Abstract
Heat stress (HS) is still the essential environmental agent influencing the poultry industry. Research on HS in poultry has progressively acquired growing interest because of increased attention to climate alteration. Poultry can survive at certain zone of environmental temperatures, so it could be considered homoeothermic. In poultry, the normal body temperature is essential to enhance the internal environment for growth, which is achieved by normal environmental temperature. Recently, many studies have revealed that HS could cause mitochondrial dysfunction in broilers by inducing redox dysfunction, increasing uncoupling protein, boosting lipid and protein oxidation, and oxidative stress. Moreover, HS diminished the energy suppliers supported by mitochondria activity. A novel strategy for combating the negative influences of HS via boosting the mitochondria function through enrichment of the diets with mitochondria enhancers was also described in this review. Finally, the current review highlights the mitochondria dysfunction induced by HS in broilers and attempts to boost mitochondria functionality by enriching mitochondria enhancers to broiler diets.
Keywords: heat stress, broiler, mitochondria dysfunction, mitochondria enhancer
INTRODUCTION
International poultry meat production increased due to its elevated nutritive significance, cheap cost, and lack of cultural objections. Climate change represents an important environmental menace to poultry health (Uyanga et al., 2023). Therefore, several adaptation trials have been exerted to aim at the increased global temperatures (World Bank, 2013). A prolonged increase in international heat is predicted to create marked harmful influences on the ecosystem, animals, and humans. High ambient temperature is a growing dangerous issue in the poultry industry (Uyanga et al., 2023). The optimum thermal zone for broilers is 16°C to 26°C and if the external temperature surpasses the thermoneutral zone, it causes heat stress (HS) in poultry (Bin-Jumah et al., 2020). Additionally, HS can adversely affect several poultry species’ health, growth, reproduction, and production (Kim et al., 2021).
So, nutritional strategies are done to alleviate the influences of the increased temperature of the environment on the nutrient intake of poultry. These manipulations are indispensable to recovering the decreased feed intake, reducing heat production, and lowering metabolic alterations (Abd El-Hack et al., 2020). Moreover, adding antioxidants to the diet is essential to enhance performance, decrease internal thermal elevation, and ameliorate the harmful influences of elevated environmental temperature (Abdelnour et al., 2020; Bin-Jumah et al., 2020). Heat stress activates oxidative stress leading to mitochondrial injury (Mujahid et al., 2006; Rhoads et al., 2013). A significant exertion in animal biology exploration is intended to understand how animals adapt and respond to environmental stimuli, including high ecological temperature (Chen et al., 2023b; Schreier, 2023), and thus the eventual aim to improve these responses for animal production (Xu et al., 2019).
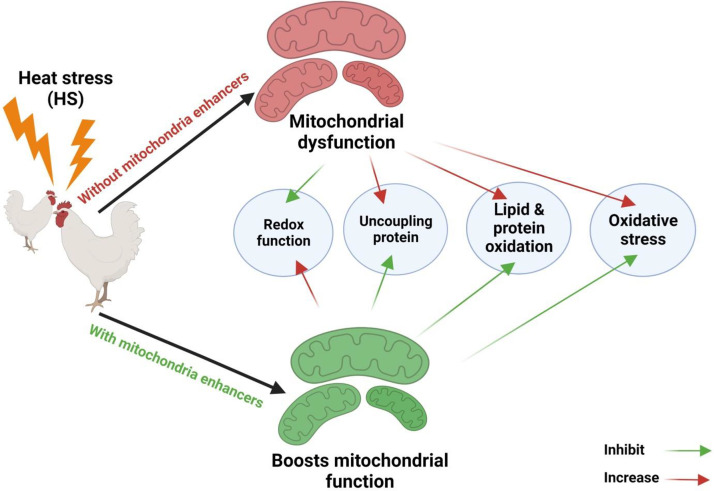
Mitochondria are pivotal in energy metabolism, comprising oxidative phosphorylation (OXPHOS) and substrate oxidation. Under HS, impaired mitochondria resulting from high temperature (Chen et al., 2023a) or elevated reactive oxidative stress (ROS) production may lead to energy-metabolic alterations (Mujahid et al., 2006). Mitochondrial malfunction may reduce the mitochondrial oxidation of carbohydrates and lipids. Many investigations proved that changes markedly influence meat quality in energy metabolism (Chen et al., 2022, 2023a). The major resource of cellular ROS is the electron transport chain of the mitochondria induced by HS. The suppression of the respiratory chain by mutation, damage, loss of cytochrome c, or ischemia will result in ROS release (Hu et al., 2023) (Figure 1).
Figure 1.
Exposing the broiler to heat stress conditions instigated mitochondria dysfunction via generating mitochondria oxidative stress, altering uncoupling proteins, redox imbalance and induced lipid and protein oxidation.
The reduction of the avian uncoupling protein (avUCP) synthesis and the decrease of the mitochondrial respiratory chain complex activity are the mechanisms by which ROS produced in chickens (Hu et al., 2023) and may be accompanied by mitochondrial injury (Zhang et al., 2018a; Ouyang et al., 2022; Chen et al., 2023a,b). In chicken, recent investigations have revealed that excess ROS production from mitochondria under HS conditions may lead to a reduction in mitochondria function, promoted substrate oxidation, and down-regulation of avUCP and change some linked genes related to mitochondria competence (Li et al., 2019; Liu et al., 2019; Muvhulawa et al., 2022; Ouyang et al., 2022). The objective of the current criticism is to scrutinize the HS influences on the mitochondria function and highlight the potentiality of using some mitochondria enhancers for promoting thermotolerance in heat-exposed chickens.
SEARCH STRATEGY
For this review, we searched for published articles on HS affecting poultry from PubMed, ScienceDirect, Google Scholar, and Web of Science up to 2023. All keywords were clustered into 7 groups which included investigations on “HS,” “oxidative stress,” “broiler,” “immune response,” “mitochondria function,” “mitophagy,” and “mitochondria enhancers” as current research hotspots.
MITOCHONDRIA FUNCTION
Mitochondria produce up to 90% of the energy (i.e., Adenosine triphosphate (ATP)) essential for the cellular system through OXPHOS. They are indispensable in sustaining cellular homeostasis (Li et al., 2019). Furthermore, they play a role in coordinating nutrient partitioning during growth, intracellular Ca2+ metabolism, and egg formation, modifying cellular stress responses, and controlling cell apoptosis (Zhang et al., 2016; Uyanga et al., 2022).
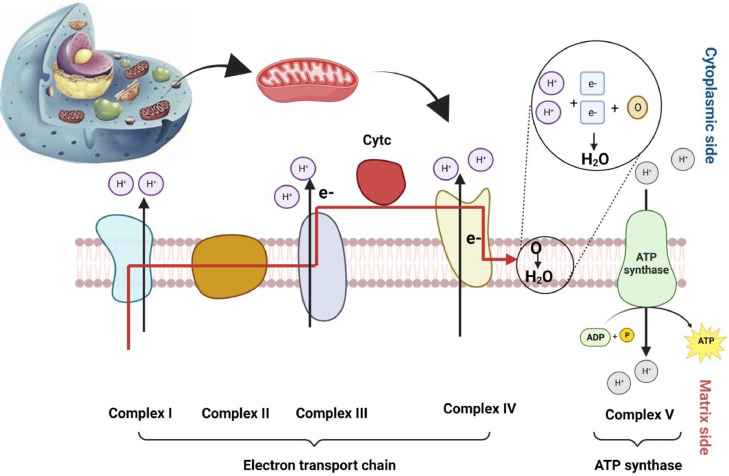
Mitochondria are the energy factories of the eukaryotes, which produce ATP through the OXPHOS cycle, including F1FO-ATP synthase and 5 complexes. Cytochrome c oxidase (CCO; complex IV) activates the transport of electrons from reduced cytochrome c to molecular oxygen (the last electron acceptor) (Figure 2) (Dhar and Wong-Riley, 2009; Zhang et al., 2016). The enzyme activity in the respiratory chain and mitochondrial biogenesis increases in stressed cells (Liao et al., 2022). Therefore, the mitochondrial protein amount and activity of CCO could be utilized as markers of mitochondrial role in animals (Zhang et al., 2016; Uyanga et al., 2022). Acute HS induced a marked reduction in the mitochondrial proteins in the broiler (Zhang et al., 2016).
Figure 2.
The oxidative phosphorylation process for generating ATP molecules in the mitochondria system.
HEAT STRESS INDUCES MITOCHONDRIAL DYSFUNCTION
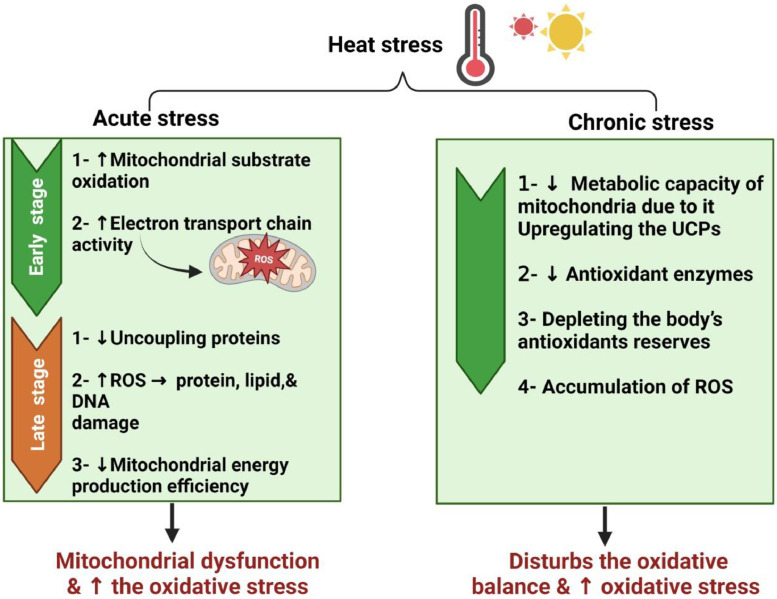
Heat stress causes oxidative stress, which results in mitochondrial dysfunction (Tables 1 and 2). Firstly, acute HS increases the activity of the electron transport chain and levels of mitochondrial substrate oxidation, leading to excess ROS production. After that, acute HS downregulates uncoupling proteins (UCPs) and exaggerated ROS deteriorates the DNA, lipid, and protein, causing mitochondrial dysfunction. However, chronic HS lowers the mitochondrial metabolic capacity because of the antioxidant enzymes’ downregulation, UCPs’ upregulation, and depletion of the body's antioxidants, which accumulate ROS, activating oxidative stress (Chen et al., 2023a) (Figure 3). Liao al. (2022) suggested that mitochondrial dysfunction has marked influences on the broiler industry due to the depression of the mitochondrial respiratory chain by the acute HS (Chen et al., 2023a), which results in excessive ROS leading to oxidative stress (Yang et al., 2010).
Table 1.
The main findings summarized from previous literature are related to the effects of heat stress on lipid and protein peroxidations, reactive oxygen species, redox balance and mitochondria function in broilers.
| Item | HS condition | Main findings | References |
|---|---|---|---|
| Lipid peroxidation | Broiler kept at control (23°C) and HS group (38°C); RH 70%, for 7 d | •Significantly increased in hepatic MDA compared to the control group. | Tan et al. (2010) |
| Broiler exposed to (36°C ± 2°C; 8 h/d lasted for 7-14 d. | •Broiler exposed to HS exhibited higher values of MDA in breast muscle compared to the control group. | Lu et al. (2017) | |
| Acute HS (34°C ± 1°C, 24 h) | •The values of MDA were significantly increased by 37.3%, 12.5%, and 40% in serum and both tissues of the liver and ileum of broiler after exposure to HS, respectively. | Ouyang et al. (2022) | |
| Embryo chicken exposed to HS conditions (39.5°C for 6 h) | •HS conditions enhanced the MDA levels in the hepatocytes of chicken embryo. | Zhang et al. (2022) | |
| Broiler exposed to 34°C ± 1°C for 8 h/d | •The levels of MDA were significantly increased by 20%.•Superior levels of MDA and hydrogen peroxide were also observed in the serum of HS group. | Deng et al. (2023) | |
| Broiler exposed to HS (34°C) | •Broiler exposed to HS had higher levels of MDA than the treated group (rutin 0.5 g/kg feed).•The levels of MDA were significantly increased by 20% in the hepatic broiler's control group after exposure to HS (35°C ± 2°C). | Chen et al. (2023b) | |
| Broiler exposed to cyclic HS (34°C for 8 h and 24°C for 16 h) | •High-temperature conditions (cyclic HS) increased (P < 0.05) hepatic MDA values in broilers. | Bai et al. (2023) | |
| Protein oxidation | Broiler exposed to HS (38°C; RH 70%) |
•Elevation in hepatic protein carbonyl was noticed in the HS group.•An increase in the protein carbonyl of hepatic tissues after exposure to HS. | Saracila et al. (2023), Tan et al. (2010) |
| Chicken embryo hepatocytes (39.5°C heat stress for 6 h) | •HS conditions significantly increased the protein carbonyl levels in chicken embryo hepatocytes. | Zhang et al. (2015), Zhang et al. (2022) | |
| ROS | Broilers exposed to high temperature (34°C ± 1°C for 8 h and 22°C ± 1°C for the remaining time) for 20 d | •Acute HS instigated a marked ROS release.•Broilers exposed to high temperatures significantly elevated ROS in the hepatocytes. | Zhang et al. (2018a), Zhang et al. (2018b) |
| HS (7 d, 32°C) | •Compared to the control group, there were significantly higher values of ROS in broiler breast muscle exposed to HS. | Lu et al. (2017) | |
| Broiler exposed to HS (36°C ± 2°C; 8 h/d lasted for 1 or 2 wk) | •HS induced superior values of ROS than the control group (25°C ± 2°C; 24 h/d) in broilers. | Wang et al. (2019) | |
| HS (39.5°C for 6 h) | •HS conditions enhanced the ROS and 8-OHdG (DNA marker damage) levels in chicken embryo hepatocytes. | Zhang et al. (2022) | |
| Mitochondria redox balance | Broiler chickens (34°C for 8 h) | •Mitochondrial GSH levels were reduced significantly in the livers of heat-stressed broilers.•HS diminished the antioxidant reactions through the NF-E2-related factor 2 (Nrf2) signaling pathway.•HS inhibited the gene expression of Prx3, TrxR2, and Trx2, which are related to redox balance in mitochondria. | Zhang et al. (2018a), Zhang (2018b) |
| Acute HS (34°C ± 1°C, 24 h) | •HS diminished the activities of GSH-Px, CAT, SOD, and TAC in hepatic and ileum tissues of stressed broilers.•Acute HS exposure resulted in elevated expression of GPx1 and Nrf2 in the ileum of the small intestine and HO-1, NQO1, and Nrf2 in the hepatic tissues compared with the control group. | Ouyang et al. (2022) | |
| HS (34°C) | •Stressed broiler had inferior SOD, GPx, and TAC levels than the treated group (rutin 0.5 g/kg feed). | Chen et al. (2023a) | |
| HS 35°C ± 2°C | •Stressed broiler had inferior hepatic antioxidant indices such SOD (↓33.3%), and GPx (↓38.46%), as compared with the control group (23°C ± 2°C).•HS induced (34°C ± 1°C for 8 h/d) intestinal barrier dysfunction and antioxidant capacity in broilers. | Ding et al. (2023) | |
| Heat stress conditions (39.5°C HS for 6 h) | •Chicken exposed to high temperature showed inferior SOD, TAC, GSH-Px, CuZn-SOD, and CAT activities. | Zhang et al. (2022) | |
| Mitochondrial activity | – | •Stressed broilers experienced significant ATP levels reductions, complexes I and III activities, relative MMP (mitochondrial membrane potential), and swelling. | Liao et al. (2022) |
| Acute heat stress | •Upregulated the mitochondrial membrane potential (increased the acceleration of energy consumption and activated mitochondria to produce more energy).•Acute heat stress caused an upregulation in the broilers’ mitochondrial membrane potential due to increased energy consumption and mitochondrial activation, resulting in increased energy production. | Uyanga et al. (2022) |
Table 2.
The main consequences of using some mitochondria enhancers on stressed broiler.
| Mitochondria enhancers | HS condition and doses | Main findings | References |
|---|---|---|---|
| L-citrulline | Acute HS (34°C ± 1°C, 24 h); broiler-given basal diets enriched with 1% L-citrulline |
|
Uyanga et al. (2022) |
| Curcumin | Broiler given 50, 100, or 200 mg/kg diet under HS conditions |
|
Zhang et al. (2018a), Zhang et al. (2018b) |
| Resveratrol | Stressed broiler given 400 mg/kg diet/ 35°C ± 2°C for 7 d |
|
Chen et al. (2023a), Ding et al. (2023) |
| Tryptophan | Stressed broiler received 0.18% |
|
Ouyang et al. (2022) |
Figure 3.
The effect of acute and chronic heat stress on the mitochondrial functions.
Lipid Peroxidation
In broiler, several investigations have reported that acute and chronic HS initiated significant elevation of lipid peroxidation represented by increased malondialdehyde (MDA) in the plasma (Uyanga et al., 2022) (Figure 4). The amount of lipid peroxidation was considerably augmented in both mitochondria and muscles in the stressed broiler (P < 0.05) (Liao et al., 2022). In an earlier investigation, Tan et al. (2010) found that exposing the broiler to a high temperature (23°C–38°C with RH = 70%) induced a significant elevation in hepatic MDA compared with the control (25°C). Broiler exposed to HS (7 d, 32°C) exhibited higher MDA values in breast muscle compared to the control group (Lu et al., 2017).
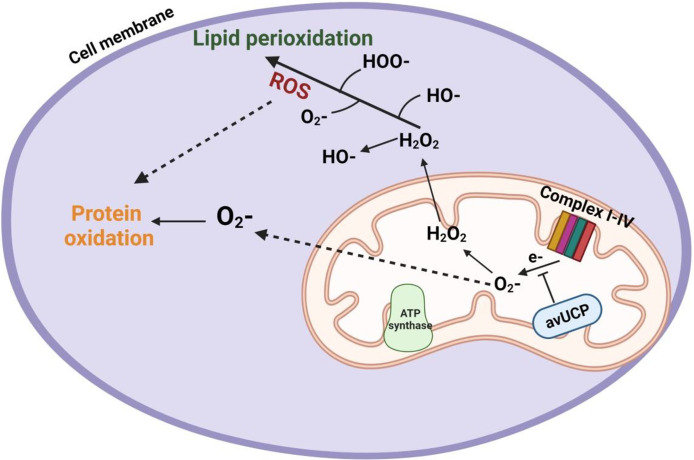
Figure 4.
Involvement of heat stress-induced lipid peroxidation, oxidative stress, and protein oxidation at the mitochondria cavity.
Broiler exposed to HS (36°C ± 2°C; 8 h/d lasted for 1 or 2 wk) had superior values of MDA than the control group (25°C ± 2°C; 24 h/d) (Wang et al., 2019). It has been clarified that the MDA values were increased significate by 37.3, 12.5, and 40% in serum, and both tissues of the liver and ileum of broiler after exposure to HS, respectively (Ouyang et al., 2022). In a recent investigation by Chen et al. (2023a), they found that the broiler exposed to HS (34°C) had higher levels of MDA than the treated group (rutin 0.5 g/kg feed). Moreover, the MAD values were significantly increased by 20% compared to the hepatic broiler group after exposure to HS (35°C ± 2°C) (Ding et al., 2023). Moreover, Deng et al. (2023) indicated that broiler reared under high temperatures (34°C ± 1°C for 8 h/d) had superior MDA levels and hydrogen peroxide (H2O2) as compared with the control one (24°C ± 1°C). HS conditions (39.5°C for 6 h) enhanced the MDA levels in chicken embryo hepatocytes (Zhang et al., 2022). High-temperature conditions (cyclic HS at 34°C for 8 h and 24°C for 16 h) increased (P < 0.05) hepatic MDA values in broilers (Bai et al., 2023). Totally, HS induced significant levels of MDA, while studying lipidomics as a new tool of omics is needed.
Reactive Oxygen Species
Heat stress generates high ROS, deactivating the body's antioxidant systems (Abd El-Hack et al., 2020). The substrate end of the respiratory chain in the inner membrane of mitochondria is one of the major ROS resources (Abdelnour et al., 2019), where the electrons’ transfer to O2 is done by mitochondrial electron transport chain complexes (Sheiha et al., 2020). The results of Yang et al. (2010) on broilers exposed to acute stress have revealed that acute HS initiated a marked ROS release. Broilers exposed to high temperature (34°C ± 1°C for 8 h and 22°C ± 1°C for the remaining time) for 20 d had a significant elevation in ROS. They significantly decreased the mitochondrial membrane potential (MMP) in the hepatocytes (Zhang et al., 2018a). Broiler exposed to HS (7 d, 32°C) exhibited higher ROS values in breast muscle compared to the control group (Lu et al., 2017). Moreover, broiler exposed to HS (36°C ± 2°C; 8 h/d lasted for 1 or 2 wk) had superior values of ROS than the control group (25°C ± 2°C; 24 h/d) (Wang et al., 2019). Heat stress conditions (39.5°C HS for 6 h) enhanced the ROS and 8-OHdG (DNA marker damage) levels in chicken embryo hepatocytes (Zhang et al., 2022).
Protein Oxidation
Protein carbonyls are the main biomarker for the oxidative destruction of protein. Subsequently, they highpoint cellular impairment instigated via diverse kinds of ROS (Bozaykut et al., 2013). Heat stress might boost the protein oxidation in the cellular system, thus increasing the ROS in the mitochondria matrix, thus causing mitochondria damage. A study by Liao et al. (2022) found that HS induced a higher extent of protein oxidation in both mitochondria and muscles of stressed broiler (P < 0.05). Previously, Tan et al. (2010) found that exposing the broiler to high temperature (23°C –38°C with RH = 70%) induced a significant elevation in hepatic protein carbonyl (PC) compared with control (25°C). Saracila et al. (2023) found that significant increase in the PC of the hepatic tissues of the broiler after exposure to HS (32°C ± 2°C). HS conditions (39.5°C HS for 6 h) enhanced the PC levels in chicken embryo hepatocytes (Zhang et al., 2022). The same results were evidenced by Bai et al. (2023), who found that cyclic HS (34°C for 8 h and 24°C for 16 h) decreased significantly (P < 0.05) hepatic superoxide dismutase (SOD), total antioxidant capacity (TAC), and glutathione peroxidase (GPx) values in broilers.
Understanding the proteomics alterations induced by HS is needed for further clarifications for detecting the signaling pathways related to this matter and could discover a suitable strategy for alleviating HS in animals.
Redox Status of Mitochondria
As previously indicated by Liao et al. (2022), HS diminished the antioxidant system in the mitochondria of muscle in the stressed broiler. Moreover, mitochondrial glutathione (GSH) levels were reduced significantly in the livers of heat-stressed broilers (Zhang et al., 2018b). Furthermore, the same authors indicated that HS diminished the antioxidant reactions through the NF-E2-related factor 2 (Nrf2) signaling pathway (Zhang et al., 2018b). The endogenous antioxidant system of mitochondria relies on both the thioredoxin (TRX)/peroxiredoxin (Prx) and glutathione/glutaredoxin families. The Trx/peroxiredoxin system comprises Trx2, Prx3, and TrxR2, which are the particular locations in the mitochondria providing the initial defense mechanism against hydrogen peroxide and superoxide released by ROS (Patenaude et al., 2004). Pérez et al. (2008) indicated that the Trx2 overexpression directly resisted mitochondrial dysfunction in oxidative-stressed cells. In broiler, Zhang et al. (2018a) reported that HS inhibited Prx3, TrxR2, and Trx2 gene expression. HS diminished the GSH, CAT, SOD, and TAC in hepatic and ileum tissues of stressed broilers (Ouyang et al., 2022). Acute HS exposure resulted in elevated expression of Gpx1 and Nrf2 in the ileum of the small intestine; heme oxygenase 1 (HO-1), NADPH quinone oxidoreductase (NQO1), and nuclear factor E2-related factor 2 (Nrf2) in the hepatic tissues compared with the control group (P < 0.05) (Ouyang et al., 2022). The same results have been clarified by Chen et al. (2023a), who found that the broiler exposed to HS (34°C) had inferior levels of SOD, GPx, and TAC than the treated group (rutin 0.5 g/kg feed). Broiler exposed to HS (35°C ± 2°C) had inferior hepatic antioxidant indices such SOD (↓33.3%), and GPx (↓38.46%), as compared with the control group (23°C ± 2°C) (Ding et al., 2023). Moreover, Deng et al. (2023) clarified that HS-induced (34°C ± 1°C for 8 h/d) intestinal barrier dysfunction and antioxidant status in broilers. Moreover, chicken exposed to HS (39.5°C HS for 6 h) showed inferior SOD, TAC, GSH-Px, CuZn-SOD, and CAT activities (Zhang et al., 2022).
Mitochondrial Activity
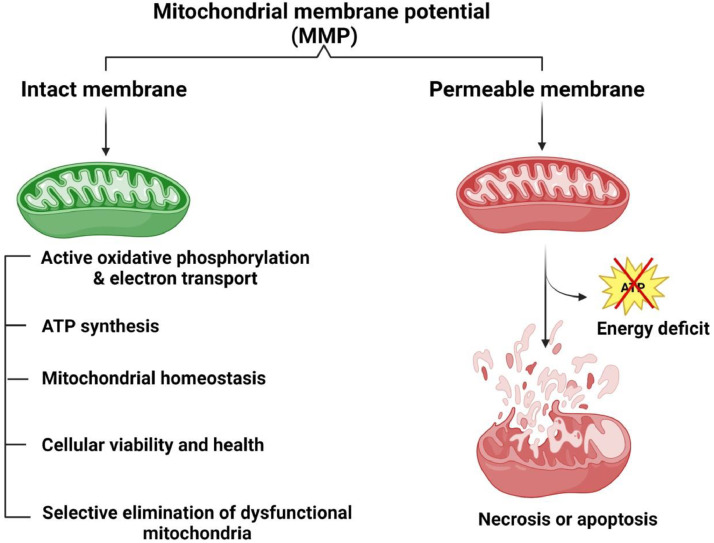
The primary indicator of mitochondrial function is the MMP, as it represents the activities of OXPHOS and electron transport, which are responsible for the production of ATP. The MMP is crucial for maintaining mitochondrial balance, cellular viability, and overall health by removing damaged mitochondria. If severe energy deficits occur, MMP dissipation or loss may lead to cell death through necrosis or trigger apoptosis (Iijima, 2006) (Figure 5). As noted by Liao et al. (2022), stressed broilers experienced significant reductions in ATP levels, relative MMP, and activities of complexes I and III. According to Ouyang et al. (2022), acute HS exposure caused a marked rise (P < 0.05) in the liver's MMP of broiler chickens as opposed to the control group. Mitochondria are the primary energy generator and crucial in maintaining proper functioning. the membrane potential of mitochondria is an essential parameter that reflects mitochondrial health, and a decrease in the potential is an early sign of apoptosis (Kikusato and Toyomizu, 2013). In this work of Ouyang et al. (2022), acute HS caused an upregulation in the broilers’ MMP due to increased energy consumption and mitochondrial activation, resulting in increased energy production. Previous studies on birds and pigs that may experience stress under certain conditions have reported similar findings (Itami et al., 2018).
Figure 5.
Heat stress-induced changes in mitochondria activity (MMP) could affect the mitochondria homeostasis, viability and induce necrosis and apoptosis, limiting the production of ATP. MMP, mitochondrial membrane potential.
Mitochondrial Function-Modulating Genes Expression
Energy Supplies (ATP Synthase F1 Subunit Beta)
Mitochondria are frequently pronounced as the “powerhouse” of the cell. They comprise the molecular pathways that administrate several distinctive metabolic paths within these organelles, counting Krebs cycle, fatty acid β-oxidation, pyruvate oxidation, and OXPHOS (Hafen et al., 2018). Moreover, it helps the cellular system to regulate homeostasis, redox balance (Campos et al., 2017), regulation of bioenergetics paths and cellular metabolism (Senyilmaz et al., 2015). The multivariable function of mitochondria has increasingly emphasized the excessive importance of such organelle in biomedicine. In this sense, mitochondrial function modulates touch-instigated fluctuations in genetic regulation directly via altered regulatory complexes and indirectly via altering energy supplies for cellular metabolism (Campos et al., 2017). Heat stress can induce changes in the mitochondria functionality via modifying genetic regulation.
Uyanga et al. (2022) studied the effects of HS on broilers and observed a downregulation (P < 0.05) in the peroxisome proliferator-activated receptor γ coactivator 1-α and ATP synthase F1 subunit beta (ATP5β) mRNA levels. Broilers exposed to HS also had lower ATP levels than the control group, as reported by Zhang et al. (2018a). Additionally, Zheng et al. (2023) found a positive correlation between ATP levels and mitochondrial DNA (mtDNA) number, resulting from mitochondrial dysfunction in the liver. The final stage of the mitochondrial respiration chain involves cyclooxygenase (COX) and Cyt-c and impacts the generation of ATP and ROS and their elimination (Sena and Chandel, 2012). However, AMP-activated protein kinase (AMPKα) was significantly reduced under HS conditions, accompanied by a decrease in acetyl-CoA carboxylase (ACC) phosphorylation level and mast cell protease-1 (M-CPT1) mRNA expression (Huang et al., 2021), indicating that mitochondrial fatty-acid oxidation was diminished in HS experienced group. This ultimately decreased ATP synthesis and reduced energy supply (Harley et al., 2022). Using proteomics analysis, Wang et al. (2023) found that exposed chicken embryonic to acute HS (42°C for 4 h) exhibited significant changes in the multiplicity of biological regulatory pathways affected by electron transport chain and mitochondrial ATP synthesis, which in term that HS instigated anomalous energy metabolism of primary chick embryonic (Wang et al., 2023). The changes in the cellular proteins under HS conditions might support the discovery of a new strategy to mitigate these detrimental effects, which will be a significant mission for researchers.
Uncoupling Proteins
The uncoupling protein in mammals has been widely researched for several years. It belongs to a transporter group of proteins localized in the inner membrane of mitochondria (Mujahid et al., 2006). In addition, UCP can disperse the proton gradient in mitochondria, causing uncoupling of respiration from ATP synthesis, and is a key regulator of thermogenesis in small rodents (Claypool, 2009). The uncoupling protein 1 is exclusively found in brown adipose tissue and is pivotal in generating heat, especially heat production, due to exposure to cold temperatures and dietary factors (Ferramosca and Zara, 2013). Recently, researchers discovered avUCP. This protein has a 71% to 73% similarity in its amino acid structure to UCP2 and UCP3 in the skeletal muscles of chicken (Davoodi et al., 2023). The avUCP expression increased in chickens and ducklings adapted to cold temperatures (Mujahid et al., 2006). In male birds, the increased expression of avUCP may be accompanied by elevated stress receptivity due to the fast growth rate of males (Mujahid et al., 2006). In another experiment, it was suggested that the proper manifestation of avUCP could reduce the superoxide excessive generation by mitochondrial and assists birds in adapting to oxidative stressors caused by sudden HS (Mujahid et al., 2007). Uyanga et al. (2022) found that during HS conditions, avUCP mRNA expression in the muscle of broiler breast was downregulated (P < 0.05). This reduction in avUCP expression was consistent with investigations showing that HS can decrease UCP gene expression in chickens (Zeng et al., 2022). The avUCP has been found to ameliorate the redox state by reducing the membrane potential of mitochondria through leaking protons on the inner membrane of mitochondria. This process enhances the efficiency of ATP synthesis. In contrast, avUCP expression is downregulated during HS to increase ATP synthesis efficiency in the HS group after 7 d of heat exposure (Zhang et al., 2022). It was previously suggested that increased avUCP expression could limit mitochondrial ROS generation by enhancing proton backflow in the mitochondrial sheet (Lu et al., 2017).
Mitochondrial Respiration
According to Tan et al. (2010), broilers exposed to high ambient temperatures of 32°C to 38°C with 70% relative humidity exhibited impaired mitochondrial respiratory chain function compared to the thermoneutral zone. The authors found that respiratory chain complex activities, especially CCO and NADH-cytochrome c reductase (NCCR), but not succinate-cytochrome c reductase (SCCR), were significantly hindered in the hepatic tissues of broilers exposed to these high temperatures. Wang et al. (2023) explored chick embryonic myocardial cells exposed to HS condition (42°C for 4 h) and exhibited OXPHOS, metabolism, and apoptosis changes (Wang et al., 2023). Moreover, these differentially expressed proteins are involved in a multiplicity of biological controlling pathways, which considerably influenced the electron transport chain, OXPHOS, and mitochondrial ATP synthesis, which implicit that HS instigated abnormal energy metabolism of chick embryonic (Wang et al., 2023).
MITOCHONDRIAL FUNCTION GENES
According to Uyanga et al. (2022), exposure to high temperatures caused a downregulation (P < 0.05) in mRNA expressions of CCO subunit 3, mitochondrial transcription factor A, and ATP5β across muscle of broiler breast. This suggests that sudden exposure to HS can decrease mitochondrial respiratory chain activity, as previously noted by Yang et al. (2010). In another work, Zhang et al. (2015) reported that exposure to HS can reduce the mRNA expressions proliferator-activated receptor γ coactivator 1α of peroxisome, nuclear respiratory factor 1 and 2, and mitochondrial transcription factor A in broilers while causing a significant increase in heat shock protein 70 (HSP70) in breast muscle. Mitochondrial biosynthesis is dependent on the synchronized operation of genetic material from both the nucleus and the mitochondria., with PGC-1α being a crucial transcription factor in the process of generating mitochondria and regulating energy metabolism, whereas TFAM and Nrf1 are necessary for both transcription and replication of mtDNA (Dhar and Wong-Riley, 2009).
Sirtuin 1 is a deacetylase that enhances the activity and functionality of mitochondria (Bai et al., 2011). NAD+ is a metabolite of TRP that is crucial in many enzymatic redox statuses, mitochondrial structures, health, and performance. The dietary addition of certain natural molecules to broiler diets has been reported to prevent impairment of biogenesis and improve mitochondria function (Itami et al., 2018). In hepatic tissues of broilers raised under acute HS, the mRNA expressions peroxisome proliferator-activated receptor-γ coactivator-1α (PGC-1α), Cyt-c, COX1, Cytochrome C Oxidase Subunit 5A, and Sirtuin 1 genes were decreased (Yue et al., 2017; Ouyang et al., 2022).
MITOCHONDRIA DNA
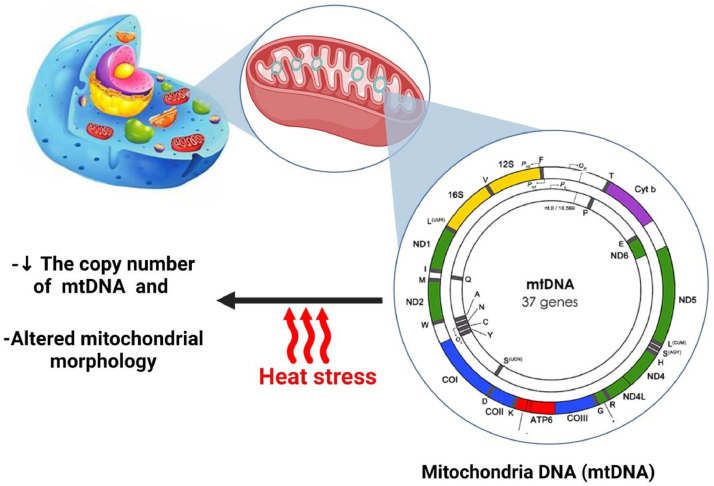
The 37 genes in mitochondrial DNA are all essential for regular mitochondrial function, and studies have displayed that mitochondrial dysfunction is accompanied by reduced mtDNA quality (Zheng et al., 2023). This suggests that mitochondrial dysfunction may be a potential reason for the reduced growth performance and feed efficiency detected in heat-stressed broilers (Chen et al., 2023a). Heat stress can induce oxidative stress in mtDNA (Chen et al., 2023b), reducing mitochondrial efficiency and repeated ROS eruptions, further aggravating mitochondrial dysfunction (Zheng et al., 2023). Therefore, mtDNA changes are the main mechanism of mitochondrial dysfunction (Chen et al., 2023b). Studies have shown that HS can reduce mtDNA and disrupt mtDNA replication (Li et al., 2019; Chen et al., 2023b). Additionally, acute HS effects on the broilers’ livers were found to decrease the mtDNA copy number (Ouyang et al., 2022), but this effect was prevented by tryptophan (TRP) supplementation. Another recent study showed that HS at 34°C caused mitochondrial damage in broilers by decreasing mtDNA number and altering mitochondrial morphology (Chen et al., 2023a) (Figure 6).
Figure 6.
Mitochondria DNA (mtDNA) and heat stress.
MITOCHONDRIA-ASSOCIATED GENES
According to Lu et al. (2017), the exposure of broilers to 32°C heat for 7 d had elevated mRNA expressions of ACC, Pyruvate Dehydrogenase Kinase 4 (PDK4), and FAS while significantly decreasing M-CPT1 mRNA expression in breast muscle. Carvalho and Moreira (2023) found that a 7-d heat exposure activated AMPK and increased CS activity and the M-CPT1 mRNA expression, increasing energy production by activating the tricarboxylic acid cycle and the β-oxidation of fatty acids. However, Shahcheraghi et al. (2023) noted that increased mitochondrial energy generation inevitably led to higher ROS levels due to the high potential difference across the mitochondrial membrane and oxygen levels. Wang et al. (2022) reported that mitochondrial fatty-acid oxidation is partially controlled via AMPK pathways, which inactivate mitochondrial ACC through phosphorylation, leading to raised Carnitine palmitoyltransferase I (CPT I) activity, reduced malonyl-CoA concentration, and an enhanced influx of long-chain fatty acids to undergo oxidation into mitochondria. Wang et al. (2019) noted an upsurge increase in the expression of ABCG2 mRNA in broilers at the end of the second week of HS. Chen et al. (2023a) reported that HS significantly augmented the expression of mitophagy-associated genes and proteins, including Parkin, PTEN-induced putative kinase 1 (PINK1), and LC3-II in broilers. Additionally, Zhang et al. (2022) found that broilers exposed to HS had lower levels of HSP70.
MITOCHONDRIA ENHANCERS
L-Citrulline
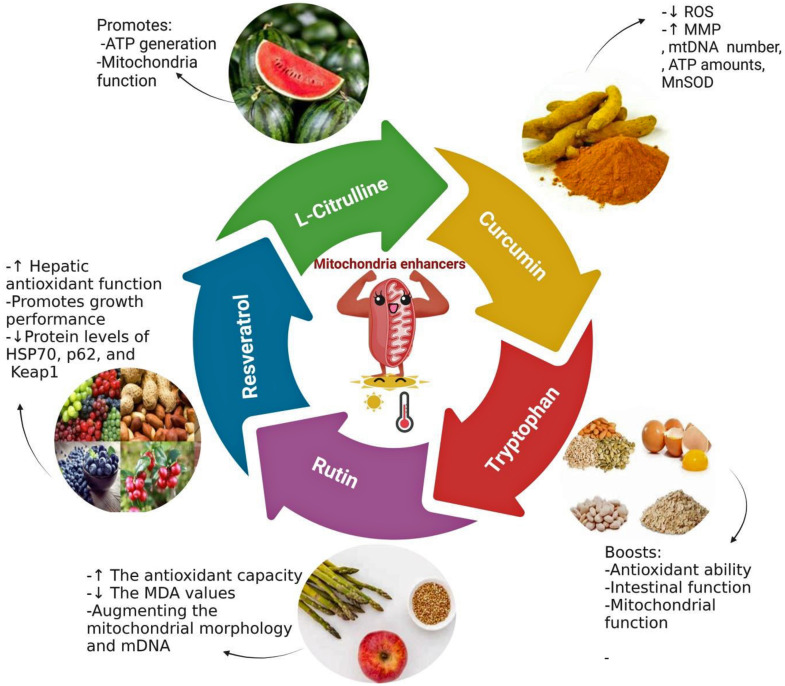
L-Citrulline is an amino acid that does not form part of proteins and helps recycle arginine and muscle protein synthesis. According to Uyanga et al. (2022), in the heat-stressed broiler, the expression of CCO subunit 3, mitochondrial transcription factor A, and ATP5β mRNA were significantly reduced (P < 0.05) (Uyanga et al., 2022). On the other hand, the same study found that adding L-Citrulline to the diet of broiler chickens during HS can reduce body temperature, limit lipid peroxidation, and enhance immune function (Uyang et al., 2022). L-Citrulline may also promote ATP production and improve mitochondrial function in the muscles of broilers exposed to HS (Figure 7).
Figure 7.
The potential of using some mitochondria enhancers to enhance the mitochondria functionality under heat stress conditions in broilers. These natural molecules could improve the antioxidant capabilities, reduce lipid oxidation promotes the mitochondria function (MMP, mtDNA, ATP amounts, and antioxidant-related genes) in the stressed broiler. MMP, mitochondrial membrane potential; mtDNA, mitochondria DNA.
Curcumin
Curcumin is a bioactive substance extracted from turmeric, known for its pharmacological properties, such as antioxidant and hepatoprotective activities (Gayathri et al., 2023). In broilers exposed to HS, curcumin increased Nrf2 expression, GSH-Px, GST, HO-1, and γ-GCLc in the hepatic tissue and improved Cu/ZnSOD and CAT expression in a dose-dependent manner (Zhang et al., 2018b). Curcumin also decreased ROS production while increasing MMP, mtDNA copy number, ATP amounts, and MnSOD in the hepatocytes of heat-stressed broilers. These effects were due to the stimulation of mitochondrial Trx2/Prx3 family members and MnSOD, which improved mitochondrial function against stressors (Zhang et al., 2018a).
Tryptophan
Tryptophan is a functional amino acid crucial in broilers’ maintenance, reproduction, and immunity (Mund et al., 2020). Increasing dietary intake of TRP improved broiler growth, feed efficiency, and immunity while mitigating oxidative and sudden chronic stress (Yue et al., 2017). Similarly, in piglets exposed to HS, TRP supplementation raised the antioxidant capacity and function of the intestine and mitochondria (Liu et al., 2019). Ouyang et al. (2022) reported that adding 0.18% TRP to the diet of broilers provides beneficial effects in defending against oxidative stress induced by sudden exposure to high temperature and mitochondrial malfunction. This protective effect is achieved by modulating antioxidant levels and increasing the expression of mitochondrial function-related genes.
Rutin
Rutin is a light yellow or light green needle or crystalline powder that is found in many foods and medicinal plants. It possesses therapeutic properties such as immunomodulatory, anti-inflammatory, antioxidant, and anticancer effects (Muvhulawa et al., 2022). According to Chen et al. (2022), including rutin in the diet improved broilers’ intestinal function, immunity, and antioxidant capabilities, enhancing their growth and feed efficiency. When broilers were fed rutin (500 mg/kg) under stress conditions (34°C), the antioxidant capacity (SOD, GPx, TAC) was enhanced and MDA values were decreased (Chen et al., 2023a). Moreover, rutin alleviated HS-induced mitochondrial impairment by promoting mitochondrial morphology and mDNA (Chen et al., 2023a). The same authors also found that dieting rutin greatly raised certain mRNA molecules related to creating mitochondria, including PGC-1α, Nuclear respiratory factor 1 (NRF1), and mitochondrial transcription factor (TFAM). This effect was achieved via the AMPK signaling pathway.
Resveratrol
Resveratrol is a plant-derived polyphenol with anti-inflammatory and antioxidant properties commonly used to suppress oxidative stress-related diseases. According to a study by Ding et al. (2023), feeding broilers with resveratrol at a concentration of 400 mg/kg in their diet can boost hepatic antioxidant capacity by activating the signaling pathway of Nrf2-Keap1, leading to better broiler growth under HS conditions (35 ± 2°C for 7 d). Additionally, resveratrol was detected to lower hepatic oxidative stress by enhancing the Nrf2 and HO-1 gene and protein levels, increasing the levels of NQO1 and SOD1 genes, and reducing the HSP70, p62, and Keap1 protein levels, thereby improving hepatic damage in heat-stressed broilers (Ding et al., 2023).
CONCLUSION
Heat stress is a severe stressor that can cause mitochondria dysfunction, negatively affecting broiler production. Mitochondrial dysfunction is closely linked to high oxidative stress, low antioxidant capacity, and a reduction in the supply of energy to the mitochondria. Moreover, HS could reduce mtDNA, MMP, and mitochondria respiration and boost autophagy, causing mitochondrial damage in broilers. Recently, some therapeutic mitochondria support such as rutin, resveratrol, curcumin, L-citrulline, and TRP have been widely used to combat HS's detrimental effects on broilers. These molecules used for this purpose could sustain the productivity of the broiler under high environmental conditions. However, these phyto-molecules can improve the function of mitochondria function and thus enhance performance and profitability of broiler production in stressful environments, further molecules should be investigated mainly based on proteomics screening. For future directions, it is essential to optimize the method of concentration and administration and ensure the stability of flavonoids in the environment for their use as an additive. Also, more investigations should be discovered to clarify the potential roles of nano-phytomolecules for targeting the mitochondria functions in neutralizing the HS conditions due to its superior bioavailability, stability and effectivity.
Acknowledgments
ACKNOWLEDGMENTS
This research was also supported in part by funds provided by USDA-NIFA, Sustainable Agriculture Systems, grant no. 2019-69012-29905. Title of project: Empowering U.S. Broiler Production for Transformation and Sustainability USDA-NIFA (Sustainable Agriculture Systems): no. 2019-69012-29905.
DISCLOSURES
The authors declare no conflict of interest.
REFERENCES
- Abd El-Hack M.E., Abdelnour S.A., Taha A.E., Khafaga A.F., Arif M., Ayasan T., Swelum A.A., Abukhalil M.H., Alkahtani S., Aleya L. Herbs as thermoregulatory agents in poultry: an overview. Sci. Total Environ. 2020;703 doi: 10.1016/j.scitotenv.2019.134399. [DOI] [PubMed] [Google Scholar]
- Abdelnour S.A., Abd El-Hack M.E., Khafaga A.F., Arif M., Taha A.E., Noreldin A.E. Stress biomarkers and proteomics alteration to thermal stress in ruminants: a review. J. Therm. Biol. 2019;79:120–134. doi: 10.1016/j.jtherbio.2018.12.013. [DOI] [PubMed] [Google Scholar]
- Abdelnour S.A., Abd El-Hack M.E., Alagawany M., Taha A.E., Elnesr S.S., Abd Elmonem O.M., Swelum A.A. Useful impacts of royal jelly on reproductive sides, fertility rate and sperm traits of animals. J. Anim. Physiol. Anim. Nutr. 2020;104:1798–1808. doi: 10.1111/jpn.13303. [DOI] [PubMed] [Google Scholar]
- Bai P., Canto C., Brunyánszki A., Huber A., Szántó M., Cen Y., Yamamoto H., Houten S.M., Kiss B., Oudart H., Gergely P., Menissier-de Murcia J., Schreiber V., Sauve A.A., Auwerx J. PARP-2 regulates SIRT1 expression and whole-body energy expenditure. Cell Metab. 2011;13:450–460. doi: 10.1016/j.cmet.2011.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai X., Wang K., Khan R.U., Zhang C., Hu H. Effect of glutamine on the growth performance, oxidative stress, and NRF2/P38 MAPK expression in the livers of heat-stressed broilers. Animals. 2023;13:652. doi: 10.3390/ani13040652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bin-Jumah M., Abd El-Hack M.E., Abdelnour S.A., Hendy Y.A., Ghanem H.A., Alsafy S.A., Khafaga A.F., Noreldin A.E., Shaheen H., Samak D., Momenah M.A., Allam A.A., AlKahtane A.A., Alkahtani S., Abdel-Daim M.M., Aleya L. Potential use of chromium to combat thermal stress in animals: a review. Sci. Total Environ. 2020;707 doi: 10.1016/j.scitotenv.2019.135996. [DOI] [PubMed] [Google Scholar]
- Bozaykut P., Sozen E., Kaga E., Ece A., Ozaltin E., Ek B., Ozer N.K., Grune T., Bergquist J., Karademir B. The role of heat stress on the age related protein carbonylation. J. Proteomics. 2013;89:238–254. doi: 10.1016/j.jprot.2013.06.025. [DOI] [PubMed] [Google Scholar]
- Campos J.C., Queliconi B.B., Bozi L.H., Bechara L.R., Dourado P.M., Andres A.M., Jannig P.R., Gomes K.M., Zambelli V.O., Rocha-Resende C. Exercise reestablishes autophagic flux and mitochondrial quality control in heart failure. Autophagy. 2017;13:1304–1317. doi: 10.1080/15548627.2017.1325062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvalho C., Moreira P.I. Metabolic defects shared by Alzheimer's disease and diabetes: a focus on mitochondria. Curr. Opin. Neurobiol. 2023;79 doi: 10.1016/j.conb.2023.102694. [DOI] [PubMed] [Google Scholar]
- Chen S., Liu H., Zhang J., Zhou B., Zhuang S., He X., Wang T., Wang C. Effects of different levels of rutin on growth performance, immunity, intestinal barrier and antioxidant capacity of broilers. Ital. J. Anim. Sci. 2022;21:1390–1401. [Google Scholar]
- Chen S., Liu H., Zhang J., Zhou B., He X., Wang T., Wang C. Dietary rutin improves breast meat quality in heat-stressed broilers and protects mitochondria from oxidative attack via the AMPK/PINK1–Parkin pathway. J. Sci. Food Agric. 2023;103:2367–2377. doi: 10.1002/jsfa.12431. [DOI] [PubMed] [Google Scholar]
- Chen Z., Sun X., Li X., Liu N. Oleoylethanolamide alleviates hyperlipidaemia-mediated vascular calcification via attenuating mitochondrial DNA stress triggered autophagy-dependent ferroptosis by activating PPARα. Biochem. Pharmacol. 2023;208 doi: 10.1016/j.bcp.2022.115379. [DOI] [PubMed] [Google Scholar]
- Claypool S.M. Cardiolipin, a critical determinant of mitochondrial carrier protein assembly and function. Biochim. Biophys. Acta Biomembr. 2009;1788:2059–2068. doi: 10.1016/j.bbamem.2009.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davoodi P., Ghaderi-Zefrehei M., Dolatabady M.M., Razmkabir M., Kianpour S., Esfahani E.N., Smith J. In silico investigation of uncoupling protein function in avian genomes. Front. Vet. Sci. 2023;9:1–18. doi: 10.3389/fvets.2022.1085112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng C., Zheng J., Zhou H., You J., Li G. Dietary glycine supplementation prevents heat stress-induced impairment of antioxidant status and intestinal barrier function in broilers. Poult. Sci. 2023;102 doi: 10.1016/j.psj.2022.102408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhar S.S., Wong-Riley M.T. Coupling of energy metabolism and synaptic transmission at the transcriptional level: role of nuclear respiratory factor 1 in regulating both cytochrome c oxidase and NMDA glutamate receptor subunit genes. J. Neurosci. 2009;29:483–492. doi: 10.1523/JNEUROSCI.3704-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding K.-N., Lu M.-H., Guo Y.-N., Liang S.-S., Mou R.-W., He Y.-M., Tang L.-P. Resveratrol relieves chronic heat stress-induced liver oxidative damage in broilers by activating the Nrf2-Keap1 signaling pathway. Ecotoxicol. Environ. Saf. 2023;249 doi: 10.1016/j.ecoenv.2022.114411. [DOI] [PubMed] [Google Scholar]
- Ferramosca A., Zara V. Biogenesis of mitochondrial carrier proteins: molecular mechanisms of import into mitochondria. Biochim. Biophys. Acta, Mol. 2013;1833:494–502. doi: 10.1016/j.bbamcr.2012.11.014. [DOI] [PubMed] [Google Scholar]
- Gayathri K., Bhaskaran M., Selvam C., Thilagavathi R. Nano formulation approaches for curcumin delivery: a review. J. Drug Deliv. Sci. Technol. 2023;82 [Google Scholar]
- Hafen P.S., Preece C.N., Sorensen J.R., Hancock C.R., Hyldahl R.D. Repeated exposure to heat stress induces mitochondrial adaptation in human skeletal muscle. J. Appl. Physiol. 2018;125:1447–1455. doi: 10.1152/japplphysiol.00383.2018. [DOI] [PubMed] [Google Scholar]
- Harley G., Katerelos M., Gleich K., de Souza D.P., Narayana V.K., Kemp B.E., Power D.A., Mount P.F. Blocking AMPK signalling to acetyl-CoA carboxylase increases cisplatin-induced acute kidney injury and suppresses the benefit of metformin. Biomed. Pharmacother. 2022;153 doi: 10.1016/j.biopha.2022.113377. [DOI] [PubMed] [Google Scholar]
- Hu H., Chen L., Huang Y., Wang K., Bai X., Pan H. CUT&tag-seq analysis of heat stress response in broiler liver provides novel insights into the improved thermotolerance by dietary phloretin. Ann. Agric. Sci. 2023;68:12–20. [Google Scholar]
- Huang Y., Xie H., Pan P., Qu Q., Xia Q., Gao X., Zhang S., Jiang Q. Heat stress promotes lipid accumulation by inhibiting the AMPK-PGC-1α signaling pathway in 3T3-L1 preadipocytes. Cell Stress Chaperones. 2021;26:563–574. doi: 10.1007/s12192-021-01201-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iijima T. Mitochondrial membrane potential and ischemic neuronal death. Neurosci. Res. 2006;55:234–243. doi: 10.1016/j.neures.2006.04.005. [DOI] [PubMed] [Google Scholar]
- Itami N., Shirasuna K., Kuwayama T., Iwata H. Short-term heat stress induces mitochondrial degradation and biogenesis and enhances mitochondrial quality in porcine oocytes. J. Therm. Biol. 2018;74:256–263. doi: 10.1016/j.jtherbio.2018.04.010. [DOI] [PubMed] [Google Scholar]
- Kikusato M., Toyomizu M. Crucial role of membrane potential in heat stress-induced overproduction of reactive oxygen species in avian skeletal muscle mitochondria. PLoS One. 2013;8:e64412. doi: 10.1371/journal.pone.0064412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim D.Y., Kim J.H., Choi W.J., Han G.P., Kil D.Y. Comparative effects of dietary functional nutrients on growth performance, meat quality, immune responses, and stress biomarkers in broiler chickens raised under heat stress conditions. Anim. Biosci. 2021;34:1839. doi: 10.5713/ab.21.0230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X.C., Peris D., Hittinger C.T., Sia E.A., Fay J.C. Mitochondria-encoded genes contribute to evolution of heat and cold tolerance in yeast. Sci. Adv. 2019;5:eaav1848. doi: 10.1126/sciadv.aav1848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao H., Zhang L., Li J., Xing T., Gao F. Acute stress deteriorates breast meat quality of Ross 308 broiler chickens by inducing redox imbalance and mitochondrial dysfunction. J. Anim. Sci. 2022;100:skac221. doi: 10.1093/jas/skac221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J., Zhang Y., Li Y., Yan H., Zhang H. L-tryptophan enhances intestinal integrity in diquat-challenged piglets associated with improvement of redox status and mitochondrial function. Animals. 2019;9:266. doi: 10.3390/ani9050266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Z., He X., Ma B., Zhang L., Li J., Jiang Y., Zhou G., Gao F. Chronic Heat stress impairs the quality of breast-muscle meat in broilers by affecting redox status and energy-substance metabolism. J. Agric. Food Chem. 2017;65:11251–11258. doi: 10.1021/acs.jafc.7b04428. [DOI] [PubMed] [Google Scholar]
- Mujahid A., Sato K., Akiba Y., Toyomizu M. Acute heat stress stimulates mitochondrial superoxide production in broiler skeletal muscle, possibly via downregulation of uncoupling protein content. Poult. Sci. 2006;85:1259–1265. doi: 10.1093/ps/85.7.1259. [DOI] [PubMed] [Google Scholar]
- Mujahid A., Akiba Y., Toyomizu M. Acute heat stress induces oxidative stress and decreases adaptation in young white leghorn cockerels by downregulation of avian uncoupling protein. Poult. Sci. 2007;86:364–371. doi: 10.1093/ps/86.2.364. [DOI] [PubMed] [Google Scholar]
- Mund M.D., Riaz M., Mirza M.A., Rahman Z.u., Mahmood T., Ahmad F., Ammar A. Effect of dietary tryptophan supplementation on growth performance, immune response and antioxidant status of broiler chickens from 7 to 21 days. Vet. Med. Sci. 2020;6:48–53. doi: 10.1002/vms3.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muvhulawa N., Dludla P.V., Ziqubu K., Mthembu S.X., Mthiyane F., Nkambule B.B., Mazibuko-Mbeje S.E. Rutin ameliorates inflammation and improves metabolic function: a comprehensive analysis of scientific literature. Pharmacol. Res. 2022;178 doi: 10.1016/j.phrs.2022.106163. [DOI] [PubMed] [Google Scholar]
- Ouyang J., Zhou H., Li Q., Zheng J., Chen C., Guo S., You J., Li G. Tryptophan alleviates acute heat stress-induced impairment of antioxidant status and mitochondrial function in broilers. Front. Vet. Sci. 2022;9 doi: 10.3389/fvets.2022.863156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patenaude A., Ven Murthy M.R., Mirault M.E. Mitochondrial thioredoxin system: effects of TrxR2 overexpression on redox balance, cell growth, and apoptosis. J. Biol. Chem. 2004;279:27302–27314. doi: 10.1074/jbc.M402496200. [DOI] [PubMed] [Google Scholar]
- Pérez V.I., Lew C.M., Cortez L.A., Webb C.R., Rodriguez M., Liu Y., Qi W., Li Y., Chaudhuri A., Van Remmen H., Richardson A., Ikeno Y. Thioredoxin 2 haploinsufficiency in mice results in impaired mitochondrial function and increased oxidative stress. Free Radic. Biol. Med. 2008;44:882–892. doi: 10.1016/j.freeradbiomed.2007.11.018. [DOI] [PubMed] [Google Scholar]
- Rhoads R.P., Baumgard L.H., Suagee J.K., Sanders S.R. Nutritional interventions to alleviate the negative consequences of heat stress. Adv. Nutr. 2013;4:267–276. doi: 10.3945/an.112.003376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saracila M., Panaite T.D., Predescu N.C., Untea A.E., Vlaicu P.A. Effect of dietary salicin standardized extract from salix alba bark on oxidative stress biomarkers and intestinal microflora of broiler chickens exposed to heat stress. Agriculture. 2023;13:698. [Google Scholar]
- Schreier H. Open Access Repositorium der Universität Ulm und Technischen Hochschule Ulm; 2023. Crosstalk Between Mitochondria and the Nucleus: A New Role of Polymerase ζ in Mitochondrial DNA Maintenance. Dissertation, Accessed Nov. 2023. [DOI] [Google Scholar]
- Sena L.A., Chandel N.S. Physiological roles of mitochondrial reactive oxygen species. Mol. Cell. 2012;48:158–167. doi: 10.1016/j.molcel.2012.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senyilmaz D., Virtue S., Xu X., Tan C.Y., Griffin J.L., Miller A.K., Vidal-Puig A., Teleman A.A. Regulation of mitochondrial morphology and function by stearoylation of TFR1. Nature. 2015;525:124–128. doi: 10.1038/nature14601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shahcheraghi S.H., Salemi F., Small S., Syed S., Salari F., Alam W., Cheang W.S., Saso L., Khan H. Resveratrol regulates inflammation and improves oxidative stress via Nrf2 signaling pathway: therapeutic and biotechnological prospects. Phytother. Res. 2023;37:1590–1605. doi: 10.1002/ptr.7754. [DOI] [PubMed] [Google Scholar]
- Sheiha A.M., Abdelnour S.A., Abd El-Hack M.E., Khafaga A.F., Metwally K.A., Ajarem J.S., Maodaa S.N., Allam A.A., El-Saadony M.T. Effects of dietary biological or chemical-synthesized nano-selenium supplementation on growing rabbits exposed to thermal stress. Animals. 2020;10:430. doi: 10.3390/ani10030430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan G.Y., Yang L., Fu Y.Q., Feng J.H., Zhang M.H. Effects of different acute high ambient temperatures on function of hepatic mitochondrial respiration, antioxidative enzymes, and oxidative injury in broiler chickens. Poult. Sci. 2010;89:115–122. doi: 10.3382/ps.2009-00318. [DOI] [PubMed] [Google Scholar]
- Uyanga V.A., Zhao J., Wang X., Jiao H., Onagbesan O.M., Lin H. Effects of dietary L-citrulline supplementation on nitric oxide synthesis, immune responses and mitochondrial energetics of broilers during heat stress. J. Therm. Biol. 2022;105 doi: 10.1016/j.jtherbio.2022.103227. [DOI] [PubMed] [Google Scholar]
- Uyanga V.A., Musa T.H., Oke O.E., Zhao J., Wang X., Jiao H., Onagbesan O.M., Lin H. Global trends and research frontiers on heat stress in poultry from 2000 to 2021: a bibliometric analysis. Front. Physiol. 2023;14 doi: 10.3389/fphys.2023.1123582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J., Xue X., Liu Q., Zhang S., Peng M., Zhou J., Chen L., Fang F. Effects of duration of thermal stress on growth performance, serum oxidative stress indices, the expression and localization of ABCG2 and mitochondria ROS production of skeletal muscle, small intestine and immune organs in broilers. J. Therm. Biol. 2019;85 doi: 10.1016/j.jtherbio.2019.102420. [DOI] [PubMed] [Google Scholar]
- Wang R., Guo Y., Shi Z., Qin S. A quantitative proteomic analyses of primary myocardial cell injury induced by heat stress in chicken embryo. J. Therm. Biol. 2023;112 doi: 10.1016/j.jtherbio.2023.103461. [DOI] [PubMed] [Google Scholar]
- Wang Y., Yu W., Li S., Guo D., He J., Wang Y. Acetyl-CoA carboxylases and diseases. Front. Oncol. 2022;12 doi: 10.3389/fonc.2022.836058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Bank Global Financial Development Report . 2014. Financial Inclusion. 2013, World Bank Publications. The World Bank Group, 1818 H Street NW, Washington, DC 20433, USA. [Google Scholar]
- Xu Y., Berkowitz O., Narsai R., De Clercq I., Hooi M., Bulone V., Van Breusegem F., Whelan J., Wang Y. Mitochondrial function modulates touch signalling in Arabidopsis thaliana. Plant. J. 2019;97:623–645. doi: 10.1111/tpj.14183. [DOI] [PubMed] [Google Scholar]
- Yang L., Tan G.-Y., Fu Y.-Q., Feng J.-H., Zhang M.-H. Effects of acute heat stress and subsequent stress removal on function of hepatic mitochondrial respiration, ROS production and lipid peroxidation in broiler chickens. Comp. Biochem. Physiol. C: Toxicol. Pharmacol. 2010;151:204–208. doi: 10.1016/j.cbpc.2009.10.010. [DOI] [PubMed] [Google Scholar]
- Yue Y., Guo Y., Yang Y. Effects of dietary L-tryptophan supplementation on intestinal response to chronic unpredictable stress in broilers. Amino acids. 2017;49:1227–1236. doi: 10.1007/s00726-017-2424-3. [DOI] [PubMed] [Google Scholar]
- Zeng H.-F., Xu J., Wang X.-L., Li S.-J., Han Z.-Y. Nicotinamide mononucleotide alleviates heat stress-induced oxidative stress and apoptosis in BMECs through reducing mitochondrial damage and endoplasmic reticulum stress. Ecotoxicol. Environ. Saf. 2022;235 doi: 10.1016/j.ecoenv.2022.113441. [DOI] [PubMed] [Google Scholar]
- Zhang J., Bai K.W., He J., Niu Y., Lu Y., Zhang L., Wang T. Curcumin attenuates hepatic mitochondrial dysfunction through the maintenance of thiol pool, inhibition of mtDNA damage, and stimulation of the mitochondrial thioredoxin system in heat-stressed broilers. J. Anim. Sci. 2018;96:867–879. doi: 10.1093/jas/sky009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J.F., Bai K.W., Su W.P., Wang A.A., Zhang L.L., Huang K.H., Wang T. Curcumin attenuates heat-stress-induced oxidant damage by simultaneous activation of GSH-related antioxidant enzymes and Nrf2-mediated phase II detoxifying enzyme systems in broiler chickens. Poult. Sci. 2018;97:1209–1219. doi: 10.3382/ps/pex408. [DOI] [PubMed] [Google Scholar]
- Zhang J.F., Hu Z.P., Lu C.H., Yang M.X., Zhang L.L., Wang T. Dietary curcumin supplementation protects against heat-stress-impaired growth performance of broilers possibly through a mitochondrial pathway. J. Anim. Sci. 2015;93:1656–1665. doi: 10.2527/jas.2014-8244. [DOI] [PubMed] [Google Scholar]
- Zhang S.-s., Su H.-g., Zhou Y., Li X.-m., Feng J.-h., Zhang M.-h. Effects of sustained cold and heat stress on energy intake, growth and mitochondrial function of broiler chickens. J. Integrat. Agricult. 2016;15:2336–2342. [Google Scholar]
- Zhang Y., Xie L., Ding X., Wang Y., Xu Y., Li D., Liang S., Wang Y., Zhang L., Fu A., Zhan X. Mechanisms underlying the protective effect of maternal zinc (Znso4 or Zn-Gly) against heat stress-induced oxidative stress in chicken embryo. Antioxidants. 2022;11:1699. doi: 10.3390/antiox11091699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng X., Chang S., Liu Y., Dai X., You C. Human mitochondrial protein hspd1 binds to and regulates the repair of deoxyinosine in DNA. J. Proteome Res. 2023;22:1339–1346. doi: 10.1021/acs.jproteome.2c00854. [DOI] [PubMed] [Google Scholar]