Abstract
Objective
Some studies suggest that hypothyroidism is associated with increased oxidative stress. Urinary excretion of 8-oxo-7,8-dihydroguanosine (8-oxoGuo) and 8-oxo-7,8-dihydro-2′-deoxyguanosine (8-oxodG) represents whole-body RNA and DNA oxidation, respectively. These biomarkers have only been explored sparsely in patients with thyroid disorders.
Methods
In 45 Danish women with newly diagnosed hypothyroidism, we compared 8-oxoGuo and 8-oxodG before or shortly after initiating levothyroxine with the excretion rates at euthyroidism. We also compared the excretion of 8-oxoGuo and 8-oxodG in the patients after restored euthyroidism with 18 healthy control subjects.
Results
Compared with baseline, none of the biomarkers changed significantly in the patients after becoming euthyroid. The geometric mean of 8-oxoGuo was 1.63 (95% CI: 1.49–1.78) nmol/mmol creatinine at baseline and 1.67 nmol/mmol at euthyroidism (95% CI: 1.53–1.83) (P = 0.39), while that of 8-oxodG was 1.28 nmol/mmol creatinine at baseline (95% CI: 1.14–1.44) and 1.32 nmol/mmol at euthyroidism (95% CI: 1.18–1.48), respectively (P = 0.47). The relative mean differences were 0.97 (95% CI: 0.91–1.04) for 8-oxoGuo and 0.97 (95% CI: 0.88–1.06) for 8-oxodG. At baseline, multiple linear regression revealed a positive association between free thyroxine and both biomarkers (8-oxoGuo, P < 0.001; 8-oxodG, P = 0.04). Furthermore, 8-oxoGuo was positively associated with age (P = 0.04) and negatively associated with thyrotropin (P = 0.02). In the control group, the geometric mean of 8-oxoGuo was 1.23 nmol/mmol creatinine (95% CI: 1.07–1.42), while that of 8-oxodG was 1.04 nmol/mmol creatinine (95% CI: 0.88–1.23). Thus, compared with control subjects, euthyroid patients showed a significantly higher level of both 8-oxoGuo (P < 0.001) and 8-oxodG (P = 0.03).
Conclusion
In hypothyroid women, no significant effect of levothyroxine treatment on the oxidative stress biomarkers 8-oxoGuo and 8-oxodG could be demonstrated. However, the excretion of these biomarkers was significantly higher than in healthy controls.
Keywords: oxidative stress; 8-oxo-7,8-dihydroguanosine; 8-oxoGuo; 8-oxo-7,8-dihydro-2′-deoxyguanosine; 8-oxodG; hypothyroidism
Introduction
Hypothyroidism, most often caused by autoimmune thyroiditis, is a common disease with a prevalence of approximately 3% in the European population (1). Epidemiological studies have shown that hypothyroidism is associated with an increased risk of comorbidity (2, 3, 4), which impacts ability to work (5) and quality of life (6) and leads to a shorter life span, at least in younger individuals (7, 8). The pathogenesis behind this impact on health is unresolved.
An imbalance between oxidative and antioxidative processes, in favor of the oxidative processes, leads to a disruption of redox signaling and/or molecular damage, and is defined as oxidative stress (9). Prooxidants include reactive oxygen species (ROS) that can be generated from oxidative phosphorylation in the mitochondria. Cells also harbor antioxidative countermeasures. Oxidation of the genome and other macromolecules is either part of the cells’ regulatory functions and adaption, or it can be pathogenic. Some chemical modifications of DNA and RNA might trigger mutagenesis, which can result in accelerated aging or malignant transformation (10). If cellular structures other than DNA and RNA are degenerated, it might result in cell dysfunction and apoptosis (11). Regarding thyroid function, the impact of oxidative stress is of particular interest, since thyroid hormones are crucial for oxidative metabolism and energy expenditure (12).
There are numerous ways of measuring oxidative stress. The most reliable methods do not only focus on the amounts of prooxidants or antioxidants but measure the integrated effect of the oxidative stress burden. The stability of the genome is crucial for the normal function of the cell. The guanine nucleoside derivatives 8-oxo-7,8-dihydroguanosine (8-oxoGuo) and 8-oxo-dihydro-2′-deoxyguanosine (8-oxodG) result from oxidative modifications to RNA and DNA, respectively (13). These biomarkers reflect the aggregated oxidative stress load in the organism (14). 8-oxoGuo and 8-oxodG are excreted into the urine and can therefore be measured noninvasively (13). Increased excretion rates of 8-oxoGuo and 8-oxodG have been identified in cardiovascular diseases and various conditions like hypertension and cancer (11). The excretion of these biomarkers also increases with smoking and aging (11) and is predictive of mortality in patients with type 2 diabetes (15, 16) and cancer development (17, 18).
Oxidized guanine nucleosides have only been explored sparsely in thyroid disorders. We recently demonstrated that 8-oxoGuo and 8-oxodG decrease by 10–25% after treatment of hyperthyroidism (19). Whether the same applies to the hypothyroid state is unknown. Thus, in the present study, we aimed to investigate the effect of levothyroxine (LT4) treatment on the oxidative stress load in hypothyroid women. To this end, we determined the urinary excretions of 8-oxoGuo and 8-oxodG and compared the excretion rates of these biomarkers with those in healthy individuals.
Methods
Study population and design
Hypothyroid patients from two different cohorts were included. Cohort A was part of a longitudinal prospective cohort study of the impact of hypothyroidism on bone microarchitecture, as described elsewhere (20). In brief, newly diagnosed hypothyroid women were recruited from the outpatient clinic at the Department of Endocrinology, Odense University Hospital, Denmark, between May 2011 and June 2016. The patients had to be 20–85 years of age and diagnosed with autoimmune thyroiditis to meet the inclusion criteria. Autoimmune thyroiditis was defined by positive antibodies against thyroid peroxidase (TPOAb) or thyroglobulin in serum. Hypothyroidism was defined as overt (plasma thyrotropin (TSH) above the reference range of 0.3–4.0 mIU/L and total plasma thyroxine (TT4) below the reference range of 60–130 nmol/L) or mild (plasma TSH above the reference range, and plasma TT4 within the reference range). Exclusion criteria were pregnancy or planned pregnancy during the study period, treatment with glucocorticoids, or reduced kidney function (serum creatinine: >100 μmol/L). Patients with known osteoporosis and treatment with antiosteoporotic agents were excluded. The study was approved by the Regional Research Ethics Committee of Southern Denmark (S-2011-0018), and is registered on www.clinicaltrials.gov (NCT02005250).
Cohort B was part of a study aiming to evaluate changes in appetite and food intake in newly diagnosed hypothyroid patients during the first 6 months after starting LT4, as described elsewhere (21). Participants were included from the Copenhagen General Population Study (22) or recruited from the outpatient clinic at the Department of Endocrinology, Herlev-Gentofte Hospital, Denmark, between August 2015 and February 2018. The participants had to be 20–75 years of age and had a TSH >10.0 mIU/L at diagnosis as well as TPOAb positivity. Exclusion criteria were severe comorbidity, pregnancy, previous thyroidectomy, amiodarone treatment, and inability to understand and speak Danish. The study was approved by the Regional Research Ethics Committee of the Capital Region of Denmark (H-15001954) and is registered on www.clinicaltrials.gov (NCT02993562).
In both cohorts, the urinary excretion of the oxidized guanine nucleosides 8-oxoGuo and 8-oxodG was monitored. Thus, all patients were examined twice, shortly after diagnosis and when euthyroidism had been restored by LT4 treatment for at least 6 months. The dose of LT4 was adjusted according to routine clinical practice and aimed at a plasma TSH level within the reference range.
Healthy female volunteers were recruited from a Danish website (https://www.forsoegsperson.dk) used to advertise medical research. The control group was matched by age and body mass index (BMI) with the patients enrolled in cohort B. Informed written consent was obtained from all participants.
Oxidative stress markers
Spot urine samples from both study visits were stored at −80°C until analysis using a validated method of ultraperformance liquid chromatography–tandem mass spectrometry (23). The concentrations of the biomarkers 8-oxoGuo and 8-oxodG were normalized against the urinary creatinine concentration. Chromatographic separation was performed using PerkinElmer Series 200 HPLC with two pumps. The HPLC columns were a Phenomenex Prodigy ODS column (100 × 2 mm, 3 μm) and a C18 ODS guard column (4 × 2 mm), both from Phenomenex (Torrance, CA, USA). The mass spectrometry detection was performed on an API 3000 triple quadrupole mass spectrometer (Sciex, Toronto, Ontario, Canada) equipped with an electrospray ionization ion source (Turbospray) operated in the positive mode (24). Urinary creatinine was measured using an in-house Jaffe’s method (25).
Blood samples
In cohort A, fasting blood samples from all study visits were drawn and stored at −20°C until analysis. After study completion, plasma was analyzed for TSH and free T4 (FT4) by a two-site chemiluminescent immunometric assay on Cobas 8000 (Roche Diagnostics). The coefficients of variation (CVs) for TSH were 8.3% and 4.2% at levels of 0.084 and 11.3 mIU/L, respectively; for FT4, the CVs were 7.1% and 4.4% at levels of 17 and 33 pmol/L, respectively (20).
In cohort B, blood samples were analyzed during each visit. TSH and FT4 were analyzed by a chemiluminescent immunoassay on ADVIA Centaur XP or Centaur CP (Siemens). The CVs for TSH were 8.17% and 6.95% at levels of 0.287 and 6.94 mIU/L, respectively; for FT4, the CVs were 8.0% and 3.1% at levels of 11.7 and 25.5 pmol/L, respectively.
TPOAb were analyzed using different commercial methods employed routinely at the local hospital laboratories. In cohort A, TPOAb were measured using AutoDELFIA (PerkinElmer). In cohort B, TPOAb were determined by a chemiluminescent immunoassay on ADVIA Centaur XP (Siemens), and the CV was 5.7%. The cutoff value defining a positive value was 60 kIU/L.
Statistical analysis
Numerical variables are presented as mean ± s.d. or median with full range where appropriate. Categorical variables are shown as numbers and ratios. Differences in excretion rates of the oxidative stress markers between baseline and follow-up were evaluated using a paired t-test. To compare the oxidative stress markers between hypothyroid women and controls, an independent t-test was used. Categorical and numerical baseline characteristics were compared with Fisher’s exact test and independent t-test, respectively. The normal distribution of data was evaluated with the Shapiro–Wilk test. If data were not normally distributed, logarithmic transformation was applied. Accordingly, the geometric mean with a 95% CI was calculated for the oxidative stress biomarkers, as these were not normally distributed.
The relationship between baseline characteristics and the baseline excretion of the oxidative stress biomarkers was evaluated with a forward stepwise multiple linear regression analysis; variables included in the full model were age, BMI, TPOAb, and TSH or FT4. Because of the correlation between TSH and FT4, separate models were fitted, including only one of these variables to avoid multicollinearity. As BMI, TSH, and TPOAb were not normally distributed, a logarithmic transformation was applied before being included in the regression analysis.
To account for the fact that smoking increases the generation of the oxidized guanine nucleosides (26), we made a sensitivity analysis of the independent t-test (comparing the oxidative stress markers between hypothyroid women and controls), excluding smokers and those with unknown smoking status.
Statistical tests were two-sided, and statistical significance was defined as P < 0.05. Statistical analyses were performed using the IBM Statistical Package for the Social Sciences Statistics (SPSS) for Macintosh, version 28.0.1.0 (released 2021; IBM Corp, Armonk, NY, USA).
Results
Characteristics of participants
Thirty-two women newly diagnosed with autoimmune thyroiditis were included in cohort A. Three women were excluded during the follow-up period due to pregnancy (n = 1), diagnosis of an eating disorder (n = 1), and donation of a kidney (n = 1). One participant was lost to follow-up, leaving 28 patients from cohort A for the final analysis. In cohort B, 19 women with newly diagnosed autoimmune thyroiditis were included. Two women were excluded due to missing oxidative stress markers at baseline (n = 1) and self-exclusion (n = 1), leaving 17 patients from cohort B for the final analysis. Thus, 45 patients in total completed the study. In addition, 18 control subjects were recruited and included in the analysis.
Relevant demographic, clinical, and laboratory characteristics of the study participants are presented in Table 1. Twenty-two participants were diagnosed with mild (subclinical) hypothyroidism, and 23 with overt hypothyroidism. The median time between baseline (at diagnosis) and follow-up (at euthyroidism) was 442 days, ranging from 183 to 1354 days. There were no significant differences in age, BMI, and smoking status between the patients and the control subjects (Table 1).
Table 1.
Characteristics of the hypothyroid women and healthy control subjects. Data are presented as ratios, n (%), mean ± s.d., or median (full range).
| Patients | Controls | Pa | |
|---|---|---|---|
| n | 45 | 18 | |
| Mild:overt hypothyroidism | 22:23 | ||
| Age (years) | 47.4 ± 11.4 | 45.2 ± 13.1 | 0.51 |
| Body mass index (kg/m2) | 28.4 ± 6.0 | 29.4 ± 5.5 | 0.58 |
| Smoking, n (%) | |||
| Yes | 6 (13.3) | 0 | 0.23 |
| No | 37 (82.2) | 18 (100) | 0.23 |
| Unknown | 2 (4.5) | 0 | 0.23 |
| TPOAb at diagnosis (kIU/L) | 590 (2–13,000)b | ||
| Time between baseline and follow-up (days) | 442 (183–1354) | ||
| LT4 dose at follow-up (µg/day) | 114 (0–200)c |
aDifference between hypothyroid participants and control subjects; bThree patients were negative for TPOAb but positive for anti-thyroglobulin antibodies (>60 mIU/L); cOne participant, initially hypothyroid, regained euthyroidism spontaneously, and the LT4 treatment could be discontinued.
LT4, levothyroxine; TPOAb, thyroid peroxidase antibodies.
Table 2 shows the thyroid hormone status at baseline and follow-up. The median LT4 dose for achieving euthyroidism was 114 μg/day. One participant, initially hypothyroid, regained euthyroidism spontaneously, and the LT4 treatment could be discontinued.
Table 2.
Thyroid function parameters and urinary excretion of oxidative stress markers in hypothyroid women and healthy control subjects. Thyroid function parameters are given as mean ± s.d., and the oxidative stress markers are presented as geometric mean with a 95% CI. The mean difference (with 95% CI) between baseline and follow-up is a relative measure as the oxidative stress markers are logarithmically transformed.
| Patients (n = 45) | Controls (n = 18) | Pb | ||||
|---|---|---|---|---|---|---|
| Baseline | Follow-up | Mean difference | Pa | |||
| TSH (mIU/L) | 21.89 ± 35.04 | 2.11 ± 1.84 | <0.001 | 1.74 ± 0.98 | ||
| FT4 (pmol/L) | 14.00 ± 5.01 | 18.10 ± 3.25 | <0.001 | 14.47 ± 1.68 | ||
| 8-oxoGuo (nmol/mmol Cr) | 1.63 (1.49–1.78) | 1.67 (1.53–1.83) | 0.97 (0.91–1.04) | 0.39 | 1.23 (1.07–1.42) | <0.001 |
| 8-oxodG (nmol/mmol Cr) | 1.28 (1.14–1.44) | 1.32 (1.18–1.48) | 0.97 (0.88–1.06) | 0.47 | 1.04 (0.88–1.23) | 0.03 |
aPaired t-test comparing hypothyroid participants at baseline and follow-up; bIndependent t-test comparing oxidative stress markers in hypothyroid participants at follow-up with control subjects.
8-oxoGuo, 8-oxo-7,8-dihydroguanosine; 8-oxodG, 8-oxo-7,8-dihydro-2′-deoxyguanosine; Cr, creatinine; FT4, free thyroxine; TSH, thyrotropin.
Urinary excretion of 8-oxoGuo and 8-oxodG
The geometric mean of the excretion of 8-oxoGuo was 1.63 nmol/mmol creatinine at baseline and 1.67 nmol/mmol at euthyroidism (P= 0.39), while that of 8-oxodG was 1.28 nmol/mmol at baseline and 1.32 nmol/mmol at euthyroidism, respectively (P = 0.47) (Table 2 and Fig. 1). Thus, no significant change in either 8-oxoGuo or 8-oxodG was seen after restored euthyroidism, i.e. the relative mean differences were 0.97 (95% CI: 0.91–1.04) and 0.97 (95% CI: 0.88–1.06), respectively.
Figure 1.
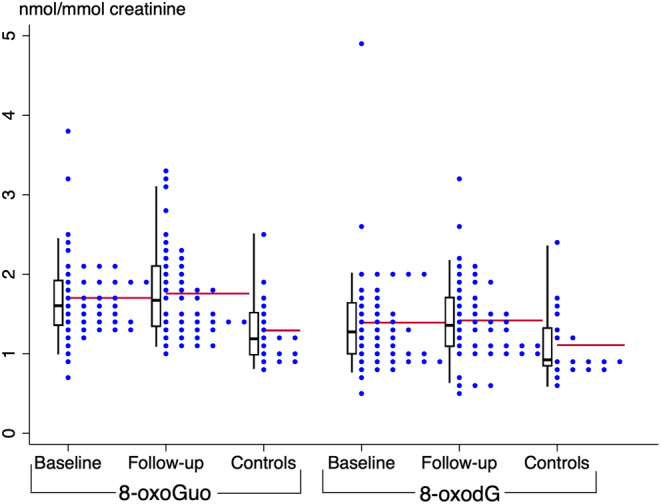
Individual urinary excretion rates of the biomarkers 8-oxo-7,8-dihydro-guanosine (8-oxoGuo) and 8-oxo-7,8-dihydro-2′-deoxyguanosine (8-oxodG) in hypothyroid women at diagnosis and at euthyroidism when treated with levothyroxine, and in the healthy control subjects. Box-and-whisker plots illustrate the 5th, 25th, 50th (median), 75th, and 95th percentile. The red lines represent mean values.
The individual changes in 8-oxoGuo and 8-oxodG are shown in Fig. 2. Twenty-five patients exhibited an increase, while 20 patients showed a decrease in 8-oxoGuo. The corresponding numbers for 8-oxodG were 26 and 19, respectively. Patients demonstrating a reduction in the biomarkers did not differ in baseline characteristics from those who showed an increase (data not shown).
Figure 2.
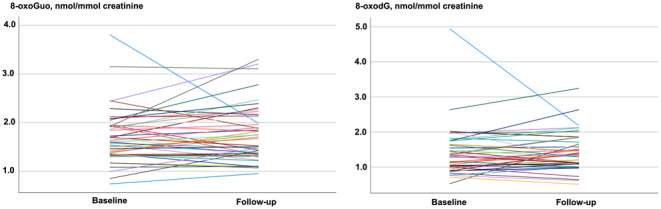
The urinary excretion of 8-oxoGuo and 8-oxodG in 45 hypothyroid women at diagnosis and at euthyroidism when treated with levothyroxine. Each line represents one individual. Urinary samples were taken before or shortly after initiating levothyroxine (baseline) and again after euthyroidism was achieved (follow-up). 8-oxoGuo, 8-oxo-7,8-dihydroguanosine; 8-oxodG, 8-oxo-7,8-dihydro-2′-deoxyguanosine.
The geometric mean of the urinary excretion of 8-oxoGuo and 8-oxodG in the control subjects was 1.23 (95% CI: 1.07–1.42) and 1.04 (95% CI: 0.88–1.23) nmol/mmol creatinine, respectively. Significantly higher excretions of 8-oxoGuo (P < 0.001) and 8-oxodG (P = 0.03) were found in the patients at euthyroidism compared to the control subjects (Table 2 and Fig. 1). In our study, six patients were smokers, and none were in the control group. We carried out a sensitivity analysis, excluding smokers and those with unknown smoking status, whereby there were no significant changes in our results (data not shown).
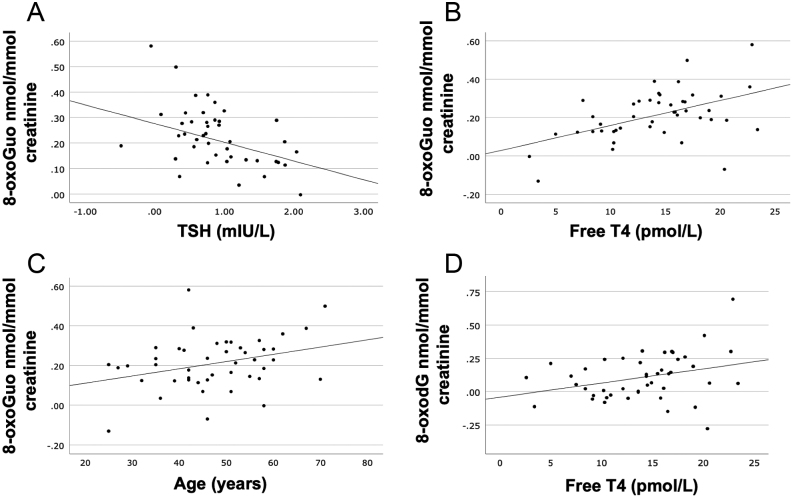
Both the full and the stepwise multiple regression analyses (Table 3) demonstrated a negative correlation between 8-oxoGuo and baseline TSH (P = 0.015) and positive correlations between 8-oxoGuo and baseline FT4 (P < 0.001) and age (P = 0.035) (Fig. 3). There was no correlation between 8-oxoGuo and BMI or TPOAb. The excretion of 8-oxodG did not correlate significantly with TSH at baseline (P = 0.119), nor were there any significant associations with age, BMI, or TPOAb. However, a positive correlation between baseline FT4 and 8-oxodG (P = 0.038) was demonstrated (Table 3 and Fig. 3).
Table 3.
Multiple regression associations of baseline excretion of 8-oxoGuo and 8-oxodG with demographic characteristics and baseline thyroid function test in 45 hypothyroid women. TPOAb, BMI, and TSH were not normally distributed, and a logarithmic transformation was applied.
| 8-oxoGuo (n = 45) | 8-oxodG (n = 45) | |||||
|---|---|---|---|---|---|---|
| β coefficienta | P | R2 | β coefficienta | P | R2 | |
| Variables included | 0.230 | 0.057 | ||||
| Age | 0.309 | 0.049 | 0.169 | 0.321 | ||
| BMI | 0.107 | 0.459 | 0.042 | 0.791 | ||
| TPOAb | 0.021 | 0.899 | 0.202 | 0.276 | ||
| TSH | −0.382 | 0.019 | −0.217 | 0.215 | ||
| Variables included | 0.331 | 0.164 | ||||
| Age | 0.277 | 0.055 | 0.155 | 0.328 | ||
| BMI | 0.157 | 0.252 | 0.101 | 0.505 | ||
| TPOAb | 0.063 | 0.680 | 0.276 | 0.112 | ||
| FT4 | 0.525 | <0.001 | 0.429 | 0.012 | ||
| By stepwise multiple regression | 0.219 | |||||
| Age | 0.297 | 0.035 | ||||
| TSH | −0.348 | 0.015 | ||||
| By stepwise multiple regression | ||||||
| FT4 | 0.491 | <0.001 | 0.241 | 0.310 | 0.038 | 0.096 |
aStandardized coefficient.
8-oxoGuo, 8-oxo-7,8-dihydroguanosine; 8-oxodG, 8-oxo-7,8dihydro-2′-deoxyguanosine; BMI, body mass index; TSH, thyrotropin; FT4, free thyroxine; TPOAb, thyroid peroxidase antibodies.
Figure 3.
Correlations between the oxidative stress markers 8-oxoGuo and 8-oxodG and the predictor variables age, thyrotropin (TSH), and free thyroxine (T4) in hypothyroid women. A: Correlation between baseline 8-oxoGuo and baseline TSH (R2 = 0.130, P = 0.015). B: Correlation between baseline 8-oxoGuo and baseline free T4 (R2 = 0.241, P < 0.001). C: Correlation between baseline 8-oxoGuo and age (R2 = 0.098, P = 0.015). D: Correlation between baseline 8-oxodG and baseline free T4 (R2 = 0.0096, P = 0.038). Logarithmic transformation was applied for 8-oxoGuo, 8-oxodG, and TSH. 8-oxoGuo, 8-oxo-7,8-dihydroguanosine; 8-oxodG, 8-oxo-7,8-dihydro-2′-deoxyguanosine.
Discussion
The present study is the first to evaluate, using a validated method, the effect of LT4 treatment in hypothyroid patients on the urinary excretion of the oxidized guanine nucleosides 8-oxoGuo and 8-oxodG (27, 28) and to compare the results with a healthy control group. No significant effect of LT4 treatment on the excretion of either 8-oxoGuo or 8-oxodG was demonstrated. However, a significantly higher oxidative stress level in hypothyroid patients than in healthy individuals persisted after restoration of euthyroidism. In accordance with previous studies, we found that age was positively correlated to the excretion of 8-oxoGuo (19, 29).
Increased oxidative stress has been associated with aging, various diseases, and mortality (11) and might also be a contributor to the increased morbidity (2, 3, 4) and mortality documented in hypothyroid patients (7, 8). However, several interacting factors seem to be involved. The lower energy expenditure resulting from hypothyroidism should, in theory, lead to a reduced generation of ROS but may be counterbalanced by a diminished antioxidant capacity. Complexity is underlined by the observation that untreated hypothyroidism, unless severe, may benefit elderly people in terms of longevity (30), although controversies exist (8). The underlying mechanism is obscure, but less oxidative stress burden in the hypothyroid condition may play an important and protective role. Such a theory is supported by our present study, considering the inverse correlation between the severity of hypothyroidism and the excretion of 8-oxoGuo.
Numerous studies using different methods have investigated oxidative stress in hypothyroidism, with conflicting results. Some methods assessed the oxidative impact on cell membranes, lipids, or proteins, while others measured the antioxidant capacity or amount of prooxidants. The hyperlipidemia resulting from hypothyroidism increases the risk of lipid peroxidation (31), which in turn leads to the formation of malondialdehyde and thiobarbituric acid reactive substances. Compared to healthy control subjects, elevated concentrations of thiobarbituric acid reactive substances (32, 33) and malondialdehyde (34, 35, 36, 37, 38) have been reported in both overt and mild hypothyroidism. In a study by Baskol et al. (39), the malondialdehyde level decreased after LT4 treatment for 6 months. Still, it remained elevated compared to healthy individuals (39), in accordance with the findings of the present study.
Regarding the antioxidant capacity in hypothyroidism, results are ambiguous (40). One study demonstrated a decrease in superoxide dismutase (41), an enzyme that inactivates ROS, in hypothyroid patients compared to healthy controls. At variance, other studies found opposite results (32, 42) or could not demonstrate any difference in superoxide dismutase activity between hypothyroid patients and healthy individuals (35, 39, 42). Other antioxidant enzymes include catalase and glutathione peroxidase, which act by preventing the production of ROS or inactivating these species. Elevated concentrations of catalase (32, 36) and glutathione peroxidase (33, 41, 42) in hypothyroid patients, compared to healthy individuals, have been reported, while other studies found no difference (41, 43), or lower concentrations of catalase (42). Studies of other markers, including vitamin E (32, 35), total antioxidant status (44, 45), paraoxonase 1, and arylesterase, also showed ambiguous results (39, 44, 46, 47).
The conflicting results in previous studies underline that measuring a single component in the oxidative or antioxidative pathways does not reliably assess the oxidative burden due to counteracting mechanisms. In contrast, the urinary excretion of the oxidized guanine nucleosides, as measured in the present study, reflects the overall damage to DNA and RNA, being pivotal in the processes of cell aging, degeneration, and apoptosis. Thus, measurement of 8-oxodG and 8-oxoGuo can be considered the biologically most relevant method for assessing whole-body oxidative stress (14).
Recently, we investigated with the same laboratory method the 8-oxoGuo and 8-oxodG urinary excretions in hyperthyroid patients before and after treatment (19). Both biomarkers decreased by 10–25% after the patients were rendered euthyroid by treatment. The oxidative stress markers were higher in patients with Graves’ disease than in those with toxic nodular goiter (19), implying that the degree of hyperthyroidism is most pronounced in the former disease. Still, the presence of autoimmunity per se may also be an important factor.
It is unclear why hyperthyroid patients benefit from treatment with respect to oxidative stress while hypothyroid patients do not. A possible explanation might be that the hyperthyroid state is characterized by markedly elevated energy expenditure and oxygen turnover, exceeding the antioxidant capacity. Treatment will reverse these processes, leading to measurable changes in oxidative stress biomarkers. In the hypothyroid state, changes in the energy expenditure may be more subtle and below the threshold at which changes in the biomarkers are detectable. This is challenged by the fact that we found a positive correlation between plasma FT4 and the oxidative stress biomarkers at baseline. However, in this context, FT4 may be considered merely a surrogate marker for the severity of thyroid autoimmunity, leaving the oxidative stress level unchanged after treatment. Thus, the underlying pathophysiological condition, i.e. thyroid autoimmunity, might also explain why the level of oxidative stress biomarkers in our patients remained higher than that in the control subjects, despite biochemical euthyroidism obtained by LT4 substitution. Such a hypothesis is supported by previous observations (43). We found no influence of the TPOAb level per se on the level of oxidative stress, but this may rely on the fact that TPOAb shows huge variations among patients with autoimmune thyroiditis. Finally, it cannot be excluded that LT4 is unable to restore complete euthyroidism within the cell, in line with the ongoing debate about the ideal treatment of hypothyroid patients (48). If true, hypothyroid individuals will not improve their oxidative stress level following LT4 treatment, in contrast to the effects seen when treating hyperthyroid patients (19). Whether LT4 combined with liothyronine proves better for the oxidative stress burden remains to be shown, ideally in the setting of a randomized controlled trial.
The presence of comorbidity may potentially contribute to increased oxidative stress (11). Most of our patients did not suffer from disorders other than hypothyroidism. However, in cohort A, four participants had coexisting chronic diseases such as type 2 diabetes mellitus, asthma, fibromyalgia, and previous ovarian cancer without evidence of residual disease, respectively. Drugs may also affect whole-body oxidative stress, although data are scarce. Previous reports support that drugs of different kinds have either a favorable or a neutral effect on the oxidative stress level (49, 50, 51, 52, 53, 54). Thus, any effect of coadministered drugs would probably tend to ameliorate the oxidative burden in our patients. If such a drug-induced effect was excluded, the difference in the oxidative stress biomarkers would be even more pronounced between patients and control subjects. No patient received statins, while five patients were treated with proton pump inhibitors or antihypertensive drugs. When excluding these individuals and those with comorbidity, our results remained significant (data not shown).
Our study has some limitations. The population comprised selected patients from two cohorts, and the study was not designed specifically for the present purpose. Further, the duration between the two study visits varied between patients, and we cannot exclude the fact that the burden of oxidative stress may depend on the length of the euthyroid state. We did not consistently monitor the use of antioxidant supplements, e.g. selenium, which potentially may have affected the oxidative stress level (55) and a number of other variables (56). However, as the use of supplements most likely remained unchanged during the study period, both in patients and in controls, any impact on our results is probably negligible. Finally, the urine and blood samples from the two study cohorts were not analyzed simultaneously, which is probably of little or no importance, as the oxidative stress biomarkers show high chemical stability and no diurnal variation (26, 57).
In conclusion, the whole-body oxidative stress biomarkers 8-oxoGuo and 8oxodG were inversely correlated with the severity of hypothyroidism in patients with newly diagnosed autoimmune thyroiditis. No significant change in these biomarkers could be demonstrated after restoring euthyroidism. Importantly, despite treatment, we demonstrated a persistently higher level of oxidative stress in our patients than in healthy control subjects. The mechanism behind the latter observation remains to be clarified. Finally, whether a causal link exists between these biomarkers of oxidative stress and the increased morbidity observed in patients with hypothyroidism remains to be elucidated.
Declaration of interest
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
Funding
For the inclusion of cohort A, funding was received from ‘The Music Publishers Agnes and Knut Mørks Foundation’, the ‘Danish Thyroid Patient Federation’, and research grants at Odense University Hospital. Cohort B received funding from ‘The Music Publishers Agnes and Knut Mørks Foundation’; Johannes Fogs Foundation; Hede-Nielsen Foundation; Department of Internal Medicine, Herlev Hospital; and research grants at Herlev Hospital. HEP and ELL received an unrestricted research grant from Boehringer Ingelheim for an unrelated study. KRR was supported by a PhD scholarship funded by the Danish Medicines Agency, the Region of Southern Denmark (grant number: 20/14658), and a grant from ‘Overlægerådets Forskningsfond’ at Odense University Hospital (grant number: A4641).
Author contribution statement
SJB, THB, LH, and BN contributed to the study conception and design. SJB, THB, CBL, BM, FKK, CZJ, and BN conducted the data collection. HEP were involved in the development of methodology (biomarkers). KRR was responsible for data analysis. KRR and SJB interpreted the data. KRR wrote the first draft of the manuscript. All authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Data availability
Some or all datasets generated during and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Acknowledgements
We thank technicians at the Osteoporosis Clinic, Odense University Hospital, for helping in study coordination. We also thank the Copenhagen General Population Study for recruitment support. We thank chemist Trine Henriksen and laboratory technician Katja R Christensen for conducting the urine analysis for 8-oxodG, 8-oxoGuo, and creatinine.
References
- 1.Madariaga AG Santos Palacios S Guillén-Grima F & Galofré JC. The incidence and prevalence of thyroid dysfunction in Europe: a meta-analysis. Journal of Clinical Endocrinology and Metabolism 201499923–931. ( 10.1210/jc.2013-2409) [DOI] [PubMed] [Google Scholar]
- 2.Thvilum M Brandt F Almind D Christensen K Brix TH & Hegedüs L. Increased psychiatric morbidity before and after the diagnosis of hypothyroidism: a nationwide register study. Thyroid 201424802–808. ( 10.1089/thy.2013.0555) [DOI] [PubMed] [Google Scholar]
- 3.Lillevang-johansen M Abrahamsen B Jørgensen HL Brix TH & Hegedüs L. Duration of over- and under-treatment of hypothyroidism is associated with increased cardiovascular risk. European Journal of Endocrinology 2019180407–416. ( 10.1530/EJE-19-0006) [DOI] [PubMed] [Google Scholar]
- 4.Thvilum M Brandt F Almind D Christensen K Brix TH & Hegedüs L. Type and extent of somatic morbidity before and after the diagnosis of hypothyroidism. A nationwide register study. PLoS One 20138e75789. ( 10.1371/journal.pone.0075789) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thvilum M Brandt F Brix TH & Hegedüs L. Hypothyroidism is a predictor of disability pension and loss of labor market income: a Danish register-based study. Journal of Clinical Endocrinology and Metabolism 2014993129–3135. ( 10.1210/jc.2014-1407) [DOI] [PubMed] [Google Scholar]
- 6.Hegedus L Bianco AC Jonklaas J Pearce SH Weetman AP & Perros P. Primary hypothyroidism and quality of life. Nature Reviews. Endocrinology 202218230–242. ( 10.1038/s41574-021-00625-8) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Laulund AS Nybo M Brix TH Abrahamsen B Jørgensen HL & Hegedüs L. Duration of thyroid dysfunction correlates with all-cause mortality. The OPENTHYRO register cohort. PLoS One 20149e110437. ( 10.1371/journal.pone.0110437) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lillevang-Johansen M Abrahamsen B Jørgensen HL Brix TH & Hegedüs L. Over- and under-treatment of hypothyroidism is associated with excess mortality: a register-based cohort study. Thyroid 201828566–574. ( 10.1089/thy.2017.0517) [DOI] [PubMed] [Google Scholar]
- 9.Sies H Berndt C & Jones DP. Oxidative stress. Annual Review of Biochemistry 201786715–748. ( 10.1146/annurev-biochem-061516-045037) [DOI] [PubMed] [Google Scholar]
- 10.Giorgio M Dellino GI Gambino V Roda N & Pelicci PG. On the epigenetic role of guanosine oxidation. Redox Biology 202029101398. ( 10.1016/j.redox.2019.101398) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Evans MD Dizdaroglu M & Cooke MS. Oxidative DNA damage and disease: induction, repair and significance. Mutation Research 20045671–61. ( 10.1016/j.mrrev.2003.11.001) [DOI] [PubMed] [Google Scholar]
- 12.Morshed SA & Davies TF. Understanding thyroid cell stress. Journal of Clinical Endocrinology and Metabolism 2020105e66–e69. ( 10.1210/clinem/dgz193) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Poulsen HE Nadal LL Broedbaek K Nielsen PE & Weimann A. Detection and interpretation of 8-oxodG and 8-oxoGua in urine, plasma and cerebrospinal fluid. Biochimica et Biophysica Acta 20141840801–808. ( 10.1016/j.bbagen.2013.06.009) [DOI] [PubMed] [Google Scholar]
- 14.Frijhoff J, Winyard PG, Zarkovic N, Davies SS, Stocker R, Cheng D, Knight AR, Taylor EL, Oettrich J, Ruskovska T, et al. Clinical relevance of biomarkers of oxidative stress. Antioxidants and Redox Signaling 2015231144–1170. ( 10.1089/ars.2015.6317) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Broedbaek K Køster-Rasmussen R Siersma V Persson F Poulsen HE & de Fine Olivarius N. Urinary albumin and 8-oxo-7,8-dihydroguanosine as markers of mortality and cardiovascular disease during 19 years after diagnosis of type 2 diabetes: a comparative study of two markers to identify high risk patients. Redox Biology 201713363–369. ( 10.1016/j.redox.2017.06.005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Broedbaek K, Siersma V, Henriksen T, Weimann A, Petersen M, Andersen JT, Jimenez-Solem E, Stovgaard ES, Hansen LJ, Henriksen JE, et al. Urinary markers of nucleic acid oxidation and long-term mortality of newly diagnosed type 2 diabetic patients. Diabetes Care 2011342594–2596. ( 10.2337/dc11-1620) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Loft S Olsen A Møller P Poulsen HE & Tjønneland A. Association between 8-oxo-7,8-dihydro-2′-deoxyguanosine excretion and risk of postmenopausal breast cancer: nested case-control study. Cancer Epidemiology, Biomarkers and Prevention 2013221289–1296. ( 10.1158/1055-9965.EPI-13-0229) [DOI] [PubMed] [Google Scholar]
- 18.Loft S Svoboda P Kasai H Tjønneland A Vogel U Møller P Overvad K & Raaschou-Nielsen O. Prospective study of 8-oxo-7,8-dihydro-2′-deoxyguanosine excretion and the risk of lung cancer. Carcinogenesis 2006271245–1250. ( 10.1093/carcin/bgi313) [DOI] [PubMed] [Google Scholar]
- 19.Larsen CB Riis KR Winther KH Larsen EL Ellervik C Hegedüs L Brix TH Poulsen HE & Bonnema SJ. Treatment of hyperthyroidism reduces systemic oxidative stress, as measured by markers of RNA and DNA damage. Journal of Clinical Endocrinology and Metabolism 2021106e2512–e2520. ( 10.1210/clinem/dgab273) [DOI] [PubMed] [Google Scholar]
- 20.Obling ML Nicolaisen P Brix TH Winther KH Hansen S Hegedüs L Hermann AP & Bonnema SJ. Restoration of euthyroidism in women with Hashimoto’s thyroiditis changes bone microarchitecture but not estimated bone strength. Endocrine 202171397–406. ( 10.1007/s12020-020-02398-y) [DOI] [PubMed] [Google Scholar]
- 21.Medici BR, Nygaard B, la Cour JL, Krakauer M, Brønden A, Sonne MP, Holst JJ, Rehfeld JF, Vilsbøll T, Faber J, et al. Effects of levothyroxine substitution therapy on hunger and food intake in individuals with hypothyroidism. Endocrine Connections 202312. ( 10.1530/EC-23-0314) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fuchs A Mejdahl MR Kühl JT Stisen ZR Nilsson EJP Køber LV Nordestgaard BG & Kofoed KF. Normal values of left ventricular mass and cardiac chamber volumes assessed by 320-detector computed tomography angiography in the Copenhagen General Population Study. European Heart Journal. Cardiovascular Imaging 2016171009–1017. ( 10.1093/ehjci/jev337) [DOI] [PubMed] [Google Scholar]
- 23.Rasmussen ST Andersen JT Nielsen TK Cejvanovic V Petersen KM Henriksen T Weimann A Lykkesfeldt J & Poulsen HE. Simvastatin and oxidative stress in humans: a randomized, double-blinded, placebo-controlled clinical trial. Redox Biology 2016932–38. ( 10.1016/j.redox.2016.05.007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Weimann A Belling D & Poulsen HE. Quantification of 8-oxo-guanine and guanine as the nucleobase, nucleoside and deoxynucleoside forms in human urine by high-performance liquid chromatography-electrospray tandem mass spectrometry. Nucleic Acids Research 200230E7. ( 10.1093/nar/30.2.e7) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Henriksen T Weimann A Larsen EL & Poulsen HE. Quantification of 8-oxo-7,8-dihydro-2′-deoxyguanosine and 8-oxo-7,8-dihydro-guanosine concentrations in urine and plasma for estimating 24-h urinary output. Free Radical Biology and Medicine 2021172350–357. ( 10.1016/j.freeradbiomed.2021.06.014) [DOI] [PubMed] [Google Scholar]
- 26.Loft S Vistisen K Ewertz M Tjønneland A Overvad K & Poulsen HE. Oxidative DNA damage estimated by 8-hydroxydeoxyguanosine excretion in humans: influence of smoking, gender and body mass index. Carcinogenesis 1992132241–2247. ( 10.1093/carcin/13.12.2241) [DOI] [PubMed] [Google Scholar]
- 27.Poulsen HE Weimann A Henriksen T Kjær LK Larsen EL Carlsson ER Christensen CK Brandslund I & Fenger M. Oxidatively generated modifications to nucleic acids in vivo: measurement in urine and plasma. Free Radical Biology and Medicine 2019145336–341. ( 10.1016/j.freeradbiomed.2019.10.001) [DOI] [PubMed] [Google Scholar]
- 28.Garratt LW, Mistry V, Singh R, Sandhu JK, Sheil B, Cooke MS, Sly PD. & ARESTCF. Interpretation of urinary 8-oxo-7,8-dihydro-2’-deoxyguanosine is adversely affected by methodological inaccuracies when using a commercial ELISA. Free Radical Biology and Medicine 2010481460–1464. ( 10.1016/j.freeradbiomed.2010.02.017) [DOI] [PubMed] [Google Scholar]
- 29.Jørs A Lund MAV Jespersen T Hansen T Poulsen HE & Holm JC. Urinary markers of nucleic acid oxidation increase with age, obesity and insulin resistance in Danish children and adolescents nucleotide oxidation in childhood obesity. Free Radical Biology and Medicine 202015581–86. ( 10.1016/j.freeradbiomed.2020.05.009) [DOI] [PubMed] [Google Scholar]
- 30.Gussekloo J Van Exel E De Craen AJM Meinders AE Frölich M & Westendorp RGJ. Thyroid status, disability and cognitive function, and survival in old age. JAMA 20042922591–2599. ( 10.1001/jama.292.21.2591) [DOI] [PubMed] [Google Scholar]
- 31.Nanda N Bobby Z & Hamide A. Oxidative stress and protein glycation in primary hypothyroidism. Male/female difference. Clinical and Experimental Medicine 20088101–108. ( 10.1007/s10238-008-0164-0) [DOI] [PubMed] [Google Scholar]
- 32.Santi A Duarte MMMF Moresco RN Menezes C Bagatini MD Schetinger MRC & Loro VL. Association between thyroid hormones, lipids and oxidative stress biomarkers in overt hypothyroidism. Clinical Chemistry and Laboratory Medicine 2010481635–1639. ( 10.1515/CCLM.2010.309) [DOI] [PubMed] [Google Scholar]
- 33.Nanda N Bobby Z Hamide A Koner BC & Sridhar MG. Association between oxidative stress and coronary lipid risk factors in hypothyroid women is independent of body mass index. Metabolism: Clinical and Experimental 2007561350–1355. ( 10.1016/j.metabol.2007.05.015) [DOI] [PubMed] [Google Scholar]
- 34.Torun AN Kulaksizoglu S Kulaksizoglu M Pamuk BO Isbilen E & Tutuncu NB. Serum total antioxidant status and lipid peroxidation marker malondialdehyde levels in overt and subclinical hypothyroidism. Clinical Endocrinology 200970469–474. ( 10.1111/j.1365-2265.2008.03348.x) [DOI] [PubMed] [Google Scholar]
- 35.Erdamar H Demirci H Yaman H Erbil MK Yakar T Sancak B Elbeg S Biberoğlu G & Yetkin I. The effect of hypothyroidism, hyperthyroidism, and their treatment on parameters of oxidative stress and antioxidant status. Clinical Chemistry and Laboratory Medicine 2008461004–1010. ( 10.1515/CCLM.2008.183) [DOI] [PubMed] [Google Scholar]
- 36.Lassoued S Mseddi M Mnif F Abid M Guermazi F Masmoudi H El Feki A & Attia H. A comparative study of the oxidative profile in Graves’ disease, Hashimoto’s thyroiditis, and papillary thyroid cancer. Biological Trace Element Research 2010138107–115. ( 10.1007/s12011-010-8625-1) [DOI] [PubMed] [Google Scholar]
- 37.Erem C Suleyman AK Civan N Mentese A Nuhoglu İ Uzun A Coskun H & Deger O. The effect of L-thyroxine replacement therapy on ischemia-modified albümin and malondialdehyde levels in patients with overt and subclinical hypothyroidism. Endocrine Research 201641350–360. ( 10.3109/07435800.2016.1163722) [DOI] [PubMed] [Google Scholar]
- 38.Haribabu A Reddy VS Pallavi Ch Bitla AR Sachan A Pullaiah P Suresh V Rao PVLNS & Suchitra MM. Evaluation of protein oxidation and its association with lipid peroxidation and thyrotropin levels in overt and subclinical hypothyroidism. Endocrine 201344152–157. ( 10.1007/s12020-012-9849-y) [DOI] [PubMed] [Google Scholar]
- 39.Baskol G Atmaca H Tanriverdi F Baskol M Kocer D & Bayram F. Oxidative stress and enzymatic antioxidant status in patients with hypothyroidism before and after treatment. Experimental and Clinical Endocrinology and Diabetes 2007115522–526. ( 10.1055/s-2007-981457) [DOI] [PubMed] [Google Scholar]
- 40.Mancini A Di Segni C Raimondo S Olivieri G Silvestrini A Meucci E & Currò D. Thyroid hormones, oxidative stress, and inflammation. Mediators of Inflammation 20162016;Epub 6757154. ( 10.1155/2016/6757154) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Reddy VS Gouroju S Suchitra MM Suresh V Sachan A Srinivasa Rao PVLN & Bitla AR. Antioxidant defense in overt and subclinical hypothyroidism. Hormone and Metabolic Research 201345754–758. ( 10.1055/s-0033-1348262) [DOI] [PubMed] [Google Scholar]
- 42.Gerenova J & Gadjeva V. Oxidative stress and antioxidant enzyme activities in patients with Hashimoto’s thyroiditis. Comparative Clinical Pathology 200716259–264. ( 10.1007/s00580-007-0689-8) [DOI] [Google Scholar]
- 43.Nanda N Bobby Z & Hamide A. Oxidative Stress in Anti thyroperoxidase antibody positive hypothyroid patients. Asian Journal of Biochemistry 2011754–58. ( 10.3923/ajb.2012.54.58) [DOI] [Google Scholar]
- 44.Ates I Altay M Yilmaz FM Topcuoglu C Yilmaz N Berker D & Guler S. The impact of levothyroxine sodium treatment on oxidative stress in Hashimoto’s thyroiditis. European Journal of Endocrinology 2016174727–734. ( 10.1530/EJE-15-1061) [DOI] [PubMed] [Google Scholar]
- 45.Ates I Yilmaz FM Altay M Yilmaz N Berker D & Güler S. The relationship between oxidative stress and autoimmunity in Hashimoto’s thyroiditis. European Journal of Endocrinology 2015173791–799. ( 10.1530/EJE-15-0617) [DOI] [PubMed] [Google Scholar]
- 46.Ates I Arikan MF Altay M Yilmaz FM Yilmaz N Berker D & Guler S. The effect of oxidative stress on the progression of Hashimoto’s thyroiditis. Archives of Physiology and Biochemistry 2018124351–356. ( 10.1080/13813455.2017.1408660) [DOI] [PubMed] [Google Scholar]
- 47.Azizi F Raiszadeh F Solati M Etemadi A Rahmani M & Arabi M. Serum paraoxonase 1 activity is decreased in thyroid dysfunction. Journal of Endocrinological Investigation 200326703–709. ( 10.1007/BF03347350) [DOI] [PubMed] [Google Scholar]
- 48.Porcelli T & Salvatore D. Targeting the right population for T3 + T4 combined therapy: where are we now and where to next? Endocrine 202069244–248. ( 10.1007/s12020-020-02391-5) [DOI] [PubMed] [Google Scholar]
- 49.Lee J Lee M Kim JU Song KI Il Choi YS & Cheong SS. Carvedilol reduces plasma 8-hydroxy-2′-deoxyguanosine in mild to moderate hypertension: a pilot study. Hypertension 200545986–990. ( 10.1161/01.HYP.0000164569.63160.24) [DOI] [PubMed] [Google Scholar]
- 50.Ogawa S Mori T Nako K & Ito S. Combination therapy with renin-angiotensin system inhibitors and the calcium channel blocker azelnidipine decreases plasma inflammatory markers and urinary oxidative stress markers in patients with diabetic nephropathy. Hypertension Research 2008311147–1155. ( 10.1291/hypres.31.1147) [DOI] [PubMed] [Google Scholar]
- 51.Abe M Maruyama N Okada K Matsumoto S Matsumoto K & Soma M. Additive antioxidative effects of azelnidipine on angiotensin receptor blocker olmesartan treatment for type 2 diabetic patients with albuminuria. Hypertension Research 201134935–941. ( 10.1038/hr.2011.67) [DOI] [PubMed] [Google Scholar]
- 52.Broedbaek K, Henriksen T, Weimann A, Petersen M, Andersen JT, Afzal S, Jimenez-Solem E, Persson F, Parving HH, Rossing P, et al. Long-term effects of irbesartan treatment and smoking on nucleic acid oxidation in patients with type 2 diabetes and microalbuminuria: an irbesartan in patients with type 2 diabetes and microalbuminuria (IRMA 2) substudy. Diabetes Care 2011341192–1198. ( 10.2337/dc10-2214) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.De Jonge PJF Siersema PD Van Breda SGJ Van Zoest KPM Bac DJ Leeuwenburgh I Ouwendijk RJT Van Dekken H Kusters JG & Kuipers EJ. Proton pump inhibitor therapy in gastro-oesophageal reflux disease decreases the oesophageal immune response but does not reduce the formation of DNA adducts. Alimentary Pharmacology and Therapeutics 200828127–136. ( 10.1111/j.1365-2036.2008.03699.x) [DOI] [PubMed] [Google Scholar]
- 54.Sørensen AL Hasselbalch HC Nielsen CH Poulsen HE & Ellervik C. Statin treatment, oxidative stress and inflammation in a Danish population. Redox Biology 201921101088. ( 10.1016/j.redox.2018.101088) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tian X Li N Su R Dai C & Zhang R. Selenium supplementation may decrease thyroid peroxidase antibody titer via reducing oxidative stress in euthyroid patients with autoimmune thyroiditis. International Journal of Endocrinology 202020209210572. ( 10.1155/2020/9210572) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Winther KH Rayman MP Bonnema SJ & Hegedüs L. Selenium in thyroid disorders—essential knowledge for clinicians. Nature Reviews. Endocrinology 202016165–176. ( 10.1038/s41574-019-0311-6) [DOI] [PubMed] [Google Scholar]
- 57.Grew IS Cejvanovic V Broedbaek K Henriksen T Petersen M Andersen JT Jimenez-Solem E Weimann A & Poulsen HE. Diurnal variation of urinary markers of nucleic acid oxidation. Scandinavian Journal of Clinical and Laboratory Investigation 201474336–343. ( 10.3109/00365513.2014.891258) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Some or all datasets generated during and/or analyzed during the current study are available from the corresponding author upon reasonable request.



 This work is licensed under a
This work is licensed under a