Abstract
Objective: Describe characteristics, daily care and outcomes of patients with coronavirus disease 2019 (COVID-19) acute respiratory distress syndrome (ARDS).
Design: Case series of 73 patients.
Setting: Large tertiary hospital in Milan.
Participants: Mechanically ventilated patients with confirmed COVID-19 admitted to the intensive care unit (ICU) between 20 February and 2 April 2020.
Main outcome measures: Demographic and daily clinical data were collected to identify predictors of early mortality.
Results: Of the 73 patients included in the study, most were male (83.6%), the median age was 61 years (interquartile range [IQR], 54–69 years), and hypertension affected 52.9% of patients. Lymphocytopenia (median, 0.77 × 103 per mm3; IQR, 0.58–1.00 × 103 per mm3), hyperinflammation with C-reactive protein (median, 184.5 mg/dL; IQR, 108.2–269.1 mg/dL) and pro-coagulant status with D-dimer (median, 10.1 μg/m; IQR, 5.0–23.8 μg/m) were present. Median tidal volume was 6.7 mL/kg (IQR, 6.0–7.5 mL/kg), and median positive end-expiratory pressure was 12 cmH2O (IQR, 10–14 cmH2O). In the first 3 days, prone positioning (12–16 h) was used in 63.8% of patients and extracorporeal membrane oxygenation in five patients (6.8%). After a median follow-up of 19.0 days (IQR, 15.0–27.0 days), 17 patients (23.3%) had died, 23 (31.5%) had been discharged from the ICU, and 33 (45.2%) were receiving invasive mechanical ventilation in the ICU. Older age (odds ratio [OR], 1.12; 95% CI, 1.04–1.22; P = 0.004) and hypertension (OR, 6.15; 95% CI, 1.75–29.11; P = 0.009) were associated with mortality, while early improvement in arterial partial pressure of oxygen (Pao2) to fraction of inspired oxygen (Fio2) ratio was associated with being discharged alive from the ICU (P = 0.002 for interaction).
Conclusions: Despite multiple advanced critical care interventions, COVID-19 ARDS was associated with prolonged ventilation and high short term mortality. Older age and pre-admission hypertension were key mortality risk factors.
Trial registration:ClinicalTrials.gov identifier: NCT04318366
In December 2019, several cases of atypical pneumonia caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) were reported in Wuhan, China.1, 2, 3 This novel virus has now become responsible for a pandemic (coronavirus disease 2019 [COVID-19]). On 20 February 2020, the first cluster of cases was detected near Milan, Italy. The number of cases increased rapidly and dramatically. As of 21 April 2020, a total of 183 957 patients had been infected and 24 648 (13.4%) have died in Italy.
In most cases, COVID-19 is a self-limited lower respiratory tract illness. However, in some patients, it may cause acute respiratory distress syndrome (ARDS), shock, myocardial injury, acute kidney injury, and multi-organ failure. The absolute number of patients requiring invasive mechanical ventilation can quickly overwhelm an unprepared health care system, as seen in Europe and the United States.4, 5, 6
At this time, three studies from Western countries have reported data on COVID-19- induced critical illness.4, 5, 6 Published single-centre studies from China have reported mortality rates of 81% and 97% in patients who develop critical illness,7, 8, 9 while case series from the US have reported mortality rates greater than 60%,4, 5 and a case series from Italy reported a mortality rate of 26%.6 However, none have specifically focused on invasively ventilated patients with COVID-19 ARDS. In addition, the largest case series published to date did not report the outcome of patients receiving mechanical ventilation specifically,6 there is no daily information on ventilatory variables, vital signs and laboratory tests, and no information on duration of follow-up and cause of death.
The above dismal findings, if confirmed in patients with COVD-19 ARDS, may lead to much tighter restrictions for ICU admission and mechanical ventilation. Therefore, larger and up-to-date clinical data from Western health care systems are essential for planning, prognosis, treatment, resource allocation, and trial development. Accordingly, we obtained data on the clinical characteristics, treatment, and outcomes of patients with COVID-19 ARDS admitted to a referral hospital in Milan, Italy.
Methods
Study oversight and design
The COVID-BioB study is an observational investigation performed at Istituto di Ricovero e Cura a Carattere Scientifico (IRCCS) San Raffaele Scientific Institute — a 1350-bed university hospital in Milan, Italy. The study was approved by the hospital ethics committee (protocol No. 34/int/2020) and was registered on ClinicalTrials.gov (NCT04318366). All the authors reviewed the manuscript and vouch for the accuracy and completeness of the data and adherence to the study protocol (Online Appendix).
Enrolment criteria
We included all patients aged 18 years or over admitted to an ICU at IRCCS San Raffaele Scientific Institute with confirmed SARS-CoV-2 infection. Confirmed infection was defined as positive real-time reverse-transcriptase polymerase chain reaction from a nasal and/or throat swab together with signs, symptoms and radiological findings suggestive of COVID-19 pneumonia.
Patient management
Reorganisation of our institution to face the COVID-19 pandemic has been recently described (Online Appendix).10 Briefly, our hospital rapidly organised a separate pathway for emergency department (ED) patients with influenza-like respiratory symptoms. All were deemed to have COVID-19. The ED could admit up to 70 patients requiring oxygen therapy or non-invasive ventilation. Four ventilators were available for patients requiring immediate invasive mechanical ventilation. After initial triage, patients were discharged home or admitted to the ward or directly to the ICU.
ICU capacity was 32 beds on 24 February 2020 (four ICUs). The number of ICUs progressively increased to eight, with 74 beds as of 30 March 2020, and 56 beds dedicated to patients with COVID-19 (Online Appendix, figures S9 and S10). In addition, the total number of non-ICU COVID-19 beds progressively increased to 270. Elective surgical activity was rapidly reduced and then stopped.
ICUs were managed by anaesthetists/intensivists, while a multidisciplinary team of infectious diseases and internal medicine specialists managed the general wards, supported by medical emergency teams providing intensive care support for patients requiring non-invasive ventilation or deteriorating on the ward.11
The following general management principles were applied to all patients with COVID-19:
-
•
aim for a negative fluid balance in the first few days from admission;
-
•
deliver oxygen therapy whenever necessary; and
-
•
deliver potential antiviral treatment with hydroxychloroquine in all patients and darunavir–cobicistat or remdesivir in selected patients.
Lopinavir–ritonavir was routinely used until the publication of a negative randomised trial.12 In addition, we used immunosuppressive therapy with anakinra, tocilizumab, sarilumab, mavrilimumab, reparixin, or high dose steroids in patients who displayed a hyperinflammatory laboratory profile (serum C-reactive protein level > 15 × the reference interval, or ferritin level > 2.5 × the reference interval).
General ward patients could receive non-invasive ventilation, usually continuous positive airway pressure and were treated with prone positioning while receiving non-invasive ventilation in selected cases.
For mechanically ventilated patients in the ICU, we adopted current recommendations for mechanical ventilation in patients with ARDS. We initially administered deep venous thrombosis prophylaxis with subcutaneous enoxaparin unless specific indications for therapeutic anticoagulation were present. However, after becoming aware of reports of arterial and venous thromboembolic episodes, we switched to therapeutic anticoagulation. Our clinical management protocol is provided in the Online Appendix.
Data collection
Medical records were used for data collection. We obtained data on contact exposure, onset of symptoms and presenting symptoms, medical history and current medications at time of symptoms onset, daily clinical and laboratory data, treatment data and outcome data. All data were collected by trained investigators independent from the clinical teams. Before analysis, an extensive round of data cleaning was performed by a dedicated data manager, together with clinicians, to check for data accuracy. In this article, we report vital status at 2 April 2020. Study definitions are shown in the Online Appendix.
Statistical analysis
Univariable comparisons were used to compare survivors and non-survivors, and patient discharged alive versus not discharged from the ICU. Continuous variables related to daily process of care were averaged as the median in the first 3 days and categorical variables as present or absent. Groups were compared and the effect estimate was reported as median differences with interquartile range (IQR) calculated from a quantile regression based on the asymmetric Laplace distribution for continuous variables, and as odds ratio (OR) with 95% confidence interval (CI) calculated from a generalised linear model with binomial distribution.
To further expand the impact of age on the risk of hospital mortality and the chance of being discharged alive from the ICU, a simple generalised linear model considering a binomial distribution was used and the results are presented as a marginal effect plot. In addition, key characteristics and outcomes were compared (using the same univariable models described above) in patients with hypertension receiving angiotensin-converting enzyme (ACE) inhibitors and/or angiotensin II receptor blockers (ARBs) versus patients with hypertension not treated with these drugs.
Due to the nature of the study, and since the sample size is small and the number of events is low, all analyses should be considered exploratory. All analyses were conducted in R v.3.6.3 (R Foundation).13
Results
Demographic and clinical characteristics of the patients
From 25 February to 2 April 2020, we admitted 701 COVID-19 patients with pneumonia and an arterial partial pressure of oxygen (Pao2) to fraction of inspired oxygen (Fio2) ratio below 300 mmHg. Of the 609 patients managed in general wards and step-down units only, at 2 April, 91 (14.9%) had died, 281 (46.1%) had been discharged home and 237 (38.9%) were still in hospital (16 patients [6.7%] were under close evaluation for ICU transfer and 46 patients [19.4%] were receiving positive pressure ventilation). Overall, 92 patients (13.1%) received invasive mechanical ventilation in the ICU and 73 patients with COVID-19 ARDS completed the required 7-day follow-up and were included in the study (Online Appendix, figure S1).
Pre-admission demographic and clinical characteristics are shown in the Online Appendix (table S1). Most patients were male, the median age was 61 years, and over 25% of patients had obesity. The most frequent comorbidities were hypertension (52.9%) and diabetes (13.6%). ACE inhibitors were used by 12.9% and ARBs were used by 17.7% of patients. Only 2% of patients were smokers.
The median time from symptoms to hospital admission and ICU admission was 6.0 (IQR, 4.0–10.0 days) and 9.0 days (IQR, 7.0–13.0 days) respectively (Table 1). At hospital admission, fever was present in 57.6% of patients, the median oxygen saturation measured by pulse oximetry (Spo2) was 94% (IQR, 85–96%), and the median respiratory rate was 28 breaths per minute (IQR, 24–33 breaths per minute). Uncommon clinical symptoms were present in around 7% of patients (Online Appendix, table S2).
Table 1.
Clinical characteristics at hospital admission
| Clinical characteristics | Values |
|
|---|---|---|
| n (%)* | Median (IQR) | |
| Total number of patients | 73 | |
| Duration from symptoms (days) | ||
| To hospital admission | 6.0 (4.0–10.0) | |
| To ICU admission | 9.0 (7.0–13.0) | |
| History of contact | ||
| Closed contact with a confirmed case | 11 (15.1%) | |
| Presence in a health care facility treating patients with COVID-19 | 3 (4.1%) | |
| Vital signs | ||
| Awake | 55/68 (80.9%) | |
| Oriented | 55/67 (82.1 %) | |
| Temperature (°C) | 38.0 (37.4–38.8) | |
| Fever (temperature > 37.8°C) | 34/59 (57.6%) | |
| Spo2 (%) | 94 (85–96) | |
| At room air | 37/70 (50.7%) | 94 (85–96) |
| With oxygen supplementation | 33/70 (45.2%) | 93 (86–97) |
| Systolic blood pressure (mmHg) | 129 (115–140) | |
| Diastolic blood pressure (mmHg) | 70 (60–80) | |
| Heart rate (beats per min) | 100 (86–113) | |
| Respiratory rate (breaths per min) | 28 (24–33) | |
| At room air | 37/70 (50.7%) | 31 (28–40) |
| With oxygen supplementation | 33/70 (45.2%) | 28 (24–31) |
| History of symptoms | ||
| Fever in the previous 14 days | 68/70 (97.1%) | |
| Cough | 43/57 (75.4%) | |
| With sputum production | 6/40 (15.0%) | |
| Sore throat | 6/46 (13.0%) | |
| Arthralgia | 1/45 (2.2%) | |
| Fatigue | 9/47 (19.1%) | |
| Shortness of breath | 45/61 (73.8%) | |
| Altered level of consciousness | 1/46 (2.2%) | |
| Imaging | ||
| Chest x-ray | 73 (100.0%) | |
| Infiltrates | 73 (100.0%) | |
| Chest computed tomography | 11 (15.1%) | |
| Interstitial pneumonia | 11/11 (100.0% | |
| Haemoglobin (g/dL) | 13.4 (12.3–14.9) | |
| White blood cell count (× 103 per mm3) | 9.20 (5.65–11.95) | |
| Lymphocyte count (× 103 per mm3) | 0.77 (0.58–1.00) | |
| Lymphocytopenia | 44/60 (73.3%) | |
| Total bilirubin (mg/dL) | 0.6 (0.4–1.0) | |
| Urea (mg/dL) | 52.0 (33.5–77.5) | |
| Creatinine (mg/dL)† | 1.10 (0.87–1.33) | |
| Lactate (mmol/L) | 1.5 (1.2–2.2) | |
| C-reactive protein (mg/dL) | 184.5 (108.2–269.1) | |
COVID-19 = coronavirus disease 2019; ICU = intensive care unit; IQR = interquartile range; Spo2 = oxygen saturation measured by pulse oximetry.
Percentages may not total 100 because of rounding.
To convert the values for creatinine to μmol/L, multiply by 88.4.
Imaging and laboratory findings
All patients had bilateral infiltrates (Table 1) mainly in the lower left quadrant (Online Appendix, table S2). Interstitial pneumonia was seen in all patients (15.1%) with chest computed tomography scans. Lymphocytopenia was present in 73.3% of patients and the median C-reactive protein was 184.5 mg/dL (IQR, 108.2–269.1 mg/dL) (Table 1). Median creatinine was 1.10 mg/dL (IQR, 0.87–1.33 mg/dL), median lactate 1.5 mmol/L (IQR, 1.2–2.2 mmol/L), and D-dimer 10.1 μg/m (IQR, 5.0-23.8 μg/m).
Daily data, mechanical ventilation and organ support
The median Pao2/Fio2 ratio on Day 1 was 110.0 mmHg (IQR, 80.0–158.5 mmHg) (Table 2 and Online Appendix, figure S2). Most patients were ventilated with volume-controlled ventilation, with a median tidal volume of 6.7 mL/kg (IQR, 6.0–7.5 mL/kg) of predicted body weight and a median positive end-expiratory pressure of 12 cmH2O (IQR, 10–14 cmH2O).
Table 2.
Daily characteristics of study patients*
| Characteristics | Day 1 | Day 2 | Day 3 |
|---|---|---|---|
| Total number of patients | 73 | 73 | 73 |
| Laboratory tests, median (IQR) | |||
| Pao2/Fio2 (mmHg) | 110.0 (80.0–158.5) | 141.6 (104.7–177.2) | 156.8 (113.3–193.8) |
| Pao2 (mmHg) | 76.0 (62.7–89.4) | 78.5 (67.1–89.7) | 74.5 (66.2–85.6) |
| Paco2 (mmHg) | 46.4 (40.0–51.3) | 48.5 (43.3–53.0) | 47.8 (43.3–52.7) |
| Arterial pH | 7.38 (7.31–7.444) | 7.41 (7.35–7.46) | 7.43 (7.36–7.48) |
| Vital signs, median (IQR) | |||
| Mean arterial pressure | 79 (66–93) | 82 (71–94) | 88 (75–97) |
| Urine output (24 h) | 2180 (1300–3150) | 3535 (2402–4687) | 3467 (2336–4695) |
| Ventilatory support | |||
| Previous use of non-invasive ventilation | 20/70 (28.6%) | na | na |
| Mode of ventilation | |||
| Controlled | 64/70 (91.4%) | 54/70 (77.1%) | 51/67 (76.1%) |
| Assisted | 6/70 (8.6%) | 16/70 (22.9%) | 16/67 (23.9%) |
| Tidal volume (mL/kg PBW), median (IQR) | 6.7 (6.0–7.5) | 6.7 (6.0–7.5) | 6.7 (6.1–7.4) |
| PEEP (cmH2O), median (IQR) | 12 (10–14) | 12 (10–14) | 12 (10–14) |
| Fio2 (mmHg), median (IQR) | 0.70 (0.52–0.80) | 0.60 (0.50–0.70) | 0.50 (0.40–0.65) |
| Peak airway pressure (cmH2O), median (IQR) | 28.5 (25.2–30.0) | 26.0 (20.5–29.5) | 26.0 (23.8–30.0) |
| Driving pressure (cmH2O),† median (IQR) | 12.0 (7.0–16.5) | 10.0 (6.0–15.0) | 11.0 (9.0–11.0) |
| Dynamic compliance (mL/cmH2O),‡ median (IQR) | 28.6 (21.8–34.0) | 31.7 (25.4–39.6) | 30.0 (25.7–34.6) |
| Clinical support | |||
| ECMO§ | 4 (5.5%) | 4 (5.5%) | 4 (5.5%) |
| Tracheostomy | 0/72 (0.0%) | 1/69 (1.4%) | 1/70 (1.4%) |
| Renal replacement therapy | 0/72 (0.0%) | 3/68 (4.4%) | 4/69 (5.8%) |
| Vasopressor or inotropic therapy | 59/72 (81.9%) | 58/70 (82.9%) | 59/70 (84.3%) |
| Norepinephrine | 42/72 (58.3%) | 48/70 (68.6%) | 39/70 (55.7%) |
|
0.15 (0.10–0.20) | 0.10 (0.05–0.20) | 0.10 (0.08–0.20) |
| Angiotensin II | 35/72 (48.6%) | 36/70 (51.4%) | 37/70 (52.9%) |
|
10.0 (5.0–20.0) | 10.0 (5.0–20.0) | 10.0 (5.0–20.0) |
| Neuromuscular blocking agents | 53/70 (75.7%) | 43/69 (62.3%) | 39/70 (55.7%) |
| Prone positioning | 28/70 (40.0%) | 36/70 (51.4%) | 29/69 (42.0%) |
| Duration (h), median (IQR) | 12 (9–18) | 16 (9–18) | 14 (9–16) |
ECMO = extracorporeal membrane oxygenation; Fio2 = fraction of inspired oxygen; IQR = interquartile range; na = not applicable; Paco2 = arterial partial pressure of carbon dioxide; Pao2 = arterial partial pressure of oxygen; PBW = predicted body weight; PEEP = positive end-expiratory pressure.
Percentages may not total 100 because of rounding.
Calculated as plateau pressure minus PEEP and available only in seven patients (9.6%) on Day 1, in 13 patients (17.8%) on Day 2 and in 13 patients (17.8%) on Day 3.
Calculated as tidal volume divided by the [peak pressure minus PEEP] and available only in 22 patients (30.1%) on Day 1, in 26 patients (35.6%) on Day 2 and in 33 patients (45.2%) on Day 3.
All but one patient receiving ECMO in the first 3 days were transferred from other centres already receiving mechanical ventilation for a period.
In the first 3 days, 5% of patients needed extracorporeal membrane oxygenation (ECMO) and 5% needed renal replacement therapy (RRT) (Table 2). All patients receiving ECMO, except one, were transferred already on ventilation from other centres. Most patients received vasopressor support at an initial median dose of norepinephrine 0.15 μg/kg/min (IQR, 0.10–0.20 μg/kg/min). Half of the patients received angiotensin II infusion as vasopressor, and 75.7% received neuromuscular blockade. Prone positioning was used in most patients (session duration 12–16 h). During the first 7 days, there was a progressive decrease in the use of controlled ventilation, neuromuscular blocking agents, vasopressors and prone positioning (Online Appendix, figure S3). Additional daily laboratory data are shown in the Online Appendix (table S3).
Outcomes
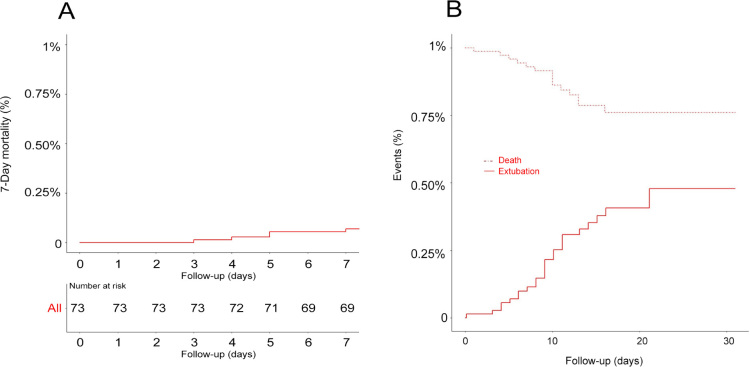
At 2 April 2020, the median follow-up time was 19.0 days (IQR, 15.0–27.0 days). At this time, 17 patients (23.3%) had died, 23 patients (31.5%) had been discharged from the ICU, and 33 patients (45.2%) were still on invasive mechanical ventilation. Only five patients (6.8%) had been discharged home (Table 3). The median duration of invasive mechanical ventilation and ICU length of stay were 10.1 (IQR, 8.0–14.3 days) and 10.5 days (IQR, 8.0–15.0 days) respectively (Table 3 and Figure 1). The cause of death in the 17 deceased patients is reported in the Online Appendix. The most common cause of death was multi-organ failure (11/17, 64.7%), mainly due to superimposed bacterial infection (5/11, 45.4%). Two patients (18.2%) died from a massive pulmonary embolism, while other two (18.2%) died from refractory hypoxaemia.
Table 3.
Clinical outcomes at the latest follow-up*
| Outcomes | Values |
|---|---|
| Total number of patients | 73 |
| Follow-up (days), median (IQR) | 19.0 (15.0–27.0) |
| Clinical outcomes | |
| Status at Day 7 | |
| Alive in the hospital | 6 (8.2%) |
| Alive in the ICU | 61 (83.6%) |
| Death | 6 (8.2%) |
| Status at the latest follow-up | |
| Alive discharged home | 5 (6.8%) |
| Alive discharged to other facilities | 5 (6.8%) |
| Alive in the hospital | 13 (17.8%) |
| Alive in the ICU | 33 (45.2%) |
| Death | 17 (23.3%) |
| ICU mortality | 14 (19.2%) |
| Duration of mechanical ventilation (days), median (IQR) | 10.1 (8.0–14.3) |
| In survivors | 10.6 (8.0–15.8) |
| ICU length of stay (days), median (IQR) | 10.5 (8.0–15.0) |
| In survivors | 11.0 (8.0–16.0) |
| Hospital length of stay (days), median (IQR) | 16.0 (13.0–24.0) |
| In survivors | 18.0 (14.8–25.2) |
| Support at the latest follow-up | |
| ECMO | 5 (6.8%) |
| Tracheostomy | 20 (27.4%) |
| Prone positioning | 55/72 (76.4%) |
| Renal replacement therapy | 16 (21.9%) |
| Complications at the latest follow-up | |
| Bacterial pneumonia | 9 (12.3%) |
| Pneumothorax or pneumomediastinum | 7 (9.6%) |
| Stroke | 1 (1.4%) |
| Heart failure | 7 (9.6%) |
| Cardiac arrhythmia | 15 (20.5%) |
| Acute myocardial infarction | 1 (1.4%) |
| Myocarditis/pericarditis | 0 (0.0%) |
| Cardiac arrest | 6 (8.2%) |
| Bacteraemia | 27 (37.0%) |
| Other secondary infection | 3 (4.1%) |
| Disseminated intravascular coagulation | 2 (2.7%) |
| Transfusion of > 2 units of red blood cells | 25 (34.2%) |
| Rhabdomyolysis | 2 (2.7%) |
| Acute kidney injury | 55 (75.3%) |
| Stage 1 | 23/55 (41.8%) |
| Stage 2 | 15/55 (27.3%) |
| Stage 3 | 17/55 (30.9%) |
| Minor gastrointestinal haemorrhage | 1 (1.4%) |
| Gastrointestinal perforation | 1 (1.4%) |
| Liver dysfunction | 16 (21.9%) |
| Hyperglycaemia | 14 (19.2%) |
| Acute thrombosis (other than pulmonary embolism) | 1 (1.4%) |
| Limb ischaemia | 2 (2.7%) |
| Pulmonary embolism | 4 (5.5%) |
| Minor (non-life threatening) bleeding | 7 (9.6%) |
| Fungal infection | 12 (16.4%) |
ECMO = extracorporeal membrane oxygenation; ICU = intensive care unit; IQR = interquartile range.
Latest follow-up at 1 April 2020. Percentages may not total 100 because of rounding.
Figure 1.
Kaplan–Meier curves for 7-day mortality (A) and cumulative incidence function of extubation and intensive care unit mortality (B)*
* Data were censored at the latest follow-up (2 April 2020).
At 2 April, ECMO was used in five patients (6.8%), prone positioning in 76.4% of patients, tracheostomy in 27.4% of patients and RRT in 21.9% of patients. The two most common complications were acute kidney injury (75.3%) and secondary bacteraemia (37.0%). Seven patients (9.6%) had a pneumothorax or pneumomediastinum and five patients (6.8%) had thromboembolic complications. Time to complication development is shown in the Online Appendix (table S4).
Clinical characteristics associated with outcome
On univariable analyses, non-survivors were older, had a much greater incidence of hypertension, a shorter time from symptoms to ICU admission, and lower Spo2 at hospital admission (Online Appendix, table S5). Patients discharged alive from the ICU were younger, had higher Spo2 at hospital admission, and a higher median Pao2/Fio2 ratio in the first 3 days of ventilation (Online Appendix, table S6). For both outcomes, age was a key factor (Online Appendix, figure S4).
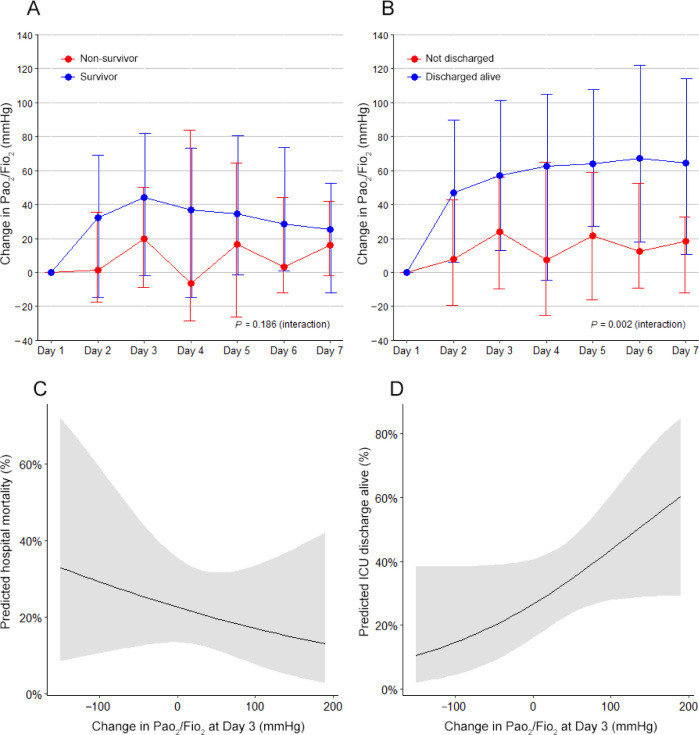
Daily characteristics and process of care according to survival or discharge are shown in the Online Appendix. Over the first 7 days, patients who were discharged alive from the ICU had a decrease in positive end-expiratory pressure and Fio2 and an increase in the Pao2/Fio2 ratio, while non-survivors did not (Online Appendix, figure S6). A sustained daily improvement of a median 10 mmHg in the Pao2/Fio2 ratio from Day 2 onwards was associated with higher chance of being discharged from the ICU alive (Figure 2).
Figure 2.
Effect of changes in Pao2/Fio2 ratio in the first 7 days on mortality and on the chance of being discharged alive from the intensive care unit (ICU) at the latest follow-up
Fio2 = fraction of inspired oxygen; Pao2 = arterial partial pressure of oxygen. Panel A shows the change in the Pao2/Fio2 ratio in the first 7 days of follow-up compared with the baseline value and according to survival at the latest follow-up. Panel B shows the change in the Pao2/Fio2 ratio in the first 7 days of follow-up compared with the baseline value and according to being discharged alive from the ICU at the latest follow-up. In both plots, circles are medians and the error bars represent the interquartile range (IQR); the P value represents the interaction between the groups and the days from an unadjusted mixed-effect quantile regression based on the asymmetric Laplace distribution for continuous variables and accounting for the repeated measurements. The median difference for the interaction is 4.19 (IQR, –2.10 to 10.50; P = 0.186) for the comparison of survivors and non-survivors, and 9.64 (IQR, 3.68–15.59; P = 0.002) for the comparison of being discharged alive or not. Panel C shows a marginal effect plot with the effect of changes in the Pao2/Fio2 ratio at Day 3 on the predicted risk of mortality at the longest follow-up from a univariable generalised linear model considering a binomial distribution. The odds ratio for the change is 0.99 (95% CI, 0.99–1.00; P = 0.441). Panel D shows a marginal effect plot with the effect of changes in the Pao2/Fio2 ratio at Day 3 on the predicted risk of being discharged alive from the ICU at the longest follow-up from a univariable generalised linear model considering a binomial distribution. The odds ratio for the change is 1.01 (95% CI, 0.99–1.02; P = 0.071).
There were no differences in key clinical characteristics and outcomes according to ACE inhibitors and/or ARBs use (Online Appendix, tables S7 and S8).
Discussion
We studied 73 invasively ventilated patients with COVID-19 ARDS in a referral centre in Milan, Italy. We found that male sex and hypertension were disproportionately common and that one in 15 patients was treated with ECMO and one in five with RRT. Most patients received vasopressors and neuromuscular blocking agents, three out of four patients were treated with prone positioning, and three in ten received a tracheostomy. At a median follow-up of about 20 days, almost one in six patients had died, with a marked increase in mortality with age above 60 years; one-third of patients were still in the ICU receiving invasive mechanical ventilation; and less than one in ten had been discharged home, for a median duration of ventilation of 10 days. Older age and a history of hypertension were key risk factors for mortality. In contrast, early short term improvement in oxygenation was associated with discharge alive from the ICU.
No previous study has specifically focused on mechanically ventilated patients with COVID-19 ARDS. Four previous studies assessed critically ill patients, with some portion of these patients receiving mechanical ventilation. One study involved 37 such patients (71%) in Wuhan, China.8 Prone positioning and ECMO were used in only six patients. At Day 28, 32 patients (86.4%) had died. A US study reported data on 21 patients, with 15 (71.4%) receiving mechanical ventilation and with a mortality rate of 52.4% in the overall cohort.4 A further American study assessed 24 patients, with 18 (75%) receiving mechanical ventilation. In this cohort, 12 patients (66%) had died at follow-up between 1 and 18 days. At one week of shortest follow-up, we found a mortality rate of 20.5%.5 However, 33 patients (45.2%) were still receiving invasive mechanical ventilation and, at the time of submission, their outcome remains uncertain. The most recent study assessed 1591 critically ill patients with 1150 patients receiving mechanical ventilation.6 In this cohort, the mortality was 26% in the overall group of patients.
Compared with the largest study published to date,6 the present study has specifically focused on invasively ventilated patients with COVID-19 ARDS. In this previous study, there is no report of the outcomes of patients receiving mechanical ventilation specifically, and also no daily information on ventilatory variables, vital signs and laboratory tests. Moreover, the rate of prone positioning (< 30%) and ECMO (< 1%) is low and probably does not reflect the care and outcomes of patients treated exclusively in referral academic centres. The present study reports the most detailed and granular data to date in this group of patients, including daily laboratory tests, ventilatory parameters and complications, and most importantly, it is the first one to report the cause of death of patients. Furthermore, the protocol was registered and reported previously and, for the first time, some predictors of outcome are assessed and reported.
In our cohort, the percentage of smokers was low, especially compared with the prevalence of smoking in Italy.14 However, this could reflect selection bias for ICU admission. Higher body mass index and obesity were common, with more than 80% of patients having overweight or obesity. In Italy, only 35.4% of the population are classified as overweight or obese15 but with an increase in body mass index with age.16 However, a higher body mass index was not associated with higher mortality.
Our study shows that COVID-19- induced ARDS treated with invasive mechanical ventilation carries a major burden of critical care interventions. Moreover, it demonstrates that invasive mechanical ventilation and ICU stay are prolonged, thus potentially leading to the progressive and, perhaps, inevitable exhaustion of ICU resources, staff, beds and ventilators. In addition, mortality appears high despite multiple high level ICU therapies. Finally, older age and a history of hypertension are key risk factors for short term mortality. In contrast, early improvement in oxygenation appears to be a marker of better prognosis in these patients.
Our study presents a greater granularity of data compared with previous studies including mechanically ventilated patients with COVID-19 ARDS together,4, 5, 6, 7 and is the only one focused on such patients with daily data. Therefore, it provides a more robust estimate of the burden of care and the expected outcome of this condition. It followed a prospective data collection protocol, with reliable and detailed data on the characteristics, treatment and outcomes of patients with COVID-19 ARDS. Finally, it presented a much more detailed set of physiological and biochemical descriptors to help characterise such patients,17 clearly identified older age and hypertension as risk factors for short term mortality, and found early improvement in oxygenation as associated with being discharged alive from the ICU.
This is a single centre study, but it is likely to reflect the experience of similar referral centres for COVID-19 ARDS in resource-rich countries. The multiple interventions described were not part of randomised controlled trials, thus no inferences can be made about their effect on outcomes. In the setting of a dramatic and overwhelming surge in clinical cases and the worst global health care crisis in a century, randomisation was not possible. Moreover, our data are likely informative in relation to expected resource utilisation and short term outcomes in Europe and the US. Finally, the identification of hypertension as a powerful risk factor for short term mortality and the steep and dramatic increase in short term mortality with older age have important implications for prognostication and resource allocation.
Conclusion
In a referral centre for COVID-19 in Milan, Italy, despite multiple advanced critical care interventions, COVID-19 ARDS was associated with prolonged ventilation and ICU stay and high short term mortality. Age and a history of hypertension were key risk factors for mortality, which increased steeply with age over 60 years. However, an early improvement in oxygenation was associated with a greater chance of being discharged alive from the ICU.
Acknowledgments
Acknowledgements
Author contributions: Giovanni Landoni and Ary Serpa Neto had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. The writing group vouches for the accuracy and completeness of the data and for the fidelity of the study to the protocol.
Concept and design: Alberto Zangrillo, Giacomo Monti, Fabio Ciceri, Ary Serpa Neto, Rinaldo Bellomo and Giovanni Landoni.
Acquisition, analyses or interpretation of data: All authors.
Drafting of the manuscript: All authors.
Critical revision of the manuscript for important intellectual content: All authors.
Statistical analyses: Ary Serpa Neto, Alessandro Belletti and Giovanni Landoni.
Administrative, technical, or material support: Alberto Zangrillo, Luigi Beretta, Fabio Ciceri, Francesco De Cobelli, Moreno Tresoldi, Lorenzo Dagna, Rinaldo Bellomo and Giovanni Landoni.
Supervision: Alberto Zangrillo, Fabio Ciceri, Rinaldo Bellomo and Giovanni Landoni.
Competing interests
Lorenzo Dagna received consultation honoraria from Roche, Sanofi-Genzyme and SOBI outside of the submitted work. Ary Serpa Neto reported receiving personal fees from Dräger outside of the submitted work.
Funding/support
The study was supported by departmental funds only. The funding source had no role in the conduct of the present study or previous trials; collection, management, analysis and interpretation of the data; preparation, review or approval of the manuscript; and decision to submit the manuscript for publication.
Institution where the work was performed
Department of Anesthesia and Intensive Care, IRCCS San Raffaele Scientific Institute, Milan, Italy.
COVID-BioB Study Group investigators
Beccaria Paolo Federico, Leggieri Carlo, Mamo Daniela, Moizo Elena, Mucci Milena, Plumari Valentina Paola, Borghi Giovanni, Dossi Roberto, Pasculli Nicola, La Bruna Alessia, Pintaudi Margherita, Colnaghi Eleonora, Cristallo Edoardo, Deni Francesco, Turi Stefano, Geddo Alessandro, Ajello Silvia, Calabrò Maria Grazia, De Luca Monica, Di Tomasso Nora, Fano Greta, Franco Annalisa, Frau Giovanna, Gerli Chiara, Pieri Marina, Mucchetti Marta, Melisurgo Giulio, Oriani Alessandro, Di Prima Ambra Licia, Licheri Margherita, Cornero Guglielmo, Mattioli Cristina, Barucco Gaia, Casiraghi Giuseppina Maria, Nuzzi Massimiliano, Baiardo Redaelli Martina, Carcò Francesco, Castella Alberto, Dalessandro Veronica, Alba Ada Carla, Formicola Giuseppe Luca, Notte Carlotta, Saleh Omar, Sartini Chiara, Tamà Simona, Tornaghi Anna, Corrao Francesco, Recca Gaia, Denaro Giuseppe, Sordoni Stella, Cuffaro Raffaele, Ortalda Alessandro, Scarparo Elena, Frassanito Claudia, Filippini Martina, Lombardi Gaetano, Pallanch Ottavia, Fenu Giulia, Tozzi Margherita, Campofreda Irene, Marzaroli Matteo, De Donato Francesco, Ferrara Barbara, Ortolani Ginevra, Chiodega Isabella, Anselmi Alberto, Nisi Francesco Giuseppe, Lazzari Stefano, Senarighi Giacomo, Palermo Paola, Barberio Cristina, Maimeri Nicolò, Faustini Carolina, Valsecchi Gabriele, Di Piazza Martina, Bellantoni Antonio, Fresilli Stefano, Todaro Gabriele, Fedrizzi Monica, Gattarello Simone, Conte Francesca, Losi Davide, Scquizzato Tommaso, Lembo Rosalba, Dalessandro Giuseppe, Giardina Giuseppe, Galbiati Carola, Bozzolo Enrica, Campochiaro Corrado, Carlucci Michele, Cavalli Giulio, D'Angelo Armando, Davalli Alberto, Dell'Acqua Antonio, Della Torre Emanuel, De Luca Giacomo, Guglielmi Barbara, Scarpellini Paolo, Spessot Marzia and Turla Giancarlo.
Supplementary Information

References
- 1.Lu H., Stratton C.W., Tang Y.W. Outbreak of pneumonia of unknown etiology in Wuhan China: the mystery and the miracle. J Med Virol. 2020;92:401–402. doi: 10.1002/jmv.25678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hui D.S., Azhar E.I., Madani T.A., et al. The continuing 2019nCoV epidemic threat of novel coronaviruses to global health — the latest 2019 novel coronavirus outbreak in Wuhan. China. Int J Infect Dis. 2020;91:264–266. doi: 10.1016/j.ijid.2020.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Huang C., Wang Y., Li X., et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arentz M., Yim E., Klaff L., et al. Characteristics and outcomes of 21 critically ill patients with COVID-19 in Washington State. JAMA. 2020 doi: 10.1001/jama.2020.4326. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bhatraju P.K., Ghassemieh B.J., Nichols M., et al. Covid-19 in critically ill patients in the Seattle region — case series. N Engl J Med. 2020 doi: 10.1056/NEJMoa2004500. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Grasselli G., Zangrillo A., Zanella A., et al. Baseline characteristics and outcomes of 1591 patients infected with SARS-CoV-2 admitted to ICUs of the Lombardy region, Italy. JAMA. 2020 doi: 10.1001/jama.2020.5394. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhou F., Yu T., Du R., et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yang X., Yu Y., Xu J., et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet. Respir Med. 2020 doi: 10.1016/S2213-2600(20)30079-5. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guan W.J., Ni Z.Y., Hu Y., et al. clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020 doi: 10.1056/NEJMoa2002032. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zangrillo A., Beretta L., Silvani P., et al. Fast reshaping of intensive care unit facilities in a large metropolitan hospital in Milan, Italy: facing the COVID-19 pandemic emergency. Crit Care Resusc. 2020 doi: 10.51893/2020.2.pov1. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cabrini L., Idone C., Colombo S., et al. Medical emergency team and non-invasive ventilation outside ICU for acute respiratory failure. Intensive Care Med. 2009;35:339–343. doi: 10.1007/s00134-008-1350-y. [DOI] [PubMed] [Google Scholar]
- 12.Cao B., Wang Y., Wen D., et al. A trial of lopinavir-ritonavir in adults hospitalized with severe Covid-19. N Engl J Med. 2020 doi: 10.1056/NEJMoa2001282. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.R Core Team . R Foundation for Statistical Computing; Vienna, Austria: 2019. R: A language and environment for statistical computing.https://www.R-project.org (viewed Apr 2020) [Google Scholar]
- 14.Statista. Number of individuals who smoke in Italy in 2018, by age and gender. https://www.statista.com/statistics/501615/italy-smokers-by-age-and-gender/ (viewed Apr 2020).
- 15.Statista. Distribution of adult body mass index (BMI) in Italy in 2018. https://www.statista.com/statistics/586182/distribution-of-adult-body-mass-index-bmi-italy (viewed Apr 2020).
- 16.Statista. Distribution of body-mass-index (BMI) in Italy in 2018, by age. https://www.statista.com/statistics/727866/distribution-of-body-mass-index-by-age-italy/ (viewed Apr 2020).
- 17.Ciceri F., Beretta L., Scandroglio A.M., et al. Microvascular COVID-19 lung vessels obstructive thromboinflammatory syndrome (MicroCLOTS): an atypical acute respiratory distress syndrome working hypothesis. Crit Care Resusc. 2020 doi: 10.51893/2020.2.pov2. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials