Abstract
Objective: To describe pain assessment and analgesic management practices in patients in intensive care units (ICUs) in Australia and New Zealand.
Design, setting and participants: Prospective, observational, multicentre, single-day point prevalence study conducted in Australian and New Zealand ICUs. Observational data were recorded for all adult patients admitted to an ICU without a neurological, neurosurgical or postoperative cardiac diagnosis. Demographic characteristics and data on pain assessment and analgesic management for a 24-hour period were collected.
Main outcome measures: Types of pain assessment tools used and frequency of their use, use of opioid analgesia, use of adjuvant analgesia, and differences in pain assessment and analgesic management between postoperative and non-operative patients.
Results: From the 499 patients enrolled from 45 ICUs, pain assessment was performed at least every 4 hours in 56% of patients (277/499), most commonly with a numerical rating scale. Overall, 286 patients (57%) received an opioid on the study day. Of the 181 mechanically ventilated patients, 135 (75%) received an intravenous opioid, with the predominant opioid infusion being fentanyl. The median dose of opioid infusion for ventilated patients was 140 mg oral morphine equivalents. Of the 318 non-ventilated patients, 41 (13%) received patient-controlled analgesia and 76 (24%) received an oral opioid, with the predominant opioid being oxycodone. Paracetamol was administered to 63 ventilated patients (35%) and 164 non-ventilated patients (52%), while 2% of all patients (11/499) received a non-steroidal anti-inflammatory drug. Ketamine infusion and regional analgesia were used in 15 patients (3%) and 17 patients (3%), respectively. Antineuropathic agents (predominantly gabapentinoids) were used in 53 patients (11%).
Conclusions: Although a majority of ICU patients were frequently assessed for pain with a validated pain assessment tool, cumulative daily doses of opioids were high, and the use of multimodal adjuvant analgesia was low. Our data on current pain assessment and analgesic management practices may inform further research in this area.
Acute pain is common in critically ill patients, with more than 50% of intensive care unit (ICU) survivors recalling moderate to severe pain during their ICU admission.1 Treatment of acute pain in an ICU setting can lead to improved outcomes and decreased length of ICU intervention,2 while inadequate analgesia can lead to chronic physical, psychological and social morbidity.3 The aetiology of acute pain in experienced in ICUs is multifactorial. Potential causes include the ICU admission diagnosis (eg, sepsis, trauma, burns, surgery), devices and procedures used to facilitate supportive therapy (eg, endotracheal tube, urinary catheter, prolonged immobility), excess medication (eg, opioids [which can induce hyperalgesia], sedatives), and procedures such as turning for pressure area care, suctioning and vascular access.4,5 Potential psychosocial factors that exacerbate acute pain include delirium, sleep deprivation, fear, social isolation, depression, anxiety and post-traumatic stress disorder.5, 6
Effective management of acute pain in ICUs is difficult. In addition to multiple potential causes of acute pain, there are barriers to effective pain assessment and multimodal analgesic management.7 Barriers to effective assessment include inability of patients to communicate due to altered consciousness, presence of an endotracheal tube, haemodynamic instability, and history of substance misuse. Barriers to analgesic management include altered pharmacodynamics and pharmacokinetics, end-organ dysfunction, and adverse effects of analgesics including respiratory depression, immunosuppression, acute kidney injury and constipation.8 This was highlighted in the recent PADIS (pain, agitation, delirium, immobility and sleep) guidelines, which are endorsed by the Society of Critical Care Medicine and are aimed at improving delivery of adequate pain assessment and management to critically ill patients.9, 10 These guidelines recommend that first-line pain management utilises opioids with analgesic adjuncts (eg, paracetamol, non-steroidal anti-inflammatory drugs [NSAIDs], ketamine, antineuropathic agents) to minimise opioid use. These recommendations were supported by moderate to low level evidence.9, 10
Despite recommendations in the PADIS guidelines, there are limited local data detailing the tools used for assessment of pain, the frequency of pain assessment, and the pharmacological management of pain in critically ill patients. To address this knowledge gap, we conducted a point prevalence study with the aim of describing the types of pain assessment tools used, the frequency of pain assessment, and analgesic pharmacotherapy, specifically opioid and adjunct analgesia, in ventilated and non-ventilated general ICU patients (mixed medical and surgical) in Australia and New Zealand. We also aimed to compare the tools used for assessment of pain, the frequency of pain assessment and analgesic pharmacotherapy in postoperative and non-operative ICU patients.
Methods
Study design
A binational, prospective, observational, multicentre, single-day point-prevalence study was conducted on one of two study days in August and November 2016 to examine how pain was assessed and managed in ICUs in Australia and New Zealand. Participating sites are listed online (Online Appendix, Table S1). The study was conducted within the Point Prevalence Program coordinated by the Australian and New Zealand Intensive Care Society Clinical Trials Group and the Critical Care Division of the George Institute for Global Health. Human research ethics committee or institutional approval for a waiver of informed consent for individual patients was obtained for all participating sites.
Item development
An external panel reached consensus on research items investigating assessment of pain and pharmacological management of pain in ICUs; the panel comprised the chief investigator, two pain specialists and a pain clinical nurse consultant. Items were based on pain assessments tools that are available in ICUs,11 current guidelines,9 and analgesic pharmacotherapies that are available in Australia and New Zealand. Items in the case report form were then reviewed by researchers in the Point Prevalence Program before inclusion in the study.
Main outcome measures
The main outcome measures were the types of pain assessment tools used and their frequency of use, use of opioid analgesia, use of adjuvant analgesia, and differences in pain assessment and analgesic management between postoperative and non-operative patients.
Data collection
Data were collected on all patients aged > 16 years who had been admitted to a participating ICU without a neurological, neurosurgical or postoperative cardiac diagnosis and were occupying a bed in the participating ICU at 10:00am on the study day. Cumulative data were collected for the 24-hour period corresponding to the chart day that included the 10:00 am time point. Each site collected data on a single day and entered the data into a database via Research Electronic Data Capture (REDCap) software.12 Data were collected based on variables defined in the data dictionary at each site by designated trained ICU clinical or research staff, using patient observation charts, medication charts and medical records.
Demographic data collected included age, sex, admission source, admission diagnosis, Acute Physiology and Chronic Health Evaluation (APACHE) II score13 and the use of invasive mechanical ventilation. Data were collected on ICU pain assessment tool use; validated tools were the critical care pain observation tool (CPOT),14 behavioural pain score (BPS),15 numerical rating scale (NRS), faces pain scale,16 and non-verbal pain scale (NVPS).17 Data were also collected on the frequency of pain assessment, parenteral and enteral use of opioid and adjunct analgesics (paracetamol, NSAIDs, ketamine, antineuropathic medications, regional analgesia). Opioid doses were converted to oral morphine equivalents (OME) using a conversion table from the Faculty of Pain Medicine of the Australian and New Zealand College of Anaesthetists18 as shown online (Online Appendix, Table S2) with the cumulative daily dose representing the point prevalence study day. Data were deidentified before submission to the coordinating centre.
Statistical analysis
Descriptive statistics are used to present demographic and clinical data. Normally distributed variables are reported as mean (standard deviation) and non-normally distributed variables as median (interquartile range). Categorical variables are reported as frequencies with percentages. To compare the distribution of clinical variables between postoperative and non-operative patients, the Mann- Whitney U test was used for numerical variables, and the test of proportions, χ2 test or Fisher exact test were used for categorical variables. No assumptions or imputations were made about missing data. Statistical analysis was performed using R version 4.1.0 (R Foundation for Statistical Computing) and RStudio version 1.4.1714. (RStudio, PBC, Boston, MA, USA). Packages used for analysis included tidyverse,19 ggplot220 and gtsummary.21 The complete analysis code is available online (https://github.com/BLMoran/PAIN-ICU_Point_Prevalence:Study).
Results
Demographics
In total, 499 patients from 45 tertiary referral, metropolitan and rural ICUs in Australia and New Zealand were enrolled in the point prevalence study (Table 1). Forty-five per cent of patients (223/499) were women, and the mean APACHE II score was 19 (SD, 8). Thirty-six per cent of patients (181/499) were mechanically ventilated and 78% (391/499) had a non-operative admission diagnosis. Thirty-eight per cent of patients (189/499) were admitted from an emergency department and 25% (124/499) from an operating theatre.
Table 1.
Demographic characteristics of patients included in the study
| Characteristic | Overall (N = 499) | Ventilated (n = 181) | Non-ventilated (n = 318) |
|---|---|---|---|
| Age in years, median (IQR) | 62 (49-74) | 59 (47-70) | 65 (50-77) |
| Female, number (%) | 223 (45%) | 67 (37%) | 156 (49%) |
| APACHE II score, median (IQR) | 17 (13-24) | 19 (15-26) | 17 (12-22) |
| Admission diagnosis, number (%) | |||
| Cardiovascular | 71 (15%) | 26 (15%) | 45 (15%) |
| Respiratory | 132 (28%) | 57 (34%) | 75 (25%) |
| Sepsis | 65 (14%) | 23 (14%) | 42 (14%) |
| Trauma | 16 (3%) | 3 (2%) | 13 (4%) |
| Other medical | 73 (16%) | 23 (14%) | 50 (17%) |
| Postoperative | 108 (23%) | 36 (21%) | 72 (24%) |
| Admission source, number (%) | |||
| Emergency department | 189 (38%) | 68 (38%) | 121 (38%) |
| Operating theatre | 124 (25%) | 44 (24%) | 80 (25%) |
| Hospital ward | 113 (23%) | 33 (18%) | 80 (25%) |
| Other | 73 (15%) | 36 (20%) | 37 (12%) |
APACHE = Acute Physiology and Chronic Health Evaluation; IQR = interquartile range.
Assessment of pain
Assessment tool
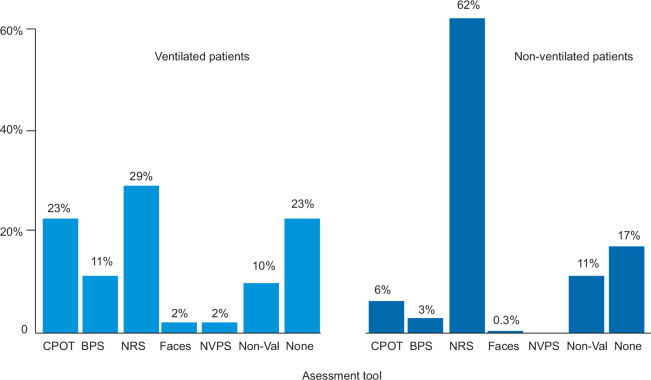
In total, 81% of patients (403/499) were assessed for pain at any time on the study day; 77% of ventilated patients (139/181) and 83% of non-ventilated patients (264/318) were assessed. For the ventilated patients who were assessed, the most common pain assessment tools were the NRS (53/181 [29%]), the CPOT (41/181 [23%]) and the BPS (20/181 [11%]). For the non-ventilated patients who were assessed, the most common assessment tools were the NRS (198/318 [66%]), the CPOT (20/318 [6%]) and non-validated pain scores (36/318, 11%) (Figure 1). Non-validated pain assessment tools included asking patients to respond with "yes" or "no", asking patients to respond by nodding or shaking their head, RASS scores and nursing opinion to determine whether a patient was in pain. In the ventilated group, the proportion of patients who were assessed for pain was not different for those who received opioids compared with those who did not (31/38 v 108/143; P = 0.57). However, in the non-ventilated group, the proportion of patients who were assessed for pain differed based on whether patients received an opioid or not (130/143 v 134/175; P < 0.01).
Figure 1.
Pain assessment tools used for ventilated and non-ventilated patients
CPOT = critical care pain observation tool; BPS = behavioural pain scale; NRS = numerical rating scale; Faces = faces pain scale; NVPS = non-verbal pain scale; Non-Val = non-validated assessment tool.
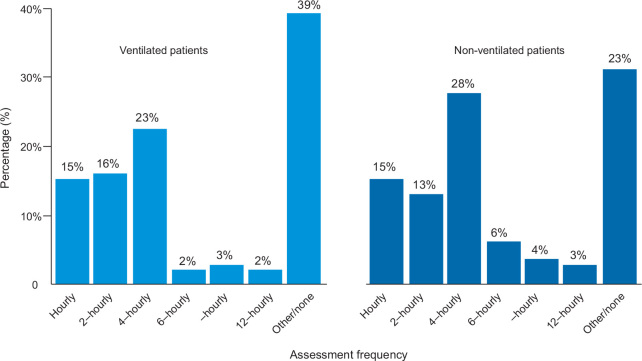
Frequency of assessment
More than half of the patients (277/499 [56%]) were assessed for pain every 4 hours or more often. Fifty-four per cent (98/181) and 56% (179/318) of ventilated and non-ventilated patients, respectively, were assessed at least every 4 hours. Thirty-nine per cent of ventilated patients (71/181) were assessed once per day or not at all (Figure 2).
Figure 2.
Frequency of pain assessment for ventilated and non-ventilated patients
Analgesic management
Opioids
In total, 57% (286/499) of patients received an opioid on the study day. The predominant route of administration was intravenous (232/499 [46%]); 16% of patients (82/499) received an enteral opioid. In the ventilated group, 79% of patients (143/181) received an opioid and 75% (135/181) received an intravenous opioid. The predominant opioid infusion in this group was fentanyl (89/181 [49%]) followed by morphine (22/181 [12%]) (Figure 3). The remainder did not receive an opioid (38/181, 21%). Oxycodone was the most common enteral opioid administered in the overall study population (57/499 [11%]) and in both ventilated patients (9/181 [5%]) and non-ventilated patients (48/318 [15%]).
Figure 3.
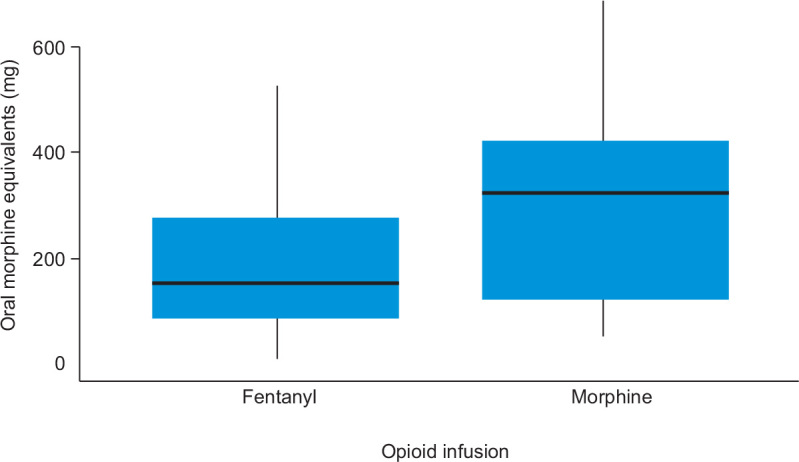
Cumulative opioid infusion doses for ventilated patients*
For the 143 ventilated patients who received an opioid, the median daily dose of opioid was 140 mg OME. For the 22 ventilated patients who received morphine, the median daily dose was 322.5 mg OME. In contrast, the median daily dose for the 89 ventilated patients who received fentanyl was 148.0 mg OME. The median daily dose in non-ventilated patients was 37 mg OME. The total daily doses were predominantly comprised of parenteral opioids (Figure 4). A breakdown of opioid type and dose for ventilated and non-ventilated patients is provided online (Online Appendix, Tables S3-S5).
Figure 4.
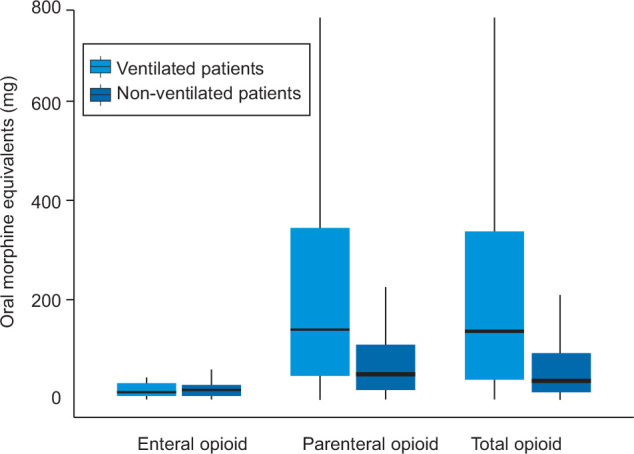
Total opioid doses for ventilated and non-ventilated patients who received opioids
* Median values are represented by the horizontal bar in each box, interquartile ranges by the upper and lower limits of each box, and ranges by whiskers.
Simple analgesia
Overall, paracetamol was administered to 45% of patients (227/499); 35% of ventilated patients (63/181) and 52% of non-ventilated patients (164/318) received paracetamol. Only 2% of all patients (11/499) and 1% of ventilated patients (2/181) received an NSAID. The predominant route of administration of simple analgesia was enteral.
Analgesic adjuncts
A ketamine infusion was administered to 3% of all patients (15/499), most of whom were non-ventilated (10/318 [3%]). Regional analgesia was used in 3% of all patients (17/499), and predominantly in non-ventilated patients (13/318 [4%]). Antineuropathic agents were used in 11% of all patients (53/499) and were used in similar proportions of ventilated patients (20/181 [11%]) and non-ventilated patients (33/318 [10%]). Gabapentinoids (gabapentin and pregabalin) were the most used antineuropathic agents. Proportions of analgesic adjuncts used are shown in Table 2.
Table 2.
Analgesic adjuncts used in the study population
| Analgesic adjunct | Overall (N = 499) | Ventilated (n = 181) | Non-ventilated (n = 318) |
|---|---|---|---|
| Paracetamol | 227 (45%) | 63 (35%) | 164 (52%) |
| Non-steroidal anti-inflammatory drugs | 11 (2%) | 2 (1%) | 9 (3%) |
| Ketamine infusion | 15 (3%) | 5 (3%) | 10 (3%) |
| Regional analgesia | 17 (3%) | 4 (2%) | 13 (4%) |
| Antineuropathic agents | 53 (11%) | 20 (11%) | 33 (10%) |
Postoperative versus non-operative patients
The proportions of pain assessment tool types used in postoperative patients were similar to those used in non-operative patients. The frequency of pain assessment was similar in postoperative and non-operative patients who were ventilated. In the non-ventilated group, postoperative patients received more frequent pain assessment than non-operative patients. There were similar proportions of type of opioid administered, both intravenous and enteral, for postoperative and non-operative patients who were ventilated and non-ventilated. The total cumulative opioid dose was similar between the postoperative and non-operative patients in the ventilated group (142 mg v 132 mg; P = 0.52) and between the postoperative and non-operative patients in the non-ventilated group (30 mg v 52 mg, P = 0.15). Proportions of simple analgesia administration in the postoperative group were similar to those in non-operative group with the exception of paracetamol use, which was higher in the postoperative non-ventilated group than in the non-operative non-ventilated group (41/82 v 105/236, P = 0.018). Patients in the postoperative group were more likely than those in the non-operative group to receive a ketamine infusion (11/127 v 4/372, P < 0.01) and to have regional analgesia (14/127 v 3/372, P < 0.001). There was no difference in the use of antineuropathic medications between the patients in the postoperative and non-operative groups.
Discussion
Key findings
In a mixed cohort of critically ill patients, we observed that more than two-thirds of patients were assessed for pain using a validated pain assessment tool, and that these assessments were used frequently. However, almost half of the ventilated patients were either not assessed for pain or had only one assessment on the study day.
Overall, more than half of the patients received an opioid on the study day, with fentanyl and oxycodone being the most common choices in ventilated and non-ventilated patients, respectively. Ventilated patients who received an opioid received a large daily dose. About half of patients received paracetamol, but use of NSAIDs and analgesic adjuncts was low. Patients with a postoperative admission to ICU had a similar pain assessment and opioid management profile to non-operative patients but were more likely to receive a ketamine infusion or regional analgesia.
Comparison to previous studies
Despite the focus on pain assessment in the PADIS guidelines,10 the proportion of ventilated patients in our study who underwent regular pain assessment was low. This is consistent with previous findings, where the frequency of assessment has been reported as 23.8%, 37–42%, 46% and 60%.22, 23, 24, 25 Our data on the types of pain assessment tool used are also consistent with previous findings; in a study of patients with coronavirus disease 2019 (COVID-19), those assessed for pain were assessed using the CPOT (37%), BPS (28%) and NRS (36%).25 This is in contrast to a study conducted in the United Kingdom, where a verbal descriptive scale was the predominant tool used in both ventilated and non-ventilated patients, and where there was minimal use of the BPS (1.1%) and CPOT (2.1%) in ventilated patients.24 Regular pain assessments with validated pain assessment tools have been shown to decrease pain intensity, reduce duration of mechanical ventilation and reduce incidence of ventilator-associated pneumonia.26 Hence, improvements relating to regular pain assessment with validated tools may improve outcomes in critically ill patients, although the optimal frequency and tool are yet to be determined.
In our study, 75% of ventilated patients received an intravenous opioid, which is consistent with previously reported findings.27, 28 Similar to findings from previous studies conducted in Australia, New Zealand and other countries,22, 23, 25, 29, 30, 31, 32, 33 fentanyl was the predominant opioid infusion used in ventilated patients in our study. A recently conducted randomised trial found that patients receiving fentanyl had more ventilator-free days and a shorter ICU length of stay compared with those receiving morphine.34 However, it should be noted that opioid infusions were titrated to target light sedation, rather than relief from pain, which makes extrapolation of opioid type for analgesia in this cohort problematic.
The cumulative daily opioid dose given to mechanically ventilated patients that we observed in our study is consistent with findings from recent studies. In an Australian randomised controlled trial comparing fentanyl to morphine, daily cumulative doses reported were 159 mg OME (IQR, 72–312 mg) for morphine and 192 mg OME (IQR, 70–367 mg) for fentanyl.34 These doses are substantially lower than those seen in French observational studies, where titration of opioid infusions to validated pain assessment scores resulted in mean daily opioid doses of 84-105 mg OME for morphine and 500-700 mg OME for fentanyl.2, 23, 32 Despite the lower daily doses seen in our study, prolonged infusions of high dose opioids, especially to opioid-naive patients, may increase the risk of opioid-related complications such as delirium, ICU-acquired infections, acute opioid tolerance and iatrogenic withdrawal, and opioid-induced hyperalgesia.35
We found that there was low use of adjunct analgesic medications in ventilated patients. This is consistent with findings from a previous observational study of ventilated patients, in which low levels of use were reported for paracetamol (24.7%), NSAIDs (6.5%), gabapentinoids (6.4%) and epidural analgesia (2.4%).30 The use of ketamine in our cohort was low (3%), and substantially lower than its reported use in a single tertiary ICU, where 18.6% of patients received a ketamine infusion (40% use in surgical ICU patients).33 However, more frequent use of ketamine infusions in a surgical ICU is consistent with our findings in both ventilated and non-ventilated postoperative patients.
Adjuvant analgesic use in ICU patients has the potential to improve outcomes. Paracetamol use in postoperative ICU patients has been shown to reduce pain intensity, opioid consumption and time to extubation, while the addition of NSAIDs has been shown to reduce pain intensity and opioid consumption after major abdominal surgery, and reduce the incidence of attributable pneumonia and duration of ventilation in patients with rib fractures.36, 37 its use in Guillain-Barré syndrome has been shown to result in decreased pain scores and opioid consumption.38, 39 Observational studies investigating continuous infusions of ketamine have shown variable effects on opioid consumption, including a decrease in opioid consumption with improved pain intensity seen,39, 40, 41 contrasting with no difference or an increase in opioid dose.42, 43, 44 Other studies have shown that the use of truncal regional analgesia can result in improved analgesia and a reduction in opioid consumption.45, 46, 47, 48 Together, these findings show that the use of analgesic adjuncts can result in improved outcomes relating to pain, but that analgesic adjunct use is low and warrants further investigation.
Implications and significance
Our study has identified that, despite specific guidelines,10 frequent use pain assessment using validated tools was moderately low, particularly in ventilated patients. This, in addition to the use of high daily doses of opioids, increases the risk of patients developing opioid-associated complications. This is compounded by the infrequent use of adjuncts as part of multimodal analgesia, except for paracetamol. This baseline description of analgesic assessment and management practices may inform further trials aimed at improving pain assessment and analgesic management for critically ill patients.
Strengths and limitations
The main strength of this study is that it provides data from a prospective, multicentre, binational observational evaluation of analgesic practices in ICU patients. Our findings add to the evidence base on pain assessment types and frequency, and provide details on use of opioid therapy and analgesic adjuncts in both ventilated and non-ventilated ICU patients. The limitations of our study include the point prevalence design, which precludes longitudinal analysis of analgesic practices, specifically the relationship to pain assessment. Also, given that extubation on the study day was possible, the route of opioid administration (eg, enteral or patient-controlled analgesia) may have changed for some patients, potentially resulting in a lower cumulative opioid dose in ventilated patients. In addition, the types of pain assessments may have changed for patients after extubation, which could explain why the NRS was the predominant pain assessment tool in ventilated patients.
Conclusions
This point prevalence study in a mixed cohort of critically ill patients in Australia and New Zealand provides detailed data on current pain assessment and management practices. Although most ICU patients were assessed for pain, those receiving mechanical ventilation were administered large cumulative opioid doses with relatively low use of analgesic adjuncts, so the analgesic and opioid-sparing benefits of multimodal analgesia were not realised. However, understanding current practice may inform the design of future observational and interventional studies that aim to investigate the effects of analgesic management on patient-specific outcomes.
Competing interests
All authors declare that they do not have any potential conflict of interest in relation to this manuscript.
Acknowledgements
This study was undertaken as part of the Australian and New Zealand Intensive Care Society Clinical Trials Group (ANZICS CTG) Point Prevalence Program and has been endorsed by the ANZICS CTG. It was supported by funding from the Australian and New Zealand Intensive Care Foundation. We thank the study site research coordinators and research staff for collecting the data and staff from the George Institute for Global Health for managing and cleaning the data. Site-based contributors are listed online (Supplementary Appendix, Table S1). We also thank those who reviewed the manuscript during the peer-review process for their comments and suggestions.
Footnotes
Median values are represented by the horizontal bar in each box, interquartile ranges by the upper and lower limits of each box, and ranges by whiskers.
Supplementary Information

References
- 1.van de Leur J.P., van der Schans C.P., Loef B.G., et al. Discomfort and factual recollection in intensive care unit patients. Crit Care. 2004;8:R467–R473. doi: 10.1186/cc2976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Payen J.F., Bosson J.L., Chanques G., et al. Pain assessment is associated with decreased duration of mechanical ventilation in the intensive care unit: a post hoc analysis of the DOLOREA study. Anesthesiology. 2009;111:1308–1316. doi: 10.1097/ALN.0b013e3181c0d4f0. [DOI] [PubMed] [Google Scholar]
- 3.Chanques G., Jaber S., Barbotte E., et al. Impact of systematic evaluation of pain and agitation in an intensive care unit. Crit Care Med. 2006;34:1691–1699. doi: 10.1097/01.CCM.0000218416.62457.56. [DOI] [PubMed] [Google Scholar]
- 4.Puntillo K.A., Morris A.B., Thompson C.L., et al. Pain behaviors observed during six common procedures: results from Thunder Project II. Crit Care Med. 2004;32:421–427. doi: 10.1097/01.CCM.0000108875.35298.D2. [DOI] [PubMed] [Google Scholar]
- 5.Kyranou M., Puntillo K. The transition from acute to chronic pain: might intensive care unit patients be at risk? Ann Intensive Care. 2012;2:36. doi: 10.1186/2110-5820-2-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Azzam P.N., Alam A. Pain in the ICU: a psychiatric perspective. J Intensive Care Med. 2013;28:140–150. doi: 10.1177/0885066611432417. [DOI] [PubMed] [Google Scholar]
- 7.Sigakis M.J., Bittner E.A. Ten myths and misconceptions regarding pain management in the ICU. Crit Care Med. 2015;43:2468–2478. doi: 10.1097/CCM.0000000000001256. [DOI] [PubMed] [Google Scholar]
- 8.Rababa M., Al-Sabbah S., Hayajneh A.A. Nurses' perceived barriers to and facilitators of pain assessment and management in critical care patients: a systematic review. J Pain Res. 2021;14:3475–3491. doi: 10.2147/JPR.S332423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Barr J., Fraser G.L., Puntillo K., et al. Clinical practice guidelines for the management of pain, agitation, and delirium in adult patients in the intensive care unit. Crit Care Med. 2013;41:263–306. doi: 10.1097/CCM.0b013e3182783b72. [DOI] [PubMed] [Google Scholar]
- 10.Devlin J.W., Skrobik Y., Gelinas C., et al. Clinical practice guidelines for the prevention and management of pain, agitation/sedation, delirium, immobility, and sleep disruption in adult patients in the ICU. Crit Care Med. 2018;46(e82):5–73. doi: 10.1097/CCM.0000000000003299. [DOI] [PubMed] [Google Scholar]
- 11.Stites M. Observational pain scales in critically ill adults. Crit Care Nurse. 2013;33:68–78. doi: 10.4037/ccn2013804. [DOI] [PubMed] [Google Scholar]
- 12.Harris P.A., Taylor R., Thielke R., et al. Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Knaus W.A., Draper E.A., Wagner D.P., Zimmerman J.E. APACHE II: a severity of disease classification system. Crit Care Med. 1985;13:818–829. [PubMed] [Google Scholar]
- 14.Gélinas C., Fillion L., Puntillo K.A., et al. Validation of the critical- care pain observation tool in adult patients. Am J Crit Care. 2006;15:420–427. [PubMed] [Google Scholar]
- 15.Chanques G., Payen J.F., Mercier G., et al. Assessing pain in non-intubated critically ill patients unable to self report: an adaptation of the Behavioral Pain Scale. Intensive Care Med. 2009;35:2060–2067. doi: 10.1007/s00134-009-1590-5. [DOI] [PubMed] [Google Scholar]
- 16.Gélinas C. [The faces pain thermometer: a new tool for critically ill adults] [French] Perspect Infirm. 2007;4:12–20. [PubMed] [Google Scholar]
- 17.Kabes A.M., Graves J.K., Norris J. Further validation of the nonverbal pain scale in intensive care patients. Crit Care Nurse. 2009;29:59–66. doi: 10.4037/ccn2009992. [DOI] [PubMed] [Google Scholar]
- 18.Faculty of Pain Medicine . ANZCA; Melbourne: 2021. Australian and New Zealand College and Anaesthetists. Opioid dose equivalence calculation table, PS01 (PM) (Appendix)https://www.anzca.edu.au/getattachment/6892fb13-47fc-446b-a7a2-11cdfe1c9902/PS01(PM)-(Appendix)-Opioid-Dose-Equivalence-Calculation-Table#page= (viewed Sep 2021) [Google Scholar]
- 19.Wickham H., Averick M., Bryan J., et al. Welcome to the tidyverse. J Open Source Softw. 2019;4:1686. [Google Scholar]
- 20.Wickham H. 2nd ed. Springer International Publishing; Cham: 2016. ggplot2: elegant graphics for data analysis. [Google Scholar]
- 21.Sjoberg DD, Curry M, Hannum M, et al. gtsummary: presentation-ready data summary and analytic result tables. https://CRAN.R-project.org/package=gtsummary (viewed March 2022).
- 22.Elliott D., Aitken L.M., Bucknall T.K., et al. Patient comfort in the intensive care unit: a multicentre, binational point prevalence study of analgesia, sedation and delirium management. Crit Care Resusc. 2013;15:213–219. [PubMed] [Google Scholar]
- 23.Payen J.F., Chanques G., Mantz J., et al. Current practices in sedation and analgesia for mechanically ventilated critically ill patients: a prospective multicenter patient-based study. Anesthesiology. 2007;106:687–695. doi: 10.1097/01.anes.0000264747.09017.da. quiz 891-2. [DOI] [PubMed] [Google Scholar]
- 24.Kemp H.I., Bantel C., Gordon F., et al. Pain Assessment in INTensive care (PAINT): an observational study of physician- documented pain assessment in 45 intensive care units in the United Kingdom. Anaesthesia. 2017;72:737–748. doi: 10.1111/anae.13786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu K., Nakamura K., Katsukawa H., et al. ABCDEF bundle and supportive ICU practices for patients with coronavirus disease 2019 infection: an international point prevalence study. Crit Care Explor. 2021;3 doi: 10.1097/CCE.0000000000000353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Georgiou E., Hadjibalassi M., Lambrinou E., et al. The impact of pain assessment on critically ill patients' outcomes: a systematic review. Biomed Res Int. 2015;201(5):503830. doi: 10.1155/2015/503830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wunsch H., Hill A.D., Fu L., et al. New opioid use after invasive mechanical ventilation and hospital discharge. Am J Respir Crit Care Med. 2020;202:568–575. doi: 10.1164/rccm.201912-2503OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Girard T.D., Thompson J.L., Pandharipande P.P., et al. Clinical phenotypes of delirium during critical illness and severity of subsequent long-term cognitive impairment: a prospective cohort study. Lancet Respir Med. 2018;6:213–222. doi: 10.1016/S2213-2600(18)30062-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shehabi Y., Howe B.D., Bellomo R., et al. Early sedation with dexmedetomidine in critically ill patients. N Engl J Med. 2019;380:2506–2517. doi: 10.1056/NEJMoa1904710. [DOI] [PubMed] [Google Scholar]
- 30.Burry L.D., Williamson D.R., Perreault M.M., et al. Analgesic, sedative, antipsychotic, and neuromuscular blocker use in Canadian intensive care units: a prospective, multicentre, observational study. Can J Anesth. 2014;61:619–630. doi: 10.1007/s12630-014-0174-1. [DOI] [PubMed] [Google Scholar]
- 31.Shehabi Y., Bellomo R., Kadiman S., et al. Sedation intensity in the first 48 hours of mechanical ventilation and 180-day mortality: a multinational prospective longitudinal cohort study. Crit Care Med. 2018;46:850–859. doi: 10.1097/CCM.0000000000003071. [DOI] [PubMed] [Google Scholar]
- 32.Payen J.F., Genty C., Mimoz O., et al. Prescribing nonopioids in mechanically ventilated critically ill patients. J Crit Care. 2013;28(534):e7–12. doi: 10.1016/j.jcrc.2012.10.006. [DOI] [PubMed] [Google Scholar]
- 33.Hofer M.M., Wieruszewski P.M., Nei S.D., et al. Intensive care unit sedation practices at a large, tertiary academic center. J Intensive Care Med. 2021 doi: 10.1177/08850666211067515. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 34.Casamento A.J., Serpa Neto A., Young M., et al. A phase II cluster-crossover randomized trial of fentanyl vs. morphine for analgosedation in mechanically ventilated patients. Am J Respir Crit Care Med. 2021;204:1286–1294. doi: 10.1164/rccm.202106-1515OC. [DOI] [PubMed] [Google Scholar]
- 35.Moran B.L., Myburgh J.A., Scott D.A. The complications of opioid use during and post-intensive care admission: a narrative review. Anaesth Intensive Care. 2022;50:108–126. doi: 10.1177/0310057X211070008. [DOI] [PubMed] [Google Scholar]
- 36.Oberhofer D., Skok J., Nesek-Adam V. Intravenous ketoprofen in postoperative pain treatment after major abdominal surgery. World J Surg. 2005;29:446–449. doi: 10.1007/s00268-004-7612-0. [DOI] [PubMed] [Google Scholar]
- 37.Yang Y., Young J.B., Schermer C.R., Utter G.H. Use of ketorolac is associated with decreased pneumonia following rib fractures. Am J Surg. 2014;207:566–572. doi: 10.1016/j.amjsurg.2013.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pandey C.K., Bose N., Garg G., et al. Gabapentin for the treatment of pain in Guillain-Barre syndrome: a double-blinded, placebo- controlled, crossover study. Anesth Analg. 2002;95:1719–1723. doi: 10.1097/00000539-200212000-00046. table of contents. [DOI] [PubMed] [Google Scholar]
- 39.Pandey C.K., Raza M., Tripathi M., et al. The comparative evaluation of gabapentin and carbamazepine for pain management in Guillain-Barre syndrome patients in the intensive care unit. Anesth Analg. 2005;1(01):220–225. doi: 10.1213/01.ANE.0000152186.89020.36. table of contents. [DOI] [PubMed] [Google Scholar]
- 40.Groth C., Droege C., Connor K., et al. Effects of ketamine on pain, sedation, and delirium in the intensive care unit (Ketamine-ICU Study) [abstract] Crit Care Med. 2020;48(1 Suppl):24. [Google Scholar]
- 41.Guillou N, Tanguy M le, Seguin P, et al. The effects of small-dose ketamine on morphine consumption in surgical intensive care unit patients after major abdominal surgery. Anesth Analg 2003; 843-7. [DOI] [PubMed]
- 42.Buchheit J.L., Yeh D.D., Eikermann M., Lin H. Impact of low-dose ketamine on the usage of continuous opioid infusion for the treatment of pain in adult mechanically ventilated patients in surgical intensive care units. J Intensive Care Med. 2019;34:646–651. doi: 10.1177/0885066617706907. [DOI] [PubMed] [Google Scholar]
- 43.Pendleton K., Stephenson L.E., Goeden N., et al. Ketamine infusion for ICU sedation and analgesia: a multicenter evaluation [abstract] Am J Respir Crit Care Med. 2020;201:A3582. doi: 10.1155/2022/9853344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Perbet S., Verdonk F., Godet T., et al. Low doses of ketamine reduce delirium but not opiate consumption in mechanically ventilated and sedated ICU patients: a randomised double-blind control trial. Anaesth Crit Care Pain Med. 2018;37:589–595. doi: 10.1016/j.accpm.2018.09.006. [DOI] [PubMed] [Google Scholar]
- 45.Nishimori M., Ballantyne J.C., Low J.H.S. Epidural pain relief versus systemic opioid-based pain relief for abdominal aortic surgery. Cochrane Database Syst Rev. 2006;3:CD005059. doi: 10.1002/14651858.CD005059.pub2. [DOI] [PubMed] [Google Scholar]
- 46.Stundner O., Memtsoudis S.G. Regional anesthesia and analgesia in critically ill patients: a systematic review. Reg Anesth Pain Med. 2012;37:537–544. doi: 10.1097/AAP.0b013e3182625f1a. [DOI] [PubMed] [Google Scholar]
- 47.O'Connell K.M., Quistberg D.A., Tessler R., et al. Decreased risk of delirium with use of regional analgesia in geriatric trauma patients with multiple rib fractures. Ann Surg. 2018;268:534–540. doi: 10.1097/SLA.0000000000002929. [DOI] [PubMed] [Google Scholar]
- 48.Pöpping D.M., Elia N., Van Aken H.K., et al. Impact of epidural analgesia on mortality and morbidity after surgery: systematic review and meta-analysis of randomized controlled trials. Ann Surg. 2014;259:1056–1067. doi: 10.1097/SLA.0000000000000237. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials