In Australia, extracorporeal membrane oxygenation (ECMO) is one of the most expensive diagnosis-related groups, costing $305 463 per complex admission to the intensive care unit (ICU).1, 2, 3 Mortality in this group of patients is high, about 43% for respiratory failure and 68% for cardiac failure.4, 5 ECMO is associated with significant risk to the patient and requires specialist training and expertise.1, 2, 3, 6, 7 Variation in clinical practice for patients supported with ECMO may compromise patient care and outcomes.8, 9, 10
In order to prepare for the coordination of this complex intervention in Australian ICUs, clinicians need to access accurate data on patients undergoing ECMO. International studies have reviewed resources and management required for ECMO; however, there is an absence of high quality multicentre observational data on the provision of ECMO in Australia.11, 12, 13, 14, 15, 16 Therefore, the aims of this study were to identify clinical practice guidelines, complication reporting, resource utilisation, and training practices in Australia before the commencement of a national ECMO registry.
Methods
Study design
A prospective electronic survey of ECMO practice in Australia was conducted in January 2019 (Online Appendix, table S1). Items included in the survey were selected following a literature review, and a subsequent item reduction took place using content experts to ensure the survey contained a manageable number of questions.17 Experienced ECMO clinicians at four centres piloted the survey. The pilot sites further strengthened the survey by providing feedback on data points that required clarification; for example, trainees renamed as ICU trainees in the final version. Sites were excluded if they did not have an ECMO service, were a paediatric ECMO service or were outside Australia.
Participant population
Participants were identified by snowball sampling.17 Surveys were emailed to a senior ECMO clinician at each of the 23 participating sites, with one survey completed per participating site. Each survey requested the respondent to reflect on practice and patients admitted from 1 July 2017 through 30 June 2018.
Statistical analysis
Data collected from all responses were analysed using SPSS Statistics version 25 (IBM). Categorical data were reported as a number and percentage of responses. Numerical data were reported as median and interquartile range (IQR) or mean and standard deviation (SD) where appropriate. Categorical variables were compared using χ2 test, and continuous variables were compared using Student t test, as appropriate. The data analysed did not contain any missing responses.
Results
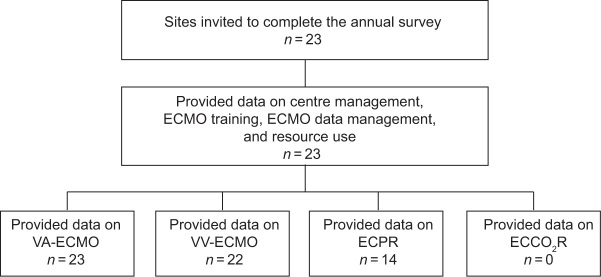
All 23 (100%) Australian hospitals that received the ECMO survey responded (Figure 1). Of these ICUs, 23 (100%) managed venoarterial (VA) ECMO, 22 (96%) managed venovenous (VV) ECMO, 14 (61%) managed patients with extracorporeal cardiopulmonary resuscitation (ECPR) and 0 ICUs (0%) managed patients with extracorporeal carbon dioxide removal (ECCO2R) (Table 1).
Figure 1.
Survey respondents
ECMO = extracorporeal membrane oxygenation; ECCO2R = extracorporeal carbon dioxide removal; ECPR = extracorporeal cardiopulmonary resuscitation; VA = venoarterial; VV = venovenous.
Table 1.
Type of extracorporeal membrane oxygenation (ECMO) support provided by state
| State | No. of survey respondents | VA (%) | VV (%) | ECPR (%) | ECCO2R (%) | No. of patients |
|---|---|---|---|---|---|---|
| New South Wales | 7 | 100% | 100% | 57% | 0% | 186 |
| Queensland | 4 | 100% | 100% | 75% | 0% | 58 |
| South Australia | 2 | 100% | 100% | 100% | 0% | 18 |
| Victoria | 8 | 100% | 88% | 38% | 0% | 142 |
| Western Australia | 2 | 100% | 100% | 100% | 0% | 23 |
| Total | 23 | 100% | 96% | 61% | 0% | 427 |
ECCO2R = extracorporeal carbon dioxide removal; ECPR = extracorporeal cardiopulmonary resuscitation; VA = venoarterial; VV = venovenous.
The median annual number of ECMO patients reported at each site was 12 (IQR, 6–16). Of the participating sites, four (17%) were considered high volume ECMO centres, reporting more than 30 ECMO patients admitted per year.18 Three of these sites (13%) reported that they admitted more than 60 patients for ECMO per year. New South Wales sites reported 186 ECMO admissions to their ICUs, representing the largest number admitted to any state (Table 1). A total of 427 ECMO patients were reported to be admitted to the 23 Australian ICUs from 1 July 2017 through to 30 June 2018. Of these sites, 17 (74%) transferred a patient to a major ECMO centre for further ECMO management. A total of 91 patients were retrieved to major ECMO centres across Australia.
Clinical practice guidelines
ECMO clinical practice guidelines were used at 19 of the 23 ICUs. Of these, there was wide variation reported for patient selection, reporting of complications to organisations outside of the hospital, training practices, credentialing and feedback. All of the four high volume centres reported they used a clinical practice guideline compared with 15 of 19 low volume centres (79%) (P = 0.32).
Data management and reporting
The ECMO-related complications that occurred were routinely reported if they occurred at 13 (57%) sites. Of these sites, seven (30%) reported all complications to the Extracorporeal Life Support Organization (ELSO) Registry. Twelve (52%) were ELSO members and seven (30%) participated in the ELSO Registry.19 Of the sites, that did not report complications to the ELSO Registry, one site (4%) measured all ECMO-related complications and four sites (17%) recorded bleeding. One site reported survival to discharge, but no complications of ECMO management.
Resource utilisation
The staff required to care for ECMO patients varied (Online Appendix, figure S1); however, intensivists, nurses and surgeons were required at all sites. The type of surgeon required depended on the site, with 17 hospitals (74%) using cardiothoracic surgeons and eight (35%) using vascular surgeons. Other staff included perfusionists, anaesthetists, cardiologists, physiotherapists, heart and lung transplant physicians, and ward support staff. The type of staff required varied depending on whether they were required for cannulation, decannulation, daily management or transport (Online Appendix, table S2). Additional staff were utilised for ECMO education or ECMO on-call services at 14 ICUs (61%).
Training practices
The type of ECMO training provided at the sites varied from site to site (Online Appendix, table S3). A written outline of the training requirements needed by staff involved with ECMO patients was present at 12 of the sites (52%). Bedside training was most frequently used, which accounted for 96% of intensivist training, 87% of ICU trainee’s training, 96% of nursing training and 52% of perfusionist training. Sites used a combination of external, internal, simulator and bedside training; accreditation; and/or credentialing. Formal feedback on ECMO activity (number, type, cannulation, survival) was provided internally to staff at 16 sites (70%).
Discussion
This study generated some important insights about ECMO clinical practice within Australia. There were 23 sites that performed ECMO, with VA ECMO being the most common type. None of the ICUs reported caring for patients who received ECCO2R and over half of the ICUs cared for ECPR patients following cardiac arrest. The survey showed variation in reported practice in Australian ECMO centres regarding clinical practice guidelines, complication reporting, resource utilisation and training practices required for patients who need ECMO. Over half the sites reported ECMO complications mostly to the ELSO Registry.
The survey results align with international literature, showing that there is considerable variation in clinical practice for ECMO patients.8, 9, 10, 11, 12, 13, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28 Two systematic reviews have found inconsistent reporting of complications and outcomes.11, 12 The reviews concluded that there was an urgent need for consensus-based definitions and a core outcome set, which has recently been published.29
The international guidelines from ELSO recommend three important factors for the successful and safe delivery of an ECMO service: the centres should have sufficient volume (at least six cases per year),19 centres should report to the ELSO Registry, and they should have an adult ICU as part of their service. A study from the United States found that significant cost savings resulted through training ICU staff to care for and manage ECMO patients compared with patients managed by perfusionists, with no change in mortality rate or adverse events.30 The ELSO guideline also states that centres should be located in a geographical area that can support a minimum of six patients per year, as high volume ECMO centres have reduced morbidity when compared with low volume ECMO centres.31 Of the sites in this survey, five (22%) did not meet this ELSO recommendation, with implications for costs of service delivery and skills maintenance.19 Seven centres (30%) reported to ELSO and all had an adult ICU as part of their service.
Strengths and limitations
All Australian ICU ECMO services approached to participate were included in this survey. It included both high and low volume ECMO sites, but there may be ECMO patients admitted to other low volume hospitals sites that were not captured in this survey. This survey did not specifically measure interdisciplinary team members, but there was an option to include these staff members under the category “other”. Information on the additional staff required for an ECMO service and other costs would also be useful to calculate.
In Australia, a recently funded partnership project (EXCEL Registry, NCT03793257) between the National Health and Medical Research Council, the National Heart Foundation of Australia, major Australian ECMO centres, the International ECMO Network (ECMONet) and the Australian and New Zealand Intensive Care Society (ANZICS) will be used to measure long term outcomes and trajectory of recovery after ECMO. This information will include specific prospective ECMO data: cannulation, mode, complications, ICU outcomes, survival, disability, return to work, health-related quality of life and costs. Several clinical trials are embedded in the EXCEL registry, including Blend to Limit Oxygen in ECMO (BLENDER) (ClinicalTrials.gov identifier: NCT03841084) and Blood Management during ECMO for Cardiac Support (OBLEX) (ClinicalTrials.gov identifier: NCT03714048). Additionally, the ANZICS Adult Patient Database has recently included ECMO variables in the database as a quality initiative. This will inform the use of ECMO on a broader scale across Australia.
Conclusion
This survey confirms that there is variation in the treatment of ECMO patients between Australian sites. Differences were found in clinical practice reported at ECMO centres across Australia and in the description of site-specific clinical practice guidelines. There is an urgent need to report current ECMO practice in a prospective national ECMO registry to measure complications, resource utilisation, training practices and long term recovery.
Acknowledgements: This work was completed with thanks to the Australian and New Zealand Intensive Care Society Clinical Trials Group; the Alfred Hospital, Melbourne, VIC: Jasmin Board, Emma Martin, Phoebe McCracken, Vincent Pellegrino, Jayne Sheldrake, Shirley Vallance and Meredith Young; Austin Hospital, Melbourne, VIC: Rinaldo Bellomo, Glenn Eastwood, Andrew Hilton, Helen Young and Leah Peck; Box Hill Hospital, Melbourne, VIC: John Dyett, Stephanie Hunter and Cheelim Liew; Epworth Richmond Hospital, Melbourne, VIC: Jonathan Barrett, Kyle Brooks and Gabrielle Hanlon; Flinders Medical Centre, Adelaide, SA: Andrew Bersten, Shailesh Bihari, Xia Jin, Elisha Matheson, Tapaswi Shrestha and Ubbo Wiersema; Fiona Stanley Hospital WA: Chris Allen, Samantha Bizzell, Ege Eroglu, Ed Litton, Annamaria Palermo and Susan Pellicano; Gold Coast University Hospital, Gold Coast, QLD: Priscilla Harvey, Mandy Tallott and James Winearls; John Hunter Hospital, Newcastle, NSW: Jorge Brieva, Ken Havill and Rachael Paton; Liverpool Hospital, Sydney, NSW: Anders Aneman, Lien Lombardo and Peter McCanny; Monash Medical Centre, Melbourne, VIC: Dhiraj Bhatia, Fereda Fazli, Chloe Peppin and Yayha Shehabi; Prince Charles Hospital. Brisbane, QLD: Amanda Corley, John Fraser, Jayshree Lavana, India Lye and Jessica Riordan; Prince of Wales Hospital, Randwick, NSW: David Collins; Princess Alexandra Hospital, Brisbane, QLD: Chris Joyce, Jason Meyer, Cassenya Preece and James Walsham; Royal Adelaide Hospital, Adelaide, SA: Kathleen Glasby, Stephanie O’Connor, Benjamin Reddi, Justine Rivett and Joannies Yap; Royal Brisbane and Women’s Hospital, Brisbane, QLD: Jason Pincus and Janine Stuart; Royal Melbourne Hospital, Melbourne, VIC: James Anstey, Deborah Barge and Kathleen Byrne; Royal North Shore Hospital, Sydney, NSW: Frances Bass, Pierre Janin, Naomi Hammond, Elliot Williams and Elizabeth Yarad; Royal Prince Alfred Hospital, Sydney, NSW: Heidi Buhr, Jennifer Coles, David Gattas, Kirsten Hammond, Maryam Sharifian Alborzi and Richard Totaro; Sir Charles Gairdner Hospital, Perth, WA: Erina Myers, Rashmi Rauniyar and Stephen Richards; St Vincent’s Hospital Melbourne, Melbourne VIC: Leanne Barbazza, Jennifer Holmes, Patricia Hurune, Yvette O’Brien and Roger Smith; St Vincent’s Hospital Sydney, Sydney, NSW: Jeff Breeding, Priya Nair, Sally Newman and Claire Reynolds; University Hospital Geelong, Geelong, VIC: Allison Bone, Joe McCaffrey and Neil Orford; Westmead Hospital, Sydeny, NSW: Ashoke Bannerjee, Josephine Ho and Anand Pupipeddi.
Competing interests
None declared.
Supplementary Information

References
- 1.Peek G.J., Mugford M., Tiruvoipati R., et al. Efficiency and economic assessment of conventional ventilatory support versus extracorporeal membrane oxygenation for severe adult respiratory failure (CESAR): a multicentre randomised controlled trial. Lancet. 2009;374:1351–1363. doi: 10.1016/S0140-6736(09)61069-2. [DOI] [PubMed] [Google Scholar]
- 2.Higgins A.M., Pettila V., Harris A.H., et al. The critical care costs of the influenza A/H1N1 2009 pandemic in Australia and New Zealand. Anaesth Intensive Care. 2011;39:384–391. doi: 10.1177/0310057X1103900308. [DOI] [PubMed] [Google Scholar]
- 3.Independent Hospital Pricing Authority . IHPA; Sydney: 2017. National hospital cost data collection, public hospitals cost report, round 19 (financial year 2014–15)https://www.ihpa.gov.au/publications/national-hospital-cost-data-collection-public-hospitals-cost-report-round-19-financial (viewed Mar 2020) [Google Scholar]
- 4.Schmidt M., Bailey M., Sheldrake J., et al. Predicting survival after extracorporeal membrane oxygenation for severe acute respiratory failure. The respiratory extracorporeal membrane oxygenation survival predication (RESP) score. Am J Respir Crit Care Med. 2014;189:1374–1382. doi: 10.1164/rccm.201311-2023OC. [DOI] [PubMed] [Google Scholar]
- 5.Scmidt M., Burrell A., Roberts L., et al. Predicting survival after ECMO for refractory cardiogenic shock: the survival after veno-arterial-ECMO (SAVE)-score. Eur Heart J. 2015;36:2246–2256. doi: 10.1093/eurheartj/ehv194. [DOI] [PubMed] [Google Scholar]
- 6.Belle L., Mangin L., Bonnet H., et al. Emergency extracorporeal membrane oxygenation in a hospital without on-site cardiac surgical facilities. Eurointervention. 2012;8:375–382. doi: 10.4244/EIJV8I3A57. [DOI] [PubMed] [Google Scholar]
- 7.Combes A., Brodie D., Bartlett R., et al. Position paper for the organization of extracorporeal membrane oxygenation programs for acute respiratory failure in adult patients. Am J Respir Crit Care Med. 2014;190:488–496. doi: 10.1164/rccm.201404-0630CP. [DOI] [PubMed] [Google Scholar]
- 8.Aubron C., Cheng A.C., Pilcher D., et al. Factors associated with outcomes of patients on extracorporeal membrane oxygenation support: a 5-year cohort study. Crit Care. 2013;17:R73. doi: 10.1186/cc12681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aubron C., Cheng A.C., Pilcher D., et al. Infections acquired by adults who receive extracorporeal membrane oxygenation: risk factors and outcome. Infect Control Hosp Epidemiol. 2013;34:24–30. doi: 10.1086/668439. [DOI] [PubMed] [Google Scholar]
- 10.Aubron C., DePuydt J., Belon F., et al. Predictive factors of bleeding events in adults undergoing extracorporeal membrane oxygenation. Ann Intensive Care. 2016;6:97. doi: 10.1186/s13613-016-0196-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tramm R., Ilic D., Davies A.R., et al. Extracorporeal membrane oxygenation for critically ill adults. Cochrane Database Syst Rev. 2015;1 doi: 10.1002/14651858.CD010381.pub2. CD010381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Burrell A.J., Serra AL Bennett V., et al. Venoarterial extracorporeal membrane oxygenation: a systematic review of selection criteria, outcome measures and definitions of complications. J Crit Care. 2019;53:32–37. doi: 10.1016/j.jcrc.2019.05.011. [DOI] [PubMed] [Google Scholar]
- 13.Abrams D., Garan A.R., Akram A., et al. Position paper for the organization of ECMO programs for cardiac failure in adults. Intensive Care Med. 2018;44:717–729. doi: 10.1007/s00134-018-5064-5. [DOI] [PubMed] [Google Scholar]
- 14.Conrad S.A., Broman L.M., Taccone F.S., et al. The Extracorporeal Life Support Organization Maastricht treaty for nomenclature in extracorporeal life support. A position paper of the Extracorporeal Life Support Organization. Am J Respir Crit Care Med. 2018;15:447–457. doi: 10.1164/rccm.201710-2130CP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Marhong J.D., Telesnicki T., Munshi L., et al. Mecahnical ventilation during extracorporeal membrane oxygenation. An international survey. Ann Am Thorac Soc. 2014;11:956–961. doi: 10.1513/AnnalsATS.201403-100BC. [DOI] [PubMed] [Google Scholar]
- 16.Fernando S.M., Qureshi D., Tanuseputro P., et al. Mortality and costs following extracorporeal membrane oxygenation in critically ill adults: a population-based cohort study. Intensive Care Med. 2019;45:1580–1589. doi: 10.1007/s00134-019-05766-z. [DOI] [PubMed] [Google Scholar]
- 17.Burns K.E., Duffett M., Kho M.E., et al. A guide to the design and conduct of self-administered surveys of clinicians. CMAJ. 2008;173:245–252. doi: 10.1503/cmaj.080372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fan E., Brodie D. Higher volumes, better outcomes: the end or just the beginning of the story for extracorporeal membrane oxygenation? Am J Respir Crit Care Med. 2015;191:864–866. doi: 10.1164/rccm.201503-0459ED. [DOI] [PubMed] [Google Scholar]
- 19.Extracorporeal Life Support Organization . ELSO; Ann Arbor, MI: 2014. ELSO Guidelines for ECMO centres (version 1.8)https://www.elso.org/Portals/0/IGD/Archive/FileManager/faf3f6a3c7cusersshyerdocumentselsoguidelinesecmocentersv1.8.pdf (viewed Mar 2020) [Google Scholar]
- 20.Kennedy L., Wrigley S., Kennedy M., Pellegrino V. Extracorporeal membrane oxygenation retrieval factors and survival to intensive care unit discharge. Emerg Med Australas. 2019;31:280–282. doi: 10.1111/1742-6723.13182. [DOI] [PubMed] [Google Scholar]
- 21.Maxwell B.G., Powers A.J., Sheikh A.Y., et al. Resource use trends in extracorporeal membrane oxygenation in adults: an analysis of the Nationwide Inpatient Sample 1998–2009. J Thorac Cardiovasc Surg. 2014;148:416–421. doi: 10.1016/j.jtcvs.2013.09.033. [DOI] [PubMed] [Google Scholar]
- 22.Cavarocchi N.C., Wallace S., Hong E.Y., et al. A cost-reducing extracorporeal membrane oxygenation (ECMO) program model: a single institution experience. Perfusion. 2015;30:148–153. doi: 10.1177/0267659114534288. [DOI] [PubMed] [Google Scholar]
- 23.Harvey M.J., Gaies M.G., Prosser L.A. US and international in-hospital costs of extracorporeal membrane oxygenation: a systematic review. Appl Health Econ Health Policy. 2015;13:341–357. doi: 10.1007/s40258-015-0170-9. [DOI] [PubMed] [Google Scholar]
- 24.Moll V., Teo E.Y., Grenda D.S., et al. Rapid development and implementation of an ECMO program. ASAIO J. 2016;62:354–358. doi: 10.1097/MAT.0000000000000331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cotza M., Carboni G., Ballotta A., et al. Modern ECMO: why an ECMO programme in a tertiary care hospital. Eur Heart J Suppl. 2016;18(Suppl):E79–E85. doi: 10.1093/eurheartj/suw016. [DOI] [PubMed] [Google Scholar]
- 26.Weems M.F., Friedlich P.S., Nelson L.P., et al. The role of extracorporeal membrane oxygenation simulation training at extracorporeal life support organization centres in the United States. Simul Healthc. 2017;12:233–239. doi: 10.1097/SIH.0000000000000243. [DOI] [PubMed] [Google Scholar]
- 27.Na S.J., Chung C.R., Choi H.J., et al. The effect of multidisciplinary extracorporeal membrane oxygenation team on clinical outcomes in patients with severe acute respiratory failure. Ann Intensive Care. 2018;8:31. doi: 10.1186/s13613-018-0375-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Komindr A., Abe R., Tateishi T., et al. Establishing extracorporeal membrane oxygenation team increased number of patients and improved data recording. J Intensive Care. 2019;7:11. doi: 10.1186/s40560-019-0366-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hodgson C., Burrell A., Engeler D., et al. Core outcome measures for research in critically ill patients receiving extracorporeal membrane oxygenation for acute respiratory or cardiac failure: an international, multidisciplinary, modified Delphi consensus study. Crit Care Med. 2019;11:1557–1563. doi: 10.1097/CCM.0000000000003954. [DOI] [PubMed] [Google Scholar]
- 30.Kim G.W., Koh Y., Lim C.M., et al. The effect of an improvement of experience and training in extracorporeal membrane oxygenation management on clinical outcomes. Korean J Intern Med. 2018;33:121–129. doi: 10.3904/kjim.2015.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Barbaro R.P., Odetola F.O., Kidwell K.M., et al. Association of hospital-level volume of extracorporeal membrane oxygenation cases and mortality. Analysis of the extracorporeal life support organization registry. Am J Respir Crit Care Med. 2015;191:894–901. doi: 10.1164/rccm.201409-1634OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials