Abstract
Objective: To quantify aerosol generation from respiratory interventions and the effectiveness of their removal by a personal ventilation hood.
Design and setting: Determination of the aerosol particle generation (in a single, healthy volunteer in a clean room) associated with breathing, speaking, wet coughing, oxygen (O2) 15 L/min via face mask, O2 60 L/min via nasal prongs, bilevel non-invasive positive-pressure ventilation (BiPAP) and nebulisation with O2 10 L/min.
Interventions: Aerosol generation was measured with two particle sizer and counter devices, focusing on aerosols 0.5–5 μm (human-generated aerosols), with and without the hood. An increase from baseline of less than 0.3 particles per mL was considered a low level of generation.
Main outcome measures: Comparisons of aerosol generation between different respiratory interventions. Effectiveness of aerosol reduction by a personal ventilation hood.
Results: Results for the 0.5–5 μm aerosol range. Quiet breathing and talking demonstrated very low increase in aerosols (< 0.1 particles/mL). Aerosol generation was low for wet coughing (0.1 particles/mL), O2 15 L/min via face mask (0.18 particles/mL), and high flow nasal O2 60 L/min (0.24 particles/mL). Non-invasive ventilation generated moderate aerosols (29.7 particles/mL) and nebulisation very high aerosols (1086 particles/mL); the personal ventilation hood reduced the aerosol counts by 98% to 0.5 particles/mL and 8.9 particles/mL respectively.
Conclusions: In this human volunteer study, the administration of O2 15 L/min by face mask and 60 L/min nasal therapy did not increase aerosol generation beyond low levels. Non-invasive ventilation caused moderate aerosol generation and nebulisation therapy very high aerosol generation. The personal ventilation hood reduced the aerosol counts by at least 98%.
The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has caused the coronavirus disease 2019 (COVID-19) pandemic, affecting millions of people worldwide.1 The World Health Organization has indicated that 14% of patients with COVID-19 require hospitalisation with oxygen (O2) support, and 5% of patients require intensive care unit (ICU) admission.1 SARS-CoV-2 is transmitted by droplet spread and contact routes, although aerosol spread is considered possible.2, 3, 4, 5 Suspended liquid and solid particles in the air generated by human respiration, communication and coughing are generally around 1 μm, but range from 0.5 μm to 20 μm in size.2, 6 Particles with diameters less than 5 μm are labelled aerosols2, 3, 6 and can remain suspended in the air for some hours. Particles greater than 5 μm are considered droplets and fall out of the air more quickly.2, 7
Many respiratory interventions performed in the ICU, such as the nebulised administration of drugs, intubation and extubation, high flow nasal O2, and non-invasive ventilation, are considered aerosol generating.8, 9 In the ICU and general hospital setting, interventions to protect health care workers and other patients from cross-infection are routinely used. These include the use of personal protective equipment, standard isolation rooms (Class S) and airborne infection isolation rooms (Class N). Negative pressure rooms are recommended for the management of patients with confirmed or suspected infectious diseases with airborne spread and for the conduct of aerosol generating prodcedures.10 In Victoria, Australia, Class N isolation rooms operate at negative 30 Pa and provide at least 12 air exchanges per hour.10 While the number of negatively pressured rooms varies between hospitals and ICUs, they are a relatively scarce resource.
We describe a novel personal ventilation hood that provides a physical barrier between the patient and environment, negative pressure within the hood, and high efficiency particulate air (HEPA) filtration of expired air.11 We hypothesised that the personal ventilation hood had the potential to reduce the transmission to health care workers of infectious diseases spread by droplets and aerosols and to allow the safer provision to patients of treatments considered aerosol generating. To test these hypotheses, we determined the degree of aerosol and droplet production during standard face O2 therapy, high flow nasal O2 therapy, non-invasive ventilation, and nebulizer therapy, and the effect of the personal ventilation hood on aerosol and droplet environmental contamination.
Methods
Aerosol particle measurements
Data were obtained from an Aerodynamic Particle Sizer (APS) spectrometer (TSI APS 3220) and a Scanning Mobility Particle Sizer (SMPS) spectrometer (TSI SMPS 3080, with 3081 column). The APS counts aerosol numbers and sizes of aerosol with aerodynamic diameters between 0.5 μm and 20 μm (most of the respiratory size range)6 by measuring the time of flight between two laser beams of the aerosols in an accelerated air flow. The SMPS determines particle size distribution between 10 nm and 660 nm based on particle electrical mobility.12 Both instruments are more sensitive than those routinely used for clean room monitoring.13, 14, 15 Aerosol samples were measured through a 0.25 inches silicon inlet tubing (length = 2300 mm to APS and 2590 mm to SMPS). With inlet flow rates of 5 L/min and 0.3 L/min for APS and SMPS respectively, the transmission of aerosols larger than 5 μm was impossible. Our results were thus limited to particles smaller than 5 μm, although more than 95% of all human aerosols (by number) are smaller than 5 μm.6
Aerosol measurements occurred in a clean room (volume 34 m3, HEPA-filtered with six air changes per hour) at the Department of Mechanical Engineering, University of Melbourne, Australia. Clean rooms are defined by the International Organization for Standardization (ISO: 14644-1: 2015)16 according to aerosol levels. We used the clean room as a negative control; the aerosol test results were compared with the background aerosol loadings stable and minimised.
To our knowledge, no agreed definition of the degree of aerosol particle generation related to medical procedures exists. The ISO clean room standard defines that the maximum number of particles 0.5 μm or greater in an ISO 7 (pharmaceutical) clean room is 0.352 particles per mL.16 We conservatively defined low aerosol generation as an increase in mean particle concentration 0.3 particles per mL (cm–3).
The personal ventilation hood
The hood (Figure 1) consists of a mobile steel and plastic frame, a fan and a standard HEPA H13 filter (rated to 99.97% clearance of 0.3 μm particles). The plastic barrier opens out to a hood of about 1.3 m3 volume. The barrier contains droplets, while the ventilation reduces aerosol escape. Air is drawn from the sides and front near the patient's legs up to the rear, and routinely away from the health care worker (principally at the front and sides of the patient). The air passes through the fan (Westaflex, Melbourne, Australia; airflow = 40 L/s) and a HEPA H13 filter (Techtronic Industries, Hong Kong, China), thereafter returning to surrounding air.
Figure 1.
Photo of the personal protective ventilation hood*
* Air flows from the left hand side (lower body) to the rear of the hood to a fan and a high efficiency particulate air (HEPA) filter at the rear (right hand side). Location: clean room at the Department of Mechanical Engineering, University of Melbourne. Jason Monty (pictured) has given consent for this photo to be published. Aspects of the pram hood's development are found at https://www.youtube.com/watch?v=McQGJpEIqGk&feature=youtu.be
Volunteer testing
Ethics approval for this study of droplet and aerosol (aerosol) measurements from one healthy, male volunteer (first author) was deemed not required by the Western Health Research Ethics manager. Only the volunteer entered the clean room; the scientist sat immediately outside monitoring aerosol concentrations. Aerosol concentrations rose upon the volunteer's room entry. Experiments did not commence until levels returned to baseline; typically after 10–20 minutes. Measurements were performed on 9 and 14–16 April 2020 and in the first week of May 2020.
The aerosol inlet sampled air at 6.8 L/min from outside of where the plastic hood folded down, about at average face height for a health care worker. The inlet was 18 cm rightward of the bed at a floor height of 155 cm, 65 cm directly away from the patient's head and 80 cm from the hood articulation point (where the plastic hood is pulled down).
We measured aerosolisation in seven scenarios:
-
•
quiet breathing;
-
•
talking (counting loudly continuously);
-
•
wet coughing;
-
•
dry face mask at 15 L/min O2 flow;
-
•
high flow O2 (30 L/min and 60 L/min) humidified nasal cannulae;
-
•
bilevel non-invasive positive-pressure (BiPAP) (5 positive end-expiratory pressure [PEEP], plus 10 continuous positive airway pressure [CPAP]) with humidification; and
-
•
5 mL nebulised saline with face mask at O2 10 L/min.
All seven tests were performed over 12 minutes each. Each subsequent test occurred after the stabilisation of background aerosol levels. The SMPS scanned its aerosol size range every 2 minutes, while the APS scanned every second, averaged to 19-second measurements. All scenarios were studied twice, once with the hood open and the fan off, and once with hood closed and the fan on.
Breathing, talking and coughing were directed towards the measurement port (worst case scenario of a patient–health care worker interaction). Wet cough was achieved with 5 seconds of forced coughing, 25 seconds resting, and drinking 25 mL water, then repeated. High flow humidified nasal prongs therapy was administered by an Airvo (F&P 950; Fisher and Paykel, Auckland, New Zealand). Non-invasive ventilation was administered using a face mask by a Dräger Oxylog 3000 (Lübeck, Germany) with humidification with an F&P 950 humidifier. The non-invasive ventilation mask was deliberately set to be "leaky", to simulate a worst case aerosol generation scenario, by inserting a nasogastric tube 15 cm within the mask.
Repeated nebuliser aerosolisation against the personal ventilation hood
To test the ventilation hood further, we used compressed air and the same nebuliser mask alone (no volunteer) as an aerosol source placed 50 cm above bed level. We ran two experiments: one with the aerosol generation similar to the nebuliser and one at a similar level to BiPAP; 15 tests planned for each experimental group. We added 5 mL 0.9% saline to the nebuliser with each 15-minute testing run. We performed 15 test pairs (hood down/fan on; hood up/fan off) for each source strength.
Data analysis
Descriptive statistics were calculated for the volunteer's testing and for the 30 planned, repeated nebulisation tests: mean, median, total particle numbers, and particle sizes. The particle size distribution with 25th–75th and 10th-90th percentiles were displayed as box-and-whisker (Tukey) plots. The mean aerosol concentrations from the prior background count were subtracted to determine the perturbations above background for each test.
Results
Background aerosol counts testing
We used a clean room level equivalent to ISO-14644-1: 2015 Level 7: less than 0.2 particles per mL for particles greater than 0.5 μm.16 All background periods were chosen such that we had at least 10 minutes of stable concentrations in the APS total concentrations before commencing the next testing sequence. The background aerosol conditions that were achieved within the clean room can be found in the Online Appendix, section 1.
Volunteer testing
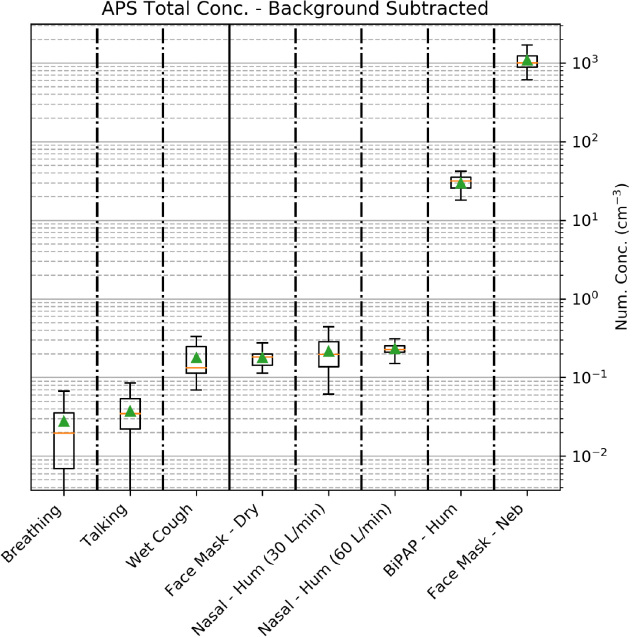
Figure 2 displays the APS results on a logarithmic y-axis from breathing and talking through to nebulisation. Aerosol generation was similar from wet coughing, dry O2 at 15 L/min via face mask, and high flow (30 L/min or 60 L/min) humidified air via nasal prongs. All were substantially less than one aerosol particle per mL. Humidified, leaky BiPAP (10 cmH20 CPAP, 5 cmH20 PEEP) had about a 100-fold greater aerosol generation compared with high flow nasal cannulae. Nebulised 0.9% saline (a source of aerosols) with O2 at 10 L/min increased aerosol generation a further 30-fold beyond BiPAP.
Figure 2.
Total concentrations of aerosols from the respiratory interventions*
APS = Aerodynamic Particle Sizer spectrometer; BiPAP = bilevel non-invasive positive-pressure; conc = concentrations; hum = humidified; neb = nebulised. * APS (TSI APS 3220) data only. Background concentrations have been subtracted from these results. No hood (ie, hood open), no fan. For the logarithmic y-axis, number concentrations (cm-3) = aerosol number per cm3 (mL). The box-and-whiskers plot (standard Tukey) shows the integrated aerosol number concentrations measured by the APS with the mean background concentration subtracted from all values. Green triangles represent means, orange lines are medians, boxes are 25th and 75th percentiles, and whiskers are the 10th and 90th percentiles respectively. Aerosol generation from different treatment scenarios: patient sitting on bed breathing; patient talking (counting 1–100 and back); patient coughing for 5 seconds, every 30 seconds taking an aliquot of water before each coughing fit; patient fitted with a humidified nasal cannula; patient with a face mask on with dry oxygen flowing; patient with a leaky BiPAP mask fitted; patient with a face mask on with a nebuliser operating.
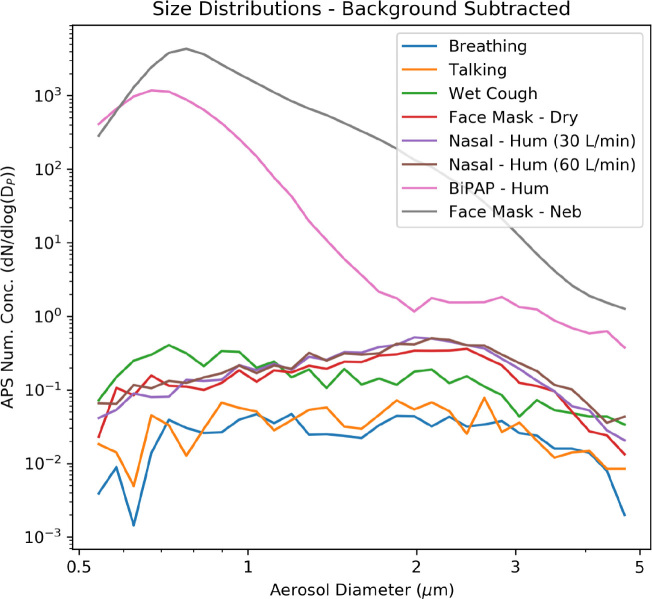
The distribution of aerosol particle sizes is shown in Figure 3. Aerosol counts at all sizes are dominated by nebulisation, followed by non-invasive ventilation. Wet coughing, dry O2 at 15 L/min via face mask, and high flow (30 L/min or 60 L/min) humidified air via nasal prongs all had similar aerosol quantities and size distributions.
Figure 3.
Aerosol size distributions from respiratory interventions*
APS = Aerodynamic Particle Sizer spectrometer; BiPAP = bilevel non-invasive positive-pressure; conc = concentrations; hum = humidified; neb = nebulised. * APS (TSI APS 3220) data only. Background concentrations subtracted from results. Hood open, no fan. For the logarithmic y-axis, number concentrations-3 = aerosol number per cm3. As for Figure 2 but showing the aerosol size distributions, in log scale, from 0.5 μm to 5 μm. The upper limit (5 μm) was restricted to aerosol sizes where a measurable change was observed above background; that is, very few aerosols greater than 5 μm were generated by any respiratory intervention. Note that size distributions represent the mean of the experiment period, with the mean of the immediately preceding background period subtracted in each size bin.
The Online Appendix, section 2, presents detailed results for the aerosol counts and diameters for all aerosol generating scenarios:
-
•
breathing quietly;
-
•
talking;
-
•
wet coughing;
-
•
dry face mask at 15 L/min O2 flow;
-
•
high air flow (30 L/min and 60 L/min) via nasal cannulae;
-
•
leaky (nasogastric tube inserted) non-invasive ventilation (10 CPAP plus 5 PEEP); and
-
•
10 L/min via nebuliser.
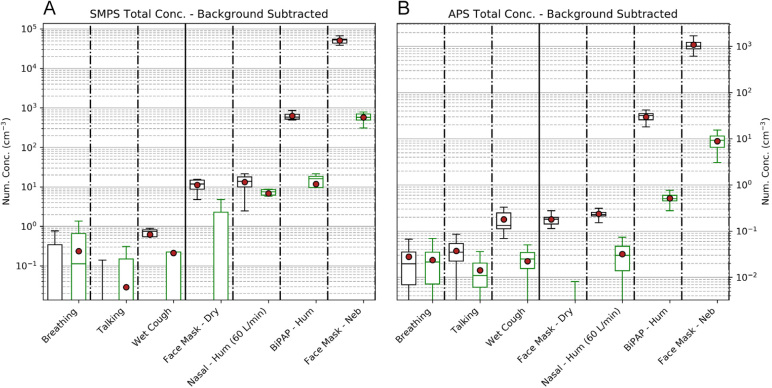
The effectiveness of the personal ventilation hood on SMPS and APS aerosol measurements is shown in Figure 4 — Table 1 presents the data contained within Figure 4. The aerosol generation for breathing and talking was minimal. For procedures associated with low aerosol generation (coughing, dry O2 via face mask, and high flow nasal cannulae), the hood reduced the aerosol count to the very low levels observed during breathing and talking without the hood.
Figure 4.
Hood effectiveness against aerosol generation*
APS = Aerodynamic Particle Sizer spectrometer; BiPAP = bilevel non-invasive positive-pressure; conc = concentrations; hum = humidified; neb = nebulised; SMPS = scanning mobility particle sizer spectrometer. * Background concentrations have been subtracted from results. Hood open, fan off. For the logarithmic y-axis, number concentrations –3 = aerosol number per cm3. SMPS (TSI SMPS 3080) and APS (TSI APS 3220) aerosol data with box-and-whisker plots (means as red dots) with the backgrounds subtracted. Size distributions represent the mean of the experiment period, with the mean of the immediately preceding background period subtracted in each size bin. Panel A: Aerosols with diameters 80–660 nm measured using the SMPS. Panel B: Aerosols with diameters 0.5–5 μm measured using the APS. The upper limit was restricted to aerosol sizes where a measurable change above background count was observed (5 μm). For each of the seven respiratory interventions, there are two box-and-whisker plots (black and green). The black, left handed sided box is for hood open/fan off. The green right hand sided box is for hood closed/fan on. Because of the logarithmic y-axis, the ranges (box sizes) appear greater for respiratory interventions such as breathing and talking, although these in fact have smaller aerosol numbers.
Table 1.
Aerosol generating procedures and the effects of the personal ventilation hood
| SMPS* data: particle counts (particles/mL) |
APS† data: particle counts (particles/mL) |
|||
|---|---|---|---|---|
| No hood Mean (25–75%) | Hood Mean (25–75%) | No hood Mean (25–75%) | Hood Mean (25–75%) | |
| Breathing | 0.0 | 0.2 | 0.03 | 0.02 |
| (–0.5 to 0.3) | (–0.2 to 0.7) | (0.01–0.04) | (0.01–0.03) | |
| Talking | –0.6 | 0.0 | 0.04 | 0.01 |
| (–0.9 to –0.4) | (–0.1 to 0.2) | (0.02–0.05) | (0.01–0.02) | |
| Wet cough | 0.6 | 0.2 | 0.18 | 0.02 |
| (0.6–0.8) | (–0.0 to 0.2) | (0.11–0.25) | (0.02–0.03) | |
| Dry face mask | 11.2 | –0.10 | 0.18 | –0.02 |
| (8.7–14.9) | (–2.5 to 2.3) | (0.10–0.20) | (–0.03 to –0.01) | |
| 60 L/min (nasal, humidified) | 13.2 | 6.8 | 0.24 | 0.03 |
| (10.0–17.8) | (6.1–8.5) | (0.21–0.25) | (0.01–0.05) | |
| BiPAP (humidified) | 630 | 11.8 | 29.7 | 0.51 |
| (520–690) | (9.6–18.3) | (25.7–35.2) | (0.45–0.59) | |
| Nebuliser | 51 000 | 570 | 1080 | 8.9 |
| (43 000–54 000) | (500–700) | (880–1200) | (6.6–11.6) | |
APS = Aerodynamic Particle Sizer spectrometer; BiPAP = bilevel non-invasive positive-pressure; SMPS = scanning mobility particle sizer spectrometer.
TSI SMPS 3080.
TSI APS 3220.
The application of the hood during non-invasive ventilation and nebuliser therapy reduced aerosol counts measured by SMPS and APS at a typical location of a health care worker by over 98% to a low level (Table 1).
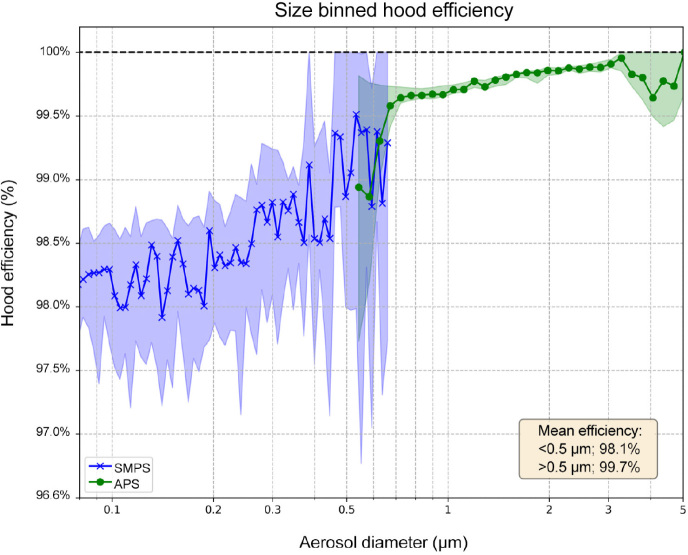
From the originally planned sample of 30 pairs of tests, one APS and three SMPS measurements were excluded because experimental conditions did not equilibrate. Efficiencies were calculated as the averages of these 29 (APS) and 27 (SMPS) tests. Figure 5 shows the percentage of hood efficiency (ie, percentage of nebulised aerosols captured by the hood) and associated data. For Experiment 1 (nebuliser levels), the APS/SMPS aerosol counts per mL for inside and outside the ventilation hood were 2.9/1100 and 1100/77 000 respectively. For experiment 2 (nebuliser aerosolisation similar to BiPAP levels), the APS/SMPS counts per mL for inside and outside the ventilation hood were 0.49/120 and 280/12 000 respectively. The efficiencies were calculated individually for each experiment pair with the different source strengths, then averaged together to create the final efficiencies and the size versus efficiency curve. Overall, the hood was shown to remove more than 99.7% of particles larger than 0.5 μm (those most relevant to human generation) and more than 98.1% of those smaller than 0.5 μm.
Figure 5.
The personal ventilation hood’s effectiveness against repeated nebuliser aerosol generation
APS = Aerodynamic Particle Sizer spectrometer (> 0.5 μm aerosols); SMPS = scanning mobility particle sizer spectrometer (< 0.5 μm aerosols). * Aerosols measured consecutively from inside and outside the hood. Hood efficiency = 1 (number of particles measured outside hood/total number of particles introduced in the hood) as a percentage. Blue (SMPS [TSI SMPS 3080] data) and green (APS [TSI APS 3220] data) curves represent mean results. Blue and green shading represent the 10–90% ranges. The efficiencies were calculated individually for each experiment pair with the two different aerosol source strengths, then averaged together to create the final efficiencies and size versus efficiency curve.
Discussion
For the first time, to our knowledge, we evaluated aerosol generation associated with common respiratory therapies in a clean room. We found that non-invasive ventilation and nebuliser therapy were significant aerosol generating procedures. However, high flow, humidified nasal O2 (60 L/min) and O2 via face mask (15 L/min) only caused a slight increase in the aerosol count. A novel personal ventilation hood reduced the very large number of 0.5–5 μm aerosols (most of the normal human range)6 generated by repeated nebuliser therapy by 98%.
Several recent studies exist of the effects of high flow nasal cannulae on aerosol spread. Loh and colleagues17 showed that high flow nasal cannulae increased food dye (droplet) spread when coughing occurred. Leung et al,18 however, indicated no increase in bacterial contamination of nearby surfaces when patients with gram-negative pneumonia received high flow nasal cannulae in lieu of standard O2 masks.18 Recently, Iwashyna and colleagues19 reported no increase in aerosol generation during the use of humidified high flow nasal cannulae. However, their study was not conducted in a clean room and the aerosol background concentration was high and fluctuated considerably (1000–8000 particles per mL).19 A significant change in aerosol concentration could easily have been missed with such background conditions. In our study in a clean room, we observed an increase in SMPS (smaller particles) count by only 13 particles per mL with high flow nasal O2 and by 630 particles per mL with non-invasive ventilation.
Most aerosols (< 5 μm diameter) and droplets (> 5 μm diameter)2 generated by humans are 0.5–20 μm, with most being less than 5 μm.6 The spread of SARS-CoV-2 is primarily by droplets, but controversy reigns over whether it spreads via aerosols.4, 5, 20 There is concern regarding the potential for health care worker infection during aerosol generating procedures. Our data indicate that O2 therapy via a face mask and high flow nasal O2 minimally increase aerosol generation. Non-invasive ventilation, however, caused moderate to high aerosol generation extensively, and unlike during nebulisation, the aerosol source was the human volunteer. We were unable to find any previous published data regarding aerosol generation with non-invasive ventilation, and postulate that it is the higher pressure and presence of a leak (worst case scenario) driving higher non-invasive ventilation aerosolisation. Our results also confirmed the clinical belief that nebulisation generates large aerosol counts.
Our pre-clinical study has limitations: we sampled the environment at one position only (albeit where a health care worker is often likely caring for a patient) and tested one healthy volunteer (not a patient). Limitations in our system meant that the sample inlet was not ideal for particle sizes larger than 5 μm. The volunteer's breathing, talking and coughing aerosol counts and distributions were similar to prior studies.6, 21 All background periods had at least 10 minutes of stable aerosol concentrations before continuing testing. We avoided fluctuating background aerosol measurements in an ICU or hospital ward, instead choosing a clean room. We studied total aerosol generation, and cannot comment on the viral load within the aerosols measurements.
Our human volunteer study provides high quality data results that may have widespread implications regarding the safe use of ubiquitous respiratory therapies. Our findings that O2 therapy by face mask up to 15 L/min and high flow nasal O2 therapy up to 60 L/min only marginally increased particle counts suggest that these procedures are unlikely to lead to clinically significant aerosol generation and support the use of contact and droplet precautions when using these therapies in patients with respiratory infectious diseases. However, non-invasive ventilation and nebulisation are high aerosol generating procedures, supporting the ongoing use of appropriate personal protective equipment for contact and airborne precautions.9
The personal ventilation hood reduces repeated nebuliser aerosolisation particle counts by 98%. The ventilation hood may reduce cross-contamination of respiratory infectious diseases beyond SARS-CoV-2 (eg, tuberculosis and influenza) more effectively than wearing an N95 mask (N95 respirator). An effective barrier between the patient and the health care worker addresses concerns about our first line of defence against cross-contamination from airborne infectious diseases, protecting the air we breathe. These preliminary effectiveness data support the evaluation of the hood in a clinical environment.
Conclusion
We confirmed that non-invasive ventilation and nebuliser therapy are high aerosol generating procedures, but standard face mask and high flow nasal O2 therapy are not. The use of a personal ventilation hood substantially reduced the increase in aerosol counts observed with both non-invasive ventilation and nebuliser therapy.
Acknowledgments
Acknowledgements:
We thank Jo Staines (Industrial Director), Geoff Duke, Max Rounds and Aditya Ramani from the Department of Mechanical Engineering, University of Melbourne; and Samantha Bates, Miriam Towns, Teresa Squillacioti, Gypsy Stevenson, Alex Angel, Annette Kakris and Marion Kainer from Western Health.
Competing interests
A patent has been filed for the personal ventilation hood by the University of Melbourne/Western Health. Forbes McGain and Jason Monty were the leads in this patent application. All other authors have no conflicts of interest.
Supplementary Information

References
- 1.World Health Organization Clinical management of severe acute respiratory infection (SARI) when COVID-19 disease is suspected: interim guidance. WHO. 2020 https://www.who.int/publications-detail/clinical-management-of-severe-acute- respiratory-infection-when-novel-coronavirus-(ncov)-infection-is-suspected (viewed Apr 2020) [Google Scholar]
- 2.Shiu E.Y., Leung N.H., Cowling B.J. Controversy around airborne versus droplet transmission of respiratory viruses: implication for infection prevention. Curr Opin Infect Dis. 2019;32:372–379. doi: 10.1097/QCO.0000000000000563. [DOI] [PubMed] [Google Scholar]
- 3.Bourouiba L. Turbulent gas clouds and respiratory pathogen emissions: potential implications for reducing transmission of COVID-19. JAMA. 2020 doi: 10.1001/jama.2020.4756. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 4.Asadi S., Bouvier N., Wexler A.S., Ristenpart W.D. The coronavirus pandemic and aerosols: does COVID-19 transmit via expiratory particles? Aerosol Sci Technol. 2020;54:635–638. doi: 10.1080/02786826.2020.1749229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Morawska L., Cao J. Airborne transmission of SARS-CoV-2: the world should face the reality. Environ Int. 2020;139 doi: 10.1016/j.envint.2020.105730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Johnson G., Morawska L., Ristovski Z., et al. Modality of human expired aerosol size distributions. J Aerosol Sci. 2011;42:839–851. [Google Scholar]
- 7.Costello A., Abbas M., Allen A., et al. Managing the health effects of climate change. Lancet. 2009;373:1693–1733. doi: 10.1016/S0140-6736(09)60935-1. [DOI] [PubMed] [Google Scholar]
- 8.Tran K., Cimon K., Severn M., et al. Aerosol generating procedures and risk of transmission of acute respiratory infections to healthcare workers: a systematic review. PLoS One. 2012;7 doi: 10.1371/journal.pone.0035797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Australian and New Zealand Intensive Care Society. COVID-19 guidelines; version 2. ANZICS; Melbourne: 2020. https://www.anzics.com.au/wp-content/uploads/2020/04/ANZI_3367_Guidelines_V2.pdf (viewed Apr 2020) [Google Scholar]
- 10.Department of Health and Human Services . State Government of Victoria; Melbourne: 2004. Design guidelines for hospitals and day procedure centres — Part E: building services and environmental design.http://www.healthdesign.com.au/vic.dghdp/ (viewed May 2020) [Google Scholar]
- 11.AusPat. Portable isolation unit (No. 2020901125). Australian Government, Intellectual Property Australia; 2020. http://pericles.ipaustralia.gov.au/ols/auspat/applicationDetails.do?applicationNo=2020901125 (viewed Apr 2020).
- 12.Shen S., Jaques P.A., Zhu Y., et al. Evaluation of the SMPS–APS system as a continuous monitor for measuring PM2.5, PM10 and coarse (PM25–10) concentrations. Atmos Environ. 2002;36:3939–3950. [Google Scholar]
- 13.Baron P.A., Mazumder M.K., Cheng Y.S. In: Aerosol measurement: principles, techniques, and applications. 2nd ed. Baron P.A., KYS Willeke, editors. Wiley and Sons; New York, USA: 2001. Chapter 17: Direct-reading techniques using particle motion and optical detection. [Google Scholar]
- 14.Flagan R.C. In: Aerosol measurement: principles, techniques, and applications. 2nd ed. Baron P.A., KYS Willeke, editors. Wiley and Sons; New York, USA: 2001. Chapter 18: Electrical techniques. Cheng YS. [Google Scholar]
- 15.Baron P.A., KYS Willeke, editors. Aerosol measurement: principles, techniques, and applications. 2nd ed. Wiley and Sons; New York, USA: 2001. Chapter 19 Condensation detection and diffusion size separation techniques. [Google Scholar]
- 16.International Organization for Standardization. ISO 14644-1 . Part 1: Classification of air cleanliness by particle concentration. 2015. — Cleanrooms and associated controlled environments.https://www.iso.org/standard/53394.html (viewed Apr 2020) [Google Scholar]
- 17.Loh N.W., Tan Y., Taculod J., et al. The impact of high-flow nasal cannula (HFNC) on coughing distance: implications on its use during the novel coronavirus disease outbreak. Can J Anaesth. 2020 doi: 10.1007/s12630-020-01634-3. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Leung C.C.H., Joynt G.M., Gomersall C.D., et al. Comparison of high-flow nasal cannula versus oxygen face mask for environmental bacterial contamination in critically ill pneumonia patients: a randomized controlled crossover trial. J Hosp Infect. 2019;101:84–87. doi: 10.1016/j.jhin.2018.10.007. [DOI] [PubMed] [Google Scholar]
- 19.Iwashyna T.J., Boehman A., Capelcelatro J., et al. Variation in aerosol production across oxygen delivery devices in spontaneously breathing human subjects [preprint] medRxiv. 2020 doi: 10.1101/2020.04.15.20066688. [DOI] [Google Scholar]
- 20.Cook T., El Boghdadly K., McGuire B., et al. Consensus guidelines for managing the airway in patients with COVID 19. Anaesthesia. 2020;75:785–799. doi: 10.1111/anae.15054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lindsley W.G., Pearce T.A., Hudnall J.B., et al. Quantity and size distribution of cough-generated aerosol particles produced by influenza patients during and after illness. J Occup Environ Hyg. 2012;9:443–449. doi: 10.1080/15459624.2012.684582. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials