Abstract
Objective
The inflammatory microenvironment has been implicated in differentiated thyroid cancer (DTC). Inflammatory stimuli induce the release of components of neutrophils into extracellular space, leading to formation of neutrophil extracellular trap (NET), which can stimulate growth and progression of cancer. Generation of activated factor XII and thrombin is also involved in cancer progression. This study attempted to determine whether the level of circulating markers of NET, activated factor XII, and endogenous thrombin potential may be useful for detecting the recurrence of DTC.
Methods
A total of 122 patients with DTC were recruited during the postoperative follow-up period. Measurement of the levels of circulating markers of NET (neutrophil elastase, histone–DNA complex, cell-free dsDNA), activated factor XII, and endogenous thrombin potential was performed.
Results
A significantly elevated level of neutrophil elastase was detected in patients with recurrence (n = 12) compared to those without recurrence (n = 110), while significant elevation of the levels of other markers was not observed. The value for area under the curve (0.717, P = 0.018) of neutrophil elastase for detecting recurrence of DTC was superior to that (0.661, P = 0.051) of serum thyroglobulin. An elevated level of neutrophil elastase was significantly associated with recurrence of DTC independent of serum thyroglobulin.
Conclusions
Because an elevated level of neutrophil elastase was detected in patients with recurrence of DTC and showed a significant association with recurrence of DTC, it can be proposed as a novel biomarker for use in detecting recurrence of DTC along with other tests.
Keywords: differentiated thyroid cancer, neutrophil elastase, recurrence, biomarker
Introduction
Differentiated thyroid cancer (DTC) is the most commonly diagnosed endocrine malignancy (1) and the risk of its recurrence varies from patient to patient, ranging from less than 5% to more than 20% (2). Efforts have been made to identify various biomarkers for detecting recurrence of DTC (3), however, serum thyroglobulin is still primarily used for follow-up of patients with DTC, particularly after total thyroidectomy and radioactive iodine ablation (4). In DTC patients receiving other treatment, the clinical utility of serum thyroglobulin can be obscured (5) and detection of cancer recurrence relies heavily on other tests such as imaging studies (2). Limitations of serum thyroglobulin assays and caveats regarding interpretation of their results have also been well recognized (6).
Inflammation is a fundamental feature of cancer and a number of studies have reported on its implications in thyroid cancer (7). Neutrophils have critical functions in the inflammatory response and their complex roles in cancer are believed to be related to the surrounding microenvironment (8). In thyroid cancer, activation and recruitment of neutrophils to tumor sites as well as their contribution to the angiogenic and inflammatory tumor microenvironment has been demonstrated (9, 10).
Upon activation, release of histones, DNA strands, and proteolytic enzymes such as neutrophil elastase by neutrophils into extracellular spaces leads to formation of neutrophil extracellular trap (NET) (11). NET has been linked with pathogenesis of cancer, including proliferation of cancer cells, angiogenesis, awakening dormant cancer cells, and metastasis (12, 13). Therefore, studies addressing the significant association of NET with risk of recurrence in several cancers have recently been reported (14, 15). However, little is known about the association of NET with recurrence of DTC.
Cancer cells have the capacity to activate coagulation factor XII (16), leading to initiation of the contact system and the subsequent pro-inflammatory process (17). Activation of the contact system by NET can also occur through contact with negatively charged surfaces of exposed DNA (18). Enhanced generation of thrombin by cancer cells, which is also well recognized (19), is related to tumor growth and progression (20). The impact of thrombin generation on cancer recurrence has been examined in several cancers (21, 22). However, it is still not known whether activated factor XII and generation of thrombin are associated with recurrence of DTC.
In this study the levels of circulating NET, activated factor XII, and thrombin generation were measured in patients with DTC during the postoperative follow-up period in order to determine whether the markers can be considered useful for detecting recurrence of DTC.
Materials and methods
Study population
Patients with DTC undergoing postoperative follow-up after total thyroidectomy or thyroid lobectomy were the subjects of this study. A total of 122 consecutive patients who visited Ulsan University Hospital for postoperative follow-up were enrolled after providing written consent. The study was approved by the hospital’s Institutional Review Board (UUH IRB 2021-08-031) and was conducted in accordance with the Declaration of Helsinki. A retrospective review of electronic medical records was conducted for collection of data on the dates and methods of operation and histopathologic findings for each patient. Staging of tumor–node–metastasis (TNM) was based on the eighth edition of the American Joint Committee on Cancer/Union for International Cancer Control (AJCC/UICC) staging system. Patients diagnosed with other malignancies were excluded. Recurrence of DTC was determined by endocrinologists based on clinical course, laboratory results, imaging studies, and histopathologic findings. Patients were classified into two groups according to recurrence status. Patients who developed recurrence prior to enrollment were classified as patients with recurrence. Patients who had not developed recurrence prior to enrollment were classified as patients without recurrence.
Measurements of NET markers, activated factor XII, and endogenous thrombin potential
Peripheral blood from each patient was collected in sodium citrate tubes (Becton Dickinson, San Jose, CA, USA). Complete blood cell count, coagulation test, thyroid function test, and measurements of serum thyroglobulin and anti-thyroglobulin antibodies were performed for each patient during postoperative follow-up. Plasma from each sample was obtained by centrifuging remnant samples at 1550 g for 15 min and stored at −70°C until thawing for use in the study. Measurement of the level of selected biomarkers was performed as follows: The level of neutrophil elastase was measured with the Human PMN-Elastase Platinum ELISA kit (eBioscience, Vienna, Austria); the level of histone–DNA complex was measured with the Cell Death Detection ELISA kit (Roche Diagnostics); the level of cell-free dsDNA was measured with the Fluoroskan Ascent microplate fluorometer (Thermo Fisher Scientific Inc.) and the Quant-iT PicoGreen dsDNA reagent (Molecular Probes); the level of activated factor XII was measured with the CoaChrom Factor XIIa test kit (CoaChrom Diagnostica GmbH, Maria Enzersdorf, Austria). Evaluation of thrombin generation can be performed using the thrombin generation assay. The assay measures endogenous thrombin potential, which reflects the amount of thrombin activated by tissue factor (23). Measurement of endogenous thrombin potential was performed using the Fluoroskan Ascent fluorometer (Thermo Labsystems, Helsinki, Finland) and the Thrombinoscope (Thrombinoscope BV, Maastricht, the Netherlands) as previously described (23).
Statistical analyses
Examination of significance of differences in variables between the two patient groups was performed with the Mann–Whitney U-test for continuous variables and Fisher's exact test for categorical variables. Receiver operating characteristics (ROC) curve analyses were performed to assess performance of NET markers, activated factor XII, and endogenous thrombin potential in detecting recurrence of DTC. Calculation of optimal cutoff values for each biomarker was also performed using ROC curve analyses. Logistic regression analyses were performed to determine association between elevated levels of selected biomarkers and recurrence of DTC. Collinearity between biomarkers was considered during the analyses and results were expressed with odds ratio (OR) and 95% CI for each biomarker. Variables that showed statistical significance in the univariable analyses were included in the multivariable analyses. In addition, linear regression analyses were performed to examine correlation between variables. Statistical significance was considered at a two-tailed P < 0.05. GraphPad Prism version 9.3.0 (GraphPad Software), MedCalc Software version 20.218 (MedCalc Software, Ostend, Belgium) and IBM SPSS Statistics version 25.0 (IBM Corp.) were used in performance of statistical analyses.
Results
Clinicopathologic characteristics of patients
Among 122 patients with DTC, 12 patients (9.8%) developed cancer recurrence. No difference in age, sex, and tumor size was observed between patients with recurrence and those without recurrence (Table 1). Twelve patients (100%) with recurrence and 99 patients (90.0%) without recurrence underwent total thyroidectomy. In both groups, T3b was the most prevalent stage, followed by T1a. Stages N1a and N1b were more common in patients with recurrence (75.0% vs 39.1%) than in those without recurrence. Distant metastasis was detected in one patient (0.9%) without recurrence. AJCC/UICC prognostic stage I showed the greatest prevalence in both groups, followed by stage II. The proportion of patients who received postoperative radioactive iodine therapy did not significantly differ between two groups.
Table 1.
Clinicopathologic characteristics of 122 patients with differentiated thyroid cancer.
| Characteristics | With recurrence (n = 12) | Without recurrence (n = 110) | P |
|---|---|---|---|
| Age (years) | 55.0 (40.5–63.8) | 58.0 (49.0–65.0) | 0.497 |
| Female sex | 12 (100.0) | 89 (80.9) | 0.125 |
| Tumor size (mm) | 12.5 (5.0–27.5) | 8.0 (6.0–13.0) | 0.305 |
| Operation | 0.599 | ||
| Total thyroidectomy | 12 (100) | 99 (90.0) | |
| Thyroid lobectomy | 0 (0.0) | 11 (10.0) | |
| Primary tumor stage | 0.100 | ||
| T1a | 4 (33.3) | 42 (38.2) | |
| T1b | 0 (0.0) | 9 (8.2) | |
| T2 | 1 (8.3) | 2 (1.8) | |
| T3a | 1 (8.3) | 0 (0.0) | |
| T3b | 6 (50.0) | 57 (51.8) | |
| T4a or T4b | 0 (0.0) | 0 (0.0) | |
| Regional lymph node stage | 0.034 | ||
| N0 | 3 (25.0) | 67 (60.9) | |
| N1a | 7 (58.3) | 35 (31.8) | |
| N1b | 2 (16.7) | 8 (7.3) | |
| Distant metastasis stage | 1.000 | ||
| M0 | 12 (100.0) | 109 (99.1) | |
| M1 | 0 (0.0) | 1 (0.9) | |
| AJCC/UICC prognostic stage | 1.000 | ||
| I | 11 (91.7) | 98 (89.1) | |
| II | 1 (8.3) | 12 (10.9) | |
| Postoperative RAI therapy | 11 (91.7) | 78 (70.9) | 0.177 |
Numbers are presented as median (interquartile range) or number of patients (percentages).
AJCC/UICC, American Joint Committee on Cancer/Union for International Cancer Control; RAI, radioactive iodine.
Difference in the levels of biomarkers according to DTC recurrence status
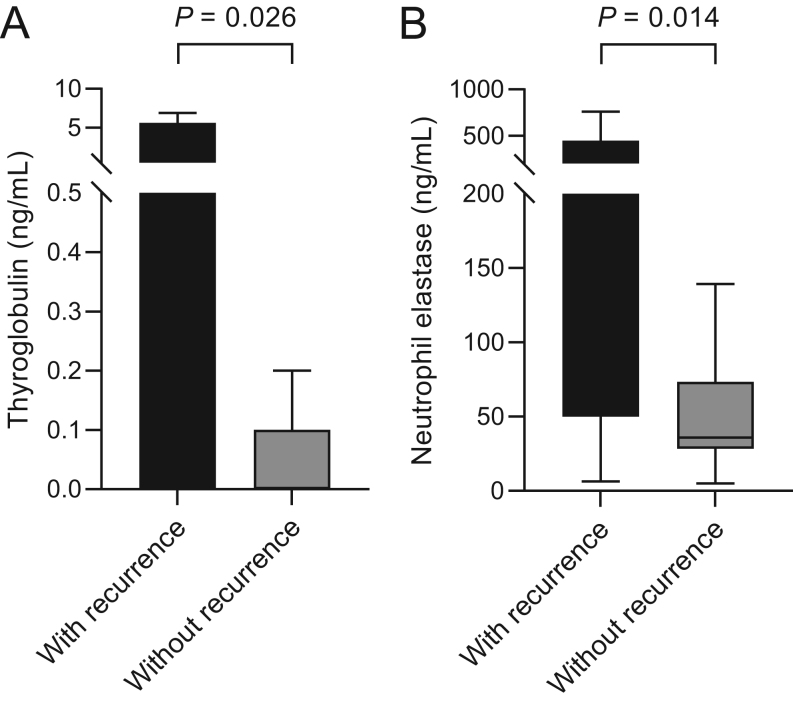
The level of serum thyroglobulin was significantly higher in patients with recurrence compared to those without recurrence (Table 2). No difference in the proportion of patients who were positive for serum anti-thyroglobulin antibody (>20.0 IU/mL) was observed between the two groups. No differences in the levels of triiodothyronine, free thyroxine, and thyroid-stimulating hormone were observed between the two groups. The level of triiodothyronine was not measured in five patients without recurrence. The level of neutrophil elastase was significantly higher in patients with recurrence (median, 116.7 ng/mL vs 35.9 ng/mL, P = 0.014) compared to those without recurrence. No difference in the levels of absolute neutrophil count, other markers of NET (histone–DNA complex and cell-free dsDNA), activated factor XII, and endogenous thrombin potential was observed between the two groups. Distribution of serum thyroglobulin and neutrophil elastase levels in patients is shown in Fig. 1.
Table 2.
Laboratory results for 122 patients with differentiated thyroid cancer.
| Tests | With recurrence (n = 12) | Without recurrence (n = 110) | P |
|---|---|---|---|
| Thyroglobulin (ng/mL) | 0.13 (0.00–5.60) | 0.00 (0.00-0.10) | 0.026 |
| Positive anti-thyroglobulin Ab (number) | 0 (0.0) | 3 (2.7) | 1.000 |
| Triiodothyronine (ng/mL)a | 1.0 (0.8–1.2) | 1.0 (0.9–1.1) | 0.781 |
| Free thyroxine (ng/mL) | 1.8 (1.4–1.8) | 1.6 (1.4–1.8) | 0.355 |
| Thyroid-stimulating hormone (mIU/mL) | 0.2 (0.0–0.7) | 0.1 (0.0–0.4) | 0.273 |
| Absolute neutrophil count (/µL) | 3537 (2808–4624) | 3192 (2731–3903) | 0.298 |
| Neutrophil elastase (ng/mL) | 116.7 (50.1–445.1) | 35.9 (28.4–73.2) | 0.014 |
| Histone–DNA complex (AU) | 125.0 (52.0–319.5) | 82.0 (50.0–156.0) | 0.624 |
| Cell-free dsDNA (ng/mL) | 99.0 (84.2–126.8) | 89.7 (76.4–119.1) | 0.206 |
| Activated factor XII (U/L) | 33.3 (22.1–59.5) | 31.3 (25.2–54.1) | 0.901 |
| Endogenous thrombin potential (nM · min) | 1532.9 (1444.2–1727.3) | 1511.3 (1381.0–1721.4) | 0.502 |
Numbers are presented as median (interquartile range) or number of patients (percentages).
aTriiodothyronine level was not measured in five patients without recurrence.
Ab, antibody; ds, double-stranded.
Figure 1.
Distribution of the level of (A) serum thyroglobulin and (B) neutrophil elastase in patients with differentiated thyroid cancer with recurrence (n = 12) and without recurrence (n = 110) shown in box and whisker plots. The median and interquartile range (IQR) are shown in the plots. Outliers (>1.5 × IQR) are included in the analyses but not shown in the plots for clarity.
Performance of biomarkers for detecting recurrence of DTC
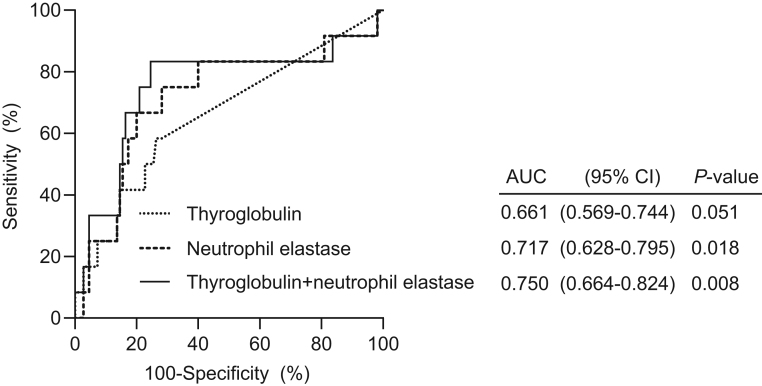
ROC curve analyses were performed to assess performance of NET markers, activated factor XII and endogenous thrombin potential in detecting recurrence of DTC, along with calculation of optimal cutoff values and values for area under the curve (AUC) (Fig. 2). Evaluation of the performance of serum thyroglobulin, a well-recognized follow-up biomarker for DTC, was also performed using ROC curve analyses for comparison. The AUC value for the neutrophil elastase level was 0.717 (95% CI, 0.628–0.795, P = 0.018), which was superior to that of serum thyroglobulin level, 0.661 (95% CI, 0.569–0.744, P = 0.051). The AUC values for other biomarkers did not show statistical significance. A combination marker model of serum thyroglobulin and neutrophil elastase was constructed using a logistic regression and the model yielded an AUC value of 0.750 (95% CI, 0.664–0.824, P = 0.008).
Figure 2.
Performance of the level of serum thyroglobulin and neutrophil elastase in detecting recurrence of differentiated thyroid cancer evaluated using receiver operating characteristic curve (ROC) analysis. Values for area under the ROC curve (AUC) for each curve are presented with the 95% CI. The biomarker ‘Thyroglobulin + neutrophil elastase’ represents a combined model of thyroglobulin and neutrophil elastase.
Based on the cutoff values calculated in ROC curve analyses, assessment for recurrence of DTC was performed using logistic regression analyses (Table 3). In univariable logistic regression analyses, an elevated level of serum thyroglobulin (>0.07 ng/mL), neutrophil elastase (>64.0 ng/mL), and histone–DNA complex (>187.0 AU) showed an association with an increased risk of DTC recurrence. Multivariable analyses were performed using biomarkers that showed statistical significance in univariable analyses and collinearity between these variables was considered. In multivariable analyses, an elevated level of neutrophil elastase (OR, 7.37, P = 0.005) and histone–DNA complex (OR, 4.96, P = 0.016) showed an independent association with an increased risk of DTC recurrence. In multivariable linear regression, the level of histone–DNA complex (β = 0.304, P < 0.001) contributed significantly to the level of neutrophil elastase (Table 4).
Table 3.
Odds ratios regarding association between biomarkers and recurrence of differentiated thyroid cancer.
| Univariable | Multivariable | Multivariable | ||||
|---|---|---|---|---|---|---|
| OR (95% CI) | P | OR (95% CI) | P | OR (95% CI) | P | |
| Thyroglobulin (>0.07 vs ≤0.07 ng/mL) | 3.91 (1.15–13.29) | 0.029 | 3.71 (1.02–13.42) | 0.046 | 4.79 (1.31–17.52) | 0.018 |
| Thyroid-stimulating hormone (>0.24 vs ≤0.24 mIU/mL) | 0.42 (0.11–1.62) | 0.205 | ||||
| Neutrophil elastase (>64.0 vs ≤64.0 ng/mL) | 7.65 (1.94–30.12) | 0.004 | 7.37 (1.83–29.78) | 0.005 | ||
| Histone–DNA complex (>187.0 vs ≤187.0 AU) | 4.00 (1.18–13.61) | 0.026 | 4.96 (1.35–18.26) | 0.016 | ||
| Cell-free dsDNA (>79.0 vs ≤79.0 ng/mL) | 6.04 (0.75–48.56) | 0.091 | ||||
| Activated factor XII (>36.7 vs ≤36.7 mg/mL) | 2.06 (0.62–6.82) | 0.239 | ||||
| Endogenous thrombin potential (>1417.6 vs ≤1417.6 nM · min) | 4.92 (0.61–39.65) | 0.134 | ||||
Multivariable logistic regression analyses were performed using statistically significant biomarkers in univariable analyses and collinearity between histone–DNA complex and neutrophil elastase was considered.
OR, odds ratio; CI, confidence interval; ds, double-stranded.
Table 4.
Contributing factors to the level of neutrophil elastase assessed by linear regression.
| Variables | Univariable | Multivariable | Multivariable | ||||||
|---|---|---|---|---|---|---|---|---|---|
| β | s.e. | P | β | S.E. | P | β | S.E. | P | |
| Age (years) | −2.038 | 1.812 | 0.263 | −0.178 | 1.426 | 0.901 | −1.462 | 1.670 | 0.383 |
| Female sex | 41.902 | 51.295 | 0.416 | 26.185 | 37.797 | 0.490 | 30.327 | 44.716 | 0.499 |
| Thyroglobulin (ng/mL) | −0.009 | 0.027 | 0.742 | −0.008 | 0.020 | 0.692 | −0.008 | 0.023 | 0.742 |
| Positive anti-thyroglobulin Ab | 194.849 | 124.107 | 0.119 | −41.532 | 109.513 | 0.705 | −9.739 | 130.053 | 0.940 |
| Triiodothyronine (ng/mL) | 84.535 | 83.127 | 0.311 | 75.355 | 74.896 | 0.317 | 104.228 | 88.707 | 0.243 |
| Free thyroxine(ng/mL) | 56.837 | 69.834 | 0.417 | 26.551 | 56.902 | 0.642 | 1.418 | 67.103 | 0.983 |
| Thyroid-stimulating hormone (mIU/mL) | −18.485 | 11.957 | 0.125 | −6.111 | 9.347 | 0.515 | −16.018 | 10.896 | 0.145 |
| Absolute neutrophil count (/uL) | 0.016 | 0.015 | 0.294 | −0.009 | 0.013 | 0.467 | −0.005 | 0.015 | 0.749 |
| Histone–DNA complex (AU) | 0.295a | 0.052 | <0.001 | 0.307a | 0.046 | <0.001 | |||
| Cell-free dsDNA (ng/mL) | 1.557a | 0.585 | 0.009 | 0.820 | 0.534 | 0.128 | |||
| Activated factor XII (U/L) | 0.729 | 0.414 | 0.081 | −0.113 | 0.336 | 0.737 | 0.593 | 0.377 | 0.118 |
| Endogenous thrombin potential (nM · min) | 0.006 | 0.061 | 0.916 | −0.015 | 0.048 | 0.746 | −0.050 | 0.056 | 0.374 |
Regression coefficients (β) and associated standard errors (SEs) are presented. Multiple linear regression analyses were performed using all variables and collinearity between NET markers was considered.
aP < 0.05
Ab, antibody; ds, double-stranded.
Discussion
The results of this study showed a significant elevation of the level of neutrophil elastase in patients with recurrence of DTC. Neutrophil elastase exerts pro-tumorigenic effects (24) and promotes dissemination and metastasis of tumor cells (25). It is involved in awakening cancer cells that are in dormancy, which has been linked to cancer recurrence (12) and has shown association with prognosis of cancer patients (26). Cancer cells have the capacity to produce neutrophil elastase (27) and upregulation of neutrophil elastase has been observed in various types of cancer (26). A high level of neutrophil elastase in breast tumor extracts is regarded as an independent indicator of poor prognosis and poor response to tamoxifen therapy (28). Because neutrophil elastase originates mainly from neutrophils (29) and its circulating level was measured in our study, neutrophils in blood circulation are its likely source. It is noteworthy that the levels of neutrophil elastase differed between patients with recurrence and those without recurrence while no difference in absolute neutrophil count was observed between the two groups. Therefore, the elevation of neutrophil elastase level observed in patients with recurrence of DTC might be due to distinctive behaviors of neutrophils in these patients, such as production of neutrophil elastase.
Thyroid cancer cells produce granulocyte–macrophage colony-stimulating factor, which leads to activation of neutrophils, and also directly induce activation of neutrophils (10). Activated neutrophils induce expression of vascular endothelial growth factor A and matrix metallopeptidase 9, thereby contributing to the angiogenic and inflammatory microenvironment of thyroid cancer (10). Thyroid cancer cells also secrete interleukin 8 for recruitment of neutrophils to tumor sites and formation of an immunosuppressive tumor microenvironment, eventually promoting cancer progression (9). Neutrophil-to-lymphocyte ratio has been examined, in this perspective, for its prognostic value in patients with thyroid cancer, and studies have yielded conflicting results (30).
During the inflammatory response, activated neutrophils can form NET consisting of histones, DNA strands, and proteolytic enzymes such as neutrophil elastase (11). An association of inflammation with recurrence of several cancer types has been reported (31, 32), therefore, it is probable that elevation of the neutrophil elastase level is a result of active formation of NET accompanied by inflammation in patients with recurrence of DTC. Except for neutrophil elastase, no significant elevation of the other NET markers, histone–DNA complex, and cell-free dsDNA was detected in patients with recurrence of DTC. This finding can likely be attributed to rapid escape of neutrophil elastase from azurophilic granules upon activation of neutrophils while formation of histone–DNA complex occurs after translocation of neutrophil elastase into the nucleus (33).
NET plays substantial roles in cancer progression through promotion of angiogenesis and proliferation of tumor cells (34). NET also contributes to cancer recurrence and metastasis by awakening dormant cancer cells (12) and capturing circulating tumor cells (35). Significant association of NET markers with recurrence and prognosis in various types of cancer has been demonstrated (36, 37). A study of patients with primary hepatic malignancies reported shorter recurrence-free survival in patients with a high level of NET markers (36). Relation of an elevated level of NET markers with poor prognosis in high-grade ovarian cancer has been reported (15). Similarly, we observed that an elevated level of neutrophil elastase was an independent marker for detecting recurrence of DTC. Our results showed that an elevated level of histone–DNA complex was also useful in detecting recurrence of DTC even though significant difference in its level was not observed between the two groups. Neutrophil elastase is required for formation of histone–DNA complex (33) and linear regression analyses showed significant correlation between the level of histone–DNA complex and neutrophil elastase. Therefore, conduct of additional studies including a larger number of patients may lead to discovery of statistically significant differences in the level of histone–DNA complex according to recurrence status.
Our results demonstrated the significant effectiveness of neutrophil elastase for detecting recurrence of DTC, which was superior to serum thyroglobulin. Serum thyroglobulin has been used for decades as a reliable marker for detecting recurrence of DTC after total thyroidectomy and radioactive iodine ablation (2). Nevertheless, it is widely acknowledged that careful interpretation of the level of serum thyroglobulin is required due to limitations of thyroglobulin assays (38). In particular, anti-thyroglobulin antibodies present in approximately 20% of patients with DTC can interfere with measurements of serum thyroglobulin and thereby hinder the use of serum thyroglobulin for follow-up (39). Treatments other than total thyroidectomy and radioactive iodine ablation draw attention to the necessity for use of other follow-up biomarkers as the utility of serum thyroglobulin is complicated by thyroglobulin from remnant thyroid tissue (5). Our results demonstrated that an elevated level of neutrophil elastase was a significant marker for detecting recurrence of DTC independent of serum thyroglobulin. Thus, additional use of neutrophil elastase may be helpful in detecting cancer recurrence in patients with DTC.
Factor XII, following activation by contact with negatively charged surfaces, drives the kallikrein–kinin system, leading to formation of bradykinin, an inflammation-promoting peptide (40). Aggregation, degranulation, and activation of neutrophils can also be induced by activated factor XII (41), contributing to the inflammatory microenvironment. Thus, association of factor XII with cancer-related inflammation and cancer progression has been reported. Regarding its effect on prognosis of patients with thyroid cancer, Luo et al. observed an association of overexpression of factor XII genes with poorer overall survival in patients with papillary thyroid cancer (42). Involvement of thrombin in angiogenesis, tumor progression, and metastasis (20) has been described and an association between generation of thrombin and cancer recurrence has been reported (21). A study of breast cancer patients reported that high endogenous thrombin potential was observed in patients with very early relapse (21). In our results, no association was observed between the level of activated factor XII, endogenous thrombin potential, and recurrence of DTC. This result is likely due to the relatively small sizes of DTC tumors in our study compared with other solid tumors. Therefore, additional studies will be necessary in order to understand the implications of activated factor XII and thrombin generation, particularly in cases of extensive metastatic DTC where greater tumor burden can be expected.
This study has several limitations. First, this study included few DTC patients with AJCC/UICC prognostic stage III and IV. It is consistent with the previous study which reported a significant decrease in the number of patients with stage III and IV after reclassification based on AJCC eighth edition (43). The significant association of the level of neutrophil elastase with cancer recurrence even in DTC patients expected to have a favorable prognosis is remarkable. Second, because the clinical utility of serum thyroglobulin level has not been well-established in follow-up of patients after thyroid lobectomy (5), there is interest in identification of new biomarkers for these patients. Unfortunately, because only 11 patients in our study population without recurrence underwent thyroid lobectomy, additional statistical analyses were unavailable. Studies on association between the level of neutrophil elastase and recurrence of DTC in patients who underwent thyroid lobectomy may provide useful information. Finally, this study was a retrospective cross-sectional study. Thus, conduct of prospective validation studies including a larger number of patients with DTC is warranted in order to validate our findings.
In summary, this study demonstrated that the level of circulating neutrophil elastase was elevated in patients with recurrence of DTC and that an elevated level of neutrophil elastase showed a significant association with recurrence of DTC independent of serum thyroglobulin.
Conclusion
Because the level of circulating neutrophil elastase has significant value for use in detecting recurrence of DTC independent of serum thyroglobulin, it can be proposed as an additional biomarker for use in follow-up of DTC. In the near future, conduct of prospective validation studies on the clinical usefulness of neutrophil elastase in detecting recurrence of DTC is warranted, along with examination of the behaviors and roles of neutrophil elastase and NET in the pathogenesis of DTC recurrence.
Declaration of interest
There are no competing financial interests.
Funding
This research was supported by a grant of the Manufacturing Human Cell-based Artificial Blood and Platform Technology Development for Transfusion, funded by the Multi-Ministrial Research Project, Republic of Korea (grant number: HX23C1718).
Author contribution statement
H.L.: formal analysis (equal); writing – original draft (equal). T.-S.K.: formal analysis (equal); writing – original draft (equal). J.-Y.G.: data curation (equal); investigation (equal). M.R.Y.: data curation (equal); investigation (equal). S.-E.L.: data curation (equal); investigation (equal). E.S.K.: conceptualization (equal); methodology (equal); writing – review and editing (equal). H.K.K.: conceptualization (equal); methodology (equal); writing – review and editing (equal).
References
- 1.Pacini F, Schlumberger M, Dralle H, Elisei R, Smit JW, Wiersinga W. & European Thyroid Cancer Taskforce. European consensus for the management of patients with differentiated thyroid carcinoma of the follicular epithelium. European Journal of Endocrinology 2006154787–803. ( 10.1530/eje.1.02158) [DOI] [PubMed] [Google Scholar]
- 2.Haugen BR, Alexander EK, Bible KC, Doherty GM, Mandel SJ, Nikiforov YE, Pacini F, Randolph GW, Sawka AM, Schlumberger M, et al.2015 American Thyroid Association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: the American Thyroid Association guidelines task force on thyroid nodules and differentiated thyroid cancer. Thyroid 2016261–133. ( 10.1089/thy.2015.0020) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nixon AM Provatopoulou X Kalogera E Zografos GN & Gounaris A. Circulating thyroid cancer biomarkers: current limitations and future prospects. Clinical Endocrinology 201787117–126. ( 10.1111/cen.13369) [DOI] [PubMed] [Google Scholar]
- 4.Pelttari H Välimäki MJ Löyttyniemi E & Schalin-Jäntti C. Post-ablative serum thyroglobulin is an independent predictor of recurrence in low-risk differentiated thyroid carcinoma: a 16-year follow-up study. European Journal of Endocrinology 2010163757–763. ( 10.1530/EJE-10-0553) [DOI] [PubMed] [Google Scholar]
- 5.Park S, Jeon MJ, Oh HS, Lee YM, Sung TY, Han M, Han JM, Kim TY, Chung KW, Kim WB, et al. Changes in serum thyroglobulin levels after lobectomy in patients with low-risk papillary thyroid cancer. Thyroid 201828997–1003. ( 10.1089/thy.2018.0046) [DOI] [PubMed] [Google Scholar]
- 6.Giovanella L, Clark PM, Chiovato L, Duntas L, Elisei R, Feldt-Rasmussen U, Leenhardt L, Luster M, Schalin-Jäntti C, Schott M, et al. Thyroglobulin measurement using highly sensitive assays in patients with differentiated thyroid cancer: a clinical position paper. European Journal of Endocrinology 2014171R33–R46. ( 10.1530/EJE-14-0148) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cunha LL Marcello MA & Ward LS. The role of the inflammatory microenvironment in thyroid carcinogenesis. Endocrine-Related Cancer 201421R85–R103. ( 10.1530/ERC-13-0431) [DOI] [PubMed] [Google Scholar]
- 8.Hedrick CC & Malanchi I. Neutrophils in cancer: heterogeneous and multifaceted. Nature Reviews. Immunology 202222173–187. ( 10.1038/s41577-021-00571-6) [DOI] [PubMed] [Google Scholar]
- 9.He J Zhou M Yin J Wan J Chu J Jia J Sheng J Wang C Yin H & He F. METTL3 restrains papillary thyroid cancer progression via m(6)A/c-Rel/IL-8-mediated neutrophil infiltration. Molecular Therapy 2021291821–1837. ( 10.1016/j.ymthe.2021.01.019) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Galdiero MR, Varricchi G, Loffredo S, Bellevicine C, Lansione T, Ferrara AL, Iannone R, di Somma S, Borriello F, Clery E, et al. Potential involvement of neutrophils in human thyroid cancer. PLoS One 201813e0199740. ( 10.1371/journal.pone.0199740) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brinkmann V Reichard U Goosmann C Fauler B Uhlemann Y Weiss DS Weinrauch Y & Zychlinsky A. Neutrophil extracellular traps kill bacteria. Science 20043031532–1535. ( 10.1126/science.1092385) [DOI] [PubMed] [Google Scholar]
- 12.Albrengues J, Shields MA, Ng D, Park CG, Ambrico A, Poindexter ME, Upadhyay P, Uyeminami DL, Pommier A, Küttner V, et al. Neutrophil extracellular traps produced during inflammation awaken dormant cancer cells in mice. Science 2018361eaao4227. ( 10.1126/science.aao4227) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rayes RF, Mouhanna JG, Nicolau I, Bourdeau F, Giannias B, Rousseau S, Quail D, Walsh L, Sangwan V, Bertos N, et al. Primary tumors induce neutrophil extracellular traps with targetable metastasis promoting effects. JCI Insight 20194e128008. ( 10.1172/jci.insight.128008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zenlander R Havervall S Magnusson M Engstrand J Ågren A Thålin C & Stål P. Neutrophil extracellular traps in patients with liver cirrhosis and hepatocellular carcinoma. Scientific Reports 20211118025. ( 10.1038/s41598-021-97233-3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim JG Kim SI Song SH Gu JY Lee M & Kim HK. Diagnostic and prognostic role of circulating neutrophil extracellular trap markers and prekallikrein in patients with high-grade serous ovarian cancer. Frontiers in Oncology 202212992056. ( 10.3389/fonc.2022.992056) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nickel KF Labberton L Long AT Langer F Fuchs TA Stavrou EX Butler LM & Renné T. The polyphosphate/factor XII pathway in cancer-associated thrombosis: novel perspectives for safe anticoagulation in patients with malignancies. Thrombosis Research 2016141(Supplement 2) S4–S7. ( 10.1016/S0049-3848(1630353-X) [DOI] [PubMed] [Google Scholar]
- 17.Pfeiler S Stark K Massberg S & Engelmann B. Propagation of thrombosis by neutrophils and extracellular nucleosome networks. Haematologica 2017102206–213. ( 10.3324/haematol.2016.142471) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Noubouossie DF Reeves BN Strahl BD & Key NS. Neutrophils: back in the thrombosis spotlight. Blood 20191332186–2197. ( 10.1182/blood-2018-10-862243) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gould TJ Vu TT Swystun LL Dwivedi DJ Mai SH Weitz JI & Liaw PC. Neutrophil extracellular traps promote thrombin generation through platelet-dependent and platelet-independent mechanisms. Arteriosclerosis, Thrombosis, and Vascular Biology 2014341977–1984. ( 10.1161/ATVBAHA.114.304114) [DOI] [PubMed] [Google Scholar]
- 20.Nierodzik ML & Karpatkin S. Thrombin induces tumor growth, metastasis, and angiogenesis: evidence for a thrombin-regulated dormant tumor phenotype. Cancer Cell 200610355–362. ( 10.1016/j.ccr.2006.10.002) [DOI] [PubMed] [Google Scholar]
- 21.Marchetti M, Giaccherini C, Masci G, Verzeroli C, Russo L, Celio L, Sarmiento R, Gamba S, Tartari CJ, Diani E, et al. Thrombin generation predicts early recurrence in breast cancer patients. Journal of Thrombosis and Haemostasis 2020182220–2231. ( 10.1111/jth.14891) [DOI] [PubMed] [Google Scholar]
- 22.Lundbech M Krag AE Christensen TD & Hvas AM. Thrombin generation, thrombin-antithrombin complex, and prothrombin fragment F1+2 as biomarkers for hypercoagulability in cancer patients. Thrombosis Research 202018680–85. ( 10.1016/j.thromres.2019.12.018) [DOI] [PubMed] [Google Scholar]
- 23.Kim N Gu JY Yoo HJ Han SE Kim YI Nam-Goong IS Kim ES & Kim HK. Contact system activation and high thrombin generation in hyperthyroidism. European Journal of Endocrinology 2017176583–589. ( 10.1530/EJE-16-0835) [DOI] [PubMed] [Google Scholar]
- 24.Houghton AM, Rzymkiewicz DM, Ji H, Gregory AD, Egea EE, Metz HE, Stolz DB, Land SR, Marconcini LA, Kliment CR, et al. Neutrophil elastase-mediated degradation of IRS-1 accelerates lung tumor growth. Nature Medicine 201016219–223. ( 10.1038/nm.2084) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Deryugina E Carré A Ardi V Muramatsu T Schmidt J Pham C & Quigley JP. Neutrophil elastase facilitates tumor cell intravasation and early metastatic events. iScience 202023101799. ( 10.1016/j.isci.2020.101799) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Seren S Joly JP Voisin P Bouchaud V Audran G Marque SRA & Mellet P. Neutrophil elastase-activatable prodrugs based on an alkoxyamine platform to deliver alkyl radicals cytotoxic to tumor cells. Journal of Medicinal Chemistry 2022659253–9266. ( 10.1021/acs.jmedchem.2c00455) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yamashita JI Ogawa M Ikei S Omachi H Yamashita SI Saishoji T Nomura K & Sato H. Production of immunoreactive polymorphonuclear leucocyte elastase in human breast cancer cells: possible role of polymorphonuclear leucocyte elastase in the progression of human breast cancer. British Journal of Cancer 19946972–76. ( 10.1038/bjc.1994.11) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Foekens JA Ries Ch Look MP Gippner-Steppert C Klijn JG & Jochum M. Elevated expression of polymorphonuclear leukocyte elastase in breast cancer tissue is associated with tamoxifen failure in patients with advanced disease. British Journal of Cancer 2003881084–1090. ( 10.1038/sj.bjc.6600813) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee WL & Downey GP. Leukocyte elastase: physiological functions and role in acute lung injury. American Journal of Respiratory and Critical Care Medicine 2001164896–904. ( 10.1164/ajrccm.164.5.2103040) [DOI] [PubMed] [Google Scholar]
- 30.Liu JF Ba L Lv H Lv D Du JT Jing XM Yang NJ Wang SX Li C & Li XX. Association between neutrophil-to-lymphocyte ratio and differentiated thyroid cancer: a meta-analysis. Scientific Reports 2016638551. ( 10.1038/srep38551) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pierce BL, Ballard-Barbash R, Bernstein L, Baumgartner RN, Neuhouser ML, Wener MH, Baumgartner KB, Gilliland FD, Sorensen BE, McTiernan A, et al. Elevated biomarkers of inflammation are associated with reduced survival among breast cancer patients. Journal of Clinical Oncology 2009273437–3444. ( 10.1200/JCO.2008.18.9068) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cheng E, Shi Q, Shields AF, Nixon AB, Shergill AP, Ma C, Guthrie KA, Couture F, Kuebler P, Kumar P, et al. Association of inflammatory biomarkers with survival among patients with Stage III colon cancer. JAMA Oncology 20239404–413. ( 10.1001/jamaoncol.2022.6911) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Papayannopoulos V Metzler KD Hakkim A & Zychlinsky A. Neutrophil elastase and myeloperoxidase regulate the formation of neutrophil extracellular traps. Journal of Cell Biology 2010191677–691. ( 10.1083/jcb.201006052) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jung HS Gu J Kim JE Nam Y Song JW & Kim HK. Cancer cell-induced neutrophil extracellular traps promote both hypercoagulability and cancer progression. PLoS One 201914e0216055. ( 10.1371/journal.pone.0216055) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Najmeh S, Cools-Lartigue J, Rayes RF, Gowing S, Vourtzoumis P, Bourdeau F, Giannias B, Berube J, Rousseau S, Ferri LE, et al. Neutrophil extracellular traps sequester circulating tumor cells via β1-integrin mediated interactions. International Journal of Cancer 20171402321–2330. ( 10.1002/ijc.30635) [DOI] [PubMed] [Google Scholar]
- 36.Kaltenmeier CT Yazdani H van der Windt D Molinari M Geller D Tsung A & Tohme S. Neutrophil extracellular traps as a novel biomarker to predict recurrence-free and overall survival in patients with primary hepatic malignancies. HPB (Oxford) 202123309–320. ( 10.1016/j.hpb.2020.06.012) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhu T Zou X Yang C Li L Wang B Li R Li H Xu Z Huang D & Wu Q. Neutrophil extracellular traps promote gastric cancer metastasis by inducing epithelialmesenchymal transition. International Journal of Molecular Medicine 202148. ( 10.3892/ijmm.2021.4960) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Perros P, Boelaert K, Colley S, Evans C, Evans RM, Ba GG, Gilbert J, Harrison B, Johnson SJ, Giles TE, et al. Guidelines for the management of thyroid cancer. Clinical Endocrinology (Oxford) 201481(Supplement1) 1–122. ( 10.1111/cen.12515) [DOI] [PubMed] [Google Scholar]
- 39.Verburg FA, Luster M, Cupini C, Chiovato L, Duntas L, Elisei R, Feldt-Rasmussen U, Rimmele H, Seregni E, Smit JW, et al. Implications of thyroglobulin antibody positivity in patients with differentiated thyroid cancer: a clinical position statement. Thyroid 2013231211–1225. ( 10.1089/thy.2012.0606) [DOI] [PubMed] [Google Scholar]
- 40.Naudin C Burillo E Blankenberg S Butler L & Renné T. Factor XII contact activation. Seminars in Thrombosis and Hemostasis 201743814–826. ( 10.1055/s-0036-1598003) [DOI] [PubMed] [Google Scholar]
- 41.Wachtfogel YT Pixley RA Kucich U Abrams W Weinbaum G Schapira M & Colman RW. Purified plasma factor XIIa aggregates human neutrophils and causes degranulation. Blood 1986671731–1737. ( 10.1182/blood.V67.6.1731.1731) [DOI] [PubMed] [Google Scholar]
- 42.Luo JH Zhang XX & Sun WH. F12 as a reliable diagnostic and prognostic biomarker associated with immune infiltration in papillary thyroid cancer. Aging (Albany NY) 2022143687–3704. ( 10.18632/aging.204037) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kim M, Kim WG, Oh HS, Park S, Kwon H, Song DE, Kim TY, Shong YK, Kim WB, Sung TY, et al. Comparison of the seventh and eighth editions of the American Joint Committee on Cancer/Union for International Cancer Control Tumor-node-metastasis staging system for differentiated thyroid cancer. Thyroid 2017271149–1155. ( 10.1089/thy.2017.0050) [DOI] [PubMed] [Google Scholar]



 This work is licensed under a
This work is licensed under a