Abstract
Heart failure with preserved ejection fraction (HFpEF) is a heterogenous syndrome with varying phenotypic expression. The phenotype chronic kidney disease (CKD) associated HFpEF is increasing in prevalence globally and is associated with increased morbidity and mortality compared to other HFpEF variants. These 2 conditions share common risk factors, including obesity, diabetes, and metabolic syndrome, as well as similar pathophysiology, including systemic inflammation, oxidative stress, elevated neurohormones, mineralocorticoid-receptor activation, and venous congestion. Given the coexistence of CKD and HFpEF, the diagnosis of HFpEF can be difficult. Moreover, treatment options for HFpEF have remained limited despite the success seen in its counterpart, heart failure with reduced ejection fraction. HFpEF encompasses complex multisystem pathophysiological perturbations beyond neurohormones, it is unlikely that a single agent can have significant benefit in this population. Recent data on sodium–glucose cotransporter 2 (SGLT2) inhibitors in HFpEF and CKD, and on glucagon-like peptide-1 (GLP-1) agonists and mineralocorticoid-receptor antagonists in metabolic syndrome, which target multiple pathways simultaneously, have led to promising therapeutics for HFpEF and CKD. In this perspective, our goal is to increase awareness of HFpEF as a multisystem disorder that shares the same disease processes seen in CKD and to emphasize that its management in individuals with CKD warrants a collective and multidisciplinary approach.
Index Words: CKD, heart failure, HFpEF, inflammation, narrative review, preserved ejection fraction, treatment
Heart failure (HF) is one of the most common chronic diseases affecting approximately 1% of individuals 65 years and older in the US and is responsible for one million hospitalizations and 3 million outpatient visits yearly.1 Currently, heart failure with preserved ejection fraction (HFpEF) accounts for approximately 50% of the HF population. However, its prevalence is increasing compared to that of heart failure with reduced ejection fraction (HFrEF), and it is becoming a global health care burden.1 More importantly, despite improvements in the prognosis of HFrEF with modern evidence-based treatments, the prognosis of patients with HFpEF has been stagnant over the same time.
One of the reasons for the disparity between advances in treatment of HFpEF and HFrEF is that the distinct pathophysiological mechanisms that cause HFpEF have not been well-elucidated. It is increasingly recognized that instead of cardiac ailment as the primary inciting event, HFpEF is a syndrome of multisystem processes that act synergistically to cause clinical expression of HFpEF. Kidney disease is highly prevalent in patients with HFpEF and is considered to play a central role in this paradigm. In this review, we examine HFpEF through the lens of its major underlying comorbidity, kidney disease, and explore the cardiorenal pathophysiology that leads to dysfunction in both the cardiac and kidney systems. Our goal is to increase awareness of HFpEF as a systemic disorder and to emphasize that its management warrants a collective and multidisciplinary approach. For the purpose of this manuscript, the discussion is focused on individuals with kidney disease not requiring maintenance dialysis or transplant.
What Is HFpEF?
The chief problem in HFpEF is diastolic dysfunction which is a functional abnormality in left ventricular relaxation due to fibrosis and negative cardiovascular remodeling of the left ventricle (LV) and the great vessels. The reduced ventricular compliance and increased passive stiffness lead to heightened sensitivity to changes in load. As a result, HFpEF patients require remarkable increases in filling pressure to increase end-diastolic volume. Therefore, while the ejection fraction may be normal at baseline, during periods of increased stress, such as exercise, tachycardia, or hypertension, end-diastolic volume is unable to increase appropriately, resulting in a fixed stroke volume that is inappropriate for stress.2, 3, 4 The clinical manifestations of decrease in left ventricular compliance are rapid onset pulmonary edema after increases in load and hypotension after small decreases in load. The suggested criteria to diagnose HFpEF include (a) clinical signs or symptoms of HF, (b) evidence of preserved or normal LV ejection fraction (EF) but elevated LV or right ventricle filling pressures and/or reduced cardiac output, and (c) evidence of a structural abnormality of the heart derived from chest radiography, electrocardiogram, assays for B-type natriuretic peptide (BNP) or N-terminal pro-BNP (NT-proBNP), or right heart catheterization.5 Figure 1 summarizes 2 noninvasive approaches called H2FPEF score and HFA-PEFF (Heart Failure Association-PEFF) score, which rely on simple clinical characteristics and echocardiography to diagnose HFpEF. These 2 scores enable discrimination of HFpEF from noncardiac causes of dyspnea, assist in determination of the need for further diagnostic testing, and can be useful for predicting future composite cardiovascular events as well as HF-related events in HFpEF patients.6,7 It is important to note that obese patients have plasma BNP values below the traditional cutoff used to diagnose congestive HF. Hence, if used in isolation, a cutoff of BNP ≤ 54 pg/mL is recommended for ruling out congestive HF in severely obese patients (body mass index [BMI] ≥ 35 kg/m2). As opposed to BNP cutoffs, relatively lower concentrations of NT-proBNP in overweight and obese patients retain their accuracy for both diagnosis and prognosis.8 Moreover, in patients with chronic kidney disease (CKD), plasma BNP and NT-proBNP concentrations are often elevated as a result of reduced clearances as well volume expansion or LV hypertrophy, therefore, higher cutoff values are suggested.9 The H2FPEF score does not use these biomarkers and can be of use in patients with obesity and kidney disease. The HFA-PEFF score takes into account the underlying BMI and kidney function while calculating the score; thus, one can assume its utility in these patients. However, definitive studies are required to confirm the validity.
Fig 1.
Noninvasive diagnostic approaches to diagnose HFpEF in patients with unexplained dyspnea. Figure reproduced with permission from: https://www.ultromics.com/articles/diagnosing-heart-failure-with-preserved-ejection-fraction-hfpef. The above figure was verified using the following reference: Abramov D, Parwani P. Diving into the diagnostic score algorithms of heart failure with preserved ejection fraction. Front Cardiovasc Med. 2021;8:665424. doi:10.3389/fcvm.2021.665424.
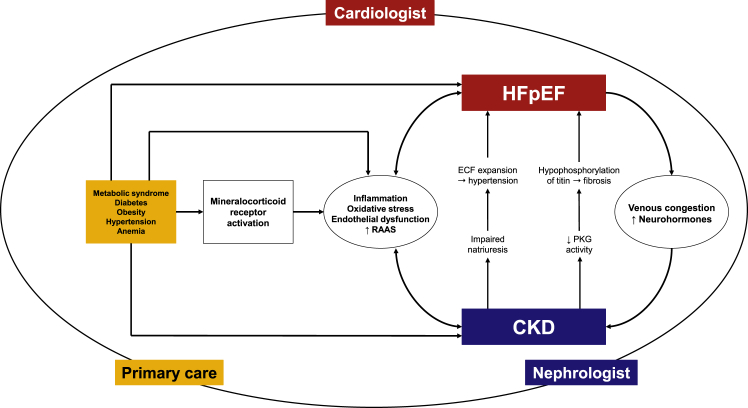
HFpEF prevalence increases with age and commonly exists with comorbid conditions, such as hypertension, obesity, metabolic syndrome, diabetes mellitus (DM), CKD, coronary artery disease, atrial fibrillation, iron deficiency anemia, chronic obstructive pulmonary disease, and hyperlipidemia. The proposed basic underlying pathophysiological mechanisms for HFpEF include (a) systemic inflammation and oxidative injury causing endothelial dysfunction, (b) elevated neurohormones, and (c) venous congestion.10 Interestingly, all these pathophysiological mechanisms are shared by CKD as well. In the following section, we discuss CKD and how these pathophysiologies interact to augment dysfunction in CKD and HFpEF (Fig2). We will also discuss the role of iron deficiency as the emerging risk factor for both CKD and HFpEF.
Fig 2.
The shared pathophysiology between heart failure with preserved ejection fraction (HFpEF) and chronic kidney disease (CKD). RAAS, renin-angiotensin-aldosterone system; ECF, extracellular fluid; PKG, protein kinase G.
The Shared Pathophysiology Between HFpEF and CKD
CKD is defined as abnormalities in kidney structure or function that occur for more than 3 months and impact health and is estimated to be present in 14% of the US adult population.11 The 3-month duration distinguishes chronic from acute kidney disease. DM and hypertension are the 2 most common causes associated with CKD. Its prevalence increases with aging and parallels that of metabolic syndrome.12
Systemic Inflammation and Oxidative Stress
CKD is accompanied by a chronic low-grade state of systemic inflammation. Inflammation is also a key component of diseases which are independent risk factors for CKD, such as obesity, insulin resistance and DM.13 Many factors contribute to the chronic inflammatory state; however, inappropriate mineralocorticoid-receptor (MR) activation has increasingly been recognized as a pivotal mechanism for increased production of proinflammatory cytokines, oxidative stress, endothelial dysfunction, and ultimately fibrosis seen in these diseases.14,15
Mineralocorticoid-Receptor Overactivation
It has been clearly shown that MR expression is not limited to epithelia but is also present in vascular endothelial cells, smooth muscle cells, cardiomyocytes, and fibroblasts as well as on immune cells. In obesity, insulin resistance, dyslipidemia, diabetes, and metabolic syndrome, all major risk factors for HFpEF and CKD, several pathways can result in MR overactivation and/or “inappropriate” MR activation. The increased MR activity could be related to modulation at the prereceptor level via ligand binding (aldosterone and cortisol) or at the receptor level due to changes in MR expression.16 There is emerging evidence that adipocytes either secrete aldosterone or produce aldosterone-secreting factors that work on the adrenal gland to generate aldosterone in excess and thus, cause MR overactivation.17 Increased aldosterone levels have been demonstrated in obesity and metabolic syndrome.17 Moreover, both aging and obesity have been shown to reduce the activity of enzyme 11β-hydroxysteroid dehydrogenase type 2 (11β-HSD2), which usually converts active cortisol to inactive cortisone, resulting in increased availability of cortisol to bind to MR.18 At the receptor level, overactivation can occur without increased ligands either due to increased synthesis or decreased degradation of MR. For instance, high glucose has been reported to stimulate MR transcriptional activity via protein kinase C signaling, and high salt causes inappropriate activation of MR possibly via oxidative stress induction and Rac1 stimulation.16,19
Activation of the MR characteristically stimulates multiple pathways producing plasminogen activator inhibitor-1, transforming growth factor-β, interleukin [IL]-6, and monocyte chemoattractant protein-1 which either contribute to inflammation and fibrosis locally or signal to other cells, such as myeloid cells.20 These myeloid cells add to the harmful cascade by releasing profibrotic molecules or promoting chemotaxis of other inflammatory cells. MR activation also enhances oxidative stress by activation of nicotinamide adenine dinucleotide phosphate oxidase-mediated generation of reactive oxygen species. All these result in increased myocardial stiffness, impaired LV relaxation, and glomerular and interstitial fibrosis in kidneys.20,21
Other Factors Contributing to Inflammation
There are 4 other key factors that contribute to the chronic inflammatory state.
Altered Metabolism of Adipose Tissue
Enlarged size and number of adipocytes increases the distance between cells and vasculature, leading to hypoxic conditions. Chronic hypoxia causes fibrosis and macrophage infiltration, leading to a cascade of events, including recruitment of immune cells and a shift from an anti-inflammatory to a proinflammatory milieu.22
Acidosis
Clinical and subclinical acidosis is commonly present in CKD due to reduced acidification capacity as a result of declines in total urinary ammonium excretion; however, ammonia production per surviving nephron markedly increases to accommodate nephron loss and ongoing acid exposure. High local intrarenal concentrations of ammonia have been shown to activate complement, NF-kb, endothelin and the renin-angiotensin-aldosterone system (RAAS), promoting proinflammatory and profibrotic mediators.23 Moreover, acidosis stimulates a broad inflammatory response in human vascular endothelial cells through activation of the proton-sensing receptor GPR4.24
Altered Phosphate-Fibroblast Growth Factor 23 (FGF23)-Klotho Endocrine Axis
Klotho expression decreases and FGF23 levels increase in CKD, earlier in response to inflammation and later due to phosphate retention. FGF23 in turn possesses proinflammatory and immune-modulatory properties by reducing the conversion of 25(OH)D to 1,25(OH)2D in monocytes/dendritic cells and promoting tumor necrosis factor-α production independent of klotho in splenocytes and macrophages.25
Intestinal Dysbiosis
In CKD patients, microbial diversity is significantly harmed with a fewer beneficial bacteria that generate short-chain fatty acids, a primary nutrient for the colonic epithelium, and more bacteria that produce uremic toxins, such as indoxyl sulfate, p-cresol sulfate, and trimethylamine-N-oxide. The impact on microbial diversity has effects in both directions. The uremic milieu negatively affects the microbiota, altering its composition and metabolism. At the same time, microbiota dysbiosis releases potential uremic toxins. Uremic milieu causes diminished transcellular tight-junction proteins, resulting in disruption of the epithelial barrier and amplified intestinal permeability. The microbial products in the blood stream trigger systemic inflammation.26, 27, 28, 29
Effects of Systemic Inflammation on Kidneys and Heart
Because kidneys are a highly vascularized organ and contain a unique microcirculatory network that is required to sustain the corticomedullary osmotic gradient, they are a vulnerable target for both systemic inflammation and hypoxia. The unique microenvironment within the kidneys is kept in a fine balance with multiple vasoactive molecules (prostaglandins, endothelins, kinins, medullipin, nitric oxide, and other molecules); however, systemic or within kidney inflammation deregulate the microvascular response to these vasoactive regulators. In addition, this inflammation promotes the production of a number of tubular toxins, including reactive oxygen species which activates intrarenal endothelial cells and leukocytes. This process results in a local amplification of proinflammatory factors and oxidative stress, which are inseparably linked.13
Inappropriate or exaggerated inflammation is a key component in both HFrEF and HFpEF; however, while the inflammatory response in HFrEF is the result of cardiomyocyte damage from infection, ischemia, or toxicity; inflammation in HFpEF is the result of extracardiac risk factors, similar to those discussed in CKD, ie, obesity, DM, salt-sensitive hypertension, causing MR activation and eventually cardiac and vascular fibrosis. Furthermore, CKD interacts synergistically with obesity and DM to amplify the inflammation in the heart by reducing nitric oxide bioavailability, cyclic guanosine monophosphate content, and protein kinase G (PKG) activity in cardiomyocytes.30,31 Low PKG activity causes hypophosphorylation of titin, a protein normally responsible for passive cardiomyocyte tension in LV.32 Hypophosphorylation of titin favors hypertrophy development, increases resting tension and thus contributes to high diastolic LV stiffness and HF development (Fig 2).31,33 Detrimental microvascular changes and endothelial dysfunction are additional consequences.31 Moreover, over the last decade, the klotho-FGF23 axis has earned much attention as the mediator of cardiorenal connections. Klotho expression is reduced as soon as kidney function starts deteriorating, generating a state of FGF23 resistance. Activation of the Wnt/β-catenin signaling pathways is considered a cause as well consequence of klotho reduction in CKD. Eventually, Wnt/β-catenin activation mediates the injury in both heart and kidney through the renin-angiotensin system, atherosclerosis, vascular calcification, endothelial dysfunction, cardiac fibrosis and hypertrophy, and kidney fibrosis.34
Elevated Neurohormones
Abnormal activation of the sympathetic nervous system, RAAS and increased vasopressin activity are the key detrimental factors contributing to HFrEF progression and are hallmarks of kidney diseases; however, these have not been extensively studied in HFpEF.35 In limited clinical studies, elevated circulating biomarkers of RAAS and sympathetic nervous system (plasma renin activity, aldosterone, and norepinephrine) and diminished tonic and reflex vagal heart rate modulation have been shown in a substantial proportion of patients with HFpEF, however, to a lesser extent than that observed in patients with HFrEF.36 Preclinical studies suggest that activation of the adrenergic system and RAAS may contribute to progressive remodeling and contractile dysfunction in HFpEF.37 As mentioned previously, MR activation from mineralocorticoids and glucocorticoids play a pivotal role in maladaptive tissue remodeling in the heart through both genomic and nongenomic actions.20 Nonetheless, current literature is not able to distinguish whether enhanced neurohormones incite HFpEF or HFpEF results in enhanced neurohormones. Once the perturbation is established, a bidirectional relationship between altered neurohormone and HFpEF develops.
Congestion
Impaired natriuresis or abnormal salt and water retention is one of the initial pathophysiological abnormalities in CKD. This occurs as a result of reduced filtration of sodium due to a declining glomerular filtration rate, which is not adequately compensated by appropriately reduced sodium absorption in the different segments of the nephron under the influence of inappropriate renin and angiotensin activation.38 In other words, an elevated renin and angiotensin system in CKD does not allow sodium transporters in the proximal and distal tubules to adequately compensate for reduced filtration of sodium, resulting in extracellular volume expansion and the development of hypertension associated with CKD. Hypertension is one of the critical inciting insults for negative cardiac remodeling resulting in concentric LV hypertrophy and stiffness. The subsequent fixed stroke volume promotes venous congestion and reduced cardiac output, which further exacerbates abnormal kidney function as a result of hypoxia, neurohormone activation and the resultant reduced renal blood flow. These effects cause further worsening of sodium and water retention and set up a vicious cycle (Figure 2).39
In a clinical trial of participants admitted with acute decompensated HF, we observed that patients with HFpEF (n = 20) had a higher BMI and more frequently diagnosed with diabetes and CKD than patients with HFrEF (n = 27). Urine sodium levels were the same in patients with HFpEF and HFrEF; however, the urine sodium to potassium ratio was much lower in patients with HFpEF.40 Of note, a low urine sodium to potassium ratio is a biomarker of MR activation in the kidney, informing increased sodium absorption and potassium secretion because of MR-mediated activation of the epithelial sodium channel and renal outer medullary potassium channel, respectively, in the principal cells in the distal nephron.41 Higher MR activation in HFpEF might be related to ligand-independent factors, such as obesity and hyperglycemia, causing MR activation in this population in addition to elevated aldosterone as the activator of MR. These clinical observations reflect the shared cardiorenal pathophysiology between CKD and HFpEF, in which inciting disease states, such as obesity and diabetes, cause MR activation, inflammation, and oxidative stress. These conditions subsequently lead to cardiac stiffness and kidney disease. The subsequent impaired natriuresis and congestion result in hypertension, allowing one organ to affect the other, thus setting up a vicious cycle of cardiorenal syndromes.
Iron Deficiency Anemia
Anemia is widely prevalent in both HF and CKD and is associated with worse quality of life and outcomes. Although there are a number of factors involved in the pathophysiological mechanisms causing anemia, iron deficiency is the most common underlying cause of anemia. In many instances, patients have iron deficiency without anemia. The interaction of iron deficiency, anemia, HF and CKD, now known as cardiorenal iron deficiency syndrome, has been of great recent interest. A large overlap in biomarker profiles has been observed, suggesting common pathways associated with these syndromes.42 Although most of the studies have included patients with HFrEF, iron deficiency is a common comorbid condition in HFpEF also and is associated with decreased exercise capacity and quality of life.43,44 The common pathways causing iron deficiency include poor nutritional intake; gastrointestinal malabsorption; inflammatory mediators, such as hepcidin and IL-6, which reduce iron uptake and mobilization of iron from its reticuloendothelial stores; and lastly, increased blood loss.45 Iron is crucial in mitochondria for the final step in producing adenosine triphosphate from glucose, fatty acids or ketones, and mitochondria are abundant in organ with high energy metabolism, such as the heart and kidneys. Therefore, iron deficiency leads to several morphological and functional changes in mitochondria in these organs, culminating in damage.46 Administration of iron in HF and advanced CKD population have shown improved composite of death and major adverse cardiovascular events in randomized clinical trials.47,48
Epidemiology of Cardiorenal Connections
Given the coexistence of hypertension, CKD, and diastolic dysfunction in cross-sectional studies, it is difficult to discern whether diastolic dysfunction is the result of CKD-associated hypertension and inflammation/fibrosis or whether diastolic dysfunction is the cause of kidney disease. Nonetheless, over the years, it has become clear that HFpEF is a heterogenous collection of pathologies and phenotypic expressions, and CKD-associated HFpEF is a subtype. In large observational cohorts, CKD (estimated glomerular filtration rate [eGFR] < 60 mL/min/1.73 m2) was present in up to 26-49% of patients with HFpEF.49 In a contemporary HFpEF population, abnormal kidney function, as defined by a reduced eGFR and albuminuria, was present in 62% of patients; of these, 26% had albuminuria with normal eGFR.50 In the Swedish Heart Failure Registry, CKD was more common in HFpEF (56%) than in HFmidrangeEF (48%) and HFrEF (45%).51 Diuretic resistance is an additional phenotype observed in HF populations related to abnormal kidney function. Depending on the definition of nonresponse, resistance to the high-dose loop-diuretics can be observed in 20-50% of patients hospitalized with acute decompensated HF. However, most of these studies included patients with HFrEF, and the prevalence of diuretic resistance in HFpEF is not well known. The pathophysiology of diuretic resistance includes a complicated interplay between heart and kidney dysfunction, with the activation of neurohormones leading to altered kidney hemodynamics and adaptation, such as distal tubule hypertrophy.52 Recently, we described that in patients hospitalized with ADHF with DR, defined as no response to a dose of 160 mg furosemide intravenous/day with at least one dose of 80 mg, HFpEF was more prevalent than HFrEF despite similar underlying kidney functions.40
Large epidemiologic studies on the prevalence of and severity of HFpEF in CKD patients are sparse. In the Chronic Renal Insufficiency Cohort (CRIC), when 2,147 participants without prior HF were followed for hospitalization for HF over 10 years, more developed HFpEF-related events than HFrEF-related events (135 vs 104 HF events).53 Decreased kidney function has independently been associated with increased risk of all-cause death, cardiovascular death, and hospitalization for all HF subtypes.54 Additionally, patients with advanced CKD have significantly worse diastolic dysfunction than patients without kidney disease.55 On the contrary, improvements in kidney function can restore cardiac function.56 The coexistence of kidney disease in HFpEF poses a large health care burden and worsens disease expression among all the other comorbid conditions prevalent in HFpEF.57
Treatment
The success seen with HFrEF treatments that primarily target and modulate neurohormones has not been observed in HFpEF patients. For example, angiotensin-converting enzyme inhibitors and angiotensin receptor blockers, highly effective treatments for both HFrEF and CKD, have not shown significant benefits for mortality in the HFpEF population; although, post hoc analysis later reported reduced hospitalization for HF with these agents.58,59 Similarly, while the angiotensin receptor–neprilysin inhibitor, sacubitril–valsartan, resulted in a lower rate of hospitalization for HF or death from cardiovascular causes in HFrEF,60 the same agent did not show benefit for death from cardiovascular causes in phase III trial in HFpEF, despite demonstrations of lower levels of NT-proBNP, reductions in left atrial size, and greater improvements in the New York Heart Association (NYHA) functional class in phase II trial in HFpEF.61,62 Of note, there was 15% reduction in hospitalization for HF and 45% improvement in NYHA class in this phase III trial with sacubitril–valsartan.61 Given the role of low nitric oxide availability and reduced cGMP content and PKG activity in the pathophysiology of HFpEF, various agents targeting these alteration have been evaluated in HFpEF. Few examples are phosphodiesterase type-5 inhibitors that reduce cGMP degradation, such as sildenafil; direct NO donors and soluble guanylate cyclase stimulators, such as vericiguat. In short-term trials, these agents have resulted in improved biventricular and left atrial filling pressures or quality of life; however, benefits on hard outcomes are yet to be reported.63 Overall, these negative hard outcome results emphasize the complex pathophysiological perturbations in HFpEF beyond neurohormones, and it is unlikely that a single agent can have significant benefit on mortality. Moreover, these trials had approximately 50% participants with CKD; however, albuminuria was not assessed. Abnormal kidney function, when considering albuminuria, is highly prevalent in HFpEF patients and amplifies these pathophysiologies, resulting in lesser benefits to the primary outcomes in HFpEF. Table 1 summarizes the results of phase III clinical trials in HFpEF with a focus on the CKD population.
Table 1.
Summary of Phase III Clinical Trials Conducted in the HFpEF Population and Subanalysis in the CKD Population
| Therapeutic Agent and Clinical Trial | Main Results | CKD Population in the Trial and Prespecified Results | Role in CKD |
|---|---|---|---|
|
CHARM-Preserved59 ARB: candesartan |
CV death and hospitalization for HF (HR 0.89; 95% CI, 0.77-1.03; P = 0.12); HF hospitalization (HR 0.84; 95% CI 0.70-1.00); P = 0.047 | <30% in both groups had eGFR < 60 mL/min/1.73 m2 No outcome results based on CKD |
Well known and established role of ARB in proteinuric CKD |
|
PARAGON-HF61 ARNI: sacubitril–valsartan |
CV death and hospitalization for HF (HR 0.87; 95% CI, 0.75-1.01; P = 0.06); 15% reduction in hospitalization for heart failure and 45% improvement in NYHA class | Mean eGFR in 2 groups 63 ± 19 and 62 ± 19 mL/min/1.73 m2; prespecified primary outcome by CKD: <60 mL/min/1.73 m2: HR 0.79 (0.66-0.95); ≥60 mL/min/1.73 m2: HR 1.01 (0.8-1.27); renal composite endpoint reduced by 50% compared with ARB in CKD subgroup (eGFR: 30-60 mL/min/1.73 m2) | No evaluation of ARNI in primary CKD |
|
TOPCAT75 MRA: spironolactone |
CV death and hospitalization for HF (HR 0.89; 95% CI, 0.77-1.04; P = 0.14); HF hospitalization (HR 0.83; 95% CI 0.69-0.99); P = 0.04 | 39% in both groups had eGFR < 60 mL/min/1.73 m2; prespecified primary outcome by CKD: <60 mL/min/1.73 m2: HR 0.95 (0.77-1.17); ≥60 mL/min/1.73 m2: HR 0.82 (0.66-1.02) | Role of novel nonsteroidal MRA is established in proteinuric diabetic kidney disease77 |
|
EMPEROR-Preserved67 SGLT2i: empagliflozin |
CV death and hospitalization for HF (HR 0.79; 95% CI, 0.69-0.9; P < 0.001); HF hospitalization (HR 0.73; 95% CI 0.61-0.88; P < 0.001) | 50% in both groups had eGFR < 60 mL/min/1.73 m2; prespecified primary outcome by CKD: <60 mL/min/1.73 m2: HR 0.78 (0.66-0.91); ≥60 mL/min/1.73 m2: HR 0.81 (0.65-1.00) | Well established role of empagliflozin in proteinuric and non proteinuric CKD with or without diabetes64 |
|
DELIVER68 SGLT2i: dapagliflozin |
CV death and hospitalization for HF (HR 0.82; 95% CI, 0.73-0.92; P < 0.001); worsening HF (HR 0.79; 95% CI, 0.69-0.91; P < 0.001). | Mean eGFR in 2 groups 63 ± 19 and 62±19 mL/min/1.73 m2; prespecified primary outcome by CKD: <60 mL/min/1.73 m2: HR 0.81 (0.69-0.94); ≥60 mL/min/1.73 m2: HR 0.84 (0.7-1.00) | Well established role of dapagliflozin in proteinuric CKD with or without diabetes66 |
CKD, chronic kidney disease; CI, confidence interval; CV, cardiovascular; eGFR, estimated glomerular filtration rate; HF, heart failure; MRA, mineralocorticoid-receptor antagonists; SGLT2i, sodium–glucose cotransporter 2 inhibitors; ARNI, angiotensin receptor–neprilysin inhibitor; ARB, angiotensin receptor blocker.
The recent triumph of sodium–glucose cotransporter 2 inhibitors (SGLT2i) in patients with cardiovascular disease and CKD, which addresses not only metabolic and bioenergetic components (weight loss, improved insulin resistance) but also the altered natriuresis, hypertension, adipokine and cytokine production, emphasizes the importance of an approach that tackles abnormal kidney function and other comorbid conditions simultaneously in HFpEF population.64, 65, 66 In EMPEROR-Preserved Trial, administration of empagliflozin to HFpEF participants (EF > 40% and NT-proBNP levels > 300 pg/mL) resulted in a 21% reduction in the primary composite outcome of cardiovascular death or hospitalization for HF; mainly related to a lower risk of hospitalization for HF in the empagliflozin group.67 In a similar trial, dapagliflozin resulted in an 18% reduction in primary outcome, again driven by reduced rates of hospitalization for HF.68 The American College of Cardiology 2022 HF guidelines recommends (Level 2a) use of SGLT2i in HFpEF patients.69 Glucagon-like peptide-1 receptor (GLP-1R) agonists are other agents with pleiotropic effects and have shown to improve abnormal LV diastolic and kidney function in initial studies.70,71 Because of their significant weight loss effects in addition to improvements in insulin resistance and diabetes, GLP-1R agonists are at advantage to improve HFpEF outcomes. In preclinical and early phase clinical studies, liraglutide significantly increased e′ and decreased E/e′ and LV end-diastolic volume, which reflects a reduction in LV filling pressure.72 The STEP HFpEF trial shows larger reductions in symptom burden and physical limitations, greater improvements in exercise function and greater decline in C-reactive protein with the use of once weekly (2.4 mg) semaglutide compared to placebo in participants with BMI ≥ 30 kg/m2 and HF with EF > 40%.73 The STEP HFpEF trial in patients with DM (NCT04916470) in underway. The secondary analyses of primary cardiovascular outcome trials which reported the beneficial effect of GLP-1R agonists on cardiovascular outcomes, have demonstrated significant benefit on albuminuria.71 The ongoing FLOW trial (NCT03819153) will shed light on the role of GLP-1R agonists in the prevention of CKD progression.
Because MR overactivation plays a central role in the development of both HFpEF and CKD, MR antagonists seem to be promising agents to improve outcomes in these patients. In the earlier ALDO-DHF study, 25 mg/day spironolactone led to improvements in LV diastolic dysfunction and LV remodeling as well as reduced levels of NT-proBNP but did not result in improved exercise capacity or quality of life measures.74 The Treatment of Preserved Cardiac Function Heart Failure With an Aldosterone Antagonist (TOPCAT) study randomized 3,445 participants with HFpEF to 15-45 mg/day spironolactone. The trial did not show a significant reduction of the primary endpoint; however, there was a small, but significant reduction in HF hospitalizations in the spironolactone group.75 A post hoc analysis of the primary endpoints based on region showed a significant decrease in both the primary endpoint and HF hospitalizations in the spironolactone group in the Americas but did not show a difference in Russia/Georgia. Notably, participants from Russia/Georgia were younger and with fewer comorbid conditions, and 89% were enrolled based on HF hospitalization criteria rather than natriuretic peptide criteria. In other words, this population did not meet HFpEF criteria and introduced bias that may have disadvantaged spironolactone.76 Two phase III clinical trials comparing spironolactone (Spironolactone in the Treatment of Heart Failure [SPIRRIT-HFpEF; NCT04727073]) and finerenone (Finerenone Trial to Investigate Efficacy and Safety Superior to Placebo in Patients with Heart Failure [FINEARTS-HF; NCT04435626]) with placebo in patients with HFpEF are currently ongoing and addressing MR activation in HFpEF, after successful results were obtained in patients with diabetic kidney disease in the FIDELITY analysis.77 Moreover, by harnessing the generalized benefits of MR antagonism, FIND-CKD is evaluating the effect of finerenone on CKD progression in nondiabetic kidney disease (NCT05047263).
Given the pathophysiological role of iron deficiency in HFpEF, there are currently two ongoing randomized clinical trials, the FAIR-HFpEF (NCT03074591) and the PREFER-HF (NCT03833336), evaluating the benefit of intravenous ferric carboxymaltose in HFpEF, after its success in patients with HFrEF.48 Finally, optimal blood pressure control, weight loss, calorie restriction, and aerobic exercise programs remain the cornerstone of management of this population.78, 79 Poor exercise tolerance in HFpEF may pose challenges for these goals. Cardiac rehabilitation programs, including exercise training, have been shown to improve exercise capacity and quality of life in HFpEF patients.80
The availability of SGLT2i, GLP-1R agonists and new MRAs have generated great excitement in the cardiorenal community given the increasing prevalence of obesity and diabetes with consequent CKD and HFpEF. However, <10% of eligible patients receive either class of drug in the US.81 In a recent study in national samples from US veterans, 11.5% of likely eligible patients with comorbid CKD, type 2 DM, and atherosclerotic CVD were prescribed SGLT2 inhibitors. Varying insurance coverage and out-of-pocket costs are important contributors of underuse. A number of patient- and clinician-level factors are additional barriers prohibiting optimal adoption outside of the endocrine community. Overstepping therapeutic boundaries (SGLT2i and GLP-1a were classically developed as hypoglycemic agents), clinical inertia, lack of knowledge about the risk of hypoglycemia, concern of urinary tract infection, acute kidney injury and hyperkalemia, and the need for increased lab monitoring are some factors contributing to under prescription by various providers. Uptake of newer medications by patients usually follows the providers’ comfort level with those medications and thus has been low.
HFpEF is a multisystem disorder; therefore its managment is the responsibility of every provider a patient interacts with, not just the cardiologists (Figure 2). A message about healthy eating habits, weight loss, increased physical activity, and optimal blood pressure control needs to be communicated to the patient at each clinic visit. A multifaceted and multilevel approach involving not only physicians, but also advanced practitioners, dieticians, pharmacists, and policy makers is essential to overcome the barriers leading to therapeutic underutilization.
Conclusion
HFpEF is best characterized as a spectrum of overlapping comorbid conditions. Of these, kidney disease is associated with the greatest morbidity and mortality and sets up a vicious cardiorenal interaction where impairment of one organ leads to malfunction of the other and vice versa. An increased understanding of the shared pathophysiologies and these interactions is the first key step to successful management. Moreover, an integrated multidisciplinary approach is required to promote risk factor reduction and increase the adoption of newer therapeutics. It has been shown that a single agent is not able to address this complex disorder. HFpEF should be considered a multispecialty disorder, not a cardiovascular disorder. The health care community needs to work in coordination, involving not only physicians, but also advanced practitioners, dieticians, pharmacists, and policy makers in a multifaceted and multilevel approach to reduce its burden in our patients.
Article Information
Authors’ Full Names and Academic Degrees
Rahul N. Patel, MD, Akash Sharma, BS, Anand Prasad, MD, and Shweta Bansal, MD.
Support
None.
Financial Disclosure
The authors declare that they have no relevant financial interests.
Peer Review
Received January 13, 2023. Evaluated by 2 external peer reviewers, with direct editorial input from the Editor-in-Chief. Accepted in revised form May 27, 2023.
Footnotes
Complete author and article information provided before references.
References
- 1.Lazzeri C., Valente S., Tarquini R., et al. Cardiorenal syndrome caused by heart failure with preserved ejection fraction. Int J Nephrol. 2011;2011 doi: 10.4061/2011/634903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kawaguchi M., Hay I., Fetics B., et al. Combined ventricular systolic and arterial stiffening in patients with heart failure and preserved ejection fraction: implications for systolic and diastolic reserve limitations. Circulation. 2003;107:714–720. doi: 10.1161/01.cir.0000048123.22359.a0. [DOI] [PubMed] [Google Scholar]
- 3.Abudiab M.M., Redfield M.M., Melenovsky V., et al. Cardiac output response to exercise in relation to metabolic demand in heart failure with preserved ejection fraction. Eur J Heart Fail. 2013;15(6):776–785. doi: 10.1093/eurjhf/hft026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Borlaug B.A., Melenovsky V., Russell S.D., et al. Impaired chronotropic and vasodilator reserves limit exercise capacity in patients with heart failure and a preserved ejection fraction. Circulation. 2006;114(20):2138–2147. doi: 10.1161/CIRCULATIONAHA.106.632745. [DOI] [PubMed] [Google Scholar]
- 5.Dunlay S.M., Roger V.L., Redfield M.M. Epidemiology of heart failure with preserved ejection fraction. Nat Rev Cardiol. 2017;14(10):591–602. doi: 10.1038/nrcardio.2017.65. [DOI] [PubMed] [Google Scholar]
- 6.Reddy Y.N.V., Carter R.E., Obokata M., et al. A simple, evidence-based approach to help guide diagnosis of heart failure with preserved ejection fraction. Circulation. 2018;138(9):861–870. doi: 10.1161/CIRCULATIONAHA.118.034646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Egashira K., Sueta D., Komorita T., et al. HFA-PEFF scores: prognostic value in heart failure with preserved left ventricular ejection fraction. Korean J Intern Med. 2022;37(1):96–108. doi: 10.3904/kjim.2021.272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Madamanchi C., Alhosaini H., Sumida A., et al. Obesity and natriuretic peptides, BNP and NT-proBNP: mechanisms and diagnostic implications for heart failure. Int J Cardiol. 2014;176(3):611–617. doi: 10.1016/j.ijcard.2014.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Anwaruddin S., Lloyd-Jones D.M., Baggish A., et al. Renal function, congestive heart failure, and amino-terminal pro-brain natriuretic peptide measurement: results from the ProBNP Investigation of Dyspnea in the Emergency Department (PRIDE) Study. J Am Coll Cardiol. 2006;47(1):91–97. doi: 10.1016/j.jacc.2005.08.051. [DOI] [PubMed] [Google Scholar]
- 10.Ter Maaten J.M., Damman K., Verhaar M.C., et al. Connecting heart failure with preserved ejection fraction and renal dysfunction: the role of endothelial dysfunction and inflammation. Eur J Heart Fail. 2016;18(6):588–598. doi: 10.1002/ejhf.497. [DOI] [PubMed] [Google Scholar]
- 11.Stevens P.E., Levin A. Kidney Disease: Improving Global Outcomes Chronic Kidney Disease Guideline Development Work Group Members. Evaluation and management of chronic kidney disease: synopsis of the kidney disease: improving global outcomes 2012 clinical practice guideline. Ann Intern Med. 2013;158:825–830. doi: 10.7326/0003-4819-158-11-201306040-00007. [DOI] [PubMed] [Google Scholar]
- 12.Jha V., Garcia-Garcia G., Iseki K., et al. Chronic kidney disease: global dimension and perspectives. Lancet. 2013;382(9888):260–272. doi: 10.1016/S0140-6736(13)60687-X. [DOI] [PubMed] [Google Scholar]
- 13.Mihai S., Codrici E., Popescu I.D., et al. Inflammation-related mechanisms in chronic kidney disease prediction, progression, and outcome. J Immunol Res. 2018;2018 doi: 10.1155/2018/2180373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lother A., Hein L. Vascular mineralocorticoid receptors: linking risk factors, hypertension, and heart disease. Hypertension. 2016;68(1):6–10. doi: 10.1161/HYPERTENSIONAHA.116.07418. [DOI] [PubMed] [Google Scholar]
- 15.Bauersachs J., Jaisser F., Toto R. Mineralocorticoid receptor activation and mineralocorticoid receptor antagonist treatment in cardiac and renal diseases. Hypertension. 2015;65(2):257–263. doi: 10.1161/HYPERTENSIONAHA.114.04488. [DOI] [PubMed] [Google Scholar]
- 16.Buonafine M., Bonnard B., Jaisser F. Mineralocorticoid receptor and cardiovascular disease. Am J Hypertens. 2018;31(11):1165–1174. doi: 10.1093/ajh/hpy120. [DOI] [PubMed] [Google Scholar]
- 17.Ayuzawa N., Fujita T. The mineralocorticoid receptor in salt-sensitive hypertension and renal injury. J Am Soc Nephrol. 2021;32(2):279–289. doi: 10.1681/ASN.2020071041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Henschkowski J., Stuck A.E., Frey B.M., et al. Age-dependent decrease in 11beta-hydroxysteroid dehydrogenase type 2 (11beta-HSD2) activity in hypertensive patients. Am J Hypertens. 2008;21(6):644–649. doi: 10.1038/ajh.2008.152. [DOI] [PubMed] [Google Scholar]
- 19.Hirohama D., Nishimoto M., Ayuzawa N., et al. Activation of Rac1-mineralocorticoid receptor pathway contributes to renal injury in salt-loaded db/db mice. Hypertension. 2021;78(1):82–93. doi: 10.1161/HYPERTENSIONAHA.121.17263. [DOI] [PubMed] [Google Scholar]
- 20.Jia G., Jia Y., Sowers J.R. Role of mineralocorticoid receptor activation in cardiac diastolic dysfunction. Biochim Biophys Acta Mol Basis Dis. 2017;1863(8):2012–2018. doi: 10.1016/j.bbadis.2016.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Luther J.M., Fogo A.B. The role of mineralocorticoid receptor activation in kidney inflammation and fibrosis. Kidney Int Suppl (2011) 2022;12(1):63–68. doi: 10.1016/j.kisu.2021.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee Y.S., Kim J.W., Osborne O., et al. Increased adipocyte O2 consumption triggers HIF-1alpha, causing inflammation and insulin resistance in obesity. Cell. 2014;157(6):1339–1352. doi: 10.1016/j.cell.2014.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wesson D.E., Buysse J.M., Bushinsky D.A. Mechanisms of metabolic acidosis-induced kidney injury in chronic kidney disease. J Am Soc Nephrol. 2020;31(3):469–482. doi: 10.1681/ASN.2019070677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dong L., Li Z., Leffler N.R., et al. Acidosis activation of the proton-sensing GPR4 receptor stimulates vascular endothelial cell inflammatory responses revealed by transcriptome analysis. PLOS ONE. 2013;8(4) doi: 10.1371/journal.pone.0061991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.David V., Francis C., Babitt J.L. Ironing out the cross talk between FGF23 and inflammation. Am J Physiol Renal Physiol. 2017;312(1):F1–F8. doi: 10.1152/ajprenal.00359.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ramezani A., Raj D.S. The gut microbiome, kidney disease, and targeted interventions. J Am Soc Nephrol. 2014;25(4):657–670. doi: 10.1681/ASN.2013080905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Carracedo M., Artiach G., Witasp A., et al. The G-protein coupled receptor ChemR23 determines smooth muscle cell phenotypic switching to enhance high phosphate-induced vascular calcification. Cardiovasc Res. 2019;115(10):1557–1566. doi: 10.1093/cvr/cvy316. [DOI] [PubMed] [Google Scholar]
- 28.Ridker P.M., MacFadyen J.G., Glynn R.J., et al. Inhibition of interleukin-1beta by canakinumab and cardiovascular outcomes in patients with chronic kidney disease. J Am Coll Cardiol. 2018;71(21):2405–2414. doi: 10.1016/j.jacc.2018.03.490. [DOI] [PubMed] [Google Scholar]
- 29.Ebert T., Pawelzik S.C., Witasp A., et al. Inflammation and premature ageing in chronic kidney disease. Toxins (Basel) 2020;12(4):227. doi: 10.3390/toxins12040227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Simmonds S.J., Cuijpers I., Heymans S., et al. Cellular and molecular differences between HFpEF and HFrEF: a step ahead in an improved pathological understanding. Cells. 2020;9(1):242. doi: 10.3390/cells9010242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Paulus W.J., Tschope C. A novel paradigm for heart failure with preserved ejection fraction: comorbidities drive myocardial dysfunction and remodeling through coronary microvascular endothelial inflammation. J Am Coll Cardiol. 2013;62(4):263–271. doi: 10.1016/j.jacc.2013.02.092. [DOI] [PubMed] [Google Scholar]
- 32.LeWinter M.M., Granzier H. Cardiac titin: a multifunctional giant. Circulation. 2010;121(19):2137–2145. doi: 10.1161/CIRCULATIONAHA.109.860171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.van Heerebeek L., Hamdani N., Falcao-Pires I., et al. Low myocardial protein kinase G activity in heart failure with preserved ejection fraction. Circulation. 2012;126(7):830–839. doi: 10.1161/CIRCULATIONAHA.111.076075. [DOI] [PubMed] [Google Scholar]
- 34.Munoz-Castaneda J.R., Rodelo-Haad C., Pendon-Ruiz de Mier M.V., et al. Klotho/FGF23 and Wnt signaling as important players in the comorbidities associated with chronic kidney disease. Toxins (Basel) 2020;12(3):185. doi: 10.3390/toxins12030185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bansal S., Lindenfeld J., Schrier R.W. Sodium retention in heart failure and cirrhosis: potential role of natriuretic doses of mineralocorticoid antagonist? Circ Heart Fail. 2009;2(4):370–376. doi: 10.1161/CIRCHEARTFAILURE.108.821199. [DOI] [PubMed] [Google Scholar]
- 36.Vergaro G., Aimo A., Prontera C., et al. Sympathetic and renin-angiotensin-aldosterone system activation in heart failure with preserved, mid-range and reduced ejection fraction. Int J Cardiol. 2019;296:91–97. doi: 10.1016/j.ijcard.2019.08.040. [DOI] [PubMed] [Google Scholar]
- 37.Verloop W.L., Beeftink M.M., Santema B.T., et al. A systematic review concerning the relation between the sympathetic nervous system and heart failure with preserved left ventricular ejection fraction. PLOS ONE. 2015;10(2) doi: 10.1371/journal.pone.0117332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bidani A.K., Griffin K.A. Pathophysiology of hypertensive renal damage: implications for therapy. Hypertension. 2004;44(5):595–601. doi: 10.1161/01.HYP.0000145180.38707.84. [DOI] [PubMed] [Google Scholar]
- 39.Colombo P.C., Doran A.C., Onat D., et al. Venous congestion, endothelial and neurohormonal activation in acute decompensated heart failure: cause or effect? Curr Heart Fail Rep. 2015;12(3):215–222. doi: 10.1007/s11897-015-0254-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sharma A., Patel R., Prasad A., et al. Diuretic resistance in acute decompensated HFpEF vs. HFrEF. Clin Nephrol. 2023;100:47–50. doi: 10.5414/CN111090. [DOI] [PubMed] [Google Scholar]
- 41.van Vliet A.A., Donker A.J., Nauta J.J., et al. Spironolactone in congestive heart failure refractory to high-dose loop diuretic and low-dose angiotensin-converting enzyme inhibitor. Am J Cardiol. 1993;71(3):21A–28A. doi: 10.1016/0002-9149(93)90241-4. [DOI] [PubMed] [Google Scholar]
- 42.Alnuwaysir R.I.S., Grote Beverborg N., Hoes M.F., et al. Additional burden of iron deficiency in heart failure patients beyond the cardio-renal anaemia syndrome: findings from the BioStat-CHF study. Eur J Heart Fail. 2022;24(1):192–204. doi: 10.1002/ejhf.2393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bekfani T., Pellicori P., Morris D., et al. Iron deficiency in patients with heart failure with preserved ejection fraction and its association with reduced exercise capacity, muscle strength and quality of life. Clin Res Cardiol. 2019;108(2):203–211. doi: 10.1007/s00392-018-1344-x. [DOI] [PubMed] [Google Scholar]
- 44.Alcaide-Aldeano A., Garay A., Alcoberro L., et al. Iron deficiency: impact on functional capacity and quality of life in heart failure with preserved ejection fraction. J Clin Med. 2020 Apr 22;9(4):1199. doi: 10.3390/jcm9041199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chopra V.K., Anker S.D. Anaemia, iron deficiency and heart failure in 2020: facts and numbers. ESC Heart Fail. 2020;7(5):2007–2011. doi: 10.1002/ehf2.12797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Alnuwaysir R.I.S., Hoes M.F., van Veldhuisen D.J., et al. Iron deficiency in heart failure: mechanisms and pathophysiology. J Clin Med. 2021:11. doi: 10.3390/jcm11010125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Macdougall I.C., White C., Anker S.D., et al. Intravenous iron in patients undergoing maintenance hemodialysis. N Engl J Med. 2019 Jan 31;380(5):447–458. doi: 10.1056/NEJMoa1810742. [DOI] [PubMed] [Google Scholar]
- 48.Ponikowski P., van Veldhuisen D.J., Comin-Colet J., et al. Beneficial effects of long-term intravenous iron therapy with ferric carboxymaltose in patients with symptomatic heart failure and iron deficiency. Eur Heart J. 2015;36(11):657–668. doi: 10.1093/eurheartj/ehu385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Unger E.D., Dubin R.F., Deo R., et al. Association of chronic kidney disease with abnormal cardiac mechanics and adverse outcomes in patients with heart failure and preserved ejection fraction. Eur J Heart Fail. 2016;18(1):103–112. doi: 10.1002/ejhf.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gori M., Senni M., Gupta D.K., et al. Association between renal function and cardiovascular structure and function in heart failure with preserved ejection fraction. Eur Heart J. 2014;35(48):3442–3451. doi: 10.1093/eurheartj/ehu254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lofman I., Szummer K., Dahlstrom U., et al. Associations with and prognostic impact of chronic kidney disease in heart failure with preserved, mid-range, and reduced ejection fraction. Eur J Heart Fail. 2017;19(12):1606–1614. doi: 10.1002/ejhf.821. [DOI] [PubMed] [Google Scholar]
- 52.Gupta R., Testani J., Collins S. Diuretic resistance in heart failure. Curr Heart Fail Rep. 2019;16(2):57–66. doi: 10.1007/s11897-019-0424-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zelnick L.R., Shlipak M.G., Soliman E.Z., et al. Prediction of incident heart failure in CKD: the CRIC study. Kidney Int Rep. 2022;7(4):708–719. doi: 10.1016/j.ekir.2022.01.1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hillege H.L., Nitsch D., Pfeffer M.A., et al. Renal function as a predictor of outcome in a broad spectrum of patients with heart failure. Circulation. 2006;113(5):671–678. doi: 10.1161/CIRCULATIONAHA.105.580506. [DOI] [PubMed] [Google Scholar]
- 55.Nardi E., Mule G., Nardi C., et al. Is echocardiography mandatory for patients with chronic kidney disease? Intern Emerg Med. 2019;14(6):923–929. doi: 10.1007/s11739-019-02028-0. [DOI] [PubMed] [Google Scholar]
- 56.Wali R.K., Wang G.S., Gottlieb S.S., et al. Effect of kidney transplantation on left ventricular systolic dysfunction and congestive heart failure in patients with end-stage renal disease. J Am Coll Cardiol. 2005;45(7):1051–1060. doi: 10.1016/j.jacc.2004.11.061. [DOI] [PubMed] [Google Scholar]
- 57.Juilliere Y., Venner C., Filippetti L., et al. Heart failure with preserved ejection fraction: A systemic disease linked to multiple comorbidities, targeting new therapeutic options. Arch Cardiovasc Dis. 2018;111(12):766–781. doi: 10.1016/j.acvd.2018.04.007. [DOI] [PubMed] [Google Scholar]
- 58.Cleland J.G., Tendera M., Adamus J., et al. The perindopril in elderly people with chronic heart failure (PEP-CHF) study. Eur Heart J. 2006;27(19):2338–2345. doi: 10.1093/eurheartj/ehl250. [DOI] [PubMed] [Google Scholar]
- 59.Rogers J.K., Pocock S.J., McMurray J.J., et al. Analysing recurrent hospitalizations in heart failure: a review of statistical methodology, with application to CHARM-Preserved. Eur J Heart Fail. 2014;16(1):33–40. doi: 10.1002/ejhf.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.McMurray J.J., Packer M., Desai A.S., et al. Angiotensin-neprilysin inhibition versus enalapril in heart failure. N Engl J Med. 2014;371(11):993–1004. doi: 10.1056/NEJMoa1409077. [DOI] [PubMed] [Google Scholar]
- 61.Solomon S.D., McMurray J.J.V., Anand I.S., et al. Angiotensin-neprilysin inhibition in heart failure with preserved ejection fraction. N Engl J Med. 2019;381(17):1609–1620. doi: 10.1056/NEJMoa1908655. [DOI] [PubMed] [Google Scholar]
- 62.Solomon S.D., Zile M., Pieske B., et al. The angiotensin receptor neprilysin inhibitor LCZ696 in heart failure with preserved ejection fraction: a phase 2 double-blind randomised controlled trial. Lancet. 2012;380(9851):1387–1395. doi: 10.1016/S0140-6736(12)61227-6. [DOI] [PubMed] [Google Scholar]
- 63.Polsinelli V.B., Shah S.J. Advances in the pharmacotherapy of chronic heart failure with preserved ejection fraction: an ideal opportunity for precision medicine. Expert Opin Pharmacother. 2017;18(4):399–409. doi: 10.1080/14656566.2017.1288717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Palmer B.F., Clegg D.J. Kidney-protective effects of SGLT2 inhibitors. Clin J Am Soc Nephrol. 2023;18(2):279–289. doi: 10.2215/CJN.09380822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Verma S., McMurray J.J.V. SGLT2 inhibitors and mechanisms of cardiovascular benefit: a state-of-the-art review. Diabetologia. 2018;61(10):2108–2117. doi: 10.1007/s00125-018-4670-7. [DOI] [PubMed] [Google Scholar]
- 66.Heerspink H.J.L., Stefansson B.V., Correa-Rotter R., et al. Dapagliflozin in patients with chronic kidney disease. N Engl J Med. 2020;383(15):1436–1446. doi: 10.1056/NEJMoa2024816. [DOI] [PubMed] [Google Scholar]
- 67.Anker S.D., Butler J., Filippatos G., et al. Empagliflozin in heart failure with a preserved ejection fraction. N Engl J Med. 2021;385(16):1451–1461. doi: 10.1056/NEJMoa2107038. [DOI] [PubMed] [Google Scholar]
- 68.Solomon S.D., McMurray J.J.V., Claggett B., et al. Dapagliflozin in heart failure with mildly reduced or preserved ejection fraction. N Engl J Med. 2022;387(12):1089–1098. doi: 10.1056/NEJMoa2206286. [DOI] [PubMed] [Google Scholar]
- 69.Heidenreich P.A., Bozkurt B., Aguilar D., et al. 2022 AHA/ACC/HFSA guideline for the management of heart failure: A report of the American College of Cardiology/American Heart Association joint committee on clinical practice guidelines. J Am Coll Cardiol. 2022;79(17):e263–e421. doi: 10.1016/j.jacc.2021.12.012. [DOI] [PubMed] [Google Scholar]
- 70.Ida S., Kaneko R., Imataka K., et al. Effects of oral antidiabetic drugs and glucagon-like peptide-1 receptor agonists on left ventricular diastolic function in patients with type 2 diabetes mellitus: a systematic review and network meta-analysis. Heart Fail Rev. 2021;26(5):1151–1158. doi: 10.1007/s10741-020-09936-w. [DOI] [PubMed] [Google Scholar]
- 71.Mosterd C.M., Bjornstad P., van Raalte D.H. Nephroprotective effects of GLP-1 receptor agonists: where do we stand? J Nephrol. 2020;33(5):965–975. doi: 10.1007/s40620-020-00738-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Tadic M., Sala C., Saeed S., et al. New antidiabetic therapy and HFpEF: light at the end of tunnel? Heart Fail Rev. 2022;27(4):1137–1146. doi: 10.1007/s10741-021-10106-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kosiborod M.N., Abildstrøm S.Z., Borlaug B.A., et al. Semaglutide in patients with heart failure with preserved ejection fraction and obesity. N Engl J Med. 2023;389:1069–1084. doi: 10.1056/NEJMoa2306963. [DOI] [PubMed] [Google Scholar]
- 74.Edelmann F., Wachter R., Schmidt A.G., et al. Effect of spironolactone on diastolic function and exercise capacity in patients with heart failure with preserved ejection fraction: the Aldo-DHF randomized controlled trial. JAMA. 2013;309(8):781–791. doi: 10.1001/jama.2013.905. [DOI] [PubMed] [Google Scholar]
- 75.Pitt B., Pfeffer M.A., Assmann S.F., et al. Spironolactone for heart failure with preserved ejection fraction. N Engl J Med. 2014;370(15):1383–1392. doi: 10.1056/NEJMoa1313731. [DOI] [PubMed] [Google Scholar]
- 76.Pfeffer M.A., Claggett B., Assmann S.F., et al. Regional variation in patients and outcomes in the Treatment of Preserved Cardiac Function Heart Failure with an Aldosterone Antagonist (TOPCAT) trial. Circulation. 2015;131(1):34–42. doi: 10.1161/CIRCULATIONAHA.114.013255. [DOI] [PubMed] [Google Scholar]
- 77.Agarwal R., Filippatos G., Pitt B., et al. Cardiovascular and kidney outcomes with finerenone in patients with type 2 diabetes and chronic kidney disease: the FIDELITY pooled analysis. Eur Heart J. 2022;43(6):474–484. doi: 10.1093/eurheartj/ehab777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.El Hajj E.C., El Hajj M.C., Sykes B., et al. Pragmatic weight management program for patients with obesity and heart failure with preserved ejection fraction. Journal of the American Heart Association. 2021;10 doi: 10.1161/JAHA.121.022930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kitzman D.W., Brubaker P., Morgan T., et al. Effect of caloric restriction or aerobic exercise training on peak oxygen consumption and quality of life in obese older patients with heart failure with preserved ejection fraction: a randomized clinical trial. Jama. 2016;315:36–46. doi: 10.1001/jama.2015.17346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Fukuta H., Goto T., Wakami K., et al. Effects of exercise training on cardiac function, exercise capacity, and quality of life in heart failure with preserved ejection fraction: a meta-analysis of randomized controlled trials. Heart Fail Rev. 2019;24(4):535–547. doi: 10.1007/s10741-019-09774-5. [DOI] [PubMed] [Google Scholar]
- 81.Nelson A.J., Pagidipati N.J., Aroda V.R., et al. Incorporating SGLT2i and GLP-1RA for cardiovascular and kidney disease risk reduction: call for action to the cardiology community. Circulation. 2021;144(1):74–84. doi: 10.1161/CIRCULATIONAHA.121.053766. [DOI] [PubMed] [Google Scholar]