Abstract
Background: Persistent psychological distress occurs frequently in family members of patients who die in an intensive care unit (ICU).
Objective: To determine the effectiveness of bereavement interventions in reducing persisting psychological distress in bereaved family members after death in an adult ICU.
Design: Systematic review and meta-analysis of studies that assessed the effect of bereavement interventions on persisting psychological distress in bereaved family members of ICU patients.
Data sources: MEDLINE and APA PsycInfo databases were searched until April 2020.
Review methods: Two of us independently screened titles and abstracts of identified studies, and then completed full text evaluation of selected studies. We assessed risk of bias using version 2 of the Cochrane risk-of-bias tool for randomised trials and the Newcastle-Ottawa Scale, which is designed to assess the quality of non-randomised studies in meta-analyses. We also used random effects meta-analysis to assess the effect of various interventions on total Hospital Anxiety and Depression Scale (HADS) scores.
Results: From 664 citations, five studies were included — three multicentre randomised clinical trials and two single centre observational studies. Three studies tested the intervention of written bereavement support materials and two studies used narration of family members’ experiences in the ICU. All studies reported HADS scores. Scores for Impact of Event Scale, Impact of Event Scale-Revised and Inventory of Complicated Grief were measured in some but not all studies. There was no effect of an intervention on HADS scores (weighted mean difference, –0.79 [95% confidence interval, –3.81 to 2.23]; I2 = 65.8%).
Conclusions: Owing to limited data, and clinical and statistical heterogeneity, there is considerable uncertainty regarding whether bereavement support strategies reduce, increase or have no effect on psychological distress in bereaved family members.
The experience of having a loved one hospitalised in an intensive care unit (ICU) is distressing and has been shown to adversely affect long-term mental health outcomes.1 Specifically, symptoms of anxiety, depression, post-traumatic stress disorder and complicated grief occur frequently.2, 3, 4, 5, 6 It has been observed that psychological distress is more common in those involved in end-of-life decision making,7 despite the fact that most family members prefer to play an active role in making these decisions to uphold their loved ones’ wishes and values.8 When psychological distress persists, the sequelae may become significant — increased burden of physical health problems, financial difficulties, alterations in quality of life, and increased risk of death have been reported in this bereaved population.3,5,9,10 In response to this substantial problem, it has been proposed that providing bereavement support to family members may ameliorate persistent symptoms.
Despite proposals to provide ICU bereavement support, there is no specific accepted intervention recommended in clinical practice guidelines; instead, there are recommendations for family-centred or holistic care.11,12 The lack of consensus may reflect inadequate empirical evidence, or inadequate collation and translation of existing data.3,13,14 An additional challenge in evaluating the impact of the ICU experience on bereaved families is uncertainty regarding optimal measurement tools and timing of assessment.15
We conducted this systematic review to evaluate all eligible observational studies and randomised controlled trials which assessed the nature and effect of ICU bereavement support strategies on the outcome of psychological distress in bereaved family members. Our hypothesis was that there would be considerable variation in interventions tested and outcomes measured, and that there would be uncertainty regarding the impact of ICU bereavement support strategies on persistent psychological stress. The research question was: what is the nature and effectiveness of interventions used to reduce persisting psychological distress in bereaved family members after a death in an adult intensive care unit?
Methods
The conduct of this review was aligned with recommendations outlined by the Preferred Reporting Items for Systematic Reviews and Meta-Analyses statement, the Cochrane handbook for systematic reviews of interventions, and Centre for Reviews and Dissemination guidance.16, 17, 18
Search strategy and data sources
We conducted a systematic search using MEDLINE (OvidSP, from 1946 to 9 April 2020) and APA PsycInfo (OvidSP, 1806 to 9 April 2020) with assistance from a librarian. Key words used are provided online (Supporting Information). We included studies if: they were randomised controlled trials or prospective observational studies published between 1990 and 9 April 2020; at least 50% of patients died in the ICU; outcomes in adult family members of adult ICU patients were examined; and pre-defined outcome measures were clearly outlined. We excluded studies if they were not available in English.
Study selection
Two of us (LIR, NYY) independently screened titles and abstracts of identified studies, and then completed full text evaluation of selected studies. Another two of us (AMD, YAA) resolved any conflicts or discrepancies. We also checked reference lists of retrieved studies for additional relevant studies which were not captured in the search strategy.
Data synthesis and statistical analysis
The outcomes of interest were: types of ICU bereavement support; timing of support; relevant tools used to measure the outcomes of support; and impact of support. Two of us (LIR, NYY) independently extracted the data, which included: study characteristics (author, year of publication, study question, study design, patient inclusion and exclusion criteria, characteristics of patients, and characteristics of participants); type and timing of the ICU bereavement support intervention; relevant tools used to measure psychological outcomes; and the impact of bereavement support. Discrepancies in data extraction forms were largely resolved by two of us (LIR, NYY), and any outstanding differences were resolved by another two of us (ADM, YAA). Once studies were deemed fit for inclusion, we contacted study authors, where necessary, and asked them to supply missing data.
The primary outcome measure for assessment of the effect of intervention was the Hospital Anxiety and Depression Scale (HADS) score, summarised as the mean and standard deviation (SD). Given the methodological differences between the studies, a random effects metaanalysis was performed, using the DerSimonian–Laird approach.19 We asked study authors to provide the mean and SD values for the total HADS outcome from their original data. For the remaining studies, we estimated the mean and SD values from the published medians and interquartile ranges (IQRs) — we used median as an estimate of mean, and estimated SD by dividing IQR by 1.35. For clarity, we grouped studies in our analysis according to data origin (ie, per author or estimated). We calculated weighted mean difference with 95% confidence interval, and used the I2 statistic as a measure of heterogeneity. We used Stata/MP 16.1 (StataCorp) for our analyses.
Quality assessments
We assessed bias in the randomised studies using version 2 of the Cochrane risk-of-bias tool for randomised trials.20 This tool assesses a specific set of domains, including the design, conduct and reporting of the trials, for risk of bias. For the observational studies, we assessed quality using the Newcastle-Ottawa Scale, which is designed to assess the quality of non-randomised studies in meta-analyses.21 This scale uses a star rating system to evaluate study group selection, comparability of groups and ascertainment of outcome of interest; the higher the number of stars awarded to a study (maximum 9 stars), the lower the risk of bias.
Results
Study selection
The database search yielded 659 non-duplicate citations, and we identified five additional records from reference lists of relevant retrieved systematic reviews and meta-analyses. Following abstract review, we excluded 648 records that did not meet all of the inclusion criteria. Following subsequent full text review for 16 articles, we excluded 11 that were not suitable for comparison with bereavement support programs — seven that did not assess family members’ psychological distress, three that examined study populations in which fewer than 50% of patients died in the ICU, and one that investigated the impact of methods for withdrawing mechanical ventilation (ie, medical strategies). We included the five remaining studies in the analysis, of which three were randomised controlled trials and two were observational studies (Figure 1).2,3,22-24
Figure 1.
Flow diagram showing selection of studies
Study and participant characteristics
The characteristics of the included studies are summarised in Table 1. In total, 580 family members or medical treatment decision makers were included from the five studies.
Table 1.
Summary of included studies evaluating the effect of bereavement support strategies
| First author (year) | Study design, location and recruitment period | Nature of intervention | Comparator | Number and composition of follow-up population (intervention, control) | Follow-up periods (bold if used in me- ta-analy- sis) | Tools used to assess psychological distress | Total HADS score mean (SD) at follow-up (control; intervention) (bold if estimated data) | Study conclusions regarding impact of intervention on total HADS score at follow-up |
|---|---|---|---|---|---|---|---|---|
| Barnato (2017)23 | Multicentre RCT, United States, June 2013 to November 2014 | Storytelling intervention and printed bereavement materials | Printed bereavement materials | 32 family members (18, 14) | 3 months, 6 months |
HADS, ICG, IES-R |
13.38 ( 1 1.36); 12.18(10.53) |
Conclusion regarding total HADS score not reported |
| Kentish-Barnes (2017)2 |
Multicentre RCT, France, December 2014 to December 201 5 | Handwritten condolence letter | Standard care, as per individual ICUs | 208 family members (109, 99) | 1 month, 6 months |
HADS, ICG, IES-R |
11.59 (9.58); 13.96(9.22) |
Intervention had no effect on total HADS score at 1 month; intervention was associated with increased (worsened) total HADS score at 6 months, P= 0.04 |
| Lautrette (2007)22 | Multicentre RCT, France, May 2005 to October 2005 | Communication strategy (end-of-life conference conducted according to specific guidelines) and printed bereavement materials | Standard care, as per individual ICUs | 126 family members (63, 63) | 3 months | HADS, IES | 17.69 (8.40); 13.28(6.68) |
Intervention was associated with reduced (improved) total HADS score, P= 0.004 |
| McAdam (2018)3 | Single centre observational trial, United States, recruitment period not stated | Printed bereavement materials, condolence card (at 1 week), telephone follow-up (at 4-5 weeks), handwritten condolence note (at 1 year) and a card on patient’s birthday | No formal bereavement program; some families received printed bereavement materials, some families received a handwritten letter of condolence at 2 weeks | 40 family members (30, 10) | 13 months | HADS, IES-R | 12.44 (9.84); 11.11 (5.28) |
Conclusion regarding total HADS score not reported |
| Schwarzkopf (2020)24 |
Single centre observational trial, Germany, December 2010 to February 2014 | Improved documentation, communication facilitator, end-of-life communication skills training for staff, debriefing sessions, ICU diary for patient’s relatives, and guiet waiting area or conference room for relatives | Standard care | 174 family members (90, 84) | 3 months | HADS, IES | 13 (11); 13 (11) | Conclusion regarding total HADS score not reported |
HADS = Hospital Anxiety and Depression Scale. ICG = Inventory of Complicated Grief. ICU = intensive care unit. IES = Impact of Event Scale. IES-R = Impact of Event Scale-Revised. RCT = randomised controlled trial. SD = standard deviation.
Risk of bias assessments
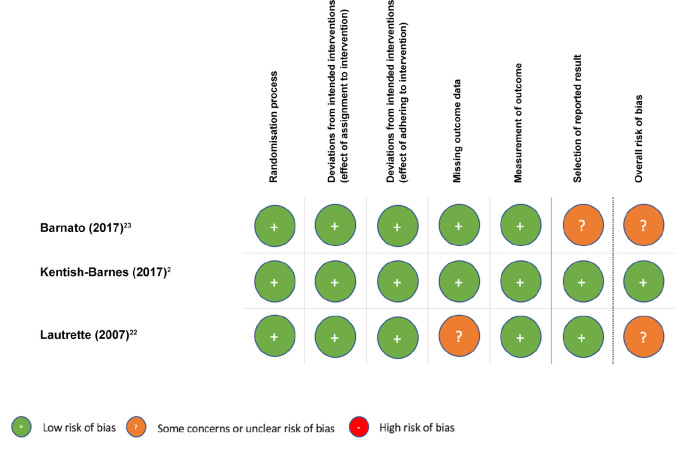
Our risk of bias assessments are shown in Figure 2, which addresses the randomised controlled trials,2, 22, 23 and in Table 2, which addresses the observational studies.3,24 Only one study was ranked as being at low risk of bias and none were ranked as being at high risk of bias. Accordingly, all five studies were included in the meta-analysis.
Figure 2.
Risk of bias in included randomised controlled trials, assessed using version 2 of the Cochrane risk-of-bias tool for randomised trials20*
* Overall risk of bias was determined as follows: low overall if all domains are rated “low risk”; some concerns or unclear overall if one or more domains are rated “some concerns or unclear”; high overall if one or more domains are rated “high risk” or if multiple domains are rated “some concerns” in a way that substantially lowers confidence in the result.
Table 2.
| Selection (maximum score: 4 stars) |
Comparability (maximum score: 2 stars) |
Outcome (maximum score: 3 stars) |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| First author (year) | Represen- tative-ness of exposed cohort | Selection of non-exposed cohort | Ascertainment of exposure | Demonstration that outcome of interest was not present at start of study | Comparability of cohorts | Assessment of outcome | Follow-up duration long enough for outcome to occur | Adequacy of follow-up | Total score; risk of bias |
| McAdam (2018)3 |
1 star | 1 star | 1 star | 0 stars | 0 stars | 1 star | 1 star | 0 stars | 5 stars; medium risk |
| Schwarzkopf (2020)24 |
1 star | 1 star | 1 star | 0 stars | 0 stars | 1 star | 1 star | 0 stars | 5 stars; medium risk |
For each study, total scores were evaluated as follows: 0–3 stars, high risk of bias (low quality); 4–6 stars, medium risk of bias (medium quality); 7–9 stars, low risk of bias (high quality)
Interventions
In four of the five studies, multifaceted interventions were assessed. The follow-up strategies in two studies considered factors before the patient’s death that might contribute to ongoing psychological distress in family members, such as staff behaviour.22,24 Communication training, debriefing and/or instructions on improving documentation in electronic medical records were delivered to staff in both studies, and a quiet waiting area for family members during the patient’s ICU stay was provided in one study. 22,24
All five studies incorporated strategies that followed the patient’s death, with three studies providing written bereavement support materials to family members.3, 22, 23 In two studies, family members were encouraged to “revisit” the patient’s time in the ICU, through active re-telling of the events of the patient’s admission or personal reflection using a detailed ICU diary provided by the unit.23,24 Written expressions of condolence were provided in the weeks following the patient’s death in two studies, either generic or handwritten and highly personalised.2,3 In the latter case, this was the only intervention tested by the investigators.2 In the former case, additional psychological support was provided via telephone 4–5 weeks after the patient’s death and by sending a card on what would have been the patient’s birthday.3
Follow-up data were obtained via telephone between 1 and 13 months after the patient’s death. In one study, patient’s death to serve as a baseline for reporting, but we patient’s death to serve as a baseline for reporting, but we did not consider baseline data in our meta-analysis.23
Clinical Outcomes
Hospital Anxiety and Depression Scale
The HADS is a 14-item self-assessment scale used to detect states of depression and anxiety in the hospital outpatient setting.25 It may be divided into two subscales — anxiety (HADS-A) and depression (HADS-D) — each of which comprise seven items. Both subscale scores and total scores are considered reliable and valid measures of emotional disorder.25
One randomised controlled trial reported that the intervention was associated with greater psychological distress, as measured by the HADS score.2 Another randomised controlled trial reported that the HADS score was reduced with the intervention.22 The remaining three studies reported neutral effects, although conclusions regarding total HADS scores were not reported in these studies.
Meta-analysis of the included studies was consistent with no observed treatment effect. The HADS scores weighted mean difference was –0.79 (95% CI, –3.81 to 2.23) (Figure 3). There was considerable statistical heterogeneity, as indicated by an I2 value of 65.8%.
Figure 3.
Forest plots showing the effect of intervention on total HADS score for studies where exact mean and SD were available from authors, studies where mean and SD were estimated from median and interquartile range, and all studies combined*
* Weights are from random effects analysis. HADS = Hospital Anxiety and Depression Scale. SD = standard deviation. WMD = weighted mean difference
Impact of Event Scale and Impact of Event Scale– Revised
Both the Impact of Event Scale and Impact of Event Scale-Revised are self-assessment scales used to detect distress experienced by an individual in relation to traumatic events.26 Although the Impact of Event Scale-Revised represents a revised version of the Impact of Event Scale, and would intuitively seem similar, the original scale lacks an assessment of hyperarousal symptoms and comprises of only 15 items. Because the Impact of Event Scale–Revised incorporates hyperarousal symptoms and comprises an additional seven items (22 items in total), the tools were deemed too dissimilar to be pooled in a meta-analysis.
Two studies reported Impact of Event Scale scores and three studies reported the Impact of Event Scale–Revised scores.2,3,22, 23, 24 Only one of the five included studies identified that the intervention was associated with statistically significantly reduced Impact of Event Scale scores when comparing the intervention with usual care (median [IQR], 27 [18–42] v 39 [25–48]; P = 0.02). 22 The remaining four studies individually reported neutral effect. 2,3,23,24
Inventory of Complicated Grief
The Inventory of Complicated Grief is considered a valid assessment of emotional distress and is associated with, yet distinct from, symptoms of depression and anxiety.27 The Inventory of Complicated Grief was developed to assess the symptoms of grief that are associated with long term functional impairments.
The Inventory of Complicated Grief was reported in two studies, neither of which demonstrated a significant effect of the intervention.2,23 We did not include these data in our meta-analysis.
Discussion
We undertook this systematic review and meta-analysis to evaluate the impact of various bereavement support distress, as measured by the HADS score.2 Another suggest that there is considerable uncertainty as to whether these interventions are beneficial, harmful or have no measurable impact on psychological distress of bereaved family members after death in an adult ICU.
The strengths of our meta-analysis include the structured search, complete retrieval of identified studies and validated methods in accordance with the Cochrane handbook for systematic reviews of interventions and Centre for Reviews and Dissemination guidance.17,18 Where available, the outcome metric for meta-analysis was supplied by the original authors, and this was only estimated where indicated. The HADS score was used as a continuous variable, permitting a more precise interpretation of intervention effect compared with an analysis based on an arbitrary cut-point where scores are treated as binary outcomes (eg, distressed versus not distressed). The studies included were conducted in multiple countries, involving patients and family members from diverse cultural backgrounds.
Our systematic review is also unique, reporting specifically on the nature of bereavement support and assessment of the associated psychological impact with validated tools. Previous systematic reviews have focused on the impact of mental health outcomes of family members of both ICU survivors and non-survivors together.6 To our knowledge, no summary of the impact of bereavement support interventions or meta-analysis of such data has previously been published. However, narrative reviews covering various aspects of end-of-life care have been published, examining bereavement support services provided to family members of patients who died in an ICU, and related topics such as decision-making interventions.28,29
Limitations of our study are inclusion of studies published in English only and potential for publication bias. The proportion of family members retained in the studies varied from 58% to 94%, and the effect of missing data on the final results is unclear. In addition, our meta-analysis reflects data derived from a small number of studies with a modest number of family members, which limits any inference. A particularly pertinent consideration relevant to all studies included in this meta-analysis is that loss to follow-up may be related to poorer mental health status, thus introducing a selection bias and reducing internal validity.
The total HADS scores for the included studies were analysed according to a random effects analysis, whereby follow-up data collected between 3 and 13 months after the patient’s death were pooled. This is expected to have contributed significantly to the heterogeneity, which at 65.8% is interpreted as substantial.17 Psychological distress experienced 3 months after a family member’s death is likely to be substantially different to that experienced 10 months later.
Furthermore, the interventions used in each study varied considerably, both in modality and timing. Interventions variably targeted staff competencies, patient care and the family members themselves. These interventions were sometimes generic, and other times highly personalised and may have been implemented before, shortly after or as much as a year after the patient’s death. In light of such considerable clinical differences, the uncertainty around the point estimate is unsurprising. It is also important to acknowledge that not all factors which affect psychological distress in bereaved family members are modifiable. There may be unavoidable components of the ICU experience that, irrespective of any intervention, contribute to poor mental health outcomes.
It should be recognised that the largest and most methodologically rigorous trial reported harm with the intervention studied.2 As a multicentre randomised controlled trial of 208 participants, the result highlights the need for adequate research before implementing bereavement support programs to limit unintended and unmeasured adverse outcomes which might arise.
While the features of anxiety and depression are measured by the HADS, the tool does not address several important psychological symptom clusters, such as post-traumatic stress symptoms or complicated grief. Therefore, not all family members who experience symptoms of psychological distress will be captured by this tool, so they would not be reflected our meta-analysis. It is unclear whether selfadministered surveys compared with telephone or face- to-face interviews would yield different results. Family members undertaking telephone or face-to-face interviews may be less forthcoming about their true feelings due to fears of judgement. However, the process of telling one’s story of loss could also help with the grieving process.30,31 In the future, administering follow-up tools online or by post, in addition to telephone interviews, may be useful.
Conclusion
We found significant variability in the design, implementation and assessment of bereavement support programs. Owing to limited numbers of studies and marked heterogeneity, both clinical and statistical, it is unclear whether bereavement support programs are beneficial, are harmful or have no measurable effect.
Competing interests
No relevant disclosures
Acknowledgements
We thank Geoff Hill, Clinical Librarian at the Royal Melbourne Hospital, who assisted with design of the search strategy and searching of the electronic databases. We also extend our thanks to the authors of the included studies for receiving and responding to our enquiries.
Availability of data and materials: Datasets used and analysed for the meta-analysis are available from the corresponding author on reasonable request.
Supplementary Information

References
- 1.Pochard F., Darmon M., Fassier T., et al. Symptoms of anxiety and depression in family members of intensive care unit patients before discharge or death. A prospective multicenter study. J Crit Care. 2005;20:90–96. doi: 10.1016/j.jcrc.2004.11.004. [DOI] [PubMed] [Google Scholar]
- 2.Kentish-Barnes N., Chevret S., Champigneulle B., et al. Effect of a condolence letter on grief symptoms among relatives of patients who died in the ICU: a randomized clinical trial. Intensive Care Med. 2017;43:473–484. doi: 10.1007/s00134-016-4669-9. [DOI] [PubMed] [Google Scholar]
- 3.McAdam J.L., Puntillo K. Pilot study assessing the impact of bereavement support on families of deceased intensive care unit patients. Am J Crit Care. 2018;27:372–380. doi: 10.4037/ajcc2018575. [DOI] [PubMed] [Google Scholar]
- 4.Kross E.K., Engelberg R.A., Gries C.J., et al. ICU care associated with symptoms of depression and posttraumatic stress disorder among family members of patients who die in the ICU. Chest. 2011;139:795–801. doi: 10.1378/chest.10-0652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kentish-Barnes N., Chaize M., Seegers V., et al. Complicated grief after death of a relative in the intensive care unit. Eur Respir J. 2015;45:1341–1352. doi: 10.1183/09031936.00160014. [DOI] [PubMed] [Google Scholar]
- 6.Zante B., Camenisch S.A., Schefold J.C. Interventions in postintensive care syndrome-family: a systematic literature review. Crit Care Med. 2020;48:e835–e840. doi: 10.1097/CCM.0000000000004450. [DOI] [PubMed] [Google Scholar]
- 7.Azoulay E., Pochard F., Kentish-Barnes N., et al. Risk of posttraumatic stress symptoms in family members of intensive care unit patients. Am J Respir Crit Care Med. 2005;171:987–994. doi: 10.1164/rccm.200409-1295OC. [DOI] [PubMed] [Google Scholar]
- 8.Nunez E.R., Schenker Y., Joel I.D., et al. Acutely bereaved surrogates’ stories about the decision to limit life support in the ICU. Crit Care Med. 2015;43:2387–2393. doi: 10.1097/CCM.0000000000001270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mitchell M., Coombs M., Wetzig K. The provision of familycentred intensive care bereavement support in Australia and New Zealand: results of a cross sectional explorative descriptive survey. Aust Crit Care. 2017;30:139–144. doi: 10.1016/j.aucc.2016.07.005. [DOI] [PubMed] [Google Scholar]
- 10.Stroebe M., Schut H., Stroebe W. Health outcomes of bereavement. Lancet. 2007;370:1960–1973. doi: 10.1016/S0140-6736(07)61816-9. [DOI] [PubMed] [Google Scholar]
- 11.Davidson J.E., Aslakson R.A., Long A.C., et al. Guidelines for family-centered care in the neonatal, pediatric, and adult ICU. Crit Care Med. 2017;45:103–128. doi: 10.1097/CCM.0000000000002169. [DOI] [PubMed] [Google Scholar]
- 12.Australian and New Zealand Intensive Care Society. ANZICS statement on care and decision-making at the end of life for the critically ill. (edition 1.0). ANZICS; Melbourne: 2014. [Google Scholar]
- 13.Bedell S.E., Cadenhead K., Graboys T.B. The doctor’s letter of condolence. N Engl J Med. 2001;344:1162–1164. doi: 10.1056/NEJM200104123441510. [DOI] [PubMed] [Google Scholar]
- 14.Downar J., Barua R., Sinuff T. The desirability of an intensive care unit (ICU) clinician-led bereavement screening and support program for family members of ICU decedents (ICU Bereave) J Crit Care. 2014;29(311):e9–16. doi: 10.1016/j.jcrc.2013.11.024. [DOI] [PubMed] [Google Scholar]
- 15.Kentish-Barnes N., Lemiale V., Chaize M., et al. Assessing burden in families of critical care patients. Crit Care Med. 2009;37:S448–S456. doi: 10.1097/CCM.0b013e3181b6e145. [DOI] [PubMed] [Google Scholar]
- 16.Moher D., Liberati A., Tetzlaff J., Altman D.G. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:332–336. [PMC free article] [PubMed] [Google Scholar]
- 17.Higgins J.P.T., Thomas J., Chandler J., et al. Cochrane; 2019. Cochrane handbook for systematic reviews of interventions, version 6.0; p. 649. http://www.training.cochrane.org/handbook (viewed Oct 2020) [Google Scholar]
- 18.Centre for Reviews and Dissemination. Systematic reviews:CRDs guidance for undertaking reviews in health care. University of York; York: 2009. http://www.york.ac.uk/inst/crd/index_guidance.htm (viewed Oct 2020) [Google Scholar]
- 19.DerSimonian R., Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 20.Sterne J.A.C., Savovi J., Page M.J., et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366 doi: 10.1136/bmj.l4898. [DOI] [PubMed] [Google Scholar]
- 21.Wells GA, Shea B, O’Connell D, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. http://www.ohri.ca/programs/ clinical_epidemiology/oxford.asp (viewed Oct 2020).
- 22.Lautrette A., Darmon M., Megarbane B., et al. A communication strategy and brochure for relatives of patients dying in the ICU. N Engl J Med. 2007;356:469–478. doi: 10.1056/NEJMoa063446. [DOI] [PubMed] [Google Scholar]
- 23.Barnato A.E., Schenker Y., Tiver G., et al. Storytelling in the early bereavement period to reduce emotional distress among surrogates involved in a decision to limit life support in the ICU: a pilot feasibility trial. Crit Care Med. 2017;45:35–46. doi: 10.1097/CCM.0000000000002009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schwarzkopf D., Pausch C., Kortgen A., et al. Quality improvement of end-of-life decision-making and communication in the ICU: effect on clinicians’ burnout and relatives’ distress. Med Klin Intensivmed Notfmed. 2020;115:600–608. doi: 10.1007/s00063-019-00632-8. [DOI] [PubMed] [Google Scholar]
- 25.Zigmond A.S., Snaith R.P. The Hospital Anxiety and Depression Scale. Acta Psychiatr Scand. 1983;67:361–370. doi: 10.1111/j.1600-0447.1983.tb09716.x. [DOI] [PubMed] [Google Scholar]
- 26.Marsella A., Wilson J.P., Tang C.S., editors. Cross-cultural assessment of psychological trauma and PTSD. Springer; New York: 2007. [Google Scholar]
- 27.Prigerson H.G., Maciejewski P.K., Reynolds C.F., III, et al. Inventory of complicated grief: a scale to measure maladaptive symptoms of loss. Psychiatry Res. 1995;59:65–79. doi: 10.1016/0165-1781(95)02757-2. [DOI] [PubMed] [Google Scholar]
- 28.Efstathiou N., Walker W., Metcalfe A., Vanderspank-Wright B. The state of bereavement support in adult intensive care: a systematic review and narrative synthesis. J Crit Care. 2019;50:177–187. doi: 10.1016/j.jcrc.2018.11.026. [DOI] [PubMed] [Google Scholar]
- 29.Bibas L., Peretz-Larochelle M., Adhikari N.K., et al. Association of surrogate decision-making interventions for critically ill adults with patient, family, and resource use outcomes: a systematic review and meta-analysis. JAMA Netw Open. 2019;2 doi: 10.1001/jamanetworkopen.2019.7229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.van der Klink M.A., Heijboer L., Hofhuis J.G.M., et al. Survey into bereavement of family members of patients who died in the intensive care unit. Intensive Crit Care Nurs. 2010;26:215–225. doi: 10.1016/j.iccn.2010.05.004. [DOI] [PubMed] [Google Scholar]
- 31.Bosticco C., Thompson T.L. Narratives and story telling in coping with grief and bereavement. Omega. 2005;51:1–16. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials