Abstract
Hypothyroidism is associated with a decreased health-related quality of life (HRQoL). We hypothesized that individuals with hypothyroidism (defined as use of thyroid hormone (TH)) and especially those having an impaired HRQoL are characterized by a high prevalence of comorbid disorders and that the impact of hypothyroidism and comorbidity on HRQoL is synergistic. Presence of comorbidity was based on data obtained using structured questionnaires, physical examination, biochemical measurements and verified medication use. Single morbidities were clustered into 14 different disease domains. HRQoL was measured using the RAND-36. Logistic regression analyses were used to determine the effect of TH use on the odds of having an affected disease domain and a lower score than an age- and sex-specific reference value for HRQoL. TH was used by 4537/14,7201 participants of the population-based Lifelines cohort with a mean (± s.d.) age of 51.0 ± 12.8 years (88% females). Eighty-five percent of the TH users had ≥1 affected disease domain in contrast to 71% of nonusers. TH use was associated with a higher odds of 13 out of 14 affected disease domains independent of age and sex. In a multivariable model, TH use was associated with a decreased HRQoL across six out of eight dimensions. No significant interactions between TH use and affected disease domains were observed. TH users with an impaired HRQoL had significantly more comorbidity than those not having an impaired HRQoL. In this large, population-based study, we demonstrated that TH users had more comorbidity than individuals not using TH. The coexistence of other chronic medical conditions in subjects with TH use led to further lowering of HRQoL in an additive manner.
Keywords: hypothyroidism, thyroid hormone, comorbidity, health-related quality of life
Introduction
Hypothyroidism is a common endocrine disorder. Treatment with levothyroxine has been considered the standard of care for patients with established primary hypothyroidism, and its use has been enforced in all international guidelines. In general, treatment is aimed at abolishing complaints related to hypothyroidism, and normalizing plasma thyroid stimulating hormone (TSH) levels (1). However, it has been shown that health-related quality of life (HRQoL) improves after 6 months of levothyroxine treatment for overt hypothyroidism, but full recovery is not always obtained (2). Furthermore, a considerable part of biochemically well-controlled patients with levothyroxine-treated hypothyroidism have persistent symptoms, such as depression and impaired mental well-being (3). Earlier, we demonstrated that HRQoL is impaired in women using levothyroxine compared with those not using levothyroxine (4).
Several possible causes of reduced HRQoL in hypothyroidism have been studied. The remaining complaints might be ascribed to the fact that replacement therapy does not completely normalize plasma free thyroxine (FT4) and free triiodothyronine (FT3) levels, despite normal TSH levels. It has been shown that in patients treated with levothyroxine monotherapy with normal TSH levels, FT4 levels were significantly higher and FT3 significantly lower than in euthyroid control subjects (4, 5). Possible explanations for this finding include impaired T4 to T3 conversion or downregulation of the deiodinase pathway (6). This hypothesis has led to multiple trials of combination therapy of levothyroxine and liothyronine, with generally negative outcomes (1). Also, variations in genes associated with thyroid hormone (TH) metabolism were hypothesized to be associated with an inferior response to levothyroxine. In an earlier paper, we showed that the Thr92Ala polymorphism was not associated with TH levels and HRQoL (4). A few studies addressed the question of whether the actual serum concentrations of FT4 and FT3 during replacement therapy influence HRQoL, with conflicting results (3, 7, 8, 9). Moreover, there are multiple factors independent of serum TH levels that could potentially contribute to the impaired HRQoL in patients treated with levothyroxine, among which are categorized as having a chronic condition, having other chronic conditions, or underlying autoimmunity (6, 10).
Most studies concerning HRQoL focus on the impact of one disease. Studies considering the impact of comorbidity are less common. The impact of multiple chronic conditions on HRQoL can be additive, synergistic or subtractive. An additive effect suggests that the combined effect of the conditions on HRQoL is approximately the sum of the independent effect of each condition. A synergistic or subtractive relationship suggest that the combined effect of the conditions is larger respectively smaller than the sum of the independent effects. Research in different disease populations demonstrated additive effects and synergistic effects of comorbidity on HRQoL (11, 12, 13, 14, 15). For example, the influence of comorbid conditions on HRQoL is additive in individuals with diabetes mellitus (DM) (13), whereas this relationship is synergistic in individuals with depression (16). However, data on the relationship between coexisting conditions and hypothyroidism on HRQoL are lacking.
In this follow-up (4) paper, we report the results obtained from our study considering the hypothesis that individuals with hypothyroidism, and especially those with an impaired HRQoL, are characterized by a high prevalence of comorbid disorders and that the impact of hypothyroidism and comorbidity on HRQoL is synergistic. The hypothesis was tested in participants using TH, who participated in a large population-based study.
Materials and methods
Lifelines cohort
We evaluated data from the Lifelines cohort study. Lifelines is a multidisciplinary prospective population-based cohort study examining in a unique three-generation design the health and health-related behaviors of 167,729 persons (aged 18 to 93 years) living in the northern parts of the Netherlands. It employs a broad range of investigative procedures in assessing the biomedical, sociodemographic, behavioral, physical and psychological factors which contribute to the health and disease of the general population, with a special focus on multimorbidity and complex genetics. Participants were enrolled between 2006 and 2013, follow-up visits were scheduled every 5 years, and in between participants received follow-up questionnaires. Participants provided written informed consent before participating in the study. The study protocol was approved by the medical ethical review committee of the University Medical Center Groningen (17, 18, 19). For current analyses, we used 147,201 adult participants with full clinical and biochemical data available.
Data collection
Physical examination
Participants were invited to visit one of the Lifelines Research sites to have a physical examination. Height was measured to the nearest 0.5 cm. Body weight was measured without shoes and with light clothing with a 0.1 kg precision. Body mass index (BMI) was calculated by dividing weight in kilograms by the squared height in meters (kg/m2). Systolic and diastolic blood pressure were measured every minute for a period of 10 min using a DINAMAP monitor. The size of the cuff was chosen according to the arm circumference. The average of the last three measurements was reported. The physical examination included spirometry using a Welch Allyn V.1.6.0.489, PC-based SpiroPerfect with Ca Workstation software.
Biomaterial collection
Blood and 24-h urine samples were collected in the morning after an overnight fast. The blood samples were placed at 4°C and transported from the Lifelines Research site to the Lifelines laboratory, under tightly controlled and continuously monitored conditions. From the Lifelines laboratory, part of the samples was directly transferred to the central laboratory of the University Medical Center Groningen, to perform routine clinical chemistry assays on fresh samples. The remaining samples were stored at −80°C and are available for future research questions. TSH, FT4 and FT3 were assayed by electrochemiluminescence immunoassay (Roche Modular E170, Roche, Switzerland). TSH has an intra- and interassay coefficient of variation range of 1.3–11.1% and 2.3–11.9%, respectively, at levels of 0.014–57.8 mIU/L. Hemoglobin concentration was determined using routine procedures on a XE2100-system (Sysmex, Japan). Total cholesterol was measured with an enzymatic colorimetric method, on the Roche Modular P chemistry analyzer. Fasting blood glucose was measured using a hexokinase method. Serum creatinine was measured on a Roche Modular P chemistry analyzer (Roche). The CDK-EPI formula was used to estimate the glomerular filtration rate (eGFR).
Morbidity
Subjects completed a self-administered questionnaire on medical history, use of medication and health behavior at home. Medication was verified by a certified research assistant and categorized using the Anatomical Therapeutic Chemical (ATC) Classification System. Furthermore, the Mini-International Neuropsychiatric Interview (MINI), a structured diagnostic interview to assess major depressive and anxiety disorders, was conducted by trained interviewers (20).
Definitions of morbidity
Data from the structured questionnaire concerning medical disorders (defined as present when they were affirmatively answered and as being absent when answered negatively or missing), medication list, physical examination, biochemical measurements and MINI were collectively used to determine the presence of disease. To decrease uncertainties concerning the sensitivity of disease registration, self-reported diseases were registered when the use of appropriate medication was verified when applicable. Single morbidities were scored according to the tenth edition of the International Statistical Classification of Diseases and Related Health Problems (ICD-10) (21). Next, single morbidities were clustered into the 14 ICD-10 disease domains. A detailed list of the single morbidities and disease domains is presented in Supplementary Table 1 (see section on supplementary materials given at the end of this article). The criteria used were based on previously published studies with data from the Lifelines cohort (22, 23, 24). Hypothyroidism was defined as the use of TH (ATC code H03A). Individuals using antithyroid preparations (ATC code H03B) were excluded (n = 118). The level of detail of the Lifelines data does not allow further refinement of reasons for TH use (25).
HRQoL
The Lifelines study used the RAND-36, which is the Dutch version of the Short Form 36 (SF-36) to measure HRQoL. The RAND-36 consists of 36 items measuring health perception among eight health dimensions. Continuous scores for each of these dimensions were calculated and ranged from 0 to 100, with 100 being the best score (26). Unfortunately, there are no data available on which score should be considered abnormal. As HRQoL scores are not normally distributed, we have chosen to generate an age- and sex-specific cutoff point at the 25th percentile of the study population not using TH. Participants with a score lower than the 25th percentile were considered to have an abnormally low score for this specific dimension (4, 27, 28). In TH users, an impaired HRQoL was defined as being in three dimensions or more in the lowest age- and sex-specific tenth percentile. Data on HRQoL were available from 138,852 participants.
Statistical analysis
Data are represented as means ± s.d., medians with interquartile ranges, or percentages. We evaluated between-group differences using one-way ANOVA, the Kruskal–Wallis test, or the chi-square test, as appropriate. Logistic regression was used to study the association between TH use and affected disease domains, adjusted for age and sex, and to determine the effect of TH use on the odds of having a lower score than the (age- and sex-specific) value for each HRQoL dimension, adjusted for smoking status, BMI and all 14 disease domains. Odds ratios were reported with 95% confidence intervals. To study the influence of thyroid hormone use on HRQoL, we developed eight regression models, with each SF-36 scale as the dependent variable for a separate model. Each model was built in three stages. The first stage assessed the effect of thyroid hormone use on HRQoL while adjusting for the influence of smoking status and BMI. The second stage assessed the independent effects of thyroid hormone use and all 14 disease domains on HRQoL while adjusting for the influence of smoking status and BMI. Finally, in the third stage, we additionally assessed interactions between thyroid hormone use and one of the 14 disease domains on HRQoL. The absence of a significant interaction term suggests that the influence of thyroid hormone use and the presence of one of the 14 disease domains was additive. The presence of a significant interaction term suggests that a synergistic or subtractive relationship existed.
As sensitivity analyses, analyses concerning HRQoL were repeated for participants being euthyroid (defined as TSH between 0.4 and 4.0 mU/L). TH level measurement was only performed between November 2009 and November 2011, and therefore was available for 1145 TH users and 38,711 nonusers. There was no clinically relevant difference in sex distribution, age and BMI between those with and without TH levels measured. Statistical analyses were performed using IBM Statistical Package for the Social Sciences Statistics software, version 23.0. We adopted a conservative α of 0.01 instead of the conventional 0.05 to reduce the risk of false-positive results.
Results
Baseline characteristics
The most relevant baseline parameters for the participants according to TH use are shown in Table 1. TH users (4537/147,201; 3.1%) were older, had a higher BMI, and were more frequently female. Mean FT3 was lower and mean FT4 was higher in the TH users, while TSH levels were comparable. Their metabolic profile was less favorable, as they had higher glucose and total cholesterol levels.
Table 1.
Baseline characteristics of the participants according to thyroid hormone use.
| Nonusers (n = 142,664) | Thyroid hormone users (n = 4537) | P | |
|---|---|---|---|
| Men/women | 60,771/81,893 | 534/4003 | |
| % women | 57.4 | 88.2 | <0.001 |
| Age (years) | 44.6 ± 13.1 | 51.0 ± 12.8 | <0.001 |
| Body mass index (kg/m2) | 26.0 ± 4.3 | 27.6 ± 5.3 | <0.001 |
| Systolic blood pressure (mmHg) | 125 ± 15 | 126 ± 16 | 0.10 |
| Diastolic blood pressure (mmHg) | 74 ± 9 | 73 ± 9 | <0.001 |
| TSH (mU/L)a | 2.1 (1.5–3.0) | 2.3 (0.8–4.2) | 0.24 |
| FT4 (pmol/L)a | 15.7 ± 2.1 | 18.2 ± 3.7 | <0.001 |
| FT3 (pmol/L)a | 5.3 ± 0.7 | 4.7 ± 1.2 | <0.001 |
| Creatinine (umol/L) | 73.6 ± 13.7 | 70.4 ± 14.4 | <0.001 |
| Fasting glucose (mmol/L) | 5.0 ± 0.8 | 5.1 ± 1.1 | <0.001 |
| Total cholesterol (mmol/L) | 5.1 ± 1.0 | 5.2 ± 1.0 | <0.001 |
| Hemoglobin (g/dL) | |||
| Men | 15.1 ± 0.9 | 15.0 ± 1.1 | <0.001 |
| Women | 13.5 ± 1.0 | 13.4 ± 1.0 | 0.005 |
| Affected disease domains | <0.001 | ||
| % with 0 affected disease domains | 29.0 | 15.4 | |
| % with one affected disease domain | 32.4 | 25.9 | |
| % with two affected disease domains | 21.0 | 23.8 | |
| % with three affected disease domains | 10.8 | 16.7 | |
| % with four affected disease domains | 4.5 | 10.2 | |
| % with five affected disease domains | 1.6 | 4.7 | |
| % with six affected disease domains | 0.6 | 2.4 | |
| % with ≥7 affected disease domains | 0.1 | 0.9 |
Data are represented as means ± s.d. (normal distribution), medians with interquartile ranges (nonnormal distribution) or percentages.
aThyroid hormone levels were available of 39,786 participants (1145 thyroid hormone users and 38,711 nonusers).
Comorbidity: affected disease domains
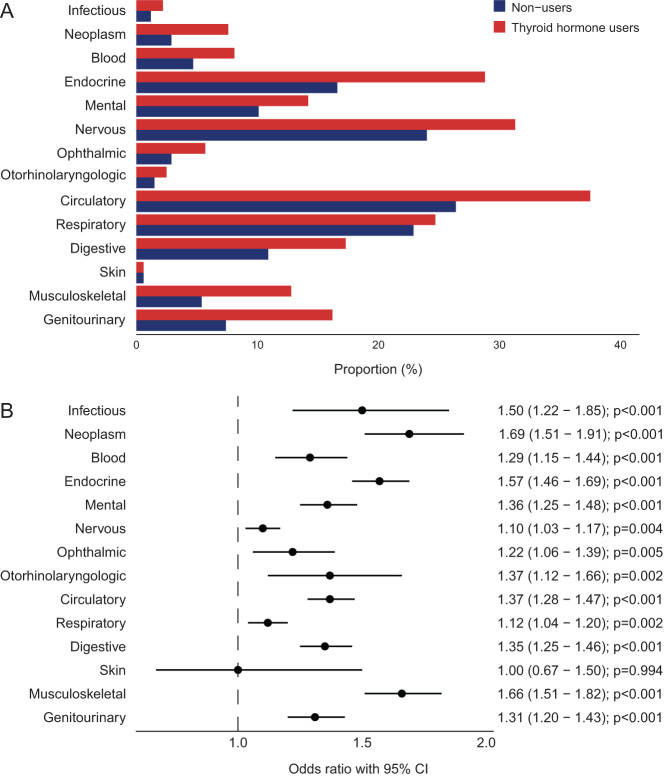
A total of 84.6% of the TH users had at least one affected disease domain compared to 71.0% in nonusers (Table 1). In nonusers, the most affected disease domains were the circulatory system (26.4%), nervous system (24.0%), respiratory system (22.9%) and endocrine system (thyroid disease excluded) (16.6%). There was a higher proportion of individuals with an affected disease domain in 13/14 disease domains in the TH users (all except skin diseases) (Fig. 1A). TH use was associated with higher odds (age- and sex-adjusted) of all those 13 disease domains (Fig. 1B). When assessing men and women separately, the associations were in general slightly stronger in males (Supplementary Fig. 1).
Figure 1.
Percentage of thyroid hormone users and nonusers with an affected disease domain (A) and forest plot demonstrating the odds ratios for having an affected disease domain according to thyroid hormone use (B). Logistic regression analyses included age and sex as covariates. Circles indicate odds ratios for each cohort, with horizontal lines corresponding to 95% confidence intervals.
Comorbidity: single morbidities
Next, we assessed which single morbidities caused the increased prevalence of affected disease domains in TH users. The largest differences between TH users and nonusers were observed for the single morbidities hypertension (37.0 vs 25.9%), hypercholesterolemia (25.3 vs 15.1%), migraine (23.7 vs 17.8%), gynecologic surgery (females: 13.0 vs 7.3%), irritable bowel disease (14.8 vs 9.2%) and fibromyalgia (8.3 vs 2.9%) (all P < 0.001). Significant differences were also observed in the prevalence of autoimmune disorders (type 1 diabetes: 1.1 vs 0.2%; celiac disease: 0.7 vs 0.2%; rheumatoid arthritis (RA): 4.3 vs 2.0%, all P < 0.001) (Supplementary Fig. 2, age- and sex-adjusted ORs in Supplementary Fig. 3). Some single morbidities were defined either by results of biochemical measurements, or by clinical evaluation, or by verified medication use, such as for example hypercholesterolemia and hypertension (Supplementary Table 1). TH users more often met the criteria based on medication use (hypercholesterolemia: 55.3 vs 42.0% and hypertension: 67.9 vs 47.5%) than nonusers.
Impact comorbidity on HRQoL
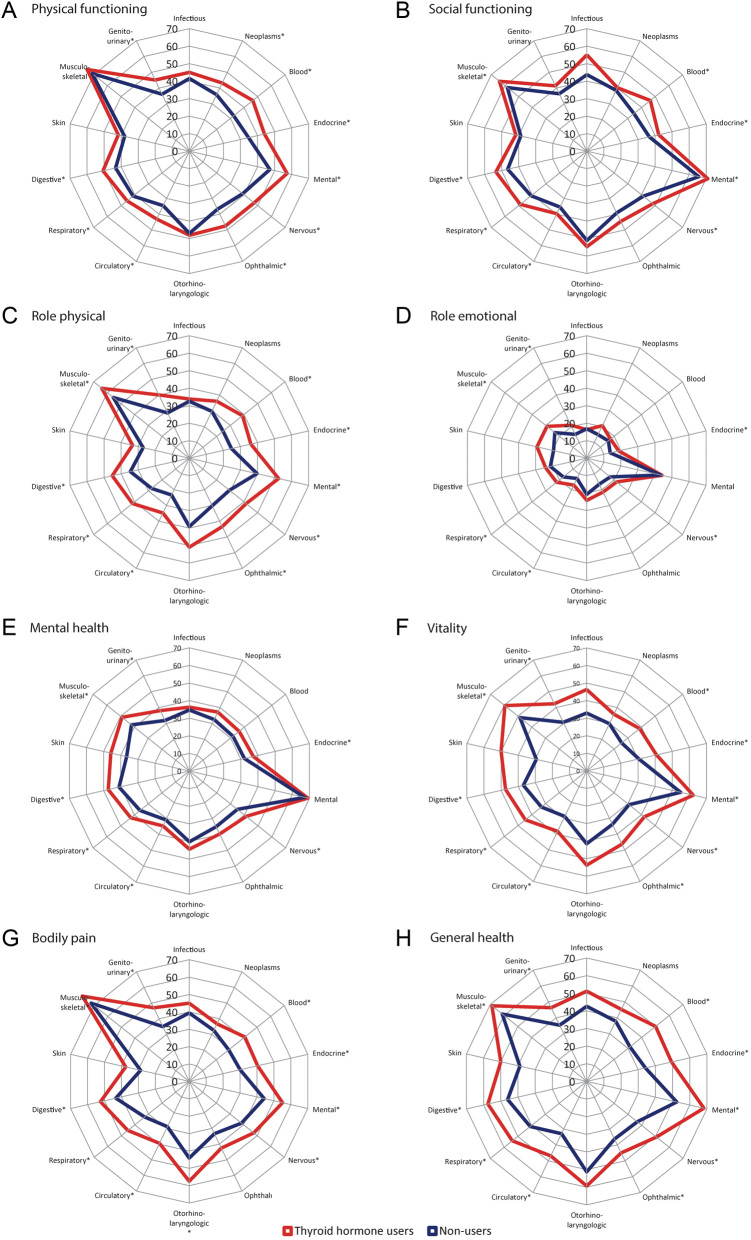
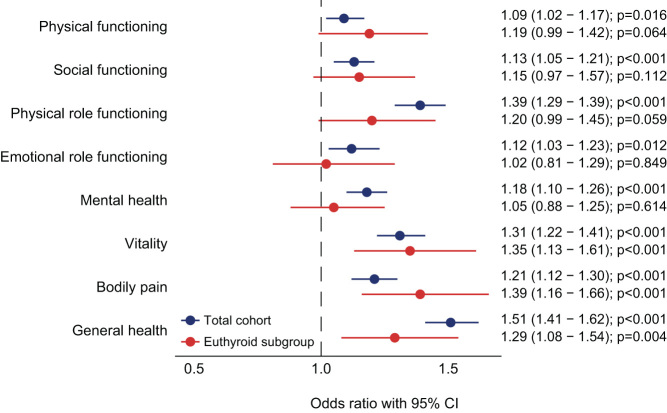
Additionally, we evaluated the impact of affected disease domains on HRQoL in TH users and nonusers. A larger proportion of TH users with an affected disease domain had a score below the age- and sex-specific value, for almost all HRQoL dimensions, as compared to nonusers with an affected disease domain (Fig. 2). This was most pronounced in the HRQoL dimensions physical role functioning, vitality and general health. Multivariable logistic regression was performed to adjust the observed associations for BMI, smoking status and the 14 disease domains. TH use remained associated with a decreased HRQoL across six dimensions (all except physical functioning and emotional role functioning). The negative impact of affected disease domains on HRQoL is shown in Supplementary Table 2. As a sensitivity analysis, the analyses were repeated for euthyroid individuals (n = 33,475, 619 TH users and 32,856 nonusers). Associations were retained in three HRQoL dimensions (bodily pain, vitality and general health) (Fig. 3). To study whether there was a subtractive, additive or synergetic effect of TH use and comorbidity on HRQoL, interactions between TH use and all 14 disease domains were assessed. No significant results were observed, meaning that the effect was additive (data not shown).
Figure 2.
Percentage of individuals, stratified according to thyroid hormone use, with a score below the age- and sex-specific cutoff values for the eight different health-related quality of life dimensions (A–H) for all 14 disease domains. An asterisk (*) indicates a significant difference in the proportion having a score below the age- and sex-specific cutoff value in thyroid hormone users as compared to nonusers.
Figure 3.
Forest plots demonstrating the odds ratios for having a lower score than the (age- and sex-specific) 25th percentile cutoff per HRQoL dimension, according to thyroid hormone use. Logistic regression analyses were performed in the total cohort and in the euthyroid subgroup and included body mass index, smoking status and 14 disease domains as covariates. Nonusers were used as the reference group. Circles indicate odds ratios for each cohort, with horizontal lines corresponding to 95% confidence intervals.
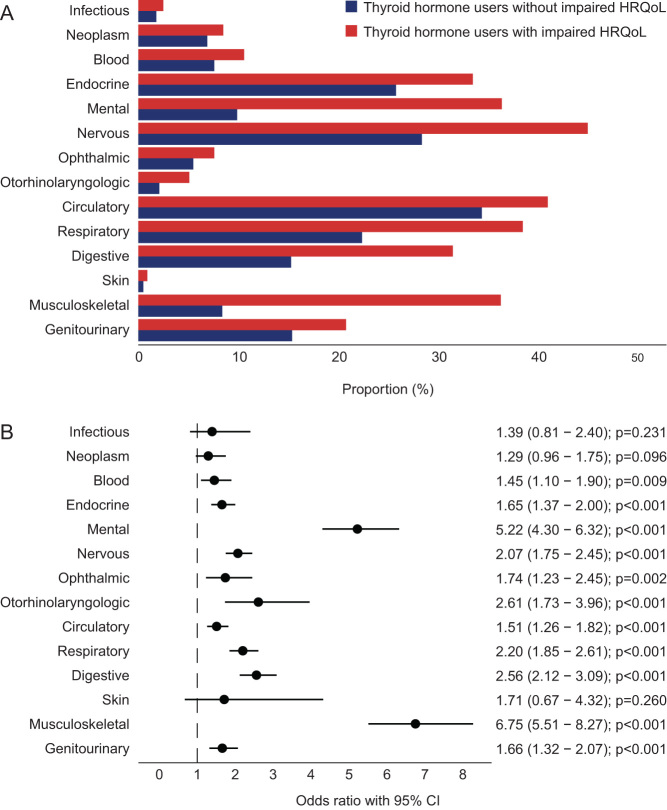
Finally, we evaluated the group TH users who had an impaired HRQoL. This resulted in a group of 686 subjects (16.8%). Among the TH users with an impaired HRQoL, 95.3% had at least one affected disease domain compared to 81.8% of TH users without impaired HRQoL (P < 0.001). In TH users with an impaired HRQoL, 10 out of 14 disease domains were more frequently affected (all except neoplasms, infectious, ophthalmic and skin diseases) compared to TH users without impaired HRQoL (Fig. 4A). This was confirmed in the multivariable model (Fig. 4B). The strongest associations were present in the disease domains of mental disorders and musculoskeletal diseases. Comparable results were obtained in the sensitivity analysis, in which the analyses were repeated for euthyroid TH users (n = 619/1051; 59%), of whom 99 (16.0%) had an impaired HRQoL. A total of 94.9% of the euthyroid TH users with an impaired HRQoL had at least one affected disease domain compared to 81.7% of TH users without impaired HRQoL (P < 0.001). Due to the limited number of TH users with a TSH value available, no analyses on disease domains could be performed.
Figure 4.
Percentage of thyroid hormone users with and without an impaired HRQoL with an affected disease domain (A) and forest plot demonstrating the odds ratios for having an affected disease domain according to an impaired HRQoL (B). Logistic regression analyses included age and sex as covariates. Circles indicate odds ratios for each cohort, with horizontal lines corresponding to 95% confidence intervals.
Discussion
In this large population-based study, we assessed the relationship between TH use, comorbidity and HRQoL. We demonstrated that comorbidity was more prevalent in TH users than in nonusers and led to further lowering of HRQoL, not in a synergistic but in an additive manner. Further, TH users with an impaired HRQoL had more comorbidity than those without an impaired HRQoL.
Few studies have assessed comorbid conditions in individuals with hypothyroidism. In contrast, several studies have been performed assessing the prevalence of thyroid dysfunction in multiple chronic disorders. A higher prevalence of hypothyroidism was demonstrated to be present in individuals with a broad range of disorders, including cardiovascular disease, rheumatic disorders, autoimmune disorders and psychiatric diseases (29, 30, 31, 32, 33).
Comorbidity was highly prevalent in both male as well as female TH users. There are several ways in which different diseases may simultaneously be present in an individual (34). First, two diseases can co-occur by chance, based on their prevalence. Second, selection bias can be an alternative explanation. As described by Berkson, disease clusters appear more frequently in patients seeking medical care compared to the general population (35, 36). This was supported in this study by the higher proportion of individuals meeting the criteria for hypercholesterolemia and hypertension based on medication use instead of based on results of physical examination or biochemical measurements. Third, all processes of TH physiology, including synthesis, release, transport, metabolism and control of TH, are susceptible to effects from drugs prescribed for other diseases. Concerning hypothyroidism, these drugs include commonly used preparations as antiarrhythmic agents (e.g. amiodarone), psychiatric medication (e.g. lithium) and glucocorticoids as well as advanced medical therapy with antineoplastic agents (e.g. checkpoint inhibitors) (37). Finally, there are different types of possible causal associations between diseases, of which several examples for hypothyroidism will be discussed. Lower levels of TH may lead to systemic and local effects. In a (severe) hypothyroid state, diastolic blood pressure increases and the pulse pressure narrows, which results in diastolic hypertension, whereas impaired cardiac muscle relaxation, decreased heart rate and decreased stroke volume may contribute to heart failure. Furthermore, a decreased expression of hepatic low-density lipoprotein (LDL) receptors and a decrease in LDL receptor activity result in hypercholesterolemia in hypothyroidism (38). Also, individuals with autoimmune thyroid disease are at a significantly increased risk of additional autoimmune diseases. In a very large Italian cohort study, the use of levothyroxine was demonstrated to be strongly associated with DM and other autoimmune diseases like celiac disease, RA, ankylosing spondylitis, lupus erythematosus, psoriatic arthritis and multiple sclerosis (39). This is in accordance with previous studies (35, 40). In our cohort, the use of TH was indeed associated with type 1 diabetes, celiac disease and RA. Additionally, it has been hypothesized that TH influences the equilibrium of neurotransmitters that are involved in the pathway of developing psychiatric diseases (41). Thvilum et al. demonstrated that hypothyroidism was associated with increased psychiatric morbidity in general, and with increased use of antidepressants, antipsychotics, and anxiolytics (42).
In our study, the relationship between TH use and other morbidities on HRQoL was not synergistic, meaning that the presence of comorbidity further reduced HRQoL in TH users in an additive manner. This is partly in concordance with the study of Rijken et al. in which the additive effect of thyroid dysfunction (n = 126, thyrotoxicosis, hypothyroidism and other thyroid disease) on mental health but not in physical health was demonstrated (14).
Especially individuals with impaired HRQoL were characterized by a high prevalence of concurrent diseases (95.3%), suggesting that comorbidity might be a contributing factor to the persistent complaints in patients with treated hypothyroidism. It is, however, beyond the scope of this article to describe all potentially involved mechanisms for persistent complaints. Examples of other factors contributing to the decreased HRQoL might include ineffective TH supplementation (43, 44). In line with this, in our study, only 59% of the TH users wer biochemically euthyroid. Only a minor part of the difference in TSH levels can be attributed to the intra- and interassay variation. The association between TH use and HRQoL was only present in three instead of six HRQoL dimensions in the sensitivity analyses, when analyzing euthyroid participants only. Besides that, TSH reference ranges obtained in a healthy population might not be a reliable indicator of the true thyroid status of an individual and consequently may not serve as an adequate target for replacement therapy (45). Also, levothyroxine use might not reverse hypothyroidism in all tissues due to tissue-specific regulation of deiodinases, which is one of the assumptions when treating with levothyroxine monotherapy (45, 46). Hashimoto’s thyroiditis, an autoimmune-mediated destruction of the thyroid gland, is the most common cause of hypothyroidism and is characterized by high levels of antibodies against thyroid antigens. Several studies demonstrated that in individuals with Hashimoto’s thyroiditis the presence of antibodies was associated with impaired HRQoL, independent of thyroid function (47, 48). Thyroid autoimmunity might also play a role in disease burden in for example fibromyalgia or depression (49, 50), which was supported by the strongest associations in the disease domains of mental and behavioral disorders and musculoskeletal system and connective tissue diseases in our cohort.
There are some strengths and limitations to this study. This is the first large-scale study investigating the role of comorbidity on HRQoL in TH users from the general population. We were able to use data from a large number of participants with a wide range in age, socioeconomic status and comorbidities. All subjects were uniformly characterized. Several potential limitations should also be acknowledged. Since this is a cross-sectional study, the analyses do not provide information about causality. Furthermore, due to the observational nature of the study, it was not possible to exclude the role of unknown or unmeasured confounding variables. A third limitation is that although the RAND-36 has proven to be highly valid for assessing HRQoL it remains a generic health status questionnaire instead of a thyroid-related questionnaire. When assessing comorbidity, our study relied mainly on (verified) medication use and self-reported disorders reported using structured questionnaires. This may have caused an over- or underestimation of the real prevalence. Finally, we were not able to study the effect of autoimmunity since anti-thyroid peroxidase antibody levels were not available.
In conclusion, in this large population-based study, we found that TH users had more comorbidity than nonusers. The coexistence of other chronic medical conditions in subjects with TH use led to further lowering of HRQoL, the effect of which was generally additive. In the subgroup of TH users with an impaired HRQoL, comorbidities were more prevalent than in TH users without impaired HRQoL. This study emphasizes the role of comorbidity in TH users concerning HRQoL. Treating physicians are encouraged to pay attention to these comorbidities when caring for patients with hypothyroidism.
Supplementary Materials
Declaration of interest
The authors have declared that no competing interests exist.
Funding
The Lifelines Biobank initiative has been made possible by subsidies from the Dutch Ministry of Health, Welfare and Sport; the Dutch Ministry of Economic Affairs; the University Medical Center Groningen (UMCG the Netherlands), University of Groningen; and the northern provinces of the Netherlands.
Data availability statement
The article is based on data from the Lifelines Cohort Study. Lifelines adheres to standards of data availability and allows access for reproducibility of the study results. The data catalogue is publicly accessible at www.lifelines.nl. The dataset supporting the conclusions of this article is available through the Lifelines organization, and all international researchers can apply for data access at the Lifelines Research office (research@lifelines.nl). For data access, a fee is required.
Acknowledgements
The authors wish to acknowledge the services of the Lifelines Cohort Study, the contributing research centers delivering data to Lifelines, and all the study participants.
References
- 1.Jonklaas J, Bianco AC, Bauer AJ, Burman KD, Cappola AR, Celi FS, Cooper DS, Kim BW, Peeters RP, Rosenthal MS, et al. Guideli nes for the treatment of hypothyroidism: prepared by the American Thyroid Association task force on thyroid hormone replacement. Thyroid 2014241670–1751. ( 10.1089/thy.2014.0028) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Winther KH Cramon P Watt T Bjorner JB Ekholm O Feldt-Rasmussen U Groenvold M Rasmussen ÅK Hegedüs L & Bonnema SJ. Disease-specific as well as generic quality of life is widely impacted in autoimmune hypothyroidism and improves during the first six months of levothyroxine therapy. PLoS One 201611e0156925. ( 10.1371/journal.pone.0156925) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Saravanan P Visser TJ & Dayan CM. Psychological well-being correlates with free thyroxine but not free 3,5,3'-triiodothyronine levels in patients on thyroid hormone replacement. Journal of Clinical Endocrinology and Metabolism 2006913389–3393. ( 10.1210/jc.2006-0414) [DOI] [PubMed] [Google Scholar]
- 4.Wouters HJ van Loon HC van der Klauw MM Elderson MF Slagter SN Kobold AM Kema IP Links TP van Vliet-Ostaptchouk JV & Wolffenbuttel BH. No effect of the Thr92Ala polymorphism of deiodinase-2 on thyroid hormone parameters, health-related quality of life, and cognitive functioning in a large population-based cohort study. Thyroid 201727147–155. ( 10.1089/thy.2016.0199) [DOI] [PubMed] [Google Scholar]
- 5.Gullo D Latina A Frasca F Le Moli R Pellegriti G & Vigneri R. Levothyroxine monotherapy cannot guarantee euthyroidism in all athyreotic patients. PLoS One 20116e22552. ( 10.1371/journal.pone.0022552) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jonklaas J. Persistent hypothyroid symptoms in a patient with a normal thyroid stimulating hormone level. Current Opinion in Endocrinology, Diabetes, and Obesity 201724356–363. ( 10.1097/MED.0000000000000355) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kelderman-Bolk N Visser TJ Tijssen JP & Berghout A. Quality of life in patients with primary hypothyroidism related to BMI. European Journal of Endocrinology 2015173507–515. ( 10.1530/EJE-15-0395) [DOI] [PubMed] [Google Scholar]
- 8.Peterson SJ McAninch EA & Bianco AC. Is a normal TSH synonymous with “euthyroidism” in levothyroxine monotherapy? Journal of Clinical Endocrinology and Metabolism 20161014964–4973. ( 10.1210/jc.2016-2660) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sowiński J Sawicka-Gutaj N Ziółkowska P & Ruchała M. Effect of free triiodothyronine concentration on the quality of life of patients treated with levothyroxine. Polskie Archiwum Medycyny Wewnetrznej 2016126293–296. ( 10.20452/pamw.3392) [DOI] [PubMed] [Google Scholar]
- 10.Bektas Uysal H & Ayhan M. Autoimmunity affects health-related quality of life in patients with Hashimoto's thyroiditis. Kaohsiung Journal of Medical Sciences 201632427–433. ( 10.1016/j.kjms.2016.06.006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.O'Neil A Stevenson CE Williams ED Mortimer D Oldenburg B & Sanderson K. The health-related quality of life burden of co-morbid cardiovascular disease and major depressive disorder in Australia: findings from a population-based, cross-sectional study. Quality of Life Research 20132237–44. ( 10.1007/s11136-012-0128-4) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hunger M Thorand B Schunk M Döring A Menn P Peters A & Holle R. Multimorbidity and health-related quality of life in the older population: results from the German KORA-age study. Health and Quality of Life Outcomes 2013953. ( 10.1186/1477-7525-9-53) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wee HL Cheung YB Li SC Fong KY & Thumboo J. The impact of diabetes mellitus and other chronic medical conditions on health-related quality of life: is the whole greater than the sum of its parts? Health and Quality of Life Outcomes 200532. ( 10.1186/1477-7525-3-2) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rijken M van Kerkhof M Dekker J & Schellevis FG. Comorbidity of chronic diseases: effects of disease pairs on physical and mental functioning. Quality of Life Research 20051445–55. ( 10.1007/s11136-004-0616-2) [DOI] [PubMed] [Google Scholar]
- 15.Fortin M Dubois MF Hudon C Soubhi H & Almirall J. Multimorbidity and quality of life: a closer look. Health and Quality of Life Outcomes 2007552. ( 10.1186/1477-7525-5-52) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gaynes BN Burns BJ Tweed DL & Erickson P. Depression and health-related quality of life. Journal of Nervous and Mental Disease 2002190799–806. ( 10.1097/00005053-200212000-00001) [DOI] [PubMed] [Google Scholar]
- 17.Scholtens S, Smidt N, Swertz MA, Bakker SJ, Dotinga A, Vonk JM, van Dijk F, van Zon SK, Wijmenga C, Wolffenbuttel BH, et al. Cohort Profile: LifeLines, a three-generation cohort study and biobank. International Journal of Epidemiology 2015441172–1180. ( 10.1093/ije/dyu229) [DOI] [PubMed] [Google Scholar]
- 18.Stolk RP Rosmalen JG Postma DS de Boer RA Navis G Slaets JP Ormel J & Wolffenbuttel BH. Universal risk factors for multifactorial diseases: LifeLines: a three-generation population-based study. European Journal of Epidemiology 20082367–74. ( 10.1007/s10654-007-9204-4) [DOI] [PubMed] [Google Scholar]
- 19.Klijs B Scholtens S Mandemakers JJ Snieder H Stolk RP & Smidt N. Representativeness of the LifeLines cohort study. PLoS One 201510e0137203. ( 10.1371/journal.pone.0137203) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sheehan DV Lecrubier Y Sheehan KH Amorim P Janavs J Weiller E Hergueta T Baker R & Dunbar GC. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. Journal of Clinical Psychiatry 199859(Supplement 20) 22–33. [PubMed] [Google Scholar]
- 21.International Classification of Diseases. International Statistical Classification of Diseases and Related Health Problems 10th Revision. Geneva, Switzerland: WHO. (available at: https://icd.who.int/browse10/2010/en) [Google Scholar]
- 22.van der Ende MY, Hartman MH, Hagemeijer Y, Meems LM, de Vries HS, Stolk RP, de Boer RA, Sijtsma A, van der Meer P, Rienstra M, et al. The LifeLines Cohort Study: prevalence and treatment of cardiovascular disease and risk factors. International Journal of Cardiology 2017228495–500. ( 10.1016/j.ijcard.2016.11.061) [DOI] [PubMed] [Google Scholar]
- 23.Meems LM, de Borst MH, Postma DS, Vonk JM, Kremer HP, Schuttelaar ML, Rosmalen JG, Weersma RK, Wolffenbuttel BH, Scholtens S, et al. Low levels of vitamin D are associated with multimorbidity: results from the LifeLines Cohort Study. Annals of Medicine 201547474–481. ( 10.3109/07853890.2015.1073347) [DOI] [PubMed] [Google Scholar]
- 24.Dekker LH de Borst MH Meems LMG de Boer RA Bakker SJL & Navis GJ. The association of multimorbidity within cardio-metabolic disease domains with dietary patterns: a cross-sectional study in 129 369 men and women from the Lifelines cohort. PLoS One 201914e0220368. ( 10.1371/journal.pone.0220368) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wouters HJCM Slagter SN Muller Kobold AC van der Klauw MM & Wolffenbuttel BHR. Epidemiology of thyroid disorders in the Lifelines Cohort Study (the Netherlands). PLoS One 202015e0242795. ( 10.1371/journal.pone.0242795) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.VanderZee KI Sanderman R Heyink JW & de Haes H. Psychometric qualities of the rand 36-Item Health survey 1.0: a multidimensional measure of general health status. International Journal of Behavioral Medicine 19963104–122. ( 10.1207/s15327558ijbm0302_2) [DOI] [PubMed] [Google Scholar]
- 27.Wouters HJCM van der Klauw MM de Witte T Stauder R Swinkels DW Wolffenbuttel BHR & Huls G. Association of anemia with health-related quality of life and survival: a large population-based cohort study. Haematologica 2019104468–476. ( 10.3324/haematol.2018.195552) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Slagter SN van Vliet-Ostaptchouk JV van Beek AP Keers JC Lutgers HL van der Klauw MM & Wolffenbuttel BH. Health-related quality of life in relation to obesity grade, type 2 diabetes, metabolic syndrome and inflammation. PLoS One 201510e0140599. ( 10.1371/journal.pone.0140599) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Razvi S Jabbar A Pingitore A Danzi S Biondi B Klein I Peeters R Zaman A & Iervasi G. Thyroid hormones and cardiovascular function and diseases. Journal of the American College of Cardiology 2018711781–1796. ( 10.1016/j.jacc.2018.02.045) [DOI] [PubMed] [Google Scholar]
- 30.Tagoe CE. Rheumatic symptoms in autoimmune thyroiditis. Current Rheumatology Reports 2015175. ( 10.1007/s11926-014-0479-7) [DOI] [PubMed] [Google Scholar]
- 31.See LC Kuo CF Yu KH Luo SF Chou IJ Ko YS Chiou MJ & Liu JR. Hyperthyroid and hypothyroid status was strongly associated with gout and weakly associated with hyperuricaemia. PLoS One 20149e114579. ( 10.1371/journal.pone.0114579) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ch’ng CL Keston Jones M & Kingham JG. Celiac disease and autoimmune thyroid disease. Clinical Medicine and Research 2007184–192. ( 10.3121/cmr.2007.738) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dayan CM & Panicker V. Hypothyroidism and depression. European Thyroid Journal 20132168–179. ( 10.1159/000353777) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Valderas JM Starfield B Sibbald B Salisbury C & Roland M. Defining comorbidity: implications for understanding health and health services. Annals of Family Medicine 20097357–363. ( 10.1370/afm.983) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Boelaert K, Newby PR, Simmonds MJ, Holder RL, Carr-Smith JD, Heward JM, Manji N, Allahabadia A, Armitage M, Chatterjee KV, et al. Prevalence and relative risk of other autoimmune diseases in subjects with autoimmune thyroid disease. American Journal of Medicine 2010123183.e1–183.e9. ( 10.1016/j.amjmed.2009.06.030) [DOI] [PubMed] [Google Scholar]
- 36.Berkson J. Limitations of the application of fourfold table analysis to hospital data. Biometrics Bulletin 194647–53. [PubMed] [Google Scholar]
- 37.Burch HB. Drug effects on the thyroid. New England Journal of Medicine 2019381749–761. ( 10.1056/NEJMra1901214) [DOI] [PubMed] [Google Scholar]
- 38.Udovcic M Pena RH Patham B Tabatabai L & Kansara A. Hypothyroidism and the heart. Methodist Debakey Cardiovascular Journal 20171355–59. ( 10.14797/mdcj-13-2-55) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Giorda CB Carnà P Romeo F Costa G Tartaglino B & Gnavi R. Prevalence, incidence and associated comorbidities of treated hypothyroidism: an update from a European population. European Journal of Endocrinology 2017176533–542. ( 10.1530/EJE-16-0559) [DOI] [PubMed] [Google Scholar]
- 40.Wiebolt J Achterbergh R den Boer A van der Leij S Marsch E Suelmann B de Vries R & van Haeften TW. Clustering of additional autoimmunity behaves differently in Hashimoto's patients compared with Graves' patients. European Journal of Endocrinology 2011164789–794. ( 10.1530/EJE-10-1172) [DOI] [PubMed] [Google Scholar]
- 41.Bauer M Goetz T Glenn T & Whybrow PC. The thyroid-brain interaction in thyroid disorders and mood disorders. Journal of Neuroendocrinology 2008201101–1114. ( 10.1111/j.1365-2826.2008.01774.x) [DOI] [PubMed] [Google Scholar]
- 42.Thvilum M Brandt F Almind D Christensen K Brix TH & Hegedüs L. Increased psychiatric morbidity before and after the diagnosis of hypothyroidism: a nationwide register study. Thyroid 201424802–808. ( 10.1089/thy.2013.0555) [DOI] [PubMed] [Google Scholar]
- 43.Vigário Pdos S Vaisman F Coeli CM Ward L Graf H Carvalho G Júnior RM & Vaisman M. Inadequate levothyroxine replacement for primary hypothyroidism is associated with poor health-related quality of life-a Brazilian multicentre study. Endocrine 201344434–440. ( 10.1007/s12020-013-9886-1) [DOI] [PubMed] [Google Scholar]
- 44.Flinterman LE, Kuiper JG, Korevaar JC, van Dijk L, Hek K, Houben E, Herings R, Franken AAM, de Graaf JP, Horikx A, et al. Impact of a forced dose-equivalent levothyroxine brand switch on plasma thyrotropin: a cohort study. Thyroid 202030821–828. ( 10.1089/thy.2019.0414) [DOI] [PubMed] [Google Scholar]
- 45.Hoermann R Midgley JEM Larisch R & Dietrich JW. Individualised requirements for optimum treatment of hypothyroidism: complex needs, limited options. Drugs in Context 20198212597. ( 10.7573/dic.212597) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Escobar-Morreale HF Obregón MJ Hernandez A Escobar del Rey F & Morreale de Escobar G. Regulation of iodothyronine deiodinase activity as studied in thyroidectomized rats infused with thyroxine or triiodothyronine. Endocrinology 19971382559–2568. ( 10.1210/endo.138.6.5212) [DOI] [PubMed] [Google Scholar]
- 47.Watt T Hegedüs L Bjorner JB Groenvold M Bonnema SJ Rasmussen AK & Feldt-Rasmussen U. Is thyroid autoimmunity per se a determinant of quality of life in patients with autoimmune hypothyroidism? European Thyroid Journal 20121186–192. ( 10.1159/000342623) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ott J Promberger R Kober F Neuhold N Tea M Huber JC & Hermann M. Hashimoto's thyroiditis affects symptom load and quality of life unrelated to hypothyroidism: a prospective case-control study in women undergoing thyroidectomy for benign goiter. Thyroid 201121161–167. ( 10.1089/thy.2010.0191) [DOI] [PubMed] [Google Scholar]
- 49.Pop VJ Maartens LH Leusink G van Son MJ Knottnerus AA Ward AM Metcalfe R & Weetman AP. Are autoimmune thyroid dysfunction and depression related? Journal of Clinical Endocrinology and Metabolism 1998833194–3197. ( 10.1210/jcem.83.9.5131) [DOI] [PubMed] [Google Scholar]
- 50.Bazzichi L Rossi A Giuliano T De Feo F Giacomelli C Consensi A Ciapparelli A Consoli G Dell'Osso L & Bombardieri S. Association between thyroid autoimmunity and fibromyalgic disease severity. Clinical Rheumatology 2007262115–2120. ( 10.1007/s10067-007-0636-8) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The article is based on data from the Lifelines Cohort Study. Lifelines adheres to standards of data availability and allows access for reproducibility of the study results. The data catalogue is publicly accessible at www.lifelines.nl. The dataset supporting the conclusions of this article is available through the Lifelines organization, and all international researchers can apply for data access at the Lifelines Research office (research@lifelines.nl). For data access, a fee is required.



 This work is licensed under a
This work is licensed under a