Abstract
Objective: Hyperammonaemia contributes to complications in acute liver failure (ALF) and may be treated with continuous renal replacement therapy (CRRT), but current practice is poorly understood.
Design: We retrospectively analysed data for baseline characteristics, ammonia concentration, CRRT use, and outcomes in a cohort of Australian and New Zealand patients with ALF.
Setting: All liver transplant ICUs across Australia and New Zealand.
Participants: Sixty-two patients with ALF.
Main outcome measures: Impact of CRRT on hyperammonaemia and patient outcomes.
Results: We studied 62 patients with ALF. The median initial (first 24 h) peak ammonia was 132 μmol/L (interquartile range [IQR], 91–172), median creatinine was 165 μmol/L (IQR, 92–263) and median urea was 6.9 mmol/L (IQR, 3.1–12.0). Most patients (43/62, 69%) received CRRT within a median of 6 hours (IQR, 2–12) of ICU admission. At CRRT commencement, three-quarters of such patients did not have Stage 3 acute kidney injury (AKI): ten patients (23%) had no KDIGO creatinine criteria for AKI, 12 (28%) only had Stage 1, and ten patients (23%) had Stage 2 AKI. Compared with non-CRRT patients, those treated with CRRT had higher ammonia concentrations (median, 141 μmol/L [IQR, 102–198] v 91 μmol/L [IQR, 54–115]; P = 0.02), but a nadir Day 1 pH of only 7.25 (standard deviation, 0.16). Prevention of extreme hyperammonaemia (> 140 μmol/L) after Day 1 was achieved in 36 of CRRT-treated patients (84%) and was associated with transplant-free survival (55% v 13%; P = 0.05).
Conclusion: In Australian and New Zealand patients with ALF, CRRT is typically started early, before Stage 3 AKI or severe acidaemia, and in the presence hyperammonaemia. In these more severely ill patients, CRRT use was associated with prevention of extreme hyperammonaemia, which in turn, was associated with increased transplant-free survival.
Acute liver failure (ALF) is a rare but important cause of critical illness that requires admission to intensive care and carries a high mortality.1 Hyperammonaemia is an inevitable complication of ALF. In high concentrations, ammonia is neurotoxic and causes cerebral oedema, leading to intracranial hypertension and neurological injury.2, 3, 4, 5, 6, 7, 8, 9 Repeat monitoring of ammonia has been suggested,3, 10, 11 but current management guidelines only advise measurement at admission to the intensive care unit (ICU) and when enteral feeding is commenced. Crucially, they provide no guidance regarding its treatment.12, 13
Interest is growing in measuring and rapidly treating hyperammonaemia in patients with ALF, despite the lack of evidence to guide the best means to achieve its correction.14, 15, 16 In this regard, however, the early initiation of continuous renal replacement therapy (CRRT),15, 16, 17, 18 may be a means to safely and effectively treat hyperammonaemia in patients with ALF.2, 15, 16, 18, 19, 20, 21, 22, 23 Unfortunately, despite its potentially beneficial application in ALF, current CRRT guidelines do not specifically advocate its use in ALF unless acute kidney injury (AKI) has also developed.
Given the above considerations, in patients with ALF treated in all Australian and New Zealand liver transplant ICUs, we conducted an exploratory evaluation of CRRT utilisation and associated biochemical monitoring in response to hyperammonaemia. Our primary hypothesis was that, in most ALF patients, CRRT initiation would occur early, before overt evidence of renal failure and in the presence of severe hyperammonaemia. We further hypothesised that such CRRT treatment would be associated with the correction or avoidance of extreme hyperammonaemia in most patients.
Method
Study design
As previously described,24 we invited all six adult liver transplant ICUs across Australia and New Zealand to share de-identified clinical data relating to the last ten or more adult patients admitted with ALF (Acute Physiology and Chronic Health Evaluation [APACHE] III diagnostic code 301.01) using a standardised collection tool. Austin Hospital Human Research Ethics Committee approval was obtained (LNR/14/Austin/676) and a Memoranda of Understanding or a Clinical Trials Research Agreement with local ethics committee oversight was implemented where required to enable sharing of de-identified data.
Data collected included patient sex, age, comorbidities, aetiology of ALF, illness severity score, components of the King’s College Criteria (KCC), the Kidney Disease: Improving Global Outcomes (KDIGO) criteria for AKI stage, biochemical and haematological test results, critical care interventions, blood product utilisation, and outcomes, including emergency liver transplantation (ELT) and death. We obtained data for interventions and investigations occurring at the time of ICU admission, 6 hours, 12 hours and 24 hours after admission, and then every day for one week. A sample size of 60 patients was considered sufficient on the basis of convenience and feasibility and as representative of about one year of practice.1 The selection of at least ten consecutive patients from each site, regardless of outcome or aetiology of ALF, was undertaken to achieve a balanced and representative sample from all centres. Comparisons were made on the basis of CRRT utilisation and outcome measures. The design and reporting of this study is aligned with the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) recommendations.25
Statistical analysis
Statistical analysis was performed using IBM SPSS statistics for Macintosh, version 25 (IBM Corporation, Armonk, NY, USA). Continuous variables are expressed as medians with interquartile ranges (IQR) and categorical variables as frequencies with percentages. Continuous data were compared using Mann–Whitney test. Categorical data were compared using χ2 or Fisher exact test as appropriate. The Kruskal–Wallis test was used to compare more than two ordinal variables between groups. Longitudinal data were assessed for normality and log-transformed where appropriate. Differences between groups over time were analysed using repeat measures analysis of variance fitting main effects for group, time and an interaction between group and time to determine if groups behaved differently over time. Results are presented as mean (standard error) or as geometric mean (95% confidence interval [CI]) in accordance with the underlying distribution of the data. A two-sided P of 0.05 was used to indicate statistical significance.
Results
Patient characteristics, aetiology of acute liver failure and clinical outcomes
We studied 62 patients with ALF of whom 33 (53%) had paracetamol overdose-related ALF, with the remaining cases having ALF due to a variety of causes (online Appendix, supplementary table 1; available at cicm.org.au/Resources/Publications/Journal).
Baseline characteristics at the time of admission to ICU are summarised in Table 1. Patients were mostly young and female, with high illness severity, and 37 (60%) fulfilled the KCC for transplantation.
Table 1.
Characteristics of patients with acute liver failure (ALF) treated with continuous renal replacement therapy (CRRT)
| Variable | All ALF patients (n = 62) | Non-CRRT patients (n = 19) | CRRT patients (n = 43) | P* |
|---|---|---|---|---|
| Baseline characteristics at time of admission to ICU | ||||
| Age (years), mean (SD) | 38 ± 12 | 38 ± 13 | 38 ± 12 | 0.79 |
| Female | 36 (58%) | 11 (58%) | 25 (58%) | 0.99 |
| Contraindications to ELT | 17 (27%) | 6 (32%) | 11 (26%) | 0.63 |
| Paracetamol overdose aetiology | 33 (53%) | 10 (53%) | 23 (53%) | 0.95 |
| Non-paracetamol overdose aetiology | 29 (47%) | 9 (47%) | 20 (47%) | 0.95 |
| Time from first presentation to ICU at ELT hospital (hours), median (IQR) | 25 (8–49) | 22 (9–47) | 25 (6–57) | 0.89 |
| APACHE III score, mean (SD) | 82 ± 36 | 54 ± 22 | 95 ± 35 | < 0.001 |
| KCC fulfilment | 37 (60%) | 6 (32%) | 31 (72%) | 0.003 |
| KDIGO stage at time of commencing CRRT (creatinine criteria) | ||||
| No AKI | 19 (31%) | 9 (47%) | 10 (23%) | 0.06 |
| Stage 1 | 17 (27%) | 5 (26%) | 12 (28%) | |
| Stage 2 | 12 (19%) | 2 (11%) | 10 (23%) | |
| Stage 3 | 14 (23%) | 3 (16%) | 11 (26%) | |
| Biochemical and clinical data over the first 24 hours in ICU | ||||
| Maximal temperature (degrees Celsius), mean (SD) | 36.9 ± 0.7 | 36.9 ± 0.45 | 36.9 ± 0.8 | 0.73 |
| Nadir pH, mean (SD) | 7.29 ± 0.16 | 7.39 ± 0.07 | 7.25 ± 0.16 | 0.001 |
| Peak arterial CO2 (mmHg), mean (SD) | 37 ± 8 | 36 ± 5 | 37 ± 9 | 0.77 |
| Peak sodium (mmol/L), mean (SD) | 142 ± 6 | 143 ± 4 | 141 ± 7 | 0.43 |
| Peak lactate (mmol/L), median (IQR) | 7.1 (4.1–11.6) | 3.9 (2.7–8.8) | 8.3 (4.2–12.5) | 0.02 |
| Peak ALT (IU), mean (SD) | 5210 ± 3206 | 6246 ± 4054 | 4745 ± 2676 | 0.09 |
| Peak GGT (IU), median (IQR) | 106 (68–205) | 136 (82–211) | 97 (67–180) | 0.39 |
| Peak ALP (IU), median (IQR) | 139 (108–184) | 121 (98–148) | 148 (108–200) | 0.04 |
| Peak bilirubin (μmol/L), median (IQR) | 95 (67–187) | 80 (60–148) | 108 (67–223) | 0.40 |
| Peak creatinine (μmol/L), median (IQR) | 165 (92–263) | 75 (65–148) | 208 (133–311) | 0.001 |
| Peak urea (mmol/L), median (IQR) | 6.9 (3.1–12.0) | 7.0 (3.1–10.8) | 6.8 (3.0–13.5) | 0.50 |
| Peak INR, mean (SD) | 6.3 ± 3.1 | 5.4 ± 2.3 | 6.7 ± 3.4 | 0.13 |
| Peak aPTT (seconds), median (IQR) | 63 (44–77) | 46 (38–57) | 72 (51–86) | < 0.001 |
| Nadir fibrinogen (g/L), median (IQR) | 1.1 (0.8–1.6) | 1.5 (1.1–2.0) | 1.0 (0.8–1.3) | 0.01 |
| Nadir platelets (× 109/L), median (IQR) | 83 (41–122) | 106 (73–159) | 77 (38–120) | 0.07 |
| Nadir haemoglobin (g/L), median (IQR) | 102 (80–118) | 111 (96–124) | 87 (78–111) | 0.01 |
| Peak ammonia (μmol/L), median (IQR) | 132 (91–172) | 91 (54–115) | 141 (102–198) | 0.02 |
| 24-Hour net fluid balance from ICU admission (mL), median (IQR) | 4178 (1755–7130) | 3867 (222–5079) | 4993 (2416–7737) | 0.06 |
AKI = acute kidney injury; ALP = alkaline phosphatase; ALT = alanine aminotransferase; APACHE = Acute Physiology and Chronic Health Evaluation; aPTT = activated partial thromboplastin time; CO2 = carbon dioxide; ELT = emergency liver transplant; GGT = γ-glutamyl transferase; ICU = intensive care unit; INR = international normalised ratio; IQR = interquartile range; KCC = King’s College Criteria; KDIGO = Kidney Disease: Improving Global Outcomes; SD = standard deviation.
Differences between CRRT and non-CRRT groups.
Use of continuous renal replacement therapy
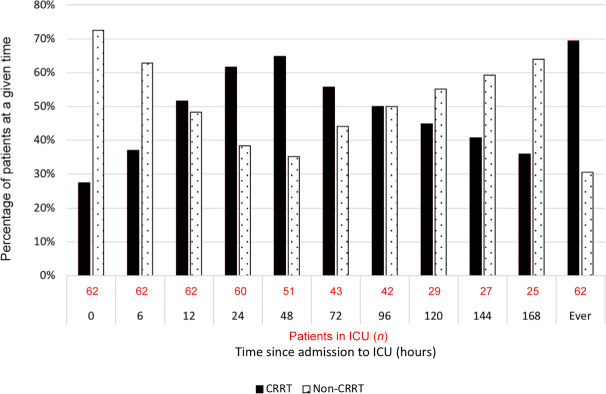
The use of CRRT over the first week of ICU management is presented in Figure 1. Overall, a total of 43 patients with ALF (69%) received CRRT. Patients treated with CRRT had greater illness severity and more patients fulfilled the KCC. They had a significantly lower pH, higher lactate concentration, worse hypofibrinogenaemia, more severe anaemia and more severe hyperammonaemia.
Figure 1.
Use of continuous renal replacement therapy (CRRT) in patients with acute liver failure over the first week in the intensive care unit (ICU)
The median time from ICU admission to commencement of CRRT was 6 hours (IQR, 2–12) and all except one patient started CRRT on the day of ICU admission. At CRRT initiation, ten patients did not meet KDIGO creatinine criteria for AKI, 12 met Stage 1, ten met Stage 2, and only 11 (26%) patients met Stage 3 AKI criteria. The median duration of CRRT therapy was 60 hours (IQR, 33–129) and the most common modality was continuous venovenous haemodiafiltration (CVVHDF), which was used in 80% of patients treated with CRRT. No patients were treated with intermittent haemodialysis.
Consistent with greater illness severity, treatment with CRRT was associated with the provision of other advanced critical care interventions (online Appendix, supplementary figure 1) and complications. Thus, 88% of CRRT patients versus 47% of non-CRRT patients were mechanically ventilated (P = 0.001), and 81% versus 42% of patients received vasopressor therapy (P = 0.002). In addition, more CRRT patients experienced a bleeding episode and received a blood transfusion or were treated with cryoprecipitate (online Appendix, supplementary table 2).
Safety of early continuous renal replacement therapy
Haemostatic parameters at the time of catheter insertion are summarised in the online Appendix (supplementary table 3). Patients commencing CRRT had a more prolonged activated partial thromboplastin time and slightly worse anaemia, and fresh frozen plasma was used to a greater extent on the day of vascular access insertion compared with non-CRRT patients (median, 2 units [IQR, 0–4.8] v 0 units [IQR, 0–2.5]; P = 0.04). Only one episode of catheter-related bleeding occurred close to the time of CRRT initiation, which required no intervention other than the administration of clotting factors.
Dynamics of biochemical parameters and continuous renal replacement therapy in acute liver failure
Peak creatinine (but not peak urea) concentration was higher in CRRT patients (Table 1), and seven patients (16%) never had a serum creatinine above the upper limit of normal. However, the peak ammonia concentration on the first day was 55% higher than in non-CRRT patients (P = 0.02) (Table 1). Despite such high levels and greater illness severity, ammonia concentrations in CRRT patients rapidly and significantly decreased over time and became similar to untreated patients. In contrast, untreated patients had no significant change over time.
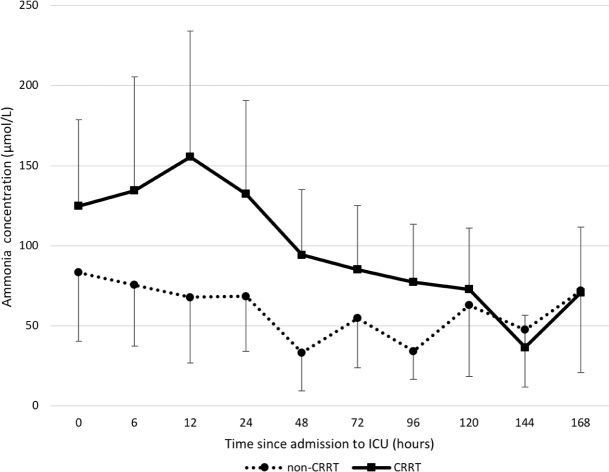
Over 7 days of ICU management, the mean peak Day 1 ammonia was reduced from 155 μmol/L (95% CI, 103–234) to 71 μmol/L (95% CI, 45–112) with CRRT; while in non-treated patients, the change over this period was from 83 μmol/L (95% CI, 40–171) to 72 μmol/L (95% CI, 21–246) (Figure 2). In addition, 14 of the 62 patients with ALF had a peak ammonia concentration greater than 140 μmol/L during the first 24 hours. However, these levels were rapidly reduced over a single day in 12 patients (92%). Moreover, among these severely hyperammonaemic patients, eight (57%) had a pH greater than 7.30. Finally, among patients with hyperammonaemia treated with CRRT, the mean nadir pH was 7.25 (SD, 0.16) (Table 1).
Figure 2.
Changes in ammonia concentrations over time on the basis of continuous renal replacement therapy (CRRT) status*
ICU = intensive care unit. * P = 0.09 (overall difference between CRRT- and non-CRRT-treated patients). Geometric mean of log-transformed data. Error bars represent 95% CI — for visual clarity, only plus or minus direction is shown for upper and lower lines respectively.
After the first ICU day, avoidance of any ammonia levels greater than 140 μmol/L was achieved in 36 CRRT patients (84%). Eight patients in total had a documented ammonia concentration greater than 140 μmol/L at least once after the first day and only one of them achieved ELT-free survival. This compares with ELT-free survival in 30 of the 54 patients without episodes of extreme hyperammonaemia after Day 1 (P = 0.05). Other key biochemical and fluid balance data are summarised in the online Appendix (supplementary figures 2–6 and supplementary table 5).
Outcomes on the basis of continuous renal replacement therapy
Clinical outcomes are summarised in the online Appendix (supplementary table 4). Patients treated with CRRT had a longer ICU length of stay than non-CRRT patients, but hospital length of stay was not significantly different between the two groups. Use of ELT in CRRT patients was not significantly greater; however, ICU mortality was nearly four times higher and ELT-free survival was nearly half that of patients not requiring CRRT.
Discussion
Key findings
In our study of patients with ALF treated in Australian and New Zealand liver transplant ICUs, CRRT was started very early in the setting of hyperammonaemia, in the absence of Stage 3 AKI, and in the overall absence of advanced acidaemia or any acidaemia in half of the patients and with a pH greater than 7.3 in most patients with extreme hyperammonaemia. Such CRRT-based treatment strategy was associated with rapid ammonia reduction to safer levels in most patients and avoidance of extreme hyperammonaemia. In contrast, there were only minor changes over time in untreated patients. Moreover, after several days of CRRT, ammonia concentrations became the same as those seen in less severely ill non-CRRT patients. Finally, avoidance of extreme hyperammonaemia was associated with ELT-free survival.
Relationship to previous studies
CRRT was used in a high proportion of Australian and New Zealand patients with ALF. Compared with previous studies, such Australian and New Zealand patients had higher illness severity at the time of admission to ICU, with a lower pH, higher international normalised ratio, higher lactate concentration, and more severe hyperammonaemia.16, 26 Half of all these patients had ICU admission blood ammonia concentrations previously associated with a high risk of neurological injury.3, 18 Despite such illness severity, outcomes in terms of survival, ELT utilisation and ELT-free survival were similar to or better than reported in resource-rich countries.1, 27, 28, 29
The optimal timing of RRT in critically ill patients is uncertain.30, 31, 32, 33 However, in patients with ALF, Australian and New Zealand clinicians initiated therapy in the absence of conventional creatinine-based indications and, in most patients, before KDIGO AKI Stage 2 or the onset of even moderate acidaemia. Australian and New Zealand clinicians appear to use CRRT to target high ammonia levels because of their potential to contribute to cerebral oedema.15, 18, 19, 34, 35 Such early initiation and catheter insertion despite coagulopathy appeared safe. Consistent with greater illness severity, bleeding episodes and blood product administration were common in CRRT patients. It is apparent that these events do not necessarily correlate with elevations of the international normalised ratio, while hypofibrinogenaemia and thrombocytopenia are associated with both and are also markers of illness severity.36, 37
Implications of study findings
Our findings suggest that in patients with ALF, Australian and New Zealand intensivists typically start CRRT early and such early commencement of therapy is safe. Moreover, our findings imply that clinicians target correction of severe hyperammonaemia even in the absence of severe AKI or severe acidaemia, suggesting that their major therapeutic target is hyperammonaemia itself. Finally, our findings imply that this approach contributes to rapid reductions of such severe hyperammonaemia to safer levels, which become similar to those of less severely ill patients with ALF.
Strengths and limitations
Our study has several strengths. All liver transplant ICUs in Australia and New Zealand contributed patients to the study ensuring the first full binational study of this aspect of ALF management. Patients with chronic liver disease were excluded, thus removing a key confounder. Data were obtained from patient records at each centre by experienced researchers thus minimising attribution errors. Finally, a clear methodology was used to ensure consistent and complete collection of available data.
Several limitations in this study warrant consideration. The study may appear relatively small, but it includes patients with ALF from every transplant unit in Australia and New Zealand and is the second largest case series worldwide of such patients in 40 years. We studied a convenience-based sample size; however, each ICU chose ten or more sequential patients based only on the diagnostic code of ALF at the time of admission, thus minimising selection bias. We did not obtain detailed data on the technical aspects of CRRT treatments, limiting our ability to ascertain whether specific techniques resulted in different outcomes. However, fluid balance and small solute (eg, ammonia) clearance is readily achieved with any of the commonly used CRRT techniques. In addition, hourly treatment intensity may not be a strong determinant of reductions in ammonia.23 Our data did not include intracranial pressure monitoring results, so we cannot correlate the control of hyperammonaemia with the control of intracranial pressure. However, it is well established that severe elevations in ammonia are strongly associated with neurological complications,3 and given that ammonia is a potent neurotoxin,38 effective control of extreme hyperammonaemia using a readily available critical care intervention that is safe and effective seems reasonable.23 We used a retrospective approach, involving review of clinical records. The accuracy of clinical records is sometimes uncertain and there is a possibility that staff may make errors during documentation. However, all patients were cared for in major ICUs with sophisticated research programs and systems for complex data management. Moreover, all submitted data are numerical, electronically recorded, not amenable to interpretation bias, and were carefully evaluated before analysis. Finally, given the retrospective design of our study, we cannot prove that hyperammonaemia was the dominant trigger of CRRT initiation. We did not collect information on the clinical reasons for commencing CRRT and acknowledge that initiation of treatment may be driven by multiple considerations (eg, acid–base control, hyperlactataemia, temperature control, tonicity control, volume control, solute control). However, the observation that severe AKI and severe acidaemia were absent in the majority of patients with hyperammonaemia treated with CRRT suggests that, in most patients, ammonia levels were an important, if not key, consideration for the start of CRRT.
Conclusion
In a binational multicentre observational study of ALF treatment across all liver transplant ICUs in Australia and New Zealand, we found that early utilisation of CRRT was common. Such treatment was typically initiated in the absence of traditional renal indications or severe acidaemia and in the presence of severe hyperammonaemia. This appeared free of bleeding complications and was associated with rapid improvements in hyperammonaemia to safer levels, which became similar to those observed in less severely ill non-CRRT treated patients with ALF. Moreover, it was associated with avoidance of extreme hyperammonaemia. Such avoidance was, in turn, associated with increased ELT-free survival. These findings are hypothesis-generating and provide the rationale for further investigation of the role of early CRRT for the treatment of severe hyperammonaemia in ALF. Future research is required to determine the optimal approach to timing, treatment intensity, duration and technical aspects, such as use of anticoagulants, for CRRT in patients with ALF. Our findings and those of future research should inform the development of evidence-based management guidelines to assist the care of these highly complex patients.
Competing interests
None declared.
References
- 1.Warrillow S., Bailey M., Pilcher D., et al. Characteristics and outcomes of patients with acute liver failure admitted to Australian and New Zealand intensive care units. Intern Med J. 2019;49:874–885. doi: 10.1111/imj.14167. [DOI] [PubMed] [Google Scholar]
- 2.Warrillow S.J., Bellomo R. Preventing cerebral oedema in acute liver failure: the case for quadruple-H therapy. Anaesth Intensive Care. 2014;42:78–88. doi: 10.1177/0310057X1404200114. [DOI] [PubMed] [Google Scholar]
- 3.Clemmesen J.O., Larsen F.S., Kondrup J., et al. Cerebral herniation in patients with acute liver failure is correlated with arterial ammonia concentration. Hepatology. 1999;29:648–653. doi: 10.1002/hep.510290309. [DOI] [PubMed] [Google Scholar]
- 4.Oja S.S., Saransaari P., Korpi E.R. Neurotoxicity of ammonia. Neurochem Res. 2017;42:713–720. doi: 10.1007/s11064-016-2014-x. [DOI] [PubMed] [Google Scholar]
- 5.Norenberg M.D., Jayakumar A.R., Rama Rao K.V., Panickar K.S. New concepts in the mechanism of ammonia-induced astrocyte swelling. Metab Brain Dis. 2007;22:219–234. doi: 10.1007/s11011-007-9062-5. [DOI] [PubMed] [Google Scholar]
- 6.Hertz L., Kala G. Energy metabolism in brain cells: effects of elevated ammonia concentrations. Metab Brain Dis. 2007;22:199–218. doi: 10.1007/s11011-007-9068-z. [DOI] [PubMed] [Google Scholar]
- 7.Bernal W., Hall C., Karvellas C.J., et al. Arterial ammonia and clinical risk factors for encephalopathy and intracranial hypertension in acute liver failure. Hepatology. 2007;46:1844–1852. doi: 10.1002/hep.21838. [DOI] [PubMed] [Google Scholar]
- 8.Bhatia V., Singh R., Acharya S.K. Predictive value of arterial ammonia for complications and outcome in acute liver failure. Gut. 2006;55:98–104. doi: 10.1136/gut.2004.061754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Norenberg M.D., Rama Rao K.V., Jayakumar A.R. Ammonia neurotoxicity and the mitochondrial permeability transition. J Bioenerg Biomembr. 2004;36:303–307. doi: 10.1023/B:JOBB.0000041758.20071.19. [DOI] [PubMed] [Google Scholar]
- 10.Chow S.L., Gandhi V., Krywawych S., et al. The significance of a high plasma ammonia value. Arch Dis Child. 2004;89:585–586. doi: 10.1136/adc.2003.036236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ferenci P. Hepatic encephalopathy. Gastroenterol Rep (Oxf) 2017;5:138–147. doi: 10.1093/gastro/gox013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wendon J., Cordoba J., Dhawan A., et al. EASL clinical practical guidelines on the management of acute (fulminant) liver failure. J Hepatol. 2017;66:1047–1081. doi: 10.1016/j.jhep.2016.12.003. [DOI] [PubMed] [Google Scholar]
- 13.Lee W.M., Stravitz R.T., Larson A.M. AASLD position paper: the management of acute liver failure: update 2011. Hepatology. 2012;55:965–967. doi: 10.1002/hep.25551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Redant S., De Bels D., Beretta-Piccoli X., et al. Is high volume haemofiltration really necessary to remove ammonia? Liver Int. 2019 doi: 10.1111/liv.14278. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 15.Slack A.J., Auzinger G., Willars C., et al. Ammonia clearance with haemofiltration in adults with liver disease. Liver Int. 2014;34:42–48. doi: 10.1111/liv.12221. [DOI] [PubMed] [Google Scholar]
- 16.Cardoso F.S., Gottfried M., Tujios S., et al. Continuous renal replacement therapy is associated with reduced serum ammonia levels and mortality in acute liver failure. Hepatology. 2018;67:711–720. doi: 10.1002/hep.29488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cardoso F.S., Karvellas C.J. Managing the patient with acute liver failure. Clin Liver Dis (Hoboken) 2017;9:89–93. doi: 10.1002/cld.628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gupta S., Fenves A.Z., Hootkins R. The role of RRT in hyperammonemic patients. Clin J Am Soc Nephrol. 2016;11:1872–1878. doi: 10.2215/CJN.01320216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Aygun F., Aygun D., Erbek Alp F., et al. The impact of continuous renal replacement therapy for metabolic disorders in infants. Pediatr Neonatol. 2018;59:85–90. doi: 10.1016/j.pedneo.2017.04.004. [DOI] [PubMed] [Google Scholar]
- 20.Aygun F., Varol F., Aktuglu-Zeybek C., et al. Continuous renal replacement therapy with high flow rate can effectively, safely, and quickly reduce plasma ammonia and leucine levels in children. Children (Basel) 2019;6 doi: 10.3390/children6040053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Braun M.C., Welch T.R. Continuous venovenous hemodiafiltration in the treatment of acute hyperammonemia. Am J Nephrol. 1998;18:531–533. doi: 10.1159/000013400. [DOI] [PubMed] [Google Scholar]
- 22.Deep A., Stewart C.E., Dhawan A., Douiri A. Effect of continuous renal replacement therapy on outcome in pediatric acute liver failure. Crit Care Med. 2016;44:1910–1919. doi: 10.1097/CCM.0000000000001826. [DOI] [PubMed] [Google Scholar]
- 23.Warrillow S., Fisher C., Bellomo R. Correction and control of hyperammonemia in acute liver failure: the impact of continuous renal replacement timing, intensity, and duration. Crit Care Med. 2019 doi: 10.1097/CCM.0000000000004153. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 24.Warrillow S., Tibballs H., Bailey M., et al. Characteristics, management and outcomes of patients with acute liver failure admitted to Australasian intensive care units. Crit Care Resusc. 2019;21:188–199. [PubMed] [Google Scholar]
- 25.von Elm E., Altman D.G., Egger M., et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet. 2007;370:1453–1457. doi: 10.1016/S0140-6736(07)61602-X. [DOI] [PubMed] [Google Scholar]
- 26.Rabinowich L., Wendon J., Bernal W., Shibolet O. Clinical management of acute liver failure: results of an international multi-center survey. World J Gastroenterol. 2016;22:7595–7603. doi: 10.3748/wjg.v22.i33.7595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Reuben A., Tillman H., Fontana R.J., et al. Outcomes in adults with acute liver failure between 1998 and 2013: an observational cohort study. Ann Intern Med. 2016;164:724–732. doi: 10.7326/M15-2211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wei G., Bergquist A., Broome U., et al. Acute liver failure in Sweden: etiology and outcome. J Intern Med. 2007;262:393–401. doi: 10.1111/j.1365-2796.2007.01818.x. [DOI] [PubMed] [Google Scholar]
- 29.Marudanayagam R., Shanmugam V., Gunson B., et al. Aetiology and outcome of acute liver failure. HPB (Oxford) 2009;11:429–434. doi: 10.1111/j.1477-2574.2009.00086.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gaudry S., Hajage D., Schortgen F., et al. Initiation strategies for renal-replacement therapy in the intensive care unit. N Engl J Med. 2016;375:122–133. doi: 10.1056/NEJMoa1603017. [DOI] [PubMed] [Google Scholar]
- 31.Barbar S.D., Clere-Jehl R., Bourredjem A., et al. Timing of renal-replacement therapy in patients with acute kidney injury and sepsis. N Engl J Med. 2018;379:1431–1442. doi: 10.1056/NEJMoa1803213. [DOI] [PubMed] [Google Scholar]
- 32.Xu Y., Gao J., Zheng X., et al. Timing of initiation of renal replacement therapy for acute kidney injury: a systematic review and meta-analysis of randomized-controlled trials. Clin Exp Nephrol. 2017;21:552–562. doi: 10.1007/s10157-016-1316-2. [DOI] [PubMed] [Google Scholar]
- 33.Liu K.D., Palevsky P.M. RRT in AKI: start early or wait? Clin J Am Soc Nephrol. 2016;11:1867. doi: 10.2215/CJN.06690616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Licari E., Calzavacca P., Warrillow S.J., Bellomo R. Life-threatening sodium valproate overdose: a comparison of two approaches to treatment. Crit Care Med. 2009;37:3161–3164. doi: 10.1097/CCM.0b013e3181b03245. [DOI] [PubMed] [Google Scholar]
- 35.Hoste E.A.J., Dhondt A. Clinical review: use of renal replacement therapies in special groups of ICU patients. Crit Care. 2012;16:201. doi: 10.1186/cc10499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Warrillow S., Fisher C., Tibballs H., et al. Coagulation abnormalities, bleeding, thrombosis, and management of patients with acute liver failure in Australia and New Zealand. J Gastroenterol Hepatol. 2019 doi: 10.1111/jgh.14876. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 37.Levi M., Opal S.M. Coagulation abnormalities in critically ill patients. Crit Care. 2006;10:222. doi: 10.1186/cc4975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Butterworth R.F. Role of circulating neurotoxins in the pathogenesis of hepatic encephalopathy: potential for improvement following their removal by liver assist devices. Liver Int. 2003;23(Suppl):5–9. doi: 10.1034/j.1478-3231.23.s.3.1.x. [DOI] [PubMed] [Google Scholar]