Abstract
Background: The NITric oxide during cardiopulmonary bypass (CPB) to improve Recovery in Infants with Congenital heart defects (NITRIC) trial, a 1320-patient, multicentre, randomised controlled trial, is aiming to improve survival free of ventilation after CPB by using nitric oxide delivered into the oxygenator of the CPB.
Objective: To provide a statistical analysis plan before completion of patient recruitment and data monitoring. Final analyses for this study will adhere to this statistical analysis plan, which details all key pre-planned analyses. Stata scripts for analyses have been prepared alongside this statistical analysis plan.
Methods: The statistical analysis plan was designed collaboratively by the chief investigators and trial statistician and builds on the previously published study protocol. All authors remain blinded to treatment allocation. Detail is provided on statistical analyses including cohort description, analysis of primary and secondary outcomes and adverse events. Statistical methods to compare outcomes are planned in detail to ensure methods are verifiable and reproducible.
Results: The statistical analysis plan developed provides the trial outline, list of mock tables, and analysis scripts. The plan describes statistical analyses on cohort and baseline description, primary and secondary outcome analyses, process of care measures, physiological descriptors, and safety and adverse event reporting. We define the pre-specified subgroup analyses and the respective statistical tests used to compare subgroups.
Conclusion: The statistical analysis plan for the NITRIC trial establishes detailed pre-planned analyses alongside Stata scripts to analyse the largest trial in the field of neonatal and paediatric heart surgery. The plan ensures standards for trial analysis validity aiming to minimise bias of analyses.
Trial registration: ACTRN12617000821392
The NITric oxide during cardiopulmonary bypass (CPB) to improve Recovery in Infants with Congenital heart defects (NITRIC) trial is the largest randomised controlled trial currently performed in the field of neonatal and paediatric heart surgery. The primary aim is to investigate whether the delivery of nitric oxide into the CPB circuit during open heart surgery leads to increased ventilator-free days (within 28 days from start of CPB) in infants under 2 years of age.
Congenital heart disease affects approximately one in 100 infants,1 most of whom will require cardiac surgery using CPB during infancy.2, 3 Side effects related to exposure of the patient’s circulation to artificial surfaces during CPB are very common and often contribute to low cardiac output syndrome, where there is failure of the cardiac output to meet the oxygen demands of organs and tissues.4, 5 Low cardiac output syndrome increases the postoperative requirement for organ support, in particular the length of invasive mechanical ventilation, and short and long term morbidity and mortality.6, 7, 8, 9, 10
The NITRIC trial design, which was informed by the encouraging pilot study data,11, 12 tests the hypothesis that nitric oxide during CPB improves ventilator-free days compared with standard care. We describe the pre-planned statistical analysis plan in detail, which has been finalised before the expected completion of patient enrolment by December 2020 and before completion and locking of the study database. The trial statistician and principal investigators wrote this statistical analysis plan and remain blinded to the treatment allocation. Elements of this statistical analysis plan have been previously published in the study protocol.13
Study design and participants
The NITRIC trial is a 1320-patient international, multicentre, randomised, double-blind trial in infants and children less than 2 years of age undergoing open heart surgery on CPB. A total of 1320 patients will be recruited across the six paediatric cardiac centres in Australia, New Zealand and The Netherlands. Eligible patients will be randomly assigned to nitric oxide at 20 ppm administered into the CPB oxygenator for the entire duration of CPB versus standard care (no nitric oxide into the CPB) in a 1:1 ratio with stratification by age (< 6 weeks; ≥ 6 weeks), single ventricle physiology (present or not present) and study site.
The primary hypothesis is to demonstrate that nitric oxide during CPB increases ventilator-free days compared with standard care in eligible infants. The full study protocol has been previously published13 and specified inclusion and exclusion criteria; randomisation and blinding procedures; interventions; study outcomes, including primary and secondary outcomes; process of care measures and physiological descriptors; adverse events; safety data monitoring; sample size; and data collection and management.
The study protocol has been approved by the Children’s Health Queensland Human Research Ethics Committee (HREC/17/QRCH/43; original submission approved on 6 March 2017). Minor modifications to the original study protocol were reviewed and approved by the HREC and are provided in the Online Appendix (table S1). This statistical analysis plan is based on version 1.4 of the study protocol.
