Abstract
Background: Targeted therapeutic mild hypercapnia may attenuate brain injury in comatose adults resuscitated from out-of-hospital cardiac arrest.
Objective: To describe the study protocol and statistical analysis plan for the Targeted Therapeutic Mild Hypercapnia after Resuscitated Cardiac Arrest (TAME) trial.
Design, setting, participants and interventions: TAME is a phase 3, multicentre, parallel-group, participant- and outcome assessor-blinded randomised controlled trial that will be conducted in intensive care units in Australia, Canada, Ireland, Saudi Arabia, New Zealand, Scandinavia, Singapore, Central and Western Europe, and the United Kingdom. A total of 1700 comatose adults resuscitated from out-of-hospital cardiac arrest will be randomly assigned to 24 hours of targeted therapeutic mild hypercapnia (arterial carbon dioxide tension 50-55 mmHg) or targeted normocapnia (arterial carbon dioxide tension 35–45 mmHg) in a 1:1 ratio. TAME recruitment began in March 2018 and is expected to be complete in September 2021.
Main outcome measures: The primary outcome measure is the proportion of participants with a favourable functional outcome at 6 months. Functional outcomes will be assessed using the Glasgow Outcome Scale-Extended (GOSE); this scale ranges from 1 to 8, with a higher value indicating a better outcome. We will define participants with a GOSE of 5 to 8 as having a favourable functional outcome. Secondary outcomes include 6-month mortality, cognitive function and quality of life. All analyses will be conducted on an intention-to-treat basis.
Results and conclusions: TAME will compare the effect of targeted therapeutic mild hypercapnia versus targeted normocapnia on functional outcomes in adults resuscitated from out-of-hospital cardiac arrest who are admitted to an intensive care unit.
Trial registration: Australian and New Zealand Clinical Trials Registry (ACTRN12617000036314p) and ClinicalTrials.gov (NCT03114033).
Out-of-hospital cardiac arrest (OHCA) is a major worldwide public health problem1, 2 associated with a high mortality rate, and with a significant risk of brain injury in patients after successful resuscitation.2, 3 Following return of spontaneous circulation (ROSC), a cascade of secondary biochemical and perfusion-related changes compound the initial hypoxic-ischaemic insult.4, 5 Specifically, the impact of early and sustained cerebral vasoconstriction and cerebral hypoxia, as demonstrated by multimodal neuromonitoring technologies,6, 7, 8, 9, 10, 11 may contribute to the poor neurological outcomes in these patients. Supported by guidelines from the International Liaison Committee on Resuscitation,12 the standard approach to arterial carbon dioxide tension (Paco2) management for resuscitated OHCA patients admitted to an intensive care unit (ICU) is to target normocapnia and avoid hypocapnia.
Observational studies have shown that early targeted mild hypercapnia (about 20% higher than the normal Paco2 range) might be beneficial.13, 14 In addition, data have shown reduced histological injury associated with mild hypercapnia in a porcine post-cardiac arrest model.15 In a phase 2 multicentre randomised controlled trial of 86 patients, delivering early targeted mild hypercapnia (Paco2 50–55 mmHg) to those who achieved ROSC was associated with reduced levels of neuron-specific enolase (a biomarker of brain injury commonly used in OHCA patients) and improved neurological recovery.16 Finally, a physiological crossover study of early cardiac arrest survivors showed that targeted mild hypercapnia after ROSC increased cerebral oxygenation as detected by near-infrared spectroscopy.17 However, the patient-centred and clinically relevant benefits of mild hypercapnia for adult patients who are resuscitated from OHCA are unknown.
Accordingly, we designed the Targeted Therapeutic Mild Hypercapnia After Resuscitated Cardiac Arrest (TAME) trial to assess whether early targeted therapeutic mild hypercapnia improves neurological outcomes at 6 months compared with targeted normocapnia in comatose patients resuscitated from OHCA. Results of our pilot safety, feasibility and biological efficacy study, the CCC trial, have been published previously; they demonstrated the feasibility of our phase 3 trial design with respect to appropriate patient selection, treatment group separation, protocol compliance, and adequacy of recruitment.16 Here, we describe the protocol and statistical analysis plan for the TAME trial to mitigate analysis bias.18, 19, 20
Methods
Trial design
The TAME trial is a multicentre, parallel-group, participant- and outcome assessor-blinded randomised controlled trial that will test the hypothesis that, among adults who are comatose following resuscitation from OHCA, targeted therapeutic mild hypercapnia improves functional outcomes at 180 days after randomisation compared with targeted normocapnia. Participants will be allocated to targeted therapeutic mild hypercapnia or targeted normocapnia in a 1:1 ratio. This two-sided superiority trial has been designed with reference to the SPIRIT (Standard Protocol Items: Recommendations for Interventional Trials) checklist.21
Setting and population
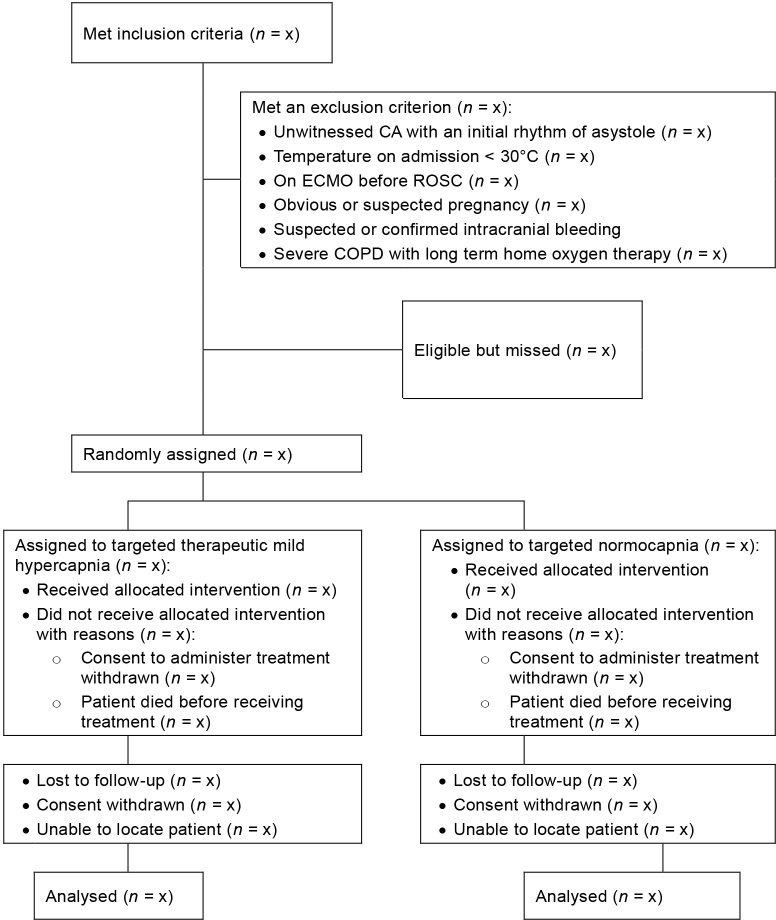
TAME will include 1700 comatose adults resuscitated from OHCA from ICUs in Australia, Canada, Ireland, Saudi Arabia, New Zealand, Scandinavia, Singapore, Central and Western Europe, and the United Kingdom. Patients are eligible for inclusion if they are aged ≥ 18 years, no more than 3 hours have passed since they achieved sustained ROSC (defined as ≥ 20 minutes without the need for chest compressions), have experienced an OHCA of a presumed cardiac or unknown cause, are unconscious and unable to obey verbal commands (score of < 4 on the Full Outline of Unresponsiveness [FOUR] scale, which ranges from 0 to 4, with higher scores indicating better motor function),22 are eligible for ICU admission without restrictions or limitations, and do not meet any of the exclusion criteria (Table 1). Recruitment commenced on 4 March 2018 and is anticipated to end in September 2021. The proposed reporting of flow of participants through the trial is shown in Figure 1.
Table 1.
Eligibility criteria
Inclusion criteria
|
Exclusion criteria
|
FOUR = Full Outline of Unresponsiveness. ROSC = return of spontaneous circulation.
FOUR scores range from 0 to 4, with higher motor response scores indicating better motor function.22
Figure 1.
Proposed reporting of flow of participants through the trial
CA = cardiac arrest. COPD = chronic obstructive pulmonary disease. ECMO = extracorporeal membrane oxygenation. ROSC = return of spontaneous circulation.
Randomisation and treatment masking (blinding)
Screening for eligibility must be performed as soon as possible, but no later than 180 minutes after ROSC, leaving an effective inclusion window of 160 minutes (180 minutes minus 20 minutes to account for the required ROSC period). After screening, participants will be randomly assigned to targeted therapeutic mild hypercapnia or targeted normocapnia. Randomisation will be performed by a clinician via a web-based system involving permuted blocks of varying sizes, stratified according to trial site. Co-enrolment in the Targeted Hypothermia Versus Targeted Normothermia After Out-of-Hospital Cardiac Arrest (TTM2) trial23 will be performed by using a two-by-two factorial allocation approach.
The assigned treatment intervention will be communicated to the bedside clinicians who implement the study intervention. The nature of the study interventions means that treatment providers cannot be blinded. However, trial participants and outcome assessors (who will conduct the functional outcome assessments) will be unaware of the trial-group assignments. In addition, during the analysis and writing process, the investigators, statisticians and authors will remain unaware of the trial-group assignments. Further, the manuscript writing will be performed in duplicate, with the groups interchanged. Importantly, group analyses of variables that might unblind the randomisation (pH, serum bicarbonate levels and respiratory rates) will not be allowed before the manuscript has been completed.
Trial intervention
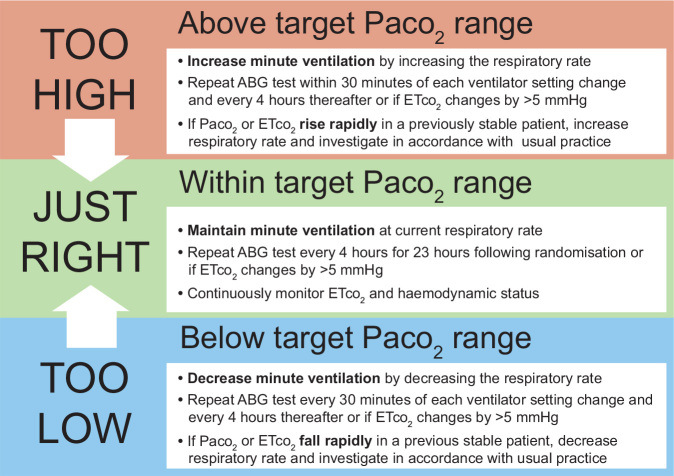
Participants will be assigned to targeted therapeutic mild hypercapnia, a Paco2 range of 50–55 mmHg (6.6–7.3 kPa), or to targeted normocapnia, a Paco2 range of 35–45 mmHg (4.6–6.0 kPa). The intervention period of 24 hours begins at the time of randomisation. Participants in both groups will be sedated to achieve deep sedation (a target score of –4 on the Richmond Agitation–Sedation Scale, which ranges from –5 [unarousable] to +4 [combative]).24 Their ventilation will be managed as described in Figure 2 and guided by arterial blood gas (ABG) data assessed after adjustment to 37°C (alpha-stat).25
Figure 2.
Ventilation management of patients allocated to targeted therapeutic mild hypercapnia or targeted normocapnia
ABG = arterial blood gas. Etco2 = end-tidal carbon dioxide. Paco2 = arterial carbon dioxide tension.
For participants allocated to targeted therapeutic mild hypercapnia, ABG and end-tidal carbon dioxide (ETco2) levels will be measured at baseline and used to guide respiratory rate adjustments of minute ventilation to remain in the mild hypercapnia range. ABG tests will be repeated every 4 hours for 24 hours following randomisation or if ETco2 values change by > 5 mmHg. For safety, if present at randomisation, severe metabolic acidosis (pH less than 7.1 and base excess less than –6 mmol/L) will be treated before delivery of targeted therapeutic mild hypercapnia begins. After the 24-hour intervention period, it is recommended that normocapnia be restored slowly over the subsequent 4 hours (total study period, 24–28 hours).
For participants allocated to targeted normocapnia, ABG and ETco2 levels will be measured at baseline and used to guide respiratory rate adjustments of minute ventilation to remain in the normocapnia range. ABG tests will be repeated every 4 hours for 24 hours following randomisation or if ETco2 values change by > 5 mmHg.
To optimise time in the allocated Paco2 range during the intervention period, the treating physicians can modify patient management, including use of sedatives, muscle relaxants and/ or paralysis agents. Each participant's treating ICU physician will decide when to discharge them from the ICU.
Neurological prognostication and withdrawal of lifesustaining therapies
As the TAME trial is aimed at evaluating an intervention that cannot be blinded to the treating clinicians, potential bias will be mitigated by using conservative and strict protocols for neuroprognostication and related decision making (ie, regarding limitations in level of care).
At 96 hours after randomisation or later, a prognosticator (neurologist, intensivist or other specialist experienced in neuroprognostication) who is unaware of the trial-group assignment will perform a neurological assessment of participants who are deemed free of the effects of sedatives and who remain in the ICU. Neuroprognostication is based on European Resuscitation Council and European Society of Intensive Care Medicine guidelines.26 Prognostication and the potential decision to withdraw active intensive care are closely related but are considered separate entities.
Withdrawal of life-sustaining therapies (WLST) based on a presumed poor neurological prognosis is not to be performed before prognostication. However, should further life-sustaining therapies be deemed unethical due to irreversible organ failure, a medical comorbidity or other reasons, life-sustaining therapies may be withdrawn before the protocolised neuroprognostication time point. For participants in whom brain death is established, WLST is defined as death rather than WLST. Intervention decisions relating to WLST are to be made by the treating physicians, together with the participant's relatives or legal surrogates, as required by local legislation. In making such decisions, the treating physician may use information from the TAME prognostication assessment. The blinded external physician will not make any recommendation on WLST.
Concomitant therapies
There are no restrictions to use of other concomitant therapies, application of local best practice policies, or application of International Liaison Committee on Resuscitation guidelines for the care of resuscitated adults admitted to an ICU — for example, optimising coronary intervention timing and applying targeted temperature management.
Outcomes
The primary outcome measure for this trial is the proportion of participants with a favourable neurological outcome at 6 months as assessed using the Glasgow Outcome Scale-Extended (GOSE).27 A GOSE score of 1 indicates death, 2 indicates vegetative state, 3 and 4 indicate severe disability, 5 and 6 indicate moderate disability, and 7 and 8 indicate good recovery. A GOSE outcome is deemed to be favourable if a participant's score is ≥ 5.
Secondary outcomes include mortality at ICU discharge and hospital discharge. Functional recovery at 6 months will be assessed using modified Rankin Scale (mRS) scores.28 An mRS score of 0 represents no symptoms, 1 represents no clinically significant disability, 2 represents slight disability, 3 represents moderate disability, 4 represents moderately severe disability, 5 represents severe disability, and 6 represents death. A mRS score is deemed to be a favourable if a participant's score is ≤ 3.28, 29 Cognitive functional recovery at 6 months will be assessed using the Montreal Cognitive Assessment-Blind (MoCA-Blind),30 Informant Questionnaire on Cognitive Decline in the Elderly31 and Symbol Digit Modalities Test.32 Lower extremity muscle strength at 6 months will be assessed using the 30-second chair test.33 Health-related quality of life at 6 months will be assessed using the visual analogue scale on the European Quality of Life 5-Dimension 5-Level questionnaire,34 which ranges from 0 to 100, with higher scores indicating better health status as assessed by the participant. Safety will be assessed by describing the proportion of pre-defined adverse events occurring in each group as reported by treating clinicians.
In addition, a nested health economic evaluation will be conducted using hospital and post-discharge estimates of costs, and a nested biomarker assessment will be conducted using baseline, 24-hour, 48-hour and 72-hour data relating to cardiac, neurological, inflammatory and mitochondrial (non-nuclear DNA) biomarkers.
Data collection and management
Trained research coordinators will collect pre-hospital and hospital data at each site. Data will be managed in a web-based case report form.
Enrolled participants will be followed up to death or 6 months after randomisation. Data on pre-hospital and cardiac arrest characteristics, along with baseline characteristics and hospital interventions and outcomes, will be collected. Detailed physiological data will be collected during the 24-hour intervention period, and daily data will be collected during the ICU stay from Day 0 (day of randomisation, which is a short day) until Day 7. Details regarding physiological and process-of-care data to be collected are shown in Table 2.
Table 2.
Physiological descriptors and process-of-care measures
Physiological descriptors
|
ABG = arterial blood gas. CA = cardiac arrest. CABG = coronary artery bypass graft. Fio2 = fraction of inspired oxygen. IABP = intra-aortic balloon pump. ICU = intensive care unit. Paco2 = arterial carbon dioxide tension. Pao2 = arterial oxygen tension. PEEP = positive end expiratory pressure. SSEP = somatosensory evoked potentials.
The primary outcome will be assessed at Day 180. Quality-of-life and cognitive assessments will be conducted in person, via telephone or by videoconference with the participant (primary candidate) or their proxy (secondary candidate) as close as possible to but after Day 180 by blinded outcome assessors (eg, occupational therapists, physicians, research nurses, professional outcome assessors). Efforts to increase inter-rater reliability include the use of psychometrically sounds measures, a written follow-up manual and training sessions (in person or by videoconference) for outcome assessors. To avoid missing data and to enable a determination on outcome status, alternative follow-up strategies (such as audit of medical notes and review of interview records) will be used as required. A full list of data being collected is shown in Table 3.
Table 3.
Data to be collected in the TAME trial
| Time of collection or type of data | Data collected |
|---|---|
| Screening |
|
| Baseline |
|
| Day 0 to Day 7 |
|
| Hospital admission |
|
| Discharge |
|
| Outcome and assessment data |
|
| Adverse events |
|
| Protocol deviations |
|
ABG = arterial blood gas. CABG = coronary artery bypass graft surgery. EQ-5D-5L = European Quality of Life 5-Dimension 5-Level questionnaire. Fio2 = fraction of inspired oxygen. GOSE = Glasgow Outcome Scale-Extended. ICU = intensive care unit. IQCODE-CA = Informant Questionnaire on Cognitive Decline in the Elderly-Cardiac Arrest. MoCA-Blind = Montreal Cognitive Assessment-Blind. mRS = modified Rankin Scale. Paco2 = arterial carbon dioxide tension. Pao2 = arterial oxygen tension. SSEP = somatosensory evoked potentials. SDMT = Symbol Digit Modality Test. TSQ = two simple questions. TST = timed stand test.
Accuracy and consistency checks of collected data will be carried out by automatic validation, and by pre-specified and ad hoc checking by personnel at the coordinating centres. On-site data monitoring or remote electronic monitoring will be performed by trained project managers and study monitors from the Australian and New Zealand Intensive Care Research Centre, Irish Critical Care Clinical Trials Network and Medical Research Institute of New Zealand for participants in Australia, Ireland and New Zealand, respectively, and by international trial representatives for participants in other countries.
For European sites, participant data will be protected by complying with European Union legislation, General Data Protection Regulation 2016/679, and all other applicable laws, regulations and codes of conduct in any jurisdiction relating to the processing of personal data and privacy. This includes guidance and codes of practice from the European Data Protection Board and Article 29 Working Party. Data management will be performed using Spiral Software.
Ethical issues and good clinical practice
The trial has been approved in Australia by the Austin Health Human Research Ethics Committee (HREC/17/Austin/209), in New Zealand by the Central Health and Disability Ethics Committee (17NTA134) and in other regions by the relevant local or national ethics review committees. By virtue of the inclusion criteria, none of the patients who are eligible for this study will be able to provide prospective informed consent. As such, depending on the circumstances, written informed consent will be deferred, obtained from a legal surrogate, obtained via a waiver of consent if applicable, or obtained from each participant who regains mental capacity. The trial is being conducted according to the standard requirements of Good Clinical Practice.35
Data safety monitoring committee and interim safety analysis
The members of the data safety monitoring committee (DSMC) for the TAME trial are Kathy Rowan (Director of Scientific and Strategic Development, Intensive Care National Audit and Research Centre [ICNARC], London), David Harrison (Honorary Professor, Head Statistician, ICNARC, London), Manu Shankar-Hari (Consultant Intensivist, Guy's and St Thomas' National Health Service Foundation Trust, London), Duncan Young (Consultant Intensivist, Nuffield Department of Clinical Neurosciences, University of Oxford, Oxford) and Paul Mouncey (Clinical Trials Unit, ICNARC). The role of the DSMC is to ensure that the rights and safety of the study participants are protected. The DSMC has reviewed and approved the study protocol and will review all reported suspected unexpected serious adverse events as they occur. A summary of protocol deviations and adverse events will also be provided to the DSMC.
A single, planned, formal interim analysis will be performed after 30-day outcome data from the first 850 participants (50% of the target sample) are available. The TAME management committee will update the DSMC on accumulating external evidence relevant to the study in a timely manner, and the DSMC will advise the TAME management committee on their opinions regarding the importance of this evidence to the ongoing conduct of the trial. The DSMC acts in an advisory capacity to the TAME steering committee, who are ultimately responsible for the conduct of the trial.
Adverse events
Consistent with established practice in academic ICU trials,36, 37 events that are part of the natural history of the primary disease process or expected complications of critical illness will not be reported as serious adverse events in this study.38 Prespecified adverse events, occurring from randomisation until Day 7 while the participant is in the ICU, will be systematically recorded; these include pneumonia, sepsis, bleeding, arrhythmia resulting in hemodynamic compromise, and skin complications related to the device used for targeted temperature management (Table 4). Specific serious adverse events that will be systematically recorded include arrythmias, suspected or raised intracranial hypertension and seizures necessitating normocapnia (Table 4). In addition, investigators will report any unexpected serious adverse events.
Table 4.
Definitions of specific adverse events and serious adverse events to be collected
| Event | Definition |
|---|---|
| Adverse event | |
| Pneumonia | The presence of increased or purulent tracheal secretions, new or progressive radiographic infiltrate and a decreased Pao2/Fio2 ratio (< 240 mmHg or < 32 kPa) |
| Sepsis and septic shock | According to the third international consensus definitions for sepsis and septic shock41 |
| Bradycardia | Requiring pacing |
| Moderate or severe bleeding | According to the GUSTO criteria42 |
| Cooling device-related skin complications | Blistering or skin necrosis in areas covered by surface device |
| Arrythmia | Resulting in haemodynamic compromise (eg, ventricular fibrillation and ventricular tachycardia) |
| Serious adverse event | |
| Arrythmia | Arrythmia necessitating normocapnia |
| Intracranial hypertension | Suspected or confirmed raised intracranial hypertension necessitating normocapnia |
| Seizures | Suspected or confirmed seizures necessitating normocapnia |
Fio2 = fraction of inspired oxygen. GUSTO = Global Use of Strategies to Open Occluded Coronary Arteries. Pao2 = arterial oxygen tension.
Sample size and power
Based on prior outcomes of our large multicentre observational study,13 phase 2 trial16 and prospective crossover study,17 and that of a prospective observational Finnish cohort study,14 we estimated that our trial would require 812 patients per arm (a total of 1624 patients) to achieve a 90% power to detect an absolute increase of 8% in the primary outcome at an of 0.05 and with an expected incidence in the control arm of 50%. We rounded this up to 1700 patients (850 in each arm) to account for a loss to follow-up and withdrawal of consent rate of 5% (a rate similar to that seen in comparable trials evaluating functional outcomes in brain-injured participants43 and follow-up studies involving OHCA cohorts).44
Statistical analysis plan
All data will initially be assessed for normality. We will analyse data on an intention-to-treat basis, defined as all randomly assigned participants except those for whom consent was withdrawn, without imputation of missing data unless specified. Baseline comparisons and comparisons of physiological descriptors and processes of care will be performed using χ2 tests to test for equal proportions, Student t tests to compare normally distributed data and Wilcoxon rank-sum tests to compare other types of data, with results reported as number (percentage), mean ± standard deviation, and median (interquartile range), respectively. Comparisons of longitudinal data will be performed using a mixed-effect linear model, fitting main effects for treatment, time and an interaction between the two, and with centres and participants entered as random effects to account for the clustering effect and for repeated measurements.
Paco2 data will be reported as time-weighted averages for each treatment group with linear interpolation. We will evaluate Paco2 separation, exposure and study protocol compliance over the intervention period by evaluating systematically obtained Paco2 recordings.
Binary outcomes, including the primary outcome, will be presented as proportions of participants in each group with the event. Unadjusted differences in proportions will be provided. Risk ratios and 95% confidence intervals will be assessed using a mixed-effect generalised linear model with a logit link, with centre as a random intercept and co-enrolment included as a fixed effect. Survival data, including time to death at ICU discharge, hospital discharge, 30 days and 180 days, will be presented in Kaplan–Meier curves and analysed using shared frailty Cox proportional hazard models, with centre included as frailty and co-enrolment included as a fixed effect. The proportional hazard assumption will be assessed through Schoenfeld residuals.
Continuous outcomes will be analysed using mixed-effect linear regression, with centre as a random intercept and co-enrolment included as a fixed effect. We expect that a large proportion of the participants will die before assessment of quality of life. So, when assessing health-related quality of life in the primary analysis, we will impute a 0 for all participants who died or could not participate in the quality-of-life assessment due to incapacitation. In a secondary analysis of quality of life, we will only include survivors at 6 months.
If more than 5% of missing data are primary or secondary outcomes data, a sensitivity analysis will be performed using multiple imputation conditional on prognostic baseline and post-baseline variables under the assumption that missing data are conditional on observed covariates and were assumed to be missing at random. As an additional sensitivity analysis for the primary outcome, GOSE data will be assessed in its original scale considering a mixed-effect cumulative logistic model (ordinal regression).
Analyses will be performed on five pre-defined subgroups irrespective of whether there is evidence of an overall treatment effect. Heterogeneity between subgroups will be determined by fitting an interaction between treatment and subgroup on the model described above. The following pre-defined subgroups will be assessed:
-
•
age ≤ 65 years versus > 65 years on admission;
-
•
female versus male;
-
•
time from cardiac arrest to ROSC higher than median versus time from cardiac arrest to ROSC lower than median;
-
•
shockable versus non-shockable initial cardiac rhythm; and
-
•
shock versus no shock on admission.
Shock on admission was defined as a systolic blood pressure of < 90 mmHg for at least 30 minutes or the need for supportive measures to maintain a systolic blood pressure of ≥ 90 mmHg and end-organ hypoperfusion (cool extremities, or urine output of < 30 mL/h and heart rate > 60 beats/min).
Analyses will also be performed on the per-protocol population and the population of participants who are not co-enrolled in the TTM2 trial.23 All analyses will be performed using SAS version 9.4 (SAS Institute) and a two-sided P value of 0.05 will be used to indicate statistical significance for the primary outcome. For all regression analyses, we will test for an interaction effect between TAME group-assignment and assignment in the TT2 trial.23 To account for multiple secondary outcomes, Holm-Bonferroni adjustments for multiplicity will be performed.
Presentation of outcome data
Our proposed tables and figures for the primary manuscript are listed in Table 5. Detailed mock tables and figures for the primary manuscript are shown in the Online Appendix. The analyses of the nested health economic evaluation and the nested biomarker assessments will be reported separately.
Table 5.
Planned tables and figures
| Proposed tables and figures for the main manuscript |
|
| Proposed tables and figures for the supplementary appendix of the main manuscript |
|
FOUR = Full Outline of Unresponsiveness. Fio2 = fraction of inspired oxygen. GOSE = Glasgow Outcome Scale-Extended. Paco2 = arterial carbon dioxide tension. Pao2 = arterial oxygen tension. PEEP = positive end expiratory pressure. TAME = Targeted Therapeutic Mild Hypercapnia after Resuscitated Cardiac Arrest. TTM2 = Targeted Hypothermia Versus Targeted Normothermia After Out-of-Hospital Cardiac Arrest.
Co-enrolment in the TTM2 trial
The TTM2 trial evaluated whether targeted temperature management to 33°C improves survival and neurological outcome at 6 months compared with a strategy of targeting normothermia and avoiding fever above 37.7°C.23 Co-enrolment in this trial is allowed for sites participating in the TTM2 trial.23 However, the option for participation in the TAME trial only has been made available for sites that are not willing or able to co-enrol. We considered co-enrolment in both trials to be an effective use of research resources.45 A recognised difficulty with co-enrolment is the potential for an interaction between interventions. However, data analysis from the original TTM trial showed no interaction between Paco2 and temperature regarding major outcomes.46 Further, as randomisation was stratified to achieve a two-by-two factorial design, any differences in primary or secondary outcomes will be monitored throughout the trial by the DSMC, which is shared by the TTM2 and TAME trials.
Data sharing
TAME trial data will be shared according to the policies and procedures of the Australian and New Zealand Intensive Care Research Centre (https://www.monash.edu/_data/assets/pdf_file/0010/1790875/terms_of_ref.pdf).
Summary
TAME is a phase 3, multicentre, parallel-group, randomised controlled trial. It trial will compare targeted therapeutic mild hypercapnia with targeted normocapnia in 1700 comatose adults resuscitated from OHCA who are admitted to an ICU. The primary outcome is favourable neurological outcomes (GOSE score, 5-8) at 6 months. This protocol and statistical analysis plan manuscript was submitted for publication before follow-up was completed.
Acknowledgements: The TAME trial is funded by grants from the National Health and Medical Research Council (Australia; APP1119855), the Health Research Board (Ireland; DIFA-2017-036), the Health Research Council of New Zealand (18/160), and an unrestricted investigator-initiated research grant from Baxter Healthcare. The funding bodies had no input into the design or conduct of the trial or into the statistical analysis plan, and they will have no input into analysis or reporting of results. The study is coordinated in Australia by the Australian and New Zealand Intensive Care Research Centre, in conjunction with the Irish Critical Care Clinical Trials Network (covering Ireland and Europe) and the Medical Research Institute of New Zealand. This study is endorsed by the Australian and New Zealand Intensive Care Society Clinical Trials Group, the Irish Critical Care Clinical Trials Group and the Australian Resuscitation Outcomes Consortium.
Competing interests
No relevant disclosures.
Footnotes
For the TAME study and the Australian and New Zealand Intensive Care Society Clinical Trials Group (ANZICS CTG), the Irish Critical Care Clinical Trials Network (ICC-CTN), and the Australian Resuscitation Outcomes Consortium (Aus-ROC)
References
- 1.Berdowski J., Berg R.A., Tijssen J.G., Koster R.W. Global incidences of out-of-hospital cardiac arrest and survival rates: systematic review of 67 prospective studies. Resuscitation. 2010;81:1479–1487. doi: 10.1016/j.resuscitation.2010.08.006. [DOI] [PubMed] [Google Scholar]
- 2.Benjamin E.J., Blaha M.J., Chiuve S.E., et al. Heart disease and stroke statistics — 2017 update. A report from the American Heart Association. Circulation. 2017;135:e146–e603. doi: 10.1161/CIR.0000000000000485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mentzelopoulos S.D., Zakynthinos S.G. Post-cardiac arrest syndrome: pathological processes, biomarkers and vasopressor support, and potential therapeutic targets. Resuscitation. 2017;121:A12–A14. doi: 10.1016/j.resuscitation.2017.10.013. [DOI] [PubMed] [Google Scholar]
- 4.Lilja G., Nielsen N., Friberg H., et al. Cognitive function in survivors of out-of-hospital cardiac arrest after target temperature management at 33°C versus 36°C. Circulation. 2015;131:1340–1349. doi: 10.1161/CIRCULATIONAHA.114.014414. [DOI] [PubMed] [Google Scholar]
- 5.Cronberg T., Lilja G., Horn J., et al. Neurologic function and health-related quality of life in patients following targeted temperature management at 33°C vs 36°C after out-of-hospital cardiac arrest a randomized controlled trial. JAMA. 2015;72:634–641. doi: 10.1001/jamaneurol.2015.0169. [DOI] [PubMed] [Google Scholar]
- 6.Buunk G., van der Hoeven J.G., Meinders A.E. Cerebral blood flow after cardiac arrest. Neth J Med. 2000;57:106–112. doi: 10.1016/s0300-2977(00)00059-0. [DOI] [PubMed] [Google Scholar]
- 7.Koch K.A., Jackson D.L., Schmiedl M., Rosenblatt J.I. Total cerebral ischemia: effect of alterations in arterial Pco2 on cerebral microcirculation. J Cereb Blood Flow Metab. 1984;4:343–349. doi: 10.1038/jcbfm.1984.51. [DOI] [PubMed] [Google Scholar]
- 8.Buunk G., van der Hoeven J.G., Meinders A.E. Prognostic significance of the difference between mixed venous and jugular bulb oxygen saturation. Resuscitation. 1999;41:257–262. doi: 10.1016/s0300-9572(99)00060-x. [DOI] [PubMed] [Google Scholar]
- 9.Sundgreen C., Larsen F.S., Herzog T.M., et al. Autoregulation of cerebral blood flow in patients resuscitated from cardiac arrest. Stroke. 2001;32:128–132. doi: 10.1161/01.str.32.1.128. [DOI] [PubMed] [Google Scholar]
- 10.Beckstead J.E., Tweed W.A., Lee J., et al. Cerebral blood flow and metabolism in man following cardiac arrest. Stroke. 1978;9:569–573. doi: 10.1161/01.str.9.6.569. [DOI] [PubMed] [Google Scholar]
- 11.Edgren E., Enblad P., Grenvik A., et al. Cerebral blood flow and metabolism after cardiopulmonary resuscitation. Resuscitation. 2003;57:161–170. doi: 10.1016/s0300-9572(03)00004-2. [DOI] [PubMed] [Google Scholar]
- 12.Nolan J.P., Soar J., Cariou A., et al. European Resuscitation Council and European Society of Intensive Care Medicine 2015 guidelines for post-resuscitation care. Intensive Care Med. 2015;41:2039–2056. doi: 10.1007/s00134-015-4051-3. [DOI] [PubMed] [Google Scholar]
- 13.Schneider A.G., Eastwood G.M., Bellomo R., et al. Arterial carbon dioxide tension and outcome in patients admitted to the intensive care unit after cardiac arrest. Resuscitation. 2013;84:927–934. doi: 10.1016/j.resuscitation.2013.02.014. [DOI] [PubMed] [Google Scholar]
- 14.Vaahersalo J., Bendel S., Reinikainen M., et al. Arterial blood gas tensions after resuscitation from out-of-hospital cardiac arrest: associations with long-term neurological outcome. Crit Care Med. 2014;42:1463–1470. doi: 10.1097/CCM.0000000000000228. [DOI] [PubMed] [Google Scholar]
- 15.Babini G., Ristagno G., Boccardo A., et al. Effect of mild hypercapnia on outcome and histological injury in a porcine post cardiac arrest model. Resuscitation. 2019;135:110–117. doi: 10.1016/j.resuscitation.2018.10.024. [DOI] [PubMed] [Google Scholar]
- 16.Eastwood G.M., Schneider A.G., Satoshi S., et al. Targeted therapeutic mild hypercapnia after cardiac arrest: a phase II multi-centre randomised controlled trial (the CCC trial) Resuscitation. 2016;104:83–90. doi: 10.1016/j.resuscitation.2016.03.023. [DOI] [PubMed] [Google Scholar]
- 17.Eastwood G.M., Tanaka A., Bellomo R. Cerebral oxygenation in mechanically ventilated early cardiac arrest survivors: the impact of hypercapnia. Resuscitation. 2016;102:11–16. doi: 10.1016/j.resuscitation.2016.02.009. [DOI] [PubMed] [Google Scholar]
- 18.Finfer S., Bellomo R. Why publish statistical analysis plans? Crit Care Resusc. 2009;11:5–6. [PubMed] [Google Scholar]
- 19.Thomas L., Peterson E.D. The value of statistical analysis plans in observational research: defining high-quality research from the start. JAMA. 2012;308:773–774. doi: 10.1001/jama.2012.9502. [DOI] [PubMed] [Google Scholar]
- 20.Billot L., Bellomo R., Gallagher M., et al. The Plasma-Lyte 148 versus Saline (PLUS) statistical analysis plan: a multicenter, randomized controlled trial of the effect of intensive care fluid therapy on mortality. Crit Care Resusc. 2021;23:24–31. doi: 10.51893/2021.1.OA2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chan A.W., Tetzlaff J.M., Altman D.G., et al. SPIRIT 2013 statement: defining standard protocol items for clinical trials. Ann Int Med. 2013;158:200–207. doi: 10.7326/0003-4819-158-3-201302050-00583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Iyer V.N., Mandrekar J.N., Danielson R.D., et al. Validity of the FOUR score coma scale in the medical intensive care unit. Mayo Clin Proc. 2009;84:694–701. doi: 10.4065/84.8.694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dankiewicz J., Cronberg T., Lilja G., et al. Hypothermia versus normothermia after out-of-hospital cardiac arrest. N Engl J Med. 2021;384:2283–2294. doi: 10.1056/NEJMoa2100591. [DOI] [PubMed] [Google Scholar]
- 24.Sessler C.N., Gosnell M.S., Grap M.J., et al. The Richmond AgitationSedation Scale. Am J Resp Crit Care. 2002;166:1338–1344. doi: 10.1164/rccm.2107138. [DOI] [PubMed] [Google Scholar]
- 25.Eastwood G.M., Nielsen N., Nichol A.D., et al. Reported practice of temperature adjustment (α-stat v pH-stat) for arterial blood gases measurement among investigators from two major cardiac arrest trials. Crit Care Resusc. 2019;21:69–71. [PubMed] [Google Scholar]
- 26.Nolan J.P., Soar J., Cariou A., et al. European Resuscitation Council and European Society of Intensive Care Medicine Guidelines for Post-resuscitation Care 2015: Section 5 of the European Resuscitation Council Guidelines for Resuscitation 2015. Resuscitation. 2015;95:202–222. doi: 10.1016/j.resuscitation.2015.07.018. [DOI] [PubMed] [Google Scholar]
- 27.Weir J., Steyerberg E.W., Butcher I., et al. Does the extended Glasgow Outcome Scale add value to the conventional Glasgow Outcome Scale? J Neurotrauma. 2012;29:53–58. doi: 10.1089/neu.2011.2137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Banks J.L., Marotta C.A. Outcomes validity and reliability of the modified Rankin scale: implications for stroke clinical trials: a literature review and synthesis. Stroke. 2007;38:1091–1096. doi: 10.1161/01.STR.0000258355.23810.c6. [DOI] [PubMed] [Google Scholar]
- 29.Haywood K., Whitehead L., Nadkarni V.M., et al. COSCA (Core Outcome Set for Cardiac Arrest) in adults: an advisory statement from the International Liaison Committee on Resuscitation. Circulation. 2018;137:e783–e801. doi: 10.1161/CIR.0000000000000562. [DOI] [PubMed] [Google Scholar]
- 30.Nasreddine Z.S., Phillips N.A., Bédirian V., et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53:695–699. doi: 10.1111/j.1532-5415.2005.53221.x. [DOI] [PubMed] [Google Scholar]
- 31.Jorm A.F., Jacomb P.A. The Informant Questionnaire on Cognitive Decline in the Elderly (IQCODE): socio-demographic correlates, reliability, validity and some norms. Psychol Med. 1989;19:1015–1022. doi: 10.1017/s0033291700005742. [DOI] [PubMed] [Google Scholar]
- 32.Smith A. Western Psychological Services; Manual. Los Angeles: 1982. Symbol Digit Modalities Test. [Google Scholar]
- 33.Csuka M., McCarty D.J. Simple method for measurement of lower extremity muscle strength. Am J Med. 1985;78:77–81. doi: 10.1016/0002-9343(85)90465-6. [DOI] [PubMed] [Google Scholar]
- 34.EuroQol Research Foundation. EQ-5D [website]. http://euroqol.org (viewed Oct 2021).
- 35.Lilja G., Nielsen N., Friberg H., et al. Cognitive function after cardiac arrest and temperature management: rationale and description of a sub-study in the Target Temperature Management trial. BMC Cardiovasc Disord. 2013;13:85. doi: 10.1186/1471-2261-13-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Longstreth W.T., Jr., Nichol G., Van Ottingham L., Hallstrom A.P. Two simple questions to assess neurologic outcome at 3 months after out-of-hospital cardiac arrest: experience from the public access defibrillation trial. Resuscitation. 2010;81:530–533. doi: 10.1016/j.resuscitation.2010.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use. Integrated Addendum to ICH E6(R1): guideline for good clinical practice (Code No. E6(R2)). 2016. https://database.ich.org/sites/default/files/E6_R2_Addendum.pdf (viewed Oct 2021).
- 38.Cooper D.J., Rosenfeld J.V., Murray L., et al. DECRA Trial Investigators; Australian and New Zealand Intensive Care Society Clinical Trials Group. Decompressive craniectomy in diffuse traumatic brain injury. N Engl J Med. 2011;364:1493–1502. doi: 10.1056/NEJMoa1102077. [DOI] [PubMed] [Google Scholar]
- 39.Mackle D., Bellomo R., Bailey M., et al. ICU-ROX Investigators the Australian and New Zealand Intensive Care Society Clinical Trials Group. Conservative oxygen therapy during mechanical ventilation in the ICU. N Engl J Med. 2020;382:989–998. doi: 10.1056/NEJMoa1903297. [DOI] [PubMed] [Google Scholar]
- 40.Cook D., Lauzier F., Rocha M.G., et al. Serious adverse events in academic critical care research. CMAJ. 2008;178:1181–1184. doi: 10.1503/cmaj.071366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Singer M., Deutschman C.S., Seymour C.W., et al. The third international consensus definitions for sepsis and septic shock (Sepsis-3) JAMA. 2016;315:801–810. doi: 10.1001/jama.2016.0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Investigators G.U.S.T.O. An international randomized trial comparing four thrombolytic strategies for acute myocardial infarction. N Engl J Med. 1993;329:673–682. doi: 10.1056/NEJM199309023291001. [DOI] [PubMed] [Google Scholar]
- 43.Cooper D.J., Nichol A.D., Bailey M., et al. Effect of early sustained prophylactic hypthermia on neurologic outcomes among patients with severe traumatic brain injury: the POLAR randomized clinical trial. JAMA. 2018;320:2211–2220. doi: 10.1001/jama.2018.17075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Smith K., Andrew E., Lijovic M., et al. Quality of life and functional outcomes 12 months after out-of-hospital cardiac arrest. Circulation. 2015;131:174–181. doi: 10.1161/CIRCULATIONAHA.114.011200. [DOI] [PubMed] [Google Scholar]
- 45.Parke R.L., McGuinness S., Eastwood G.M., et al. Co-enrolment for the TAME and TTM-2 trials: the cerebral option. Crit Care Resusc. 2017;19:99–100. [PubMed] [Google Scholar]
- 46.Ebner F., Harmon M.B.A., Aneman A., et al. Carbon dioxide dynamics in relation to neurological outcome in resuscitated out-of-hospital cardiac arrest patients: an exploratory Target Temperature Management Trial substudy. Crit Care. 2018;22:196. doi: 10.1186/s13054-018-2119-5. [DOI] [PMC free article] [PubMed] [Google Scholar]