Abstract
Background & Aims
Von Willebrand factor antigen (vWFAg), a protein measured to test the level of vWF released from the vascular endothelium has gained much attention as a marker for portal hypertension (PHT) severity. The objectives of this study were to investigate the use of vWFAg as a biomarker along with liver and spleen stiffness measurements by transient elastography as potential predictors of clinically significant varices (CSV), variceal bleeding (VB) and decompensation in children with PHT.
Methods
This observational prospective cohort study included 117 children (median age 10 [IQR 6-14] years) who underwent oesophagogastroduodenoscopy between January’2012 to November’2021 and a validation group of 33 children who underwent the same procedure between December’2021 to March’2023. Measurements of vWFAg and glycoprotein Ib binding activity of VWF (GPIbR) were available in 97 patients in the study group and in all patients in the validation group.
Results: vWFAg and GPIbR were significantly higher in children with CSV (223 IU/dl and 166 IU/dl; p = 0.015 and p = 0.04, respectively) and VB (218 IU/dl and 174 IU/dl; p = 0.077 and p = 0.03, respectively) than in those without CSV or VB, respectively. Ninety-six patients had liver and spleen stiffness measurements. Spleen stiffness was significantly higher in patients with CSV compared to those without CSV (p = 0.003). In a chronic liver disease subgroup, a predictive scoring tool based on vWFAg, GPIbR, platelet count, and spleen/liver stiffness measurements could predict CSV with an AUROC of 0.76 (p = 0.04).
Conclusions
This study suggests the predictive value of vWF for CSV and VB increases when combined with spleen stiffness, with AUROCs of 0.88 and 0.82, respectively. Hence, a combination of biomarkers could assist clinicians in diagnosing CSV, preventing unnecessary invasive procedures.
Impacts and implications
Surveillance endoscopies in children with portal hypertension (PHT) have their own risks and non-invasive markers, such as von Willebrand factor antigen, glycoprotein Ib binding activity of VWF (GPIbR), and transient elastography could be used to predict clinically significant varices, variceal bleeding and disease compensation in children with PHT. Such non-invasive markers for PHT and varices are lacking in the paediatric population. The results show that von Willebrand factor and GPIbR along with transient elastography can be used to formulate a scoring system which can be used as a clinical tool by paediatric hepatologists to monitor the progression of PHT and risk of bleeding, and hence to stratify the performance of invasive endoscopic procedures under general anaesthesia. However, there is a need to validate the scoring system in children with portal vein thrombosis and for hepatic decompensation in a multi-centre registry in the future.
Keywords: Biomarkers, Clinically significant varices, Variceal Bleeding, Liver Stiffness, Spleen Stiffness
Graphical abstract
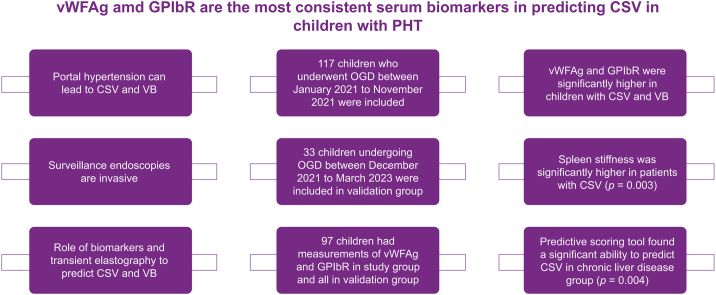
Highlights
-
•
Portal hypertension in children can lead to variceal bleeding and complications.
-
•
Surveillance endoscopies are invasive, hence non-invasive biomarkers are needed.
-
•
vWF and GPIbR are the most consistent serum biomarkers to predict significant varices.
-
•
Combination with spleen stiffness increases their predictive value.
-
•
Risk scoring prediction models need to be validated in larger numbers in the future.
Introduction
Portal hypertension (PHT) is defined as increased pressure within the portal venous system which results from altered blood flow from the portal vein to the hepatic vein. PHT can occur either at a pre-hepatic (extrahepatic portal vein obstruction), intrahepatic (pre-sinusoidal, sinusoidal or post-sinusoidal) or post-hepatic level (right heart failure, hepatic vein occlusion). Portal hypertension can lead to varices, variceal bleeding and complications like ascites, hepatic encephalopathy, hepatopulmonary syndrome, porto-pulmonary hypertension, growth failure and an overall poorer quality of life.[1], [2], [3], [4] Gastrointestinal (GI) bleeding remains a major life-threatening complication which develops from the rupture of varices in the GI tract (most commonly the oesophagus).6 GI variceal bleeding is associated with significant morbidity and varied mortality rates of between 2% and 20% in children,[7], [8], [9] however, the mortality associated with extrahepatic PHT variceal bleeding remains at about 3%.10,11 Acute decompensation clinically marked by the development of complications such as ascites, variceal haemorrhage, acute kidney injury (AKI) and overt hepatic encephalopathy are also associated with variceal bleeding.12 Hence, early diagnosis and management of PHT remains important.
A widely used approach to diagnosis PHT in adults is by direct measurement of hepatic venous pressure gradient (HVPG), but this may not be routine practice in children due to its invasive nature.5,13
In adults, PHT is defined as an HVPG measurement ≥6 mmHg and clinically significant portal hypertension (CSPH), the pressure at which varices begin to form, as an HVPG measurement ≥10 mmHg. In children, the diagnosis of PHT is commonly based on clinical/haematological findings of PHT complications such as splenomegaly, thrombocytopenia and ascites.
Management of PHT in children requires surveillance endoscopies to directly visualize and prophylactically treat varices, where clinically indicated. Surveillance endoscopies have their own risks and non-invasive markers can be helpful in the diagnosis of clinically significant varices (CSV) to prevent children from undergoing unnecessary endoscopies. Transient elastography (TE) is a non-invasive tool to measure liver fibrosis that has been validated in adults14,15 and is increasingly utilised in children.16,17
Von Willebrand factor (vWF) is a multimeric glycoprotein and a marker of vascular endothelial damage. Endothelial dysfunction has been shown to contribute to the pathogenesis and progression of PHT.18,19 The vWF antigen/thrombocyte ratio (VITRO) score has been described as a non-invasive marker to diagnose and predict CSPH in patients with cirrhosis.20
The aim of our study was to investigate the use of vWFAg as a biomarker along with liver and spleen stiffness (LS and SS) measurements by TE for the prediction of CSV, variceal bleeding (VB) and disease decompensation in children with PHT.
Patients and methods
Patients
This observational prospective cohort study included all patients between the age of 6 months to 18 years who underwent OGD (oesophagogastroduodenoscopy) in the Paediatric Liver, GI and Nutrition Centre, King’s College Hospital, London, between January 2012 and November 2021. To validate the study, we prospectively included all patients between the age of 6 months to 18 years who underwent OGD between December 2021 to March 2023. All patients selected for OGD underwent surveillance endoscopies and either primary or secondary prophylaxis, where clinically indicated, if they had evidence of PHT using the criteria of clinically and/or radiologically confirmed splenomegaly and persistent thrombocytopenia recorded on more than one occasion (platelet count below 100 x109/L) as per departmental guidelines. All children who underwent OGD without any evidence of underlying PHT were excluded. Clinical data on demographics, diagnosis, medical history including any GI bleeding episode and age of all these children (defined as age at the time of OGD) were recorded. All children had routine laboratory investigations as per departmental protocols including haematological and coagulation parameters, clinical biochemistry. vWFAg, glycoprotein Ib binding activity of VWF (GPIbR) tests and VITRO score available from the last 5 years were collected and calculated. Most children underwent TE using FibroScan to measure LS and SS either at the time of presentation to our department or at the time of OGD. Variceal prediction scores and CSV were recorded at the time of endoscopy as per British Society of Paediatric Gastroenterology, Hepatology and Nutrition (BSPGHAN) guidelines21 (https://bspghan.org.uk/wp-content/uploads/2021/12/Varices-guideline_BSPGHAN_v2.pdf).
The study was approved by the local Institutional Review Board (IRAS ID 238002). All patient records were anonymized before analysis.
vWFAg and GPIbR
Plasma levels of vWFAg and GPIbR were measured at the time of admission for endoscopy. VITRO score was calculated as defined previously17 by dividing VWFAg by platelet count (vWFAg/PLT).
Transient elastography
LSM and SSM were assessed using TE (FibroScan; Echosens, Paris, France) as described previously22 by a trained professional either at the time of presentation to our department or at the time of OGD. The median LSM and SSM values were specified in kPa.
Grading of varices
Varices were graded as grade I, II, III as per BAVENO VI consensus23 and BSPGHAN guidelines. Varices that are grade II or more than grade I with red wale signs were defined as CSV as per international consensus. Primary prophylaxis of varices is defined as treatment of varices before bleeding has occurred. Secondary prophylaxis of varices is defined as treatment of varices that have previously bled, but not during an acute bleeding episode.
Acute kidney injury
AKI was defined as either an increase in baseline creatinine >50% within 48 hours from bleeding or development of hepatorenal syndrome.24
Statistical analysis
Statistical analyses were performed using GraphPad Prism 9.4.3 for Microsoft Windows (GraphPad LLC., San Diego, CA, USA). Descriptive statistics were reported as median and IQR or percentage. Differences of median values of vWFAg, GPIbR, VITRO score, LSM, SSM and platelet count groups with and without CSPH were assessed by the Mann-Whitney U test. Receiver-operating characteristic curves were constructed for the assessment of the diagnostic accuracy of GPIbR, VITRO score, LSM, SSM and platelet count groups. AUROC, sensitivity, specificity, likelihood ratio (LR) values of non-invasive tests were calculated. We applied the multiple logistic regression model under which we calculated the AUROC for the combination of all variables including vWFAg, GPIbR, VITRO score, LSM, SSM and platelet count. The value with the best sensitivity and specificity in AUROC analysis (Youden index: sensitivity + specificity -1) was chosen as the best cut-off.
A prediction score to analyse children with CSV was derived using binary logistic regression analysis and deriving coefficients using SPSS Statistics version 28.0.1.1. This score was applied to a chronic liver disease (CLD) group and a portal vein thrombosis (PVT) group to validate the study. A Mann-Whitney U test was used to assess the difference and receiver-operating characteristic curves were constructed with a combination of all variables including vWFAg, GPIbR, VITRO score, LSM, SSM and platelet count. All p values were two-sided and p values <0.05 were considered significant.
Results
Patient characteristics
This study included 117 children, of whom 63 (54%) were males, who underwent OGD as per department protocols at a median age of 9.4 years (IQR 8 years).
The underlying diagnoses were PVT in 33 children (28%), biliary atresia (BA) in 26 (22%), cystic fibrosis in 14 (12%), ciliopathies in 9 (8%), intestinal failure-associated liver disease (IFALD) in 5 (4%), progressive familial intrahepatic cholestasis (PFIC) in 3 (2.5%), autoimmune liver disease in 3 (2.5%), and other diagnoses (including veno-occlusive disease, primary sclerosing cholangitis, Wilson’s disease and cryptogenic liver disease) in 24 (20%) children.
Seventy-seven (66%) children, including 43 (56%) males, with a median age of 9 years were found to have CSV. Forty-seven children (40%), including 28 (60%) males, with a median age of 8 years either presented with or had breakthrough GI VB. vWFAg and GPIbR measurements were available for 97 children (83%). SSM and LSM were available for 96 children (82%). The CLD subgroup (n = 52) included children with BA (n = 26, 50%), IFALD (n = 5), autoimmune liver disease (n = 3), PFIC (n = 3) and cryptogenic/other CLD (n = 10). During the study period, 70 (60%) children had CSV and underwent primary prophylaxis of varices, 39 (33%) children had previously suffered a GI bleed and had secondary prophylaxis and 8 (7%) children had OGD following an acute bleeding episode. Fifty-one (44%) children underwent band ligation for variceal treatment, 14 (12%) children underwent sclerotherapy, 44 (38%) children did not have any intervention, 4 (3%) children had both band ligation and sclerotherapy at the time of OGD and 4 (3%) children had previous treatment for varices in another hospital/country. Out of 47 children with VB, 18 (32%) children had ascites, 10 (21%) had sepsis, 5 (11%) had AKI, 8 (17%) children required paediatric intensive care unit (PICU) admission and 2 (4%) had a Sengstaken-Blakemore tube inserted for management of bleeding.
CSV group
vWFAg values, in all children, were significantly higher in the CSV positive (+ve) group (n = 77) than the CSV negative (-ve) group (n = 40) (median = 223 IU/dl and 153 IU/dl, respectively, p = 0.015), with an AUROC of 0.65 (95% CI 0.531–0.764), irrespective of the underlying diagnosis (Table 1). Using a cut-off of >168 IU/dl for vWFAg, the sensitivity and specificity for CSV were 73% and 62%, respectively, with a LR of 1.9. Using a cut-off of >200 IU/dl, the sensitivity and specificity were 60% and 73%, respectively, with a LR of 2.22. Similarly, GPIbR was significantly higher in the CSV +ve group than the CSV -ve group (median = 166 IU/dl vs. 145 IU/dl, respectively; p = 0.04), with an AUROC of 0.62 (95% CI 0.507–0.748). Using a cut-off of 175 IU/dl, the sensitivity and specificity were 46% and 74%, respectively, with a LR of 1.7. SSM was also significantly higher in the CSV +ve vs. the CSV -ve group (median = 38 kPa vs. 24.5 kPa, respectively, p = 0.003), with an AUROC of 0.68 (95% CI 0.569–0.783). Using a cut-off of 40 kPa, the sensitivity and specificity of SSM were 41% and 86%, respectively, with a LR of 3.1. Similarly, the VITRO score was significantly higher in the CSV +ve than the CSV -ve group (median = 2.85 vs. 1.91, respectively, p = 0.017), with an AUROC of 0.64 (95% CI 0.528–0.759). Using a cut-off of >2.113, the sensitivity and specificity were 72% and 62%, respectively. There were no differences between the two groups regarding platelet count and LSM (Table 2). The diagnoses of children within the CSV +ve group were PVT (n = 26), BA, CLD, IFALD and PFIC.
Table 1.
Variables that were statistically significantly different between the CSV +ve and -ve groups.
| Test | CSV +ve | CSV -ve | p value | AUROC | Cut-off | Sensitivity | Specificity | Likelihood ratio |
|---|---|---|---|---|---|---|---|---|
| All patients | n = 77 | n = 40 | ||||||
| vWFAg (IU/dl) | 223 | 153 | 0.015 | 0.65 | >200 | 60% | 73% | 2.22 |
| GPIbR (IU/dl) | 166 | 145 | 0.04 | 0.62 | >175 | 46% | 74% | 1.7 |
| VITRO score | 2.85 | 1.91 | 0.017 | 0.64 | >2.113 | 72% | 62% | |
| SSM (kPa) |
38 |
24.5 |
0.003 |
0.68 |
40 |
41% |
86% |
3.1 |
| Chronic liver disease | n = 26 | n = 26 | ||||||
| vWFAg (IU/dl) | 240 | 147 | 0.007 | 0.74 | >205 | 71% | 72% | 3.143 |
| SSM (kPa) |
38 |
23 |
0.014 |
0.71 |
>31 |
61% |
70% |
2.0 |
| Portal vein thrombosis | n = 26 | n = 7 | ||||||
| GPIbR (IU/dl) | 157 | 92 | 0.01 | 0.83 | >139 | 74% | 83% | 4.43 |
| SSM (kPa) | 21 | 3 | 0.02 | 0.91 | >16 | 85% | 100% |
Data presented as median values, percentages (%), ratio and p-value (all are significant values <0.05) using Mann-Whitney U test to compare normally distributed non-parametrically distributed data where appropriate of laboratory and TE measurements of patients in CSV +ve and CSV -ve group and chronic liver disease & portal vein thrombosis subgroup.
CSV, clinically significant varices; GPIbR, vWF ristocetin co-factor; SSM, spleen stiffness measurement; VITRO, von Willebrand factor antigen/thrombocyte ratio; vWF, von Willebrand factor; vWFAg, von Willebrand factor antigen.
Table 2.
Differences in vWF-based variables, LSM and platelet count between the CSV +ve and -ve groups.
| Test | CSV +ve | CSV -ve | p value |
|---|---|---|---|
| All patients | n = 77 | n = 40 | |
| LSM (kPa) | 15 | 12.5 | 0.58 |
| Platelet (x109) |
72 |
80 |
0.22 |
| Chronic liver disease | n = 26 | n = 26 | |
| GPIbR (IU/dl) | 219 | 147 | 0.04 |
| VITRO | 2.86 | 1.91 | 0.019 |
| LSM (kPa) | 17 | 12 | 0.27 |
| Platelet (x109) |
74 |
78 |
0.54 |
| Portal vein thrombosis | n = 26 | n = 7 | |
| vWFAg (IU/dl) | 201 | 160 | 0.11 |
| VITRO score | 2.49 | 1.23 | 0.38 |
| LSM (kPa) | 6 | 7 | 0.31 |
| Platelet (x109) | 91 | 105 | 0.61 |
Data presented as median values and p values (significant for GPIbR =0.04 and VITRO =0.019) using Mann-Whitney U test to compare normally distributed non-parametrically distributed data, of laboratory and TE measurements of patients in CSV +ve and CSV -ve group and chronic liver disease & portal vein thrombosis subgroup.
CSV, clinically significant varices; GPIbR, vWF ristocetin co-factor; LSM, liver stiffness measurement; VITRO, von Willebrand factor antigen/thrombocyte ratio; vWF, von Willebrand factor; vWFAg, von Willebrand factor antigen.
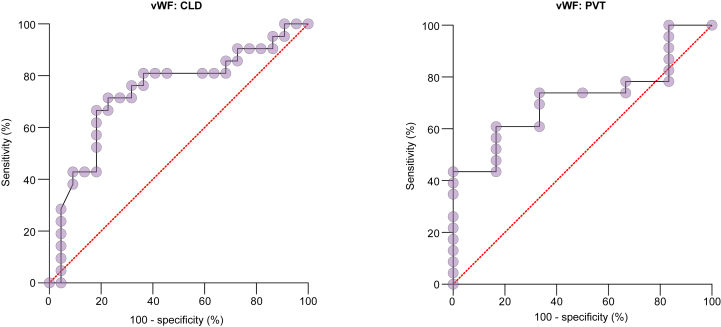
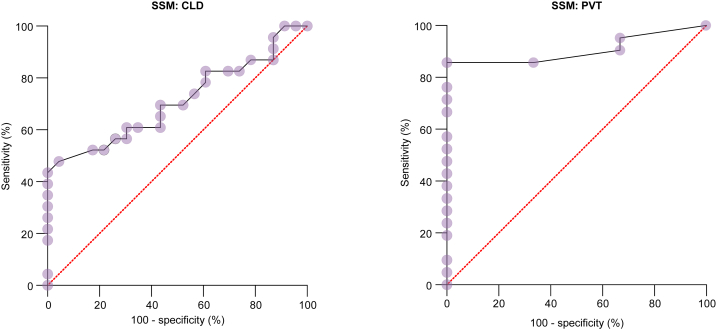
Out of 52 children with CLD, 26 (50%) were CSV +ve and 26 (50%) CSV -ve. Within the CLD group, differences in vWFAg, GPIbR, VITRO and SSM values between the CSV +ve and CSV -ve cohorts were statistically significant (Table 1), while the platelet count and LSM were not statistically different (Table 2). Using a cut-off of >205 IU/dl, vWFAg had an AUROC of 0.74 for the prediction of CSV, with a sensitivity and specificity of 71% and 72%, respectively, and a LR of 3.143 (Fig. 1). Using a cut-off of >31 kPa, SSM had an AUROC of 0.71, with a sensitivity and specificity of 61% and 70%, and a LR of 2.0 (Table 1 and Fig. 2).
Fig. 1.
ROC analysis (with 100% - specificity in x-axis and sensitivity in y-axis) shows the ability of vWF to predict CSV in children with CLD and PVT, with AUROCs of 0.74 and 0.72, respectively.
CLD, chronic liver disease; CSV, clinically significant varices; PVT, portal vein thrombosis; vWFAg, von Willebrand factor antigen.
Fig. 2.
ROC analysis (with 100% - specificity in x-axis and sensitivity in y-axis) shows the ability of SSM to predict CSV in children with CLD and PVT, with AUROCs of 0.71 and 0.91, respectively.
CLD, chronic liver disease; CSV, clinically significant varices; PVT, portal vein thrombosis; SSM, spleen stiffness measurement.
Of 33 patients with PVT, 26 (79%) were CSV +ve. Within the PVT group, GPIbR and SSM were statistically significant when compared between CSV +ve and -ve cohorts (Table 1). Using a cut-off of >139 IU/dl for GPIbR, the sensitivity and specificity was 74% and 83%, respectively, with an AUROC of 0.83 and a LR of 4.43. Similarly, using a cut-off of >16 kPa for SSM, the sensitivity and specificity were 85% and 100%, with an AUROC of 0.91. Neither vWFAg, VITRO score, nor platelet count showed statistically significant differences in this subgroup (Table 2).
Variceal bleed group
Forty-seven (40%) children had a VB in our study. vWFAg was higher amongst the VB +ve group vs. the VB -ve group but did not reach statistical significance (median = 218 IU/dl vs. 167 IU/dl, respectively; p = 0.08). Using a cut-off of >185 IU/dl the sensitivity and specificity was 69% and 62%, respectively, with an AUROC of 0.61 and LR of 2. Interestingly, GPIbR was statistically significantly different between the VB +ve group vs. the VB -ve group (median = 174 IU/dl vs. 142 IU/dl, respectively, p = 0.03). Using a cut-off of >155 IU/dl, the sensitivity and specificity was 68% and 61%, respectively, with an AUROC of 0.63 and a LR of 1.76 (Table 3). There was no statistical difference found between SSM, LSM, VITRO score or platelet count when compared between the two groups (Table 3).
Table 3.
Performance of vWF-based variables, LSM, SSM, and platelet count for the prediction of VB.
| Test | VB +ve | VB -ve | p value |
|---|---|---|---|
| All patients | n = 47 | n = 70 | |
| VITRO | 2.81 | 2.16 | 0.37 |
| Platelet (/μl) | 85 | 75 | 0.66 |
| LSM (kPa) | 15 | 11 | 0.41 |
| SSM (kPa) |
34 |
32 |
0.54 |
| Chronic liver disease | n = 15 | n = 30 | |
| vWFAg (IU/dl) | 219 | 159 | 0.17 |
| GPIbR (IU/dl) | 215 | 151 | 0.1 |
| VITRO | 2.86 | 2.13 | 0.29 |
| Platelet (/μl) | 81 | 77 | 0.89 |
| LSM (kPa) | 80 | 70 | 0.89 |
| SSM (kPa) |
24 |
33 |
0.5 |
| Portal vein thrombosis | n = 21 | n = 12 | |
| VITRO | 2.5 | 1 | 0.07 |
| Platelet (/μl) | 92 | 106 | 0.5 |
| LSM (kPa) | 4.5 | 7 | 0.08 |
| SSM (kPa) | 33 | 25 | 0.21 |
Data presented as median values and p values (all values are not significant >0.05) using Mann-Whitney U test to compare normally distributed non-parametrically distributed data of laboratory and TE measurements of patients in VB +ve and VB -ve group and chronic liver disease & portal vein thrombosis subgroup.
GPIbR, vWF ristocetin co-factor; LSM, liver stiffness measurement; SSM, spleen stiffness measurement; VB, variceal bleeding; VITRO, von Willebrand factor antigen/thrombocyte ratio; vWF, von Willebrand factor; vWFAg, von Willebrand factor antigen.
In the CLD subgroup (n = 45), 15 children (33%) had a VB. There was no significant difference in vWFAg and GPIbR between those who bled against those who did not (Table 3). There were also no statistical differences for SSM, LSM, VITRO score or platelet count (Table 3).
Within the PVT subgroup, 21 patients (64%) were VB +ve while 12 (36%) were VB -ve. There was a statistically significant difference in vWFAg between VB +ve and VB -ve children (Table 4). Using a cut-off of >185 IU/dl, the sensitivity and specificity of vWFAg were 68% and 80%, respectively, with an AUROC of 0.61 and a LR of 1.808. GPIbR was also statistically significantly different (Table 4). Using a cut-off of >155 IU/dl, the sensitivity and specificity were 63% and 80%, respectively, with an AUROC of 0.74 and a LR of 3.2 (Table 4). There was no significant difference in SSM, LSM, VITRO score or platelet count (Table 3).
Table 4.
Performance of von Willebrand factor-based variables for the prediction of VB.
| Test | VB +ve | VB -ve | p value | AUROC | Cut -off | Sensitivity | Specificity | Likelihood ratio |
|---|---|---|---|---|---|---|---|---|
| All patients | n = 47 | n = 70 | ||||||
| vWFAg (IU/dl) | 218 | 167 | 0.08 | 0.61 | >185 | 69% | 62% | 2 |
| GPIbR (IU/dl) |
174 |
142 |
0.03 |
0.63 |
>155 |
68% |
61% |
1.76 |
| Portal vein thrombosis | n = 21 | n = 12 | ||||||
| vWFAg (IU/dl) | 215 | 154 | 0.03 | 0.61 | >185 | 68% | 80% | 1.808 |
| GPIbR (IU/dl) | 159 | 126 | 0.03 | 0.74 | >155 | 63% | 80% | 3.2 |
Data presented as median values, percentages, ratio and p value (all values are significant with p <0.05) using Mann-Whitney U test to compare normally distributed non-parametrically distributed data, AUROC, sensitivity, specificity and Likelihood ratio of laboratory and TE measurements of patients in VB +ve and VB -ve group and portal vein thrombosis subgroup.
GPIbR, vWF ristocetin co-factor; LSM, liver stiffness measurement; SSM, spleen stiffness measurement; VB, variceal bleeding; VITRO, von Willebrand factor antigen/thrombocyte ratio; vWF, von Willebrand factor; vWFAg, von Willebrand factor antigen.
Complications of variceal bleeding
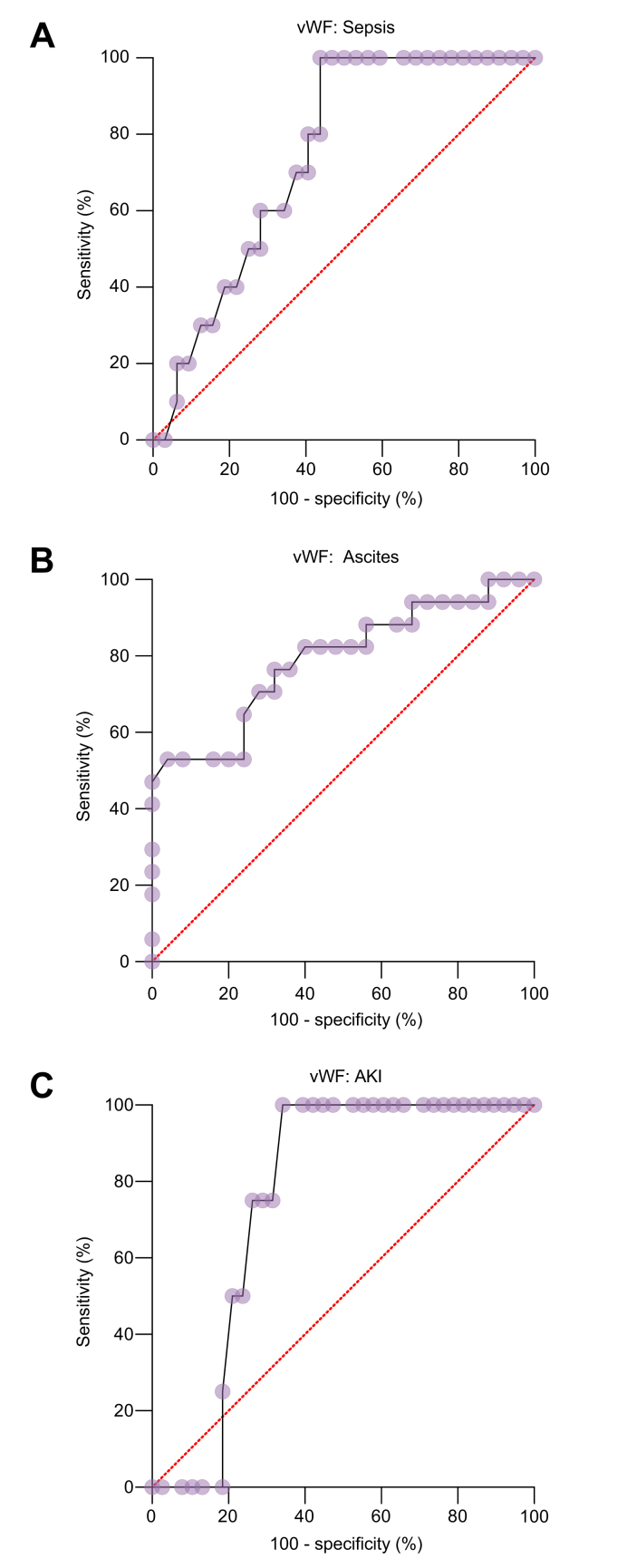
Within the VB group, we assessed the predictability of developing complications such as ascites, sepsis, AKI and admission to PICU. There was a statistically significant difference in vWF amongst children with ascites (n = 18) (p = 0.0016). Using a cut-off >208 IU/dl, the sensitivity and specificity of vWF for ascites was 82% and 60%, respectively, with an AUROC of 0.79 and a LR of 2.1 (Fig. 3). Similarly, for children with sepsis (n = 10), using a cut-off of >218 IU/dl, the sensitivity and specificity of vWF were 80% and 60%, respectively, with AUROC of 0.74 and a LR of 2. Five children developed AKI and using a cut-off of >225 IU/dl, the sensitivity and specificity of vWF were 100% and 66%, respectively, with an AUROC of 0.76 and a LR of 3 (Fig. 3). There was no positive correlation of vWF with PICU admission. We did not find any positive correlation between liver stiffness or spleen stiffness with ascites, sepsis, AKI or PICU admission.
Fig. 3.
ROC analysis (with 100% - specificity in x-axis and sensitivity in y-axis) shows the ability of vWF to predict complications in children with a variceal bleed.
Ability of vWF to predict (A) sepsis (B) ascites (C) AKI with AUROCs of 0.79, 0.74 and 0.76, respectively. AKI, acute kidney injury; vWF, von Willebrand factor.
Multiple logistic regression analysis
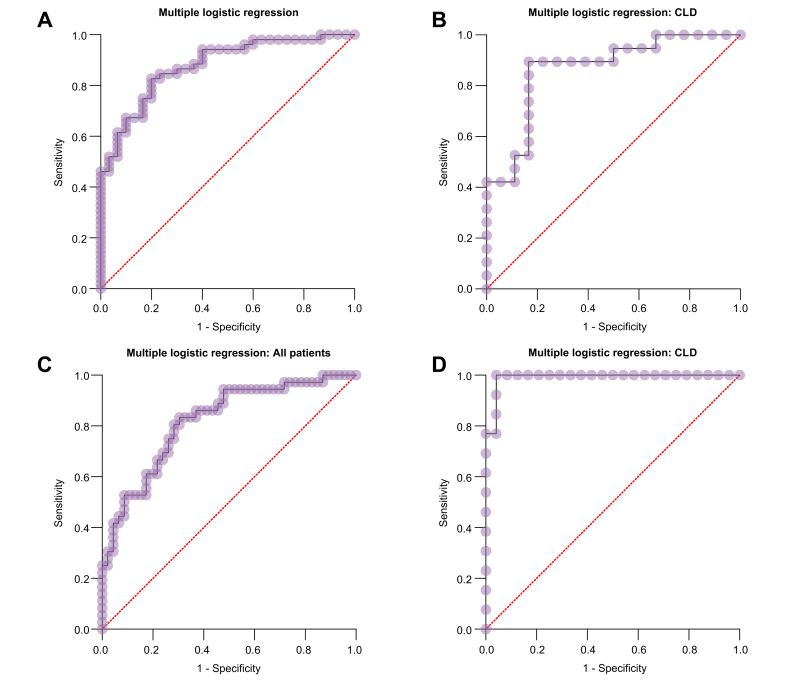
Multiple logistic regression analysis showed that when vWFAg, GPIbR, platelet count, LSM and SSM were combined, the prediction of CSV and VB improved with AUROCs of 0.88, 0.87, 0.82 and 0.99, respectively, for CSV in all study children, CSV in children with CLD, VB in all study children and VB in children with CLD (p <0.0001 in all) (Fig. 4).
Fig. 4.
ROC curve (with 1-specificity in x-axis and sensitivity in y-axis) showing predictive ability of score combining vWFAg, GPIbR, platelet count, LSM and SSM.
Score’s ability to predict (A) CSV in all children (B) CSV in children with CLD (C) VB in all patients (D) VB in children with CLD, with respective AUROCs of 0.88, 0.87, 0.82 and 0.99. CLD, chronic liver disease; CSV, clinically significant varices; GPIbR, glycoprotein Ib Binding activity of VWF; LSM, liver stiffness measurement; SSM, spleen stiffness measurement; vWFAg, von Willebrand factor antigen.
Validation group and prediction score
The validation group, albeit small due to endoscopy restrictions due to the COVID-19 pandemic, included 33 children, of whom 23 (70%) were male, who underwent OGD as per department protocols at a median age of 9.25 years. The underlying diagnoses were PVT in 6 children (18%) and BA in 10 (33%), with other diagnoses including alpha-1-antitrypsin deficiency, cryptogenic liver disease (6 [18%]), cystic fibrosis, autoimmune liver disease, mitochondrial gene mutation, Budd-Chiari syndrome, and Alagille syndrome. Of the 27 children in the CLD group, 20 (74%) were diagnosed as CSV +ve at the time of endoscopy. In the PVT group, 5/6 (83%) children were found to be CSV +ve.
A prediction score was formulated in the initial study group using binary regression analysis and deriving coefficients as follows:
| CSV = constant + vWF(Co∗) + GPIbR(Co∗) + SSM(Co∗) + LSM(Co∗) + platelet(Co∗) |
(∗Co= coefficient)
Scaling of the coefficients and intercept was done by multiplying with a factor of 20/log2 for children with CSV and 2/log2 for the PVT group. LSM was not included in the risk prediction score for PVT.
The final formulas for risk prediction score to determine CSV in children with CLD and PVT were as follows:
Children with CLD: CSV = 1/(1 + (-4.267 + 0.19xvWF - 0.008xGPIbR - 0.008xplatelet - 0.006xLSM + 0.084xSSM) and applying (20/log2) to the coefficient, the risk score was calculated as CSV = 1/(1 + (-283 + 1.3xvWF - 0.5xGPIbR - 0.5xplatelet - 0.4xLSM + 5.6xSSM)
Children with PVT: Risk score CSV = 1/(1 + (-658.572 + 1.662xvWF + 1.752xGPIbR + 0.747xplatelet + 2.349xSSM) and applying (2/log2) to the coefficient, the risk score was calculated as CSV = 1/(1 + (-4371 + 11xvWF + 12xGPIbR + 5xplatelet + 16xSSM)
In the CLD group, the median (IQR) values of vWF, GPIbR, platelets, SSM and LSM were 252.2 (123.1) IU/dl, 202.8 (99.4) IU/dl, 85 (68) x109/L, 29.1 (28.9) kPa, and 16.8 (31.8) kPa, respectively. In the PVT group, the median (IQR) values of vWF, GPIbR, platelets, and SSM were 194.6 (72.5) IU/dl, 149.5 (50.1) IU/dl, 90.5 (21.2) x109/L, and 41.7 (21.4) kPa, respectively. On application of the risk score in the validation group of 33 children, we found that in the CLD subgroup the mean prediction score was 0.00164, with an IQR of 0.0208 and an AUROC of 0.76 (95% CI 0.4828–1.000) and a significant p value of 0.04. In the PVT subgroup, the median prediction score was 0.00088, with an IQR of 0.0035 and an AUROC of 0.6000 (95% CI 0.1706–1.000, p = 0.76).
Discussion
In children with PHT, a means to reliably predict those who may have CSV and be at risk of VB has been the focus of several studies in the past.6 It has been a challenging question to address but the emergence of novel non-invasive tools has offered the opportunity to develop on previous efforts. Furthermore, in recent years, increasing emphasis is being placed not only on the direct morbidity and mortality from GI blood loss from PHT but also on the accompanying risks of liver decompensation, AKI ischaemic hepatitis, encephalopathy and liver transplantation that influence outcome and management decisions. Our study is the first paediatric study to integrate vWFAg, GPIbR and TE to predict CSV and VB in children with PHT.
Under physiological conditions, vWFAg, a multimeric glycoprotein, facilitates adhesion of platelets to the vascular sub-endothelium during vascular injury and stabilises coagulation factor VIII. However, in patients with cirrhosis levels are elevated further due to increased shear endothelial stress, bacterial infection, and induced expression of vWFAg in the cirrhotic liver itself, leading to endothelial instability.25 Therefore, endothelial dysfunction is considered to be a potential mechanism of variceal bleeding beyond the standard HVPG measurement.26 In adult patients with CSPH, markers such as vWFAg, LSM and SSM have been widely assessed and have been integrated into composite scores for prediction of CSPH or ruling out varices needing treatment.27 Furthermore, a reduction in vWFAg was demonstrated in those who received non-selective beta blocker therapy with an accompanied decrease in the risk of liver disease decompensation and death.28
Hence, vWFAg as an indicator of endothelial dysfunction, which plays an important role in the pathophysiology of both intra- and extrahepatic causes of PHT,29,30 could serve as a potential serum biomarker to predict/diagnose CSV and VB in children with PH. Our study demonstrated that vWFAg and/or GPIbR may serve as serum biomarkers for the stratification of children with CSV who can benefit from a surveillance endoscopy with regression analysis showing that prediction of CSV and VB improved on combining vWFAg, GPIbR, platelet count, LSM and SSM, with AUROCs of 0.88 and 0.82, respectively, for CSV and VB in all study children.
A recent meta-analysis of vWFAg in adults with cirrhosis and PHT showed a pooled sensitivity and specificity of 82% and 76% for CSV, with an AUC of 0.87.31 In this study, the etiologies of liver disease were significantly different to those seen in children and included alcohol-related liver disease, hepatitis B and hepatitis C infections. The sample size in these studies ranged from 42-286 patients with three studies having >200 patients. vWFAg level was significantly higher in patients with PHT at >300 IU/dl with non significant variation in sensitivity, making it a good marker for diagnosis. However, the cut-offs used in these studies varied from 226 IU/dl to 264 IU/dl, which are higher than reported in our study.
Islek et al.32 reported higher levels of vWFAg and GPIbR in children with chronic liver disease and extrahepatic PHT when compared to healthy controls with cut-off values of 171 IU/dl and 133 IU/dl, respectively. The limitation of their study was that the study population was rather limited (n = 28 in the cirrhotic group, n = 16 in the group with extrahepatic PHT). A similar study of 42 children by Beattie et al.33 also found increased levels of vWFAg in those with chronic liver disease and PVT. In our PVT subgroup, with a cut-off of >139 IU/dl for vWFAg, the sensitivity and specificity for prediction of CSV are 74% and 83%, respectively, with a LR of 4.43 and an AUROC of 0.83. Our study also found higher levels of vWFAg in children with PVT who had a VB (p = 0.03). Lower ADAMTS 13 antigen and hence higher vWFAg found in children with PVT suggests a vascular endothelial role in children with PHT without CLD.27,29
Our study included 52 children with CLD, with 26 (50%) having BA, of whom 14 (54%) required sclerotherapy or banding and 5 liver transplantation. In the CLD subgroup, vWF at a cut-off value of >205 IU/dl predicted CSV with a sensitivity and specificity of 71% and 72%, respectively, with a LR of 3.143 and an AUROC of 0.74. The advanced nature of liver disease in children who had VB might explain the higher vWFAg values and statistical significance, however, more studies are needed. Heterogeneity in diagnoses between adult studies and paediatric studies, advanced disease, concomitant inflammatory processes, or infection could explain the heterogeneity in cut-offs. The heterogeneity in diagnoses in our CLD study group needs to be acknowledged as in most such paediatric studies. Large patient registries and collaborations amongst paediatric liver centres that are focused on PHT in children, such as the IMPPHR (https://www.texaschildrens.org/international-multi-center-pediatric-portal-hypertension-registry), may help overcome such challenges.
Liver and spleen stiffness have previously been described as a reliable non-invasive tool for diagnosing PHT in children,16 with LSM showing a pooled sensitivity and specificity of 90% and 79%, respectively, for the diagnosis of PHT, with an AUROC of 0.92. However, most studies in this meta-analysis focused on LSM and only three studies[34], [35], [36] had simultaneous LS and SS measurements, with SSM being higher than LSM. Another study from our group by Sutton et al.22 showed that SSM had the best predictive value, with an AUROC of 0.92 and NPV of 80% to stratify children based on the presence of CSV. The current study shows that SSM >31 kPa has a sensitivity and specificity of 61% and 70%, with an AUROC of 0.71 for CSV in children with CLD. The sensitivity and specificity was higher at 85% and 100%, respectively, with an AUROC of 0.91 for patients with PVT. Interestingly, SSM was not significantly different in patients with VB and LSM was not statistically significantly different between bleeders or non-bleeders in the CLD subgroup. Studies by Sutton et al.22 and Vadlapudi et al.37 also did not find SSM to be statistically significantly different in bleeders. A possible explanation for the above discrepancy could be multifactorial. Difference in blood supplies to the liver, which is under dual supply, and the spleen, which is supplied by the high pressure arterial system, could possibly account for changes in SSM with a recent bleed. The complex pathophysiology of GI bleeding involving potential dysregulation in metabolic, endothelial, or coagulation pathways could be another reason. The cut-off values for SSM and LSM have not been fully defined and have varied through different studies and there is a future need to have defined cut-off values to predict CSV.38 There are several limitations to TE, including operator variability, obesity and ascites, a frequent complication of PHT. This could be mitigated by magnetic resonance elastography (MRE); however, use of MRE is still limited by high costs and limited availability. MRE-based SSM and LSM could still have future potential in this regard, with one meta-analysis in adults by Singh et al.39 showing SSM by this method to have improved specificity and overall accuracy in detecting CSPH.
Duché et al.40 reported life-threatening complications in 20% of children who bled spontaneously before first endoscopy and 10% of children died following first bleed before liver transplantation. D’souza et al.12 reported acute decompensation marked by complications such as sepsis and hepatorenal syndrome associated with VB. In our study, 32% of children developed ascites, 21% developed sepsis and 17% required PICU admission as a manifestation of hepatic decompensation following VB. We found vWFAg to be significantly correlated with ascites, sepsis and AKI, with AUROCs of 0.79, 0.74 and 0.76, respectively. This indicates the potential of vWFAg to predict CSV and complications of VB, though more prospective studies with well-defined objectives will be required. Additionally, in adults with stable decompensated cirrhosis treated with non-selective beta blockers, it has been shown that a decrease in vWFAg levels is associated with a reduced risk of decompensation, acute-on-chronic liver failure and/or death.28 Although there is no consensus regarding the use of non-selective beta blockers in children with PHT, there is potential to prospectively monitor their response in a non-invasive way.
Multiple logistic regression analysis was carried out in order to facilitate forming a non-invasive screening tool for prediction of CVS and VB in children. It showed excellent AUROC scores across all groups indicating that a prediction screening tool can be formulated with vWFAg, GPIbR, platelet count, SSM and LSM as independent variables. The scoring system can be applied as a clinical tool to monitor progression of PHT at the early stages or the risk of bleeding and complications in children with CLD during their follow up.
To validate the study, we created a risk scoring prediction model for children with CSV in CLD and PVT subgroups with similar demographics. Receiver-operating characteristic curve analysis of the risk scoring system in the CLD group was statistically significant with a p value of 0.04, thereby confirming that combining laboratory markers and ultrasonographic measurements can provide a convenient stratification method for clinicians. However, the small sample (n = 6) limited the validation of our scoring system in the PVT group. We are not able to comment on our ability to validate the prediction score for hepatic decompensation due to insufficient sample size.
This study suggests that vWFAg and GPIbR are the most consistent serum biomarkers in predicting CSV and PHT in children. When combined with SSM, the predictive value increases for CSV. A scoring model combining vWFAg, GPIbR, SSM, LSM and platelet count can predict CSV in children with CLD. There is a further need to validate the results in the PVT group and for complications arising due to hepatic decompensation in a multi-centre registry in the future.
Financial support
The authors did not receive any financial support to produce this manuscript.
Authors’ contributions
TG, RH contributed to the conception and design of the work. BT, AG, SD contributed to acquisition of data. AG, RH, TG, EK, VJ, ADh contributed to processing and analysis and interpretation of data. ADo contributed to the statistical analysis and interpretation of data. AG, RH, TG drafted the initial versions of the manuscript. All authors critically reviewed and edited the manuscript.
Data availability statement
The datasets analysed in this study are not publicly available.
Conflicts of interest
The authors of this study declare that they do not have any conflict of interest.
Please refer to the accompanying ICMJE disclosure forms for further details.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jhepr.2023.100935.
Supplementary data
The following are the supplementary data to this article:
:
References
- 1.Sharma P., Sharma B.C., Puri V., Sarin Shiv. Minimal hepatic encephalopathy in patients with extrahepatic portal vein obstruction. Am J Gastroenterol. 2008;103(6):1406–1412. doi: 10.1111/j.1572-0241.2008.01830.x. [DOI] [PubMed] [Google Scholar]
- 2.Raevens S., Geerts A., Van Steenkiste C., Verhelst X., Van Vlierberghe H. Colle I. Hepatopulmonary syndrome and portopulmonary hypertension: recent knowledge in pathogenesis and overview of clinical assessment. Liver Int. 2015;5(6):1646–1660. doi: 10.1111/liv.12791. [DOI] [PubMed] [Google Scholar]
- 3.Sarin S.K., Bansal A., Sasan S., Nigam A. Portal-vein obstruction in children leads to growth retardation. Hepatology. 1992;15(2):229–233. doi: 10.1002/hep.1840150210. [DOI] [PubMed] [Google Scholar]
- 4.Krishna Y.R., Yachha S.K., Srivastava A., Negi D., Lal R., Poddar U. Quality of life in children managed for extrahepatic portal venous obstruction. J Pediatr Gastroenterol Nutr. 2010;50(5):531–536. doi: 10.1097/MPG.0b013e3181b6a55d. [DOI] [PubMed] [Google Scholar]
- 5.Reiberger T., Püspök A., Schoder M., Baumann-Durchschein F., Bucsics T., Datz C., et al. Austrian consensus guidelines on the management and treatment of portal hypertension (Billroth III) Wien Klin Wochenschr. 2017;129(Suppl 3):135–158. doi: 10.1007/s00508-017-1262-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sutton H., Dhawan A., Grammatikopoulos T. Non-invasive markers of portal hypertension: appraisal of adult experience and potential utilisation in children. J Pediatr Gastroenterol Nutr. 2018;66(4):559–569. doi: 10.1097/MPG.0000000000001882. [DOI] [PubMed] [Google Scholar]
- 7.Eroglu Y., Emerick K.M., Whitingon P.F., Alonso E.M. Octreotide therapy for control of acute gastrointestinal bleeding in children. J Pediatr Gastroenterol Nutr. 2004;38(1):41–47. doi: 10.1097/00005176-200401000-00011. [DOI] [PubMed] [Google Scholar]
- 8.van Heurn L.W., Saing H., Tam P.K. Portoenterostomy for biliary atresia: long-term survival and prognosis after esophageal variceal bleeding. J Pediatr Surg. 2004;39(1):6–9. doi: 10.1016/j.jpedsurg.2003.09.019. [DOI] [PubMed] [Google Scholar]
- 9.Carneiro de Moura M., Chen S., Kamath B.M., Ng V.L., Ling S.C. Acute variceal bleeding causes significant morbidity. J Pediatr Gastroenterol Nutr. 2018;67(3):371–376. doi: 10.1097/MPG.0000000000002039. [DOI] [PubMed] [Google Scholar]
- 10.Chaudhary N., Mehrotra S., Srivastava M., Nundy S. Management of bleeding in extrahepatic portal venous obstruction. Int J Hepatol. 2013;2013 doi: 10.1155/2013/784842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Di Giorgio A., De Angelis P., Cheli M., Vajro P., Iorio R., Cananzi M., et al. Etiology, presenting features and outcome of children with non-cirrhotic portal vein thrombosis: a multicentre national study. Dig Liver Dis. 2019;51(8):1179–1184. doi: 10.1016/j.dld.2019.02.014. [DOI] [PubMed] [Google Scholar]
- 12.D’Souza R., Grammatikopoulos T., Pradhan A., Sutton H., Douiri A., Davenport M., et al. Acute-on-chronic liver failure in children with biliary atresia awaiting liver transplantation. Pediatr Transpl. 2019;23(2) doi: 10.1111/petr.13339. [DOI] [PubMed] [Google Scholar]
- 13.Groszmann R.J., Wongcharatrawee S. The hepatic venous pressure gradient: anything worth doing should be done right. Hepatology. 2004;39(2):280–282. doi: 10.1002/hep.20062. [DOI] [PubMed] [Google Scholar]
- 14.Augustin S., Millán L., González A., Martell M., Gelabert A., Segarra A., et al. Detection of early portal hypertension with routine data and liver stiffness in patients with asymptomatic liver disease: a prospective study. J Hepatol. 2014;60(3):561–569. doi: 10.1016/j.jhep.2013.10.027. [DOI] [PubMed] [Google Scholar]
- 15.Berzigotti A. Non-invasive evaluation of portal hypertension using ultrasound elastography. J Hepatol. 2017;67(2):399–411. doi: 10.1016/j.jhep.2017.02.003. [DOI] [PubMed] [Google Scholar]
- 16.Kim D.W., Yoon H.M., Jung A.Y., Lee J.S., Oh S.H., et al. Diagnostic performance of ultrasound elastography for evaluating portal hypertension in children: a systematic review and meta-analysis. J Ultrasound Med. 2019;38(3):747–759. doi: 10.1002/jum.14764. [DOI] [PubMed] [Google Scholar]
- 17.Tseng Y., Li F., Wang J., Chen S., Jiang W., Shen X., et al. Spleen and liver stiffness for noninvasive assessment of portal hypertension in cirrhotic patients with large esophageal varices. J Clin Ultrasound. 2018;46(7):442–449. doi: 10.1002/jcu.22635. [DOI] [PubMed] [Google Scholar]
- 18.Iwakiri Y., Groszmann R.J. Vascular endothelial dysfunction in cirrhosis. J Hepatol. 2007;46(5):927–934. doi: 10.1016/j.jhep.2007.02.006. [DOI] [PubMed] [Google Scholar]
- 19.Pomej K., Scheiner B., Balcar L., Nussbaumera R.J., Weinzierla J., Paternostro R., et al. Clinical significance of substantially elevated von Willebrand factor antigen levels in patients with advanced chronic liver disease. Dig Liver Dis. 2022 Oct;54(10):1376–1384. doi: 10.1016/j.dld.2022.06.010. [DOI] [PubMed] [Google Scholar]
- 20.Hametner S., Ferlitsch A., Ferlitsch M., Etschmaier A., Schöfl R., Ziachehabi A., et al. The VITRO score (von Willebrand factor antigen/thrombocyte ratio) as a new marker for clinically significant portal hypertension in comparison to other non-invasive parameters of fibrosis including ELF test. PLoS One. 2016 Feb;11(2) doi: 10.1371/journal.pone.0149230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.D’Antiga L., Betalli P., De Angelis P., Davenport M., Di Giorgio A., McKiernan P., et al. Interobserver agreement on endoscopic classification of oesophageal varices in children. J Pediatr Gastroenterol Nutr. 2015 Aug;61(2):176–181. doi: 10.1097/MPG.0000000000000822. [DOI] [PubMed] [Google Scholar]
- 22.Sutton H., Fitzpatrick E., Davenport M., Burford C., Alexander E., Dhawan A., et al. Transient elastography measurements of spleen stiffness as a predictor of clinically significant varices in children. J Pediatr Gastroenterol Nutr. 2018;67(4):446–451. doi: 10.1097/MPG.0000000000002069. [DOI] [PubMed] [Google Scholar]
- 23.de Franchis R., VI Faculty Baveno. Expanding consensus in portal hypertension: report of the Baveno VI Consensus Workshop: stratifying risk and individualizing care for portal hypertension. J Hepatol. 2015;63(3):743–752. doi: 10.1016/j.jhep.2015.05.022. [DOI] [PubMed] [Google Scholar]
- 24.Deep A., Saxena R., Jose B. Acute kidney injury in children with chronic liver disease. Pediatr Nephrol. 2019;34(1):45–59. doi: 10.1007/s00467-018-3893-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.La Mura V., Reverter J.C., Flores-Arroyo A., Raffa S., Reverter E., Seijo S., et al. Von Willebrand factor levels predict clinical outcome in patients with cirrhosis and portal hypertension. Gut. 2011;60(8):1133–1138. doi: 10.1136/gut.2010.235689. [DOI] [PubMed] [Google Scholar]
- 26.Bellot P., García-Pagán J.C., Francés R., Abraldes J.G., Navasa M., Pérez-Mateo M., et al. Bacterial DNA translocation is associated with systemic circulatory abnormalities and intrahepatic endothelial dysfunction in patients with cirrhosis. Hepatology. 2010;52(6):2044–2052. doi: 10.1002/hep.23918. [DOI] [PubMed] [Google Scholar]
- 27.Jachs M., Reiberger T. Prevention of variceal bleeding and rebleeding by nonselective beta-blockers: a tailored approach. Clin Liver Dis. 2021;25(2):311–326. doi: 10.1016/j.cld.2021.01.004. [DOI] [PubMed] [Google Scholar]
- 28.Jachs M., Hartl L., Simbrunner B., Bauer D., Paternostro R., Scheiner B., et al. Decreasing von Willebrand factor levels upon nonselective beta blocker therapy indicate a decreased risk of further decompensation, acute-on-chronic liver failure, and death. Clin Gastroenterol Hepatol. 2022;20(6):1362–1373 e6. doi: 10.1016/j.cgh.2021.07.012. [DOI] [PubMed] [Google Scholar]
- 29.Lancellotti S., Basso M., Veca V., Sacco M.M., Riccardi L., Pompili M., et al. Presence of portal vein thrombosis in liver cirrhosis is strongly associated with low levels of ADAMTS-13: a pilot study. Intern Emerg Med. 2016;11(7):959–967. doi: 10.1007/s11739-016-1467-x. [DOI] [PubMed] [Google Scholar]
- 30.Raffa S., Reverter J.C., Seijo S., Tassies D., Abraldes J.G., Bosch J., et al. Hypercoagulability in patients with chronic noncirrhotic portal vein thrombosis. Clin Gastroenterol Hepatol. 2012;10(1):72–78. doi: 10.1016/j.cgh.2011.09.010. [DOI] [PubMed] [Google Scholar]
- 31.Zou Z., Yan X., Li C., Li X., Ma X., Zhang C., et al. von Willebrand factor as a biomarker of clinically significant portal hypertension and severe portal hypertension: a systematic review and meta-analysis. BMJ Open. 2019 Aug;9(8) doi: 10.1136/bmjopen-2018-025656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Islek A., Ilhan D., Ozturk N., Guven B., Sag E. Altered von Willebrand factor and ADAMTS13 levels in children with cirrhosis and extrahepatic portal hypertension. J Pediatr Hematol Oncol. 2021;43(7):e951–e956. doi: 10.1097/MPH.0000000000002038. [DOI] [PubMed] [Google Scholar]
- 33.Beattie W., Magnusson M., Hardikar W., Monagle P., Ignjatovic V. Characterization of the coagulation profile in children with liver disease and extrahepatic portal vein obstruction or shunt. Pediatr Hematol Oncol. 2017;34(2):107–119. doi: 10.1080/08880018.2017.1313919. [DOI] [PubMed] [Google Scholar]
- 34.Burak Özkan M., Bilgici M.C., Eren E., Caltepe G. Diagnostic accuracy of point shear wave elastography in the detection of portal hypertension in pediatric patients. Diagn Interv Imaging. 2018;99(3):151–156. doi: 10.1016/j.diii.2017.10.009. [DOI] [PubMed] [Google Scholar]
- 35.Goldschmidt I., Brauch C., Poynard T., Baumann U. Spleen stiffness measurement by transient elastography to diagnose portal hypertension in children. J Pediatr Gastroenterol Nutr. 2014;59(2):197–203. doi: 10.1097/MPG.0000000000000400. [DOI] [PubMed] [Google Scholar]
- 36.Tomita H., Ohkuma K., Masugi Y., Hosoe N., Hoshino K., Fuchimoto Y., et al. Diagnosing native liver fibrosis and esophageal varices using liver and spleen stiffness measurements in biliary atresia: a pilot study. Pediatr Radiol. 2016;46(10):1409–1417. doi: 10.1007/s00247-016-3637-4. [DOI] [PubMed] [Google Scholar]
- 37.Vadlapudi S.S., Jagadisan B., Ananthkrishnan R., Narayanaswamy S. Splenic stiffness and platelet count to predict varices needing treatment in pediatric extrahepatic portal vein obstruction. Indian J Gastroenterol. 2020;39(6):576–583. doi: 10.1007/s12664-020-01099-8. [DOI] [PubMed] [Google Scholar]
- 38.de Franchis R., Bosch J., Garcia-Tsao G., Reiberger T., Ripoll C., Baveno VII Faculty Baveno VII - renewing consensus in portal hypertension. J Hepatol. 2022;76(4):959–974. doi: 10.1016/j.jhep.2021.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Singh R., Wilson M.P., Katlariwala P., Murad M.H., McInnes M.D.F., Low G. Accuracy of liver and spleen stiffness on magnetic resonance elastography for detecting portal hypertension: a systematic review and meta-analysis. Eur J Gastroenterol Hepatol. 2021;32(2):237–245. doi: 10.1097/MEG.0000000000001724. [DOI] [PubMed] [Google Scholar]
- 40.Duché M., Ducot B., Ackermann O., Guérin F., Jacquemin E., Olivier Bernard. Portal hypertension in children: high-risk varices, primary prophylaxis and consequences of bleeding. J Hepatol. 2017;66(2):320–327. doi: 10.1016/j.jhep.2016.09.006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
:
Data Availability Statement
The datasets analysed in this study are not publicly available.