Sample size
Pilot study data demonstrated a 66-hour (standard deviation [SD], 0.33) reduction in ventilator-free days associated with the study intervention.11 Assuming a minimally clinically significant small effect size (SD, 0.2), 90% power, two-sided α level of significance of 5%, 10% withdrawals, and 15% increase in sample size to account for a non-normal distribution of ventilator-free days, 1320 patients are required (660 per group).
Randomisation
Randomisation is conducted online through the purpose-built REDCap electronic data capture tool hosted at the University of Queensland;14, 15 the randomisation module can only be accessed by the central study coordinator and the site perfusionist, and only after a patient has been screened, is deemed to have met eligibility criteria, and has provided informed consent. A randomisation sequence using variable block randomisation with a 1:1 ratio was generated and loaded into a REDCap database before screening of the first patient. Randomisation is stratified on age (< 6 weeks, ≥ 6 weeks), cardiac physiology (univentricular, biventricular) and study site. The precise time of randomisation occurs variably before starting CPB dependent on site logistics, and the intervention begins with start of CPB.
Outcome measures
The definition and detail of calculation of outcome measures can be found in the Online Appendix (section S2), alongside the Stata scripts written to calculate these outcomes (available on GitHub16). The primary outcome is ventilator-free days within 28 days from the start of CPB. The secondary outcomes are:
-
•
individual ventilator-free days components (ie, duration of mechanical ventilation, death within 28 days after CPB start);
-
•
individual and components of composite measure of low cardiac output syndrome, and/or extracorporeal life support within 48 hours after CPB start and/or death within 28 days after CPB start;
-
•
length of paediatric intensive care unit (PICU) stay after CPB start;
-
•
length of hospital stay after CPB start;
-
•process of care measures:
-
►treatment with extracorporeal life support within 48 hours after CPB start;
-
►duration of postoperative time spent with open chest, including unplanned chest reopening after CPB start;
-
►treatment and duration of treatment using inhalational nitric oxide postoperatively after CPB start;
-
►treatment and duration of treatment of postoperative renal replacement therapy (includes peritoneal and continuous venous-venous haemodialysis) after CPB start;
-
►
-
•physiological descriptors:
-
►postoperative troponin levels during the first 24 hours after the operation;
-
►severity and duration of postoperative organ dysfunction; and
-
►postoperative acute kidney injury and serum creatinine levels measured during the first 24 hours.
-
►
For the purposes of analysis and reporting, low cardiac output syndrome within 48 hours after CPB start, extracorporeal life support within 48 hours after CPB start, death within 28 days after CPB start, and length of stay in PICU and hospital are classified as potentially patient-important secondary outcomes. The remaining secondary outcomes will be reported to describe patient physiology and processes of care only.
Data monitoring
Data monitoring is being undertaken throughout the trial, based on a data monitoring and auditing plan (DMAP) devised by the study team. The DMAP was developed in accordance with the International Council for Harmonisation E6 (R2) Good Clinical Practice Guideline17 and reflects current best practice for data monitoring practices in investigator-initiated trials. The DMAP includes the following components:
-
•
data verification on all screening data items (ie, inclusion and exclusion criteria) on a random sample of 20 ineligible patients (or all patients if < 20 ineligible patients) from each site;
-
•
data verification on all screening data items (ie, inclusion and exclusion criteria) for every enrolled patient;
-
•
data verification on the stratification used for randomisation and consent data items and data items related to calculation of the primary outcome and secondary outcomes for every enrolled patient; and
-
•
data verification on key data items relating to cohort descriptors on a random sample of 10% of enrolled patients from each site.
The original study REDCap database was enhanced to facilitate the DMAP. Each Australian and New Zealand site is being monitored by a research coordinator from a different trial site. This is being undertaken through a combination of on-site monitoring and remote monitoring using the institutional program to share desktop computer screens. All sites except one have the required information for physiological data, treatment, outcomes, laboratory values, and patient notes on an electronic health record. One site has parts of the patient data on paper, which requires digital scanning for review. During the coronavirus disease 2019 (COVID-19) pandemic, sites are only allowing remote monitoring. Each relevant data item is verified individually by comparing the entered value with the value in the source documentation. Where discrepancies are found, the site research coordinator and monitor meet to discuss and resolve the discrepancies. Once data monitoring is finalised, the patient’s REDCap data entry record is locked in preparation for analysis. The site in The Netherlands is being monitored on-site by an external monitoring company, as remote monitoring is not permitted in the country; during the COVID-19 pandemic, monitoring was paused.
Statistical analysis
Statistical analysis principles
-
•
Analyses will be conducted based on the intention-to-treat principle. Specifically, patients who are eligible, who do not meet any exclusion criteria and who undergo randomisation will be analysed based on the treatment group they were allocated to, independent of the compliance with the treatment delivered.
-
•
Participants who had more than one surgery before their second birthday will only have data analysed related to their first surgery for which study consent was available.
-
•
Statistical tests will be two-sided applying a statistical significance level of 0.05. As we are not correcting for multiplicity when comparing secondary or other outcomes, such results will be considered exploratory and will be reported as point estimates with 95% confidence intervals (CIs).
-
•
If there are missing data for the primary outcome measure for any participants, imputation methods will be used.
-
•
Continuous variables will be assessed for normality; this will be undertaken using visual inspection of histograms and Q-Q plots.
-
•
Standard descriptive statistics will be used when summarising variables; frequencies (percentages) for discrete variables, mean and SD for continuous variables, or, if continuous variables are non-normally distributed, median with interquartile range.
-
•
This analysis plan and the primary manuscript will only include analyses up to 28 days. We will present analyses of postoperative delirium, health care costs, inflammatory markers and long term outcomes (12 months and later after procedure) separately.
-
•
Pre-planned subgroup analyses will be performed including the pre-defined study strata; these will be executed regardless of any potential treatment effect on the primary or secondary outcomes in the main cohort.
-
•
To ensure transparency and reproducibility, the Stata code that will be used to analyse the final study data is available on GitHub.16
-
•
The trial statistician will be blinded to the treatment group until the analyses outlined in this statistical analysis plan have been completed.
-
•
Changes in the analysis plan by the investigators effective after publication of this statistical analysis plan will be declared as such.
Interim analyses
Two pre-planned interim analyses were performed after the primary outcome measure was finalised for 660 and 1000 patients respectively. Blinded interim analyses that detailed the primary outcome between treatment groups as well as information on recruitment and adverse events were presented to the Data and Safety Monitoring Board for their consideration. The Haybittle–Peto rule was applied (ie, a significant P < 0.001 was deemed necessary to warrant consideration of stopping the study early for benefit).18, 19 The type I error for the final study analyses has not been adjusted to allow for interim analyses.
Datasets analysed
The Consolidated Standards of Reporting Trials (CONSORT) flow diagram will be presented based on all patients who were screened for the study. All other analyses will be performed on eligible patients who underwent randomisation; that is, the intention-to-treat population independent of compliance with the protocol. If consent is not obtained or is withdrawn, data will be excluded from the analyses, unless withdrawn patients permitted the use of data up to the point of withdrawal. The primary dataset for analysis will include baseline variables, surgical data, outcomes, adverse events and protocol deviations. Following completion of the data monitoring process, data and associated data transformation code will be extracted from the study REDCap database in a Stata (StataCorp, College Station, Texas) format.
Trial profile and overview
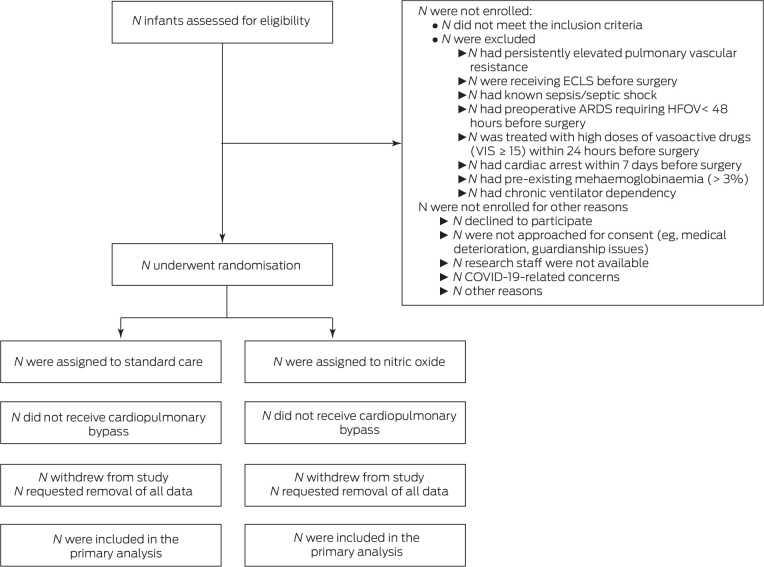
Recruitment of patients into the trial will be represented using a flow chart based on the CONSORT guidelines20 (Figure 1). This will describe screened patients, those meeting exclusion criteria, eligible patients, consent process, and those randomised into each of the study arms, with the documentation of the respective primary outcome. We will report on the start and stop date of the trial and provide the recruitment graph by month including division into the contributing sites.
Figure 1.
Draft CONSORT participant flow diagram
ARDS = Acute respiratory distress syndrome; COVID-19 = coronavirus disease 2019; ECLS = extracorporeal life support; HFOV = high frequency oscillatory ventilation; VIS = Vasoactive-Inotropic Score.
Patient baseline characteristics
Baseline characteristics at time of randomisation will be reported for each of the two treatment groups (statistical comparison between groups will not be undertaken) (Table 1).
Table 1.
Baseline characteristics of infants enrolled in the NITric oxide during cardiopulmonary bypass to improve Recovery in Infants with Congenital heart defects (NITRIC) trial
| Characteristic | Standard care N = | Nitric oxide N = |
|---|---|---|
| Age at randomisation (weeks),⁎ median (IQR) | ||
| < 6 weeks, n (%) | ||
| ≥ 6 weeks, n (%) | ||
| Weight (kg), median (IQR) | ||
| Female sex, n (%) | ||
| Ethnicity, n (%) | ||
| Caucasian | ||
| Aboriginal/Torres Strait Islander | ||
| Asian | ||
| Mãori/Pacific Islander | ||
| Mixed/other | ||
| Congenital heart disease, n (%) | ||
| Univentricular⁎ | ||
| Biventricular⁎ | ||
| History of previous cardiac surgery on CPB | ||
| Right-sided obstructive lesions | ||
| Tetralogy of Fallot | ||
| Double outlet right ventricle | ||
| Pulmonary stenosis/atresia | ||
| Tricuspid stenosis/atresia | ||
| Other | ||
| Left-sided obstructive lesions | ||
| Coarctation | ||
| Interrupted aortic arch | ||
| Hypoplastic aortic arch | ||
| HLHS | ||
| Other | ||
| Shunt lesions | ||
| ASD | ||
| VSD | ||
| AVSD | ||
| Truncus | ||
| TGA | ||
| Persistent ductus arteriosus | ||
| Other | ||
| Various lesions | ||
| Double-inlet left ventricle | ||
| TAPVD | ||
| Other | ||
| Current ICU admission and treatments before heart surgery | ||
| Intensive care admission, n (%) | ||
| Duration of ICU stay (days), median (IQR) | ||
| Treatments | ||
| Invasive ventilation, n (%) | ||
| Duration of invasive ventilation (days), median (IQR) | ||
| Tracheostomy, n (%) | ||
| Inotropes, n (%) | ||
| Prostaglandin, n (%) | ||
| Steroids, n (%) | ||
| Afterload reducing agents, n (%) | ||
| Inhaled nitric oxide, n (%) | ||
| Sildenafil, n (%) | ||
| Comorbidities, n (%) | ||
| POPC | ||
| Good/normal | ||
| Functionally normal | ||
| Mild overall disability | ||
| Moderate overall disability | ||
| Severe overall disability | ||
| Coma/vegetative state | ||
| Brain death | ||
| Unknown | ||
| Congenital syndrome | ||
| Trisomy 21 | ||
| 22q11 | ||
| Turner | ||
| Noonan | ||
| VACTERL | ||
| CHARGE | ||
| Other syndrome | ||
| Country of hospital, n (%) | ||
| Australia | ||
| New Zealand | ||
| The Netherlands | ||
| Surgical complexity | ||
| RACHS score, median (IQR) | ||
| RACHS-1, n (%) | ||
| RACHS-2, n (%) | ||
| RACHS-3, n (%) | ||
| RACHS-4, n (%) | ||
| RACHS-5, n (%) | ||
| RACHS-6, n (%) | ||
| Surgical procedure, n (%) | ||
| Tetralogy repair | ||
| Norwood procedure | ||
| Bicavopulmonary shunt | ||
| Right ventricular to pulmonary artery shunt/conduit | ||
| Fontan completion | ||
| Arterial switch operation | ||
| ASD repair | ||
| VSD repair | ||
| AVSD repair | ||
| Aortic arch repair | ||
| Coarctation repair | ||
| Truncus repair | ||
| Pulmonary artery band | ||
| Ross procedure | ||
| Left ventricular outflow tract surgery | ||
| Right ventricular outflow tract surgery | ||
| Valve surgery | ||
| Valve repair | ||
| Anomalous pulmonary vein repair | ||
| Heart transplant | ||
| Other |
ASD = atrial septal defect; AVSD = atrioventricular septal defect; CHARGE = coloboma, heart defects, atresia choanae (also known as choanal atresia), growth retardation, genital abnormalities, and ear abnormalities; CPB = cardiopulmonary bypass; HLHS = hypoplastic left heart syndrome; ICU = intensive care unit; IQR = interquartile range; POPC = Pediatric Overall Performance Category; RACHS = Risk Adjustment for Congenital Heart Surgery; TAPVD = total anomalous pulmonary venous drainage; TGA = transposition of the great arteries; VACTERL = vertebral defects, anal atresia, cardiac defects, tracheoesophageal fistula, renal anomalies, and limb abnormalities; VSD = ventricular septal defect.
Used for stratification.
Surgical procedure and intervention characteristics
We will provide details on the surgical procedure by treatment group, including concomitant therapies (Table 2). We will compare characteristics of the procedure that in principle may be altered by the intervention (eg, duration of CPB, duration of aortic cross-clamp) between the two study arms by presenting descriptive statistics along with estimate of difference and 95% CI.
Table 2.
Surgical and perioperative characteristics of infants enrolled in the NITric oxide during cardiopulmonary bypass to improve Recovery in Infants with Congenital heart defects (NITRIC) trial
| Characteristics | Nitric oxide N = | Standard care N = | Difference (95% CI) |
|---|---|---|---|
| Cardiopulmonary bypass characteristics | |||
| Blood prime, n (%) | |||
| CPB duration (min), median (IQR) | |||
| CPB < 60 min, n (%) | |||
| CPB ≥ 60 min, n (%) | |||
| Cross-clamp, n (%) | |||
| Cross-clamp (min), median (IQR) | |||
| Number of CPB runs, n (%) | |||
| 1 | |||
| 2 | |||
| 3 | |||
| ≥ 4 | |||
| Deep hypothermic arrest, n (%) | |||
| Duration of deep hypothermic arrest (min), median (IQR) | |||
| Antegrade cerebral perfusion, n (%) | |||
| Modified ultrafiltration used, n (%) | |||
| Slow continuous ultrafiltration used, n (%) | |||
| Blood products received in theatre, median (IQR) | |||
| Red blood cells (mL/kg) | |||
| Whole blood (mL/kg) | |||
| Platelets (mL/kg) | |||
| Fresh frozen plasma (mL/kg) | |||
| Cryoprecipitate (mL/kg) | |||
| Drug treatments received in theatre, n (%) | |||
| Intravenous steroids | |||
| Inhaled NO | |||
| Administration of study drug (NO) | |||
| Time from start of CPB to start of NO (min), mean (SD)/median (IQR) | |||
| Duration of NO on CPB (min), median (IQR) | |||
| Proportion of time spent on CPB with NO,⁎ mean (SD)/median (IQR) | |||
| Change in methaemoglobin level (%) between start and post-CPB, mean (SD)/median (IQR)† | |||
| Change in methaemoglobin level (%) between start and post-CPB > 3%,†n (%) |
CPB = cardiopulmonary bypass; IQR = interquartile range; NO = nitric oxide; SD = standard deviation.
If multiple CPB runs during the same surgery, summarising every CPB run individually.
Calculated as post-CPB methaemoglobin/pre-CPB methaemoglobin.
We will report on the compliance with the study drug (nitric oxide) using a number of measures, reported for patients randomised to the intervention group only (Table 2). In addition, we will list protocol deviations relating to the administration of nitric oxide in supplementary material.
Outcome measures analysis
Primary outcome measure
The primary outcome measure (ventilator-free days censored at 28 days after CPB start) will be analysed using a Mann–Whitney test, with differences between medians calculated by quantile regression using the simplex algorithm, inclusive of stratification variables (age group and physiology) and site included as fixed effects in the model. The effect estimate, corresponding 95% CI and P value will be presented. The unadjusted P value using a Mann–Whitney test will also be presented (Table 3).
Table 3.
Primary and secondary outcomes per intention-to-treat analysis
| Outcome | Standard care N = | Nitric oxide N = | Unadjusted P | Estimate of difference (95% CI) | Adjusted P |
|---|---|---|---|---|---|
| Primary outcome | |||||
| Ventilator-free days, median (IQR) | |||||
| Secondary outcomes | |||||
| Duration of invasive ventilation (days), median (IQR) | |||||
| Low cardiac output syndrome (need for ECLS, or death), n (%) | |||||
| Low cardiac output syndrome | |||||
| ECLS | |||||
| Death | |||||
| Length of stay in PICU (days), median (IQR) | |||||
| Length of stay in hospital (days), median (IQR) | |||||
| Process of care measures | |||||
| Duration of time with open chest after the operation (hours), median (IQR) | |||||
| Treated with inhaled NO after the operation, n (%) | |||||
|
|||||
| Physiological descriptors | |||||
| Organ dysfunction after the operation (PELOD-2 score), median (IQR) | |||||
|
|||||
| Troponin after the operation, median (IQR) | |||||
|
|||||
| Creatinine, mean (SD) | |||||
|
|||||
| AKI: PICU admission, n (%) | |||||
|
|||||
| AKI: 24 hours, n (%) | |||||
|
|||||
| AKI: 48 hours, n (%) | |||||
|
|||||
AKI = acute kidney injury; ECLS = extracorporeal life support; IQR = interquartile range; NO = nitric oxide; PELOD-2 = Paediatric Logistic Organ Dysfunction-2; PICU = paediatric intensive care unit; SD = standard deviation.
Secondary outcome measures
For binary outcome measures (eg, low cardiac output syndrome), logistic regression analyses will be used including age group and physiology as fixed effects and site as a random effect, with unadjusted and adjusted odds ratios and 95% CIs reported (Table 3). Survival outcomes (length of PICU stay, length of hospital stay) will be visually presented using a Kaplan–Meier plot, and a Cox proportional hazard model will be used to assess differences between treatment groups, with treatment group and stratification variables as fixed effects and site as a random effect (ie, using a shared frailty model). The hazard ratio and 95% CI will be presented as an estimate of treatment effect. Continuous outcomes (eg, Paediatric Logistic Organ Dysfunction-2 [PELOD-2] score, duration of renal replacement therapy) will be analysed sing linear regression adjusting for age group and physiology as fixed effects and site as a random effect, with mean difference and 95% CI reported. If the residuals demonstrate non-normality, quantile regression will instead be used in the same manner as for the primary outcome. Key assumptions of the models will be tested and reported on for logistic regression (specification, goodness-of-fit, absence of multicollinearity and absence of influential observations), for survival analysis (proportionality assumption and goodness-of-fit), and for linear regression (specification, distribution of residuals, homoscedasticity, absence of multicollinearity, and linearity).
Safety outcomes
Adverse drug reactions considered to be possibly, probably or definitively related to the study treatment based on the judgement of the treating local physician will be reported in the supplementary material. Additionally, the proportion of patients with at least one adverse event will be compared between the two treatment groups using logistic regression as described above for secondary outcomes.
Subgroup and sensitivity analyses
We will undertake two subgroup analyses that were pre-defined in the study protocol: age (< 6 weeks v ≥ 6 weeks) and physiology (univentricular v biventricular).
Subgroup analyses will be undertaken using the same analysis methods described for the primary and secondary outcome measures, with the addition of the subgroup variable and its related interaction term into the main regression model. For each subgroup, the relevant descriptive statistics will be presented for the primary and secondary outcomes, along with the appropriate measure of effect size (and 95% CI) and interaction effect (and 95% CI and P value). A Forest plot will be developed to present heterogeneity between the treatment group and subgroup variable, including the P value, and presented as a supplementary figure.
In addition, a sensitivity analysis for study outcomes will be undertaken including the variables treatment group, duration of CPB, surgical complexity (recorded using the Risk Adjustment for Congenital Heart Surgery [RACHS] score), blood prime use during surgery, sex, and strata variables as fixed effects and site as a random effect. Results will be presented in the same manner as primary analyses and included in the supplementary material.
Treatment of missing data
Missing data will be imputed for the primary outcome measure for any patients missing one or more components required to calculate ventilator-free days. Fully conditional specification will be used for imputation; the imputation model will include randomised treatment arm, study site and the two study strata variables. Ten sets of imputed data will be created using the methods described for the primary outcome. A pooled common effect estimate and 95% CI will be generated from the imputed datasets.
Conclusion
Cardiac surgery with CPB is associated with ischaemia-reperfusion injury and systemic inflammatory response in all infants and children. In two small, single centre pilot studies in children, administration of nitric oxide during the CPB has improved patient outcomes; the NITRIC trial is adequately powered to definitively assess the efficacy of nitric oxide during cardiopulmonary bypass and whether there are any specific groups of patients that may benefit. Application of this statistical analysis plan to the NITRIC trial will facilitate high quality, best-practice evaluation of the clinical data and will allow translation of the results into clinical practice.
Competing interests
Yves d’Udekem receives consultancy fees from Actelion (Janssen) and travel fees from Berlin Heart.
Acknowledgements
This work was supported by grants from the Heart Kids Foundation, Australia; the Children’s Health Foundation, Australia; and by a grant from the National Health and Medical Research Council (NHMRC), Australia (GNT1140322).
Yves d’Udekem and Luregn Schlapbach hold NHMRC Clinical Practitioner Fellowships and Andreas Schibler holds a Medical Research Future Fund Practitioner Fellowship. Simon Erickson is supported by a grant from the Perth Children’s Hospital Foundation. The Victorian Government’s Operational Infrastructure Support Program supported this research project. Paul Young holds a Clinical Practitioner Fellowship from the Health Research Council of New Zealand. The funding sources had no involvement in study design, analyses, or interpretation of the results.
Mallinckrodt Pharmaceuticals is providing nitric oxide delivery devices to Australian and New Zealand study sites, and The Netherlands site has a NO-A nitric oxide delivery system on loan from EKU Elektronik during the study period. Neither company have any involvement in study design, conduct or analyses.
Author contribution statement
The statistical analysis plan first draft was designed by KG, LJS and AS. KG reviewed all sections on statistical analyses. SH, DL, JB, SE, MF, YdU, NA, DW, KJ, CD, KvL, BG, JF, PY, AB, MJ and WB reviewed the manuscript and approved the final version. KG prepared the final manuscript which was reviewed and approved by all authors.
Supplementary Information

References
- 1.Triedman J.K., Newburger J.W. Trends in congenital heart disease: the next decade. Circulation. 2016;133:2716–2733. doi: 10.1161/CIRCULATIONAHA.116.023544. [DOI] [PubMed] [Google Scholar]
- 2.Marelli A.J., Ionescu-Ittu R., Mackie A.S., et al. Lifetime prevalence of congenital heart disease in the general population from 2000 to 2010. Circulation. 2014;130:749–756. doi: 10.1161/CIRCULATIONAHA.113.008396. [DOI] [PubMed] [Google Scholar]
- 3.Marelli A.J., Mackie A.S., Ionescu-Ittu R., et al. Congenital heart disease in the general population: changing prevalence and age distribution. Circulation. 2007;115:163–172. doi: 10.1161/CIRCULATIONAHA.106.627224. [DOI] [PubMed] [Google Scholar]
- 4.Zahler S., Massoudy P., Hartl H., et al. Acute cardiac inflammatory responses to postischemic reperfusion during cardiopulmonary bypass. Cardiovasc Res. 1999;41:722–730. doi: 10.1016/s0008-6363(98)00229-6. [DOI] [PubMed] [Google Scholar]
- 5.McElhinney D.B., Wernovsky G. Outcomes of neonates with congenital heart disease. Curr Opin Pediatr. 2001;13:104–110. doi: 10.1097/00008480-200104000-00003. [DOI] [PubMed] [Google Scholar]
- 6.Ballweg J.A., Wernovsky G., Gaynor J.W. Neurodevelopmental outcomes following congenital heart surgery. Pediatr Cardiol. 2007;28:126–133. doi: 10.1007/s00246-006-1450-9. [DOI] [PubMed] [Google Scholar]
- 7.Marino B.S. New concepts in predicting, evaluating, and managing neurodevelopmental outcomes in children with congenital heart disease. Curr Opin Pediatr. 2013;25:574–584. doi: 10.1097/MOP.0b013e328365342e. [DOI] [PubMed] [Google Scholar]
- 8.Marino B.S., Lipkin P.H., Newburger J.W., et al. Neurodevelopmental outcomes in children with congenital heart disease: evaluation and management: a scientific statement from the American Heart Association. Circulation. 2012;126:1143–1172. doi: 10.1161/CIR.0b013e318265ee8a. [DOI] [PubMed] [Google Scholar]
- 9.Brown M.D., Wernovsky G., Mussatto K.A., Berger S. Long-term and developmental outcomes of children with complex congenital heart disease. Clin Perinatol. 2005;32(1043-57):xi. doi: 10.1016/j.clp.2005.09.008. [DOI] [PubMed] [Google Scholar]
- 10.Kaltman J.R., Andropoulos D.B., Checchia P.A., et al. Report of the pediatric heart network and national heart, lung, and blood institute working group on the perioperative management of congenital heart disease. Circulation. 2010;121:2766–2772. doi: 10.1161/CIRCULATIONAHA.109.913129. [DOI] [PubMed] [Google Scholar]
- 11.James C., Millar J., Horton S., et al. Nitric oxide administration during paediatric cardiopulmonary bypass: a randomised controlled trial. Intensive Care Med. 2016;42:1744–1752. doi: 10.1007/s00134-016-4420-6. [DOI] [PubMed] [Google Scholar]
- 12.Checchia P.A., Bronicki R.A., Muenzer J.T., et al. Nitric oxide delivery during cardiopulmonary bypass reduces postoperative morbidity in children — a randomized trial. J Thorac Cardiovasc Surg. 2013;146:530–536. doi: 10.1016/j.jtcvs.2012.09.100. [DOI] [PubMed] [Google Scholar]
- 13.Schlapbach L.J., Horton S.B., Long D.A., et al. Study protocol: NITric oxide during cardiopulmonary bypass to improve Recovery in Infants with Congenital heart defects (NITRIC trial): a randomised controlled trial. BMJ Open. 2019;9 doi: 10.1136/bmjopen-2018-026664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Harris P.A., Taylor R., Minor B.L., et al. The REDCap consortium: building an international community of software platform partners. J Biomed Inform. 2019;95 doi: 10.1016/j.jbi.2019.103208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Harris P.A., Taylor R., Thielke R., et al. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gibbons K.S. NITric oxide during cardiopulmonary bypass to improve Recovery in Infants with Congenital heart defects (NITRIC) trial: Statistical Analysis Code. GitHub. 2020 doi: 10.51893/2021.1.OA4. https://github.com/kgibbons44/NITRICAnalysis/ (viewed Dec 2020) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.ICH harmonised guideline: integrated addendum to ICH E6 (R1): guideline for good clinical practice E6 (R2) 2016. International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use. [Google Scholar]
- 18.Haybittle J.L. Repeated assessment of results in clinical trials of cancer treatment. Br J Radiol. 1971;44:793–797. doi: 10.1259/0007-1285-44-526-793. [DOI] [PubMed] [Google Scholar]
- 19.Peto R., Pike M.C., Armitage P., et al. Design and analysis of randomized clinical trials requiring prolonged observation of each patient. I. Introduction and design. Br J Cancer. 1976;34:585–612. doi: 10.1038/bjc.1976.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schulz K.F., Altman D.G., Moher D., Group C CONSORT 2010 statement: updated guidelines for reporting parallel group randomised trials. BMJ. 2010;340 doi: 10.4103/0976-500X.72352. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials