Abstract
Objective: To study the cardiovascular effect over 30 minutes following the end of fluid bolus therapy (FBT) with 20% albumin in patients after cardiac surgery.
Design: Prospective observational study.
Setting: Intensive care unit of a tertiary university-affiliated hospital.
Participants: Twenty post-cardiac surgery mechanically ventilated patients with a clinical decision to administer FBT.
Intervention: FBT with a 100 mL bolus of 20% albumin.
Main outcome measures: Cardiac index (CI) response was defined by a ≥ 15% increase, while mean arterial pressure (MAP) response was defined by a ≥ 10% increase.
Results: The most common indication for FBT was hypotension (40%). Median duration of infusion was 7 minutes (interquartile range [IQR], 3–9 min). At the end of FBT, five patients (25%) showed a CI response, which increased to almost half in the following 30 minutes and dissipated in one patient. MAP response occurred in 11 patients (55%) and dissipated in five patients (45%) by a median of 6 minutes (IQR, 6–10 min). CI and MAP responses coexisted in four patients (20%). An intrabolus MAP response occurred in 17 patients (85%) but dissipated in 11 patients (65%) within a median of 7 minutes (IQR, 2–11 min). On regression analysis, faster fluid bolus administration predicted MAP increase at the end of the bolus.
Conclusion: In post-cardiac surgery patients, CI response to 20% albumin FBT was not congruous with MAP response over 30 minutes. Although hypotension was the main indication for FBT and a MAP response occurred in most of patients, such response was maximal during the bolus, dissipated in a few minutes, and was dissociated from the CI response.
Fluid bolus therapy (FBT) is ubiquitous in the intensive care unit (ICU). It is typically given to treat haemodynamic instability.1 This may be especially common in cardiac surgery patients,2 where the aim is to expand intravascular volume in order to increase cardiac index (CI) and mean arterial pressure (MAP).3 However, much uncertainty remains in terms of the timing of FBT administration, the speed of delivery, the type and volume of fluid, the clinical indications and physiological targets,4 and the evaluation of response.5, 6 In particular, only 50% of ICU patients with haemodynamic instability appear FBT responsive,7 and although crystalloids are frequently used in post-cardiac surgical patients,2 previous studies reported dissipation of their cardiovascular effect within 10 minutes8 following the end of fluid infusion after surgery. Artificial colloids, such as starch and gelatine, may have a longer lasting effect on haemodynamics, but they have been associated with significant risks.9, 10, 11, 12
On the other hand, after exclusion of patients with traumatic brain injury,13 human albumin solutions have repeatedly been shown to be safe and may achieve longer lasting haemodynamic effects.14, 15, 16 Furthermore, fluid resuscitation with hyperoncotic (20%) albumin solutions have been associated with decreased fluid requirements and less fluid accumulation in critically ill patients17 compared with iso-oncotic (4–5%) albumin.14 Such beneficial effect on fluid balance has been recently confirmed in cardiac surgery patients.18 However, no study has addressed the cardiovascular pharmacodynamics of a rapid 20% albumin FBT in cardiac surgery patients. Accordingly, we conducted a detailed assessment of the cardiovascular effect of a rapid 20% albumin bolus in a cohort of post-cardiac surgery patients. Our primary hypothesis was that, in such patients, a CI response would occur in most cases.
Methods
The study was approved by the Austin Health Human Research Ethics Committee (LNR/16/Austin/358). The need for informed consent was waived due to the observational nature of the study.
Study design
This study was a prospective, single-centre, observational study performed in the ICU of a tertiary Australian university-affiliated hospital between July 2017 and May 2018. All patients aged 18 years or over, who were admitted to the ICU following on-pump cardiac surgery and were mechanically ventilated, were considered for inclusion in the study. Patients were included by the research team if the attending clinician decided to administer a 20% albumin FBT to treat haemodynamic instability in the first 12 hours of ICU admission and if there was a member of the research team available to observe the patient. Patients were excluded if they did not have invasive blood pressure and CI monitoring by pulmonary artery catheter (PAC), or if any intervention affecting haemodynamics (eg, change of sedatives, vasopressors, mechanical ventilation settings, patient’s position) was performed immediately before or during the observational period.
Patients who were known to be pregnant or who required mechanical haemodynamic support (intra-aortic balloon pump or extracorporeal membrane oxygenation) were also excluded.
Haemodynamic data
All patients were monitored using Philips Intelliview MP70 (Philips Healthcare, Best, Netherlands) bedside monitors, with continuous systemic arterial blood pressure monitoring from either a radial or brachial artery catheter. Central venous pressure (CVP), pulmonary artery pressures, and blood temperature were recorded from the PAC (Edwards Lifesciences, Irvine, CA, USA), inserted via the internal jugular vein. The correct position of the PAC was confirmed on chest x-ray at ICU admission. Depending on the type of PAC, intermittent or semi-continuous measurements of CI (equal to cardiac output divided by body surface area) were recorded, using the thermodilution technique. When patients did not have a PAC capable of semi-continuous cardiac output monitoring, CI was measured at the following specific time points: before the fluid bolus, immediately after completion of the bolus, and then at 15 minutes and 30 minutes post-FBT.
Baseline haemodynamic parameters were recorded over a minimum of 3 minutes before commencement of FBT. Systolic arterial pressure (SAP), diastolic arterial pressure (DAP), MAP, CVP, systolic pulmonary arterial pressure, diastolic pulmonary arterial pressure, and mean pulmonary arterial pressure measurements were referenced to the intersection of the anterior axillary line and the fifth intercostal space (phlebostatic level). We also collected further haemodynamic data, such as heart rate and peripheral oxygen saturation (Spo2). Finally, mean systemic perfusion pressure (MSPP), as a determinant of systemic blood flow,19 was defined as equal to the difference between MAP and CVP. Pulse pressure was defined as equal to the difference between SAP and DAP. Stroke volume was defined as equal to the ratio between CI and heart rate, multiplied for body surface area.
All haemodynamic data were exported live on a second-by-second basis, using the MediCollector data logging software (MediCollector, Boston, MA, USA) connected to the bedside monitors.
Fluid bolus therapy characteristics
FBT consisted of 100 mL of room temperature 20% albumin, which was given using an intravenous set with a hand pump. The clinical indication for FBT was collected before the FBT. A member of the research team was present at the bedside throughout the FBT administration and for 30 minutes afterwards. We truncated the study observational period at 30 minutes after the end of FBT administration due to the practical impossibility to obtain confounder-free observations over a longer period of time in this population, and in accordance with the observational feature of the study. Furthermore, the researcher contemporaneously recorded the nature of any intervention performed during the study period by electronic annotation added to the MediCollector data file.
Haemodynamic response definitions
Patients were classified as early CI responsive to FBT if their CI increased by 15% or greater above baseline immediately after the end of the FBT infusion.6 They were classified as early MAP responsive if their MAP increased by 10% or greater above baseline at that same time point.20 We defined delayed responsiveness if such changes were seen at 30 minutes after the completion of the bolus.
We then arbitrarily defined “time to dissipation” of FBT effect as the time from the end of the FBT to the time when a patient’s CI was no greater than 5% above baseline for at least 2 consecutive minutes and, for blood pressure response, when a patient’s MAP was no greater than 3 mmHg above baseline for at least 2 consecutive minutes.
The primary study hypothesis was that more than 50% of patients would have a persistent increase in CI greater than 15% at 30 minutes after the administration of 20% albumin. We also tested several secondary hypotheses focused on the effect of the FBT on other haemodynamic parameters as described in our online pre-published protocol and statistical analysis plan.21
Pre-morbid and baseline data
We recorded demographic characteristics (age, sex, body surface area), type of surgery and duration of the extracorporeal bypass. Baseline ventilator settings, arterial blood gases and lactates, doses of sedatives, vasoactive drugs and inotropes (if any) were also noted. Arterial blood gas results from within 2 hours before the fluid bolus were also recorded.
Statistical analysis
A statistical analysis plan related to the protocol was published online ahead of data collection.21 All analyses were performed using the R software, version 3.3.1 (The R Foundation for Statistical Computing, Vienna, Austria), with the package lme4. A P value below 0.05 was considered statistically significant. Continuous variables were expressed as median with interquartile range (IQR), and categorical variables as count with percentage. A convenience sample size of 20 patients for whom the observational period of the study was free from haemodynamic confounders was analysed. All baseline characteristics were compared between responders and non-responders, using Fisher exact test for categorical variables and the Wilcoxon–Mann–Whitney test for continuous variables. Variables collected over the observation period in all patients were first compared with baseline values, using linear mixed effects regression models, accounting for the repetition of measurements in a given individual. Then, we performed an overall comparison of variables (either absolute values or relative change from baseline) collected over the observation period between responders and non-responders, using similar methodology. If significant, we performed a post hoc analysis to examine the significance of the difference at each time point, accounting for the inflation in the a risk using the Tukey adjustment method. Finally, time to dissipation of haemodynamic response was assessed using Kaplan–Meier curves. A P below 0.05 was considered statistically significant.
Results
Patients characteristics
Of the 35 patients screened, 15 patients were excluded from analysis because of haemodynamic confounders (online Appendix, eFigure 1; available at cicm.org.au/Resources/Publications/Journal). The characteristics of the 20 patients included in the study are displayed in Table 1. Most patients had paced cardiac rhythm and rate, and only a few were on vasopressor or inotropic support at ICU admission. There were no significant differences of demographic, surgical and baseline haemodynamic characteristics between either early CI or MAP responders and non-responders.
Table 1.
Demographic, surgical and baseline characteristics of patients by early haemodynamic responses
|
CI early response⁎ |
MAP early response† |
||||||
|---|---|---|---|---|---|---|---|
| Whole cohort (n = 20) | CI non-responders (n = 15) | CI responders (n = 5) | P | MAP non-responders (n = 9) | MAP responders (n = 11) | P | |
| Age (years), median (IQR) | 67 (64.2–75.8) | 69 (60.5–78.5) | 67 (66–67) | 0.97 | 70 (66–79) | 66 (60.5–71) | 0.20 |
| Gender (female) | 4 (20%) | 2 (13%) | 2 (40%) | 0.25 | 2 (22%) | 2 (18%) | >0.99 |
| Body surface area (m2), median (IQR) | 2 (1.8–2.1) | 2 (1.7–2.2) | 1.9 (1.9–2) | 0.60 | 1.9 (1.7–2) | 2.1 (1.9–2.2) | 0.12 |
| Type of surgery | >0.99 | 0.84 | |||||
| CABG | 12 (60%) | 9 (60%) | 3 (60%) | 5 (56%) | 7 (64%) | ||
| Valves | 5 (25%) | 4 (27%) | 1 (20%) | 2 (22%) | 3 (27%) | ||
| CABG + valves | 3 (15%) | 2 (13%) | 1 (20%) | 2 (22%) | 1 (9%) | ||
| Post-bypass LV function | >0.99 | 0.11 | |||||
| Normal | 11 (55%) | 8 (53%) | 3 (60%) | 7 (78%) | 4 (36%) | ||
| Dysfunction | 6 (30%) | 5 (33%) | 1 (20%) | 1 (11%) | 5 (45%) | ||
| Post-bypass RV function | >0.99 | >0.99 | |||||
| Normal | 16 (80%) | 12 (80%) | 4 (80%) | 8 (89%) | 8 (73%) | ||
| Dysfunction | 1 (5%) | 1 (7%) | 0 (0%) | 0 (0%) | 1 (9%) | ||
| Bypass duration (min), median (IQR) | 114.5 (89.5–144.8) | 116 (88–137.5) | 113 (96–180) | 0.63 | 113 (85–121) | 116 (93.5–173.5) | 0.32 |
| Heart rhythm | 0.50 | 0.81 | |||||
| Paced | 11 (55%) | 7 (47%) | 4 (80%) | 5 (56%) | 6 (55%) | ||
| Sinus rhythm | 8 (40%) | 7 (47%) | 1 (20%) | 3 (33%) | 5 (45%) | ||
| Atrial fibrillation | 1 (5%) | 1 (7%) | 0 (0%) | 1 (11%) | 0 (0%) | ||
| Cl measuring technique | 0.06 | 0.20 | |||||
| Discontinuous | 12 (60%) | 7 (47%) | 5 (100%) | 7 (78%) | 5 (45%) | ||
| Semi-continuous | 8 (40%) | 8 (53%) | 0 (0%) | 2 (22%) | 6 (55%) | ||
| Mechanical ventilator setting, median (IQR) | |||||||
| Tidal volume (mL/IBW) | 7.9 (6.8–8.6) | 8 (6.9–8.4) | 7.5 (6.8–8.5) | >0.99 | 8 (5.9–8.3) | 7.8 (7.2–8.7) | 0.41 |
| Peak pressure (cmH20) | 19 (16.5–21) | 19 (17–21) | 18 (15–21) | 0.73 | 19 (17–21) | 19 (16–20.5) | >0.99 |
| PEEP | 5 (5-5) | 5 (5–5) | 5 (5–5) | 0.90 | 5 (5–5) | 5 (5–5) | >0.99 |
| Fio2 | 0.5 (0.3–0.6) | 0.5 (0.3–0.6) | 0.5 (0.3–0.5) | 0.69 | 5 (5–5) | 0.6 (0.4–0.8) | 0.06 |
| Biochemistry findings, median (IQR) | |||||||
| Lactate (mmol/L) | 1 (0.9–1.4) | 1.1 (0.9–1.4) | 0.9 (0.8–1) | 0.16 | 1.1 (0.9–1.5) | 1 (0.9–1.4) | 0.65 |
| Active external warming | |||||||
| Use of external warming | 8 (40%) | 6 (40%) | 2 (40%) | > 0.99 | 3 (33%) | 5 (45%) | > 0.99 |
| Haemodynamic support | |||||||
| Vasopressors | 7 (35%) | 6 (40%) | 1 (20%) | 0.61 | 4 (44%) | 3 (27%) | 0.64 |
| Inotropes | 2 (13%) | 0 (0%) | > 0.99 | 1 (11%) | 1 (9%) | > 0.99 | |
| Number of fluid boluses received before study | |||||||
| 0 | 18 (90% | 14 (93%) | 4 (80%) | 9 (100%) | 9 (82%) | ||
| 1 | 1 (5%) | 0 (0%) | 1 (20%) | 0 (%) | 1 (9%) | ||
| ≥ 2 | 1 (5%) | 1 (7%) | 0 (0%) | 0 (%) | 1 (9%) | ||
CABG = coronary artery bypass graft; CI = cardiac index; Fio2 = fraction of inspired oxygen; IBW = ideal body weight; IQR = interquartile range; LV = left ventricle; MAP = mean arterial pressure; PEEP = positive end-expiratory pressure; RV = right ventricle.
Early CI response to fluid bolus therapy (FBT) was verified when CI increased by ≥ 15% above baseline immediately after the end of the FBT infusion.
Early MAP response to FBT was verified when MAP increased by ≥ 10% above baseline immediately after the end of the FBT infusion. Dissipation of CI response to FBT was verified when CI was no greater than 5% above baseline for at least 2 consecutive minutes. Dissipation of MAP response to FBT was verified when MAP was no greater than 3 mmHg above baseline for at least 2 consecutive minutes.
Fluid bolus therapy description
Table 2 describes the FBT characteristics along with haemodynamic outcomes. The most common indication for FBT was hypotension (n = 8, 40%). The median duration of infusion was 7 minutes (IQR, 3–9 min).
Table 2.
Fluid bolus characteristics, outcomes and comparison based on haemodynamic responses
| Whole cohort (n = 20) |
CI early response⁎ |
P |
MAP early response† |
P | |||
|---|---|---|---|---|---|---|---|
| CI non-responders (n = 15) |
CI responders (n = 5) |
MAP non-responders (n = 9) | MAP responders (n = 11) | ||||
| Fluid bolus characteristics | |||||||
| Indication | 0.29 | 0.87 | |||||
| Tachycardia | 1 (5%) | 1 (7%) | 0 (0%) | 0 (0%) | 1 (9%) | ||
| Low CI | 6 (30%) | 3 (20%) | 3 (60%) | 2 (22%) | 4 (36%) | ||
| Low filling pressures | 3 (15%) | 3 (20%) | 0 (0%) | 2 (22%) | 1 (9%) | ||
| Hypotension | 8 (40%) | 7 (47%) | 1 (20%) | 4 (44%) | 4 (36%) | ||
| Other | 2 (10%) | 1 (7%) | 1 (20%) | 1 (11%) | 1 (9%) | ||
| Infusion duration (min), median (IQR) | 7 (3–9) | 7 (4–9) | 3 (3–7) | 0.23 | 8 (7–10) | 4 (3–7) | 0.05 |
| CI response | |||||||
| Early response | 5 (25%) | 0 (0%) | 5 (100%) | < 0.01 | na | na | na |
| At 15 min after bolus | 1 (5%) | 1 (7%) | na | na | na | na | na |
| At 30 min after bolus | 8 (40%) | 5 (33%) | 3 (60%) | 0.35 | na | na | na |
| Dissipation of CI response‡ | |||||||
| Number of dissipators | 1 (5%) | na | 1 (20%) | na | na | na | na |
| Time to dissipation (min), median (IQR) | na | na | 30 (30–30) | na | na | na | na |
| MAP response | |||||||
| Early response | 11 (55%) | na | na | na | 0 (0%) | 11 (100%) | < 0.01 |
| At 15 min after bolus | 7 (35%) | na | na | na | 2 (22%) | 5 (45%) | 0.37 |
| At 30 min after bolus | 5 (25%) | na | na | na | 1 (11%) | 4 (36%) | 0.32 |
| Dissipation of MAP response§ | |||||||
| Number of dissipators | 5 (25%) | na | na | na | na | 5 (45%) | na |
| Time to dissipation (min), median (IQR) | na | na | na | na | na | 6 (6–10) | na |
CI = cardiac index; IQR = interquartile range; MAP = mean arterial pressure; na = not applicable.
Early CI response to fluid bolus therapy (FBT) was verified when CI increased by ≥ 15% above baseline immediately after the end of the FBT infusion.
Early MAP response to FBT was verified when MAP increased by ≥ 10% above baseline immediately after the end of the FBT infusion.
Dissipation of CI response to FBT was verified when CI was no greater than 5% above baseline for at least 2 consecutive minutes.
Dissipation of MAP response to FBT was verified when MAP was no greater than 3 mmHg above baseline for at least 2 consecutive minutes.
Pharmacodynamic analysis according to early cardiac index response
Early CI response was observed in five patients (25%) (Table 2) and the absolute increase from the baseline was significantly larger in early CI responders than in non-responders (Figure 1). The prevalence of CI responsiveness increased over the observational period and, by 30 minutes, it was still present in three early CI responders (15%) and in a further five patients (25%), which showed a delayed CI response. Among other haemodynamic parameters, only MSPP was significantly higher in early CI responders (Table 3), and on univariate regression analysis, a greater MSPP was associated with CI response (online Appendix, eTable 1). At 30 minutes, body temperature showed smaller changes in early CI responders than in non-responders (online Appendix, eFigure 2 and eFigure 3).
Figure 1.
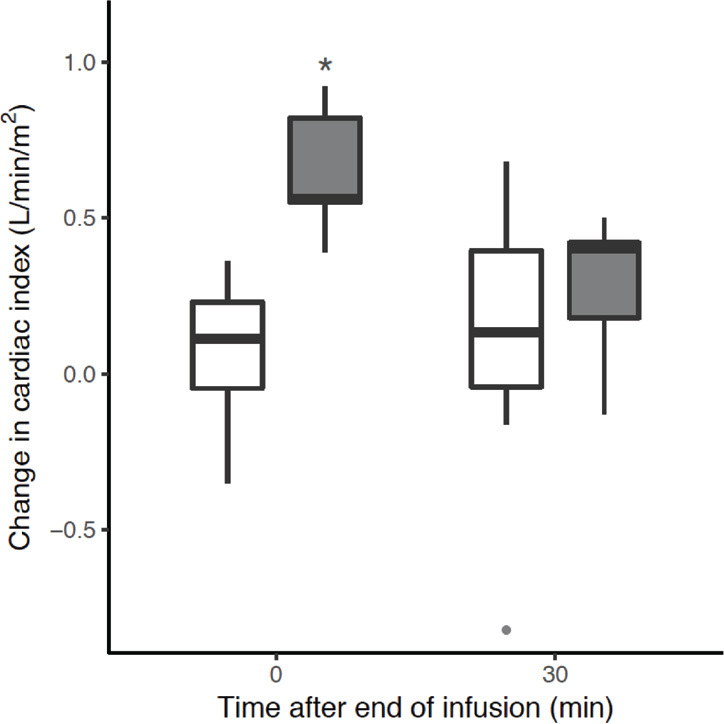
Change in cardiac index (CI) from its baseline value, based on CI early response
The boxplots represent the observed change in CI from baseline in CI responders (grey boxes) and non-responders (open boxes) over the observation period. The asterisk reflects a significant difference between groups at the specific time point, resulting from the pairwise comparisons analysis.
Table 3.
Main haemodynamics during the observation period, and comparison based on haemodynamic response
| Whole cohort (n = 20) Median (IQR) |
CI early response⁎ |
MAP early response† |
|||
|---|---|---|---|---|---|
| Non–responders (n = 15) Median (IQR) | Responders (n = 5) Median (IQR) | Non–responders (n = 9) Median (IQR) | Responders (n = 11) Median (IQR) | ||
| CI (L/min/m2) | |||||
| Baseline | 2.3 (1.9–2.9) | 2.4 (2–2.9) | 1.7 (1.7–2.8) | 2.1 (2–2.8) | 2.5 (1.8–2.9) |
| End | 2.6 (2.1–3.2)‡ | 2.6 (2.1–3.1) | 2.6 (2.3–3.4) | 2.4 (2.1–3.4) | 2.6 (2.1–3.1) |
| 15 min | 2.6 (2.4–3) | 2.6 (2.4–3) | na | 1.9 (1.8–2.7) | 2.7 (2.4–3) |
| 30 min | 2.6 (2.2–3.1)‡ | 2.7 (2.3–3.1) | 2.2 (2.1–2.7) | 2.5 (2.1–3.1) | 2.7 (2.2–3) |
| Mean arterial pressure (mmHg) | |||||
| Baseline | 68 (63–75) | 67 (63–70) | 77 (67–82) | 67 (67–70) | 68 (62–80) |
| End | 76 (68–84)‡ | 73 (68–79) | 91 (75–95) | 69 (68–73) | 80 (76–93) |
| 15 min | 71 (65–81)‡ | 69 (65–78) | 82 (75–89) | 69 (65–75) | 77 (67–83) |
| 30 min | 71 (65–81)‡ | 69 (64–76) | 85 (71–91) | 69 (65–71) | 73 (65–88) |
| Heart rate (per min) | |||||
| Baseline | 88 (80–92) | 88 (76–96) | 88 (80–88) | 88 (78–96) | 88 (80–90) |
| End | 88 (79–92) | 88 (77–96) | 88 (80–88) | 88 (78–96) | 88 (80–90) |
| 15 min | 88 (80–92) | 88 (78–96) | 88 (80–88) | 88 (78–95) | 88 (80–90) |
| 30 min | 88 (78–91) | 88 (78–96) | 88 (80–88) | 88 (78–94) | 88 (79–90) |
| Systolic arterial pressure (mmHg) | |||||
| Baseline | 102 (93–111) | 97 (87–108) | 106 (104–112) | 106 (94–111) | 100 (92–108) |
| End | 111 (99–127)‡ | 108 (98–123) | 124 (122–136) | 101 (93–113) | 124 (108–133) |
| 15 min | 105 (96–117) | 101 (96–115) | 112 (108–119) | 101 (93–106) | 112 (99–117) |
| 30 min | 102 (93–118) | 97 (92–115) | 115 (98–122) | 97 (92–109) | 114 (96–122) |
| Diastolic arterial pressure (mmHg) | |||||
| Baseline | 52 (47–59) | 52 (49–55) | 63 (45–64) | 50 (48–52) | 53 (48–63) |
| End | 57 (51–67)‡ | 53 (51–62) | 68 (54–78) | 52 (50–53) | 66 (58–70) |
| 15 min | 56 (49–62)‡ | 53 (49–60) | 62 (61–70) | 52 (49–58) | 61 (52–64) |
| 30 min | 54 (48–63) | 53 (47–60) | 64 (54–68) | 53 (48–54) | 61 (50–65) |
| Central venous pressure (mmHg) | |||||
| Baseline | 7 (6–10) | 7 (6–9) | 8 (5–10) | 7 (5–10) | 8 (7–8) |
| End | 10 (8–11)‡ | 10 (9–11) | 10 (8–12) | 10 (7–11) | 10 (9–10) |
| 15 min | 9 (7–10)‡ | 9 (8–10) | 9 (6–11) | 8 (6–11) | 9 (8–10) |
| 30 min | 9 (7–10)‡ | 9 (7–10) | 9 (7–11) | 8 (6–11) | 9 (8–10) |
| Systolic pulmonary arterial pressure (mmHg) | |||||
| Baseline | 26 (22–30) | 26 (22–34) | 23 (22–27) | 27 (24–33) | 23 (22–28) |
| End | 28 (26–34)‡ | 28 (25–39) | 27 (26–31) | 31 (26–37) | 27 (25–32) |
| 15 min | 27 (25–32)‡ | 27 (24–35) | 27 (25–30) | 28 (26–33) | 26 (24–31) |
| 30 min | 27 (24–33)‡ | 26 (24–34) | 29 (25–31) | 28 (26–32) | 26 (24–32) |
| Diastolic pulmonary arterial pressure (mmHg) | |||||
| Baseline | 13 (10–16) | 13 (10–17) | 12 (11–14) | 13 (11–15) | 12 (10–17) |
| End | 15 (12–17)‡ | 15 (13–18) | 14 (11–16) | 15 (12–17) | 15 (12–19) |
| 15 min | 14 (12–17)‡ | 14 (12–18) | 13 (13–14) | 14 (13–17) | 13 (11–18) |
| 30 min | 14 (12–18)‡ | 14 (11–18) | 14 (13–16) | 14 (12–17) | 14 (11–18) |
| Mean pulmonary arterial pressure (mmHg) | |||||
| Baseline | 18 (16–22) | 19 (16–24) | 18 (17–18) | 19 (17–22) | 18 (16–22) |
| End | 21 (18–25)‡ | 21 (18–27) | 20 (20–21) | 21 (19–25) | 20 (19–25) |
| 15 min | 19 (18–23)‡ | 20 (17–25) | 19 (19–20) | 20 (18–22) | 19 (17–24) |
| 30 min | 19 (18–24)‡ | 20 (17–25) | 19 (19–23) | 21 (18–23) | 19 (17–24) |
| Body temperature (°C) | |||||
| Baseline | 35.8 (35.4–37) | 36.9 (35.4–37) | 35.6 (35.4–36) | 37 (35.4–37.1) | 35.7 (35.3–36.9) |
| End | 35.7 (35.4–37)‡ | 36.7 (35.4–37) | 35.5 (35.3–35.8) | 36.8 (35.5–37) | 35.6 (35.2–36.9) |
| 15 min | 35.9 (35.5–37.1)‡ | 36.9 (35.6–37.2) | 35.5 (35.4–35.9) | 36.9 (35.6–37.2) | 35.8 (35.4–37) |
| 30 min | 36 (35.6–37.3)‡ | 37 (35.8–37.3) | 35.7 (35.5–36) | 37 (35.8–37.4) | 35.9 (35.5–37.1) |
| Systemic perfusion pressure (mmHg) | |||||
| Baseline | 62 (56–65)§ | 60 (54–64) | 69 (65–75) | 63 (57–65) | 61 (56–73) |
| End | 64 (59–71)‡ | 63 (59–69) | 82 (75–87)§ | 62 (59–64) | 75 (66–85) |
| 15 min | 68 (58–72)‡ | 63 (57–69) | 75 (73–79)§ | 62 (58–69) | 71 (63–77) |
| 30 min | 63 (57–74) | 60 (56–66) | 76 (73–80)§ | 60 (57–67) | 74 (63–78) |
| Pulse pressure (mmHg) | |||||
| Baseline | 48 (43–54) | 47 (40–54) | 52 (48–54) | 48 (47–59) | 48 (43–53) |
| End | 53 (47–63)‡ | 53 (47–60) | 54 (52–65) | 50 (40–56) | 57 (50–65) |
| 15 min | 51 (46–58) | 51 (44–56) | 50 (48–60) | 48 (38–53) | 52 (47–58) |
| 30 min | 51 (44–58) | 50 (43–58) | 52 (49–55) | 50 (38–51) | 52 (48–58) |
CI = cardiac index; IQR = interquartile range; MAP = mean arterial pressure; na = not applicable.
Early CI response to FBT was verified when CI increased by ≥ 15% above baseline immediately after the end of the FBT infusion.
Early MAP response to fluid bolus therapy (FBT) was verified when MAP increased by ≥ 10% above baseline immediately after the end of the FBT infusion.
P < 0.05 compared with baseline value.
P < 0.05 compared with CI non-responders. P < 0.05 compared with MAP non-responders. Dissipation of CI response to FBT was verified when CI was no greater than 5% above baseline for at least 2 consecutive minutes. Dissipation of MAP response to FBT was verified when MAP was no greater than 3 mmHg above baseline for at least 2 consecutive minutes.
Pharmacodynamic analysis according to early mean arterial pressure response
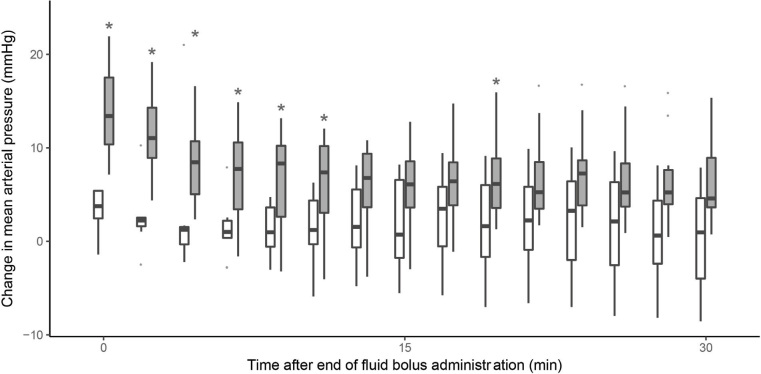
Early MAP response was observed in 11 patients (55%) (Table 2) and their absolute MAP increase from the baseline was significantly greater (Figure 2) within the first 10 minutes from the end of the bolus. However, by 30 minutes, the response had dissipated in seven patients (64%) (median time to dissipation, 6 min after bolus completion; IQR, 6–10 min), while one early non-responder (11%) showed a delayed response. Thus, only five patients (25%) were responders at 30 minutes.
Figure 2.
Change in mean arterial pressure from its baseline value, based on mean arterial pressure (MAP) early response
The boxplots represent the observed change in MAP from baseline in MAP responders (grey boxes) and non-responders (open boxes) over the observation period. The asterisk reflects a significant difference between groups at the specific time point, resulting from the pairwise comparisons analysis.
Among other haemodynamic parameters, SAP, MAP, DAP and MSPP were significantly higher in early MAP responders (Table 3) but not at subsequent time points. Changes in SAP, MSPP and pulse pressure and DAP are shown in the online Appendix (eFigure 4 and eFigure 5). The dissipation rate is presented in the online Appendix (eFigure 6). On univariate regression, a shorter FBT duration was associated with early MAP response (online Appendix, eTable1).
When taking into account the intrabolus period, the number of MAP responder increased to 17 (85%). However, in 11 of these patients (65%) the MAP effect dissipated, at a median of 7 minutes (IQR, 2–11 min) of FBT start (online Appendix, eTable2). The probability of effect persistence (non-dissipation) of MAP response as a function of time is shown in the online Appendix (eFigure 7).
Secondary endpoints and other haemodynamic effects
A CVP increase greater than +2 mmHg was observed in seven patients (35%) at the end of the FBT and in three patients (15%) at 30 minutes. MSPP increased more than 5% in ten patients (50%) at the end of the FBT, and this increase was preserved in nine patients (45%) at 30 minutes after FBT.
Post hoc analysis
Early CI and MAP response coexisted in only four patients (20%), and the majority of early MAP responders were not CI responders (n = 7, 64%) (online Appendix, eFigure 8). Delayed CI and MAP response coexisted only in two patients (10%), and the majority of delayed MAP responses were not CI responders (n = 3, 60%) (online Appendix, eFigure 9). We did not observe any correlation between relative or absolute CI and MAP changes from baseline, nor at the end of the FBT or at 30 minutes afterwards (online Appendix, eFigure 10 and eFigure 11 respectively). We did not observe any further correlation between relative changes in stroke volume and pulse pressure at the same time points (online Appendix, eFigure 12).
Finally, although we observed drain output increase and haemoglobin concentration decrease one hour after FBT, the absolute changes were not significant (online Appendix, eTable3).
Discussion
Key findings
We performed a detailed observational study of the magnitude and duration of the haemodynamic effect of 100 mL 20% albumin FBT in post-cardiac surgery patients for whom a fluid bolus was deemed necessary by attending clinicians. We found that, at the end of the bolus, only a quarter of the patients had a significant CI response, but also that this percentage increased to almost half in the following 30 minutes, with only one responder showing CI effect dissipation. Hypotension was the most common indication for FBT, and more than 50% of patients had a significant early MAP response to it. However, such MAP effect was short-lived and dissipated within about 10 minutes in most responders. Faster fluid administration predicted early MAP response, and such MAP increase peaked in most patients before the end of the bolus. Finally, the CI response and the MAP response were dissociated in most cases. FBT was not associated with significant drain output and haemoglobin concentration changes one hour after its administration.
Relationship to previous studies
Early CI response to FBT in our study was lower than previously described in patients with haemodynamic instability due to sepsis or major surgery.5, 6, 7 For example, in a prospective observational study of 20 mostly septic critically ill patients with circulatory shock and taking vasopressors, who received 500 mL of crystalloids over 30 minutes,22 an early CI response was detected in 13 patients (65%). However, this effect dissipated within 60 minutes. In another study8 of FBT (250 mL of crystalloid over 5 min) as part of a goal-directed therapy protocol in 26 post-operative critically ill patients, early CI response was detected in 13 patients (50%), with peak effect at one minute and dissipation within 10 minutes. Compared with other studies of compound sodium lactate8 and Ringer’s lactate or sodium chloride 0.9% solution,22 most of our patients became CI responsive at 30 minutes after FBT and CI dissipation was less. This aligns with evidence in septic patients that the effect of 20% albumin may be almost twice that of the infused volume, reaching its peak at 30 minutes,23 possibly related to its hyperoncotic properties.24
Hypotension is the most common indication for FBT and the MAP response is frequently used as a surrogate of CI responsiveness.2,4 However, the available evidence shows a dissociation between CI and MAP response after FBT administration,25, 26 and demonstrates that MAP increases are not a reliable surrogate of CI improvements after FBT. In contrast with CI responsiveness, which has been reported in more than 30 studies,6 MAP responsiveness has only been previously studied once27 and, to our knowledge, never in post-cardiac surgery patients. In our study, MAP increased immediately, even after the completion of the FBT, even in CI non-responders. This phenomenon requires further investigation; however, we hypothesise that this may be related to the scavenging effect of albumin on nitric oxide28 and/or to systemic vasoconstriction induced by relative hypothermia secondary to the administration of a room temperature FBT.29
Study implications
Our study implies that small volume FBT with 20% albumin is safe and leads to similar early effects to those reported for larger volumes of other fluids. It also implies that, unlike other fluids, its CI effect increases over time, possibly related to the movement of fluid into intravascular compartment in response to an increase in oncotic pressure. Moreover, it implies that a MAP response does not signify a CI response and that the early MAP response should not be expected to last. Finally, the impact of speed on MAP effect implies that 20% albumin can achieve rapid stabilisation of hypotensive patients even before the bolus is completed and with a minimum amount of fluid. However, in most cases, such effect only provides an approximate 10-minute window to diagnose the underlying state and deliver other targeted interventions to increase MAP if necessary.
Study strengths and limitations
This study has several strengths. It involved an intervention of clinical interest in post-cardiac surgery patients.18 It was conducted in a homogeneous sample of patients to minimise confounders. We selected a baseline period that was shorter than previously studied in other clinical trials (eg, 10 min8 or 15 min30) with the aim of taking a real snapshot of the proximate haemodynamic conditions leading to FBT in patients where such parameters change quickly over time. We investigated the pharmacodynamics of 20% albumin 100 mL in an everyday clinical context of haemodynamic instability and free from any other confounding intervention. We did not dictate the decision to give FBT, thus minimising research-dependent selection bias. We measured multiple relevant haemodynamic parameters in great detail, thus providing the first detailed analysis of the haemodynamic effect of 20% albumin.
Our study has some limitations. We designed a single-centre observational study and included a small number of patients. However, we are the first to investigate the haemodynamic effect of 20% albumin in this population and we collected detailed haemodynamic information. Moreover, such detailed monitoring is extremely demanding to execute and, for practical and logistic reasons, cannot be performed in hundreds of patients. We did not use pulse pressure variation or stroke volume variation to trigger FBT. However, we wished to study clinical practice as is currently applied; the application of such dynamic indices in clinical practice remains uncommon4 and of limited clinical value in post cardiac surgical patients,31,32 for whom the FBT challenge remains a common intervention.1,33 Furthermore, the majority of patients had paced cardiac rhythm, which may have limited the heart rate response to the FBT. However, such clinical condition is frequent after cardiac surgery and represents a common limitation in this group of patients, which supports the external validity of our results. We used arbitrary definition for CI and MAP response dissipation. However, we are the first to systematically characterise this phenomenon in these patients with this type of fluid and no consensus exists on this concept. Finally, we did not compare the haemodynamic effect of 20% albumin administration with other fluids; however, we provided the physiologic background for such studies.
Conclusion
In post-cardiac surgery patients treated with a 100 mL 20% albumin bolus according to clinical judgement, only a quarter had a significant CI response. However, this percentage doubled in the following 30 minutes. Similarly, more than half of patients had a significant early MAP response to it, which was dependent on speed of delivery, and often occurred even before the bolus was complete. However, such MAP effect dissipated within about 10 minutes in most responders and was dissociated from the CI response. These findings provide the first detailed pharmacodynamic assessment of the effect of FBT with 100 mL of 20% albumin and inform clinicians considering its use in such patients.
Competing interests
None declared.
Acknowledgements:
We thank all nurses at Austin Hospital for the help and support provided to the investigators for this research.
Supplementary Information

References
- 1.Cecconi M., De Backer D., Antonelli M., et al. Consensus on circulatory shock and hemodynamic monitoring Task force of the European Society of Intensive Care Medicine. Intensive Care Med. 2014;40:1795–1815. doi: 10.1007/s00134-014-3525-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Parke R., McGuinness S., Gilder E., et al. Intravenous fluid use after cardiac surgery: a multicentre, prospective, observational study. Crit Care Resusc. 2014;16:164–169. [PubMed] [Google Scholar]
- 3.Vincent J., De Backer D. Circulatory Shock. N Engl J Med. 2013;369:1726–1734. doi: 10.1056/NEJMra1208943. [DOI] [PubMed] [Google Scholar]
- 4.Cecconi M., Hofer C., Teboul J., et al. Fluid challenges in intensive care: the FENICE study. Intensive Care Med. 2015;41:1529–1537. doi: 10.1007/s00134-015-3850-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Toscani L., Aya H., Antonakaki D., et al. What is the impact of the fluid challenge technique on diagnosis of fluid responsiveness? A systematic review and meta-analysis. Crit Care. 2017;21:207. doi: 10.1186/s13054-017-1796-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Glassford N., Eastwood G., Bellomo R. Physiological changes after fluid bolus therapy in sepsis: a systematic review of contemporary data. Crit Care. 2014;18:696. doi: 10.1186/s13054-014-0696-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bentzer P., Griesdale D., Boyd J., et al. Will this hemodynamically unstable patient respond to a bolus of intravenous fluids? JAMA. 2016;316:1298–1309. doi: 10.1001/jama.2016.12310. [DOI] [PubMed] [Google Scholar]
- 8.Aya H., Ster I., Fletcher N., et al. Pharmacodynamic analysis of a fluid challenge. Crit Care Med. 2016;44:880–891. doi: 10.1097/CCM.0000000000001517. [DOI] [PubMed] [Google Scholar]
- 9.Myburgh J., Finfer S., Bellomo R., et al. Hydroxyethyl starch or saline for fluid resuscitation in intensive care. N Engl J Med. 2012;367:1901–1911. doi: 10.1056/NEJMoa1209759. [DOI] [PubMed] [Google Scholar]
- 10.Perner A., Haase N., Guttormsen A., et al. Hydroxyethyl starch 130/0.42 versus Ringer’s acetate in severe sepsis. N Engl J Med. 2012;367:124–134. doi: 10.1056/NEJMoa1204242. [DOI] [PubMed] [Google Scholar]
- 11.Haase N., Perner A., Hennings L., et al. Hydroxyethyl starch 130/0.38-0.45 versus crystalloid or albumin in patients with sepsis: systematic review with meta-analysis and trial sequential analysis. BMJ. 2013;346 doi: 10.1136/bmj.f839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lewis S., Pritchard M., Evans D., et al. Colloids versus crystalloids for fluid resuscitation in critically ill people. Cochrane Database Syst Rev. 2018;8:CD000567. doi: 10.1002/14651858.CD000567.pub7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.The SAFE Study Investigators Saline or albumin for fluid resuscitation in patients with traumatic brain injury. N Engl J Med. 2007;357:874–884. doi: 10.1056/NEJMoa067514. [DOI] [PubMed] [Google Scholar]
- 14.Mårtensson J., Bihari S., Bannard-Smith J., et al. Small volume resuscitation with 20% albumin in intensive care: physiological effects. Intensive Care Med. 2018;44:1797–1806. doi: 10.1007/s00134-018-5253-2. [DOI] [PubMed] [Google Scholar]
- 15.The SAFE Study Investigators A comparison of albumin and saline for fluid resuscitation in the intensive care unit. N Engl J Med. 2004;350:2247–2256. doi: 10.1056/NEJMoa040232. [DOI] [PubMed] [Google Scholar]
- 16.Bihari S., Wiersema U., Perry R., et al. Efficacy and safety of 20% albumin fluid loading in healthy subjects: a comparison of four resuscitation fluids. J Appl Physiol. 1985;2019(126):1646–1660. doi: 10.1152/japplphysiol.01058.2018. [DOI] [PubMed] [Google Scholar]
- 17.Caironi P., Tognoni G., Masson S., et al. Albumin replacement in patients with severe sepsis or septic shock. N Engl J Med. 2014;370:1412–1421. doi: 10.1056/NEJMoa1305727. [DOI] [PubMed] [Google Scholar]
- 18.Wigmore G., Anstey J., St John A., et al. 20% Human albumin solution fluid bolus administration therapy in patients after cardiac surgery (the HAS FLAIR study) J Cardiothorac Vasc Anesth. 2019;33:2920–2927. doi: 10.1053/j.jvca.2019.03.049. [DOI] [PubMed] [Google Scholar]
- 19.Wong B., Chan M., Glassford N., et al. Mean arterial pressure and mean perfusion pressure deficit in septic acute kidney injury. J Crit Care. 2015;30:975–981. doi: 10.1016/j.jcrc.2015.05.003. [DOI] [PubMed] [Google Scholar]
- 20.Glassford N., Mårtensson J., Eastwood G., et al. Defining the characteristics and expectations of fluid bolus therapy: A worldwide perspective. J Crit Care. 2016;35:126–132. doi: 10.1016/j.jcrc.2016.05.017. [DOI] [PubMed] [Google Scholar]
- 21.Cutuli S, Osawa E, Bellomo R. Fluid bolus of 20% albumin in post-cardiac surgical patient: a prospective observational study of effect duration. Melbourne: Monash University, 2018. https://www.monash.edu/__data/assets/pdf_file/0004/1337935/Protocol-SAP_2018-04-04_Cutuli_Fluid-bolus-of-20-Albumin. pdf (viewed Dec 2019).
- 22.Nunes T.S., Ladeira R.T., Bafi A.T., et al. Duration of hemodynamic effects of crystalloids in patients with circulatory shock after initial resuscitation. Ann Intensive Care. 2014;4:25. doi: 10.1186/s13613-014-0025-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Margarson M.P., Soni N.C. Changes in serum albumin concentration and volume expanding effects following a bolus of albumin 20% in septic patients. Br J Anaesth. 2004;92:821–826. doi: 10.1093/bja/aeh111. [DOI] [PubMed] [Google Scholar]
- 24.Jacob M., Chappell D., Hofmann-Kiefer K., et al. The intravascular volume effect of Ringer’s lactate is below 20%: a prospective study in humans. Crit Care. 2012;16:R86. doi: 10.1186/cc11344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pierrakos C., Velissaris D., Scolletta S., et al. Can changes in arterial pressure be used to detect changes in cardiac index during fluid challenge in patients with septic shock? Intensive Care Med. 2012;38:422–428. doi: 10.1007/s00134-011-2457-0. [DOI] [PubMed] [Google Scholar]
- 26.Le Manach Y., Hofer C., Lehot J., et al. Can changes in arterial pressure be used to detect changes in cardiac output during volume expansion in the perioperative period? Anesthesiology. 2012;117:1165–1174. doi: 10.1097/ALN.0b013e318275561d. [DOI] [PubMed] [Google Scholar]
- 27.Natalini G., Rosano A., Militano C., et al. Prediction of arterial pressure increase after fluid challenge. BMC Anesthesiol. 2012;12:3. doi: 10.1186/1471-2253-12-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Taverna M., Marie A., Mira J., et al. Specific antioxidant properties of human serum albumin. Ann Intensive Care. 2013;3:4. doi: 10.1186/2110-5820-3-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wall O., Ehrenberg L., Joelsson-Alm E., et al. Haemodynamic effects of cold versus warm fluid bolus in healthy volunteers: a randomised crossover trial. Crit Care Resusc. 2018;20:277–284. [PubMed] [Google Scholar]
- 30.Gondos T., Marjanek Z., Ulakcsai Z., et al. Short-term effectiveness of different volume replacement therapies in postoperative hypovolaemic patients. Eur J Anaesthesiol. 2010;27:794–800. doi: 10.1097/EJA.0b013e32833b3504. [DOI] [PubMed] [Google Scholar]
- 31.Suzuki S., Woinarski N., Lipcsey M., et al. Pulse pressure variation-guided fluid therapy after cardiac surgery: a pilot before-and-after trial. J Crit Care. 2014;29:992–996. doi: 10.1016/j.jcrc.2014.07.032. [DOI] [PubMed] [Google Scholar]
- 32.Aneman A., Brechot N., Brodie D., et al. Advances in critical care management of patients undergoing cardiac surgery. Intensive Care Med. 2018;44:799–810. doi: 10.1007/s00134-018-5182-0. [DOI] [PubMed] [Google Scholar]
- 33.Vincent J., Weil M. Fluid challenge revisited. Crit Care Med. 2006;34:1333–1337. doi: 10.1097/01.CCM.0000214677.76535.A5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials