Abstract
Background: The effect of conservative versus liberal oxygen therapy on 90-day in-hospital mortality in patients who require unplanned invasive mechanical ventilation in an intensive care unit (ICU) is uncertain and will be evaluated in the mega randomised registry trial research program (Mega-ROX).
Objective: To summarise the protocol and statistical analysis plan for Mega-ROX.
Design, setting and participants: Mega-ROX is a 40 000-patient parallel-group, registry-embedded clinical trial in which adults who require unplanned invasive mechanical ventilation in an ICU will be randomly assigned to conservative or liberal oxygen therapy. Within this overarching trial research program, three nested parallel randomised controlled trials will be conducted. These will include patients with suspected hypoxic ischaemic encephalopathy (HIE) following resuscitation from a cardiac arrest, patients with sepsis, and patients with non-HIE acute brain injuries or conditions.
Main outcome measures: The primary outcome is in-hospital allcause mortality up to 90 days from the date of randomisation. Secondary outcomes include duration of survival, duration of mechanical ventilation, ICU length of stay, hospital length of stay, and proportion of patients discharged home.
Results and conclusions: Mega-ROX will compare the effect of conservative versus liberal oxygen therapy on 90-day in-hospital mortality in critically ill adults who receive unplanned invasive mechanical ventilation in an ICU. The protocol and a pre-specified approach to analyses are reported here to mitigate analysis bias.
Trial registration: Australian and New Zealand Clinical Trials Registry (ANZCTRN 12620000391976).
Patients with acute life-threatening illnesses requiring intensive care generally receive invasive mechanical ventilation. Delivery of supplemental oxygen to these patients often exposes them to a high fraction of inspired oxygen (Fio2) and supraphysiological arterial oxygen partial pressures. Several recent clinical trials have investigated so-called conservative strategies of oxygen administration in intensive care unit (ICU) patients, designed to limit exposure to high levels of oxygen.1, 2, 3, 4, 5 Overall, these studies suggest that such conservative oxygen strategies are probably safe, but the comparative effectiveness of conservative and liberal oxygen strategies on mortality in ICU patients is unclear.6 Supplemental oxygen therapy, which often exposes patients to hyperoxaemia, is ubiquitous in ICU patients who receive unplanned invasive mechanical ventilation, and many hundreds of thousands of patients receive invasive mechanical ventilation in ICUs each year. Thus, even small absolute mortality differences attributable to oxygen therapy regimens would be of profound global public health importance.6,7 High quality data to guide clinical decision making are particularly relevant in situations in which oxygen supplies are limited or when demand for oxygen is very high. This has been the case in many countries during the coronavirus disease 2019 (COVID-19) pandemic.6
It is possible that conservative oxygen therapy will be best for patients with some acute conditions, while liberal oxygen will be best for patients with other diagnoses (ie, there may be heterogeneity of treatment effect).2,7, 8, 9 To address the possibility of such heterogeneity, we are conducting three parallel nested trials within an overall 40 000 sample size envelope. Each of these nested trials will evaluate a pre-specified hypothesis about oxygen therapy regimens in a specific cohort of critically ill patients. Protocols for these nested trials will be prepared and published separately from this article. Here we describe the protocol for the mega randomised registry trial research program comparing conservative versus liberal oxygenation targets in adults receiving unplanned invasive mechanical ventilation in the ICU (Mega-ROX), focusing on the overarching trial.
Methods
Trial design
Mega-ROX is a 40 000-participant international, multicentre, randomised, parallel-group, two-sided, superiority trial. It has been designed to test the hypothesis that among adult ICU patients who receive unplanned invasive mechanical ventilation, conservative oxygen therapy reduces inhospital all-cause mortality up to 90 days from the date of randomisation by at least 1.5 percentage points when compared with liberal oxygen therapy. The relationship between Mega-ROX and the nested randomised controlled trials, which will be conducted in the pre-specified subgroups of patients with suspected hypoxic ischaemic encephalopathy, sepsis, and acute brain injuries or conditions other than hypoxic ischaemic encephalopathy, is shown in Figure 1. It is anticipated, based on data from the Intensive Care Unit Randomized Trial Comparing Two Approaches to Oxygen Therapy (ICU-ROX),2 that about 26 000 of the overall 40 000 patients in Mega-ROX will be enrolled in these nested trials. Patients who are not enrolled in one of the nested trials are likely to have a range of conditions, including conditions requiring emergency surgery, major trauma, pancreatitis, pulmonary embolism, haemorrhage, myocardial infarction, and respiratory failure due to non-infection-related causes. In the overarching trial, we have pre-specified what we consider to be the most likely overall effect of conservative oxygen therapy on 90-day in-hospital mortality. This hypothesis accords with beneficial mortality effects reported in a meta-analysis of acutely ill adults,10 and reflects the fact that heterogeneity of treatment effect in our pre-specified subgroups is hypothesised but as not yet proven.
Figure 1.

Overview of Mega-ROX
HIE = hypoxic ischaemic encephalopathy. Mega-ROX = mega randomised registry trial research program comparing conservative versus liberal oxygenation targets in adults receiving unplanned invasive mechanical ventilation in the ICU. RCT = randomised controlled trial.
Setting and population
Mega-ROX will be conducted in about 100 ICUs worldwide and is expected to include patients from low, middle and high income countries. The first patient was enrolled in June 2020. Patients aged ≥ 18 years who receive invasive mechanical ventilation in an ICU following an emergency (unplanned) ICU admission and those in whom mechanical ventilation is initiated in an ICU (ie, patients intubated in an ICU) will be eligible for inclusion. Where enrolment is not considered in a particular patient’s best interests by the treating clinician, that patient will be excluded. Operationally, this criterion will exclude all patients for whom either of the oxygen regimens being tested are considered by clinicians to be indicated or contraindicated, and patients for whom death is deemed imminent. Patients who have previously been enrolled in the study will also be excluded. Patients must be enrolled within 12 hours of fulfilling the eligibility criteria. When a patient is not enrolled within this timeframe, they will be described as eligible but missed, rather than excluded, for the purposes of describing participant flow.
Randomisation and blinding
Treatment assignment will be performed using a secure, centralised, web-based, randomisation interface. Participants will be enrolled in the study by ICU doctors, nurses and research staff. The assigned intervention will be communicated to the bedside nurse and/or respiratory therapist who will implement the study intervention.
A novel feature of this trial is that it will use adaptive randomisation. This will subtly increase the probability that trial participants in pre-specified subgroups of interest are allocated to the oxygen regimen that appears to be associated with the lowest mortality risk for their condition based on accumulating trial data. At a study population level, this approach is designed to create a potential benefit to trial participation. The conditions of interest are suspected hypoxic ischaemic encephalopathy following resuscitation from cardiac arrest, sepsis, and acute brain injury or condition other than hypoxic ischaemic encephalopathy. Patients with none of these conditions will be included in a fourth group. If ICU clinical staff are enrolling a patient who fits into more than one of the first three groups, they will be assigned to one group for the purposes of allocating study treatment during randomisation. This will be achieved by using a hierarchy determined by the magnitude of the absolute risk difference between observed treatment groups for that subgroup. This approach is based on the rationale that a larger risk difference corresponds to a smaller number needed to treat. Given the point estimates of treatment effect suggested by data from ICU-ROX,9 the hierarchy at the beginning of the trial will be hypoxic ischaemic encephalopathy, then sepsis, and then acute brain injury or condition other than hypoxic ischaemic encephalopathy. At the beginning of the trial, participants within each group will be preferentially randomised, in a ratio of 1.05:1, to the oxygen regimen that was associated with the lowest in-hospital mortality risk for that particular patient group in ICU-ROX.2 Therefore, patients with hypoxic ischaemic encephalopathy will be more likely to be assigned to conservative oxygen rather than liberal oxygen, patients with sepsis will be more likely to be assigned to liberal oxygen rather than conservative oxygen, and patients with an acute brain injury or condition other than hypoxic ischaemic encephalopathy will be more likely to be assigned to liberal oxygen rather than conservative oxygen. Patients who have none of these diagnoses will be assigned to conservative oxygen or liberal oxygen in a 1:1 ratio.
The oxygen regimen which is favoured at randomisation in a ratio of 1.05:1 for each patient group and the hierarchy-based randomisation will be reviewed and may be adapted at interim analyses. The maximum randomisation imbalance of 1.05:1 has been chosen to create a potential trial participation advantage for patients by ensuring that each trial participant benefits from information derived from previous trial participants, and to make sure that loss in power due to unequal sizes of treatment groups is trivial. If observed mortality rates at an interim analysis are similar in a particular group of patients (ie, if the point estimate of the relative risk of mortality with conservative versus liberal oxygen incorporating adjustment for site is between 0.9 and 1.1), a randomisation ratio of 1:1 will be used for that subgroup. After the first interim analysis, randomisation ratios for each patient group will not be known by the study management committee or the investigators. Only the data monitoring committee (DMC) and information technology company that will program the study website to implement adaptive randomisation will be provided this information during the study.
The randomisation sequence used to implement specified randomisation ratios will be determined by the information technology company providing the trial software. They will use computer-generated random numbers to determine the sequence. Because of complexities associated with adaptive randomisation and the large planned sample size, no stratification or permuted block randomisation is will be used. The randomisation sequence will be concealed from clinical staff, the management committee, and the investigators.
Study treatments
This study is designed to compare two approaches to providing oxygen therapy to adults who require mechanical ventilation in an ICU in emergency circumstances. These approaches are based on those tested in ICU-ROX2 but have been modified slightly to maximise treatment separation and minimise the risk of contamination (ie, to prevent implementation of conservative oxygen therapy in patients assigned to liberal oxygen therapy). The study treatments will be provided as soon as allocated. The treatment arm will not be blinded to trial participants, bedside nurses or research staff.
One treatment arm is a conservative approach to oxygen therapy (Figure 2), which aims to minimise unnecessary exposure to hyperoxaemia and reduce exposure to higher than necessary Fio2. When a participant is assigned to conservative oxygen therapy, the Fio2 will be decreased to a minimum of 0.21 (room air) as rapidly as possible provided that the arterial oxygen saturation measured by peripheral pulse oximetry (Spo2) is greater than the default acceptable lower limit of 91% (which can be reduced to less than 91% at the discretion of the treating clinician). Spo2 levels of greater than 94% will be strictly avoided and an upper Spo2 alarm limit of 95% will apply whenever supplemental oxygen is being administered in an ICU to minimise the risk of hyperoxaemia. After extubation, the upper monitored alarm limit of acceptable Spo2 of 95% will still apply whenever supplemental oxygen is being administered in an ICU. If the Spo2 exceeds the acceptable upper limit, downward titration of supplemental oxygen will be undertaken as a high priority and supplemental oxygen will be discontinued as soon as possible.
Figure 2.
Management of patients assigned to conservative oxygen therapy and those assigned to liberal oxygen therapy
Fio2 = fraction of inspired oxygen. Mega-ROX = mega randomised registry trial research program comparing conservative versus liberal oxygenation targets in adults receiving unplanned invasive mechanical ventilation in the ICU. Pao2 = arterial partial pressure of oxygen. Sao2 = arterial oxygen saturation. Spo2 = arterial oxygen saturation measured by peripheral pulse oximetry.
The other treatment arm is a liberal approach to oxygen therapy (Figure 2), which will be administered to patients both during mechanical ventilation and after extubation, with no specific measures taken to avoid high Fio2 or high Spo2 (including no upper alarm limit for Spo2). In this treatment arm, the minimum acceptable Fio2 during mechanical ventilation in an ICU will be 0.30. In both treatment groups, lower alarm limits for Spo2 will be set at a default level of 90% (which can be reduced to less than 90% at the discretion of the treating clinician).
Irrespective of the specific lower limit chosen for a particular patient, if an arterial blood gas analysis shows that the arterial partial pressure of oxygen (Pao2) is less than 60 mmHg or the arterial oxygen saturation (Sao2) is lower than the acceptable lower limit, the Fio2 can be increased if there is clinical concern regardless of the Spo2 reading.
The duration of study therapy will be until ICU discharge or 90 days, whichever is sooner. The study intervention will be applied only when a patient is in an ICU. If patients are transported out of an ICU for radiological or other investigations, or for procedures or operations, they will receive standard (non-study) treatment. Similarly, if an increase in Fio2 is required for procedures performed in an ICU — including, but not limited to, bronchoscopy, suctioning, tracheostomy, and preparation for extubation — this is permitted in both groups. Concomitant treatments provided to patients are not restricted. In particular, the titration of positive end expiratory pressure for patients in both arms of the trial will be determined by the treating clinician. Clinicians will be specifically discouraged from reducing positive end expiratory pressure to meet oxygenation targets.
Outcomes
The primary outcome is in-hospital all-cause mortality up to 90 days from the date of randomisation. All patients who survive the index hospital admission and are discharged from the index hospital within 90 days of randomisation will be defined as alive. Secondary outcomes are duration of survival time up until either the last follow-up or 90 days (whichever is sooner), ICU length of stay, hospital length of stay, duration of invasive mechanical ventilation, proportion of patients discharged home, and 90-day all-cause mortality, which will be reported for patients where vital status after hospital discharge can be obtained from a registry data source (eg, a national death registry). Additional secondary outcomes will be explored in secondary analyses as registry data permit. Such outcomes may, for example, include rates of delirium and ventilator-associated conditions, which are collected in Canada. Except as outlined above, participants will be followed up until 90 days from randomisation or until hospital discharge, whichever is sooner.
Oxygen exposure metrics
We will report the following oxygen exposure metrics by treatment group:
-
•
median percentage of hours per participant and median number of hours per participant spent with an Spo2 of less than 88% while in ICU;
-
•
median percentage of hours per participant and median number of hours per participant spent with an Spo2 of at least 97% while in ICU;
-
•
median percentage of hours per participant and median number of hours per participant spent breathing an Fio2 of 0.21 while in ICU;
-
•
number and percentage of patients with at least one Pao2 recording of less than 60 mmHg; and
-
•
number and percentage of patients with at least one Pao2 recording of greater than 100 mmHg.
We will also compare oxygen exposure over time by treatment group in a series of figures as shown in our proposed presentation of data, which is available online (http://www.wellingtonicu.com/PubResPres/Protocols).
Data collection and management
A range of methods will be used to obtain study data. At most sites, the majority of data will be obtained from existing databases, such as regional and national ICU registries. Patients who are enrolled in the study will be identified in these databases using variables that are recorded on the study website at the time of randomisation (Figure 3). Other data that will be collected at randomisation are shown in Table 1.
Figure 3.
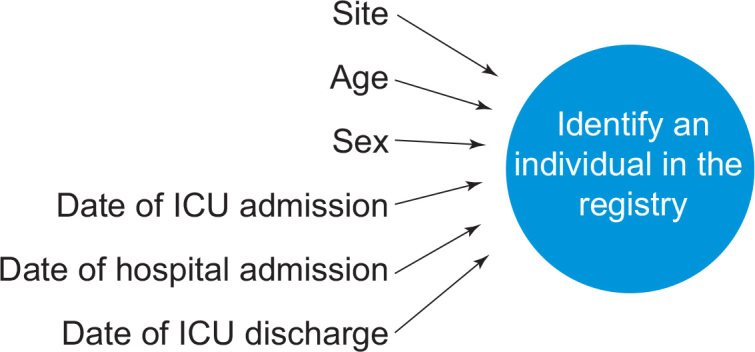
Data recorded via the study website and then used to identify patients in registry databases
ICU = intensive care unit.
Table 1.
Data that will be collected via the study website at randomisation
| Variable | Explanation |
|---|---|
| Patient initials | Used to identify patients at sites (not exported to the study database). |
| Date of birth | Used to calculate patient age for the purposes of matching to other data sources (see Figure 3). |
| Sex at birth | Used for the purposes of matching to other data sources (see Figure 3). |
| Date of hospital and ICU admission | Used for the purposes of matching to other data sources (see Figure 3). |
| Admission diagnosis | The patient’s diagnosis recorded using the ICNARC coding method.11 The code obtained by this method is hierarchical, with diagnosis coded as: type (surgical/non-surgical); body system (eg, respiratory); anatomical site (eg, lungs); physiological or pathological process (eg, inflammation, infection); and the specific condition. Data on whether the patient has a suspected hypoxic brain injury following resuscitation from a cardiac arrest, another acute brain injury or condition, known or suspected COVID-19 infection, and/or sepsis will also be entered at randomisation. |
| Possible hypoxic ischaemic encephalopathy (yes/no) | Instructions are provided on the study website to answer “yes” to this question if a patient has not obeyed commands following resuscitation from a cardiac arrest and there is clinical concern about possible brain damage. |
| Confirmed or strongly suspected sepsis (yes/no) | Instructions are provided on the study website to answer “yes” to this question if a patient is confirmed or strongly suspected to have life-threatening organ dysfunction due to a dysregulated host response to infection. |
| Acute brain injury or condition other than hypoxic ischaemic encephalopathy (yes/no) | Instructions are provided on the study website to answer “yes” to this question if a patient has an injury or condition such as a traumatic brain injury, an ischaemic or haemorrhagic stroke, a central nervous system infection, an autoimmune central nervous system condition, or a subarachnoid haemorrhage. The website also notes that seizures occurring in the absence of one of these injuries or conditions and neurological manifestations of non-central nervous system conditions (eg, hepatic encephalopathy or delirium) are not considered to be acute brain injuries or conditions. |
| COVID-19 infection (yes/no) | Includes both known and suspected COVID-19 infection. |
| Pao2 and Fio2 | The Pao2 from the last arterial blood gas analysis for the period before randomisation will be recorded (where available) along with the concurrent Fio2. |
COVID-19 = coronavirus disease 2019. Fio2 = fraction of inspired oxygen. ICNARC = Intensive Care National Audit and Research Centre. ICU = intensive care unit. Pao2 = arterial partial pressure of oxygen.
Data relating to physiological variables and process-of-care measures will be collected and reported as outlined in Table 2. These will be collected from patients’ medical records specifically for the study unless they can be obtained directly from electronic health records in an automated fashion. Data that will be obtained from existing data sources (for most sites) are shown in Table 3. The primary outcome data (Table 4) will be obtained from hospital medical records for all trial participants, even if they are available in a registry. Sites that do not contribute to a data registry, and have no means of obtaining study data from an existing data source, can still participate in Mega-ROX by collecting the data points outlined in Table 3 from patients’ medical records using a conventional trial case report form. It is anticipated that not all variables will be available in existing databases, but a pre-requisite to study participation is that primary outcome data can be reliably ascertained.
Table 2.
Physiological outcomes and process-of-care measures
| Outcome | Method by which outcome will be ascertained |
|---|---|
| Physiological outcomes | |
| Proportion of hours in ICU in the first 7 days following randomisation when the Spo2 is ≥ 97% | Based on all values (up to a maximum of one per hour) recorded on the ICU flow chart or electronic health record in the first 7 days following randomisation. |
| Total number of hours in ICU in the first 7 days following randomisation with an Spo2 ≥ 97% | Based on all values (up to a maximum of one per hour) recorded on the ICU flow chart or electronic health record in the first 7 days following randomisation. |
| Proportion of hours in ICU in the first 7 days following randomisation when the Spo2 is < 88% | Based on all values (up to a maximum of one per hour) recorded on the ICU flow chart or electronic health record in the first 7 days following randomisation. |
| Total number of hours in ICU in the first 7 days following randomisation with an Spo2 < 88% | Based on all values (up to a maximum of one per hour) recorded on the ICU flow chart or electronic health record in the first 7 days following randomisation. |
| Mean daily Pao2 in ICU in the first 7 days following randomisation | Based on values recorded 6-hourly on the ICU flow chart (at 06:00, 12:00, 18:00 and 24:00) from randomisation until Day 7. |
| Highest daily Pao2 in ICU in the first 7 days following randomisation | Based on values recorded daily from randomisation until Day 7 and determined from all recorded Pao2 values (not just those used to calculate mean daily Pao2). |
| Lowest daily Pao2 in ICU in the first 7 days following randomisation | Based on values recorded daily from randomisation until Day 7 and determined from all recorded Pao2 values (not just those used to calculate mean daily Pao2). |
| Process-of-care measures | |
| Proportion of hours in ICU in the first 7 days when the Fio2 is 0.21 | Based on all values (up to a maximum of one per hour) recorded on the ICU flow chart or electronic health record in the first 7 days following randomisation |
| Total number of hours in ICU in the first 7 days when the Fio2 is 0.21 | Based on all values (up to a maximum of one per hour) recorded on the ICU flow chart or electronic health record in the first 7 days following randomisation. |
| Mean daily Fio2 (while ventilated) in the first 7 days in ICU | Based on values recorded 6-hourly on the ICU flow chart (at 06:00, 12:00, 18:00 and 24:00) from randomisation until Day 7. |
| Highest daily Fio2 (while ventilated) in the first 7 days in ICU | Based on values recorded daily from randomisation until Day 7 and determined from all recorded Fio2 values on the ICU flow chart (not just those used to calculate mean daily Fio2). |
| Lowest daily Fio2 (while ventilated) in the first 7 days in ICU | Based on values recorded daily from randomisation until Day 7 and determined from all recorded Fio2 values on the ICU flow chart (not just those used to calculate mean daily Fio2). |
Fio2 = fraction of inspired oxygen. ICU = intensive care unit. Pao2 = arterial partial pressure of oxygen. Spo2 = arterial oxygen saturation measured by peripheral pulse oximetry.
Table 3.
Baseline data that will be obtained from existing databases for most sites
| Variable | Explanation |
|---|---|
| Weight | Weight (body mass) of the patient measured in kilograms. |
| Height | Height of the patient measured in centimetres. |
| Time from hospital admission to randomisation | Calculated using the year, month, day and time when the patient was admitted to hospital for the episode of ICU care in which they were randomly assigned for Mega-ROX. |
| Time from ICU admission to randomisation | Calculated using the year, month, day and time when the patient was admitted to ICU for the episode of ICU care in which they were randomly assigned for Mega-ROX. |
| ICU admission source | The mechanism by which a patient was admitted to ICU for the episode of ICU care in which they were randomly assigned for Mega-ROX, represented by a code. For patients admitted to an ICU from a procedure room (eg, catheterisation laboratory or radiology unit), their location before such rooms should be regarded as the source of ICU admission. The only caveat to this rule is if a patient receives a general anaesthetic during their procedure; these patients should be coded with an ICU admission source of operating theatre or recovery room. An exception will be made for out-of-hospital cardiac arrest patients who go directly from an emergency department to a cardiac catheterisation laboratory while intubated and sedated; these patients should be coded with an ICU admission source of emergency department. Also, if a patient is admitted to an ICU from an operating theatre or recovery room without having undergone a surgical procedure (eg, if the procedure was cancelled or not initiated), then the patient should be considered non-operative and their ICU admission source should be the their location before the operating theatre or recovery room; an example would be a patient who has anaphylaxis following anaesthesia that is given before surgery. However, if surgery begins, the patient should be considered post-operative. |
| Chronic respiratory disease before this hospitalisation (yes/no) | Defined as at least one of the following: chronic restrictive, obstructive disease resulting in severe exercise restriction (unable to climb stairs or perform household duties); documented chronic hypoxia, hypercapnia, secondary polycythaemia or severe pulmonary hypertension (mean pulmonary artery pressure > 40 mmHg); or ventilator dependency. |
| Chronic cardiac disease before this hospitalisation (yes/no) | Defined as New York Heart Association Class IV heart failure: angina or symptoms at rest or on minimal exertion (while getting dressed or during self-care). |
| Chronic liver disease before this hospitalisation (yes/no) | Defined as either biopsy-proven cirrhosis and documented portal hypertension or past episodes of upper gastrointestinal bleeding attributed to portal hypertension. If the patient has a functioning transplanted liver, this chronic health item will not apply. |
| Chronic renal disease before this hospitalisation (yes/no) | Defined as receiving haemodialysis or peritoneal dialysis on a long term basis. |
| Immunosuppression secondary to disease before this hospitalisation (yes/no) | Defined as immunosuppressive disease that is sufficiently advanced to suppress resistance to infection; examples include leukaemia, AIDS, lymphoma, severe autoimmune disease and documented diffuse metastatic cancer. |
| Immunosuppression secondary to therapy before this hospitalisation (yes/no) | Defined as therapy that has suppressed resistance to infection; examples include immunosuppression, chemotherapy within 4 weeks of admission, radiation therapy, high-dose steroid treatment (eg, > 1.5 mg/kg methylprednisolone or equivalent for ≥ 5 days) and long term treatment with > 20 mg/day steroid. |
| Pregnancy status | Collected for women at the time of ICU admission, using the following categories: currently pregnant, not pregnant and postpartum (within 42 days of giving birth). |
| Frailty | Collected at the time of ICU admission for the patient’s first ICU admission within the hospital admission in which the patient was randomly assigned for Mega-ROX, based on the patient’s level of physical function in the 2 months before this admission, as previously described. |
| Illness severity | Recorded for the first 24 hours of ICU admission using APACHE II scores. |
APACHE = Acute Physiology and Chronic Health Evaluation. ICU = intensive care unit. Mega-ROX = mega randomised registry trial research program comparing conservative versus liberal oxygenation targets in adults receiving unplanned invasive mechanical ventilation in the ICU.
Table 4.
Outcome data that will be obtained from existing databases for most sites
| Variable | Explanation |
|---|---|
| Time from randomisation to ICU discharge (ICU length of stay) | Calculated using the year, month, day and time when the patient was discharged from ICU for the episode of ICU care in which they were randomly assigned for Mega-ROX. |
| Time from randomisation to hospital discharge (hospital length of stay) | Calculated using the year, month, day and time when the patient was discharged from the hospital for the hospital admission corresponding to the episode of ICU care in which they were randomly assigned for Mega-ROX. |
| Proportion of patients discharged home | Obtained from the patient’s hospital outcome status and categorised as one of the following: died, home, nursing home/chronic care/palliative care, other hospital ICU, other acute hospital, rehabilitation, mental health care facility, hospital in the home, or other. |
| In-hospital all-cause mortality up to 90 days from the date of randomisation | Calculated using the patient’s vital status at discharge from hospital for the hospital admission corresponding to the episode of ICU care in which they were randomly assigned for Mega-ROX and their date of hospital discharge. Collecting this variable from existing databases will verify accuracy of the primary outcome data collected from medical records. Any discrepancies will be checked and data accuracy will be verified. |
| Hours of invasive ventilation in ICU | Includes the ICU admission when the patient was randomly assigned for Mega-ROX but does not include any readmission episodes. Any form of positive pressure ventilation delivered through an artificial airway, such as an oral or nasal endotracheal tube or a tracheostomy tube, will be considered invasive ventilation. It will include all modes of mandatory ventilation, spontaneous pressure support ventilation and continuous positive airway pressure when delivered through an artificial airway. |
| 90-day all-cause mortality | At study sites where data on vital status at Day 90 are available in a database for patients who survive beyond hospital discharge, these data will be used to calculate all-cause mortality at Day 90. Patients who survive to hospital discharge at these sites and are not identified as having died in the relevant database will be considered alive at Day 90. |
| Duration of survival | For patients who die in hospital, duration of survival will be calculated using their date of death, which will be defined as the year, month, day and time of hospital discharge. For patients who survive beyond hospital discharge and have a date of death within 90 days available in a database, this date will be used to calculate duration of survival. Patients who survive to hospital discharge at sites that have data on death within 90 days after discharge and are not identified as having died in the relevant database will be considered alive at Day 90. |
ICU = intensive care unit. Mega-ROX = mega randomised registry trial research program comparing conservative versus liberal oxygenation targets in adults receiving unplanned invasive mechanical ventilation in the ICU.
Ethics approval
Research ethics approval will be obtained before the start of the study at each institution from the responsible local and/or national human research ethics committee. As most patients enrolled in Mega-ROX will lack capacity to give consent at the time of trial enrolment, the following consent options are acceptable:
-
•
a priori consent from the patient;
-
•
a priori consent by a substitute decision maker;
-
•
delayed consent from a substitute decision maker;
-
•
delayed consent from the patient;
-
•
waiver of consent;
-
•
consent provided by an ethics committee, guardianship board or other legal authority; and
-
•
opt out as an alternative to consent.
The availability of options at individual participating sites will be determined by the relevant ethics committee and/or administrative tribunals and subject to applicable laws in each participating country.
Data management
Data management and monitoring will be conducted in accordance with a pre-specified plan, which is available online (http://www.wellingtonicu.com/PubResPres/Protocols).
Data monitoring committee
An independent DMC consisting of experts in intensive care medicine clinical research and biostatistics was established before the first trial participant was enrolled. The DMC members are Anders Perner (DMC chair, and Professor of Intensive Care, University of Copenhagen), Manu Shankar-Hari (Professor of Critical Care Medicine, University of Edinburgh) and Laurent Billot (DMC statistician and Professor of Medicine, UNSW). A set of DMC guidelines and a DMC charter were prepared by the study management committee and signed by the members of the DMC before the trial commenced. The key responsibility of the DMC is to follow interim analyses as required, which will fulfil two purposes: to make recommendations to the study management committee with reference to pre-specified stopping criteria for the overall trial and the nested trials; and to coordinate changes to the hierarchy of subgroups and adaptive randomisation ratios according to the pre-specified study plan.
Adverse events
We recognise that the intensive care patient population will experience several common aberrations in laboratory values, signs and symptoms due to the severity of underlying disease and the impact of standard therapies. Intensive care patients will frequently develop life-threatening organ failure(s) unrelated to study interventions and despite optimal management. Therefore, consistent with established practice in academic ICU trials,12 events that are part of the natural history of the primary disease process or expected complications of critical illness will not be reported as serious adverse events in this study. All adverse events which are potentially causally related to the study intervention or are otherwise of concern in the investigator’s judgement will be reported and reviewed by the management committee and DMC.
Sample size and power
Assuming a mortality rate of 29.7% in the liberal oxygen group (based on ICU-ROX data2), a sample size of 38 420 provides 90% power to detect an absolute mortality difference of 1.5 percentage points using a two-tailed hypothesis at an alpha of 0.05. The sample size has been inflated to 40 000 to account for losses to follow-up, consent withdrawal, and the minor trial inefficiency that results from unequal group sizes that may occur because of adaptive randomisation.
An absolute mortality difference between groups of 1.5 percentage points would equate to 1500 lives lost or saved for every 100 000 patients treated. It is biologically plausible that conservative oxygen therapy could reduce mortality in patients who require unplanned invasive mechanical ventilation in an ICU. A treatment effect on mortality of this magnitude attributable to conservative versus liberal oxygen therapy has not been excluded by any previous clinical trial. While there is no established minimal clinically important difference in 90-day in-hospital mortality for ICU patients, if there is a zero percentage point absolute mortality difference between treatment groups in Mega-ROX, 95% confidence intervals would be expected to exclude the possibility of an absolute increase or decrease in mortality of well under one percentage point. In the absence of heterogeneity of treatment response, this could reasonably be considered as excluding the possibility of a clinically important effect of conservative oxygen therapy on 90-day in-hospital mortality in this patient population.6
Overview of planned statistical analyses
Analysis and reporting principles
We will analyse data on an intention-to-treat basis, whereby all patients assigned to a treatment group will be analysed according to the group to which they were assigned, without imputation of missing data except where pre-specified. The intention-to-treat population will be defined as all patients enrolled in the trial except for those patients for whom consent for use of study data is withdrawn. A P value of less than 0.05 (two-tailed) will be used to indicate statistical significance for the primary outcome variable. For the six secondary clinical outcomes, we will control the family-wise error rate by applying a Holm–Bonferroni correction. All analyses will be performed using Stata 16 or a later version (StataCorp) or SAS 9.4 (SAS Institute).
The study team includes a blinded statistician who is a member of the study management committee and an unblinded statistician who is independent of the study management committee. The unblinded statistician will conduct interim analyses and will provide these to the DMC. Once study data are available for the entire study population, the unblinded statistician will assign mock treatment codes to study participants. Analyses using actual study data but with mock treatment codes will be run by the blinded statistician using the general approach outlined in this document. Any data queries that arise from these initial analyses will be addressed. Any changes to the approach outlined here that are needed will be specified in the formal stand-alone statistical analysis plan which will be publicly available before final study database lock or unmasking of true study treatment assignments. Analyses of the final study dataset will be untaken by two study statisticians independently with any discrepancies between findings resolved through consensus and, when required, discussion with the management committee.
Analyses of the primary outcome
Analysis of the primary outcome will be done using a log-binomial model, adjusting for site and the presence or absence of each of the following at randomisation: suspected hypoxic ischaemic encephalopathy following resuscitation from a cardiac arrest, sepsis, and acute brain injury or condition other than hypoxic ischaemic encephalopathy. The numbers at risk in each group, and the number and proportion of events observed, will be reported. In addition, the equivalent absolute risk difference and relative risk ratio, and corresponding 95% CIs, will be reported.
Sensitivity analyses accounting for site and any clinically meaningful baseline imbalances will be performed using log-binomial regression. In addition, adjustment for the independent covariates of age, sex and APACHE II score will be incorporated. Adjustment for baseline imbalances in the numbers of patients with hypoxic ischaemic encephalopathy, sepsis, and other acute brain injury or condition, and which are expected due to adaptive randomisation, will also be incorporated into all secondary analyses. Missing baseline characteristics will be imputed via single mean imputation using centre-specific means.13
The main sensitivity analyses for the impact of missing primary outcomes will involve imputing outcomes under worst-best and best-worst case scenarios. In the worst-best scenario, a worst outcome event (ie, in-hospital death within 90 days) is assigned to all patients missing the outcome in one treatment group, and a best outcome event (ie, survival to hospital discharge within 90 days) is assigned to all patients missing the outcome in the other treatment group. The best-worst scenario is the exact opposite assignment of outcomes. If substantively different conclusions do not arise from these two analyses, no further missing data assessments will be performed for that outcome. If a substantively different conclusion does arise, then multiple imputation will be undertaken for that outcome. Missing outcomes will be imputed separately by randomised group, using chained equations and predictive mean matching, using the five nearest neighbours.
In the low and middle income countries participating in this study, it is not uncommon for patients to be discharged from an ICU when discharge is not considered medically indicated (eg, because of the high cost of care and/or because death is anticipated). We will undertake two sensitivity analyses to account for patients categorised as discharged from ICU when discharge was not considered medically indicated. In the first analysis, these patients will be defined as dead when assigned to conservative oxygen and will be defined as alive when assigned to liberal oxygen. In the second analysis, these patients will be defined as alive when assigned to conservative oxygen and will be defined as dead when assigned to liberal oxygen.
Analyses of secondary outcomes
The effect of treatment allocation on the proportion of patients discharged home and 90-day mortality will be assessed in the same way as the primary outcome. To account for the competing risk of death, we will analyse duration of invasive mechanical ventilation, ICU length of stay and hospital length of stay using subdistribution hazard regression models and present the results using cumulative incidence functions. As lengths of stay are typically well approximated by log-normal distributions, for increased transparency they will also be reported as geometric means with 95% CIs for survivors and non-survivors separately, and differences between treatment groups will be reported as a ratios with 95% CIs. Survival times according to treatment group will be displayed as Kaplan–Meier curves and analysed using a log-rank test. Estimates of hazard ratios for survival with 95% CIs will be obtained from the Cox proportional hazards models incorporating treatment group alone, and additionally using independent covariates used in the log-binomial models described in relation to the primary outcome.
Analyses of oxygen exposure metrics
For analyses that compare differences in the median percentage of hours per participant and the median number of hours per participant above and below specific Pao2 thresholds, and those that compare the median percentage of hours per participant and the median number of hours per participant spent breathing an Fio2 of 0.21 while in ICU, we will calculate differences and medians with 95% CIs using quantile regression. These analyses will be adjusted for site and for the presence or absence of each of the following at randomisation: suspected hypoxic ischaemic encephalopathy following resuscitation from a cardiac arrest, sepsis, and acute brain injury or condition other than hypoxic ischaemic encephalopathy.
Analyses that compare the proportion of patients with at least one Pao2 recording of less than 60 mmHg with the proportion of patients with at least one Pao2 recording of greater than 100 mmHg will be conducted via log-binomial models. These analyses will be adjusted for site and the presence or absence of each of the following at randomisation: suspected hypoxic ischaemic encephalopathy following resuscitation from a cardiac arrest, sepsis, and acute brain injury or condition other than hypoxic ischaemic encephalopathy. The numbers at risk in each group, the numbers and proportions of events observed, and the relative risks with 95% CIs will also be reported.
Interim analyses
Interim efficacy and safety analyses will be conducted after 1000, 8000, 16 000, 24 000 and 32 000 patients have been enrolled, using registry data for the primary outcome that are available when each enrolment threshold is passed.
The primary purpose of the initial interim analysis is to ensure that the recruitment rate achieved is rapid enough to enable enrolment of 40 000 participants within 8 years by rolling the trial out to 100 ICUs. The other purposes of the initial interim analysis are to update trial randomisation ratios to reflect accumulating trial data, and to ensure significant separation in oxygen exposure by treatment group. At all interim analyses, an additional model will be fit, including an interaction term between treatment group and subgroup, and point estimates of the relative risks for each subgroup will be obtained. For each subgroup, if the point estimate of the relative risk of mortality with conservative versus liberal oxygen incorporating adjustment for site is between 0.9 and 1.1, a randomisation ratio of 1:1 will be used for that subgroup. Otherwise, the randomisation ratio of 1.05:1 will be used, favouring the randomised group with fewer deaths.
At the second and subsequent interim analyses, available primary outcome data will be analysed as described for the final analysis of the primary outcome. These interim analyses will be done on an intention-to-treat basis. A Haybittle–Peto symmetric stopping boundary of P < 0.001 will be applied, and the trial will be stopped if the P value for the effect of treatment is less than 0.001.
Details of stopping rules that will be used for nested subgroup trials are outlined in respective protocol manuscripts for these trials. In brief, an interim analysis to consider early stopping of a nested trial will only be conducted where there is evidence of heterogeneity of treatment response (P < 0.05) involving the subgroup of patients included in that trial. If such an analysis is undertaken, stopping rules will be determined by a Haybittle–Peto symmetric stopping boundary of P < 0.001. We do not plan to stop for futility because we consider that, given how commonly oxygen therapy is used in invasively mechanically ventilated patients in ICUs, providing the most precise estimates of the likely plausible range of mortality treatment effects is important.
Description of baseline variables
Baseline characteristics will be summarised by randomised group as appropriate: means and standard deviations for continuous variables that appear to be distributed about symmetrically; medians and interquartile ranges for other continuous variables; and counts and percentages for categorical variables.
Subgroup analyses
Analyses will be performed on six pre-defined subgroup pairs irrespective of whether there is evidence of a mortality treatment effect. Heterogeneity between subgroups will be determined by fitting an interaction between treatment and subgroup for the primary outcome (90-day in-hospital mortality). The subgroup pairs are:
-
•
suspected hypoxic ischaemic encephalopathy following resuscitation from a cardiac arrest versus not;9
-
•
sepsis versus not;8
-
•
acute brain injury or condition other than hypoxic ischaemic encephalopathy versus not;
-
•
confirmed or clinically suspected COVID-19 versus not;
-
•
recruited in a high income country versus a low or middle income country; and
-
•
time from ICU admission to randomisation less than 2 hours versus greater than or equal to 2 hours.
The first three subgroups align with the subgroups being evaluated in the nested trials.
Occult hypoxaemia (ie, a Pao2 < 88% despite an oxygen saturation of 92–96% on pulse oximetry) appears to be more common in patients who identify as black than in patients who identify as white.14 To evaluate the potential clinical importance of this observation, interested study sites will be invited to participate in a skin tone substudy where we will collect baseline data on skin tone using the Fitzpatrick skin tone scale.15 In this study, which will be published separately to the main study manuscript, we will evaluate associations between skin tone and outcomes in patients allocated to conservative or liberal oxygen therapy.
Presentation of outcome data
A complete set of mock tables and figures is available online (http://www.wellingtonicu.com/PubResPres/Protocols). The methods used to obtain study data and the individual variables that are available for all sites using registry data sources will be reported.
Summary
Mega-ROX is a 40 000-participant phase 3 international, multicentre, randomised, parallel-group, two-sided superiority trial designed to test the hypothesis that among adult ICU patients who receive unplanned invasive ventilation, conservative oxygen therapy reduces in-hospital all-cause mortality up to 90 days from the date of randomisation by at least 1.5 percentage points when compared with liberal oxygen therapy. This protocol and statistical analysis plan article was submitted for publication before the first interim efficacy and safety analysis was undertaken.
Acknowledgments
Acknowledgements:
Mega-ROX is funded by grants from the Health Research Council of New Zealand and by an unrestricted donation from the Alpha Charitable Trust. The Low Oxygen Intervention for Cardiac Arrest Injury Limitation (LOGICAL) Mega-ROX substudy is funded by the Australian National Health and Medical Research Council. In Canada, Mega-ROX has received funding from the Pragmatic Trials Platform – Alberta Strategy for Patient-Oriented Research (SPOR) Support Unit. The funding bodies have had no input into the design or conduct of the trial or into the statistical analysis plan, and will have no input into analysis or reporting of the results. The study is coordinated in New Zealand by the Medical Research Institute of New Zealand and in Australia by the Australian and New Zealand Intensive Care Research Centre. The study is coordinated in Ireland by the Irish Critical Care Clinical Trials Network, which is supported by the Health Research Board. The study is coordinated in Canada by the University of Alberta. The study is coordinated in Japan by Jikei University. The study is coordinated in Asia by the Critical Care Asia Network and in Africa by the Critical Care Africa Network (parts of the National Intensive Care Surveillance, Mahidol–Oxford Tropical Medicine Research Unit [NICS-MORU] collaboration), which are supported by a Wellcome Innovations grant (215522). This study is endorsed by the Australia and New Zealand Intensive Care Society Clinical Trials Group, the Irish Critical Care Clinical Trials Group, and the Alberta Health Services Critical Care Strategic Clinical Network.
Competing interests
All authors declare that they do not have any potential conflict of interest in relation to this manuscript.
References
- 1.Schjorring O.L., Klitgaard T.L., Perner A., et al. Lower or higher oxygenation targets for acute hypoxemic respiratory failure. N Engl J Med. 2021;384:1301–1311. doi: 10.1056/NEJMoa2032510. [DOI] [PubMed] [Google Scholar]
- 2.ICU-ROX Investigators and the Australian and New Zealand Intensive Care Society Clinical Trials Group. Conservative oxygen therapy during mechanical ventilation in the ICUN Engl J Med. 2020;382:989–998. doi: 10.1056/NEJMoa1903297. [DOI] [PubMed] [Google Scholar]
- 3.Barrot L., Asfar P., Mauny F., et al. Liberal or conservative oxygen therapy for acute respiratory distress syndrome. N Engl J Med. 2020;382:999–1008. doi: 10.1056/NEJMoa1916431. [DOI] [PubMed] [Google Scholar]
- 4.Girardis M., Busani S., Damiani E., et al. Effect of conservative vs conventional oxygen therapy on mortality among patients in an intensive care unit: the oxygen-ICU randomized clinical trial. JAMA. 2016;316:1583–1589. doi: 10.1001/jama.2016.11993. [DOI] [PubMed] [Google Scholar]
- 5.Panwar R., Hardie M., Bellomo R., et al. Conservative versus liberal oxygenation targets for mechanically ventilated patients. A pilot multicenter randomized controlled trial. Am J Respir Crit Care Med. 2016;193:43–51. doi: 10.1164/rccm.201505-1019OC. [DOI] [PubMed] [Google Scholar]
- 6.Young P.J. Effect of oxygen therapy on mortality in the ICU. N Engl J Med. 2021;384:1361–1363. doi: 10.1056/NEJMe2101538. [DOI] [PubMed] [Google Scholar]
- 7.Young P.J., Bagshaw S.M., Bailey M., et al. O2, do we know what to do? Crit Care Resusc. 2019;21:230–232. [PubMed] [Google Scholar]
- 8.Young P., Mackle D., Bellomo R., et al. Conservative oxygen therapy for mechanically ventilated adults with sepsis: a post hoc analysis of data from the intensive care unit randomized trial comparing two approaches to oxygen therapy (ICU-ROX) Intensive Care Med. 2020;46:17–26. doi: 10.1007/s00134-019-05857-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Young P., Mackle D., Bellomo R., et al. Conservative oxygen therapy for mechanically ventilated adults with suspected hypoxic ischaemic encephalopathy. Intensive Care Med. 2020;46:2411–2422. doi: 10.1007/s00134-020-06196-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chu D.K., Kim L.H.Y., Young P.J., et al. Mortality and morbidity in acutely ill adults treated with liberal versus conservative oxygen therapy (IOTA): a systematic review and meta-analysis. Lancet. 2018;391:1693–1705. doi: 10.1016/S0140-6736(18)30479-3. [DOI] [PubMed] [Google Scholar]
- 11.Young J.D., Goldfrad C., Rowan K. Development and testing of a hierarchical method to code the reason for admission to intensive care units: the ICNARC Coding Method. Intensive Care National Audit & Research Centre. Br J Anaesth. 2001;87:543–548. doi: 10.1093/bja/87.4.543. [DOI] [PubMed] [Google Scholar]
- 12.Cook D., Lauzier F., Rocha M.G., et al. Serious adverse events in academic critical care research. CMAJ. 2008;178:1181–1184. doi: 10.1503/cmaj.071366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.White I.R., Thompson S.G. Adjusting for partially missing baseline measurements in randomized trials. Stat Med. 2005;24:993–1007. doi: 10.1002/sim.1981. [DOI] [PubMed] [Google Scholar]
- 14.Sjoding M.W., Dickson R.P., Iwashyna T.J., et al. Racial bias in pulse oximetry measurement. N Engl J Med. 2020;383:2477–2478. doi: 10.1056/NEJMc2029240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fitzpatrick T.B. The validity and practicality of sun-reactive skin types I through VI. Arch Dermatol. 1988;124:869–871. doi: 10.1001/archderm.124.6.869. [DOI] [PubMed] [Google Scholar]



