Abstract
Background: Contemporary glucose management of intensive care unit (ICU) patients with type 2 diabetes is based on trial data derived predominantly from patients without type 2 diabetes. This is despite the recognition that patients with type 2 diabetes may be relatively more tolerant of hyperglycaemia and more susceptible to hypoglycaemia. It is uncertain whether glucose targets should be more liberal in patients with type 2 diabetes.
Objective: To detail the protocol, analysis and reporting plans for a randomised clinical trial — the Liberal Glucose Control in Critically Ill Patients with Pre-existing Type 2 Diabetes (LUCID) trial — which will evaluate the risks and benefits of targeting a higher blood glucose range in patients with type 2 diabetes.
Design, setting, participants and intervention: A multicentre, parallel group, open label phase 2B randomised controlled clinical trial of 450 critically ill patients with type 2 diabetes. Patients will be randomised 1:1 to liberal blood glucose (target 10.0–14.0 mmol/L) or usual care (target 6.0–10.0 mmol/L).
Main outcome measures: The primary endpoint is incident hypoglycaemia (< 4.0 mmol/L) during the study intervention. Secondary endpoints include biochemical and feasibility outcomes.
Results and conclusion: The study protocol and statistical analysis plan described will delineate conduct and analysis of the trial, such that analytical and reporting bias are minimised.
Trial registration: This trial has been registered on the Australian New Zealand Clinical Trials Registry (ACTRN No. 12616001135404) and has been endorsed by the Australian and New Zealand Intensive Care Society Clinical Trials Group.
It is advocated that blood glucose concentrations are closely monitored and maintained within a range considered safe during critical illness. Following the publication of a single centre, open-label randomised clinical trial of surgical intensive care unit (ICU) patients that reported a reduction in mortality with an intensive insulin treatment regimen,1 many guidelines recommended targeting blood glucose concentrations below 6.1 mmol/L.2, 3 However, this beneficial effect on mortality was not reproduced in a general ICU population by the same research group4 nor by other researchers.5, 6, 7
The Normoglycemia in Intensive Care Evaluation — Survival Using Glucose Algorithm Regulation (NICE-SUGAR) trial8 was a multinational randomised clinical trial comparing intensive insulin therapy (4.5–6.0 mmol/L) with conventional glucose control (6.0–10.0 mmol/L) in a heterogeneous cohort of critically ill patients.8 The study indicated that targeting a blood glucose below 6.1 mmol/L increased 90-day all-cause mortality when compared with targeting 6.0–10.0 mmol/L.8 The results from NICE-SUGAR have been incorporated into all major critical care and diabetes guidelines, with recommendations for insulin to be administered at blood glucose of 10.0 mmol/L or greater and titrated to a target below 10.0 mmol/L, regardless of pre-existing glycaemic status.9, 10
Type 2 diabetes is a common comorbidity in critically ill patients,11, 12, 13, 14, 15 and the observational data strongly support the concept that there is a signal of benefit from higher blood glucose in patients with type 2 diabetes.16, 17, 18, 19 Conversely, hypoglycaemia (absolute and relative) and increased fluctuations in blood glucose concentrations known as glycaemic variability, which are more likely to occur with administration of insulin, are strongly associated with increased mortality in patients with type 2 diabetes.20, 21
The outcomes of single centre sequential period studies, which have compared a so-called liberal approach to glycaemic control (insulin initiated when blood glucose > 14.0 mmol/L; target 10.0–14.0 mmol/L) with usual care (insulin initiated when blood glucose > 10.0 mmol/L; target 6.0–10.0 mmol/L), suggest that a more liberal strategy is beneficial.22, 23, 24 Kar and colleagues23 studied 52 patients with pre-existing type 2 diabetes (4047 hours) receiving usual care and 31 patients (3244 hours) receiving liberal target care. Time-weighted blood glucose concentrations were predictably greater during the liberal period. The primary outcome of moderate to severe hypoglycaemia (< 4.0 mmol/L) occurred for 61 hours during the usual care period and for 12 hours during the liberal period. Participants allocated to the liberal approach were less likely to experience episodes of moderate to severe hypoglycaemia, with five compared with 18 participants during the usual care period (relative risk [RR], 0.47; 95% CI, 0.19–1.13).23 Luethi and colleagues24 studied 700 patients with type 2 diabetes who received either a liberal or usual approach to glycaemic management. In patients with poor pre-morbid blood glucose control (glycated haemoglobin [HbA1c] > 53 mmol/mol), hypoglycaemia occurred in 9.6% of patients receiving standard care and in 4.1% of patients with liberal glucose targets (P = 0.053).24 A liberal approach to glycaemia was not associated with an increased risk of hospital-acquired infectious, cardiovascular, renal or neurological complications.25
Despite the recognition that critically ill patients with type 2 diabetes may benefit from a more liberal approach to management of hyperglycaemia with insulin compared with current recommended glycaemic control,26, 27 this hypothesis has not been tested within a randomised controlled clinical trial. The objectives of the Liberal Glucose Control in Critically Ill Patients with Pre-existing Type 2 Diabetes (LUCID) trial are to evaluate the acute physiological effects of a liberal approach to glucose lowering with insulin and to determine whether a phase 3 randomised controlled trial of a liberal approach compared with usual care in critically ill patients with type 2 diabetes is appropriate and feasible.
Methods
Design
Multicentre, parallel group, open-label phase 2B randomised controlled clinical trial.
Setting
LUCID will be conducted in 23 ICUs in Australia and New Zealand.
Intervention
The trial will compare two blood glucose thresholds with complementary target ranges for the initiation and management of insulin therapy in critically ill patients with type 2 diabetes.
Participants assigned to the intervention of a liberal approach will have insulin commenced at a blood glucose level greater than 14.0 mmol/L and titrated to a target blood glucose in the range 10.0–14.0 mmol/L. If the blood glucose is below 10.0 mmol/L, no attempt to lower or increase blood glucose will be made, with the exception of local protocols for management of hypoglycaemia.
Participants assigned to the usual care group will have the usual care for the institution, which will be aligned to the NICE-SUGAR results, with insulin initiated at a blood glucose level greater than 10.0 mmol/L and titrated to a target blood glucose level in the range of 6.0–10.0 mmol/L.
At each site, the approach to maintaining blood glucose within the relevant ranges will be informed by local practice and will employ local institutional blood glucose and insulin algorithms rather than a standardised protocol across all sites. This pragmatic approach will facilitate external validity and enable real-world comparisons.
Screening
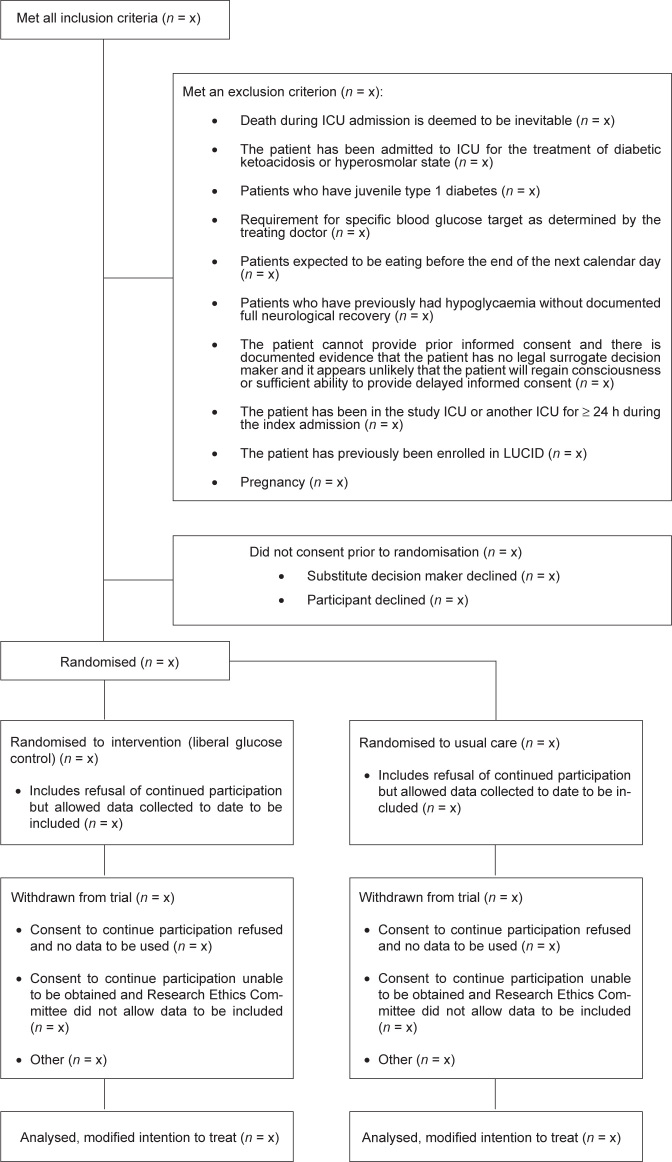
All patients admitted to a participating ICU will be considered for enrolment. Patients will be eligible if they fulfil all the inclusion criteria and none of the exclusion criteria (Table 1). Inclusion and exclusion of patients (including reasons for exclusion) will be reported according to the Consolidated Standards for Reporting of Trials (CONSORT) guidelines (Figure 1).28
Table 1.
Study inclusion and exclusion criteria
| Description | |
|---|---|
| Inclusion criteria |
|
| Exclusion criteria |
|
hCG = human chorionic gonadotropin; ICU = intensive care unit;
LUCID = Liberal Glucose Control in Critically Ill Patients with Pre-existing Type 2 Diabetes trial.
Figure 1.
Consolidated Standards for Reporting of Trials (CONSORT): study flow diagram
ICU = intensive care unit; LUCID = Liberal Glucose Control in Critically III Patients with Pre-existing Type 2 Diabetes trial.
Assignment of intervention
Randomisation will be performed using a secure, web-based interface, with allocation concealment maintained using a permuted, variable size block randomisation stratified by site. Randomisation will not be performed until a participant fulfils all eligibility criteria and can be assigned to study treatment. Group assignment will be unblinded for all involved in the trial.
Baseline data
Baseline data will be recorded and presented (Online Appendix).
Outcome data
The primary outcome is incident hypoglycaemia defined as blood glucose below 4.0 mmol/L. Other outcomes, broadly categorised as feasibility, physiological and clinical outcomes, and processes of care will be reported (Table 2). When using the term “blood glucose”, we are referring to “point of care blood glucose” or “laboratory plasma glucose”, given that the test used for each glucose concentration may vary, the measurement technique of each sample is being collected.
Table 2.
Study outcomes
| Description | |
|---|---|
| Primary outcome |
|
| Secondary outcomes | |
| Feasibility outcomes |
|
| Physiological outcomes |
|
| Clinical outcomes |
|
| Processes of care |
|
CoV = coefficient of variation; ICU = intensive care unit; SD = standard deviation.
On Days 1–7, blood glucose will be recorded as the nearest sample to four time points (00:00 h, 06:00 h, 12:00 h and 18:00 h). If no sample is taken within 3 hours of the designated interval, data will be recorded as missing. If the daily minimum or maximum blood glucose concentration occurred outside of these periods, these will be recorded separately. On study days 8–14, the blood glucose closest to 08:00 hours will be recorded. Blood glucose will not be recorded after Day 14.
Hypoglycaemia will be defined as a blood glucose level below 4.0 mmol/L, obtained from arterial, capillary or venous blood and measured using point-of-care glucometer, arterial blood gas analyser or hospital laboratory testing. An incident event will be defined as hypoglycaemia in the absence of recorded hypoglycaemia in the preceding 4 hours. Because recurrent hypoglycaemia may cause greater harm than a single episode,20, 29 the number of episodes of hypoglycaemia per patient and the proportion of patients experiencing episodes will be reported. Relative hypoglycaemia will also be recorded and defined as a more than a 30% reduction from pre-morbid estimated average glucose, which will be calculated by the formula: (mmol/L) = 1.59 x HbA1c (%) – 2.59.20, 21 Glycaemic variability will be reported using both the coefficient of variation (CoV) and standard deviation (SD) over the first 7 study days.30 Maximum, minimum and group mean glucose will also be reported.
Feasibility outcomes include recruitment and consent rates. The number of study participants assigned to usual care who subsequently receive insulin and the number of overall participants in whom blood glucose is 10.0 mmol/L or greater will be reported, given that insulin-induced hypoglycaemia and glycaemic variability are proposed as key mechanisms underlying harm of usual care.29 Time within blood glucose range and protocol adherence will also be reported. The time outside of blood glucose range does not equate to non-adherence to the protocol. Rather, protocol non-adherence will be restricted to episodes when the assigned blood glucose is no longer being targeted. Non-adherence will be recorded using a categorisation process to discriminate between clinical (eg, the clinician determines that the assigned blood glucose target is no longer in the patient’s best interest) and research-related (eg, consent withdrawal) reasons.
Criteria for discontinuing or modifying allocated intervention
Study participants will continue to receive the intervention while in the ICU or censored at 28 days from randomisation. Glucose management outside the ICU will be at the discretion of the treating physician. The intervention will cease if consent is withdrawn before Day 28, the treating clinician determines that it is in the patient’s best interest to cease the trial intervention, or the treating clinician wishes to transition the participant to an alternative regimen, such as long-acting insulin or oral agents, before discharge from ICU.
Clinical outcomes
Clinical outcomes include 90-day all-cause mortality; length of ICU and hospital stay, with death as a competing risk; hospital discharge destination; and location at Day 90. Infectious complications will be recorded as the number of patients with established blood stream infections and sternal wound infections in cardio-thoracic surgical patients up to Day 28 (Online Appendix).31 To evaluate for a potential difference in infectious complications that may not be apparent as blood stream infections, the highest daily white blood cell count and C-reactive protein concentration will be reported if collected as part of routine care.
Protocol registration and endorsement
The concept for the trial was presented at the Australian and New Zealand Intensive Care Society Clinical Trials Group (ANZICS-CTG) 2016 Annual Meeting on Clinical Trials in Intensive Care. The protocol was subsequently drafted, registered with the Australian New Zealand Clinical Trials Registry (2 August 2016, Trial ID: ACTRN12616001135404), and endorsed as an ANZICS-CTG trial (10 November 2016).
Funding and support
The trial has received funding from four separate project grants:
-
•
the Royal Adelaide Hospital Research Committee Project Grant (2017);
-
•
the Intensive Care Foundation Fisher and Paykel Research Project Grant (2017);
-
•
the Diabetes Australia Research Trust Project Grant (2018); and
-
•
the Melbourne Academic Centre for Health Rapid Applied Research Translation Grant (2019).
Alexis Poole enrolled in a PhD program and will include these data. He receives a University Postgraduate Scholarship (Faculty of Health Sciences Divisional Scholarship and Royal Adelaide Hospital Research Committee Dawes Top-up Scholarship) to support his involvement. The trial is managed within the Centre of Research Excellence in Translating Nutritional Science to Good Health, University of Adelaide. The members of the Management Committee and participating sites are listed in the Online Appendix.
Participant safety
Patients will be withdrawn from the trial if the treating clinician determines that it is in the patient’s best interest to cease the trial intervention. Adverse and serious adverse events will be recorded along with relationship to therapy and action taken (Online Appendix).
Analysis and reporting of results
Data management
Study data will recorded on paper case report forms and then entered into REDCap — a secure web-based data capture tool.32 On-site source monitoring will be conducted by the coordinating centre and will include 25% source data verification for the primary endpoint. Source data verification will be completed for all data points for the first two patients at each centre and partial source data verification will be completed randomly thereafter (20% of total recruitment).
Presentation of outcome data
The proposed table and figures are shown in Table 3. A complete set of mock tables and figures is provided in the Online Appendix.
Table 3.
Planned tables and figures
| Proposed tables and figures | Table/figure | Description |
|---|---|---|
| For the manuscript |
Table 1 Table 2 Figure 1 Figure 2 |
Baseline patient characteristics (by treatment group) Primary and secondary outcomes (by treatment group) Flow of participants through the trial (see Figure 1) Population-averaged mean blood glucose (by treatment) |
| For the online Appendix | Table S1 | Description of consent process |
| Table S2 | Process of care measured in ICU (blood glucose measurement technique, insulin nutrition, corticosteroids and catecholamines administered) | |
| Table S3 | Subgroup analysis (primary and secondary outcomes for HbA1c ≥ 53 mmol/mol | |
| Table S4 | Summary of protocol deviations/adverse events | |
| Figure S1 | Insulin administration v time (units per day) | |
| Figure S2 | Population-averaged mean blood glucose (by treatment) for subgroup HbA1c ≥ 53 mmol/mol | |
| Figure S3 | Cumulative incident plots for the subhazards (ICU or hospital discharge), with death as a competing risk |
HbA1c = glycated haemoglobin; ICU = intensive care unit.
Sample size
The sample size was based on pilot data from a single-centre exploratory study of liberal glucose control against usual care, with the relative risk of hypoglycaemia being 0.47,23 and assumed a baseline rate for incident hypoglycaemia of 17.5% from NICE-SUGAR.8 A sample size of 408 participants would provide 80% power (α 0.05; ∆ 9.3%) to determine a reduction in hypoglycaemic episodes. An additional 10% was added to account for refused consent, loss to follow-up and an unexpected short period of observation. Accordingly, 450 participants will be included in the trial.
Analysis of primary and secondary outcomes
Data will be presented as n/N (%), mean (SD) or median (interquartile range [IQR]), with between-group comparisons using χ2, t test or rank-sum test as indicated. Because of the consent model, the main analyses will be conducted on a modified intention to treat basis (Figure 1).33
The primary outcome will be reported as the incident rate with corresponding 95% confidence interval (CI) and as the raw number of events per group and the proportion of individuals experiencing one or more events. Secondary outcomes will be presented as point estimates with 95% CI. Group point estimates and confidence intervals will be adjusted for within-subject correlation using generalised estimating equations regression with robust standard errors. The incident rate will be standardised to a defined ICU exposure interval; for example, incident rate = X (95% CI) events per N ICU days.
Mortality at Day 90 will be analysed by χ2 test and adjusted for pre-set covariates (age, sex, Acute Physiology and Chronic Health Evaluation [APACHE] II, invasive mechanical ventilation and post-operative admission) by logistic regression, with standard errors adjusted for ICU site.
Pre-defined subgroup analyses
An exploratory subgroup analysis will be conducted based on HbA1c 53 mmol/mol or greater, taken to reflect chronic hyperglycaemia or suboptimal glycaemic control. Stratified randomisation based on this subgroup will not be employed, as this information will frequently be unavailable at randomisation.34
Interim analysis
An interim safety analysis will be conducted after 200 patients are enrolled. An independent Data Safety Monitoring Board (DSMB), composed of an experienced clinical researcher and biostatistician without other connection to the LUCID trial, will operate under a charter based on the recommendations of the DAMOCLES Study Group35 (Online Appendix). Analysis will include primary, secondary, feasibility, clinical and safety outcomes, although not outcomes of interest for the final dataset, ICU and hospital mortality will be included, in addition to 90-day mortality, to facilitate the interim analysis time frame.
Missing data
No imputation will be undertaken for missing data. Rates for missing data will be reported in the supplement when more than 10% values are missing.
Ethical considerations
Ethics approval
The Royal Adelaide Hospital/Central Adelaide Local Heath Network Human Research Ethics Committee has approved the current protocol version 3 dated 26 May 2017 (HREC/16/ RAH/220 and Online Appendix). Under the National Mutual Acceptance (NMA) Scheme, this covers all sites in South Australia, Victoria, New South Wales and Queensland, except for the Alfred Hospital in Victoria. The Alfred Hospital Human Research Ethics Committee has approved a modified protocol allowing only prior written informed consent (Project No. 411/17). The protocol has been approved by the Central Australian Human Research Ethics Committee (Alice Springs Hospital, HREC-16-446) and by the Northern A Health and Disability Ethics Committee in Auckland for sites in New Zealand (ethics reference No. 18/NTA/144).
Consent process
As many patients eligible for this trial will be too unwell to provide informed consent, the approach to obtaining consent in Australia will be based on that developed from the guidelines in Chapter 4.4 of the National Health and Medical Research Council National Statement36 and is consistent with local laws. The approach is a hierarchical consent model. For competent patients, informed consent will be obtained before enrolment. For patients who do not have capacity to consent, the approach to consent will be via the medical treatment decision maker. For patients who do not have capacity and for whom there is no immediately available medical treatment decision maker, patients can be enrolled and consent to continue participation obtained. Consent to continue participation will be obtained at the earliest opportunity and the time will be recorded. The approach to inform the substitute decision maker of study participation if the patient dies before this process is completed is provided in the Online Appendix.
In New Zealand, we will use an approach consistent with section 7.4 of the Health and Disability Code,37 which outlines the appropriate approach to providing treatment to patients who are unable to consent for themselves. The specific approach will be:
-
•
to consider whether participation is in the best interest of each individual patient; and
-
•
as soon as it is practical and reasonable to do so, to seek the advice of persons interested in the patient’s welfare to establish that study participation is consistent with the patient’s wishes.
All participants who recover sufficiently will be given the opportunity to provide informed consent for ongoing study participation and for the use of data collected for the study.
Approach to co-enrolment
The ANZICS-CTG policy on co-enrolment will be followed.38 Site investigators may co-enrol participants in LUCID and other trials, as long as the intervention in other trials is unrelated to glycaemic control and does not require a specific blood glucose target. Trials with co-enrolment approval are listed in the Online Appendix.
Knowledge translation
Data sharing statement
De-identified individual participant data reported in this trial will be made available to researchers who provide a written, methodologically sound proposal between 3 and 7 years after publication. Proposals should be directed to the Principal Investigator. If approved, requestors will be required to enter into a data access and confidentiality agreement.
Information distribution
After completion of the trial, results will be presented at relevant national and international meetings and published in a peer-reviewed journal.
Summary
This study will provide important information to inform future research on the management of patients with type 2 diabetes admitted to an ICU. Our pre-specified statistical analysis plan was prepared before the completion of recruitment and data collection. This published plan provides a detailed description of the principles and methods for analysis and reporting of the study results.
Competing interests
None declared.
Contributor Information
Alexis P. Poole, Email: Alexis.Poole@adelaide.edu.au.
Adam M. Deane, Email: Adam.Deane@mh.org.au.
Supplementary Information

References
- 1.Van den Berghe G., Wouters P., Weekers F., et al. Intensive insulin therapy in critically ill patients. N Engl J Med. 2001;345:1359–1367. doi: 10.1056/NEJMoa011300. [DOI] [PubMed] [Google Scholar]
- 2.ACE/ADA Task Force on Inpatient Diabetes American College of Endocrinology and American Diabetes Association Consensus statement on inpatient diabetes and glycemic control. Diabetes Care. 2006;29:1955–1962. doi: 10.2337/dc06-9913. [DOI] [PubMed] [Google Scholar]
- 3.Dellinger R.P., Carlet J.M., Masur H., et al. Surviving Sepsis Campaign guidelines for management of severe sepsis and septic shock. Intensive Care Med. 2004;30:536–555. doi: 10.1007/s00134-004-2210-z. [DOI] [PubMed] [Google Scholar]
- 4.Van den Berghe G., Wilmer A., Milants I., et al. Intensive insulin therapy in mixed medical/surgical intensive care units: benefit versus harm. Diabetes. 2006;55:3151–3159. doi: 10.2337/db06-0855. [DOI] [PubMed] [Google Scholar]
- 5.De La Rosa Gdel C., Donado J.H., Restrepo A.H., et al. Strict glycaemic control in patients hospitalised in a mixed medical and surgical intensive care unit: a randomised clinical trial. Crit Care. 2008;12:R120. doi: 10.1186/cc7017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brunkhorst F.M., Engel C., Bloos F., et al. Intensive insulin therapy and pentastarch resuscitation in severe sepsis. N Engl J Med. 2008;358:125–139. doi: 10.1056/NEJMoa070716. [DOI] [PubMed] [Google Scholar]
- 7.Arabi Y.M., Dabbagh O.C., Tamim H.M., et al. Intensive versus conventional insulin therapy: a randomized controlled trial in medical and surgical critically ill patients. Crit Care Med. 2008;36:3190–3197. doi: 10.1097/CCM.0b013e31818f21aa. [DOI] [PubMed] [Google Scholar]
- 8.Finfer S., Chittock D.R., Su S.Y., et al. Intensive versus conventional glucose control in critically ill patients. N Engl J Med. 2009;360:1283–1297. doi: 10.1056/NEJMoa0810625. [DOI] [PubMed] [Google Scholar]
- 9.Dellinger R.P., Levy M.M., Rhodes A., et al. Surviving sepsis campaign: international guidelines for management of severe sepsis and septic shock: 2012. Crit Care Med. 2013;41:580–637. doi: 10.1097/CCM.0b013e31827e83af. [DOI] [PubMed] [Google Scholar]
- 10.Moghissi E.S., Korytkowski M.T., DiNardo M., et al. American Association of Clinical Endocrinologists and American Diabetes Association consensus statement on inpatient glycemic control. Diabetes Care. 2009;32:1119–1131. doi: 10.2337/dc09-9029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Falciglia M., Freyberg R.W., Almenoff P.L., et al. Hyperglycemia-related mortality in critically ill patients varies with admission diagnosis. Crit Care Med. 2009;37:3001–3009. doi: 10.1097/CCM.0b013e3181b083f7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hermanides J., Bosman R.J., Vriesendorp T.M., et al. Hypoglycemia is associated with intensive care unit mortality. Crit Care Med. 2010;38:1430–1434. doi: 10.1097/CCM.0b013e3181de562c. [DOI] [PubMed] [Google Scholar]
- 13.Krinsley J.S. Understanding glycemic control in the critically ill: three domains are better than one. Intensive Care Med. 2011;37:382–384. doi: 10.1007/s00134-010-2110-3. [DOI] [PubMed] [Google Scholar]
- 14.Krinsley J.S., Egi M., Kiss A., et al. Diabetic status and the relation of the three domains of glycemic control to mortality in critically ill patients: an international multicenter cohort study. Crit Care. 2013;17:R37. doi: 10.1186/cc12547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Plummer M.P., Bellomo R., Cousins C.E., et al. Dysglycaemia in the critically ill and the interaction of chronic and acute glycaemia with mortality. Intensive Care Med. 2014;40:973–980. doi: 10.1007/s00134-014-3287-7. [DOI] [PubMed] [Google Scholar]
- 16.Egi M., Bellomo R., Stachowski E., et al. Blood glucose concentration and outcome of critical illness: the impact of diabetes. Crit Care Med. 2008;36:2249–2255. doi: 10.1097/CCM.0b013e318181039a. [DOI] [PubMed] [Google Scholar]
- 17.Krinsley J.S. Glycemic control, diabetic status, and mortality in a heterogeneous population of critically ill patients before and during the era of intensive glycemic management: six and one-half years experience at a university-affiliated community hospital. Semin Thorac Cardiovasc Surg. 2006;18:317–325. doi: 10.1053/j.semtcvs.2006.12.003. [DOI] [PubMed] [Google Scholar]
- 18.Rady M.Y., Johnson D.J., Patel B.M., et al. Influence of individual characteristics on outcome of glycemic control in intensive care unit patients with or without diabetes mellitus. Mayo Clin Proc. 2005;80:1558–1567. doi: 10.4065/80.12.1558. [DOI] [PubMed] [Google Scholar]
- 19.Whitcomb B.W., Pradhan E.K., Pittas A.G., et al. Impact of admission hyperglycemia on hospital mortality in various intensive care unit populations. Crit Care Med. 2005;33:2772–2777. doi: 10.1097/01.ccm.0000189741.44071.25. [DOI] [PubMed] [Google Scholar]
- 20.Investigators N.-S.S., Finfer S., Liu B., et al. Hypoglycemia and risk of death in critically ill patients. N Engl J Med. 2012;367:1108–1118. doi: 10.1056/NEJMoa1204942. [DOI] [PubMed] [Google Scholar]
- 21.Fahy B.G., Sheehy A.M., Coursin D.B. Glucose control in the intensive care unit. Crit Care Med. 2009;37:1769–1776. doi: 10.1097/CCM.0b013e3181a19ceb. [DOI] [PubMed] [Google Scholar]
- 22.Di Muzio F., Presello B., Glassford N.J., et al. Liberal versus conventional glucose targets in critically ill diabetic patients: an exploratory safety cohort assessment. Crit Care Med. 2016;44:1683–1691. doi: 10.1097/CCM.0000000000001742. [DOI] [PubMed] [Google Scholar]
- 23.Kar P., Plummer M.P., Bellomo R., et al. Liberal glycemic control in critically ill patients with type 2 diabetes: an exploratory study. Crit Care Med. 2016;44:1695–1703. doi: 10.1097/CCM.0000000000001815. [DOI] [PubMed] [Google Scholar]
- 24.Luethi N., Cioccari L., Biesenbach P., et al. Liberal glucose control in ICU patients with diabetes: a before-and-after study. Crit Care Med. 2018;46:935–942. doi: 10.1097/CCM.0000000000003087. [DOI] [PubMed] [Google Scholar]
- 25.Luethi N., Cioccari L., Eastwood G., et al. Hospital-acquired complications in intensive care unit patients with diabetes: a before-and-after study of a conventional versus liberal glucose control protocol. Acta Anaesthesiol Scand. 2019;63:761–768. doi: 10.1111/aas.13354. [DOI] [PubMed] [Google Scholar]
- 26.Krinsley J.S., Preiser J.C. Is it time to abandon glucose control in critically ill adult patients? Curr Opin Crit Care. 2019;25:299–306. doi: 10.1097/MCC.0000000000000621. [DOI] [PubMed] [Google Scholar]
- 27.Poole A.P., Anstey J., Bellomo R., et al. Opinions and practices of blood glucose control in critically ill patients with pre-existing type 2 diabetes in Australian and New Zealand intensive care units. Aust Crit Care. 2019;32:361–365. doi: 10.1016/j.aucc.2018.09.001. [DOI] [PubMed] [Google Scholar]
- 28.Schulz K.F., Altman D.G., Moher D. CONSORT 2010 statement: updated guidelines for reporting parallel group randomised trials. BMJ. 2010;340 doi: 10.4103/0976-500X.72352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kar P., Jones K.L., Horowitz M., Deane A.M. Management of critically ill patients with type 2 diabetes: the need for personalised therapy. World J Diabetes. 2015;6:693–706. doi: 10.4239/wjd.v6.i5.693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mackenzie I.M., Whitehouse T., Nightingale P.G. The metrics of glycaemic control in critical care. Intensive Care Med. 2011;37:435–443. doi: 10.1007/s00134-010-2103-2. [DOI] [PubMed] [Google Scholar]
- 31.Hall K.K., Lyman J.A. Updated review of blood culture contamination. Clin Microbiol Rev. 2006;19:788–802. doi: 10.1128/CMR.00062-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Harris P.A., Taylor R., Thielke R., et al. Research electronic data capture (REDCap) — a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chapman M., Peake S.L., Bellomo R., et al. Energy-dense versus routine enteral nutrition in the critically ill. N Engl J Med. 2018;379:1823–1834. doi: 10.1056/NEJMoa1811687. [DOI] [PubMed] [Google Scholar]
- 34.Weinel L.M., Summers M.J., Finnis M.E., et al. Are point-of-care measurements of glycated haemoglobin accurate in the critically ill? Aust Crit Care. 2019;32:465–470. doi: 10.1016/j.aucc.2018.11.064. [DOI] [PubMed] [Google Scholar]
- 35.DAMOCLES Study Group, NHS Health Technology Assessment Programme A proposed charter for clinical trial data monitoring committees: helping them to do their job well. Lancet. 2005;365:711–722. doi: 10.1016/S0140-6736(05)17965-3. [DOI] [PubMed] [Google Scholar]
- 36.National Health and Medical Research Council, Australian Research Council, Universities Australia National statement on ethical conduct in human research 2007 (updated 2018) Commonwealth of Australia. 2018 https://www.nhmrc.gov.au/about-us/publications/national-statement-ethical-conduct-human-research-2007-updated-2018 (viewed Mar 2020) [Google Scholar]
- 37.Health and Disability Commissioner Health and Disability Commissioner (Code of Health and Disability Services Consumers’ Rights) Regulations. 1996. https://www.hdc.org.nz/your-rights/about-the-code/code-of-health-and-disability-services-consumers-rights/ (viewed Mar 2020)
- 38.Australian and New Zealand Intensive Care Society ANZICS statement on care and decision-making at the end of life for the critically ill; ed. 1.0. Melbourne: ANZICS. 2014. https://www.anzics.com.au/wp-content/uploads/2018/08/ANZICS-Statement-on-Care-and-Decision-Making-at-the-End-of-Life- for-the-Critically-Ill.pdf (viewed Mar 2020)
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials