Abstract
Background: Intravenous vitamin C is known to interfere with some point-of-care blood glucose meters. We aimed to determine the concentrations at which ascorbate interferes with glucose concentrations measured using a point-of-care blood glucose meter. We also compared the point-of-care meter and an arterial blood gas (ABG) analyser in the intensive care unit with laboratory glucose monitoring in septic patients receiving intravenous vitamin C infusions.
Methods: Blood samples containing normal, depleted and supplemented glucose and increasing concentrations of ascorbate (0.1-1.0 mmol/L) were tested using an Accu-Chek Inform II (Roche Diagnostics, USA) glucometer. For the in vivo study, 41 individual blood samples were drawn daily from septic patients (n = 16) receiving infusions of 25 mg/kg of vitamin C every 6 hours. The glucose values of matched blood samples were assessed using Accu-Chek, ABG and laboratory glucose methods.
Results: For every 1 mmol/L of ascorbate added, the glucose concentration measured by the point-of-care monitor increased by 1.4 mmol/L (95% CI, 1.0-1.8; P < 0.001). Analysis of matched blood samples collected following intravenous vitamin C infusion indicated that 98% of the ABG and 83% of the Accu-Chek values met the International Organization for Standardization (ISO) 15197:2013 accuracy criteria. One patient had severe renal impairment, which contributed to elevated plasma vitamin C concentrations (median, 0.95 mmol/L; range, 0.64-1.10 mmol/L), resulting in elevated Accu-Chek readings and presenting a moderate clinical risk for the highest value.
Conclusions: Vitamin C concentrations < 0.8 mmol/L do not interfere with point-of-care glucose monitoring. Intravenous vitamin C infusion of 25 mg/kg every 6 hours does not interfere with point-of-care glucose monitoring unless the patient has renal impairment, in which case laboratory glucose tests should be used.
Due to the recent increase in clinical trials investigating intravenous vitamin C as a potential therapy in critically ill patients with sepsis and septic shock, safety issues have been raised. It is well known that high (10-110 g) doses of intravenous vitamin C, typically administered to oncology patients and patients with severe burns, can interfere with some point-of-care glucose meters, particularly if measured during or immediately after the intravenous vitamin C infusion.1,2 Therefore, caution is required for patients needing regular glucose monitoring. However, it is less certain whether the lower (1.5 g or 25 mg/kg every 6 hours) doses of intravenous vitamin C that are typically administered to critically ill patients interfere to the same extent. Preliminary evidence indicates that critically ill patients with renal impairment (oliguria) may exhibit spuriously high point-of-care glucose values following low dose intravenous vitamin C infusion.3, 4, 5
Intravenous vitamin C can interfere with many point-of-care glucose meters, even at low gram doses.6 Point-of-care glucose meters utilise a variety of different detection methods, including electrochemical detection using glucose dehydrogenase or glucose oxidase.6 Vitamin C can donate up to two electrons per molecule and potentially elevate point-of-care readings, depending on the biochemistry used in the monitor. Intravenous vitamin C, however, does not interfere with the laboratory-based hexokinase method, which uses spectrophotometric rather than electrochemical detection.2, 5, 6
In this study, we aimed to determine the concentrations at which ascorbate begins to interfere with the Accu-Chek Inform II (Roche Diagnostics, USA) point-of-care glucose meter (which utilises glucose dehydrogenase amphoteric detection) using three different baseline glucose concentrations. We also compared matched arterial blood samples tested with the Accu-Chek, an arterial blood gas (ABG) analyser located in the intensive care unit, and the laboratory glucose method in septic patients receiving intravenous vitamin C infusions (at a dose of 25 mg/kg every 6 hours) with measured plasma vitamin C concentrations. Finally, we utilised the surveillance error grid analysis to assess the magnitude of clinical risk from analytically inaccurate data.7
Methods
In vitro study
Venous blood was drawn from two healthy volunteers — ethics approval was obtained from the New Zealand Health and Disability Ethics Committee (#URA/06/12/083). Some samples were depleted of glucose by overnight blood storage at ambient temperature. Other samples had additional glucose added at a final concentration of 1.7 mmol/L (30 mg/dL). A stock solution of ascorbate was prepared in phosphate-buffered saline, and the concentration was confirmed spectrophotometrically.8 Ascorbate was added in a minimal volume to the three types of blood samples (normal, glucose-depleted, and glucose-supplemented) to give final concentrations of added ascorbate ranging from 0.1 mmol/L to 1.0 mmol/L. An Accu-Chek Inform II glucose meter and a newly opened package of test strips were used to test the glucose concentrations. Before beginning each experiment, a control test was performed using Accu-Chek Performa control solutions (Roche Diagnostics, USA). Each measurement was carried out in duplicate.
In vivo study
Blood samples were prospectively collected from a subset of critically ill patients with septic shock (n = 16) who were enrolled in a registered vitamin C intervention trial (ACTRN12617001184369; Northern A Health and Disability Ethics Committee #16NTA238) receiving intravenous vitamin C infusions (25 mg/kg every 6 hours for up to 4 days). Matched blood samples were collected for 1-4 days for each participant (a total of 41 individual samples). These were analysed using the Accu-Chek meter, an ABL90 FLEX PLUS blood gas analyser (Radiometer Pacific, Auckland, New Zealand) and the laboratory glucose hexokinase enzyme assay using an Abbott c series analyser (carried out at Canterbury Health Laboratories, an international accreditation New Zealand laboratory).
The timing of intermittent blood glucose monitoring was based on routine clinical practice, not relative to the timing of the intravenous vitamin C infusions. Therefore, the timing of the closest intravenous vitamin C infusion before the glucose monitoring was recorded. The vitamin C content of the plasma samples 6 h after infusion (trough) was determined using high-performance liquid chromatography (HPLC) with electrochemical detection as described previously.9 Urine output was recorded (oliguria was defined as < 400 mL/24 h), and serum creatinine was determined using the classical Jaffe reaction using an Abbott c series analyser (Canterbury Health Laboratories; reference range, 45-90 μmol/L for females, 50-110 μmol/L for males). The estimated glomerular filtration rate (eGFR) was calculated using serum creatinine, age and sex (kidney dysfunction was defined as eGFR < 60 ml/min/1.73 m2). Data are presented as the median and interquartile range (IQR) or mean and 95% confidence interval (CI).
The correlation of the ABG and Accu-Chek glucose values with the laboratory glucose test was determined using the Pearson correlation coefficient, and Bland-Altman analysis was used to assess the agreement between the ABG or point-of-care and laboratory assays.10 The accuracy of the ABG and Accu-Chek values with respect to the International Organization for Standardization (ISO) 15197:2013 criteria for blood glucose was evaluated. These criteria specify that at least 95% of measurements must fall within ± 15 mg/dL (0.8 mmol/L) of the laboratory reference result if the blood glucose level is < 100 mg/dL (5.6 mmol/L), and within ± 15% of the laboratory reference result if the blood glucose level is ≥ 100 mg/dL (5.6 mmol/L).11 The surveillance error grid analysis tool was used to assess the magnitude of clinical risk from analytically inaccurate data.12 The error grid is divided into five risk grades (A-E), signifying the degree of risk associated with an incorrect measurement: no risk, slight, moderate, great, or extreme risk of hypo- or hyperglycaemia.
Results
In vitro findings
The addition of increasing concentrations of ascorbate (up to 1.0 mmol/L) to blood from healthy volunteers containing different concentrations of glucose resulted in concentrationdependent increases in blood glucose readings using the Accu-Chek monitor (Figure 1). Regression analysis indicated that for every 1 mmol/L of added ascorbate, the glucose measured increased by an estimated 1.4 mmol/L (95% CI, 1.0-1.8; P < 0.001).
Figure 1.
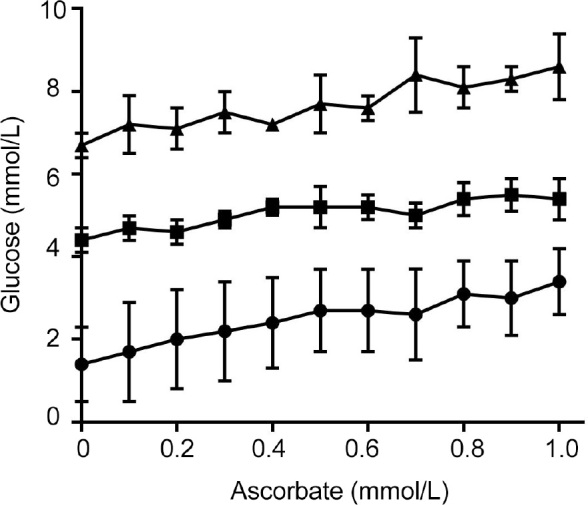
Effect of added ascorbate on Accu-Chek (Roche Diagnostics, USA) glucose measurements*
*Ascorbate was spiked into peripheral blood collected from healthy volunteers (■), depleted of glucose over night at ambient temperature (●), or spiked with 1.7 mmol/L (30 mg/dL) glucose (▲). Each Accu-Chek measurement was carried out in duplicate and data represent mean and range of two independent experiments. Point-of-care glucose monitors typically exhibit greater inaccuracy at lower glucose levels
In vivo findings
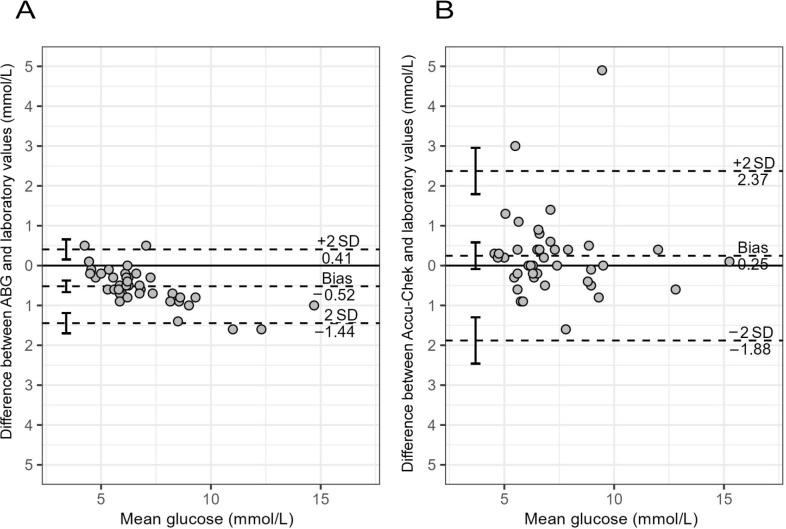
The characteristics of the participants included in this study are shown in Table 1. The median dose of intravenous vitamin C administered to the 16 participants was 2.1 g (IQR, 1.8-2.2 g) every 6 hours, resulting in median plasma vitamin C concentrations of 0.31 mmol/L (IQR, 0.21-0.42 mmol/L). The laboratory glucose concentration of the cohort was a median of 6.4 mmol/L (IQR, 5.8-8.2 mmol/L; n = 41 individual samples). Linear correlation analysis of the ABG and Accu-Chek meter glucose concentrations relative to laboratory glucose measurements provided Pearson coefficients of r = 0.99 and r = 0.89, respectively (P < 0.0001). Bland-Altman analysis indicated that ABG monitoring gave readings that were slightly lower (on average 0.4 mmol/L) than the laboratory values, but, after taking this bias into account, were more consistent (high Pearson correlation) with the laboratory measures (Figure 2, A). On average, the Accu-Chek values were closer to the laboratory values, but had greater variability, resulting in less precision (Figure 2, B).
Table 1.
Participant characteristics
| Participants | |
|---|---|
| Total number or participants | 16 |
| Sex, male | 13 (81%) |
| Age, years, median (IQR) | 69 (64-75) |
| Weight, kg, median (IQR) | 82 (72-86) |
| Source of sepsis | |
| Abdominal | 6 (38%) |
| Skin/soft tissue | 4 (25%) |
| Pulmonary | 2 (12.5%) |
| Bloodstream | 2 (12.5%) |
| Urinary tract | 2 (12.5%) |
| Other/unknown | 2 (12.5%) |
| SAPS 2, median (IQR) | 52 (41-59) |
| APACHE III score, median (IQR) | 85 (76-98) |
| SOFA score, median (IQR) | 9.0 (6.8-11.2) |
| PaO2/FiO2, median (IQR) | 223 (154-309) |
| Lactate, mmol/L, median (IQR) | 2.2 (1.1-3.1) |
| Creatinine, μmol/L, median (IQR) | 256 (114-309) |
APACHE = Acute Physiology and Chronic Health Evaluation; FiO2 = fraction of inspired oxygen; IQR = interquartile range; PaO2 = arterial partial pressure of oxygen; SAPS = Simplified Acute Physiology Score; SOFA = Sequential Organ Failure Assessment.
Figure 2.
Bland–Altman plots of differences between testing methods*
ABG = arterial blood gas analyser; SD = standard deviation. *Matched samples were assessed using laboratory glucose test and (A) ABG or (B) bedside Accu-Chek monitor (Roche Diagnostics, USA). The timing of the blood tests was a median of 4 hours after intravenous vitamin C infusion (range, 45 min to 6.1 h).
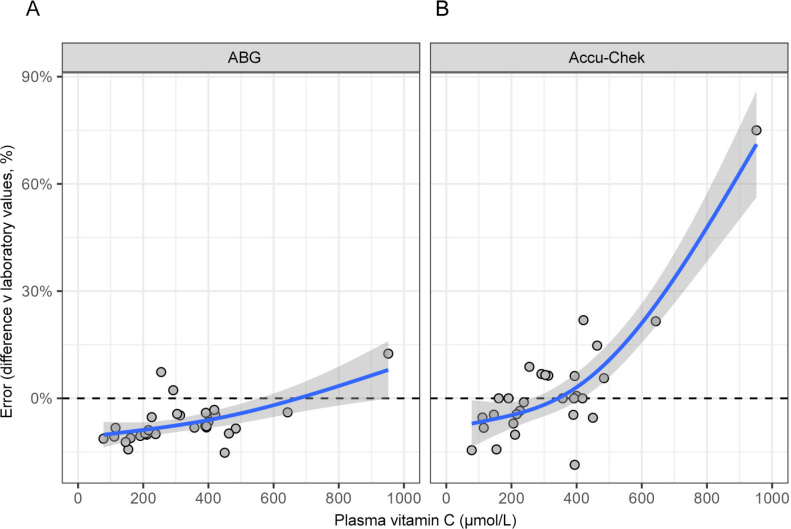
The timing of the blood tests was a median of 4 hours after vitamin C infusion (range of 45 min to 6 h). There was no correlation between Accu-Chek discrepancies and the timing of vitamin C infusions (r2 = 0.068; P = 0.1). Assessing the relationship between plasma vitamin C concentrations and the measurement error for ABG and Accu-Chek monitoring indicated that both the ABG and Accu-Chek underestimated the glucose concentration compared with the laboratory values when vitamin C levels were low. However, the Accu-Chek got closer to and eventually overestimated the laboratory values as plasma vitamin C concentrations increased (Figure 3).
Figure 3.
Effect of vitamin C concentration on discrepancy between laboratory and (A) arterial blood gas analyser (ABG) or (B) Accu-Chek (Roche Diagnostics, USA) bedside monitor glucose values
Points indicate individual observations, blue lines and shaded areas indicate smoothed mean with 95% CI.
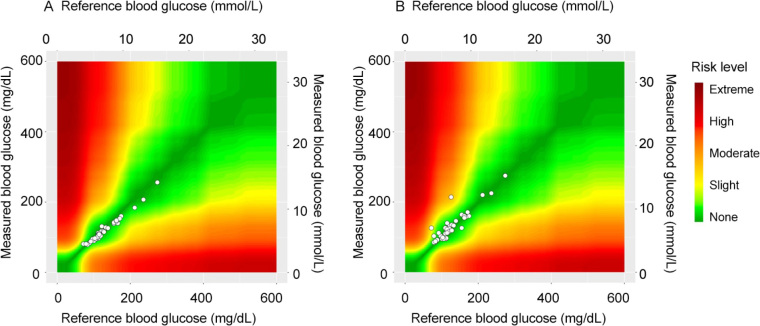
Assessment of the accuracy of the ABG and Accu-Chek indicated that 98% and 83%, respectively, of the analysed blood samples met ISO 15197:2013 accuracy criteria relative to laboratory glucose values (recommendation is ≥ 95%; Table 2). In addition, using the surveillance error grid analysis tool, we determined that 100% and 98% of the ABG and Accu-Chek values had no or only slight clinical risk (Table 3 and Figure 4).
Table 2.
Accuracy of arterial blood gas analyser (ABG) or Accu-Chek (Roche Diagnostics, USA) relative to laboratory glucose data assessed against International Organization for Standardization (ISO) criteria for blood glucose meters
| ISO range* | ABG | Accu-Chek |
|---|---|---|
| ≤ 5% or ≤ 5 mg/dL | 13 (32%) | 18 (44%) |
| > 5-10% or >5-10 mg/dL | 16 (39%) | 12 (29%) |
| > 10-15% or > 10-15 mg/dL | 11 (27%) | 4 (9.8%) |
| > 15-20% or > 15-20 mg/dL | 1 (2.4%) | 3 (7.3%) |
| > 20% or > 20 mg/dL | 0 | 4 (9.8%) |
Difference between blood glucose monitoring (BGM) and laboratory test reference (REF) as percentage of REF for REF > 100 mg/dL (5.6 μmol/L) and in mg/dL for REF ≤ 100 mg/dL (5.6 μmol/L) (https://www.iso.org/standard/54976.html).
Table 3.
Clinical risk of inaccurate arterial blood gas analyser (ABG) and Accu-Chek (Roche Diagnostics, USA) glucose values determined using the surveillance error grid analysis tool
Key: A = none; B = slight; C = moderate, D = high, E = extreme (https://www.diabetestechnology.org/seg/).
Figure 4.
Clinical risk of (A) arterial blood gas analyser (ABG) and (B) Accu-Chek (Roche Diagnostics, USA) values relative to laboratory glucose values*
*Assessed using the surveillance error grid analysis tool (https://www.diabetestechnolog.org/seg.shtml).
One patient (a 70-year-old woman) had Accu-Chek readings of up to 4.9 mmol/L higher than the laboratory glucose test 2 hours after vitamin C infusion. In addition, this patient had elevated serum creatinine (median 656 μmol/L; range, 472-835 μmol/L), oliguria (urine output, median 253 mL/day; range, 183-364 mL/day), and minimal eGFR (median 5 mL/ min/1.73 m2; range, 4-7 mL/min/1.73 m2), indicating severe renal impairment. This attenuated the patient's ability to clear the infused vitamin C, resulting in elevated median plasma vitamin C concentrations of 0.95 mmol/L (range, 0.64-1.10 mmol/L) and a significant correlation with serum creatinine concentrations (r2 = 0.737; P < 0.0001; Figure 5). These elevated vitamin C concentrations can interfere with Accu-Chek glucose readings, and surveillance error grid analysis indicated moderate risk from the highest reading. One other patient had elevated serum creatinine and vitamin C concentrations at one time point (48 hours), but this patient underwent dialysis, and both values subsequently decreased.
Figure 5.
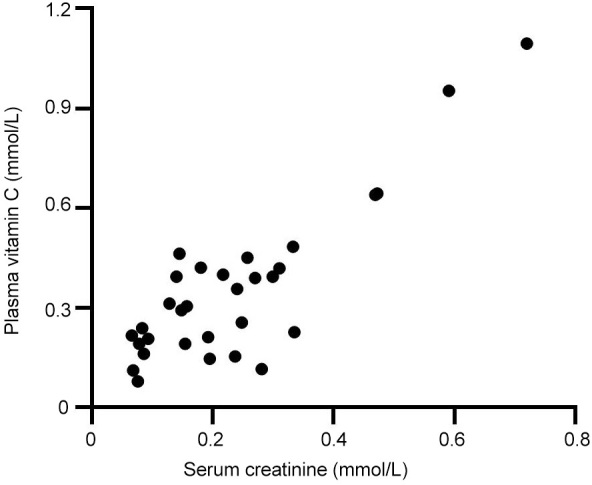
Correlation between plasma vitamin C and serum creatinine concentrations of the participants’ blood samples (r2 = 0.737; P < 0.0001)
Discussion
Point-of-care glucose testing often lacks accuracy in critically ill patients due to specific physiological issues common to this patient group, such as hypotension requiring vasopressor support, decreased haematocrit due to fluid resuscitation, variable blood oxygenation, and administered medications (eg, acetaminophen, dopamine), sugars (eg, maltose, galactose) and antioxidants (eg, vitamin C, thiol compounds).4, 6, 13, 14 We have shown that plasma vitamin C concentrations < 0.8 mmol/L do not elevate Accu-Chek Inform II glucose values adversely. Other studies have found interference with Accu-Chek Inform monitoring at vitamin C concentrations ≥ 0.55 mmol/L15, 16 and ≥ 0.85 mmol/L17 and other Accu-Chek point-of-care monitors at concentrations ≥ 0.5 mmol/L6 and ≥ 0.6 mmol/L in glucose-depleted samples,13 the latter of which is unlikely to result in clinical risk.
We found that intravenous vitamin C administration of approximately 2 g every 6 hours to septic patients resulted in median plasma concentrations < 0.5 mmol/L, which did not interfere with point-of-care glucose monitoring. The exception was a patient with renal dysfunction (oliguria) who had attenuated clearance of the administered vitamin C resulting in plasma concentrations of up to 1 mmol/L, which produced significantly elevated Accu-Chek glucose values. Despite this, there was only moderate clinical risk at the highest value, as the patient's baseline glucose values were within the normal range. The clinical risk would be more apparent in a patient with a high baseline glucose concentration, as spuriously elevated glucose readings may result in aggressive insulin therapy, leading to iatrogenic hypoglycaemia. Two retrospective analyses of critically ill patients who received 1.5 g intravenous vitamin C every 6 hours indicated that those with oliguria and end-stage renal disease had spuriously elevated glucose readings using Accu-Chek Inform II monitoring.3,4 A case report of a 73-year-old man receiving peritoneal dialysis for end-stage renal disease and enrolled in a septic shock trial administering intravenous vitamin C (at a dose of 50 mg/kg every 6 hours) exhibited factitious hyperglycaemia, resulting in a short term iatrogenic coma because of hypoglycaemia secondary to insulin administration.18 Although dialysis can decrease ascorbate concentrations by approximately 50%,19 this may not be sufficient for patients receiving higher dose intravenous vitamin C. Of note, ABG glucose readings were less adversely affected by elevated vitamin C concentrations.
Conclusion
Vitamin C infusions that provide < 0.8 mmol/L plasma ascorbate concentrations do not interfere with point-of-care glucose monitoring. Low dose vitamin C infusion of 25 mg/kg every 6 hours (~2 g every 6 hours) does not interfere with point-of-care glucose monitoring unless the patient has renal impairment, in which case, laboratory glucose tests should be used to monitor blood glucose concentrations.
Acknowledgments
Acknowledgements:
The study received funding through a New Zealand Health Research Council Sir Charles Hercus Health Research Fellowship (#16/037 to AC) and a Canterbury Medical Research Foundation project grant (PRO16/01 to AC).
Author contributions:
PR and CW, in vitro study; PR and GS, in vivo study; ES, vitamin C analyses; JW, statistical analyses; AC, study coordination, data analyses, paper preparation.
Competing interests
All authors declare that they do not have any potential conflict of interest in relation to this manuscript.
References
- 1.Carr A.C., Cook J. Intravenous vitamin C for cancer therapy -identifying the current gaps in our knowledge. Front Physiol. 2018;9:1182. doi: 10.3389/fphys.2018.01182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kahn S.A., Lentz C.W. Fictitious hyperglycemia: point-of-care glucose measurement is inaccurate during high-dose vitamin C infusion for burn shock resuscitation. J Burn Care Res. 2015;36:e67–e71. doi: 10.1097/BCR.0000000000000141. [DOI] [PubMed] [Google Scholar]
- 3.Stephenson E., Hooper M. Marik P Vitamin C and point of care glucose measurements: a retrospective, observational study. Chest. 2018;154(Suppl):255A. [Google Scholar]
- 4.Howell A.P., Parrett J.L., Malcom D.R. Impact of high-dose intravenous vitamin C for treatment of sepsis on point-of-care blood glucose readings. J Diabetes Sci Technol. 2021;15:309–316. doi: 10.1177/1932296819889638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.He J., Zheng G., Qian X., et al. Effect of high-dose intravenous vitamin C on point-of-care blood glucose level in septic patients: a retrospective, single-center, observational case series. Curr Med Res Opin. 2021;37:555–565. doi: 10.1080/03007995.2021.1887832. [DOI] [PubMed] [Google Scholar]
- 6.Tang Z., Du X., Louie R.F., Kost G.J. Effects of drugs on glucose measurements with handheld glucose meters and a portable glucose analyzer. Am J Clin Pathol. 2000;113:75–86. doi: 10.1309/QAW1-X5XW-BVRQ-5LKQ. [DOI] [PubMed] [Google Scholar]
- 7.Klonoff D.C., Lias C., Vigersky R., et al. The surveillance error grid. J Diabetes Sci Technol. 2014;8:658–672. doi: 10.1177/1932296814539589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pullar J.M., Bayer S., Carr A.C. Appropriate handling, processing and analysis of blood samples is essential to avoid oxidation of vitamin C to dehydroascorbic acid. Antioxidants (Basel) 2018;7:29. doi: 10.3390/antiox7020029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carr A.C., Pullar J.M., Moran S., Vissers M.C.M. Bioavailability of vitamin C from kiwifruit in non-smoking males: determination of 'healthy' and 'optimal' intakes. J Nutr Sci. 2012;1 doi: 10.1017/jns.2012.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bland J.M., Altman D.G. Measuring agreement in method comparison studies. Stat Methods Med Res. 1999;8:135–160. doi: 10.1177/096228029900800204. [DOI] [PubMed] [Google Scholar]
- 11.Smith K.E., Brown C.S., Manning B.M., et al. Accuracy of point-of-care blood glucose level measurements in critically ill patients with sepsis receiving high-dose intravenous vitamin C. Pharmacotherapy. 2018;38:1155–1161. doi: 10.1002/phar.2182. [DOI] [PubMed] [Google Scholar]
- 12.Kovatchev B.P., Wakeman C.A., Breton M.D., et al. Computing the surveillance error grid analysis: procedure and examples. J Diabetes Sci Technol. 2014;8:673–684. doi: 10.1177/1932296814539590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ceriotti F., Kaczmarek E., Guerra E., et al. Comparative performance assessment of point-of-care testing devices for measuring glucose and ketones at the patient bedside. J Diabetes Sci Technol. 2015;9:268–277. doi: 10.1177/1932296814563351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Grzych G., Pekar J.D., Chevalier-Curt M.J., et al. Antioxidants other than vitamin C may be detected by glucose meters: immediate relevance for patients with disorders targeted by antioxidant therapies. Clin Biochem. 2021;92:71–76. doi: 10.1016/j.clinbiochem.2021.03.007. [DOI] [PubMed] [Google Scholar]
- 15.Ten Berge D., Muller W., Beishuizen A., et al. Significant interference on specific point-of-care glucose measurements due to high dose of intravenous vitamin C therapy in critically ill patients. Clin Chem Lab Med. 2021;59:e197–e199. doi: 10.1515/cclm-2020-1445. [DOI] [PubMed] [Google Scholar]
- 16.Karon B.S., Griesmann L., Scott R., et al. Evaluation of the impact of hematocrit and other interference on the accuracy of hospitalbased glucose meters. Diabetes Technol Ther. 2008;10:111–120. doi: 10.1089/dia.2007.0257. [DOI] [PubMed] [Google Scholar]
- 17.Cho J., Ahn S., Yim J., et al. Influence of vitamin C and maltose on the accuracy of three models of glucose meters. Ann Lab Med. 2016;36:271–274. doi: 10.3343/alm.2016.36.3.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lachance O., Goyer F., Adhikari N.K.J., et al. High-dose vitamin-C induced prolonged factitious hyperglycemia in a peritoneal dialysis patient: a case report. J Med Case Rep. 2021;15:297. doi: 10.1186/s13256-021-02869-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Story D.A., Ronco C., Bellomo R. Trace element and vitamin concentrations and losses in critically ill patients treated with continuous venovenous hemofiltration. Crit Care Med. 1999;27:220–223. doi: 10.1097/00003246-199901000-00057. [DOI] [PubMed] [Google Scholar]