Abstract
-
•
Albumin is the most abundant and perhaps most important protein in human blood. Research has identified many of albumin’s possible roles in modulating acid-base balance, modifying inflammation, maintaining vascular endothelial integrity, and binding endogenous and exogenous compounds.
-
•
Albumin plays a key role in the homeostasis of vascular endothelium, offering protection from inflammation and damage to the glycocalyx.
-
•
Albumin binds a diverse range of compounds. It transports, delivers and clears drugs, plus it helps with uptake, storage and disposal of potentially harmful biological products.
-
•
The biological effects of albumin in critical illness are incompletely understood, but may enhance its clinical role beyond use as an intravenous fluid. In this article, we summarise the evidence surrounding albumin’s biological and physiological effects beyond its use for plasma volume expansion, and explore potential mechanistic effects of albumin as a disease modifier in patients with critical illness.
Albumin is widely used in critical care and other conditions to expand plasma volume, replace losses, and restore serum albumin levels toward normal in hypoalbuminaemic states.1, 2, 3 This use is driven by the concept that albumin’s oncotic pressure effects have primacy over any other biological, physiological or clinical effects.1 However, as a naturally occurring protein, albumin has other important biological properties beyond its oncotic and volume expansion effects. These effects may enhance its clinical role beyond use as an intravenous fluid.4 Such effects should be understood by clinicians to fully appreciate the potential effects of human albumin solution therapy.
Albumin physiology
Human albumin constitutes some 50% of plasma protein present in normal healthy individuals. It is a 66-kilodalton protein, which is small compared with other plasma proteins.5 Albumin is highly water soluble, has an elliptical shape and has low intrinsic viscosity. It is synthesised in polysomes bound to the endoplasmic reticulum of hepatocytes. Albumin is not stored in the liver and there is no reserve for release on demand.6 However, under basal physiological circumstances only 20–30% of hepatocytes produce albumin; so with factors such as change in interstitial colloid oncotic pressure, synthesis can be increased if required by a factor of 200–300%.3
Despite being the main protein in plasma, albumin is predominantly an extravascular protein. Specifically, its serum concentration is about 40 g/L, equating to a total intravascular mass of about 120 grams. In contrast, its interstitial concentration is lower (about 14 g/L) and varies between different anatomical regions. However, its estimated total extravascular mass is about 160 grams. A proportion of this albumin can be easily mobilised from loose interstitial tissue, while some is tightly bound, particularly in the skin. There appears to be a circular flow of albumin from the intravascular to extravascular space, followed by return to the circulation via lymphatic vessels. Such movement has been objectively measured and represents a circulation halflife of about 16–18 hours.5
Evidence also suggests that albumin has effects independent of its oncotic effect. These include protection of the glycocalyx,7 improvement in endothelial integrity,8 inhibition of endothelial apoptosis,9 modulation of nitric oxide pathways,10, 11 anti-inflammatory effects,12, 13 antioxidant effects,14, 15, 16 modulation of acid-base status,17 binding of drugs and other plasma substances,18, 19 activation of intracellular processes,20, 21 regulation of electrolyte shifts, and changes in intracellular volume.22, 23, 24 In this review, we examine these biological effects of albumin that go beyond its intravascular volume expansion properties.
Protection of the glycocalyx
The glycocalyx is a complex gel that lies between flowing blood and the endothelial cell wall, and interacts with both plasma proteins and lipids.25 It contributes to the epithelial barrier, and its degradation is sufficient to increase lung permeability.26 Similarly, the endothelial glycocalyx is known to contribute to critical vascular functions, such as endothelial barrier integrity, transduction of shear stress, regulation of vascular tone, and inhibition of leucocyte adhesion.27, 28 Damage to the glycocalyx structure causes increased vascular permeability, leading to tissue oedema, uncovering adhesion molecules for blood leucocyte and platelet activation, and initiating local inflammation plus proaggregatory and procoagulatory conditions.29, 30
Albumin attenuates glycocalyceal shedding during cold ischaemia and reperfusion, reducing interstitial oedema and intracoronary adhesion of leucocytes.7 This makes it an important and beneficial ingredient of a preservation solution used during heart transplantation.7 In an isolated perfused organ model, albumin, in contrast to artificial colloids, leads to a significant increase in coronary flow through its interaction with the glycocalyx.31 Furthermore, human albumin, through its interaction with glycocalyceal hyaluronan,8 improves endothelial integrity, alleviating postischemic intracoronary retention of granulocytes. This effect is confirmed by a morphologically intact endothelial glycocalyx and a markedly decreased coronary venous release of glycocalyx constituents.32 This protective effect of albumin might be mediated by a protein-bound substance that inhibits matrix metalloproteinase cleavage of the endothelial glycocalyx, such as the lipid mediator sphingosine 1-phosphate.4
The role of the glycocalyx in fluid shift across the endothelium, as a determinant of hydraulic conductivity and in the revised Starling model, is well recognised.33 A low- protein environment has long been known to cause a rapid breakdown, or shedding, of the endothelial glycocalyx.34 In preclinical studies, this phenomenon is independent of the effect on osmotic pressure, and albumin is more effective than semisynthetic colloids at preserving and restoring the endothelial glycocalyx, reducing vascular permeability, and reducing platelet and leucocyte adhesion.35, 36 However the ability for albumin to protect glycocalyx integrity in clinical studies has yet to be examined.
Protection of endothelium
Albumin inhibits endothelial apoptosis9, 37 via a G-coupled protein PI3K (phosphoinositide 3-kinases)-dependent mechanism.38 It modulates arachidonic acid release and membrane fluidity, and protects against ischaemia and reperfusion injury.21, 39 Albumin inhibits apoptosis triggered by oxidative stress by scavenging reactive oxygen species in cultured macrophages, neutrophils, lymphocytes, and kidney tubular epithelial cells.14, 15, 16 This protective activity depends on radical scavenging, primarily through albumin’s cysteine residue at position 34 (Cys34) and its binding of lysophosphatidic acid, an abundant serum lipid.14, 15, 16, 40
Albumin prevents flow-induced vasoconstriction41 and suppresses angiotensin-converting enzyme activity,42 and its administration increases angiopoietin-1 to angiopoietin-2 ratio43 — an effect which has been associated with endothelial integrity.44 It significantly inhibits tumour necrosis factor-α (TNF-α)-induced adhesion of THP-1 (human leukaemia monocytic cell line) cells and dose-dependently inhibits TNF-α-induced mRNA and protein expression of vascular cell adhesion molecule-1.20 Moreover, it inhibits activation and nuclear translocation of nuclear factor kappa-light-chain-enhancer of activated B cells (a transcription factor) in a dose-dependent manner.
Since excessive endothelial apoptosis may contribute to vascular disease,45 it is possible that albumin’s protective effect on the vascular endothelium is due to its antiapoptotic activity.9, 37 Albumin may directly influence vascular integrity, by binding to the interstitial matrix and subendothelium and altering the permeability of these layers to large molecules and solutes.22
In patients with congenital analbuminaemia — a rare, inherited, autosomal recessive disorder with about 90 patients known worldwide — there is low or complete absence of albumin. However, due to a compensatory increase in other plasma proteins, these patients have mild oedema, reduced blood pressure and chronic fatigue.46 However, even very low concentrations of albumin, as seen in these patients, help maintain endothelial barrier function.8
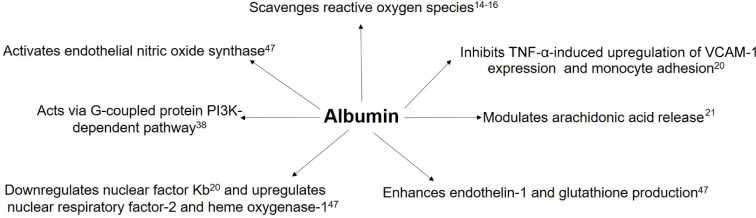
Studies have demonstrated a protective effect of 4% human serum albumin treatment on endothelial dysfunction which is achieved by inhibiting inflammatory and oxidative stress pathways induced by endotoxins in animal models.47 Multiple mechanisms have been proposed to explain these effects (Figure 1), but there is a lack of data to support any such improvement in endothelial function in critically ill humans — an aspect of human albumin biology that requires investigation.
Figure 1.
Mechanisms by which albumin protects endothelial cells
Nuclear factor Kb = nuclear factor kappa-light-chain-enhancer of activated B cells; PI3K = phosphoinositide 3-kinases; TNF-α = tumour necrosis factor-α; VCAM-1 = vascular cell adhesion molecule-1.
Nitric oxide modulation
Human serum albumin plays a key role in storing, scavenging and transporting nitric oxide in humans.10, 11 Hypoalbuminaemia is viewed as a causal factor in the pathogenesis of endothelial dysfunction and cardiovascular disease in patients with proteinuric chronic kidney disease; low albumin levels have been shown to increase endothelial nitric oxide production and decrease vascular sensitivity to nitric oxide.48
Albumin is the major extracellular source of reduced sulphydryl groups, which are avid scavengers of reactive oxygen and nitrogen species, especially superoxide hydroxyl and peroxynitrite radicals. Albumin can also limit the production of these reactive species through its binding of free copper, an ion known to be particularly important in accelerating production of free radicals.5 Similarly, the anticoagulant effect of albumin is possibly due to its capacity to bind nitric oxide to form S-nitrosothiols, thereby inhibiting the rapid inactivation of nitric oxide and allowing prolongation of its inhibitory effects on platelets.49
Influence on inflammation
When inflammation-related reactive oxygen species are absorbed, stored and transported by albumin, albumin’s Cys34 becomes oxidised. This results in a structural change in the albumin molecule — from its reduced form mercaptoalbumin (HMA) to its oxidised form nonmercaptoalbumin (HNA). In a small study of dialysisdependent patients receiving oral calcitriol for secondary hyperparathyroidism due to chronic kidney disease, a correlation between HNA to HMA ratios and serum levels of C-reactive protein and interleukin (IL)-6 was found.12 In another observational study, which included 87 patients admitted to a Brazilian intensive care unit (ICU), levels of serum albumin were inversely associated with inflammatory cytokines including IL-6, IL-7, IL-8, IL-10, TNF-α and interferon-α.13 These data support the hypothesis that albumin may be a significant component of the proteindependent response to oxidative stress during inflammation. Thus, hypoalbuminaemia may result in an overall reduction in plasma antioxidant capacity.
In healthy individuals, serum albumin is not glycated. Glycation of albumin has been associated with inflammation and chronic diseases such as diabetes, kidney disease and eye disease.50 Levels of glycated albumin have also been correlated with progression of these diseases, their complications, and even mortality.51 In patients with diabetes, levels of glycated haemoglobin and glycated albumin closely correlate. However, as albumin has a shorter half-life than haemoglobin, changes in glycated albumin reflect short term glycaemic control (2–3 weeks) as opposed to glycated haemoglobin, which reflects long term glycaemic control (2–3 months). On the other hand, while glycated albumin may be the result of hyperglycaemia, there is also evidence that it may, itself, further exacerbate insulin resistance.52
Influence on acid–base status
Acute critical illness is often characterised by acidosis. Albumin is a weak acid comprising the majority of total extracellular acid in human plasma. According to Stewart’s model of acid-base physiology, such acids are an independent variable controlling acid–base status.17 During episodes of acute inflammation, albumin behaves as a negative acute phase reaction protein. The subsequent hypoalbuminaemia and reduction in total extracellular acid contributes to metabolic alkalosis and attenuates any acute illness-associated metabolic acidosis. This phenomenon is often seen in patients with chronic critical illness long after their initial acidifying insult (eg, lactic acidosis in septic shock) has resolved. In theory, the intravenous administration of albumin should result in an increase in serum albumin concentration and thus total extracellular acid, causing acidification of blood. However, albumin for infusion is presented as sodium chloride solution at 4-5% (iso- oncotic) concentration or chloride-poor solution at 20–25% (hyperoncotic) concentration. Thus 4-5% albumin solutions can be expected have different acid-base effects compared with 20–25% albumin solutions. In this regard, a secondary analysis of a sepsis trial in African children revealed that fluid resuscitation with 4% albumin, compared with no bolus fluid therapy, resulted in hyperchloraemic acidosis.53 In contrast, infusion of hyperoncotic albumin, with its low chloride content, results in a fall in serum chloride and no overall change in blood pH or base excess.54 Studies directly comparing 4-5% and 20% albumin solutions also confirm that the use of 4-5% preparations results in increased serum chloride levels and a tendency for metabolic acidosis.55, 56 Thus the concentration of albumin solutions has a differential effect on acid-base status. Finally, there are strong relationships with inflammation and acid-base status — albumin appears to have independent influence over both. However, the relationship between these acid–base variables and inflammation is not entirely understood or explained by illness severity alone.13
Binding of drugs and other ligands
Albumin has a strong negative charge, enabling it to reversibly bind other charged particles. In particular, it has strong binding capacity for water, calcium, sodium and trace elements. Albumin also plays a vital role in the storage and transport of endogenous compounds including fatty acids, bilirubin and hormones, plus a range of (exogenous) drugs. Albumin’s physical structure is based around three homologous domains (I, II and III) with each containing two subdomains (A and B). This modular structure is held together by disulphide bonds and provides multiple sites for the binding of ligands (Figure 2). Sudlow and colleagues first described the most important drug-binding sites in 1975, after identifying two specific locations in subdomains IIA and IIIA based on patterns of fluorescent probe displacement by competitive drugs.18, 19 Albumin also possesses esterase capability, allowing it to hydrolyse ester bonds of bound drugs and subsequently modify their pharmacological effects. Non-ester drugs tend to bind albumin at Sudlow site I, whereas drugs with an ester bond have a greater affinity at Sudlow site II.58
Figure 2.
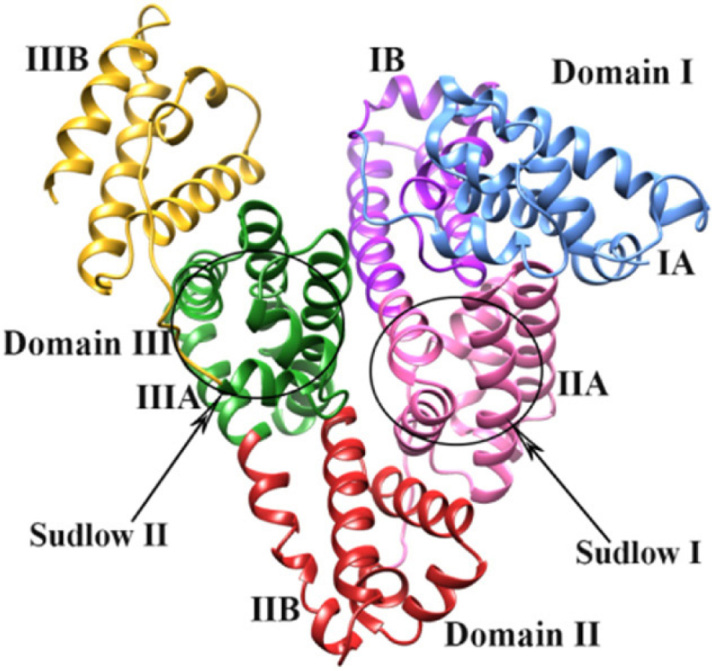
Physical structure of human albumin, including its domains (I-III), subdomains (A and B) and Sudlow binding sites (I and II)*
* Reproduced from Lakshmi et al,57 under the Creative Commons Attribution 3.0 Unported license (available at https://creativecommons.org/licenses/by/3.0)
The combination of albumin’s binding capacity, esterase activity and abundance in human blood is responsible for its influence on the clinical efficacy and safety of several drugs. For example, drugs such as warfarin and phenytoin compete for the same albumin binding sites, meaning they will displace each other, and this renders their subsequent pharmacodynamic effects unpredictable and potentially harmful. If such drugs are used in critical illness, they should be titrated carefully according to established therapeutic drug monitoring procedures. In addition, the use of common drugs (such as non-steroidal anti-inflammatories and oral hypoglycaemic agents) alongside fluoroquinolone antibiotics results in an increased free fraction of antibiotics,59 which may result in increased risk of important clinical adverse effects such as peripheral neuropathy.60 A lot of the data concerning drug-binding interactions with albumin come from in vitro experiments, as it is difficult to replicate such measurements in vivo, especially in critical illness. However, there have been observations of the potential increased free drug fraction in hypoalbuminaemic states, such as faster onset of sedation in patients given midazolam.61
Many other factors influence drug binding to serum albumin, including acid-base status, inflammatory states (acute and chronic), sex, age and pathological condition. In the context of critical illness, however, it is unlikely that any one factor has a large enough effect on drug binding to cause any significant clinical impact.62
Significance of hypoalbuminaemia
Hypoalbuminaemia is a common feature of chronic kidney diseases and chronic liver disease and, when combined with episodes of systemic inflammation, has been associated with increased mortality.63,64 Recently, two trials have found that albumin treatment reduced systemic inflammation and cardiocirculatory dysfunction in patients with decompensated cirrhosis.65 Although hypoalbuminaemia is commonly seen in patients with acute conditions such as burns and sepsis, this is more likely to be because of increased transcapillary escape and the dilutional effects of exogenous fluid administration rather than increased consumption or clearance. In this regard, in vivo kinetics studies using iodine-labelled albumin have demonstrated increased transcapillary escape during sepsis and after major surgery.66, 67 While malnutrition is characterised by hypoalbuminaemia, observational data in burns patients associates hypoalbuminaemia with increasing inflammation as measured by C-reactive protein rather than the provision of adequate nutrition.68 In fact, acute systemic inflammation actually stimulates albumin production to increase the body’s functional synthetic rate,69 but such increased production is unable to keep up with transudation, catabolism or both. Clinical measurements of serum albumin concentration, therefore, do not reflect the complexity of its bodily kinetics during acute inflammatory states or the variety of aetiological factors at play.70
Hypoalbuminaemia commonly seen in critical illness can result in an increased free fraction of drugs that bind albumin. This may increase a drug’s volume of distribution and reduce its efficacy. In addition, some drugs rely on albumin for more than binding and transport. Frusemide, for example, binds to albumin, which appears to facilitate its secretion into the tubular lumen. Clinical trials have shown enhanced diuresis and improved oxygenation when frusemide is given in combination with albumin in both chronic and acute disease states.71, 72 Other physiological factors, such as temperature and pH, have also been shown to alter the structure of binding sites and their subsequent ability to bind drugs.13, 73
Current clinical use of albumin
Albumin is clinically available as iso-oncotic albumin (4% or 5%) or hyperoncotic albumin (20% or 25%). Hyperoncotic albumin contains minimal sodium and chloride content and is available in smaller volumes. Current albumin solutions have excellent safety records, and substantial improvement in purity and tolerability has been achieved in the past 20 years.74
Albumin is commonly used for fluid resuscitation in critically ill patients. The Saline versus Albumin Fluid Evaluation (SAFE) study, which randomly assigned 6997 critically ill patients to 4% albumin or isotonic sodium chloride solution for fluid resuscitation during the 28 days after ICU admission, found no differences in 28-day all-cause mortality, ICU or hospital length of stay, or duration of mechanical ventilation or renal replacement therapy. However, in the subgroup of 1218 patients with severe sepsis, albumin use was associated with a trend toward reduced mortality.75 More recently in the Albumin Italian Outcome Sepsis (ALBIOS) trial, patients with severe sepsis were randomly assigned to receive daily supplementation with 20% albumin or crystalloid fluid alone, but mortality did not differ significantly between groups.76 However, in the subgroup of 1121 patients with septic shock at enrolment, there was a lower risk of death for those receiving 20% albumin even after adjustment for baseline covariates, supporting the rationale for use of albumin as a drug in these patients. Large clinical trials examining the use of albumin in septic patients are currently underway (ClinicalTrials.gov identifiers NCT01337934 and NCT03869385), and the results will further inform albumin’s role in patients with sepsis.
Albumin replacement is a common practice in patients with liver failure. Long term albumin administration prolongs overall survival and might act as a disease-modifying treatment in patients with decompensated chronic liver disease77 and hepatorenal syndrome.78 Similarly, albumin replacement is commonly used in large volume paracenteses, to prevent effective hypovolaemia, where albumin (6–8 grams per litre of fluid removed) is administered.79 Finally, albumin is used as a replacement fluid for therapeutic plasma exchanges.80
Albumin solutions, however, can also be harmful. In a substudy of a large double-blind randomised controlled trial involving critically ill patients with traumatic brain injury, fluid resuscitation with 4% hypotonic albumin was associated with higher mortality rates than resuscitation with saline.81 However, this injurious effect did not appear secondary to the albumin molecule itself but rather to the low tonicity of the solution containing it. In particular, when compared with saline or an isotonic albumin solution, hypotonic albumin solution increased intracranial pressure in normal animals, while the other two solutions did not.82, 83
Recently, the use of small-volume resuscitation with hyperoncotic albumin has been investigated84 and shown to be safe and effective in ICU patients.55 More specifically, in patients after cardiac surgery, it results in less positive cumulative fluid balance and offers several haemodynamic and potential ICU treatment advantages such as reduced risk of postoperative acute kidney injury and requirement of vasopressors.85, 86 A phase 2 randomised controlled trial of 20% albumin resuscitation in cardiac surgery patients is about to start in Australia (Australian New Zealand Clinical Trials Registry registration number ACTRN12620000137998). Despite some early concerns that the plasma volume-expanding effect of albumin relative to that of crystalloids might be decreased under conditions characterised by increased permeability, such as cardiac surgery, this has not been shown to be correct under experimental conditions.87 Hyperoncotic albumin causes long-lasting plasma volume expansion of similar magnitude in postoperative patients and volunteers23, 24, 88 and its bolus administration is effective and safe in healthy subjects when compared with other commonly available crystalloids and colloidal solution.43
Finally, there are substantial financial implications with the use of albumin-based solutions. Even though it is available free of cost in Australia, there is societal cost of requiring blood donors, and the cost in countries outside Australia is quite notable when compared with crystalloid solutions.89
Conclusion
Albumin may be considered the most important protein in human blood. It plays a key role in the homeostasis of vascular endothelium, offering protection from inflammation and damage to the glycocalyx. Albumin’s ability to bind a diverse range of compounds enables the transport, delivery and clearance of drugs, as well as the uptake, storage and eventual disposal of potentially harmful biological products. Its interplay with acid–base balance and inflammatory processes is only partially understood, with data on this role mainly coming from basic science experiments and clinical trials of patients with chronic disease. Pilot studies suggest a beneficial effect in cardiac surgery patients. Much evidence from randomised controlled trials suggests that albumin improves outcomes in conditions related to chronic liver disease. Similarly, and more relevant to critically ill patients, data from the ALBIOS and SAFE studies suggest beneficial effects on survival in patients with septic shock and/or severe sepsis. As a consequence, several randomised controlled studies are currently underway or being planned to further test the effect of hyperoncotic albumin solutions in septic shock and cardiac surgery patients. The role of albumin therapy in critical illness is yet to be fully explored.
Competing interests
None declared.
References
- 1.Caironi P., Gattinoni L. The clinical use of albumin: the point of view of a specialist in intensive care. Blood Transfus. 2009;7:259–267. doi: 10.2450/2009.0002-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vincent J.L., Russell J.A., Jacob M., et al. Albumin administration in the acutely ill: what is new and where next? Crit Care. 2014;18:231. doi: 10.1186/cc13991. Erratum in: Crit Care 2014; 18: 630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Garcovich M., Zocco M.A., Gasbarrini A. Clinical use of albumin in hepatology. Blood Transfus. 2009;7:268–277. doi: 10.2450/2008.0080-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Adamson R.H., Clark J.F., Radeva M., et al. Albumin modulates S1P delivery from red blood cells in perfused microvessels: mechanism of the protein effect. Am J Physiol Heart Circ Physiol. 2014;306:H1011–H1017. doi: 10.1152/ajpheart.00829.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Evans T.W. Review article: albumin as a drug — biological effects of albumin unrelated to oncotic pressure. Aliment Pharmacol Ther. 2002;16(Suppl 5):6–11. doi: 10.1046/j.1365-2036.16.s5.2.x. [DOI] [PubMed] [Google Scholar]
- 6.Redman C.M. Biosynthesis of serum proteins and ferritin by free and attached ribosomes of rat liver. J Biol Chem. 1969;244:4308–4315. [PubMed] [Google Scholar]
- 7.Jacob M., Paul O., Mehringer L., et al. Albumin augmentation improves condition of guinea pig hearts after 4 hr of cold ischemia. Transplantation. 2009;87:956–965. doi: 10.1097/TP.0b013e31819c83b5. [DOI] [PubMed] [Google Scholar]
- 8.Stevens A.P., Hlady V., Dull R.O. Fluorescence correlation spectroscopy can probe albumin dynamics inside lung endothelial glycocalyx. Am J Physiol Lung Cell Mol Physiol. 2007;293:L328–L335. doi: 10.1152/ajplung.00390.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zoellner H., Höfler M., Beckmann R., et al. Serum albumin is a specific inhibitor of apoptosis in human endothelial cells. J Cell Sci. 1996;109:2571–2580. doi: 10.1242/jcs.109.10.2571. [DOI] [PubMed] [Google Scholar]
- 10.Ishima Y. Albumin-based nitric oxide traffic system for the treatment of intractable cancers. Biol Pharm Bull. 2017;40:128–134. doi: 10.1248/bpb.b16-00867. [DOI] [PubMed] [Google Scholar]
- 11.Garcia-Martinez R., Caraceni P., Bernardi M., et al. Albumin: pathophysiologic basis of its role in the treatment of cirrhosis and its complications. Hepatology. 2013;58:1836–1846. doi: 10.1002/hep.26338. [DOI] [PubMed] [Google Scholar]
- 12.Nasif W.A., Mukhtar M.H., El-Emshaty, Alwazna A.H. Redox state of human serum albumin and inflammatory biomarkers in hemodialysis patients with secondary hyperparathyroidism during oral calcitriol supplementation for vitamin D. Open Med Chem J. 2018;12:98–110. doi: 10.2174/1874104501812010098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zampieri F.G., Kellum J.A., Park M., et al. Relationship between acid–base status and inflammation in the critically ill. Crit Care. 2014;18:R154. doi: 10.1186/cc13993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kouoh F., Gressier B., Luyckx M., et al. Antioxidant properties of albumin: effect on oxidative metabolism of human neutrophil granulocytes. Farmaco. 1999;54:695–699. doi: 10.1016/s0014-827x(99)00082-8. [DOI] [PubMed] [Google Scholar]
- 15.Moran E.C., Kamiguti A.S., Cawley J.C., Pettitt A.R. Cytoprotective antioxidant activity of serum albumin and autocrine catalase in chronic lymphocytic leukaemia. Br J Haematol. 2002;116:316–328. [PubMed] [Google Scholar]
- 16.Iglesias J., Abernethy V.E., Wang Z., et al. Albumin is a major serum survival factor for renal tubular cells and macrophages through scavenging of ROS. Am J Physiol. 1999;277:F711–F722. doi: 10.1152/ajprenal.1999.277.5.F711. [DOI] [PubMed] [Google Scholar]
- 17.Stewart P.A. Modern quantitative acid-base chemistry. Can J Physiol Pharmacol. 1983;61:1444–1461. doi: 10.1139/y83-207. [DOI] [PubMed] [Google Scholar]
- 18.Sudlow G., Birkett D.J., Wade D.N. The characterization of two specific drug binding sites on human serum albumin. Mol Pharmacol. 1975;11:824–832. [PubMed] [Google Scholar]
- 19.Yamasaki K., Hyodo S., Taguchi K., et al. Long chain fatty acids alter the interactive binding of ligands to the two principal drug binding sites of human serum albumin. PLoS One. 2017;12 doi: 10.1371/journal.pone.0180404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang W.-J., Frei B. Albumin selectively inhibits TNF alpha-induced expression of vascular cell adhesion molecule-1 in human aortic endothelial cells. Cardiovasc Res. 2002;55:820–829. doi: 10.1016/s0008-6363(02)00492-3. [DOI] [PubMed] [Google Scholar]
- 21.Beck R., Bertolino S., Abbot S.E., et al. Modulation of arachidonic acid release and membrane fluidity by albumin in vascular smooth muscle and endothelial cells. Circ Res. 1998;83:923–931. doi: 10.1161/01.res.83.9.923. [DOI] [PubMed] [Google Scholar]
- 22.Ramirez-Vick J., Vargas F.F. Albumin modulation of paracellular permeability of pig vena caval endothelium shows specificity for pig albumin. Am J Physiol. 1993;264:H1382–H1387. doi: 10.1152/ajpheart.1993.264.5.H1382. [DOI] [PubMed] [Google Scholar]
- 23.Hahn R.G., Zdolsek M., Hasselgren E., et al. Fluid volume kinetics of 20% albumin. Br J Clin Pharmacol. 2019;85(1303-1):1. doi: 10.1111/bcp.13897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zdolsek M., Hahn R.G., Zdolsek J.H. Recruitment of extravascular fluid by hyperoncotic albumin. Acta Anaesthesiol Scand. 2018;62:1255–1260. doi: 10.1111/aas.13150. [DOI] [PubMed] [Google Scholar]
- 25.Gouverneur M., Spaan J.A.E., Pannekoek H., et al. Fluid shear stress stimulates incorporation of hyaluronan into endothelial cell glycocalyx. Am J Physiol Heart Circ Physiol. 2006;290:H458–H462. doi: 10.1152/ajpheart.00592.2005. [DOI] [PubMed] [Google Scholar]
- 26.Haeger S.M., Liu X., Han X., et al. Epithelial heparan sulfate contributes to alveolar barrier function and is shed during lung injury. Am J Respir Cell Mol Biol. 2018;59:363–374. doi: 10.1165/rcmb.2017-0428OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dull R.O., Dinavahi R., Schwartz L., et al. Lung endothelial heparan sulfates mediate cationic peptide-induced barrier dysfunction: a new role for the glycocalyx. Am J Physiol Lung Cell Mol Physiol. 2003;285:L986–L995. doi: 10.1152/ajplung.00022.2003. [DOI] [PubMed] [Google Scholar]
- 28.Schmidt E.P., Yang Y., Janssen W.J., et al. The pulmonary endothelial glycocalyx regulates neutrophil adhesion and lung injury during experimental sepsis. Nat Med. 2012;18:1217–1223. doi: 10.1038/nm.2843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jacob M., Bruegger D., Rehm M., et al. Contrasting effects of colloid and crystalloid resuscitation fluids on cardiac vascular permeability. Anesthesiology. 2006;104:1223–1231. doi: 10.1097/00000542-200606000-00018. [DOI] [PubMed] [Google Scholar]
- 30.Pries A.R., Kuebler W.M. Normal endothelium. Handb Exp Pharmacol. 2006:1–40. doi: 10.1007/3-540-32967-6_1. [DOI] [PubMed] [Google Scholar]
- 31.Jacob M., Rehm M., Loetsch M., et al. The endothelial glycocalyx prefers albumin for evoking shear stress-induced, nitric oxidemediated coronary dilatation. J Vasc Res. 2007;44:435–443. doi: 10.1159/000104871. [DOI] [PubMed] [Google Scholar]
- 32.Rehm M., Zahler S., Lötsch M., et al. Endothelial glycocalyx as an additional barrier determining extravasation of 6% hydroxyethyl starch or 5% albumin solutions in the coronary vascular bed. Anesthesiology. 2004;100(121):1–23. doi: 10.1097/00000542-200405000-00025. [DOI] [PubMed] [Google Scholar]
- 33.Milford E.M., Reade M.C. Resuscitation fluid choices to preserve the endothelial glycocalyx. Crit Care. 2019;23:77. doi: 10.1186/s13054-019-2369-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zeng Y., Adamson R.H., Curry F.R.E., Tarbell J.M. Sphingosine-1-phosphate protects endothelial glycocalyx by inhibiting syndecan-1 shedding. Am J Physiol Heart Circ Physiol. 2014;306:H363–H372. doi: 10.1152/ajpheart.00687.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jacob M., Bruegger D., Rehm M., et al. The endothelial glycocalyx affords compatibility of Starling’s principle and high cardiac interstitial albumin levels. Cardiovasc Res. 2007;73:575–586. doi: 10.1016/j.cardiores.2006.11.021. [DOI] [PubMed] [Google Scholar]
- 36.Adamson R.H., Clark J.F., Radeva M., et al. Albumin modulates S1P delivery from red blood cells in perfused microvessels: mechanism of the protein effect. Am J Physiol Heart Circ Physiol. 2014;306:H1011–H1017. doi: 10.1152/ajpheart.00829.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zoellner H., Hou J.Y., Lovery M., et al. Inhibition of microvascular endothelial apoptosis in tissue explants by serum albumin. Microvasc Res. 1999;57:162–173. doi: 10.1006/mvre.1998.2126. [DOI] [PubMed] [Google Scholar]
- 38.Bolitho C., Bayl P., Hou J.Y., et al. The anti-apoptotic activity of albumin for endothelium is mediated by a partially cryptic protein domain and reduced by inhibitors of G-coupled protein and PI-3 kinase, but is independent of radical scavenging or bound lipid. J Vasc Res. 2007;44:313–324. doi: 10.1159/000101777. [DOI] [PubMed] [Google Scholar]
- 39.Watts J.A., Maiorano P.C. Trace amounts of albumin protect against ischemia and reperfusion injury in isolated rat hearts. J Mol Cell Cardiol. 1999;31:1653–1662. doi: 10.1006/jmcc.1999.1001. [DOI] [PubMed] [Google Scholar]
- 40.Koh J.S., Lieberthal W., Heydrick S., Levine J.S. Lysophosphatidic acid is a major serum noncytokine survival factor for murine macrophages which acts via the phosphatidylinositol 3-kinase signaling pathway. J Clin Invest. 1998;102:716–727. doi: 10.1172/JCI1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hoogerwerf N., Zijlstra E.J., van der Linden P.J., et al. Endothelium function is protected by albumin and flow-induced constriction is independent of endothelium and tone in isolated rabbit femoral artery. J Vasc Res. 1992;29:367–375. doi: 10.1159/000158953. [DOI] [PubMed] [Google Scholar]
- 42.Fagyas M., Úri K., Siket I.M., et al. New perspectives in the rennin-angiotensin-aldosterone system (RAAS) II: albumin suppresses angiotensin converting enzyme (ACE) activity in human. PLoS One. 2014;9 doi: 10.1371/journal.pone.0087844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bihari S., Wiersema U.F., Perry R., et al. Efficacy and safety of 20% albumin fluid loading in healthy subjects: a comparison of four resuscitation fluids. J Appl Physiol. 2019;126:1646–1660. doi: 10.1152/japplphysiol.01058.2018. [DOI] [PubMed] [Google Scholar]
- 44.Imhof B.A., Aurrand-Lions M. Angiogenesis and inflammation face off. Nat Med. 2006;12:171–172. doi: 10.1038/nm0206-171. [DOI] [PubMed] [Google Scholar]
- 45.Stefanec T. Endothelial apoptosis: could it have a role in the pathogenesis and treatment of disease? Chest. 2000;117:841–854. doi: 10.1378/chest.117.3.841. [DOI] [PubMed] [Google Scholar]
- 46.Minchiotti L., Caridi G., Campagnoli M., et al. Diagnosis, phenotype, and molecular genetics of congenital analbuminemia. Front Genet. 2019;10:336. doi: 10.3389/fgene.2019.00336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kremer H., Baron-Menguy C., Tesse A., et al. Human serum albumin improves endothelial dysfunction and survival during experimental endotoxemia: concentration-dependent properties. Crit Care Med. 2011;39:1414–1422. doi: 10.1097/CCM.0b013e318211ff6e. [DOI] [PubMed] [Google Scholar]
- 48.Bevers L.M., van Faassen E.E., Vuong T.D., et al. Low albumin levels increase endothelial NO production and decrease vascular NO sensitivity. Nephrol Dial Transplant. 2006;21:3443–3449. doi: 10.1093/ndt/gfl443. [DOI] [PubMed] [Google Scholar]
- 49.Marelli D., Paul A., Samson R., et al. Does the addition of albumin to the prime solution in cardiopulmonary bypass affect clinical outcome? A prospective randomized study. J Thorac Cardiovasc Surg. 1989;98:751–756. [PubMed] [Google Scholar]
- 50.Roohk H.V., Zaidi A.R., Patel D. Glycated albumin (GA) and inflammation: role of GA as a potential marker of inflammation. Inflamm Res. 2018;67:21–30. doi: 10.1007/s00011-017-1089-4. [DOI] [PubMed] [Google Scholar]
- 51.Nathan D.M., McGee P., Steffes M.W., Lachin J.M., DCCT/EDIC Research Group Relationship of glycated albumin to blood glucose and HbA1C values and to retinopathy, nephropathy, and cardiovascular outcomes in the DCCT/EDIC study. Diabetes. 2014;63 doi: 10.2337/db13-0782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Song F., Schmidt A.M. Glycation and insulin resistance: novel mechanisms and unique targets? Arterioscler Thromb Vasc Biol. 2012;32:1760–1765. doi: 10.1161/ATVBAHA.111.241877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Levin M., Cunnington A.J., Wilson C., et al. Effects of saline or albumin fluid bolus in resuscitation: evidence from re-analysis of the FEAST trial. Lancet Resp Med. 2019;7:581–593. doi: 10.1016/S2213-2600(19)30114-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mallat J., Meddour M., Lemyze M., et al. Effects of a rapid infusion of 20% human serum albumin solution on acid-base status and electrolytes in critically ill patients. Int Care Med. 2016;42:128–129. doi: 10.1007/s00134-015-4080-y. [DOI] [PubMed] [Google Scholar]
- 55.Mårtensson J., Bihari S., Bannard-Smith J., et al. Small volume resuscitation with 20% albumin in intensive care: physiological effects: the SWIPE randomised clinical trial. Intensive Care Med. 2018;44:1797–1806. doi: 10.1007/s00134-018-5253-2. [DOI] [PubMed] [Google Scholar]
- 56.Bannard-Smith J., Alexander P., Glassford N., et al. Haemodynamic and biochemical responses to fluid bolus therapy with human albumin solution, 4% versus 20%, in critically ill adults. Crit Care Resusc. 2015;17:122–128. [PubMed] [Google Scholar]
- 57.Lakshmi P., Mondal M., Ramadas K., Natarajan S. Molecular interaction of 2,4-diacetylphloroglucinol (DAPG) with human serum albumin (HSA): The spectroscopic, calorimetric and computational investigation. Spectrochim Acta A Mol Biomol Spectrosc. 2017;183:90–102. doi: 10.1016/j.saa.2017.04.012. [DOI] [PubMed] [Google Scholar]
- 58.Narwal M., Kumar D., Mukherjee T.K., et al. Molecular dynamics simulation as a tool for assessment of drug binding property of human serum albumin. Mol Biol Rep. 2018;45:1647–1652. doi: 10.1007/s11033-018-4308-3. [DOI] [PubMed] [Google Scholar]
- 59.Seedher N., Agarwal P. Competitive binding of fluoroquinolone antibiotics and some other drugs to human serum albumin: a luminescence spectroscopic study. Luminescence. 2013;28:562–568. doi: 10.1002/bio.2494. [DOI] [PubMed] [Google Scholar]
- 60.Morales D., Pacurariu A., Slattery J., et al. Association between peripheral neuropathy and exposure to oral fluoroquinolone or amoxicillin-clavulanate therapy. JAMA Neurol. 2019;76:827–833. doi: 10.1001/jamaneurol.2019.0887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Reves J.G., Newfield P., Smith L.R. Influence of serum protein, serum albumin concentrations and dose on midazolam anaesthesia induction times. Can Anaesth Soc J. 1981;28:556–560. doi: 10.1007/BF03007152. [DOI] [PubMed] [Google Scholar]
- 62.Fanali G., di Masi A., Trezza V., et al. Human serum albumin: from bench to bedside. Mol Aspects Med. 2012;33:209–290. doi: 10.1016/j.mam.2011.12.002. [DOI] [PubMed] [Google Scholar]
- 63.Alves F.C., Sun J., Qureshi A.R., et al. The higher mortality associated with low serum albumin is dependent on systemic inflammation in end-stage kidney disease. PLoS One. 2018;13 doi: 10.1371/journal.pone.0190410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Arroyo V., Garcia-Martinez R., Salvatella X. Human serum albumin, systemic inflammation, and cirrhosis. J Hepatol. 2014;61:396–407. doi: 10.1016/j.jhep.2014.04.012. [DOI] [PubMed] [Google Scholar]
- 65.Fernández J., Clària J., Amorós A., et al. Effects of albumin treatment on systemic and portal hemodynamics and systemic inflammation in patients with decompensated cirrhosis. Gastroenterology. 2019;157:149–162. doi: 10.1053/j.gastro.2019.03.021. [DOI] [PubMed] [Google Scholar]
- 66.Fleck A., Raines G., Hawker F., et al. Increased vascular permeability: a major cause of hypoalbuminaemia in disease and injury. Lancet. 1985;1:781–784. doi: 10.1016/s0140-6736(85)91447-3. [DOI] [PubMed] [Google Scholar]
- 67.Komaromi A., Estenberg U., Hammarqvist F., et al. Simultaneous assessment of the synthesis rate and transcapillary escape rate of albumin in inflammation and surgery. Critical Care. 2016;20:370. doi: 10.1186/s13054-016-1536-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ishida S., Hashimoto I., Seike T., et al. Serum albumin levels correlate with inflammation rather than nutrition supply in burns patients: a retrospective study. J Med Invest. 2014;61:361–368. doi: 10.2152/jmi.61.361. [DOI] [PubMed] [Google Scholar]
- 69.Barle H., Hammarqvist F., Westman B., et al. Synthesis rates of total liver protein and albumin are both increased in patients with an acute inflammatory response. Clin Sci. 2006;110:93–99. doi: 10.1042/CS20050222. [DOI] [PubMed] [Google Scholar]
- 70.Komaromi A., Estenberg U., Hammarqvist F., et al. Simultaneous assessment of the synthesis rate and transcapillary escape rate of albumin in inflammation and surgery. Crit Care. 2016;20:370. doi: 10.1186/s13054-016-1536-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Phakdeekitchareon B., Boonyawat K. The added-up albumin enhances the diuretic effect of furosemide in patients with hypoalbuminaemic chronic kidney disease: a randomized controlled study. BMC Neph. 2012;13:92. doi: 10.1186/1471-2369-13-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Martin G.S., Moss M., Wheeler A.P., et al. A randomized, controlled trial of furosemide with or without albumin in hypoproteinemic patients with acute lung injury. Crit Care Med. 2005;33:1681–1687. doi: 10.1097/01.ccm.0000171539.47006.02. [DOI] [PubMed] [Google Scholar]
- 73.Urien S., Nguyen P., Berlioz S., et al. Characterization of discrete classes of binding sites of human serum albumin by application of thermodynamic principles. Biochem J. 1994;302:69–72. doi: 10.1042/bj3020069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Matejtschuk P., Dash C.H., Gascoigne E.W. Production of human albumin solution: a continually developing colloid. Br J Anaesth. 2000;85:887–895. doi: 10.1093/bja/85.6.887. [DOI] [PubMed] [Google Scholar]
- 75.Study Investigators S.A.F.E., Finfer S., McEvoy S., Bellomo R., et al. Impact of albumin compared with saline on organ function and mortality of patients with severe sepsis. Intensive Care Med. 2011;37:86–96. doi: 10.1007/s00134-010-2039-6. [DOI] [PubMed] [Google Scholar]
- 76.Caironi P., Tognoni G., Masson S., et al. ALBIOS Study Investigators. Albumin replacement in patients with severe sepsis or septic shock. N Engl J Med. 2014;370:1412–1421. doi: 10.1056/NEJMoa1305727. [DOI] [PubMed] [Google Scholar]
- 77.Caraceni P., Riggio O., Angeli P., et al. Long-term albumin administration in decompensated cirrhosis (ANSWER): an open-label randomised trial. Lancet. 2018;391:2417–2429. doi: 10.1016/S0140-6736(18)30840-7. [DOI] [PubMed] [Google Scholar]
- 78.Cavallin M., Kamath P.S., Merli M., et al. Terlipressin plus albumin versus midodrine and octreotide plus albumin in the treatment of hepatorenal syndrome: a randomized trial. Hepatology. 2015;62:567–574. doi: 10.1002/hep.27709. [DOI] [PubMed] [Google Scholar]
- 79.Bernardi M., Caraceni P., Navickis R.J., et al. Albumin infusion in patients undergoing large-volume paracentesis: a meta-analysis of randomized trials. Hepatology. 2012;55(1):172–181. doi: 10.1002/hep.24786. [DOI] [PubMed] [Google Scholar]
- 80.Connelly-Smith L., Dunbar N.M. The 2019 guidelines from the American Society for Apheresis: what’s new? Curr Opin Hematol. 2019;26:461–465. doi: 10.1097/MOH.0000000000000534. [DOI] [PubMed] [Google Scholar]
- 81.SAFE Study Investigators, Australian and New Zealand Intensive Care Society Clinical Trials Group, Australian Red Cross Blood Service, George Institute for International Health, Myburgh J., Cooper D.J., Finfer S., et al. Saline or albumin for fluid resuscitation in patients with traumatic brain injury. N Engl J Med. 2007;357:874–884. doi: 10.1056/NEJMoa067514. [DOI] [PubMed] [Google Scholar]
- 82.Cooper D.J., Myburgh J., Heritier S., et al. Albumin resuscitation for traumatic brain injury: is intracranial hypertension the cause of increased mortality? J Neurotrauma. 2013;30:512–518. doi: 10.1089/neu.2012.2573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Iguchi N., Kosaka J., Bertolini J., et al. Differential effects of isotonic and hypotonic 4% albumin solution on intracranial pressure and renal perfusion and function. Crit Care Resusc. 2018;20:48–53. [PubMed] [Google Scholar]
- 84.Myburgh J.A. The evidence for small-volume resuscitation with hyperoncotic albumin in critical illness. Crit Care. 2008;12:143. doi: 10.1186/cc6882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lee E.-H., Kim W.-J., Kim J.-Y., et al. Effect of exogenous albumin on the incidence of postoperative acute kidney injury in patients undergoing off-pump coronary artery bypass surgery with a preoperative albumin level of less than 4.0 g/dl. Anesthesiology. 2016;124:1001–1011. doi: 10.1097/ALN.0000000000001051. [DOI] [PubMed] [Google Scholar]
- 86.Wigmore G.J., Anstey J.R., St John A., et al. 20% human albumin solution fluid bolus administration therapy in patients after cardiac surgery (the HAS FLAIR Study) J Cardiothorac Vasc Anesth. 2019;33:2920–2927. doi: 10.1053/j.jvca.2019.03.049. [DOI] [PubMed] [Google Scholar]
- 87.Bansch P., Statkevicius S., Bentzer P. Plasma volume expansion with 5% albumin compared with Ringer’s acetate during normal and increased microvascular permeability in the rat. Anesthesiology. 2014;121:817–824. doi: 10.1097/ALN.0000000000000363. [DOI] [PubMed] [Google Scholar]
- 88.Hasselgren E., Zdolsek M., Zdolsek J.H., et al. Long intravascular persistence of 20% albumin in postoperative patients. Anesth Analg. 2019;129:1232–1239. doi: 10.1213/ANE.0000000000004047. [DOI] [PubMed] [Google Scholar]
- 89.Primack W.A., Estes K. Fluid resuscitation in the intensive care unit. N Engl J Med. 2004:1905–1908. author reply 1905-8. [PubMed] [Google Scholar]