risk of Death model. J Crit Care. 2013;28:935–941. doi: 10.1016/j.jcrc.2013.07.058. [DOI] [PubMed] [Google Scholar]
- 29.Pilcher D., Paul E., Bailey M., Huckson S. The Australian and New Zealand Risk of Death (ANZROD) model: getting mortality prediction right for intensive care units. Crit Care Resusc. 2014;16:3–4. [PubMed] [Google Scholar]
- 30.Williams T.A., Ho K.M., Dobb G.J., et al. Effect of length of stay in intensive care unit on hospital and long-term mortality of critically ill adult patients. Br J Anaesth. 2010;104:459–464. doi: 10.1093/bja/aeq025. [DOI] [PubMed] [Google Scholar]
- 31.Mahesh B., Choong C.K., Goldsmith K., et al. Prolonged stay in intensive care unit is a powerful predictor of adverse outcomes after cardiac operations. Ann Thorac Surg. 2012;94:109–116. doi: 10.1016/j.athoracsur.2012.02.010. [DOI] [PubMed] [Google Scholar]
- 32.Ng P.Y., Eikermann M. The obesity conundrum in sepsis. BMC Anesthesiol. 2017;17:147. doi: 10.1186/s12871-017-0434-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhao Y., Li Z., Yang T., et al. Is body mass index associated with outcomes of mechanically ventilated adult patients in intensive critical units? A systematic review and meta-analysis. PLoS One. 2018;13 doi: 10.1371/journal.pone.0198669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dixon J.B. Joining the dots for the management of clinically severe obesity. Med J Aust. 2015;202:472–473. doi: 10.5694/mja14.01753. [DOI] [PubMed] [Google Scholar]
- 35.Gribsholt S.B., Pedersen L., Richelsen B., et al. Body mass index of 92 027 patients acutely admitted to general hospitals in Denmark: associated clinical characteristics and 30-day mortality. PLoS One. 2018;13 doi: 10.1371/journal.pone.0195853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nie W., Zhang Y., Jee S.H., et al. Obesity survival paradox in pneumonia: a meta-analysis. BMC Med. 2014;12:61. doi: 10.1186/1741-7015-12-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sakr Y., Alhussami I., Nanchal R., et al. Being overweight is associated with greater survival in ICU patients: results from the Intensive Care Over Nations Audit. Crit Care Med. 2015;43:2623–2632. doi: 10.1097/CCM.0000000000001310. [DOI] [PubMed] [Google Scholar]
- 38.Ni Y.N., Luo J., Yu H., et al. Can body mass index predict clinical outcomes for patients with acute lung injury/acute respiratory distress syndrome? A meta-analysis. Crit Care. 2017;21:36. doi: 10.1186/s13054-017-1615-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pepper D.J., Demirkale C.Y., Sun J., et al. Does obesity protect against death in sepsis? A retrospective cohort study of 55 038 adult patients. Crit Care Med. 2019;47(5):643–650. doi: 10.1097/CCM.0000000000003692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tobias D.K., Hu F.B. Does being overweight really reduce mortality? Obesity. 2013;21:1746–1749. doi: 10.1002/oby.20602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schooling C.M., Au Yeung S.L. “Selection bias by death” and other ways collider bias may cause the obesity paradox. Epidemiology. 2017;28:e16. doi: 10.1097/EDE.0000000000000591. e7. [DOI] [PubMed] [Google Scholar]
- 42.Twig G., Yaniv G., Levine H., et al. Body-mass index in 2.3 million adolescents and cardiovascular death in adulthood. N Engl J Med. 2016;374:2430–2440. doi: 10.1056/NEJMoa1503840. [DOI] [PubMed] [Google Scholar]
- 43.Zheng Y., Manson J.E., Yuan C., et al. Associations of weight gain from early to middle adulthood with major health outcomes later in life. JAMA. 2017;318:255–269. doi: 10.1001/jama.2017.7092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Khan S.S., Ning H., Wilkins J.T., et al. Association of body mass index with lifetime risk of cardiovascular disease and compression of morbidity. JAMA Cardiol. 2018;3:280–287. doi: 10.1001/jamacardio.2018.0022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yang Y., Dugue P.A., Lynch B.M., et al. Trajectories of body mass index in adulthood and all-cause and cause-specific mortality in the Melbourne Collaborative Cohort Study. BMJ Open. 2019;9 doi: 10.1136/bmjopen-2019-030078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gearon E., Tanamas S.K., Stevenson C., et al. Changes in waist circumference independent of weight: Implications for population level monitoring of obesity. Prev Med. 2018;111:378–383. doi: 10.1016/j.ypmed.2017.11.030. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials