Graphical abstract
Keywords: Chaga mushroom, Triterpenoid, Cytotoxicity, Cancer, IC50
Highlights
-
•
Chaga mushroom contain pharmaceutical and nutraceutical against cancer.
-
•
Triterpenoid constituents of Chaga mushrooms possess cytotoxicity to cancer cells.
-
•
Inotodiol and 3-β-22 α-dihydroxylanosta-8, 25-diene-24-one are the most potent.
-
•
Potential exists for triterpenoid supplementation as an adjunct to cancer treatment.
Abstract
Cancer has become the second leading cause of death in the world. Integrative cancer therapy management is continuously evolving to enhance treatment outcomes. Chaga mushroom (Inonotus obliquus) is a parasitic fungus acclaimed to contain pharmaceutical and nutraceutical value in the fight against cancer. In particular, triterpenoid constituents derived from Chaga mushrooms have been recognized for their anti-cancer activity after distinguished cytotoxicity was repeatedly observed in cancer cells treated in vitro with lipophilic fractions of extract compared to aqueous ones. Studies that investigate the anti-cancer activity of Chaga mushroom triterpenoids are reviewed in this article to determine which cancer cell lines demonstrate the greatest susceptibility to them while highlighting the structure–activity relationships that are involved. Triterpenoid supplementation as an adjunct to cancer treatment may be a viable option as inotodiol and 3-β-22 α-dihydroxylanosta-8, 25-diene-24-one have been shown to exhibit anti-cancer activity similar to that of conventional drugs. Advances in addressing bioavailability challenges are also included in this review as studies include in vivo components.
Introduction
Chaga mushroom (Inonotus obliquus) is a parasitic white rot fungus in the Hymenochaetaceae family under the Basidiomycota division (Agnestisia et al., 2019, Lee et al., 2008). Visible sterile conks or sclerotia of densely aggregated mycelia form on the trunks of broad-leaved deciduous angiosperms, namely birch, that contain a vast range of metabolites of nutraceutical and pharmaceutical value (Kim et al., 2011, Campbell and Davidson, 1938; Lee et al., 2008, Jiang et al., 2020, Balandaykin and Zmitrovich, 2015, Kim et al., 2020, Lau and Abdullah, 2016, Willetts and Bullock, 1992). After a suitable living host tree incurs an injury, Chaga mushroom penetrates the wound where decay spreads throughout the heartwood for years during the anamorphic stage (Lee et al., 2008). The spore-forming sexual reproducing resupinate layers of Chaga mushroom initiate underneath the bark of dead wood (Lee et al., 2008) and are rarely detected as they promptly attract mycophagous beetles such as Orchesia cultriformis that may act as vectors to disperse viable spores elsewhere (Bunyard, 2015). Chaga mushroom is widely available in the cold circumboreal regions of the northern hemisphere (Lee et al., 2008), and has been consumed for centuries, especially by Khanty people in Russia, for health maintenance and hygiene (Saar, 1991).
Chaga mushroom sclerotium is typically harvested during the winter months for the retrieval of bioactive compounds which have been acclaimed to exhibit protective effects against diabetes (Zhang et al., 2018), inflammation (Kou et al., 2021), hepatoxicity (Alzand et al., 2018), and cancer (Alzand et al., 2018, Zhao et al., 2015; Drąg-Zalesińska et al., 2017; Wold et al., 2020, Ma et al., 2013, Wang et al., 2022, Lee et al., 2021, Tanaka et al., 2011, Li et al., 2010).
Trending research has led to the corroboration of traditional claims on the efficacy of Chaga mushroom-derived compounds in exhibiting anti-cancer effects (Zhao and Zheng, 2021). In Japan, the habitual intake of Chaga mushroom infusion is estimated to be around 6 mg/kg/day, and tumor growth was suppressed in mice administered Chaga mushroom extract at this dose for 3 weeks prior to and 16 days after Lewis lung carcinoma (3LL) cell implantation (Arata et al., 2016). Bioactive constituents found in Chaga mushroom extract include polysaccharides (Jiang et al., 2020), organic acids (Glamočlija et al., 2015), an extensive array of phenolic and isoprenoid compounds (Glamočlija et al., 2015), and most notably, triterpenoids (Zhao et al.,2015). While inhibitory effects against cancer exist for aqueous extracts (Arata et al., 2016, Géry et al., 2018), Chaga mushroom triterpenoids and sterols have taken center stage in current research as studies share similar findings regarding their prominent cytotoxicity toward various cancer cell types without impacting normal cells (Kang et al., 2015, Ma et al., 2013, Kim et al., 2020, Nomura et al., 2008). Zhao and Zheng (2021) reviewed the antitumoral potential of Chaga mushroom extracts and concluded the potential of their bioactive metabolites to reduce the incidence of tumorigenesis in healthy people. These findings are encouraging as cancer remains a serious public health concern (Ferlay et al., 2021), and prognoses may be exacerbated following the use of conventional therapies alone (Schirrmacher, 2018).
To address the increasing need for alternative cancer treatments, research has further sought to identify the most effective triterpenoids in Chaga mushroom extract to exhibit anti-cancer activity and associate them with their most susceptible cancer cell lines. To date, the cancer cell lines A549 (Zhao et al., 2015, Wang et al., 2022), 4 T1 (Zhao et al., 2016), MCF-7 (Zhao et al., 2016), HT29-MTX (Li et al., 2010, Wold et al., 2020), Me-45 (Drąg-Zalesińska et al., 2017), KB (Alzand et al., 2018), BEL-7402 (Alzand et al., 2018), L1210 (Tanaka et al., 2011), MDA-MD-231 (Lee et al., 2021), SK-BR 3 (Lee et al., 2021), and PC3 (Ma et al., 2013) have been used to demonstrate significant susceptibility to triterpenoid and steroid derivatives isolated from Chaga mushroom with half maximal inhibitory concentrations (IC50) ≤ 10 µM (Table 1).
Table 1.
Half maximal inhibitory concentration (IC50 ≤ 10 µM) results after treatment with Chaga mushroom triterpenoids and steroid derivatives.
| Triterpenoid or steroid derivative | Cancer cell line | Cancer type | Incubation time (h) |
IC50 (µM) |
Reference |
|---|---|---|---|---|---|
| 3-β-22 α-dihydroxylanosta-8, 25-diene-24-one | A549 KB |
Human lung cancer Human squamous cell carcinoma (SCC) |
96 96 |
5.39 9.60 |
Zhao et al., 2015, Alzand et al., 2018 |
| Betulinic acid | HT29-MTX NCI-H460 |
Human colon cancer Human lung cancer |
48 24 |
0.8 2.1 |
Li et al., 2010, Wold et al., 2020 |
| Betulin |
NCI-H460 HT29-MTX |
Human lung cancer Human colon cancer |
24 24 |
2.8 1.6 |
Wold et al., 2020 |
| Inonotsutriol A | A549 | Human lung cancer | 96 | 2.34 | Zhao et al., 2015 |
| Inonotsutriol D | A549 L1210 |
Human lung cancer Mouse lymphocytic leukemia |
96 72 |
8.39 10 |
Zhao et al., 2015, Tanaka et al., 2011 |
| Inonotsutriol E | A549 | Human lung cancer | 96 | 1.63 | Zhao et al., 2015 |
| Inotodiol | HT29-MTX NCI-H460 SK-BR3, MDA-MD-231, MCF-7 |
Human colon cancer Human lung cancer Human breast cancer Human breast cancer Human breast cancer |
24 24 48 48 48 |
3.8 4.7 4.06 4.74 |
Wold et al., 2020, Lee et al., 2021 |
| Inonotusane D | 4 T1 | Mouse breast cancer | 48 | 9.4 | Zhao et al., 2016 |
| 4- (3,4 dihydroxyphenyl) but-3-en-2-one | BEL-7402 | Human liver cancer | 96 | 4.5 | Alzand et al., 2018 |
| Ornithine ester of betulin | Me-45 | Human melanoma | 72 | 2.47 | Drąg-Zalesińska et al., 2017 |
| Lysine ester of betulin | Me-45 | Human melanoma | 72 | 2.46 | Drąg-Zalesińska et al., 2017 |
| 3° amine group betulin at C-28 |
MGC-803 PC3 Bcap-37 MCF-7 |
Human gastric cancer Human prostate cancer Human breast cancer Human breast cancer |
72 72 72 72 |
8.5 9.6 6.7 9.2 |
Yang et al., 2015 |
| Pyrrolidine group betulin C-28 |
MGC-803 PC3 Bcap-37 |
Human gastric cancer Human prostate cancer Human breast cancer |
72 72 72 |
8.9 8.3 7.9 |
Yang et al., 2015 |
| Piperidine group betulin at C-28 |
MGC-803 PC3 Bcap-37 A375 MCF-7 |
Human gastric cancer Human prostate cancer Human breast cancer Human melanoma Human breast cancer |
72 72 72 72 72 |
4.3 4.5 5.2 7.5 5.2 |
Yang et al., 2015 |
| Heterocyclic group betulin at C-28 |
MGC-803 Bcap-37 |
Human gastric cancer Human breast cancer |
72 72 |
9.2 6.4 |
Yang et al., 2015 |
| Inonotsutriol C | 4 T1 | Mouse breast cancer | 48-72a | 8.90 | Wang et al., 2022 |
| Inonotusol G | KB | SCC | 96 | 9.9 | Liu et al., 2014 |
| (20R,21S,24S)21,24-cyclopenta-3β,21,25-trihydroxylanosta-8-ene | A549 4 T1 |
Human lung cancer Mouse breast cancer |
48-72a 48-72a |
7.80 8.20 |
Wang et al., 2022 |
| (23E)-Lanosta-8,23-diene-3β,25-dihydroxy-21-al | A549 | Human lung cancer | 48-72a | 7.80 | Wang et al., 2022 |
| Saponaceoic acid I | MCF-7 4 T1 |
Human breast cancer Mouse breast cancer |
48 48 |
8.35 7.79 |
Zhao et al., 2016 |
| 9,11-dehydroergosterol peroxide | MCF-7 4 T1 |
Human breast cancer Mouse breast cancer |
48 48 |
8.40 9.31 |
Zhao et al., 2016 |
| Ergosterol peroxide | MCF-7 | Human breast cancer | 48 | 9.06 | Kim et al., 2011 |
| Ergosterol | PC3 | Human prostate cancer | 72b | 9.82 | Ma et al., 2013 |
IC50 denotes a 50% reduction in cell viability compared to untreated cells after incubation. IC50 values are expressed in µM (µmol/L). a resazurin reduction test bMTS assay.
Due to the diverse nature of triterpenoids, this review takes a closer look at the structure–activity relationships that exist for anticancer, anti-inflammatory, and immunomodulatory capabilities. Factors that influence bioavailability and application as a potential adjunct to minimally invasive cancer therapies are also discussed.
Nutritional value and bioactive compounds
Chaga mushroom sclerotia contain a variety of myco chemicals with medicinal value and nutraceutical properties. Chaga mushroom composition varies, but some extracts have been found to contain trace amounts of oxygenated aliphatic substances like linoleic acid, the presence of fat-soluble vitamin E, approximately 7 % protein, and a fair assortment of minerals including calcium, sodium, magnesium, and boron (Petrović et al., 2019, Ayoub et al., 2009; Abu-Reidah et al., 2021). Extracts of Chaga mushroom have been reported to contain as much as 57–70 % polysaccharides (Jiang et al., 2020), many of which make up insoluble fibers with cross-linked chitin and β-1,3-glucans that support its unyielding cell walls (Rhee et al., 2008). A wide range of phenolic compounds exist in Chaga mushroom sclerotia including but not limited to gallic acid, protocatechuic acid, 3,4–di-hydroxybenzaldehyde, caffeic acid, 2,5-dihydroxy terephthalic acid, syringic acid, and vanillic acids (Abu-Reidah et al., 2021; Hwang et al., 2019, Alzand et al., 2018, Glamočlija et al., 2015). The sterile conks have a rough textured exterior that appears to resemble burnt charcoal (Géry et al., 2018), which can be attributed to the high melanin content of the 1,8-dihydroxynaphthalene (DHN) type (Wold et al., 2020). Melanin deposition normally occurs during sclerotium maturation as seen in other fungal species besides Chaga mushroom (Willetts and Bullock, 1992).
Chaga mushroom contains a variety of triterpenoids, mainly of the lanostane type, that have been observed to exhibit anticancer activity. Inotodiol was found to be the most abundant triterpenoid extracted from Chaga mushrooms followed by trametenolic acid (Kim et al., 2020, Zhao et al., 2016). The dark texturized outer layer of Chaga mushroom tends to be discarded during harvest but might prove serviceable in the extraction process as Kim et al. (2020) discovered that it contains higher concentrations of betulinic acid and inotodiol in relation to the interior. More triterpenoid structures isolated from Chaga mushroom extract are being recognized for their biological activity. Kou et al. (2021) isolated seven additional lanostane triterpenes named inonotusols H-N (1–7) with 21, 24-cyclopentanol moiety in the side chain from Chaga mushroom ethanol extract that showed considerable inhibitory activity by Griess reaction of nitric oxide generation in lipopolysaccharide (LPS)-induced BV-2 microglial cells with IC50 ranging from 2.3 to 23.8 µM. Even triterpenoids from other natural sources are being acknowledged for their antineoplastic properties. An oleanane-type triterpenoid saponin, known as raddeanin A, isolated from Anemone raddeana Regal was found to have cytotoxic effects against different cancer cell types (Naz et al., 2020). Garádi et al. (2021) recently studied a lesser-known species closely related to Chaga mushroom, named Inonotus nidus-pici, containing ergostane-type triterpenoids that were found to exhibit cytotoxic activity towards MES-SA, MES-SA/Dx5, and A431 cells with IC50 between 36.2 and 83.2 µM. Betulin and betulinic acid are lupane-type triterpenoids that developed in Chaga mushrooms through convergent evolution (Safronov, 2022). Chaga mushrooms generally contain more betulinic acid than birch bark and vice versa (Safronov, 2022). Triterpenoid profile and content extracted from Chaga mushrooms and other sources vary significantly depending on environmental factors such as the geographical location of harvest (Géry et al., 2018, Zhou et al., 2021). For instance, Chaga mushroom from Normandy (northern France) was found to contain more betulin and betulinic acid, but less inotodiol when compared to their Canadian counterparts (Géry et al., 2018). Harsh growth conditions and environmental stress were shown to increase triterpenoid concentration and diversity while cultured Chaga mushroom mycelia in a medium consisted of fewer bioactive compounds (Zheng et al., 2007, Razumov et al., 2019).
While inhibitory effects against cancer exist for aqueous extracts (Arata et al., 2016, Géry et al., 2018), Chaga mushroom triterpenoids have been recognized for their anti-cancer activity as distinguished cytotoxicity was repeatedly observed in cancerous cells treated with lipophilic fractions of hexane, petroleum ether, chloroform, and dichloromethane, displaying IC50 compared to aqueous fractions (Kang et al., 2015, Kim et al., 2020, Baek et al., 2018, Nomura et al., 2008). Triterpenes are routinely identified as being the main constituents in these fractions due to their relatively low water solubility (Glamočlija et al., 2015). Quantification of triterpenoids is generally completed with the use of liquid chromatography-mass spectrometry (HPLC-MS), and 1H nuclear magnetic resonance (NMR) data are often referred to for structural elucidation. Triterpenoid compounds continue to be discovered in Chaga mushroom extracts as studies optimize fractionation procedures, employ advanced analytical techniques, and maximize extraction efficiency (Alzand et al., 2018).
Formation of triterpenes and their classification
Isoprenes are the basic constituents that form terpenoids, a diverse group of ubiquitous organic compounds involved in metabolic processes, biological activities, and the formation of structures in cellular membranes. A monoterpene is an isoprene dimer, and the number of terpenes that exist in an organic compound is denoted by a prefix. Hence, triterpenes contain six isoprene units and a total of 30 carbons. Terpenoids are derivatives of organic terpene hydrocarbons that contain characteristic functional groups, while triterpenoid saponins refer to triterpene glycosides (Zhao et al., 2010).
The pattern of isoprene head-to-tail addition in the formation of higher molecular terpenoid structures, discovered by Leopold Ruzika, is known as the isoprene rule (Hillier and Lathe, 2019). The active isoprene building block, isopentyl pyrophosphate, is first synthesized in the mitochondria via the mevalonate pathway or the cytoplasm by the non-mevalonate pathway depending on the organism or physiological conditions (Zhao et al., 2010, Kushiro and Ebizuka, 2010). Pacbio genome sequencing of Chaga mushroom and associated birch from the Merikarvia region in Finland revealed CYP450 monooxygenase enzymes involved in mevalonate pathways for betulinate biosynthesis (Safronov et al., 2021). Cytochrome CYP505 monooxygenases are responsible for encoding enzymes that facilitate the formation of terpenoid metabolites in Chaga mushrooms as well as other fungi (Safronov, 2022). Terpene and terpenoid biosynthesis occur through a series of enzymatic reactions that are initiated when isopentyl diphosphate and its isomer dimethylallyl diphosphate are joined through condensation to form the monoterpene or isoprene dimer (C10), geranyl diphosphate (Faylo et al., 2021, Gill and Kumar, 2016). The addition of another active isopentyl pyrophosphate generates farnesyl diphosphate (C15), which can act solely as a precursor for sesquiterpenes (C15) or create squalene (C30) by dimerization (Gill and Kumar, 2016, Brodelius et al., 2002). Squalene is a triterpenoid precursor that undergoes cyclization to form a variety of ring systems with a vast combination of terpene skeletal diversity (Brodelius et al., 2002; Kushiro and Ebizuka, 2010, Xu et al., 2004). Triterpenes are subdivided by the number of rings present in their structure and further differentiated by their stereochemical configuration. Tetracyclic and pentacyclic triterpenoids (4 and 5 ring containing triterpenoids) are abundantly found in nature (Xu et al., 2004) and are studied extensively for their anti-cancer activity (Gill and Kumar, 2016). While many dicotyledonous plants synthesize triterpenoids in response to infection for host defense (Osbourn, 1996), parasitic fungi metabolize triterpenoids from their host and synthesize others to modulate plant growth and provide protection against environmental stressors (Jan et al., 2021).
Anti-cancer activity of triterpenoids
It has been deduced that triterpenoids are responsible for the antineoplastic properties of Chaga mushroom extracts as significant dose-dependent cytotoxicity was repeatedly observed in cancerous cells treated with lipophilic fractions of hexane, petroleum ether, chloroform, ethyl acetate, and dichloromethane, displaying IC50 compared to aqueous fractions (Kang et al., 2015, Kim et al., 2020, Baek et al., 2018, Ma et al., 2013). Methanol and ethanol extracts are generally the primary solvents used for crude extraction prior to the fractionization of Chaga mushroom triterpenoid constituents. Solvent-partitioned fractions of hexane and dichloromethane featured greater cytotoxicity on cancer cells than crude methanol extract (Kahlos et al., 1987, Baek et al., 2018). Ma et al. (2013) recognized the increased cytotoxicity of petroleum ether fractions against PC3 and MDA-MB-231 cancer cells tested in vitro. Compared to hexane and ethyl acetate, dichloromethane fractions were found to be superior at inhibiting proliferation through cell cycle arrest at the G1 phase in HT-29 cells (Lee et al., 2015). Cytotoxic concentrations of chloroform-extracted fractions were found to be similar to inotodiol (Nomura et al., 2008). The anti-cancer activity of Chaga mushroom-extracted triterpenoids has been routinely observed through in vitro growth inhibition assays, usually by MTT colorimetric method (Ni et al., 2009). Half maximal inhibitory concentrations reported to be less than 10 µM in cancer cells treated with triterpenoids are outlined in Table 1. Chaga mushroom-derived triterpenoids have been widely studied for their inhibitory effect on cancer cell types such as lung adenocarcinoma cells (Baek et al., 2018, Chung et al., 2010, Wold et al., 2020, Li et al., 2010, Zhao et al., 2020, Wang et al., 2022), colorectal adenocarcinoma cells (Wold et al., 2020, Zhao et al., 2015, Kim et al., 2020, Li et al., 2010, Tsai et al., 2017), hepatoma cell lines (Zhuo et al., 2018, Li et al., 2010, Alzand et al., 2018), human epithelial carcinoma cells (Alzand et al., 2018), melanoma cancer cells (Drąg-Zalesińska et al., 2017), cervical cancer cells (Chung et al., 2010, Wang et al., 2022, Zhang et al., 2019, Li et al., 2010), breast cancer cells (Chung et al., 2010, Lee et al., 2021, Li et al., 2010, Wang et al., 2022), prostate cancer cells (Li et al., 2010, Kim et al., 2020), leukemia cells (Nomura et al., 2008, Li et al., 2010, Zhuo et al., 2018, Handa et al., 2012), and gastric cancer cells (Chung et al., 2010, Wang et al., 2022, Kim et al., 2020).
In some cases, triterpenoids have demonstrated inhibitory concentrations similar to conventional drugs alone (Alzand et al., 2018, Zhang et al., 2019). HeLa cells treated with inotodiol were inhibited almost to the same extent as those treated with 5-fluorouracil, a chemotherapy drug (Zhang et al., 2019). Similarly, 3-β-22 α-dihydroxylanosta-8, 25-diene-24-one and 5-fluorouracil displayed cytotoxic activity against KB epithelial cell types producing IC50 of 9.6 µM and 8.1 µM, respectively (Alzand et al., 2018). The effects of standard breast cancer treatment drugs tamoxifen, lapatinib, and doxorubicin combined with inotodiol- and trametenolic acid-enriched fractions displayed synergistic or additive cytotoxic effects (Lee et al., 2021). An additional lanostane triterpenoid was recently found to have a cytostatic effect on gastric adenocarcinoma cell line BCG-823 with an average IC50 of 12.0 µM, which is lower than that of suberoylanilide hydroxamic acid at 12.4 µM (Wang et al., 2022).
Research has progressed in identifying bioactive constituents that contain anti-cancer activity by producing consistent reports on similar cancer cell types. For instance, there are numerous reports on the antiproliferative effects of Chaga mushroom triterpenoids on breast cancer MCF-7 cells. Cytotoxic effects were observed on MCF-7 cell lines at 300 µg/mL from a triterpenoid fraction consisting of betulin, betulinic acid, trametenolic acid, and inotodiol (Kim et al., 2020). An even greater reduction in cell viability was observed by Lee et al. (2021) in MCF-7 cell lines treated with fractions of inotodiol and trametemolic acid at concentrations lower than 10 µg/mL. Inotodiol-enriched fractions were three times more cytotoxic than trametenolic acid against this cancer cell lines with IC50 between 1.8 and 2.1 µg/mL and 6–7 µg/mL, respectively (Lee et al., 2021). Silica gel CC eluted with chloroform, methanol, and water (13:0.95:0.05) combined with RP-HPLC with methanol and water (83:17) by Zhao et al. (2016) yielded a fraction containing saponaceoic acid I that inhibited MCF-7 cells with an IC50 concentration of 8.35 µM (Table 1). The cytotoxicity of chaga mushroom-derived triterpenoids differs in magnitude and specificity. Although saponaceoic acid I may demonstrate significant cytotoxicity towards MCF-7 cells, no effect was observed in lung cancer cell lines Calu-6, H1299, A549, and H1264 (Baek et al., 2018).
Chaga mushroom triterpenoids are further described here in relation to one another in terms of their cytotoxicity starting with the tetracyclic triterpenoid 3b-hydroxylanosta-8,24 -dien-21-al that has exclusively been attributed to chaga mushrooms since initial reports in 1984 (Kahlos et al., 1984, Wold et al., 2020). It demonstrates greater water solubility compared to other Chaga mushroom-extracted triterpenoids and was found to exhibit stronger inhibitory activity against cancer cell lines A549, AGS, MCF-7, and HeLa than subfractions containing inotodiol and lanosterol (Chung et al., 2010, Kahlos et al., 1984). In vivo observations from the same study conducted by Chung et al. (2010) coincided with in vitro findings where 3b-hydroxylanosta-8, 24-dien-21-al inhibited tumor growth to a greater extent than inotodiol and lanosterol with a 33.7 % reduction in tumor weight compared to the control group in Balbc/c mice bearing Sarcoma-180 cells after oral administration at 0.2 mg per mouse per day for 20 days. Further, inotodiol was not detected to have any effect against lung adenocarcinoma cells compared to 3b-hydroxylanosta-8, 24-dien-21-al (IC50 range of 75–128 µM), trametenolic acid (IC50 range of 184–227 µM), and chagabusone A (IC50 range of 82–141 µM) (Baek et al., 2018). Lung cancer cell lines H1264, Calu 6, and A549 were observed to be highly susceptible to 3b-hydroxylanosta-8, 24-dien-21-al whereas H1299 was significantly inhibited by the triterpenoid identified as chagabusone A to a greater extent in comparison (Baek et al., 2018). This could be due to the fact that H1299 cells are derived from lymph tissue as opposed to lung tissue, and they have been found to express the tumor-modulating ubiquitin C-terminal hydrolase (UCH-L1) protein (Liu et al., 2014). Future studies should investigate the anti-cancer effects of 3b-hydroxylanosta-8, 24-dien-21al due to its enhanced water solubility for in vivo applications.
As studies optimize fractionation procedures with different combinations of solvent eluent ratios, a number of inonotsutriols (A-E) have been extracted from Chaga mushroom (Baek et al., 2018, Wang et al., 2022, Taji et al., 2008, Zhao et al., 2015). In a study conducted by Tanaka et al. (2011), inonotsutriol D was shown to have a broad cytotoxic effect against three leukemia cell lines (P388, HL-60, L1210) and an epidermoid carcinoma cell line (KB) with IC50 between 10 and 24 µM. Murine leukemia cell line L1210 was more vulnerable to Chaga mushroom triterpenoids when compared to P388, HL-60, and KB cells (Tanaka et al., 2011). Inonotsutriol E, D and A were found to strongly inhibit A549 cells (IC50 1.6, 8.4, and 2.3 µM, respectively) by Zhao et al., 2015. However, in another study conducted by Baek et al. (2018), inhibition was not detected in A549 cells treated with inonotsutriol E or A. Inonotsutriols should be further studied to confirm the cytotoxic effects, especially against A549 lung cancer cell lines.
The biological mechanisms involved in the antiproliferative effects of chaga mushroom triterpenoids have been investigated alongside reports of cytotoxicity.
One of the hallmarks of cancerous cells is the ability to evade apoptosis and proliferate uncontrollably (Matsuura et al., 2016, Merlin et al., 2021). Using western blot analysis, several apoptotic regulatory protein expression observations confirm the cascading effects involved in the mitochondrial pathway of induced apoptosis in cancer cells treated with Chaga mushroom-derived triterpenoids (Li et al., 2010, Hordyjewska et al., 2019). As such, protein expression and the nature of cancer cells can be strong indicators for targeted triterpenoid susceptibility. It is becoming more established that many Chaga mushroom triterpenoids induce caspase 3-mediated mitochondrial apoptosis through p53 expression in susceptible cancer cell lines (Nomura et al., 2008, Baek et al., 2018, Chung et al., 2010, Li et al., 2010). In addition to apoptosis, Chaga mushroom triterpenoids have been shown to disrupt the cell cycle by eliciting inhibitory signals at certain phases (Lee et al., 2015, Zhuo et al., 2018). Colorectal carcinoma (HCT-116), lung cancer (HKULC2), and glioblastoma (U87, A172) cell lines treated with Chaga mushroom extracts were found to exit the cell cycle at G0/G1 (Li et al., 2022, Tsai et al., 2017) and in G1 phase (Zhao et al., 2020). The derivative 3,28‑di‑(2‑nitroxy‑acetyl)‑oxy‑betulin was found to inhibit the transition from G2/M phase in Huh7 cells (Zhuo et al., 2018). Some triterpenoids have immunomodulatory effects that may target tumor cells aggressively. Intensified dendritic cell maturity, a greater expression of major histocompatibility (MHC)-II and costimulatory molecules, was observed after treatment with inotodiol through phosphatidylinositol-3-kinase activation without a significant increase of inflammatory cytokine promotion (Maza et al., 2021).
Overall, lung adenocarcinoma, murine breast cancer, human breast cancer, methotrexate-resistant colorectal adenocarcinoma, melanoma, epidermal carcinoma of nasopharynx, hepatocellular carcinoma, murine lymphocytic leukemia, and prostate cancer cells have demonstrated significant susceptibility to triterpenoid treatment at (IC50) ≤ 10 µM (Table 1) Additional research is needed to confirm the level of cytotoxicity in the listed triterpenoids, especially using the MTT colorimetric method with multiple incubation times. Highlighting the structure–function relationships that appear as an indication of anti-cancer activity could aid in the prediction of other strongly associated triterpenoids with cancer cell lines that are more prone to susceptibility.
Structure-function relationships of triterpenoids and biological significance
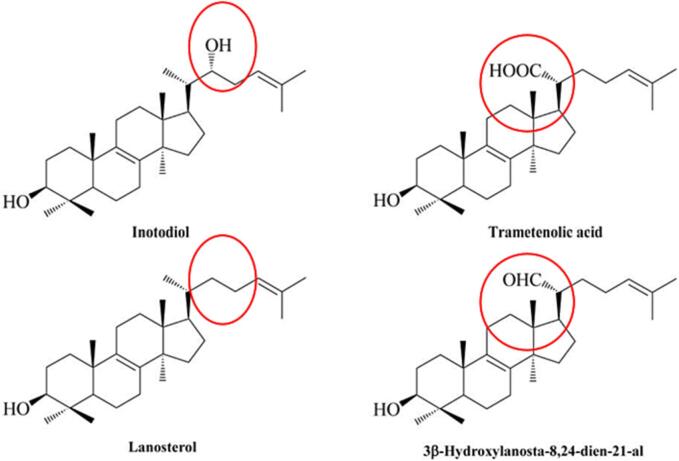
Triterpenoids, while classified broadly, interact uniquely depending on their structure, stereochemical configuration, and polarity (Gill and Kumar, 2016; Hillier and Lathe, 2019). To illustrate, the absence of a hydroxyl group in lanosterol at carbon 22 compared to inotodiol appears to eliminate the capacity of lanosterol to influence dendritic cell maturity (Fig. 1) (Maza et al., 2021). In terms of stereochemistry, the antiproliferative activity between epimeric triterpenoids inonotsutriol E and inonotsutriol D demonstrated IC50 1.6 and 8.7 µM, respectively, against A549 cells (Zhao et al., 2015). Due to the lack of functional groups in lanosterol (Fig. 1), the anticancer activity is hardly reported to the same extent as other Chaga mushroom-derived triterpenoids against cells tested in vitro. Aside from murine leukemia L1210 cell line IC50 37.2 (Zhao et al., 2015), most cancer cell lines fail to exhibit susceptibility to lanosterol. Triterpenoids that share similar polarities such as 3b-hydroxylanosta-8, 24-dien-2-al and inotodiol were found to activate human complement thereby inhibiting serum-induced hemolysis of sheep erythrocytes, whereas trametenolic acid failed to initiate the same cascading effect (Wold et al., 2020). Oncogenic capabilities of the complement system may exist through the facilitation of cellular proliferation and regeneration by dysregulating mitogenic signaling pathways and preventing apoptosis (Rutkowski et al., 2010).
Fig. 1.
Four lanostane-type triterpenoids commonly isolated from Chaga mushrooms (Kim et al., 2020, Zhao et al., 2016). Functional groups present at C-20 and C-22 have been shown to influence biological activity (Maza et al., 2021, Nakata et al., 2007).
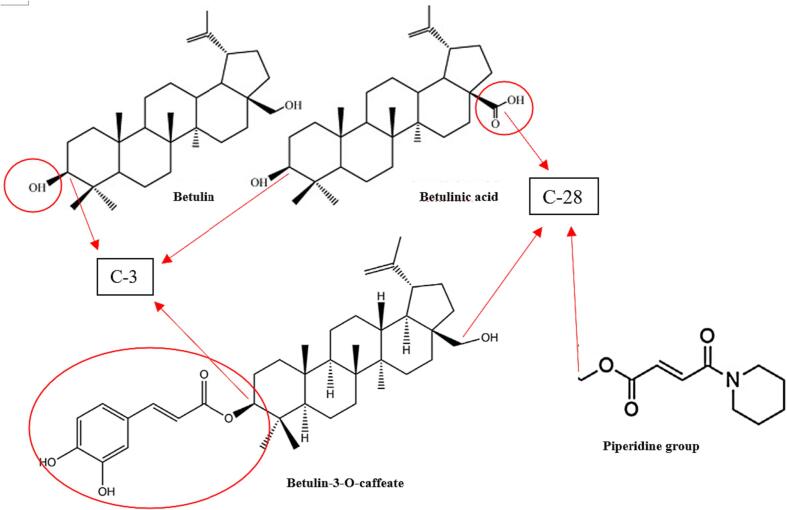
Under certain conditions, characteristic patterns of moiety side chains in triterpenoids can be a strong indicator of biological activity (Yang et al., 2015). In two separate studies, betulin-3-O-caffeate, morolic acid 3-O-caffeate, and betulinic acid 3-O-caffeate were observed to have significant anti-inflammatory effects by reducing nitric oxide levels in murine primary macrophages and LPS-stimulated RAW264.7 cells without impacting cell viability, while betulin alone did not (Jeong et al., 2009; Wold et al., 2020). Moreover, C-21 aldehyde groups existing in lanostane-type triterpenoids (Fig. 1) have been observed to enhance inhibitory effects on the formation of Epstein-Barr virus early antigens activated by tumor promotors (Nakata et al., 2007). Inonotusols with a hydroxyl group at C-28 had a less inhibitory activity of nitric oxide production except when accompanied by a conjugated diene system (Kou et al., 2021). In some cases, biological activities exhibited by triterpenoids may be inversely related. Inotodiol, betulinic acid, and betulin failed to exhibit anti-inflammatory activity, yet demonstrated the greatest cytotoxicity towards colorectal cell line HT29-MTX and lung cancer cell line NCI-460, contrary to 3 beta-22-alpha-dihydroxylanosta-8, 25-diene-24-one and trametenolic acid (Wold et al., 2020). Pentacyclic triterpenoids with polar constituents tend to have higher pharmacological activity. Modifications made to C-3 and C-28 carbon positions in betulin, especially with an added piperidine group at C-28, enhanced cytotoxicity up to four times as much as betulin toward cancer cell lines MGC-803, PC3, Bcap-37, A375, and MCF-7 (Yang et al., 2015). A semi-synthetic derivative of betulin (3, 28 – di-(2-nitroxy-acetly)-oxy–betulin) demonstrated enhanced cytotoxicity toward hepatocellular carcinoma Huh7 cells with an IC50 of 13 ± 1.4 µM (Zhuo et al., 2018). Unlike inotodiol (IC50 of 14 µM), the lanostane triterpenoids trametenolic acid and 3β-hydroxylanosta-8,24-dien-21-al were suggested not to have noticeable anti-proliferative effects for P388 cell lines due to oxygen substitution at C-21 and the lack of a hydroxyl group at C-22 (Nomura et al., 2008). Interestingly, inotodiol with an additional OH group at C-24 (inonotsutriol D, IC50 10 µM) had greater anti-proliferative than insotodiol for P388 cells (Tanaka et al., 2011, Nomura et al., 2008). However, in the study by Tanaka et al. (2011), inotodiol, trametenolic acid, and 3β-hydroxylanosta-8,24-dien-21-al displayed similar cytotoxicity for P388 cells (IC50 to 51 µM).
Chaga mushroom sclerotium contains bioactive secondary metabolites that signify host-pathogen associations as well as convergent evolution (Safronov, 2022, Géry et al., 2018). Betulin and betulinic acid are highly characteristic lupane-type pentacyclic triterpenoids of Chaga mushrooms since they are naturally present in birch bark (Géry et al., 2018; Hordyjewska et al., 2019). Betulin was previously thought to be a relatively inactive constituent. However, betulin and betulinic acid both inhibit cancer cell growth via separate mechanisms (Li et al., 2010). The anticancer effect of betulinic acid has been observed over a wide range of cancer cell types due to the direct effect it has on mitochondrial membrane permeability transition pore (MPTP), whereas betulin cytotoxicity depends on the expression patterns of apoptosis regulating proteins (Li et al., 2010). Compounds that negatively impact MPTP may exhibit toxicity raising safety concerns; thus betulinic acid requires further investigation. Treatment of isolated mitochondria with betulin and betulinic acid confirms this as only betulinic acid-treated mitochondria result in cytochrome c release (Li et al., 2010). Cytotoxic cancer drugs have been reported to facilitate apoptosis by initiating the cytochrome c/Apaf-1/caspase-9-dependent pathway (Druškovič et al., 2006). Enhanced expression of caspase-9 in cancer cell lines such as hepatocellular carcinoma HepG2 was shown to increase their susceptibility to treatment with betulin (Li et al., 2010). In the same study, SK-HEP-1 cells constructed with overexpression of caspase-9 similar to HepG2 cells had enhanced susceptibility to betulin treatment compared to original SK-HEP-1 cells. Li et al. (2022) found betulinic acid to exhibit antiproliferative effects against U87 cells in a dose-dependent manner with IC50 of 12 µg/mL or 26 µM. Furthermore, betulinic acid treatment at 13 µM resulted in approximately 80 % PC3 cell inhibition (Shin et al., 2011). The same study by Shin et al. (2011) found that treatment with 10 µM betulinic acid prevented hypoxia-induced angiogenesis in PC3 cells by ameliorating the signal transducer and activator of transcription-3 (STAT3) and hypoxia-inducible factor (HIF)-1α interaction with vascular endothelial growth factor (VEGF) promotor, thereby reducing VEGF production. Methotrexate-treated (MTX) HT29 colorectal cell lines tend to produce more mucus and inhibit the permeability of lipophilic compounds, but this was not the case for betulinic acid (Behrens et al., 2001, Wold et al., 2020). In fact, betulinic acid treatment of colorectal HT29-MTX resulted in an IC50 of 0.8 µM, which makes this triterpenoid-cancer cell type association the most inhibitory one reported to date.
Betulinic acid is formed through betulin oxidation where the hydroxyl at C-28 is replaced with a carboxyl group as illustrated in Fig. 2. Comparative analysis of betulin and betulinic acid highlights the significance of functional groups and substituents in addition to overall structure when it comes to modulating anti-cancer activity. As noted previously, treatment with inotodiol (tetracyclic), betulin, and betulinic acid (pentacyclic) on colorectal cell line HT29-MTX and lung cancer cell line NCI-460 shared cytotoxic characteristics despite having different skeletal backbones (Wold et al., 2020). In some cases, generalized characteristics of moiety side chains in triterpenoids as an indicator of biological activity could lead to false assumptions. In general, inonotusols that include 21,24-cyclopental moiety in their structure have shown reduced cytotoxicity towards lung cancer cells in vitro compared to triterpenoids that do not, such as inonotsutriol G (Kou et al., 2021, Liu et al., 2014). However, this was not the case for inonotsutriol E, which has demonstrated prominent cytotoxicity toward A549 cells (Zhao et al., 2015).
Fig. 2.
Characteristic lupane-type Chaga mushrooms derived triterpenoids (Safronov et al., 2021; Safronov, 2022; Géry et al., 2018). Esterified functional amine groups at C-28 were found to enhance the cytotoxicity of betulin (Yang et al., 2015). The presence of moiety 3-O-caffeate may increase anti-inflammatory effects by reducing nitric oxide levels (Wold et al., 2020, Jeong et al., 2009). Modifications at C-3 and C-28 have also been made to improve aqueous solubility (Zhao et al., 2020).
Biochemical mechanism(s) of anti-cancer activity
The biochemical mechanism(s) of triterpenoids of Chaga mushroom is dependent on the specific compounds and their various interactions with cell signalling associated with proliferation, apoptosis, and autophagy (Table 2). For example, betulin and betulinic acid derivatives could exert their anticancer effects through different mechanisms, inclusive of induction of apoptosis and autophagy, antiangiogenesis, inhibition of invasion and migration, cell cycle arrest and multidrug resistance reversal (Li et al., 2010, Shin et al., 2011, Wold et al., 2020). However. current understanding of the toxicological mechanism of various triterpenoids and their mixtures exhibit their selective cytotoxicity to various cancer cells is very limited, thus in-depth investigations are required.
Table 2.
Anti-cancer and pharmacological mechanisms of triterpenoid of Chaga mushroom.
| Triterpenoid or steroid derivative | Experimental model | Proposed anticancer mechanism | Reference |
|---|---|---|---|
| Inotodiol and tramatanolic acid-rich extract | Breast cancer cells in vitro and 4T1-tumor-bearing mice | Induced autophagy by activating AMPK and inhibiting the mTOR signaling pathway | Lee et al., 2021 |
| Inotodiol | HeLa cells in vitro | Inhibition of cyclin E and bcl-2 | Zhao et al., 2014 |
| Inotodiol extract | A549 cells in vitro | Arresting cell cycle in the S phase and inhibition of bcl-2 | Zhong et al., 2011 |
| Inonotsutriol E | Various breast cancer cell lines | Inhibition of JAK2/STAT3 signaling pathway | Shan et al., 2023 |
| Betulin | HeLa cells in vitro | Mitochondrial cytochrome c release through expression of apoptosis regulating proteins such as caspases | Li et al., 2010 |
| Betulinic acid | Hypoxic PC3 cell model | Anti-angiogenic effect through disturbing the binding of STAT3 and HIF-1α to the VEGF promotor, thereby reducing VEGF production. | Shin et al., 2011 |
| Betulin and betulinic acid | HT29-MTX in vitro | Anti-proliferative activity | Wold et al., 2020 |
| Ergosterol peroxide | AOM/DSS-treated mice and human colorectal cancer cell lines | Tumor suppression and anti-proliferative activity through down-regulation of β-catenin signaling | Kang et al., 2015 |
A549, human lung cancer cells, 4T1, mouse breast cancer cells; HeLa, human cervical cancer cells; PC3, human prostate cancer cells; HT29-MTX, methotrexate-resistant colon adenocarcinoma cells, bcl2, Antiapoptotic B cell leukemia/lymphoma 2; JAK, Janus kinase; STAT3, signal transducer and activator of transcription 3; HIF-1α, hypoxia-inducible factor-1α; VEGF, vascular endothelial growth factor; AOM/DSS, azoxymethane/dextran sodium sulphate.
Bioavailability of triterpenoids
While numerous in vitro studies report the anti-proliferative activity of Chaga mushrooms extracted triterpenoid compounds on various cancer cells, there are some concerns over their bioavailability for effective cancer treatment (Wold et al., 2020; Kim et al., 2021). During LC-MS/MS method validation for the analysis of inotodiol levels in mouse plasma, Kim et al. (2021) found that oral administration is not an efficient delivery approach. Even after intravenous administration, a low volume distribution of inotodiol was observed within tissues and organs (Kim et al., 2021). In addressing this, a study by Lu et al. (2021) utilized folic acid as a potential targeting ligand in liposomal formulation to increase the bioavailability of inotodiol for its use in treating various types of epithelial-originating cancers. Interestingly, P388-bearing mice intraperitoneally administered inotodiol at 10 mg/kg had extended survival without observed side effects compared to the control group (Nomura et al., 2008). Furthermore, mice bearing STZ-DMBA-induced mammary tumors intravenously treated with inotodiol experienced improved survival and limited tumorigenesis through reduced total and phosphorylated β-catenin signaling (Zhang et al., 2018). In addition to inonotiol, the low absorption following oral administration of betulin and betulinic acid also presents a challenge. Derivatives of betulinic acid and betulin have been synthesized for enhanced bioavailability and cytotoxicity. A study found that synthesized betulinic acid nanoparticles were able to significantly inhibit HKULC2, H1299, and H23 by 67 %, 72 %, and 76 %, respectively after treatment with 10 µM (Zhao et al., 2020). Moreover, betulinic acid nanoparticles developed by Li et al. (2022) were able to successfully cross the blood–brain barrier to increase their cellular uptake in central nervous system glioblastoma tumors. In 2017, Drag-Zalesinska et al. developed amino acid ester derivatives of betulin to increase their aqueous solubility while maintaining cytotoxicity toward melanoma Me-45 cells after 72 h of incubation. The greatest anti-cancer activity remained in the lysine and ornithine esters of betulin (Drąg-Zalesińska et al., 2017).
An increasing number of in vivo studies are investigating the anti-tumor activity of Chaga mushroom triterpenoids that show consistent cytotoxicity toward certain cancer cell lines. A study by Chung et al. (2010) with an in vivo component found 3b-hydroxylanosta-8,24-dien-21-al to inhibit tumor growth in Balb/c mice bearing sarcoma-180 cells more than lanosterol and inotodiol. Because aqueous solubility is important for clinical application, 3b-hydroxylanosta-8, 24-dien-21-al should be further studied in vivo in relation to conventional anti-cancer drugs (Chung et al., 2010, Kahlos et al., 1984). Successful encapsulation and modified release of 80 % Chaga mushroom extract in calcium alginate beads was achieved, where the extract was mostly retained at a gastric pH of 1.75 and heavily released at alkaline pH of 8.5 (Petrović et al., 2019). As more cancer studies involving Chaga mushrooms expand to include in vivo components, developments aimed at optimizing the bioavailability and pharmacokinetics of triterpenoids are essential to ensure they encounter target cells.
Conclusions
Cancer remains a global public health concern that needs to be addressed using multiple approaches. Treatment options and preventative measures that are non-invasive, successful, and easy to administer could promote the global healthcare system, economic recovery, and sustainable work productivity in response to the increasing incidences of cancer worldwide. Additional research is needed to develop safe and effective targeted cancer therapies to mitigate the increasing incidences of mortality and disability-adjusted life years as a result of cancer. In some cases, conventional therapies available to combat advanced stages of cancer have deleterious side effects that require extensive risk–benefit analysis prior to implementation. Among Chaga mushroom bioactives, the strongest anti-cancer activity associations were reported for betulinic acid (IC50 0.8 µM) against HT29-MTX and inonotsutriol E against A549 (IC50 1.6 µM). However, in vitro studies should be performed to provide more robust and reproducible data by expanding cell viability tests using multiple assays under different incubation times as well as assessing specificity to cancer cells by comparing the cytotoxicity to the counterpart normal cells. Future research should also focus on susceptible types of cancers using pre-clinical experimental animal models to assess Chaga mushroom triterpenoids and steroid derivatives. These studies can also aim at the biochemical mechanism(s) that can promote anti-cancer drug discovery using Chaga triterpenoids as lead molecules. Structural indicators of oncogenic capabilities largely depend on carbon positions C-3/C-28 in lupane-type and C-21/C-22 in lanostane-type triterpenoids. As aqueous solubility is important for bioavailability, functional constituents such as amino acid esters have been strategically incorporated at C-28 in betulin to improve solubility without sacrificing cytotoxic effects. The next step in the application of triterpenoids as an adjunct to minimally invasive cancer treatment is optimizing pharmacokinetics for effective targeted therapy. Introducing integrative cancer therapies that include triterpenoid supplementation as an adjuvant could enhance efficacy and efficiency to improve cancer treatment outcomes.
CRediT authorship contribution statement
Selina Plehn: Conceptualization, Writing – original draft. Sajeev Wagle: Conceptualization, Writing – original draft. H.P. Vasantha Rupasinghe: Conceptualization, Writing – review & editing, Supervision.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Data availability
No data was used for the research described in the article.
References
- Abu-Reidah I.M., Critch A.L., Manful C.F., Rajakaruna A., Vidal N.P., Pham T.H., Cheema M., Thomas R. Effects of PH and temperature on water under pressurized conditions in the extraction of nutraceuticals from chaga (inonotus obliquus) mushroom. Antioxidants. 2021;10:1322. doi: 10.3390/antiox10081322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agnestisia R., Ono A., Nakamura L., Chino R., Nodera K., Aiso-Sanada H., Nezu I., Ishiguri F., Suzuki T., Yokota S. The complete mitochondrial genome sequence of the medicinal fungus inonotus obliquus (hymenochaetaceae, basidiomycota) Mitochondrial DNA Part B. 2019;4:3504–3506. doi: 10.1080/23802359.2019.1675548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alzand K., Ünal S., Boufaris M. Lanostane-type triterpenes and abietane-type diterpene from the sclerotia of chaga medicinal mushroom, inonotus obliquus (agaricomycetes), and their biological activities. Int. J. Med. Mushrooms. 2018;20:507–516. doi: 10.1615/IntJMedMushrooms.2018026007. [DOI] [PubMed] [Google Scholar]
- Arata S., Watanabe J., Maeda M., Yamamoto M., Matsuhashi H., Mochizuki M., Kagami N., Honda K., Inagaki M. Continuous intake of the chaga mushroom (inonotus obliquus) aqueous extract suppresses cancer progression and maintains body temperature in mice. Heliyon. 2016;2:e00111. doi: 10.1016/j.heliyon.2016.e00111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayoub N., Lass D., Schultze W. Volatile constituents of the medicinal fungus chaga inonotus obliquus (Pers.: Fr.) (aphyllophoromycetideae) Int. J. Med. Mushrooms. 2009;11 doi: 10.1615/IntJMedMushr.v11.i1.60. [DOI] [Google Scholar]
- Baek J., Roh H.-S., Baek K.-H., Lee S., Lee S., Song S.-S., Kim K.H. Bioactivity-based analysis and chemical characterization of cytotoxic constituents from chaga mushroom (inonotus obliquus) that induce apoptosis in human lung adenocarcinoma cells. J. Ethnopharmacol. 2018;224:63–75. doi: 10.1016/j.jep.2018.05.025. [DOI] [PubMed] [Google Scholar]
- Balandaykin M., Zmitrovich I. Review on chaga medicinal mushroom, inonotus obliquus (higher basidiomycetes): realm of medicinal applications and approaches on estimating its resource potential. Int. J. Med. Mushrooms. 2015;17:95–104. doi: 10.1615/IntJMedMushrooms.v17.i2.10. [DOI] [PubMed] [Google Scholar]
- Behrens I., Stenberg P., Artursson P., Kissel T. Transport of lipophilic drug molecules in a new mucus-secreting cell culture model based on HT29-MTX cells. Pharm. Res. 2001;18:1138–1145. doi: 10.1023/a:1010974909998. [DOI] [PubMed] [Google Scholar]
- Brodelius M., Lundgren A., Mercke P., Brodelius P.E. Fusion of farnesyldiphosphate synthase and epi-aristolochene synthase, a sesquiterpene cyclase involved in capsidiol biosynthesis in Nicotiana Tabacum. Eur. J. of Biochem. 2002;269:3570–3577. doi: 10.1046/j.1432-1033.2002.03044.x. [DOI] [PubMed] [Google Scholar]
- Bunyard B. First record of insect mycophagy of the commercially-important “Chaga” fungus inonotus obliquus (Ach. Ex Pers.) Pilát (Hymenochaetales: Hymenochaetaceae) in North America. Proc. Entomol. Soc. of Wash. 2015;117:452–457. doi: 10.4289/0013-8797.117.4.452. [DOI] [Google Scholar]
- Campbell W.A., Davidson R.W. A poria as the fruiting stage of the fungus causing the sterile conks on birch. Mycologia. 1938;30:553–560. doi: 10.2307/3754349. [DOI] [Google Scholar]
- Chung M.J., Chung C.-K., Jeong Y., Ham S.-S. Anticancer activity of subfractions containing pure compounds of chaga mushroom (Inonotus Obliquus) extract in human cancer cells and in balbc/c mice bearing sarcoma-180 cells. Nutr. Res. Pract. 2010;4:177–182. doi: 10.4162/nrp.2010.4.3.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drąg-Zalesińska M., Drąg M., Poręba M., Borska S., Kulbacka J., Saczko J. Anticancer properties of ester derivatives of betulin in human metastatic melanoma cells (Me-45) Cancer Cell Int. 2017;17:4. doi: 10.1186/s12935-016-0369-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Druškovič M., Šuput D., Milisav I. Overexpression of caspase-9 triggers its activation and apoptosis in vitro. Croat. Med. J. 2006;47:832–840. [PMC free article] [PubMed] [Google Scholar]
- Faylo J.L., van Eeuwen T., Kim H.J., Gorbea Colón J.J., Garcia B.A., Murakami K., Christianson D.W. Structural insight on assembly-line catalysis in terpene biosynthesis. Nat. Commun. 2021;12:3487. doi: 10.1038/s41467-021-23589-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferlay J., Colombet M., Soerjomataram I., Parkin D.M., Piñeros M., Znaor A., Bray F. Cancer statistics for the year 2020: an overview. Int. J. Cancer. 2021;149:778–789. doi: 10.1002/ijc.33588. [DOI] [PubMed] [Google Scholar]
- Garádi Z., Dékány M., Móricz Á., Gaál A., Papp V., Béni S., Ványolós A. Antimicrobial, antioxidant and antiproliferative secondary metabolites from Inonotus Nidus-Pici. Molecules. 2021;26:5453. doi: 10.3390/molecules26185453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Géry A., Dubreule C., André V., Rioult J.-P., Bouchart V., Heutte N., Eldin de Pécoulas P., Krivomaz T., Garon D. Chaga (Inonotus Obliquus), a future potential medicinal fungus in oncology? A chemical study and a comparison of the cytotoxicity against human lung adenocarcinoma cells (A549) and human bronchial epithelial cells (BEAS-2B) Integr. Cancer Ther. 2018;17:832–843. doi: 10.1177/1534735418757912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill B.S., Kumar S. Navgeet triterpenes in cancer: significance and their influence. Mol. Biol. Rep. 2016;43:881–896. doi: 10.1007/s11033-016-4032-9. [DOI] [PubMed] [Google Scholar]
- Glamočlija J., Ćirić A., Nikolić M., Fernandes Â., Barros L., Calhelha R.C., Ferreira I.C.F.R., Soković M., van Griensven L.J.L.D. Chemical characterization and biological activity of chaga (Inonotus Obliquus), a medicinal “mushroom”. J. Ethnopharmacol. 2015;162:323–332. doi: 10.1016/j.jep.2014.12.069. [DOI] [PubMed] [Google Scholar]
- Handa N., Yamada T., Tanaka R. Four new lanostane-type triterpenoids from Inonotus Obliquus. Phytochem. Lett. 2012;5:480–485. doi: 10.1016/j.phytol.2012.04.010. [DOI] [PubMed] [Google Scholar]
- Hillier S.G., Lathe R. Terpenes, hormones and life: isoprene rule revisited. J. Endocrinol. 2019;242:R9–R22. doi: 10.1530/JOE-19-0084. [DOI] [PubMed] [Google Scholar]
- Hordyjewska A., Ostapiuk A., Horecka A., Kurzepa J. Betulin and betulinic acid: triterpenoids derivatives with a powerful biological potential. Phytochem. Rev. 2019;18:929–951. doi: 10.1007/s11101-019-09623-1. [DOI] [Google Scholar]
- Hwang A.Y., Yang S.C., Kim J., Lim T., Cho H., Hwang K.T. Effects of non-traditional extraction methods on extracting bioactive compounds from chaga mushroom (Inonotus Obliquus) compared with hot water extraction. LWT. 2019;110:80–84. doi: 10.1016/j.lwt.2019.04.073. [DOI] [Google Scholar]
- Jan R., Asaf S., Numan M., Lubna Plant secondary metabolite biosynthesis and transcriptional regulation in response to biotic and abiotic stress conditions. Agronomy. 2021;11, 968 doi: 10.3390/agronomy11050968. [DOI] [Google Scholar]
- Jeong W., Hong S.S., Kim N., Yang Y.T., Shin Y.S., Lee C., Hwang B.Y., Lee D. Bioactive triterpenoids from Callistemon Lanceolatus. Arch. Pharm. Res. 2009;32:845–849. doi: 10.1007/s12272-009-1605-3. [DOI] [PubMed] [Google Scholar]
- Jiang S., Shi F., Lin H., Ying Y., Luo L., Huang D., Luo Z. Inonotus Obliquus polysaccharides induces apoptosis of lung cancer cells and alters energy metabolism via the LKB1/AMPK axis. Int. J. of Biol. Macromol. 2020;151:1277–1286. doi: 10.1016/j.ijbiomac.2019.10.174. [DOI] [PubMed] [Google Scholar]
- Kahlos K., Hiltunen R., Schantz M. v 3β-hydroxy-lanosta-8,24-dien-21-al, a new triterpene from Inontus Obliquus. Planta. Med. 1984;50:197–198. doi: 10.1055/s-2007-969674. [DOI] [PubMed] [Google Scholar]
- Kahlos K., Kaila-Kangas L., Hiltunen R. Antitumor activity of some compounds and fractions from an N-hexane extract of Inonotus Obliquus in vitro. Acta. Pharm. Fennica. 1987;96:33–40. [Google Scholar]
- Kang J.H., Jang J.E., Mishra S.K., Lee H.J., Nho C.W., Shin D., Jin M., Kim M.K., Choi C., Oh S.H. Ergosterol peroxide from Chaga mushroom (Inonotus obliquus) exhibits anti-cancer activity by down-regulation of the β-catenin pathway in colorectal cancer. J. Ethnopharma. 2015;173:303–312. doi: 10.1016/j.jep.2015.07.030. [DOI] [PubMed] [Google Scholar]
- Kim J.H., Gao D., Cho C.W., Hwang I., Kim H.M., Kang J.S. A novel bioanalytical method for determination of inotodiol isolated from Inonotus Obliquus and its application to pharmacokinetic study. Plants. 2021;10:1631. doi: 10.3390/plants10081631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Y.J., Park J., Min B.S., Shim S.H. Chemical constituents from the sclerotia of Inonotus Obliquus. J. Korean Soc. Appl. Biol. Chem. 2011;54:287–294. doi: 10.3839/jksabc.2011.045. [DOI] [Google Scholar]
- Kim J., Yang S.C., Hwang A.Y., Cho H., Hwang K.T. Composition of triterpenoids in Inonotus Obliquus and their anti-proliferative activity on cancer cell lines. Molecules. 2020;25:4066. doi: 10.3390/molecules25184066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kou R.-W., Han R., Gao Y.-Q., Li D., Yin X., Gao J.-M. Anti-Neuroinflammatory polyoxygenated lanostanoids from chaga mushroom Inonotus Obliquus. Phytochemistry. 2021;184 doi: 10.1016/j.phytochem.2020.112647. [DOI] [PubMed] [Google Scholar]
- Kushiro, T.; Ebizuka, Y. 1.18 - Triterpenes. In Comprehensive Natural Products II; Liu, H.-W. (Ben), Mander, L., Eds.; Elsevier: Oxford, 2010; pp. 673–708 ISBN 978-0-08-045382-8.
- Lau, B.F.; Abdullah, N. Chapter 7 - sclerotium-forming mushrooms as an emerging source of medicinals: current perspectives. In mushroom biotechnology; Petre, M., Ed.; Academic Press: San Diego, 2016; pp. 111–136 ISBN 978-0-12-802794-3.
- Lee M.-W., Hur H., Chang K.-C., Lee T.-S., Ka K.-H., Jankovsky L. Introduction to distribution and ecology of sterile conks of Inonotus Obliquus. Mycobiology. 2008;36:199–202. doi: 10.4489/MYCO.2008.36.4.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H.S., Kim E.J., Kim S.H. Ethanol extract of inonotus obliquus (Chaga Mushroom) induces G1 cell cycle arrest in HT-29 human colon cancer cells. Nutr. Res. Pract. 2015;9:111–116. doi: 10.4162/nrp.2015.9.2.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee M.-G., Kwon Y.-S., Nam K.-S., Kim S.Y., Hwang I.H., Kim S., Jang H. Chaga mushroom extract induces autophagy via the AMPK-MTOR signaling pathway in breast cancer cells. J. Ethnopharmacol. 2021;274 doi: 10.1016/j.jep.2021.114081. [DOI] [PubMed] [Google Scholar]
- Li Y., He K., Huang Y., Zheng D., Gao C., Cui L., Jin Y.-H. Betulin induces mitochondrial cytochrome c release associated apoptosis in human cancer cells. Mol. Carcinog. 2010;49:630–640. doi: 10.1002/mc.20638. [DOI] [PubMed] [Google Scholar]
- Li Y., Wang Y., Gao L., Tan Y., Cai J., Ye Z., Chen A.T., Xu Y., Zhao L., Tong S., et al. Betulinic acid self-assembled nanoparticles for effective treatment of glioblastoma. J. Nanobiotechnology. 2022;20:39. doi: 10.1186/s12951-022-01238-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C., Zhao C., Pan H.-H., Kang J., Yu X.-T., Wang H.-Q., Li B.-M., Xie Y.-Z., Chen R.-Y. Chemical constituents from Inonotus Obliquus and their biological activities. J. Nat. Prod. 2014;77:35–41. doi: 10.1021/np400552w. [DOI] [PubMed] [Google Scholar]
- Lu Y., Jia Y., Xue Z., Li N., Liu J., Chen H. Recent developments in Inonotus obliquus (Chaga mushroom) polysaccharides: isolation, structural characteristics, biological activities and application. Polymers. 2021;13(9):1441. doi: 10.3390/polym13091441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma L., Chen H., Dong P., Lu X. Anti-inflammatory and anticancer activities of extracts and compounds from the mushroom Inonotus Obliquus. Food Chem. 2013;139:503–508. doi: 10.1016/j.foodchem.2013.01.030. [DOI] [PubMed] [Google Scholar]
- Matsuura, K.; Canfield, K.; Feng, W.; Kurokawa, M. Chapter Two - Metabolic Regulation of Apoptosis in Cancer. In Int. Rev. Cell and Mol. Biol.; Jeon, K.W., Galluzzi, L., Eds.; Academic Press, 2016; Vol. 327, pp. 43–87. [DOI] [PMC free article] [PubMed]
- Maza P.A.M.A., Lee J.-H., Kim Y.-S., Sun G.-M., Sung Y.-J., Ponomarenko L.P., Stonik V.A., Ryu M., Kwak J.-Y. Inotodiol from Inonotus Obliquus chaga mushroom induces atypical maturation in dendritic cells. Front. Immunol. 2021;12 doi: 10.3389/fimmu.2021.650841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merlin, J.P;, Rupasinghe, H.P.V.; Dellaire, G.; Murphy, K. Role of dietary antioxidants in p53-mediated cancer chemoprevention and tumor suppression. Oxidative Medicine and Cellular Longevity, 2021. 2021, Article ID 9924328. doi.org/10.1155/2021/9924328. [DOI] [PMC free article] [PubMed]
- Nakata T., Yamada T., Taji S., Ohishi H., Wada S.-I., Tokuda H., Sakuma K., Tanaka R. Structure determination of inonotsuoxides A and B and in vivo anti-tumor promoting activity of inotodiol from the sclerotia of Inonotus Obliquus. Bioorg. Med. Chem. 2007;15:257–264. doi: 10.1016/j.bmc.2006.09.064. [DOI] [PubMed] [Google Scholar]
- Naz I., Ramchandani S., Khan M.R., Yang M.H., Ahn K.S. Anticancer potential of raddeanin A, a natural triterpenoid isolated from anemone Raddeana Regel. Molecules. 2020;25:1035. doi: 10.3390/molecules25051035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ni G., Zhang Q.-J., Zheng Z.-F., Chen R.-Y., Yu D.-Q. 2-Arylbenzofuran derivatives from Morus Cathayana. J. Nat. Prod. 2009;72:966–968. doi: 10.1021/np800789y. [DOI] [PubMed] [Google Scholar]
- Nomura M., Takahashi T., Uesugi A., Tanaka R., Kobayashi S. Inotodiol, a lanostane triterpenoid, from Inonotus obliquus inhibits cell proliferation through caspase-3-dependent apoptosis. Anticancer Res. 2008 [PubMed] [Google Scholar]
- Osbourn A. Preformed antimicrobial compounds and plant defense against fungal attack. Plant Cell. 1996;8:1821–1831. doi: 10.1105/tpc.8.10.1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrović P., Ivanovic K., Octrue C., Tumara M., Jovanović A., Vunduk J., Niksic M., Pjanovic R., Branko B., Klaus A. Immobilization of chaga extract in alginate beads for modified release: simplicity meets efficiency. Hem. Ind. 2019;73 [Google Scholar]
- Razumov E., Safin R., Mukhametzyanov S.R., Hučko B., Martin P., Gomba G.K., Bazant V. Influence of development conditions on qualitative indicators of fungus chaga. IOP Conf. Ser.: Mater. Sci. Eng. 2019;666 doi: 10.1088/1757-899X/666/1/012084. [DOI] [Google Scholar]
- Rhee S.J., Cho S.Y., Kim K.M., Cha D.-S., Park H.-J. A Comparative study of analytical methods for alkali-soluble β-glucan in medicinal mushroom, chaga (Inonotus Obliquus) LWT - Food Sci. Technol. 2008;41:545–549. doi: 10.1016/j.lwt.2007.03.028. [DOI] [Google Scholar]
- Rutkowski M.J., Sughrue M.E., Kane A.J., Mills S.A., Parsa A.T. Cancer and the complement cascade. Mol. Cancer Res. 2010;8:1453–1465. doi: 10.1158/1541-7786.MCR-10-0225. [DOI] [PubMed] [Google Scholar]
- Saar M. Fungi in khanty folk medicine. J. Ethnopharmacol. 1991;31:175–179. doi: 10.1016/0378-8741(91)90003-V. [DOI] [PubMed] [Google Scholar]
- Safronov O. University of Helsinki, Finland; Doctorate: 2022. Chaga Genome and Convergent Evolution of Betulinate Biosynthesis in Host (Betula Pendula) and the Pathogen (Inonotus Obliquus) [Google Scholar]
- Safronov O., Bal G.L., Sipari N., Wilkens M., Safdari P., Smolander O.-P., Lihavainen J., Silvan N., Rajaraman S., Laine P.K., et al. Convergent evolution of mevalonate pathway in Inonotus Obliquus and Betula Pendula. Evol. Biol. 2021 doi: 10.1101/2021.11.28.470225. [DOI] [Google Scholar]
- Schirrmacher V. From chemotherapy to biological therapy: a review of novel concepts to reduce the side effects of systemic cancer treatment (review) Int. J. Oncol. 2018;54:407–419. doi: 10.3892/ijo.2018.4661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shan P., Wang C., Chen H., Yu J., Zhang H. Inonotsutriol E from Inonotus obliquus exhibits promising anti breast cancer activity via regulating the JAK2/STAT3 signaling pathway. Bioorg. Chem. 2023;139 doi: 10.1016/j.bioorg.2023.106741. [DOI] [PubMed] [Google Scholar]
- Shin J., Lee H.-J., Jung D.-B., Jung J.H., Lee H.-J., Lee E.-O., Lee S.-G., Shim B., Ko S.-G., Ahn K., et al. Suppression of STAT3 and HIF-1 alpha mediates anti-angiogenic activity of betulinic acid in hypoxic PC-3 prostate cancer cells. PLoS One. 2011;6:e21492. doi: 10.1371/journal.pone.0021492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taji S., Yamada T., Tanaka R. Three new lanostane triterpenoids, inonotsutriols A, B, and C, from Inonotus Obliquus. Hel. Chim. Acta. 2008;91:1513–1524. doi: 10.1002/hlca.200890165. [DOI] [Google Scholar]
- Tanaka R., Toyoshima M., Yamada T. New lanostane-type triterpenoids, inonotsutriols D, and E, from Inonotus Obliquus. Phytochem. Letters. 2011;4:328–332. doi: 10.1016/j.phytol.2011.07.001. [DOI] [Google Scholar]
- Tsai C.-C., Li Y.-S., Lin P.-P. Inonotus Obliquus extract induces apoptosis in the human colorectal carcinoma’s HCT-116 cell line. Biomed. & Pharmacother. 2017;96:1119–1126. doi: 10.1016/j.biopha.2017.11.111. [DOI] [PubMed] [Google Scholar]
- Wang C., Wang L., Wang J., Li Y.-P., Zhang J.-S., Shan P., Zhang H. 21,24-cyclolanostanes revisited: structural revision and biological evaluation. Fitoterapia. 2022;156 doi: 10.1016/j.fitote.2021.105101. [DOI] [PubMed] [Google Scholar]
- Willetts H.J., Bullock S. Developmental biology of sclerotia. Mycol. Res. 1992;96:801–816. doi: 10.1016/S0953-7562(09)81027-7. [DOI] [Google Scholar]
- Wold C.W., Gerwick W.H., Wangensteen H., Inngjerdingen K.T. Bioactive triterpenoids and water-soluble melanin from inonotus obliquus (chaga) with immunomodulatory activity. J. Func. Foods. 2020;71 doi: 10.1016/j.jff.2020.104025. [DOI] [Google Scholar]
- Xu R., Fazio G.C., Matsuda S.P.T. On the origins of triterpenoid skeletal diversity. Phytochemistry. 2004;65:261–291. doi: 10.1016/j.phytochem.2003.11.014. [DOI] [PubMed] [Google Scholar]
- Yang S.-J., Liu M.-C., Xiang H.-M., Zhao Q., Xue W., Yang S. Synthesis and in vitro antitumor evaluation of betulin acid ester derivatives as novel apoptosis inducers. Eur. J. Med. Chem. 2015;102:249–255. doi: 10.1016/j.ejmech.2015.08.004. [DOI] [PubMed] [Google Scholar]
- Zhang X., Bao C., Zhang J. Inotodiol suppresses proliferation of breast cancer in rat model of type 2 diabetes mellitus via downregulation of β-catenin signaling. Biomed. & Pharmacother. 2018;99:142–150. doi: 10.1016/j.biopha.2017.12.084. [DOI] [PubMed] [Google Scholar]
- Zhang S.-D., Yu L., Wang P., Kou P., Li J., Wang L.-T., Wang W., Yao L.-P., Zhao X.-H., Fu Y.-J. Inotodiol inhibits cells migration and invasion and induces apoptosis via P53-dependent pathway in HeLa cells. Phytomedicine. 2019;60 doi: 10.1016/j.phymed.2019.152957. [DOI] [PubMed] [Google Scholar]
- Zhao C.L., Cui X.M., Chen Y.P., Liang Q. Key enzymes of triterpenoid saponin biosynthesis and the induction of their activities and gene expressions in plants. Nat. Prod. Commun. 2010;5 doi: 10.1177/1934578X1000500736. [DOI] [PubMed] [Google Scholar]
- Zhao F., Mai Q., Ma J., Xu M., Wang X., Cui T., Qiu F., Han G. Triterpenoids from Inonotus Obliquus and their antitumor activities. Fitoterapia. 2015;101:34–40. doi: 10.1016/j.fitote.2014.12.005. [DOI] [PubMed] [Google Scholar]
- Zhao H., Mu X., Zhang X., You Q. Lung cancer inhibition by betulinic acid nanoparticles via adenosine 5′-triphosphate (ATP)-binding cassette transporter G1 gene downregulation. Med. Sci. Monit. 2020;26 doi: 10.12659/MSM.922092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao F., Xia G., Chen L., Zhao J., Xie Z., Qiu F., Han G. Chemical constituents from inonotus obliquus and their antitumor activities. J. Nat. Med. 2016;70:721–730. doi: 10.1007/s11418-016-1002-4. [DOI] [PubMed] [Google Scholar]
- Zhao Y., Zheng W. Deciphering the antitumoral potential of the bioactive metabolites from medicinal mushroom Inonotus obliquus. J. Ethnopharmacology. 2021;265 doi: 10.1016/j.jep.2020.113321. [DOI] [PubMed] [Google Scholar]
- Zhao L.W., Zhong X.H., Yang S.Y., Zhang Y.Z., Yang N.J. Inotodiol inhabits proliferation and induces apoptosis through modulating expression of cyclinE, p27, bcl-2, and bax in human cervical cancer HeLa cells. Asian Pac. J. Cancer Prev. 2014;15(7):3195–3199. doi: 10.7314/apjcp.2014.15.7.3195. [DOI] [PubMed] [Google Scholar]
- Zheng W., Liu T., Xiang X., Gu Q. Sterol composition in field-grown and cultured mycelia of inonotus obliquus. Yao. Acta Pharm. Sin. b. 2007;42:750–756. [PubMed] [Google Scholar]
- Zhong X.H., Wang L.B., Sun D.Z. Effects of inotodiol extracts from Inonotus obliquus on proliferation cycle and apoptotic gene of human lung adenocarcinoma cell line A549. Chinese J. Integr. Med. 2011;17:218–223. doi: 10.1007/s11655-011-0670-x. [DOI] [PubMed] [Google Scholar]
- Zhou M., Quek S.Y., Shang X., Fang S. Geographical variations of triterpenoid contents in Cyclocarya Paliurus leaves and their inhibitory effects on hela cells. Ind. Crops and Prod. 2021;162 doi: 10.1016/j.indcrop.2021.113314. [DOI] [Google Scholar]
- Zhuo Z.-J., Xiao M.-J., Lin H.-R., Luo J., Wang T. Novel betulin derivative induces anti-proliferative activity by G2/M phase cell cycle arrest and apoptosis in Huh7 cells. Oncol. Lett. 2018;15:2097–2104. doi: 10.3892/ol.2017.7575. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data was used for the research described in the article.