Abstract
Objectives: We aimed to investigate the use of sedation in patients with severe traumatic brain injury (TBI), focusing on the choice of sedative agent, dose, duration, and their association with clinical outcomes.
Design: Multinational, multicentre, retrospective observational study.
Settings: 14 trauma centres in Europe, Australia and the United Kingdom.
Participants: A total of 262 adult patients with severe TBI and intracranial pressure monitoring.
Main outcome measures: We described how sedative agents were used in this population. The primary outcome was 60-day mortality according to the use of different sedative agents. Secondary outcomes included intensive care unit and hospital length of stay, and the Extended Glasgow Outcome Scale at hospital discharge.
Results: Propofol and midazolam were the most commonly used sedatives. Propofol was more common than midazolam as first line therapy (35.4% v 25.6% respectively). Patients treated with propofol had similar Acute Physiology and Chronic Health Evaluation (APACHE) II and International Mission for Prognosis and Analysis of Clinical Trials in Traumatic Brain Injury (IMPACT) scores to patients treated with midazolam, but lower Injury Severity Score (ISS) (median, 26 [IQR, 22–38] v 34 [IQR, 26–44]; P = 0.001). The use of propofol was more common in heavier patients, and midazolam use was strongly associated with opioid co-administration (OR, 12.9; 95% CI, 3.47–47.95; P < 0.001). Sixty-day mortality and hospital mortality were predicted by a higher IMPACT score (P < 0.001) and a higher ISS (P < 0.001), but, after adjustment, were not related to the choice of sedative agent.
Conclusions: Propofol was used more often than midazolam, and large doses were common for both sedatives. The first choice was highly variable, was affected by injury severity, and was not independently associated with 60-day mortality.
Sedative agents are commonly administered to critically ill adults with severe traumatic brain injury (TBI) to facilitate mechanical ventilation,1 prevent agitation2 and reduce intracranial pressure.3 Sedatives have been reported to improve intracranial pressure by reducing cerebral metabolic rate (CMR), cerebral blood flow, and volume.4 However, sedatives may decrease arterial blood pressure and, thus, cerebral perfusion pressure,5 which may contribute to secondary brain injury.6 Furthermore, sedatives may interfere with neurological assessment and may accumulate and prolong the length of intensive care unit (ICU) and hospital stay.7
Propofol and midazolam are the most used sedative agents in the ICU. Propofol has a rapid onset and short duration of action and relatively fast recovery even after prolonged sedation. It may also protect against cerebral oedema and ischaemia, reduce intracranial pressure, decrease the risk of seizures, and preserve cerebrovascular autoregulation,8 with minimal effects on cerebral blood flow and CMR of oxygen (CMRO2) coupling.9 Despite its useful anticonvulsive properties and a lower risk of arterial hypotension,4 the use of midazolam results in a less pronounced reduction of CMRO2, cerebral blood flow, and intracranial pressure4 compared with propofol.
Regrettably, there is very limited knowledge on the current use of these agents in patients with TBI admitted to trauma ICUs, their use in isolation, their combination with opioid drugs, their dosage, or their association with intracranial pressure and clinical outcome. This is problematic because an understanding of current practice is necessary to inform the design of randomised controlled trials aimed at improving the quality of sedation in patients with TBI. Therefore, we conducted a multinational, multicentre, retrospective observational study of patients with severe TBI. We aimed to investigate the early use (first 24-72 hours after ICU admission) of sedative agents, the variability in choice of sedative, dose, and duration of such therapy, and to assess whether such therapy carried any association with clinical outcomes.
Methods
Study design and data collection
We retrospectively analysed data from 14 tertiary ICUs. The data collection period was from March 2013 to September 2017. Two centres were from Australia, two from the United Kingdom, and the other ten from Europe (Innsbruck, Austria; Brussels, Belgium; Paris and Nice, France; Berlin, Germany; Monza, Italy; Rotterdam, Netherlands; Valencia, Spain; Stockholm, Sweden; Lausanne, Switzerland).
The purpose of the dataset was to identify differences in practice in the management of patients with severe TBI between major TBI centres internationally. Ethics approval for this study (QA2016096) was provided by the Human Research Ethics Committee of the Royal Melbourne Hospital, Melbourne, Australia. Ethics approval for contribution to this international dataset was obtained locally by each centre.
The eligibility criteria included patients aged 18 years and over, intracranial pressure monitoring for at least 96 hours, mechanical ventilation, survival beyond the first 48 hours of ICU admission, and either isolated TBI or head injury associated with multitrauma. Centres collected data from up to 20 patients each.
For each patient, we collected baseline demographic data, vital signs, indicators of TBI severity (ie, Glasgow Coma Scale [GCS] before sedation, pupillary reactivity and initial computed tomography [CT] scan findings summarised by the Marshall score), Injury Severity Score (ISS),10 and Acute Physiology and Chronic Health Evaluation (APACHE) II score.11 We calculated the extended International Mission for Prognosis and Analysis of Clinical Trials in Traumatic Brain Injury (IMPACT) score (lab model)12 as a prognostic tool. Intraparenchymal catheters and/or extraventricular drainage were used as part of monitoring of intracranial pressure, with the intracranial pressure value recorded every 4 hours.
For this study, we considered sedative agents to be midazolam and propofol. Information about concurrent opioid doses was also collected. We asked centres to record infusion rates at midday each day. We took these doses to be representative of the average hourly dosing for the whole day. Then, we converted these doses to a weightbased equivalent.
Outcomes measures
The primary outcome measure of this exploratory analysis was 60-day mortality according to “early use” (first 24 hours after ICU admission) of propofol and midazolam. The secondary outcomes included length of stay in the ICU, length of stay in hospital, and Extended Glasgow Outcome Scale (GOS-E)13, 14 at hospital discharge.
Statistical analysis
We analysed the use of sedative agents in the first 24 hours after ICU admission (intention-to-treat analysis). We defined four subpopulations of interest according to which sedative was used: patients treated with propofol only, midazolam only, a combination of the two, or no sedatives.
All data were assessed for normality. Results were reported as the number of events and as a percentage of the total. Normally distributed variables were presented as a mean and standard deviation (SD) and non-normally distributed variables were presented as a median and interquartile range (IQR). Unadjusted analyses among subpopulations included χ2 or Fisher exact test to compare categorical variables and the Kruskal-Wallis test to compare continuous variables. Patients receiving propofol only and midazolam only as sedation in the first 24 hours were identified as the “propofol-only group” and the "midazolam-only group” respectively.
We defined a "non-midazolam-only group” as those patients treated with propofol, midazolam and propofol combined, or no sedative in the first 24 hours. We defined a "non-propofol-only group” as those patients treated with midazolam, midazolam and propofol combined, or no sedative in the first 24 hours. A sensitivity analysis was performed using sedation treatment on day 2 and 3 to confirm or refute the findings of the intention-to-treat analysis using data from day 1.
We used multivariable logistic regression models to predict which characteristics were associated with the use of propofol and/or midazolam and to predict which variables were associated with no sedation. Sex, weight, ISS, IMPACT score, hypoxia (peripheral oxygen saturation [SpO2] ≤ 90%) and hypotension (systolic blood pressure ≤ 90 mmHg) before admission and the use of any opioid or other sedative agents in the first 24 hours after ICU admission were considered as possible explanatory variables.
We used intracranial pressure data collected over the first week by comparing the propofol-only group with the non-propofol-only group and the midazolam-only group with the non-midazolam-only group using linear mixed effects models, accounting for IMPACT score and ISS as fixed effects, hospital as random effect, and within-subject repeated measures treating time as a continuous variable.
The time to death in the first 60 days was illustrated by a Kaplan-Meier curve, and a log-rank test was performed to assess statistical significance. A Cox proportional hazards regression model adjusted for the use of propofol and midazolam in the first 24 hours, ISS, and IMPACT score was performed to analyse 60-day mortality — the primary outcome — censored at hospital discharge. We performed a logistic regression analysis to predict hospital mortality, adjusting for gender, weight, ISS, IMPACT score, use of opioids and use of sedatives as explanatory variables.
Missing data for baseline characteristics and for use of sedatives were included in Table 1 and Table 2 respectively. The percentage of missing values across the baseline variables of interest varied between 0% and 14.9%. However, the statistical analysis was restricted to the complete cases only.
Table 1.
Baseline characteristics and rescue therapies
| Variables | Overall | Missing |
|---|---|---|
| Number of patients | 262 | |
| Age (years), median (IQR) | 44 (29–62) | |
| Sex, male | 202 (77.1 %) | |
| Body weight (kg), median (IQR) | 75 (68–85) | |
| GCS at hospital admission, median (IQR) | 3 (3–7) | 0.4 |
| Pupillary reactivity at hospital admission | 6.9 | |
| Both reactive | 159 (65.2%) | |
| One reactive | 40 (16.4%) | |
| Non-reactive | 45 (18.4%) | |
| Pre-hospital hypoxia (SpO2 < 90%) | 46 (19.3%) | 9.2 |
| Pre-hospital hypotension (SBP < 90 mmHg) | 41 (16.9%) | 7.3 |
| Mass lesion on first CT scan* | 180 (68.7%) | |
| Traumatic SAH on CT scan | 186 (71%) | |
| Marshall score, median (IQR) | 3 (2–5) | 14.9 |
| IMPACT score (lab model), median (IQR) | 25 (14–40) | 0.4 |
| APACHE II score, median (IQR) | 20 (15–26) | 1.1 |
| Injury Severity Score, median (IQR) | 30 (25–42) | 0.4 |
| Use of barbiturate† | 34 (13%) | |
| Surgery for clot evacuation† | 94 (36%) | 0.4 |
| Decompressive craniectomy† | 78 (29.8%) | |
| External ventricular drainage† | 84 (32.1%) |
APACHE = Acute Physiology and Chronic Health Evaluation; CT = computed tomography; GCS = Glasgow Coma Scale; IMPACT = International Mission for Prognosis and Analysis of Clinical Trials in Traumatic Brain Injury; IQR = interquartile range; SAH = subarachnoid haemorrhage; SBP = systolic blood pressure; SpO2 = peripheral oxygen saturation.
Mass lesion indicates the presence of subdural haemorrhage, extradural haemorrhage, and/or intracerebral haemorrhage.
At any time point.
Table 2.
Sedative drug administration in the first 24 hours of intensive care unit admission
| Variables | Propofol-only | Midazolam-only | Propofol and midazolam | No sedation | P* | Missing |
|---|---|---|---|---|---|---|
| Number of patients | 93 (35.4%) | 67 (25.6%) | 18 (6.9%) | 84 (32.1%) | ||
| Age (years), median (IQR) | 40 (28-58) | 41 (28-63.5) | 37.5 (26-49.5) | 53.5 (32.3-68) | 0.029 | |
| Sex, male | 73 (78.5%) | 56 (83.6%) | 15 (83.3%) | 58 (69%) | 0.159 | |
| Body weight (kg), median (IQR) | 79.2 (69.4-90) | 75 (67.5-82.8) | 74.5 (68.5-88.8) | 75 (65-80.5) | 0.338 | |
| GCS at hospital admission, median (IQR) | 3 (3-6) | 6 (3-9) | 3 (3-3) | 4 (3-7) | 0.017 | 0.4 |
| Pupillary reactivity at hospital admission | 0.372 | 6.9 | ||||
| Both reactive | 53 (58.9%) | 47 (74.6%) | 10 (62.5%) | 49 (65.3%) | ||
| One reactive | 17 (18.9%) | 5 (7.9%) | 3 (18.8%) | 15 (20%) | ||
| Non-reactive | 20 (22.2%) | 11 (17.5%) | 3 (18.8%) | 11 (14.7%) | ||
| Pre-hospital hypoxia (SpO2 < 90%) | 17 (21.2%) | 17 (27%) | 2 (13.3%) | 10 (12.5%) | 0.151 | 9.2 |
| Pre-hospital hypotension (SBP < 90 mmHg) | 11 (13.6%) | 13 (20.3%) | 4 (25%) | 13 (15.9%) | 0.577 | 7.3 |
| Mass lesion on first CT scan† | 61 (65.6%) | 54 (80.6%) | 12 (66.7%) | 53 (63.1 %) | 0.107 | |
| Traumatic SAH on CT scan | 65 (69.9%) | 45 (67.2%) | 12 (66.7%) | 64 (76.2%) | 0.616 | |
| Marshall score, median (IQR) | 3 (2-5) | 3 (2-5) | 3 (2-5) | 5 (2-5) | 0.393 | 14.9 |
| IMPACT score (lab model), median (IQR) | 22 (13-37) | 23 (11-33) | 21 (13.2-41.8) | 31 (19.5-46.5) | 0.029 | 0.4 |
| APACHE II score, median (IQR) | 20 (15-25) | 20 (16-24) | 18.5 (16.2-25.8) | 21 (16-29) | 0.51 | 1.1 |
| Injury Severity Score, median (IQR) | 26 (22-38) | 34 (26-44) | 29 (22-42) | 33 (26-40) | 0.001 | 0.4 |
| Use of barbiturate‡ | 12 (12.9%) | 8 (11.9%) | 3 (16.7%) | 11 (13.1%) | 0.963 | |
| Surgery for clot evacuation‡ | 34 (36.6%) | 20 (29.9%) | 7 (38.9%) | 33 (39.8%) | 0.640 | 0.4 |
| Decompressive craniectomy‡ | 30 (32.3%) | 14 (20.9%) | 10 (55.6%) | 24 (28.6%) | 0.035 | |
| External ventricular drainage‡ | 24 (25.8%) | 21 (31.3%) | 6 (33.3%) | 33 (39.3%) | 0.294 | |
| Opioid administration in the first 24 hours | 77 (82.8%) | 65 (97%) | 17 (94.4%) | 50 (59.5%) | < 0.001 |
APACHE = Acute Physiology and Chronic Health Evaluation; CT = computed tomography; GCS = Glasgow Coma Scale; IMPACT = International Mission for Prognosis and Analysis of Clinical Trials in Traumatic Brain Injury; IQR = interquartile range; SAH = subarachnoid haemorrhage; SBP = systolic blood pressure; SpO2 = peripheral oxygen saturation.
Comparison of the four groups: χ2 test and Kruskal-Wallis test for categorical and continuous variables respectively.
Mass lesion indicates the presence of subdural haemorrhage, extradural haemorrhage, and/or intracerebral haemorrhage.
At any time point.
We performed all statistical analyses using R Studio Software (version 4.0.2). As this was an exploratory analysis aimed to identify potential and previously unknown associations and represented the results of multiple comparisons, we chose a two-sided P value below 0.05 to indicate statistical significance and provide an acceptable balance between discovery (avoiding type II error) and incorrect identification of spurious associations (type I error) within the framework of an exploratory study.
Results
Baseline characteristics of the study cohort
We studied 262 patients with severe TBI (Table 1). Most were men (n = 77.1%) with a median age of 44 years (IQR, 29–62 years) and a median GCS on admission of 3 (IQR, 3–7). Both pupils were reactive at hospital admission in 65.2% of patients.
Propofol versus midazolam
Propofol and midazolam were the most used sedative agents (Table 2). Patients treated only with propofol as first line (intention to treat) sedation (n = 93, 35.4%) had a similar APACHE II and IMPACT score to patients treated with midazolam only (n = 67, 25.6%), but a lower ISS (median, 26 [IQR, 22–38] v 34 [IQR, 26–44]; P = 0.001).
The midazolam-only group had a similar age to the propofol-only group (median, 41 [IQR, 28-63.5] v 40 [IQR, 28-58] years respectively), but more such patients received opioids (97% v 82.8%; P < 0.001).
Propofol and midazolam versus combination versus no sedation
A small number of patients (n = 18, 6.9%) received both propofol and midazolam on the first day, whereas 84 patients (32%) did not receive either sedative agent. Patients treated with propofol only and patients who received both sedatives in the first 24 hours had a lower median GCS at hospital admission (median, 3 [IQR, 3-6] v 3 [IQR, 3-3] respectively) compared with patients treated with midazolam only (median, 6; IQR, 3-9) or no sedation (median, 4; IQR, (3-7); P = 0.17) (Table 2). Moreover, numerically, more patients (55.6%) treated with both sedatives received decompressive craniectomy compared with patients treated with no sedative (28.6%; P = 0.35).
In addition, patients treated with no sedative were numerically older than the other groups, had the highest ISS, the lowest frequency of a mass lesion on the first CT scan, the highest rate of subarachnoid haemorrhage on CT scan, the highest Marshall score, and, together with the midazolam-only group, the highest IMPACT score. Furthermore, opioid administration was significantly more common when midazolam was given either alone or in combination with propofol.
The planned sensitivity analysis confirmed and extended the results seen in the first 24 hours for the same subpopulations treated and the same agents to day 2 and day 3 after ICU admission (Online Appendix, table 1 and table 2). By day 2, male patients were more likely to receive propofol only, and patients treated with midazolam had a higher GCS score. Furthermore, higher body weight was associated with propofol-only sedation. However, patients who received no sedation, or had their sedation stopped or switched, had a significantly higher IMPACT score compared with patients treated with propofol only or midazolam only. Moreover, patients who remained on propofol had a significantly and consistently lower ISS (P < 0.001).
Sedative use and dose
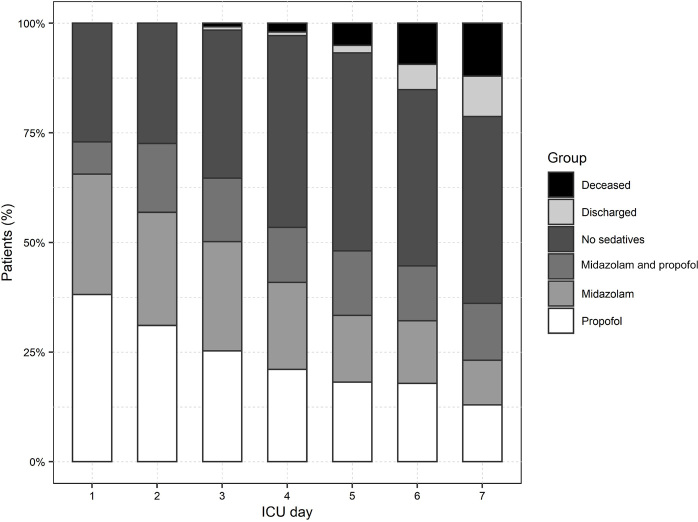
The use of propofol and midazolam decreased over time during the first week (Figure 1). The median duration of sedation with propofol and midazolam was 4 days (IQR, 2-6 days) and 3 days (IQR, 2-5 days) respectively.
Figure 1.
Sedative agent use over the first week
ICU = intensive care unit. Propofol was the most common sedative agent used as first line sedation after ICU admission (35.4% of patients). Midazolam was less common (25.6%). A minority of patients were treated with a combination of both (6.9%). Some patient received no sedation (32.1%). The use of propofol and midazolam decreased over time during the first week.
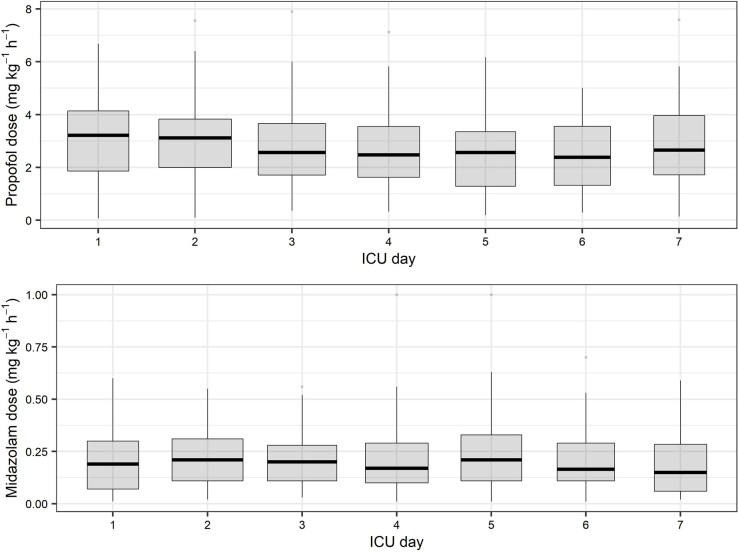
The sedative doses are described in Figure 2. The cumulative median doses over the first week of propofol and midazolam were 402.8 mg kg-1 h-1 (IQR, 344.6-449.3 mg kg-1 h-1) and 28.3 mg kg-1 h-1 (IQR, 24.2-32.3 mg kg-1 h-1) respectively.
Figure 2.
Propofol and midazolam doses over the first week
ICU = intensive care unit. The median initial rescue dose of propofol was 3.2 mg kg-1 h-1 (interquartile range [IQR], 1.9–4.1 mg kg-1 h-1), with a range from 0.07 to 6.7 mg kg-1 h-1. The median initial rescue dose of midazolam was 0.2 mg kg-1 h-1 (IQR, 0.07–0.3 mg kg-1 h-1), with a range from 0.01 to 0.6 mg kg-1 h-1.
Additional use of opioids
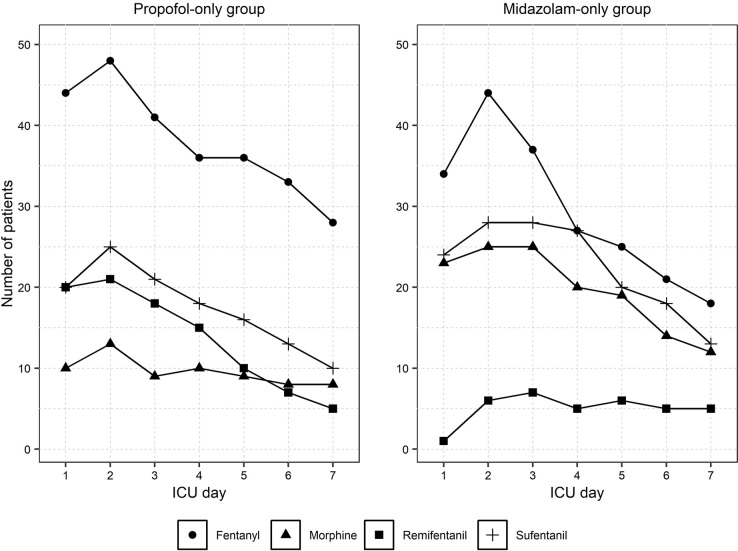
Different patterns of opioid and sedative use are described in Figure 3.
Figure 3.
Patterns of opioid and sedative use over the first week
ICU = intensive care unit. Opioids were administered in combination with sedatives. The most common was fentanyl in both groups. Morphine and sufentanil were common in patients receiving midazolam. Use of opioids decreased from day 2.
Sedation and changes in intracranial pressure
When analysing intracranial pressure changes over time (Figure 4), the midazolam-only and non-midazolam-only groups as well as the propofol-only and the nonpropofol-only groups showed no significant group effect nor interaction between time and group. We found similar results when we analysed the midazolam-only and propofol- only groups.
Figure 4.
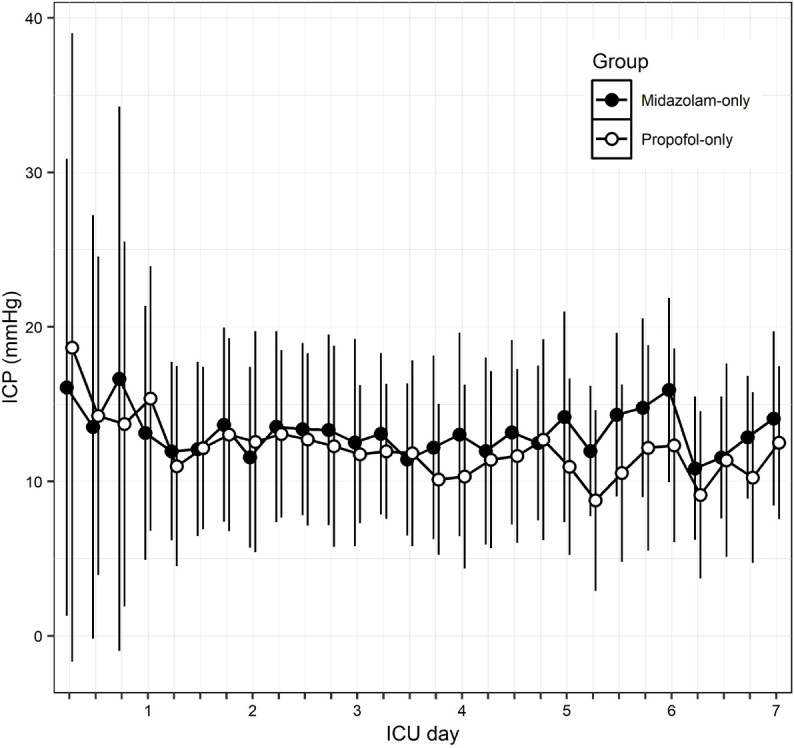
Intracranial pressure by sedative group over the first week
ICU = intensive care unit; ICP = intracranial pressure. Comparison of ICP between the midazolam-only and the propofol-only group. ICP values were collected every 6 hours. Each dot and bar represent the mean ICP (± standard deviation) in the time point. There was no significant group effect nor interaction effect between time and group on ICP.
Predictors of sedative choice
Factors associated with propofol and midazolam administration as the first line sedatives are presented in the Online Appendix, table 3 and table 4 respectively. The dominant predictors of propofol use were greater body weight, lower ISS, and the use of opioids. In contrast, administration of midazolam made the use of propofol markedly less likely. The dominant predictors of midazolam were a lower IMPACT score and the use of opioids. The association with opioid use was particularly striking, with an adjusted odds ratio (OR) of almost 13 and five times greater than with propofol. The use of propofol predicted avoidance of midazolam.
Treatment with no sedation was predicted by a higher IMPACT score (OR, 1.03; 95% CI, 1.01–1.05; P = 0.006) and was weakly associated with the use of opioids (OR, 0.24; 95% CI, 0.11–0.49; P < 0.001) (Online Appendix, table 5).
Outcomes
As shown in Table 3, the overall ICU and hospital mortality rates were 24.4% and 27.9% respectively. The median length of stay in the ICU and in hospital was 14 days (IQR, 8–20 days) and 21 days (IQR, 11-34 days) respectively. However, the ICU length of stay was longer for patients treated with midazolam or with the combination of midazolam and propofol in the first 24 hours. Moreover, patients treated with midazolam had better neurological outcomes at hospital discharge in the unadjusted analysis.
Table 3.
Outcomes: overall and by sedative agent used in the first 24 hours after intensive care unit (ICU) admission
| Outcomes | Overall | Propofol only | Midazolam only | Propofol and midazolam | No sedation | P* | Missing |
|---|---|---|---|---|---|---|---|
| Number of patients | 262 | 93 (35.5%) | 67 (25.6%) | 18 (6.9%) | 84 (32.1%) | ||
| ICU mortality | 64 (24.4%) | 19 (20.4%) | 18 (26.9%) | 3 (16.7%) | 24 (28.6%) | 0.496 | |
| Hospital mortality | 73 (27.9%) | 21 (22.6%) | 20 (29.9%) | 3 (16.7%) | 29 (34.5%) | 0.221 | |
| ICU LOS (days), median (IQR) | 14 (8-20) | 13 (7-19) | 16 (9-24) | 17 (13-24) | 13 (7-21) | 0.048 | |
| Hospital LOS (days), median (IQR) | 21 (11-34) | 21 (14-34) | 21 (12-31) | 25 (18-36) | 18 (8-35) | 0.268 | |
| GOS-E at hospital discharge | 0.031 | 36.3 | |||||
| Favourable (5–8) | 64 (38.3%) | 18 (28.1%) | 25 (47.2%) | 3 (30%) | 18 (45%) | ||
| Unfavourable (2–4) | 62 (37.1%) | 34 (53.1%) | 13 (24.5%) | 5 (50%) | 10 (25%) | ||
| Deceased (1) | 41 (24.6%) | 12 (18.8%) | 15 (28.3%) | 2 (20%) | 12 (30%) |
GOS-E = Extended Glasgow Outcome Scale; IQR = interquartile range; LOS = length of stay.
Comparison of the four groups: χ2 test and Kruskal-Wallis test for categorical and continuous variables respectively.
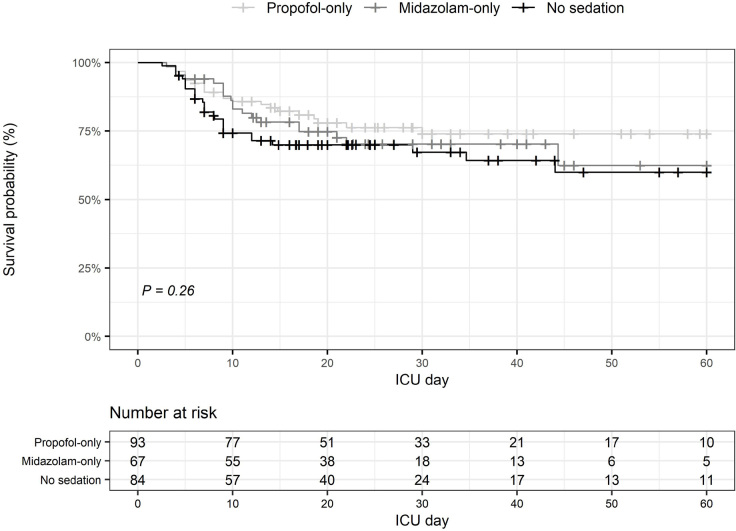
A Kaplan-Meier survival plot comparing patient groups according to sedative choice is presented in Figure 5. The Cox proportional hazard model showed no difference in the time to death between patients treated with propofol and patients treated with midazolam in the first 24 hours (P = 0.726). Moreover, at each time point, the ISS (hazard ratio [HR], 1.06; 95% CI, 1.03–1.08; P < 0.001) and the IMPACT score (HR, 1.02; 95% CI, 1–1.03; P = 0.01) were associated with higher mortality rate.
Figure 5.
Survival probability by group censored at 60 days
ICU = intensive care unit. Kaplan–Meier survival plots for hospital mortality censored at 60 days showed no difference in time to death for patients treated with propofol in the first 24 hours compared with patients treated with midazolam or treated without sedatives in the first 24 hours (log-rank test; P = 0.26).
After adjustment for key covariates (as detailed in the Methods section), the only predictors of 60-day hospital mortality were the IMPACT score (OR, 1.05; 95% CI, 1.031.07; P < 0.001) and the ISS (OR, 1.06; 95% CI, 1.03–1.09; P < 0.001). Early sedation with propofol only (OR, 0.93; 95% CI, 0.44–1.97; P = 0.845) or with midazolam only (OR, 1.2; 95% CI, 0.54–2.68; P = 0.65) was not independently associated with hospital mortality (Online Appendix, table 6).
Discussion
Key findings
We analysed the early use of sedatives using a multicentre, international dataset describing the management of patients with severe TBI in the ICU who had an intracranial pressure monitor. We found that propofol was the most common sedative agent, simultaneous use of midazolam and propofol was uncommon, and opioids were used in addition to sedation in most patients, but much more so when midazolam was the chosen sedative. Moreover, we found that illness severity was associated with the choice of sedative agent, with propofol being prescribed to less severely injured and heavier patients, and that one-third of patients (who appeared to be the most severely injured) did not receive any sedative agents. The dose of sedatives was highly variable and reached remarkably high doses in some patients. There was no independent association between the choice of sedative agent and mortality.
Relationship to previous studies
To our knowledge, very few studies have compared sedative agents in patients with TBI in relation to choice of sedative, dose, patient characteristics, and outcomes, and none of them have been published as part of the CENTER-TBI Project.15 A meta-analysis performed to compare the safety and efficacy of propofol with midazolam was based on only four studies, with two reporting on GOS-E and mortality, and with a total study population of 57 patients (less than a quarter of our study population).16 There were no significant findings. Our study increases our understanding of current sedation practice in these patients.
A systematic review analysed 13 randomised control trials of sedative use in 380 trauma patients.17 Three trials (including 130 patients with severe TBI) showed that propofol or midazolam led to no significant difference in intracranial pressure and cerebral perfusion pressure control or haemodynamics. However, two studies found that patients treated with propofol were more likely to be switched to alternate therapy secondary to hypertriglyceridaemia or inability to obtain an adequate level of sedation despite maximum doses.18, 19 Our findings, however, show that in the 14 trauma units studied, propofol was the dominant sedative for patients with TBI.
Implications of study findings
Our findings imply that propofol and midazolam are the most common sedatives for patients with TBI but that propofol is more common. They also imply that both are typically given together with an opioid, but also that such combined use is markedly more likely when midazolam is prescribed. The choice of agent is generally affected by illness severity, such that patients treated with propofol have lower illness severity and those with highest illness severity are less likely to receive any sedation. Irrespective of the agent of choice, they also suggest that a quarter of patients may receive high sedative doses (≥ 320 mg h-1 of propofol and ≥ 25 mg h-1 of midazolam in a typical patient weighing 80 kg) and even doses of up to 536 mg h-1 and 48 mg h-1 respectively.
Study strengths and limitations
Our study has several strengths. It is an international multicentre study, thus presenting the only current perspective on the practice of sedative therapy for TBI in centres from Europe, Australia and the United Kingdom. It presents the largest dataset describing the use of sedatives in patients with TBI. Moreover, it provides novel insights about practice variability with regard to choice of sedative agent, predictors, dose, and duration of therapy. Finally, it supplies the largest dataset on the independent association between sedative use or choice and patient outcomes. We acknowledge some limitations. This is not a randomised controlled trial and, thus, any associations described cannot be used to infer causality. We cannot exclude the impact of unknown or unmeasured confounders. However, before randomised controlled trials of sedation can be designed and conducted, an understanding of usual care is essential. The study was limited to 14 centres in Europe, Australia and the United Kingdom and to 262 patients. Therefore, no inferences can be made on current practice and practice variability and sedative choices in North American trauma centres, and the findings are exposed to a degree of type I and type II errors. However, the study centres are large trauma centres in their respective countries and likely to reflect practice in similar centres in Europe, Australia and the United Kingdom. The study is by far the largest to date and, to our knowledge, no equivalent data from North America have ever been published. Our study was retrospective in nature, with all the inherent limitations of such studies. However, the data were collected by medical staff experienced in TBI care and without specific bias in relation to sedation therapy. We only used the dose at midday each day as a representative of likely patterns of dosage during the remainder of the day. We did not collect data on depth of sedation, nor the reasons for sedative use. We did not include use of a-agonists (clonidine and dexmedetomidine) in the study. The sedation regimens were centre-specific; however, the number of events for each centre was too low to study a centre effect as an explanatory variable in the multivariable analysis. We cannot exclude use of sedatives outside of ICU or as bolus therapy. We did not have data on long term neurological outcomes. However, we were able to assess the association of sedative agents with hospital mortality censored at 60 days — a clinically meaningful outcome.
Conclusions
Propofol and midazolam are the current dominant sedatives for patients with TBI, with the use of propofol being more common. They are typically combined with an opioid, markedly more so if midazolam is used. Moreover, the choice of agent is affected by illness severity such that patients treated with propofol have less illness severity and those treated with no sedation appear to have the highest level of injury. In addition, large doses of sedatives are common for both midazolam and propofol. The choice of initial sedative did not appear independently associated with patient-centred outcomes but was associated with a greater probability of ICU discharge (propofol) or hospital discharge (both agents). These observations supply the epidemiological basis for the design of interventional studies of sedation in patients with TBI to test the hypothesis that propofol-based sedation decreases the duration of ICU stay.
Competing interests
All authors declare that they do not have any potential conflict of interest in relation to this manuscript.
Supplementary Information

References
- 1.Picetti E., Pelosi P., Taccone F.S., et al. VENTILatOry strategies in patients with severe traumatic brain injury: the VENTILO Survey of the European Society of Intensive Care Medicine (ESICM) Crit Care. 2020;24:158. doi: 10.1186/s13054-020-02875-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Devlin J.W., Skrobik Y., Gelinas C., et al. Clinical practice guidelines for the prevention and management of pain, agitation/sedation, delirium, immobility, and sleep disruption in adult patients in the ICU. Crit Care Med. 2018;46:e825–e873. doi: 10.1097/CCM.0000000000003299. [DOI] [PubMed] [Google Scholar]
- 3.Urwin S.C., Menon D.K. Comparative tolerability of sedative agents in head-injured adults. Drug Saf. 2004;27:107–133. doi: 10.2165/00002018-200427020-00003. [DOI] [PubMed] [Google Scholar]
- 4.Opdenakker O., Vanstraelen A., De Sloovere V., Meyfroidt G. Sedatives in neurocritical care: an update on pharmacological agents and modes of sedation. Curr Opin Crit Care. 2019;25:97–104. doi: 10.1097/MCC.0000000000000592. [DOI] [PubMed] [Google Scholar]
- 5.Xie Q., Wu H.B., Yan Y.F., et al. Mortality and outcome comparison between brain tissue oxygen combined with intracranial pressure/cerebral perfusion pressure-guided therapy and intracranial pressure/cerebral perfusion pressure-guided therapy in traumatic brain injury: a meta-analysis. World Neurosurg. 2017;100(1):18–27. doi: 10.1016/j.wneu.2016.12.097. [DOI] [PubMed] [Google Scholar]
- 6.Stocker R.A. Intensive care in traumatic brain injury including multimodal monitoring and neuroprotection. MedSci. 2019;7:37. doi: 10.3390/medsci7030037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Marklund N. The neurological wake-up test-a role in neurocritical care monitoring of traumatic brain injury patients? Front Neurol. 2017;8:540. doi: 10.3389/fneur.2017.00540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Flower O., Hellings S. Sedation in traumatic brain injury. Emerg Med Int. 2012;2012 doi: 10.1155/2012/637171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Johnston A.J., Steiner L.A., Chatfield D.A., et al. Effects of propofol on cerebral oxygenation and metabolism after head injury. Br J Anaesth. 2003;91:781–786. doi: 10.1093/bja/aeg256. [DOI] [PubMed] [Google Scholar]
- 10.Baker S.P., O'Neill B., Haddon W., Long W.B. The Injury Severity Score: a method for describing patients with multiple injuries and evaluating emergency care. J Trauma. 1974;14:187–196. [PubMed] [Google Scholar]
- 11.Knaus W.A., Draper E.A., Wagner D.P., Zimmerman J.E. APACHE II: a severity of disease classification system. Crit Care Med. 1985;13:818–829. [PubMed] [Google Scholar]
- 12.Steyerberg E.W., Mushkudiani N., Perel P., et al. Predicting outcome after traumatic brain injury: development and international validation of prognostic scores based on admission characteristics. PLoS Med. 2008;5:1251–1261. doi: 10.1371/journal.pmed.0050165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wilson J.T.L., Pettigrew L.E.L., Teasdale G.M. Structured interviews for the Glasgow Outcome Scale and the Extended Glasgow Outcome Scale: guidelines for their use. J Neurotrauma. 1998;15:573–580. doi: 10.1089/neu.1998.15.573. [DOI] [PubMed] [Google Scholar]
- 14.Lu J., Marmarou A., Lapane K., et al. A method for reducing misclassification in the extended Glasgow Outcome Score. J Neurotrauma. 2010;27:843–852. doi: 10.1089/neu.2010.1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.CENTER-TBI. CENTER-TBI publications. https://www.center-tbi.eu/publications (viewed Oct 2022).
- 16.Gu J.W., Yang T., Kuang Y.Q., et al. Comparison of the safety and efficacy of propofol with midazolam for sedation of patients with severe traumatic brain injury: a meta-analysis. J Crit Care. 2014;29:287–290. doi: 10.1016/j.jcrc.2013.10.021. [DOI] [PubMed] [Google Scholar]
- 17.Roberts D.J., Hall R.I., Kramer A.H., et al. Sedation for critically ill adults with severe traumatic brain injury: a systematic review of randomized controlled trials. Crit Care Med. 2011;39:2743–2751. doi: 10.1097/CCM.0b013e318228236f. [DOI] [PubMed] [Google Scholar]
- 18.Sanchez-Izquierdo-Riera J.A., Caballero-Cubedo R.E., Perez-Vela J.L., et al. Propofol versus midazolam: safety and efficacy for sedating the severe trauma patient. Anesth Analg. 1998;86:1219–1224. doi: 10.1097/00000539-199806000-00016. [DOI] [PubMed] [Google Scholar]
- 19.Camps A.S., Sanchez-Izquierdo Riera J.A., Vazquez D.T., et al. Midazolam and 2% propofol in long-term sedation of traumatized, critically ill patients: Efficacy and safety comparison. Crit Care Med. 2000;28:3612–3619. doi: 10.1097/00003246-200011000-00009. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials