Significant concern exists regarding the risk of transmission of severe acute respiratory syndrome coronavirus 2 (SARS-Cov-2) to health care workers during aerosol generating procedures.1, 2 The risk of viral transmission to health care workers during tracheostomy insertion is unknown.
To address this uncertainty, we continuously measured aerosol particle concentration during a planned, semi-elective surgical tracheostomy insertion on a SARS-CoV-2-positive patient. The procedure was performed in April 2020 at Western Health, Melbourne, Australia. Written informed consent for tracheostomy was obtained from the patient’s legally approved representative. The Western Health Human Research Ethics Committee advised that ethics approval was not required to conduct this environmental study.
We used two spectrometers to measure aerosol particles: the portable Mini Wide Range Aerosol Sizer 1371 (MiniWRAS) (GRIMM Aerosol Technik, Ainring, Germany) and the Aerodynamic Particle Sizer (APS) (TSI, Shoreview, MN, USA). The APS spectrometer detected larger aerosols (> 0.37 μm) and the MiniWRAS spectrometer measured smaller particles (0.01–0.35 μm). Human-generated respiratory air particles range mostly from 0.5 μm to 20 μm.3 We measured operating theatre aerosol counts for 24 hours before the procedure to obtain baseline background aerosol concentration. During the procedure, the aerosol detector inlet was positioned 30 cm directly above the patient’s neck, representing the surgeon’s breathing air space.
Total intravenous anaesthesia was maintained with propofol and fentanyl, and neuromuscular blockade with cisatracurium. Synchronised intermittent mandatory volume controlled ventilation was delivered throughout. Surgeons used bipolar diathermy for electrocautery during dissection of the neck tissues. Several minutes before tracheal incision, the endotracheal tube (ETT) was advanced under apnoeic conditions towards the carina to avoid accidental cuff damage when tracheal incision occurred. Ventilation was recommenced to allow for several minutes of pre-oxygenation before tracheal incision. At this point, the high pressure alarm occurred. This was clinically suggestive of inadvertent endobronchial intubation; however, due to the risk of aerosolisation this was not verified via bronchoscopy. The high pressure alarm was rectified by withdrawing the ETT by 2 cm. Tracheal incision and tracheostomy tube insertion were conducted under apnoeic conditions. A 5-second period of accidental circuit disconnection occurred before completion of surgery.
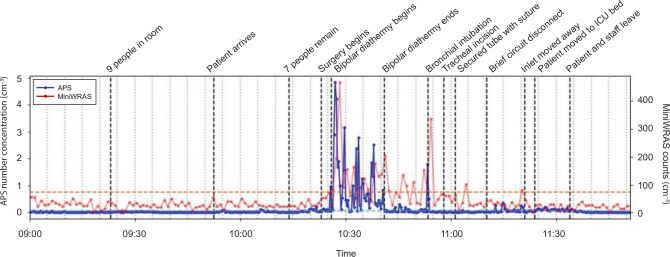
Figure 1 provides a time series of particle concentrations detected by the APS (larger size) and MiniWRAS (smaller size) spectrometers. Table 1 details the total particle concentrations measured by the spectrometers at different times during the procedure. Particle counts for the majority of the tracheostomy procedure were low; similar to delivering oxygen at 15 L/min via a simple face mask.4 Tracheal incision and brief circuit disconnection did not cause a significant rise in aerosol counts. The most significant increase in particle counts was detected during diathermy (50-fold increase) and at the time of inadvertent bronchial intubation (30-fold increase). The sudden and sharp rise in aerosol count at this time was unexpected and could not be attributed to another clinical incident. While the high peak airway pressure alarm was being investigated, the peak pressure limit was increased temporarily. We suspect that this led to airway pressures surpassing the ETT cuff pressure and thus allowing turbulent gas flow to bypass the ETT and generate aerosols via the patient’s upper airway. These high aerosol counts are similar to quantities detected during non-invasive ventilation4 and are greater than the chosen safe threshold for low aerosol generation.
Figure 1.
Timeline of aerosol concentration detected by Aerodynamic Particle Sizer (APS)* and Mini Wide Range Aerosol Sizer (MiniWRAS)† spectrometers‡
* GRIMM Aerosol Technik, Ainring, Germany. † TSI, Shoreview, MN, USA. ‡ The x-axis has lighter grey, vertical dotted bars indicating 5-minute intervals. Important events are labelled along the timeline as darker, black dashed bars. Pre-oxygenation occurred between bipolar diathermy ending and bronchial intubation. A useful threshold for a significant rise in particle counts is up to 3 standard deviations (99.5%) above the baseline mean of the theatre preparation period with all staff present (Table 1, Activity 2). The horizontal dashed orange line indicates this threshold for the APS measurements, while the light blue dashed line (mixed into the background blue line) represents this for the MiniWRAS measurements. Clearly, only diathermy, bronchial intubation and inadvertent circuit disconnection post-tracheal incision lead to aerosolisation above this background aerosol count. The y-axis gives the number of aerosols per cm-3 (mL), with smaller particles measured by the MiniWRAS spectrometer and larger particles by the APS spectrometer.
Table 1.
Total aerosol concentration recorded by the Aerodynamic Particle Sizer (APS)* and Mini Wide Range Aerosol Sizer (MiniWRAS)† spectrometers during the tracheostomy‡
| Activity | Particles per mL |
Number of samples taken |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean |
10th % |
90th % |
Maximum |
|||||||
| MiniWRAS | APS | MiniWRAS | APS | MiniWRAS | APS | MiniWRAS | APS | MiniWRAS | APS | |
| 1. Background empty theatre (overnight) | 29 | 0.001 | 11 | 0.000 | 46 | 0.003 | 188 | 0.019 | 360 | 1080 |
| 2. Theatre preparation (all staff present) | 32 | 0.020 | 15 | 0.009 | 50 | 0.035 | 57 | 0.057 | 23 | 69 |
| 3. Bipolar diathermy | 136 | 0.997 | 51 | 0.092 | 187 | 2.671 | 468 | 4.828 | 15 | 45 |
| 4. Bronchial intubation | 209 | 0.594 | - | 0.035 | - | 1.407 | - | 1.790 | 2 | 4 |
| 5. Tracheal incision | 48 | 0.029 | - | 0.028 | - | 0.031 | - | 0.032 | 1 | 3 |
| 6. Brief circuit disconnect | 60 | 0.018 | - | 0.013 | - | 0.023 | - | 0.025 | 1 | 3 |
| 7. Patient moved to ICU bed | 41 | 0.089 | - | 0.020 | - | 0.035 | - | 0.038 | 1 | 3 |
| 8. Patient and staff leave | 26 | 0.100 | - | 0.064 | - | 0.117 | - | 0.126 | 1 | 3 |
| 9. Background stabilised theatre 30 min after surgery | 15 | 0.001 | - | 0.087 | - | 0.115 | - | 0.120 | 1 | 3 |
ICU = intensive care unit.
GRIMM Aerosol Technik, Ainring, Germany.
TSI, Shoreview, MN, USA.
Energy devices, such as diathermy, generate aerosolised particles,5 but the risk of infection transmission via these bioaerosols is unknown. The only evidence that viral transmission is possible is in a bovine model.6 We are not aware of any evidence in humans that diathermy-generated aerosols can carry active virus.5
Our study has limitations. We only used a single sampling point, which could potentially underestimate particle concentrations; however, it was positioned in close proximity to the surgical field and likely provides a reliable estimate of aerosol burden related to the surgical procedure. We did not measure or confirm viral aerosol presence. We cannot extrapolate our findings to an emergent surgical tracheostomy or percutaneous tracheostomy.
This case demonstrates it is possible to achieve low aerosol particle generation during the majority of the conduct of a surgical tracheostomy. While diathermy generates aerosols, it is unlikely any virus survives the high energy discharge related to it. However, subclinical or clinical leakage of exhaled airway gas around the endotracheal tube also caused increased aerosol particle generation. This reinforces the need for full personal protective equipment with contact and aerosol precautions. Our data suggest that particle generation dangers during a surgical tracheostomy are generally low and can be mitigated by careful health care worker collaboration. Surgical tracheostomy can be performed safely in patients with coronavirus disease 2019 (COVID-19).
Competing interests
None declared.
Acknowledgements
We thank Simon Ellul (ENT Surgeon); Paul Buso, Michael Dawe, Paige Williamson and Diljo Jacob (Sunshine Operating Theatres); and Gregg Miller and Elizabeth Hessian (Anaesthesia), who were involved in the tracheostomy. We also thank Marion Kainer and Richard Bartolo (Infectious Diseases), who provided infection prevention advice; and Melita Keywood (CSIRO) and Andrew Yule (Australian Radiation Protection and Nuclear Safety Agency), who provided technical and equipment assistance.
References
- 1.Rosenbaum L. Facing COVID-19 in Italy — ethics, logistics, and therapeutics on the epidemic’s front line. N Engl J Med. 2020;382:1873–1875. doi: 10.1056/NEJMp2005492. [DOI] [PubMed] [Google Scholar]
- 2.British Laryngological Association. COVID-19 tracheostomy guidelines (April 2020). https://www.entuk.org/sites/default/files/BLA%20Tracheostomy%20guideline%20-BLA%20April%202020%20final.pdf (viewed June 2020).
- 3.Johnson G., Morawska L., Ristovski Z., et al. Modality of human expired aerosol size distributions. J Aerosol Sci. 2011;42:839–851. [Google Scholar]
- 4.McGain F., Humphries R.S., Lee J.H., et al. Critical Care Resusc. 2020. Aerosol generation related to respiratory interventions and the effectiveness of a personal ventilation hood.32475101 [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zakka K., Erridge S., Chidambaram S., et al. Electrocautery, diathermy, and surgical energy devices: are surgical teams at risk during the COVID-19 pandemic? Annals Surg. 2020;272:e257–e262. doi: 10.1097/SLA.0000000000004112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Garden J.M., O’Banion M.K., Bakus A.D., Olson C. Viral disease transmitted by laser-generated plume (aerosol) Archives Derm. 2002;138:1303–1307. doi: 10.1001/archderm.138.10.1303. [DOI] [PubMed] [Google Scholar]