Abstract
Objective: To quantify current protein prescription and delivery in critically ill adults in Australia and New Zealand and compare it with international guidelines.
Design: Prospective, multicentre, observational study.
Setting: Five intensive care units (ICUs) across Australia and New Zealand.
Participants: Mechanically ventilated adults who were anticipated to receive enteral nutrition for ≥ 24 hours.
Main outcome measures: Baseline demographic and nutrition data in ICU, including assessment of requirements, prescription and delivery of enteral nutrition, parenteral nutrition and protein supplementation, were collected. The primary outcome was enteral nutrition protein delivery (g/kg ideal body weight [IBW] per day). Data are reported as mean ± standard deviation or n (%).
Results: 120 patients were studied (sex, 60% male; mean age, 59 ± 16 years; mean admission APACHE II score, 20 ± 8). Enteral nutrition was delivered on 88%, parenteral nutrition on 6.8%, and protein supplements on 0.3% of 1156 study days. For the 73% (88/120) of patients who had a nutritional assessment, the mean estimated protein requirements were 99 ± 22 g/day (1.46 ± 0.55 g/kg IBW per day). The mean daily protein delivery was 54 ± 23 g (0.85 ± 0.35 g/kg IBW per day) from enteral nutrition and 56 ± 23 g (0.88 ± 0.35 g/kg IBW per day) from all sources (enteral nutrition, parenteral nutrition, protein supplements). Protein delivery was ≥ 1.2 g/kg IBW per day on 29% of the total study days per patient.
Conclusions: Protein delivery as a part of current usual care to critically ill adults in Australia and New Zealand remains below that recommended in international guidelines.
Critical illness is a hypercatabolic state which causes marked loss of lean muscle mass1 and persistent poor functional recovery.2 Higher protein doses may play a role in attenuating muscle loss. International guidelines for critically ill adults recommend protein delivery between 1.2 g/kg and 2.0 g/kg actual body weight (ABW) per day when body mass index (BMI) is ≤ 30 kg/m2, and between 1.2 g/kg and 2.0 g/kg ideal body weight (IBW) per day when BMI is > 30 kg/m2.3, 4 However, these recommendations are based on low quality evidence.5, 6, 7 Such limited evidence highlights the need for larger, more definitive studies to inform international recommendations.
Observational studies enrolling critically ill mechanically ventilated patients have suggested that higher protein doses (> 1.3 g/kg IBW per day) are associated with reduced mortality.8 Similarly, post hoc analyses of prospective data from an international multicentre nutrition survey reported that 60-day mortality was reduced in critically ill mechanically ventilated patients meeting ≥ 80% of protein goals compared with those not meeting goals.9, 10 Nevertheless, observational studies have a potential inherent risk of bias from unidentified confounders and do not imply causation. Conversely, there is also evidence for harm. Higher protein intakes have been associated with higher rates of muscle loss,11 and a post hoc analysis of the EPaNIC trial (n = 4640) reported an association between early amino acid administration and prolonged time to discharge from the intensive care unit (ICU).12 Further, a meta-analysis by Davies and colleagues13 failed to show a mortality benefit from higher protein doses, although the higher protein group received just 0.67 ± 0.38 g/kg per day (body weight calculations were not stipulated), well below the international recommendations.
In order to inform the standard care arm of a definitive randomised controlled clinical trial, it is important that usual clinical practice is quantified.14 In Australia and New Zealand, two multicentre observational analyses have reported protein delivery during critical illness. Bellomo et al15 reported protein doses of 0.5 ± 0.4 g/kg ABW per day delivered to critically ill patients on renal replacement therapy for severe acute kidney injury in 2005–2008.15 Similarly, in a large cohort of critically ill mechanically ventilated patients (n = 2776), Ridley and colleagues16 reported protein doses of 0.60 ± 0.35 g/kg per day (weight calculated using multiple methods) from 2007 to 2013. These data were collected before the most recent updates of international guidelines in which higher protein doses were recommended and, hence, may not be reflective of current usual practice. Therefore, the aim of this study was to quantify current protein prescription and delivery practices (in g/kg IBW per day) in Australian and New Zealand ICUs in order to inform the design of a phase 3 randomised controlled trial of augmented protein delivery in which the control arm is representative of current clinical practice.
Methods
A prospective, multicentre, observational study was conducted in five metropolitan ICUs, four in Australia and one in New Zealand. All the ICUs were mixed level 3 adult quaternary or tertiary referral sites with 14–60 beds each. The study included 120 consecutive mechanically ventilated adult patients who were about to commence enteral nutrition or had commenced enteral nutrition within the preceding 12 hours and were expected to receive enteral nutrition for at least 24 hours. These inclusion criteria were informed by a recent protein delivery feasibility trial.17 Patients were excluded if palliative treatment was being considered, death was deemed imminent by the treating intensivist, or higher than standard protein delivery (eg, burns) was deemed appropriate. No more than 30 patients were included at any individual site to ensure representative data. Ethics approval was obtained through the Central Adelaide Local Health Network Human Research Ethics Committee (HREC/19/CALHN/452), with local governance obtained for each site. Informed consent was waived as there was no intervention and the data were collected in a de-identified manner.
Baseline data variables collected at ICU admission included demographic data (age, sex, ABW, height, BMI), Acute Physiology and Chronic Health Evaluation (APACHE) II scores, and APACHE III diagnostic codes. The method used to obtain ABW was also documented (estimated, reported, or measured).
If a formal nutritional assessment was conducted by a health care professional as part of routine care during the ICU admission, the estimated calorie and protein requirements were recorded.
Nutritional data (nutrition prescription, delivery, and gastric residual volumes) were collected daily for the duration of the enteral nutrition delivery or until oral intake commenced, day 28 of ICU admission, ICU discharge or death. Nutrition prescription data included the type and volume of enteral nutrition, parenteral nutrition, and protein supplements (via any route) prescribed by the treating team at 12:00 pm on each study day. The 12:00 pm prescription was taken as indicative of the daily prescription. Nutrition delivery data included the type and total volume of enteral nutrition, parenteral nutrition, and protein supplements delivered on each calendar day. These data were used to calculate total calorie and protein delivery per day. Total gastric residual volumes aspirated and discarded on each study day were also recorded.
The primary outcome was mean enteral protein delivery in g/kg IBW per day. Clinical outcomes included ICU and hospital length of stay, and ICU and hospital mortality censored at day 28.
Statistical analysis
A pragmatic sample size of n = 120 was set to establish feasibility of recruitment at different sites and to complete the study within a suitable time frame to inform further studies on protein delivery.
Categorical data are reported as number (percentage, %) and continuous data are reported as mean ± standard deviation (SD) or median (interquartile range [IQR]). Protein data are described as g/kg per day, with weight reported in two ways: ABW, in keeping with international guidelines; and IBW, where IBW was calculated using these formulae: Men = (height (cm) – 152.4) x 0.9 + 50; Women = (height (cm) – 152.4) x 0.9 + 45.5).18
In addition, for patients who had a nutritional assessment, the daily protein deficit was calculated as the total protein received from enteral nutrition, parenteral nutrition, and protein supplements subtracted from the calculated daily protein requirements. Daily protein deficits were summed to determine the total protein deficit over the study period.
Results
Baseline characteristics
One-hundred and twenty patients were enrolled between 8 January and 15 April 2020 across five sites (12–30 patients per site) (Table 1). Mean age was 58.5 ± 15.7 years, and 60% of patients were male. Mean ABW was 86 ± 24 kg and IBW was 64 ± 10 kg, and the mean BMI was 30 ± 8 kg/m2. Mean admission APACHE II score was 20 ± 8 and the most common APACHE III diagnostic category was neurological (23%).
Table 1.
Patient baseline demographic and clinical characteristics⁎
| Characteristics | Values |
|---|---|
| Total number of patients | 120 |
| Age, years | 58.5 ± 15.7 |
| Sex, male | 72 (60%) |
| Actual body weight, kg | 86 ± 24 |
| Method used to determine weight | |
| Measured | 60 (50%) |
| Reported | 33 (27.5%) |
| Estimated | 27 (22.5%) |
| Body mass index, kg/m2⁎ | 30 ± 8 |
| Ideal body weight, kg† | 64 ± 10 |
| APACHE II score‡ | 20 ± 8 |
| APACHE III diagnostic code | |
| Neurological | 28 (23%) |
| Cardiovascular | 20 (17%) |
| Respiratory | 20 (17%) |
| Trauma | 16 (13%) |
| Gastrointestinal | 14 (12%) |
| Sepsis | 9 (7.5%) |
| Cardiothoracic surgery | 7 (5.8%) |
| Metabolic | 3 (2.5%) |
| Other | 3 (2.5%) |
| Post-operative | 42 (35%) |
| Number of patients for whom a nutritional assessment was performed | 88 (73%) |
| Length of stay (days), median (IQR) | |
| ICU | 8.4 (5.7–13.7) |
| Hospital | 20.5 (13.5–31.2) |
| Mortality | |
| ICU | 22 (18%) |
| Hospital | 27 (23%) |
APACHE = Acute Physiology and Chronic Health Evaluation; ICU = intensive care unit; IQR = interquartile range. Data are presented as mean ± standard deviation unless otherwise stated.
Actual body weight in kg/height in m2.
Ideal body weight calculated as Men = (height (cm) – 152.4) x 0.9 + 50; Women = (height (cm) – 152.4) x 0.9 + 45.5).18
Calculated on admission to the ICU.
Nutrition assessment
A formal nutrition assessment was completed by a dietitian for 73% (88/120) of patients. The mean estimated protein requirements were 99 ± 22 g per day (1.1 ± 0.2 g/kg ABW per day) (Table 2).
Table 2.
Nutrition data⁎
| Total |
||
|---|---|---|
| Characteristic | N | Mean ± SD |
| Estimated protein requirement (from nutritional assessment)† | ||
| Protein, g/day | 88 | 99 ± 22 |
| Protein, g/kg ABW per day | 88 | 1.11 ± 0.20 |
| Protein, g/kg IBW per day | 88 | 1.46 ± 0.55 |
| Protein dose prescribed | ||
| Protein, g/day | 116 | 82 ± 27 |
| Protein, g/kg ABW per day | 115 | 1.03 ± 0.39 |
| Protein, g/kg IBW per day | 115 | 1.28 ± 0.42 |
| Protein dose delivered by enteral nutrition | ||
| Protein, g/day | 119 | 54 ± 23 |
| Protein, g/kg ABW per day | 119 | 0.67 ± 0.29 |
| Protein, g/kg IBW per day | 119 | 0.85 ± 0.35 |
| Protein dose delivered from other sources, g/day | ||
| Parenteral nutrition | 10 | 63 ± 22 |
| Protein supplements | 1 | 9.4 (-) |
| Total protein dose delivered from all sources | ||
| Protein, g/day | 119 | 56 ± 23 |
| Protein, g/kg ABW per day | 119 | 0.69 ± 0.29 |
| Protein, g/kg IBW per day | 119 | 0.88 ± 0.35 |
| Percentage of study days when delivered protein was ≥ 1.2 g/kg IBW per day per patient | 80 | 29 (26%) |
| Volume of enteral nutrition, mL/day† | ||
| Prescribed | 116 | 1357 ± 410 |
| Delivered | 119 | 891 ± 336 |
| Calories delivered by enteral nutrition | ||
| kcal/day | 119 | 1106±441 |
| kcal/kg ABW per day | 119 | 14 ± 6 |
| kcal/kg IBW per day | 119 | 18 ± 7 |
| Calories delivered from other sources, kcal/day | ||
| Parenteral nutrition | 10 | 1200 ± 425 |
| Protein supplements | 1 | 297 (-) |
| Calories delivery from all sources | ||
| kcal/day | 119 | 1148±451 |
| kcal/kg ABW per day | 119 | 14 ± 6 |
| kcal/kg IBW per day | 119 | 18 ± 7 |
| Number of study days when products were delivered | ||
| Enteral nutrition | 1156 | 1012 (88%) |
| Parenteral nutrition | 1156 | 79 (6.8%) |
| Protein supplements | 1156 | 4 (0.3%) |
| Total daily gastric residual volume‡ | ||
| Measured, mL | 117 | 210 ± 207 |
| Discarded, mL | 117 | 64 ± 132 |
ABW = actual body weight; IBW = ideal body weight; SD = standard deviation.
Calculated protein requirements according to documented nutritional assessment when this study was performed.
Three patients had an intensive care unit length of stay < 48 h and thus received enteral nutrition, but prescription data were not recorded at 12:00 pm on day 0 and enteral nutrition was ceased by 12:00 pm on day 1.
The gastric residual volume was recorded in 117 out of 119 patients.
Nutrition prescription
The final analysis included 119 patients due to incomplete data collection for one patient. Of these, 97.5% (116/119) were prescribed enteral nutrition on any day, 8.4% (10/119) were prescribed parenteral nutrition, and one patient was prescribed protein supplements (Table 2). Of the 1156 total study days, enteral nutrition was prescribed on 78.8% (799/1156), parenteral nutrition on 5.4% (63/1156), and protein supplements on 0.3% (4/1156) of the study days. No nutrition was prescribed on 27.9% (323/1156) of the study days.
The mean volume of enteral nutrition prescribed was 1357 ± 410 mL/day. Fifteen different types of enteral nutrition formulae were prescribed (Table 3); the most common being Nutrison Protein Plus Multi Fibre (Nutricia Australia). Parenteral nutrition formulae prescribed included Olimel N9 (Baxter) and SmofKabiven (Fresenius Kabi) and the protein supplement prescribed was Beneprotein (Nestlé).
Table 3.
Enteral and parenteral formulae and non-oral protein supplements delivered
| Protein delivered |
||||
|---|---|---|---|---|
| Calorie content (kcal/L) | Protein content (g/L) | Frequency⁎ | Percentage of type† | |
| Enteral nutrition formulae | ||||
| Nutrison Protein Plus Multi Fibre (Nutricia) | 1280 | 63 | 419 | 41% |
| Jevity Promote (Abbott) | 1000 | 55.5 | 264 | 26% |
| Nutrison 1.0 kcal/mL (Nutricia) | 1000 | 40 | 89 | 8.8% |
| TwoCal HN (Abbott) | 2000 | 84.8 | 48 | 4.7% |
| Nutrison Protein Plus (Nutricia) | 1250 | 63 | 47 | 4.7% |
| Nutrison Energy Multi Fibre (Nutricia) | 1530 | 60 | 40 | 4.0% |
| Nutrison Concentrated (Nutricia) | 2000 | 75 | 29 | 2.9% |
| Novasource Renal (Nestlé) | 2000 | 91 | 16 | 1.6% |
| Nutrison Energy (Nutricia) | 1500 | 60 | 13 | 1.3% |
| Isosource Protein Fibre (Nestlé) | 1330 | 67 | 12 | 1.2% |
| Nutrison Multi Fibre (Nutricia) | 1030 | 40 | 7 | 0.7% |
| Nutrison Protein Intense (Nutricia) | 1260 | 100 | 7 | 0.7% |
| Ensure Plus HN (Abbott) | 1250 | 79 | 4 | 0.4% |
| Vital (Abbott) | 1500 | 67.5 | 2 | 0.2% |
| Isosource 2.0 (Nestlé) | 2000 | 84 | 1 | 0.1% |
| Other | - | 15 | 1.5% | |
| Parenteral nutrition formulae | ||||
| Olimel N9 (Baxter) | 1070 | 47 | 44 | 70% |
| SmofKabiven (Fresenius Kabi) | 1116 | 42 | 12 | 19% |
| Other | - | - | 7 | 11% |
| Protein supplement | ||||
| Beneprotein powder (Nestlé)‡ | - | 857 | 4 | 100% |
Refers to the number of study days the formula was delivered.
Refers to the percentage of total study days the formula was delivered.
Documentation on the preparation of Beneprotein was not recorded. A 7 g scoop of Beneprotein provides 6 g protein from whey protein isolate and needs to be administered with a minimum of 60 mL water per scoop.
Nutrition delivery
Of the 119 patients with data available, 100% received enteral nutrition, 8.4% (10/119) received parenteral nutrition, and one patient received protein supplements during their ICU admission. Enteral nutrition was delivered on 87.5% (1012/1156), parenteral nutrition on 6.8 % (79/1156), and protein supplements on 0.3% (4/1156) of the study days. The mean volume of enteral nutrition delivered was 891 ± 336 mL/day.
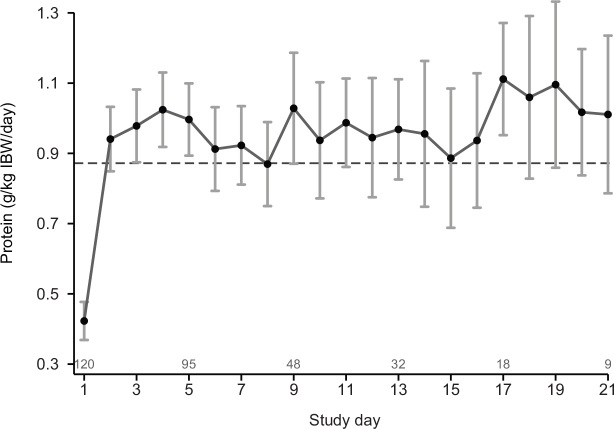
The mean amount of protein delivered to patients per day from all sources was 56 ± 23 g/day (0.88 ± 0.35 g/kg IBW per day) (Table 2). On average per patient, mean daily protein delivery was ≥ 1.2 g/kg IBW per day on 29% of the study days. The mean daily protein delivered in g/kg IBW per day from day 1 to day 21 is presented in Figure 1. The mean calorie delivery from all sources was 1148 ± 451 kcal/day (18.0 ± 6.9 kcal/kg IBW per day) (Table 2).
Figure 1.
Mean daily protein delivered in g/kg ideal body weight per day, with 95% CI adjusted for within patient correlation, for days 1–21
Dashed line = population-averaged mean value over the displayed period.
Clinical outcomes
The median ICU length of stay was 8.4 days (IQR, 5.7–13.7 days), and the median hospital length of stay was 20.5 days (IQR, 13.5–31.2 days). ICU and hospital mortality were 18% and 23% respectively.
Discussion
This prospective, multicentre, observational study quantified protein doses delivered as part of current clinical practice in five ICUs in Australia and New Zealand and may provide fundamental insights into the changes in protein delivery that have occurred since the international guidelines were updated. The results suggest that current Australian and New Zealand clinical practice delivers more protein compared with previous observational studies.
Although the international guidelines recommend IBW to calculate protein requirements, there is no consistency in published trials or in clinical practice about what weight to use. This makes it difficult to interpret and compare with previous data. Importantly, protein dose calculated and reported according to IBW will appear higher but will be less variable than when using ABW. In 2014, the RENAL trial reported protein delivery from all sources of 0.5 g/kg ABW per day during the ICU stay.15 That study recruited patients with acute kidney injury requiring renal replacement therapy in whom higher protein delivery is recommended19 and, thus, may not have represented standard care for a general ICU population. Conversely, Ridley and colleagues16 extracted data on Australia and New Zealand practice from the International Nutrition Surveys from 2007 to 2013 and reported protein delivery from all sources of 0.6 g/kg per day; body weight was calculated using a mix of methods that were not provided.16 This study included a heterogeneous critically ill mechanically ventilated population similar to our patient population, and suggests a likely true but small increase in protein delivery (from 0.6 g/kg ABW per day to 0.69 g/kg ABW per day; Table 2) between 2013 and 2020.
In pragmatic clinical trials, the aim is frequently to determine the effect of an intervention or change compared with usual care.14 Two recent randomised controlled trials have explored augmented protein delivery compared with standard care. Both of these trials reported delivery of higher protein doses in their standard care arm than reported in previous observational studies. In a European study conducted in ICU patients with obesity, 0.76 g protein per kg IBW per day was delivered to the standard care arm at day 5;20 and in an Australian study conducted in a mixed group of mechanically ventilated ICU patients, the standard care arm received 0.99 ± 0.27 g protein per kg IBW per day.17 Our current study confirms that protein delivery is higher in the five ICUs studied than reported previously and similar to the standard care arms in recent European and Australian studies. This is an important consideration for future trial design to ensure the protein dose targeted as part of usual care achieves a dose representative of current clinical practice.
The higher protein dose received in our study likely reflects a shift towards prescription of enteral formulae with higher protein content, with Nutrison Protein Plus Multi Fibre (63 g protein/L and 1.25 kcal/mL) being the predominant formula delivered in the five centres studied. It was noted in the point prevalence study from Australia and New Zealand in 201221 that the most common enteral formula prescribed and delivered was a 1 kcal/mL standard formula with 40 g protein per L. This was similar to the formula most commonly prescribed in the International Nutrition Survey between 2007 and 2013 across Australia and New Zealand.16 Hence, this signifies a likely change in practice in the centres studied. Another mechanism whereby additional protein could be delivered would be by providing more nutrition overall; however, the overall calorie delivery was similar to that reported in the International Nutrition Survey (1133 ± 572 kcal/day).16
Despite an increase in protein delivery over the past two decades, our results demonstrate that protein delivery remains below that recommended in international guidelines. In addition, there was minimal use of protein supplements or parenteral nutrition to increase protein delivery to achieve recommended doses. Although the feasibility of using protein supplements has been demonstrated to increase protein delivery22 and attenuate muscle loss,23 its widespread implementation into current clinical practice and effect on clinical outcomes remains to be explored.
The strengths of our study are that it was investigator-led, supported by independent funding, and sponsored by a university-affiliated hospital. A heterogeneous critically ill mechanically ventilated population was studied, excluding specific cohorts of patients believed to require higher protein doses (ie, burns), which improves generalisability across Australia and New Zealand. Illness severity and clinical outcome data were similar to that previously reported in ICU nutrition studies,17 suggesting a cohort of patients representative of the general Australian and New Zealand ICU population. Limitations include that data were collected from only five metropolitan ICUs in Australia and New Zealand, which may limit generalisability. Prescription data were collected at a single time point and extrapolated to a calendar day to reduce data collection burden; hence, changes in prescriptions over a 24-hour period were not accounted for. A lack of consistency in the measurement of body weight is a limiting factor in the comparison of nutrition studies. To avoid this, we and others have recently been using IBW.24, 25 Although this provides some level of reproducibility and consistency, it remains uncertain whether nutrition prescription according to IBW is optimal. Finally, partial days of data on the day of enrolment, commencement of oral nutrition, and the day of discharge or death were not adjusted for in the analysis of the total delivery over the study period.
Conclusions
This prospective, multicentre, observational study demonstrated that current protein delivery to critically ill adults in patients from five ICUs in Australia and New Zealand is higher than previously reported in observational studies but below international recommendations. These findings can inform the usual care group of future investigations.
Acknowledgements: Marianne Chapman is the recipient of a National Health and Medical Research Council (NHMRC) Project grant (APP1144496). The Royal Adelaide Hospital provided infrastructure and administrative support. TARGET Protein Observational Study Site Investigators (alphabetically by institution and all in Australia unless specified): Marianne J Chapman, Lee-anne S Chapple, Stephanie N O’Connor and Matthew J Summers from the Royal Adelaide Hospital; Deborah Barge, Kathleen M Byrne, Adam M Deane, Alana Driscoll and Madeline Haile from the Royal Melbourne Hospital; Heidi Buhr, Jennifer Coles and Suzie Ferrie from the Royal Prince Alfred Hospital; Catherine Kurenda, Sandra L Peake and Patricia J Williams from the Queen Elizabeth Hospital; and Kirsha Delaney, Charlotte Latimer-Bell, Eden Lesona, Alexandra Millington, Shaanti Olatunji, Leanlove Navarra, Mary R Sol Cruz, Raulle Sol Cruz, Chelsea Young and Paul J Young from the Wellington Regional Hospital, New Zealand. Tejaswini Arunachala Murthy is supported by an Australian Government Research Training Program scholarship. Lee-anne Chapple is supported by a NHMRC Early Career Fellowship.
Competing interests
No relevant disclosures.
References
- 1.Batt J., Herridge M., dos Santos C. Mechanism of ICU-acquired weakness: skeletal muscle loss in critical illness. Intensive Care Med. 2017;43:1844–1846. doi: 10.1007/s00134-017-4758-4. [DOI] [PubMed] [Google Scholar]
- 2.McNelly A.S., Rawal J., Shrikrishna D., et al. An exploratory study of long-term outcome measures in critical illness survivors: construct validity of physical activity, frailty, and health-related quality of life measures. Crit Care Med. 2016;44:e362–e369. doi: 10.1097/CCM.0000000000001645. [DOI] [PubMed] [Google Scholar]
- 3.McClave S.A., Taylor B.E., Martindale R., et al. Guidelines for the provision and assessment of nutrition support therapy in the adult critically ill patient: Society of Critical Care Medicine (SCCM) and American Society for Parenteral and Enteral Nutrition (ASPEN) J Parenter Enteral Nutr. 2016;40:159–211. doi: 10.1177/0148607115621863. [DOI] [PubMed] [Google Scholar]
- 4.Singer P., Blaser A.R., Berger M.M., et al. ESPEN guideline on clinical nutrition in the intensive care unit. Clin Nutr. 2019;38:48–79. doi: 10.1016/j.clnu.2018.08.037. [DOI] [PubMed] [Google Scholar]
- 5.Ferrie S., Allman Farinelli M., Daley M., Smith K. Protein requirements in the critically ill: a randomized controlled trial using parenteral nutrition. J Parent Enteral Nutr. 2016;40:795–805. doi: 10.1177/0148607115618449. [DOI] [PubMed] [Google Scholar]
- 6.Weijs P.J., Stapel S.N., de Groot S.D., et al. Optimal protein and energy nutrition decreases mortality in mechanically ventilated, critically ill patients: a prospective observational cohort study. J Parenter Enteral Nutr. 2012;36:60–68. doi: 10.1177/0148607111415109. [DOI] [PubMed] [Google Scholar]
- 7.Allingstrup M.J., Esmailzadeh N., Knudsen A.W., et al. Provision of protein and energy in relation to measured requirements in intensive care patients. Clin Nutr. 2012;31:462–468. doi: 10.1016/j.clnu.2011.12.006. [DOI] [PubMed] [Google Scholar]
- 8.Song J.H., Lee H.S., Kim S.Y., et al. The influence of protein provision in the early phase of intensive care on clinical outcomes for critically ill patients on mechanical ventilation. Asia Pac J Clin Nutr. 2017;26:234–240. doi: 10.6133/apjcn.032016.01. [DOI] [PubMed] [Google Scholar]
- 9.Nicolo M., Heyland D.K., Chittams J., et al. Clinical outcomes related to protein delivery in a critically ill population: a multicenter, multinational observation study. J Parent Enteral Nutr. 2016;40:45–51. doi: 10.1177/0148607115583675. [DOI] [PubMed] [Google Scholar]
- 10.Compher C., Chittams J., Sammarco T., et al. Greater protein and energy intake may be associated with improved mortality in higher risk critically ill patients: a multicenter, multinational observational study. Crit Care Med. 2017;45:156–163. doi: 10.1097/CCM.0000000000002083. [DOI] [PubMed] [Google Scholar]
- 11.Puthucheary Z.A., Rawal J., McPhail M., et al. Acute skeletal muscle wasting in critical illness. JAMA. 2013;310:1591–1600. doi: 10.1001/jama.2013.278481. [DOI] [PubMed] [Google Scholar]
- 12.Casaer M.P., Wilmer A., Hermans G., et al. Role of disease and macronutrient dose in the randomized controlled EPaNIC trial: a post hoc analysis. Am J Respir Crit Care Med. 2013;187:247–255. doi: 10.1164/rccm.201206-0999OC. [DOI] [PubMed] [Google Scholar]
- 13.Davies M.L., Chapple L.-A.S., Chapman M.J., et al. Protein delivery and clinical outcomes in the critically ill: a systematic review and meta-analysis. Crit Care Resusc. 2017;19:117. [PubMed] [Google Scholar]
- 14.Silverman H.J., Miller F.G. Control group selection in critical care randomized controlled trials evaluating interventional strategies: an ethical assessment. Crit Care Med. 2004;32:852–857. doi: 10.1097/01.ccm.0000114814.62759.06. [DOI] [PubMed] [Google Scholar]
- 15.Bellomo R., Cass A., Cole L., et al. Daily protein intake and patient outcomes in severe acute kidney injury: findings of the randomized evaluation of normal versus augmented level of replacement therapy (RENAL) trial. Blood Purif. 2014;37:325–334. doi: 10.1159/000363175. [DOI] [PubMed] [Google Scholar]
- 16.Ridley E.J., Peake S.L., Jarvis M., et al. Nutrition therapy in Australia and New Zealand intensive care units: an international comparison study. J Parent Enteral Nutr. 2018;42:1349–1357. doi: 10.1002/jpen.1163. [DOI] [PubMed] [Google Scholar]
- 17.LaS Chapple, Summers M.J., Bellomo R., Chapman M.J., Davies A.R., Ferrie S., et al. Use of a high protein enteral nutrition formula to increase protein delivery to critically ill patients: a randomized, blinded, parallel group, feasibility trial. J Parent Enteral Nutr. 2021;45:699–709. doi: 10.1002/jpen.2059. [DOI] [PubMed] [Google Scholar]
- 18.Brower R.G., Matthay M.A., Morris A., et al. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med. 2000;342:1301–1308. doi: 10.1056/NEJM200005043421801. [DOI] [PubMed] [Google Scholar]
- 19.Fiaccadori E., Sabatino A., Barazzoni R., et al. ESPEN guideline on clinical nutrition in hospitalized patients with acute or chronic kidney disease. Clin Nutr. 2021;40:1644–1668. doi: 10.1016/j.clnu.2021.01.028. [DOI] [PubMed] [Google Scholar]
- 20.van Zanten A.R., Petit L., De Waele J., et al. Very high intactprotein formula successfully provides protein intake according to nutritional recommendations in overweight critically ill patients: a double-blind randomized trial. Crit Care. 2018;22:1–12. doi: 10.1186/s13054-018-2070-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Peake S.L., Chapman M.J., Davies A.R., et al. Enteral nutrition in Australian and New Zealand intensive care units: a pointprevalence study of prescription practices. Crit Care Resusc. 2012;14:148. [PubMed] [Google Scholar]
- 22.Heyland D.K., Cahill N.E., Dhaliwal R., et al. Enhanced proteinenergy provision via the enteral route in critically ill patients: a single center feasibility trial of the PEP uP protocol. Crit Care. 2010;14:1–12. doi: 10.1186/cc8991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fetterplace K., Deane A.M., Tierney A., et al. Targeted full energy and protein delivery in critically ill patients: a study protocol for a pilot randomised control trial (FEED Trial) Pilot Feasibility Stud. 2018;4:52. doi: 10.1186/s40814-018-0249-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chapman M., Peake S.L., Bellomo R., et al. Energy-dense versus routine enteral nutrition in the critically ill. N Engl J Med. 2018;379:1823–1834. doi: 10.1056/NEJMoa1811687. [DOI] [PubMed] [Google Scholar]
- 25.Rice T.W., Wheeler A.P., Thompson B.T., et al. Initial trophic vs full enteral feeding in patients with acute lung injury: the EDEN randomized trial. JAMA. 2012;307:795–803. doi: 10.1001/jama.2012.137. [DOI] [PMC free article] [PubMed] [Google Scholar]