The coronavirus disease 2019 (COVID-19) pandemic caused a health care crisis worldwide.1, 2, 3 Identifying clinical predictors of disease severity and progression is important for resource allocation. Inhalation of nicotine via smoking is associated with acute respiratory distress syndrome (ARDS) adverse outcomes.4 Smokers are more likely to develop severe influenza and have higher mortality rates when affected by other coronaviruses.5, 6 We recently published a case series of 73 critically ill mechanically ventilated patients with COVID-19 and found that less than 2% of these patients were active smokers,7 compared with a 25% rate of active smokers in the Italian population.8 As of 8 May 2020, a total of 130 patients with COVID-19 were admitted to our intensive care units (ICUs) (79 with available smoking status) and we confirmed this low rate of active smokers (3/79, 3.8%).
We then aimed to determine smoking status for the first 410 consecutive patients admitted to the hospital but not to the ICU and obtained smoking status data for 274 patients. Of these, six were active smokers (2.2%).
In addition, we performed an anonymous survey among San Raffaele Scientific Institute health care workers from 16 April to 21 April 2020 to determine the number of active smokers. Of the 1964 health care workers who replied to the survey, 108 were severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)-positive. Active cigarette smoking was present in 12 (11.1%) among 108 SARS-CoV- 2-positive health care workers and in 327 (17.9%) among 1826 SARS-CoV-2-negative workers.
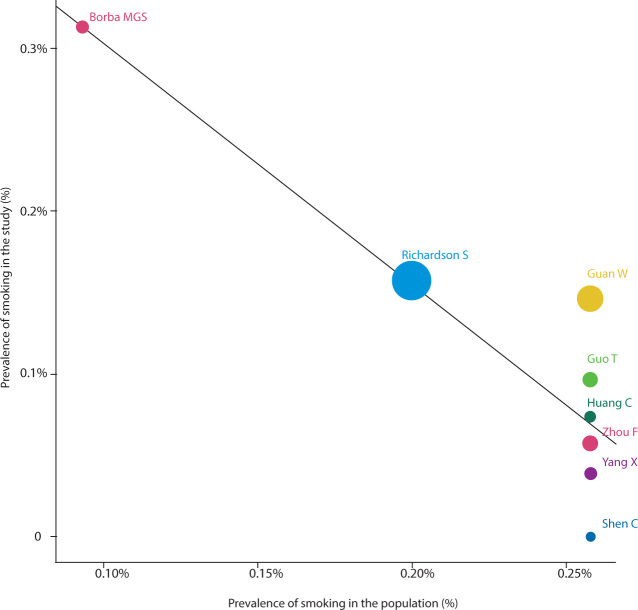
We reviewed all major studies on COVID-19 published in the three general medicine journals with the highest impact factor (The New England Journal of Medicine, The Lancet, JAMA, and their parent journals). We identified 27 articles (Online Appendix), eight of which reported data on active smoking prevalence.9, 10, 11, 12, 13, 14, 15, 16 Overall, the prevalence of active smoking ranged from 0 to 12.6% (Table 1), well below the age-adjusted smoking prevalence per country.17 We performed a meta-analysis of such studies (the methodology for the meta-analysis is available in the Online Appendix) and present data on the prevalence of smoking in hospital and ICU studies of COVID-19 (Online Appendix, supplementary figure 1) as well as the possible effect of smoking on mortality (Online Appendix, supplementary figure 2) with meta-regression for prevalence of smoking in each country. The meta-regression, within the limitations of very few data points, shows a pattern of decreased prevalence of smoking in the study according to the prevalence of smoking in the population (Figure 1). Of note, most of the studies were performed in China, thereby limiting generalisability of the results.
Table 1.
Prevalence of active smoking among patients with coronavirus disease 2019 (COVID-19) in major case series
| First author | Journal | Countries | Study sample size (n) | Patients’ age (years) | Active smokers among patients with COVID-19 (n) | Active smoking prevalence among patients with COVID-19 (%) | 2016 Country smoking age-adjusted prevalence (%)17 |
|---|---|---|---|---|---|---|---|
| Borba MGS9 | JAMA Netw Open | Brazil | 48 | 51 ± 13.9* | 4 | 8.3% | 14% |
| Guan W10 | N Engl J Med | China | 1085 | 47 (35–58)† | 137 | 12.6% | 25.2% |
| Guo T11 | JAMA Cardiol | China | 187 | 58.5 ± 14.66* | 18 | 9.6% | 25.2% |
| Huang C12 | Lancet | China | 41 | 49 (41–58)† | 3 | 7.3% | 25.2% |
| Mehra MR13 | N Engl J Med | USA, Canada, Spain, Italy, UK, Germany, France, Turkey, China, South Korea, Japan | 8910 | 49 ± 16* | 491 | 5.5% | na |
| Shen C14 | JAMA | China | 5 | 36–73‡ | 0 | 0% | 25.2% |
| Yang X15 | Lancet Respir Med | China | 52 | 59.7 ± 13.3* | 2 | 3.8% | 25.2% |
| Zhou F16 | Lancet | China | 191 | 56 (46–67)† | 11 | 5.8% | 25.2% |
na = not applicable; UK = United Kingdom; USA = United States of America.
Data presented as mean ± standard deviation.
Data presented as median (interquartile range).
Data presented as range.
Figure 1.
Meta-regression analysis to assess the impact of the prevalence of smoking in the population on the prevalence of smokers in the studies
Thus, using a large case series from a Western country, we found a low rate of current cigarette smoking in patients who developed COVID-19 requiring ICU or hospitalisation compared with the general population. We then confirmed such findings in a survey of health care workers. Finally, we reconfirmed these findings in a systematic review of manuscripts published in high quality journals.
There are several possible explanations for our findings. First, we cannot exclude selection bias. However, the percentage of smokers versus non-smokers was similar between the general wards and ICU patients. Second, smokers may have been infected at the same rate as non-smokers but died before hospital admission. This seems unlikely. Third, poor collection of medical history details in an emergency situation might have contributed to our findings. This might also apply to other published studies included in our systematic review. However, we have no reason to assume that missing patients would have a different rate of smoking versus non-smoking, and have no reason to believe inaccurate reporting by health care workers.
Others hypothesised a possible role of the nicotinic acetylcholine receptor (nAChR) in the pathophysiology of COVID-19. nAChR may act as a co-receptor for SARS-CoV-2, or might have a role in modulating angiotensin-converting enzyme 2 (ACE2),18 as also suggested for air pollution.19 Indeed, nicotine has been shown to modulate the activity or expression of ACE2 and receptors for angiotensin II in patients with cardiovascular disease,20 and rat models suggest that cigarette smoke decreases the expression of ACE2 in the lungs.21 A similar mechanism of antiviral effect through modulation of ACE2 has been hypothesised for angiotensin II.22, 23
Meta-analyses, however, have suggested that active smoking might be associated with a worse prognosis of COVID-19.24, 25 This might be related to chronic lung and vascular injury induced by smoking, in a disease characterised by a marked pro-thrombotic state and pro-inflammatory state.26, 27 Accordingly, it is possible that smoking may confer some protective factors against development of SARS-CoV-2 infection, but might be associated with worse disease once acquired. However, smoking is also a risk factor for social isolation.28 Therefore, it is possible that people who smoke are simply less exposed to potential infected contacts.
Nevertheless, despite the above findings and given the large level of heterogeneity in smoking-related observations, larger epidemiological studies are needed to confirm these observations and determine the true association between recent nicotine exposure, risk of COVID-19 development, and death.
Acknowledgments
Acknowledgements:
We thank all the personnel of San Raffaele Hospital for the dedication to these patients and for the support in data collection.
Competing interests
None declared.
Supplementary Information

References
- 1.Garcia-Castrillo L., Petrino R., Leach R., et al. European Society For Emergency Medicine position paper on emergency medical systems response to COVID-19. Eur J Emerg Med. 2020;27:174–177. doi: 10.1097/MEJ.0000000000000701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zangrillo A., Beretta L., Silvani P., et al. Fast reshaping of intensive care unit facilities in a large metropolitan hospital in Milan, Italy: facing the COVID-19 pandemic emergency. Crit Care Resusc. 2020 doi: 10.51893/2020.2.pov1. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sartini C., Tresoldi M., Scarpellini P., et al. Respiratory parameters in patients with COVID-19 after using noninvasive ventilation in the prone position outside the intensive care unit. JAMA. 2020;323:2338–2340. doi: 10.1001/jama.2020.7861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tonnesen P., Marott J.L., Nordestgaard B., et al. Secular trends in smoking in relation to prevalent and incident smoking-related disease: a prospective population-based study. Tob Induc Dis. 2019;17:72. doi: 10.18332/tid/112459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Park J.E., Jung S., Kim A. MERS transmission and risk factors: a systematic review. BMC Public Health. 2018;18:574. doi: 10.1186/s12889-018-5484-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Arcavi L., Benowitz N.L. Cigarette smoking and infection. Arch Intern Med. 2004;164:2206–2216. doi: 10.1001/archinte.164.20.2206. [DOI] [PubMed] [Google Scholar]
- 7.Zangrillo A., Beretta L., Scandroglio A.M., et al. COVID-BioB Study Group. Characteristics, treatment, outcomes and cause of death of invasively ventilated patients with COVID-19 ARDS in Milan, Italy. Crit Care Resusc. 2020 doi: 10.1016/S1441-2772(23)00387-3. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Progetto Sorveglianza PASSI. [Abitudine al fumo in Italia 2015–2018.] [Italian] https://www.epicentro.iss.it/passi/dati/fumo (viewed May 2020).
- 9.Borba M.G.S., Val F.F.A., Sampaio V.S., et al. CloroCovid-19 Team. Effect of high vs low doses of chloroquine diphosphate as adjunctive therapy for patients hospitalized with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection: a randomized clinical trial. JAMA Netw Open. 2020;3 doi: 10.1001/jamanetworkopen.2020.8857. [DOI] [PubMed] [Google Scholar]
- 10.Guan W.J., Ni Z.Y., Hu Y., et al. China Medical Treatment Expert Group for COVID-9. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guo T., Fan Y., Chen M., et al. Cardiovascular implications of fatal outcomes of patients with coronavirus disease 2019 (COVID-19) JAMA Cardiol. 2020 doi: 10.1001/jamacardio.2020.1017. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huang C., Wang Y., Li X., et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan. China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mehra M.R., Desai S.S., Kuy S., Henry T.D., Patel A.N. Cardiovascular Disease, Drug Therapy, and Mortality in Covid-19. N Engl J Med. 2020 doi: 10.1056/NEJMoa2007621. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 14.Shen C., Wang Z., Zhao F., et al. Treatment of 5 critically ill patients with COVID-19 with convalescent plasma. JAMA. 2020 doi: 10.1001/jama.2020.4783. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhou F., Yu T., Du R., et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yang X., Yu Y., Xu J., et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. 2020;8:475–481. doi: 10.1016/S2213-2600(20)30079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.World Health Organization World Health Statistics data visualizations dashboard. Tobacco smoking — last updated. 23 Mar 2018. https://apps.who.int/gho/data/node.sdg.3-a-viz?lang=en (viewed May 2020)
- 18.Changeux J.P., Amoura Z., Rey F., Miyara M. A nicotinic hypothesis for COVID-19 with preventive and therapeutic implications [preprint] Qeios. 2020 doi: 10.32388/FXGQSB. [DOI] [PubMed] [Google Scholar]
- 19.Frontera A., Cianfanelli L., Vlachos K., et al. Severe air pollution links to higher mortality in COVID-19 patients: the “double-hit” hypothesis. J Infect. 2020 doi: 10.1016/j.jinf.2020.05.031. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Oakes J.M., Fuchs R.M., Gardner J.D., et al. Nicotine and the renin-angiotensin system. Am J Physiol Regul Integr Comp Physiol. 2018;315:R895–R906. doi: 10.1152/ajpregu.00099.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Han S.X., He G.M., Wang T., et al. Losartan attenuates chronic cigarette smoke exposure-induced pulmonary arterial hypertension in rats: possible involvement of angiotensin-converting enzyme-2. Toxicol Appl Pharmacol. 2010;245:100–107. doi: 10.1016/j.taap.2010.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Busse L.W., Chow J.H., McCurdy M.T., Khanna A.K. COVID-19 and the RAAS-a potential role for angiotensin II? Crit Care. 2020;24:136. doi: 10.1186/s13054-020-02862-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zangrillo A., Landoni G., Beretta L., et al. COVID-BioB Study Group. Angiotensin II infusion in COVID19-associated vasodilatory shock: a case series. Crit Care. 2020;24:227. doi: 10.1186/s13054-020-02928-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhao Q., Meng M., Kumar R., et al. The impact of COPD and smoking history on the severity of COVID-19: a systemic review and meta-analysis. J Med Virol. 2020 doi: 10.1002/jmv.25889. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vardavas C.I., Nikitara K. COVID-19 and smoking: a systematic review of the evidence. Tob Induc Dis. 2020;18:20. doi: 10.18332/tid/119324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ciceri F., Beretta L., Scandroglio A.M., et al. Microvascular COVID-19 lung vessels obstructive thromboinflammatory syndrome (MicroCLOTS): an atypical acute respiratory distress syndrome working hypothesis. Crit Care Resusc. 2020 doi: 10.51893/2020.2.pov2. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cavalli G., De Luca G., Campochiaro C., et al. Interleukin 1 blockade with high dose intravenous anakinra in patients with COVID-19, acute respiratory distress syndrome, and hyper-inflammation: a retrospective cohort study. Lancet. Rheumatol. 2020 doi: 10.1016/S2665-9913(20)30127-2. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nagarajan D., Lee D.A., Robins L.M., Haines T.P. Risk factors for social isolation in post-hospitalized older adults. Arch Gerontol Geriatr. 2020;88 doi: 10.1016/j.archger.2020.104036. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials