Abstract
Chinese herbs have been used as feed additives and are commonly utilized in domestic intensive livestock farming. However, their impact on the production performance and intestinal health of broiler breeders has yet to be thoroughly explored. This study aimed to evaluate the effects of a Chinese herbal mixture (CHM) on the production performance of broiler breeders in terms of reproductive hormones, antioxidant capacity, immunity, and intestinal health of broiler breeders. A total of 336 thirty-wk-old hens were randomly allotted to 4 groups with 6 replicates of fourteen hens each, which fed a basal diet supplemented with 0 (CON), 500 (CHM500), 1,000 (CHM1000), and 1,500 (CHM1500) mg/kg CHM for 56 days, respectively. Our results showed that dietary supplementation with CHM1000 increased the laying rate and number of SYF and decreased the feed conversion ratio (P < 0.05). All CHM groups increased oviduct and ovarian indexes, serum E2 and T-AOC levels, and decreased serum TG and MDA levels compared with CON (P < 0.05). In comparison to the CON group, the CHM1000 and CHM1500 groups increased serum ALB, IgM, and IL-10 levels, whereas the CHM1000 group also increased serum TP and SOD levels, and the CHM1500 group increased serum P and decreased serum TNF-α (P < 0.05). The addition of CHM increased FSHR expressions in the ovary, Claudin-1 expressions in the jejunum, and SOD1 expressions in the liver and ovary, but decreased the mRNA expressions of INH in the ovary as well as IL-2 and IL-6 expressions in the jejunum (P < 0.05). Moreover, CHM500 and CHM1000 groups increased CAT, GPx, and HO-1 expression in the ovary, and SOD1 and GPx expression in the jejunum, while decreasing IL-17A expression in the jejunum (P < 0.05). In addition, CHM1000 and CHM1500 groups increased villus height, VCR, and the mRNA expressions of Nrf2, HO-1, Occludin, and MUC2 in the jejunum, and IL-10 expression in the ovary, while decreasing IL-2 and IL-17A expression in the ovary, in addition to increasing GPx, Nrf2, HO-1, NQO1, and IL-10 expression in the liver (P < 0.05). Supplementation with CHM1000 increased ESR-α, ESR-β, GnRH, Nrf2, and NQO1 expression in the ovary, but decreased IFN-γ expression in the ovary as well as crypt depth in the jejunum (P < 0.05). Supplementing CHM1500 increased NQO1 and ZO-1 expression in the jejunum and decreased IL-2 in the liver (P < 0.05). The high-throughput sequencing results showed that dietary CHM1000 supplementation altered the composition of the intestinal microbiota, as evidenced by the regulation of the genera Lactobacillus, Faecalibacterium, and Phascolarctobacterium. PICRUSt analysis revealed that metabolic pathways of bacterial chemotaxis, butanoate metabolism, and synthesis and degradation of ketone bodies were enriched in the CHM1000 group. Spearman's correlation analysis indicated that the differentiated genera were significantly associated with the production performance, serum hormone, and gut barrier-related genes. Taken together, supplementation of CHM, especially at 1,000 mg/kg, could improve production performance by regulating reproductive hormones, antioxidant capacity, immunity, and intestinal health of broiler breeders, and maybe provide insights into its application as a potential feed additive to promote the performance of broiler breeders.
Key words: broiler breeder, Chinese herbal mixture, production performance, reproductive hormone, intestinal microbiota
INTRODUCTION
With the continuous development of the modern intensive and large-scale poultry industry, laying hens are threatened by more stress diseases, including heat stress (Li et al., 2020), oxidative stress (Jian et al., 2022), and immune stress (Ploegaert et al., 2007; Matur et al., 2016), which may lead to slower growth, inflammation, a decreased egg production rate, and egg quality. In the past, antibiotics were used not only for the treatment of bacterial infections but also as feed additives in animal feeding processes (Uddin et al., 2021). However, the subsequent implementation of the policy restricting antibiotics for livestock prompted us to look for safe and effective feed additives to maintain animal health and promote the efficient production of laying hens (Seidavi et al., 2022). Natural plant (Chinese herbal medicine) extracts are known for their multicomponent, no known bacterial resistance, and low toxicity (Pan and Kong, 2018; Eldin et al., 2023).
The specific Chinese herbal mixture (CHM) used in this study consisted of Epimedium, Angelica sinensis, Rehmannia glutinosa, Bupleurum chinense, Peri-carpium citri reticulatae, Leonurus japonicus, Paeonia lactiflora, Eucommia ulmoides, Lycium barbarum, Codonopsis pilosula, and Astragalus membranaceus. Epimedium is one of the important components of the formula CHM, which has the function of regulating sex hormone levels and improving sexual dysfunction (Ma et al., 2011; Zhao et al., 2019). Flavonoids is reported as a major active component in Epimedium, which may be related to its ability to regulate estrogen metabolism. For instance, flavonoid icariin has been found to elevate estrogen levels and activate estrogen receptor alpha (ERα) (Yan et al., 2008). It is well known that normal reproductive function is inseparable from the regulation of reproductive hormones such as gonadotropin-releasing hormone (GnRH), Luteinizing hormone (LH), and follicle stimulating hormone (FSH) (Reddy et al., 2016; Hanafy and Elnesr, 2021). Nonlaying hens were considered to be associated with a reduction in serum LH and LYF and SYF numbers, which was reversed after GnRH injection (Hanafy and Elnesr, 2021). In addition, Angelica sinensis, Bupleurum chinense, Leonurus japonicus, and Paeonia lactiflora have traditionally been used to replenish, invigorate blood circulation and treat menstrual disorder (Liu et al., 2022). Consistently, Angelica sinensis and Paeonia lactiflora have been used in clinical to treat polycystic ovary syndrome (Zeng et al., 2022; Gao et al., 2023). Paeonia lactiflora extract can reduce the over-production of testosterone induced by dexamethasone in theca cells (Ong et al., 2019). Leonurus japonicus has been used in traditional Chinese medicine for 2,000 years to treat obstetrical and gynecological diseases, such as menstrual disorder, dysmenorrhea, and amenorrhea (Miao et al., 2019; Kang et al., 2021). The active compounds, terpenoids and alkaloids are thought to be closely related to the traditional functions of Leonurus japonicus (Miao et al., 2019). For example, leonurine has been identified with a variety of pharmacological activities including the treatment of cardiovascular, nervous, and uterus diseases (Miao et al., 2019; Li et al., 2020). Peri-carpium citri reticulatae and Lycium barbarum are common herbals that have the concomitant function of both medicine and foodstuff (Sun et al., 2021). Codonopsis pilosula and Astragalus membranaceus have traditionally been used as qi-regulating drug and have numerous beneficial effects, such as boosting immune and antioxidant function (Fu et al., 2014; Luan et al., 2021).
In general, a combination of herbs is considered more beneficial to the host than a single herb (Lan et al., 2017). Previous researches have shown that adding herbs (Astragalus, Epimedium, and Ligustrum lucidum) can improve the production performance and antioxidant capacity of pigeons (Zhang et al., 2023). A recent study has found that the specific compound Chinese herbal medicine additive (Astragalus, Licorice, and Radix Codonopsis) improve the performance parameters of Zi goose (Zheng et al., 2022). Moreover, synergistic interaction between the bioactive constituents of herbal mixtures has been demonstrated in vivo and in vitro (Bag and Chattopadhyay, 2015; Xie et al., 2021). Recent research also demonstrates that herbs in the diet can increase egg production and egg quality in laying hens (Yu et al., 2023). Besides, in poultry production, Chinese herbal, including compound formulas, extracts, and active compounds of herbs, are reported to have effective antioxidant activity and can regulate immunity and gut microbiota, improving the function of the intestinal barrier (Ding et al., 2022; Giannenas et al., 2022). The beneficial effects of Chinese herbals are largely accomplished through the regulation of the gut microbiota, which plays a vital role in maintaining gut health and influencing the overall production performance of laying hens (An et al., 2019; Wang et al., 2020). Gut microbiota homeostasis is an important prerequisite to ensure the growth and production performance of laying hens, which is supported by the various bacteria transplantation experiments (Liu et al., 2022; Cao et al., 2023).
Wenchang chicken is famous for its coarse grain resistance, heat resistance, and excellent meat quality (Tan et al., 2019). However, it should be noted that Wenchang chicken is an indigenous Chinese chicken breed with relatively low in egg production (Yang et al., 2016). To the best of our knowledge, little information is available about the effect of Chinese herbal mixtures as feed additives on reproductive hormones, immunity, and intestinal health in local broiler breeders. Therefore, this study used CHM as feed additives and investigated the effects of different levels of CHM on production performance, reproductive hormones, antioxidant capacity, immunity, and intestinal health of broiler breeders were evaluated.
MATERIALS AND METHODS
Preparation and Composition of Chinese Herbal Mixture
The type of CHM used in this study consisted of Epimedium, Angelica sinensis, Rehmannia glutinosa, Bupleurum chinense, Pericarpium citri reticulatae, Leonurus japonicus, Paeonia lactiflora, Eucommia ulmoides, Lycium barbarum, Codonopsis pilosula, and Astragalus membranaceus in the ratio of 2:2:1:1:1:3:1:2:2:2:2. The CHM was supplied by Anguo Qi'an Pharmaceutical Co. Ltd. (Hebei, China). The proximate chemical composition (crude protein, ether extract, crude fiber, ash, total phosphorus, and calcium) of CHM was measured according to AOAC methods (AOAC International, 2010). The detailed nutritional levels are presented in Table S1.
Experimental Birds, Diets, and Management
All experimental protocols were approved by the Animal Care and Use Committee of the South China Agricultural University (approval number: SYXK 2019–0136, Guangzhou, China).
A total of 336 healthy Wenchang broiler breeders (30-wk-old) were randomly and equally divided into 4 treatments with 6 replicates of 14 breeder hens. Hens were provided by Enping Jilong Industrial Co., Ltd. (Jiangmen, China) and were housed and fed at the Enping Jilong hens production base (Jiangmen, China). Each treatment group received a different level of CHM supplementation (CHM was mixed with the diet), namely the CON (a basal diet), CHM500 (basal diet + 500 mg/kg CHM), CHM1000 (basal diet + 1,000 mg/kg CHM), and CHM 1500 (basal diet + 1,500 mg/kg CHM) groups, respectively. The composition and ingredients of the basal diets are shown in Table S2. After 7 d of adaptation, all breeder hens were fed the assigned experimental diets for 56 d. Each hen is fed about 85 g diets daily (07:30) and given ad libitum access to water throughout the experiment. The lighting regimen used was a 16 h light and 8 h darkness cycle throughout the entire experimental period.
Sample Collection
On d 56, 6 laying hens were randomly selected from each group (one per replicate). After fasting for 12 h, blood samples (5 mL) were obtained from the wing vein and centrifuged at 3,000× g at 4°C for 10 min. The serum was stored at −80°C for further analysis. The hens were then euthanized by cervical dislocation and organ tissues (liver, oviduct, and ovarian) were removed, weighed, and calculated the organ index. Subsequently, 2 segments of each of the jejunum were excised and stored in a formalin solution for histological examination. Similarly, the tissue samples of the liver and ovarian and mucosal samples of the middle jejunum were collected and stored at −80°C for later testing. The digesta samples of the cecum were collected quickly collected on ice and then stored at −80°C for analysis of 16S RNA. The number of grade follicles, including small yellow follicles (8–10 mm in diameter) and preovulatory follicles (>10 mm in diameter), and oviduct length were measured.
Production Performance
The number of eggs, egg weight, feed consumption, and mortality were recorded during the experimental period every day. The egg production and average egg weight were calculated based on the total egg numbers and total egg weight.
Serum Biochemical Parameters
Serum concentrations of aspartate aminotransferase (ASL), alanine aminotransferases (ALT), triglycerides (TG), total cholesterol (TC), total protein (TP), albumen (ALB), glucose (GLU), calcium (Ca), and phosphorus (P) were measured using a Mindray BS-380 Fully Automated Biochemistry Analyzer (Shenzhen Mindray Bio-Medical Electronics Co., Ltd., Shenzhen, China) with the respective detection kits.
Determination of Serum Hormone, Antioxidant Capacity, and Immunologic Indices
The activities of total antioxidant capacity (T-AOC), and superoxide dismutase (SOD) as well as the concentration of malondialdehyde (MDA) in the serum by using commercial kits based on manufacturer's guidelines (Jiancheng Bioengineering Institute, Nanjing, Jiangsu, China). In parallel, serum 17β-estradiol (E2), FSH, immunoglobulin M (IgM), Interleukin-10 (IL-10), and tumor necrosis factor alpha (TNF-α) were measured using commercial ELISA kits, purchased from the Shanghai Mlbio Co., Ltd. (Shanghai, China). All measurements were performed at least in triplicate following the manufacturer's instructions.
Jejunal Villus Morphology
The tissue of jejunum was collected and fixed with 4% paraformaldehyde, embedded in paraffin, sliced, dehydrated, and stained with hematoxylin and eosin. The Image-Pro Plus (Media Cybernetics, Inc, Rockville, MD, USA) software was used to measure villus height (VH), crypt depth (CD), and the ratio of villus height to crypt depth (VCR).
Tissue RNA Extraction and qRT-PCR Analysis
Total RNA from the tissue (jejunal mucosa, liver, and ovary) was extracted using Trizol reagent (Vazyme Biotech Co., Ltd., Nanjing, China), along with a Takara PrimeScript RT Kit (Vazyme Biotech Co., Ltd.) was used to transcribe RNA into cDNA. ChamQ Universal SYBR qPCR Master Mix (Vazyme Biotech Co., Ltd.) was used for qRT-PCR following the manufacturer's instructions. All primer sequences for target genes are listed in Table S3. The fold changes were calculated after normalizing to the housekeeping gene β-actin, and the 2−ΔΔCt method was used to estimate mRNA abundance (Livak and Schmittgen, 2001).
16S rRNA Sequencing and Gut Microbiota Analysis
Gut microbiota from the collected cecum content samples were analyzed using bacterial 16S rRNA gene sequencing, as referenced in the previous study (Liu et al., 2023). DNA extraction from fecal samples was performed using a QIAamp DNA Stool Kit (Qiagen, Valencia, United States) according to the manufacturer's instructions. The V3-V4 region of the microbial 16S rRNA gene was amplified by PCR. The primer sequences were F: ACTCCTACGGGAGGCAGCA, R: GGACTACHVGGGTWTCTAAT. Sequencing was performed by Shanghai Paisano Biotechnology Co., Ltd. (Shanghai, China) using the Illumina MiSeq gene sequencing platform. Microbial analysis was performed using the Genes Cloud Platform (www.genescloud.cn).
Statistical Analysis
All data were analyzed by one-way analysis of variance (ANOVA) with Duncan's multiple comparison test using SPSS 22.0 statistical software (SPSS Institute Inc., Chicago, IL), and the GraphPad Prism 7.0 software (GraphPad, San Diego, CA) was used to generate graphs. Orthogonal polynomial comparisons were used to test the linear and quadratic responses to the CHM levels. P < 0.05 was considered statistically significant.
RESULTS
Effect of CHM on Production Performance
The production performance of broiler breeders fed different dietary supplements is presented in Table 1. Compared with the CON group, dietary CHM supplementation significantly increased the laying rate and decreased the feed conversion ratio but had no significant effect on the average egg weight or qualified egg ratio. Furthermore, broiler breeders fed with CHM1000 showed a higher laying rate and a lower feed conversion ratio than those fed without CHM (P < 0.05).
Table 1.
Effects of dietary CHM supplementation on the production performance in broiler breeders.
| Items1 | Groups |
SEM |
P value |
|||||
|---|---|---|---|---|---|---|---|---|
| CON | CHM500 | CHM1000 | CHM1500 | ANOVA | Linear | Quadratic | ||
| Laying rate (%) | 66.73b,c | 68.85a,b | 69.12a | 65.44c | 0.49 | 0.010 | 0.309 | 0.002 |
| Average egg weight (g) | 43.45 | 44.72 | 44.18 | 44.22 | 0.21 | 0.210 | 0.336 | 0.149 |
| Qualified egg ratio (%) | 87.76 | 88.45 | 88.26 | 86.97 | 0.86 | 0.943 | 0.760 | 0.601 |
| Feed conversion ratio (g/g) | 3.38a | 3.21b | 3.19b | 3.29a,b | 0.027 | 0.048 | 0.209 | 0.012 |
Means within a row lacking a common superscript differ (P < 0.05). Values are means ± SEM, n = 6.
CHM1000: basal diet supplemented with 1,000 mg/kg herbal mixture; CHM1500: basal diet supplemented with 1,500 mg/kg herbal mixture; CHM500: basal diet supplemented with 500 mg/kg herbal mixture; CON: control group, basal diet.
Effect of CHM on Reproductive Organs and Ovarian Follicle Development
As shown in Table 2, dietary CHM supplementation increased oviduct index, ovarian weight, ovarian index, and the number of SYF significantly (P < 0.05). Specifically, compared with the CON group, CHM1000, and CHM1500 could significantly increase the index of the oviduct and ovarian, while CHM1000 could significantly increase the ovarian weight and the number of SYF (P < 0.05).
Table 2.
Effects of dietary CHM supplementation on the reproductive organ and ovarian follicle development in broiler breeders.
| Items1 | Groups |
SEM |
P value |
|||||
|---|---|---|---|---|---|---|---|---|
| CON | CHM500 | CHM1000 | CHM1500 | ANOVA | Linear | Quadratic | ||
| Liver index (%) | 1.65 | 2.02 | 1.91 | 1.82 | 0.06 | 0.100 | 0.368 | 0.034 |
| Oviduct weight (g) | 43.12 | 47.57 | 46.96 | 47.84 | 0.80 | 0.121 | 0.055 | 0.241 |
| Oviduct index (%) | 2.19b | 2.42a,b | 2.47a | 2.53a | 0.05 | 0.034 | 0.007 | 0.308 |
| Oviduct length (cm) | 50.20 | 50.00 | 51.80 | 54.00 | 1.11 | 0.395 | 0.121 | 0.515 |
| Ovarian weight (g) | 35.25b | 42.21a | 42.95a | 38.21a,b | 1.03 | 0.014 | 0.210 | 0.003 |
| Ovarian index (%) | 1.79c | 2.15ab | 2.25a | 2.02b | 0.05 | 0.001 | 0.017 | 0.001 |
| Number of SYF | 15.40b | 15.60b | 23.4a | 19.20a,b | 1.06 | 0.010 | 0.018 | 0.198 |
| Number of POF | 5.00 | 6.20 | 6.60 | 5.40 | 0.29 | 0.181 | 0.516 | 0.041 |
Means within a row lacking a common superscript differ (P < 0.05). Values are means ± SEM, n = 6.
CHM1000: basal diet supplemented with 1,000 mg/kg herbal mixture; CHM1500: basal diet supplemented with 1,500 mg/kg herbal mixture; CHM500: basal diet supplemented with 500 mg/kg herbal mixture; CON: control group, basal diet; POF: preovulatory follicles; SYF: small yellow follicles.
Effect of CHM on Serum Biochemical Indices
The serum biochemical indices of the laying hens fed different dietary supplements are presented in Table 3. Compared with the CON group, dietary CHM supplementation significantly increased the level of ALB and decreased the level of TG in serum (P < 0.05). Specifically, dietary CHM1500 supplementation significantly increased the level of P in serum (P < 0.05). Meanwhile, the broiler breeders fed with CHM1000 showed lower levels of TG and higher levels of ALB and TP in serum than those fed without CHM (P < 0.05).
Table 3.
Effects of dietary CHM supplementation on the serum biochemical index in broiler breeders.
| Items1 | Groups |
SEM |
P value |
|||||
|---|---|---|---|---|---|---|---|---|
| CON | CHM500 | CHM1000 | CHM1500 | ANOVA | Linear | Quadratic | ||
| AST (U/L) | 221.66 | 232.02 | 241.30 | 223.86 | 6.30 | 0.715 | 0.791 | 0.307 |
| ALT (U/L) | 3.18 | 2.98 | 2.60 | 2.76 | 0.21 | 0.797 | 0.412 | 0.685 |
| ALB (g/L) | 10.66b | 12.16a,b | 13.00a | 12.58a | 0.31 | 0.027 | 0.011 | 0.078 |
| TP (g/L) | 42.72b | 49.52a,b | 54.72a | 46.32a,b | 1.62 | 0.043 | 0.212 | 0.014 |
| TC (mmol/L) | 3.76 | 3.25 | 3.90 | 3.20 | 0.24 | 0.684 | 0.647 | 0.853 |
| TG (mmol/L) | 13.35a | 7.80b | 6.50b | 6.55b | 1.02 | 0.036 | 0.012 | 0.122 |
| GLU (mmol/L) | 5.73 | 5.93 | 7.37 | 4.81 | 0.38 | 0.106 | 0.673 | 0.060 |
| Ca (mmol/L) | 3.71 | 3.82 | 3.87 | 3.52 | 0.10 | 0.673 | 0.600 | 0.303 |
| P (mmol/L) | 3.09b | 2.65b | 2.98b | 4.72a | 0.29 | 0.041 | 0.032 | 0.042 |
Means within a row lacking a common superscript differ (P < 0.05). Values are means ± SEM, n = 6.
ALB: albumin; AST: aspartate amino transferase; Ca: calcium; CHM1000: basal diet supplemented with 1,000 mg/kg herbal mixture; CHM1500: basal diet supplemented with 1,500 mg/kg herbal mixture; CHM500: basal diet supplemented with 500 mg/kg herbal mixture; CON: control group, basal diet; GLU: glucose; P: phosphorus; TC: total cholesterol; TG: triglyceride; TP: total protein.
Effect of CHM on Hormone Indices
As shown in Figure 1, dietary CHM supplementation significantly increased the serum levels of E2 (P < 0.05) but had no significant influence on FSH. Then, the expressions of hormone-related genes in the ovary were detected. Compared with the CON group, all CHM groups significantly increased the mRNA expression of follicle-stimulating hormone receptor (FSHR) and decreased that of INH (P < 0.05). Breeder hens fed CHM1000 had a higher level of ESR-α, ESR-β, and GnRH mRNA expression than the CON group (P < 0.05).
Figure 1.
Effects of dietary supplemented with CHM on hormone indices of broiler breeders. (A) serum E2; (B) serum FSH; (C-G) The expressions of hormone-related genes (ESR-α, ESR-β, FSHR, GnRH, and INH) in the ovary. Values are means ± SEM, n = 6. Means without a common letter differ, P < 0.05. Abbreviations: CHM, Chinese herbal mixture; E2, estradiol; ESR-α, estrogen receptor alpha; ESR-β, estrogen receptor beta; FSH, follicle stimulating hormone; FSHR, follicle-stimulating hormone receptor; GnRH, gonadotropin-releasing hormone; INH, inhibin.
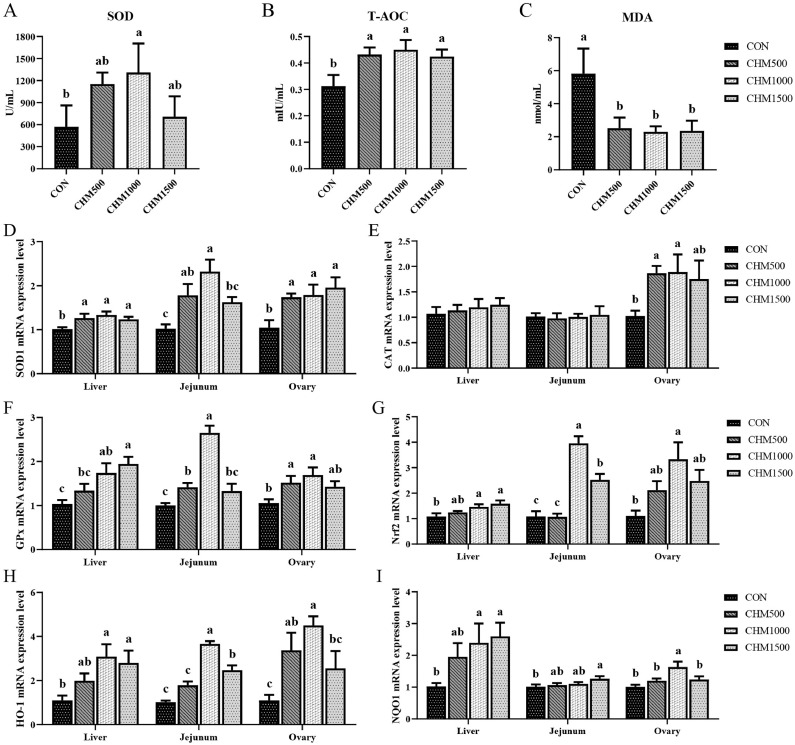
Effect of CHM on Antioxidant Capacity
As shown in Figure 2, the serum levels of SOD in the CHM1000 group significantly increased, while the serum levels of T-AOC and MDA in all CHM groups were found to be significantly increased and decreased, respectively (P < 0.05). Besides, the mRNA expressions of SOD1, GPx, Nrf2, HO-1, and NQO1 in the liver were significantly increased in the CHM1000 and CHM1500 groups (P < 0.05). In the jejunum, the mRNA expressions of SOD1 and GPx in CHM500, CHM1000 groups, Nrf2, HO-1 in CHM1000, CHM1500 groups, and NQO1 in CHM1500 group were significantly increased compared with the CON group (P < 0.05). In the ovary, the mRNA expressions of SOD1, GPx, CAT, and HO-1 in the CHM500, CHM1000 groups, Nrf2 and NQO1 in the CHM1000 group were significantly increased compared with the CON group (P < 0.05).
Figure 2.
Effects of dietary supplemented with CHM on antioxidant capacity of broiler breeders. (A) serum SOD, T-AOC, and MDA; (B-I) The expressions of antioxidant-related genes (SOD1, GPx, CAT, Nrf2, HO-1, and NQO1) in liver, jejunum, and ovary, respectively. Values are means ± SEM, n = 6. Means without a common letter differ, P < 0.05. Abbreviations: CAT, catalase; CHM, Chinese herbal mixture; GPx, glutathione peroxidase; HO-1, heme-oxygenase 1; MDA, malondialdehyde; NQO1, NAD(P)H quinone oxidoreductase 1; Nrf2, nuclear factor erythroid 2; SOD, superoxide dismutase; T-AOC, total antioxidant capacity.
Effect of CHM on Immune Function
As shown in Figure 3, dietary CHM1000 and CHM1500 supplementation significantly increased the serum levels of IgM and IL-10, and CHM1500 also decreased the serum level of TNF-α, significantly (P < 0.05). Moreover, the mRNA expression of IL-10 in the liver were increased significantly in CHM1000 and CHM1500 groups compared with the CON group (P < 0.05). Dietary CHM also decreased the mRNA expressions of IL-2 and IL-6 in the jejunum, meanwhile, the mRNA expressions of IL-17A in the CHM500, CHM1000 groups were decreased compared with the CON group (P < 0.05). In the ovary of the CHM1000 group, the mRNA expression of IL-10 significantly increased, while the mRNA expressions of IL-2, IL-17A, and IFN-γ decreased (P < 0.05).
Figure 3.
Effects of dietary supplemented with CHM on immune function in broiler breeders. (A) Serum IgM, IL-10, and TNF-α; (B–G) The mRNA expression of inflammatory genes (IL-2,IL-6,IL-10,IL-17A,IFN-γ) in liver, jejunum and ovary, respectively. Values are means ± SEM, n = 6. Means without a common letter differ, P < 0.05. Abbreviations: CHM, Chinese herbal mixture; IFN-γ, IFN-gamma; IgM, immunoglobulin M; IL-10, interleukin-10; IL-17A, interleukin-17A; IL-2, interleukin-2; IL-6, interleukin-6; TNF-α, tumor necrosis factor-alpha.
Effect of CHM on Intestinal Physical Barrier Function
As shown in Figure 4, dietary supplemented with CHM did not cause significant inflammation in the jejunal mucosa of broiler breeders. Besides, CHM1000 also significantly decreased crypt depth, increased villus height and VCR (P < 0.05). Compared with the CON group, the mRNA expression of CLDN-1, OCLN, and MUC-2 in jejunal mucosa were significantly increased in the CHM1000, CHM1500 groups, as well as ZO-1 in the CHM1500 group (P < 0.05).
Figure 4.
Effects of dietary supplemented with CHM on intestinal morphology and barrier genes in broiler breeders. (A) Representative images (×100) were stained with hematoxylin and eosin. (B) Villus height, crypt depth, and VCR of the jejunal mucosa. (C) The mRNA expressions of tight junction proteins and mucin (CLDN1, OCLN, ZO-1, and MUC-2) in jejunal mucosa. Values are means ± SEM, n = 6. Means without a common letter differ, P < 0.05. Abbreviations: CHM, Chinese herbal mixture; CLDN1, Claudin-1; MUC2, Mucin 2; OCLN, Occludin; ZO-1, Zonula occludens-1.
Microbial Diversity and Composition of the Cecal Digesta
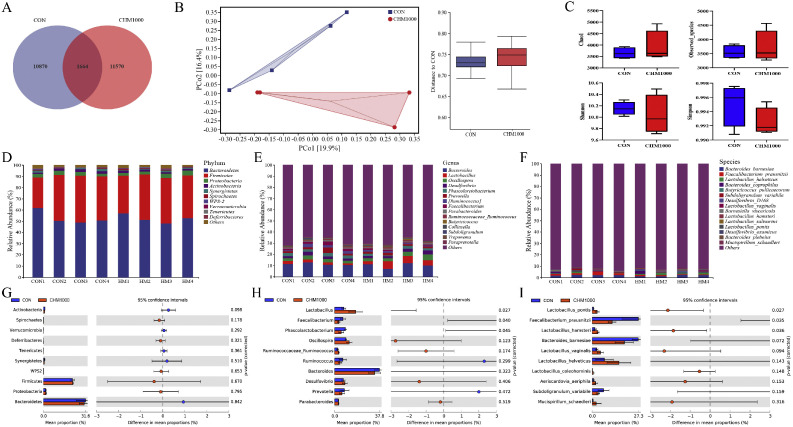
To investigate the effect of CHM on gut microbiota, we performed 16S rRNA gene sequencing on the fecal samples between CON and CHM1000 groups. After sequencing analysis, an average of 59,761 raw data per sample were retrieved and 44,561 high-quality sequences per sample were obtained after quality trimming and chimera checking. As shown in Figure 5A, there were 1,664 operational taxonomic units in the 2 groups of cecal microbiota. The specific operational taxonomic units of the CON group and CHM1000 group were 10, 870, and 11,570, respectively. The indicators of α-diversity (Figure 5C), including Chao 1, observed species, Shannon index, and Simpson index showed no significant treatment differences (P>0.05). Next, β diversity analysis was employed by Principal Coordinate Analysis using the Bray-Curtis index (Figure 5B) The Principal Coordinate Analysis scatterplot showed that CHM1000 could remarkably change the structure of the microbiota community in cecum. We then determined the relative abundances of phylum, genus, and species, respectively (Figures 5C–5E). Bacteria that were differentially enriched by T-test after CHM treatment are shown in Figures 5F to 5H. At the phylum level, Bacteroidetes, Firmicutes, Proteobacteria, and Actinobacteria were the major groups. At the genus level, Bacteroidetes, Lactobacillus, and Oscillospora were the common predominant bacteria. Lactobacillus Compared with the CON groups, supplementation with CHM increased the relative abundance of Lactobacillus (P < 0.05) but reduced the relative abundance of Faecalibacterium and Phascolarctobacterium (P < 0.05). Dietary CHM supplementation selectively enriched the abundance of Lactobacillus_pontis and Lactobacillus_hamsteri while Faecalibacterium_prausnitzii were enriched in the CON group (P < 0.05). Compared with the CON groups, the relative abundance of Bacteroides_barnesiae displayed a decreasing trend in the CHM group (P = 0.072), whereas the relative abundance of Lactobacillus_vaginalis had an upward trend in the CHM group (P = 0.094). Finally, a linear discriminant analysis (LDA) effect size (LEfSe) analysis was conducted to further identify the enriched microbial community from phylum to species between the CON and CHM1000 groups with an LDA score > 2.5 (Figures 6A and 6C). The results of LEfSe analysis showed that some bacterial groups including the family Veillonellaceae, the genera Faecalibacterium, and Phascolarctobacterium in the CON group had a higher score, while some other bacterial groups, such as the order Lactobacillales, the class Bacilli, the family Lactobacillaceae, the genera Lactobacillus, Clostridium in CHM1000 group had a higher score.
Figure 5.
Effects of dietary supplemented with CHM1000 on cecal microbiota diversity and composition in broiler breeders. (A) Veen diagram. (B) Principal component analysis (PCA) scatterplot and sample distance figure. (C) Bacterial alpha-diversity indices (Chao1, Observed_species, Shannon, and Simpson). The relative abundance of cecum microbiota at (D, G) phylum, (E, H) genus, and (F, F) species level. *P < 0.05, **P < 0.01 (n = 4).
Figure 6.
Changes in breeder hens cecal microbiota and functional prediction with CHM1000 supplementation. (A, C) LEfSe analysis and branching diagram of evolution scatterplot. (B) Functional prediction analysis. (D) Spearman's correlation analysis. *P < 0.05, **P < 0.01 (n = 4).
Functional Prediction of Cecal Microbiota
In order to predict the functional alterations of the cecal microbiota, a Phylogenetic Investigation of Communities by Reconstruction of Unobserved States was performed to analyze the possible levels of Kyoto Encyclopedia of Genes and Genomes pathways (Figure 6B). The prediction analysis identified 8 functional pathways between CON and CHM groups. Among the 8 pathways identified, broiler breeders fed with CHM1000 notably increased the microbial gene abundance of cell motility (bacterial chemotaxis), carbohydrate metabolism (butanoate metabolism), and lipid metabolism (synthesis and degradation of ketone bodies), while suppressed the microbial gene abundance of infectious diseases (epithelial cell signaling in Helicobacter pylori infection), amino acid metabolism (valine, leucine, and isoleucine biosynthesis), biosynthesis of other secondary metabolites (penicillin and cephalosporin biosynthesis), and carbohydrate metabolism (fructose and mannose metabolism and glyoxylate and dicarboxylate metabolism).
Correlations Between Cecal Microbiota and Different Indicators
The intestinal microbiota was closely associated with the production performance of broiler breeders. In this experiment, the results of spearman's correlation analysis of the cecal microbiota and other indicators such as production performance, antioxidant indicators, and gut barrier-related genes (Figure 6D) indicated that Lactobacillus showed a positive correlation with laying rate, villus height, VCR, and serum (E2, T-AOC, TP, IgM, and IL-10), as well as antioxidant-related genes (Nrf2, SOD1, HO-1, and GPx) and jejunal barrier-related genes (Claudin-1, Occludin, Mucin-2), but had a negative correlation with cytokine related genes (IL-2, IL-6, and IL-17A) expression in the jejunum. Interestingly, the genera Faecalibacterium and Phascolarctobacterium showed opposite trends compared to Lactobacillus, especially the latter. The genera Ruminococcaceae_Ruminococcus was positively correlated with ALT, SOD, GPx expression, villus height, Occludin expression, and Mucin-2 expression but negatively correlated with FCR, MDA, crypt depth, IL-2 expression, and IL-6 expression.
DISCUSSION
Natural plants contain several but less toxic bioactive components and are considered to be one of the natural alternatives to antibiotics in livestock and poultry fanning. In this study, the major active constituents of herbal we used have been reviewed in detail by other researchers. In general, polysaccharides, flavonoids and their derivatives, alkaloids, terpenoids, saikosaponin, volatile oil, lignans, etc. are thought be the major active constituents of CHM (Ma et al., 2011; He et al., 2014; Liu et al., 2019; Miao et al., 2019; Luan et al., 2021; Sun et al., 2021). Then, synergistic interaction between the bioactive components of CHM on the production performance of laying hens was evaluated in this study. Our results demonstrated that broiler breeders fed diets supplemented with CHM1000 significantly increased the laying rate and decreased the feed conversion ratio. Numerous herbs or their bioactive components have been utilized as supplements and additives that may help animals perform better in terms of growth performance.
Serum biochemical indices reflect the health and metabolic status of laying hens over time. Our results showed that different levels of CHM could increase the serum levels of ALB and TP and decrease that of TG. Among them, the performance of the CHM1000 group was prominent, indicating that CHM1000 can better regulate the protein metabolism and lipid metabolism of laying hens. Consistent with our results, Salvia miltiorrhiza polysaccharide was reported to have a good protective effect on chicken liver damage by increasing the serum levels of TP and ALB in vivo and in vitro (Han et al., 2019). In similar results, Astragali could enhance the anti-inflammatory effect via improving the albumen quality (Xie et al., 2019).
The laying rate is mainly associated with oviduct and intestine health (Nii, 2022). Previous studies supported that oviduct aging (oxidative stress) and infection not only reduce the laying rate but also influence eggs formation and result in the contamination of eggs (Raspoet et al., 2011; Wang et al., 2015). Besides, the development and maturation of follicle are also influenced by reproductive hormones. For instance, serum estrogen is positively related to the laying rate in laying hens (Liu et al., 2018). In this study, different levels of CHM significantly increased the ovarian index and the serum E2. The levels of plasma E2 are thought to be indicative of ovarian follicular development (Tanabe et al., 1979). Consistently, CHM1000 also significantly increased oviduct index, ovarian weight, and the number of SYF. Previous researches have shown that total flavonoids of Epimedium could improve hormone secretion and follicle development, promote the laying rate of laying hens (Guo et al., 2020). Both separately and in combination, daidzein and Chinese herbs improve laying efficiency and serum hormone contents in postpeak laying hens (Zhang et al., 2021). In addition, the bioactive ingredients such as flavonoids and Saikosaponin‑D could exert hormone regulatory capacity via activating ERα (Yan et al., 2008; Liu et al., 2019). The current study showed that dietary supplementation of CHM up-regulated the mRNA expressions of ESR-α, ESR-β, FSHR, and GnRH in the ovary. The FSHR plays a key role in promoting follicle maturation via the FSH-mediated cAMP pathway (Xia et al., 2020). Similar results were reported by Ding et al. (2021), who demonstrated that adding soy isoflavones increased E2 level in serum and the ESR-β expression in the ovary.
The inflammation response is an important defense of the body's immune system against external stimuli (Han and Ulevitch, 2005). Interleukin-10 is a multipotent cytokine with powerful anti-inflammatory properties that activates macrophages to release anti-inflammatory factors such as TNF-α (Saraiva and O'Garra, 2010). IL-2 is an important immunomodulatory cytokine and has dual regulatory functions by maintaining Treg cells and stimulating conventional T cells (Abbas et al., 2018). In the present study, CHM1000 and CHM1500 could enhance the immune of laying hens by increasing serum IgM, IL-10, and reducing inflammatory factors. It was reported that feeding Astragalus polysaccharide to chicks can improve their growth performance and raise the contents of immunoglobulins like IgG, IgM, and IgA in the serum (Wu, 2018). In addition, dietary CHM1000 supplementation significantly increased serum levels of IL-10 and its expression in the liver and ovary and decreased IL-2 and IL-17A expression in the jejunum and ovary. The results could be in agreement with that of Song et al. (2023), who reported that traditional Chinese prescription can promote immune functions.
Studies suggest that herbal medicine may play an antioxidant role by upregulating the mRNA expressions of antioxidant enzymes. Consistent with previous studies, our results indicated that CHM supplementation can show higher antioxidant capacity by increasing the serum levels of T-AOC and SOD and decreasing those of MDA. Previous studies have shown that supplementation with Leonurus sibiricus increases the activity of antioxidant enzymes, and reduces the serum levels of MDA in broilers (Yang et al., 2019). Besides, the combination of these plant extracts (Lonicera japonica, Astragalus membranaceus, Eucommia folium, and Codonopsis pilosula) have been reported with antioxidant and anti-inflammatory activities (Wang et al., 2021). The addition of CHM1000 to the diet significantly increased the mRNA expressions of antioxidant indexes (SOD1, GPx, Nrf2, HO-1, and NQO1) in the liver, jejunum, and ovary. The effectiveness of CHM may be attributed to its antioxidant activity through activating the Nrf2 signaling pathway (Luo et al., 2021). Based on the above results, the supplementation of CHM (especially CHM1000) to the diet can enhance the antioxidant capability of broiler breeders.
It is well known that good egg production performance is inseparable from a healthy gut. The gut is a congenital barrier that maintains the balance of the intestinal environment and prevents pathogenic bacteria and toxins. Disruption of the intestinal environment could cause intestinal inflammation, thus reducing the growth performance and laying rate of laying hens (Nii et al., 2020). The result of HE staining showed that dietary supplemented with CHM did not cause significant inflammation in the jejunal mucosa of broiler breeders. Besides, CHM1000 could promote nutrient absorption by decreased crypt depth, increased villus height, and VCR. Previous researches have shown that feeding of ducklings with Astragalus polysaccharides improved intestinal morphology (Liao et al., 2021). Mucus and intercellular tight junctions act together to maintain the integrity of the gut barrier. Our results showed that CHM also increased the mRNA expression of CLDN-1, OCLN, ZO-1, and MUC-2 in the jejunal mucosa. Previous research showed that the coexisting components in herbal mixtures improved solubility and increased the membrane permeability of enterocytes, promoting the intestinal absorption of active components (Zhao et al., 2020). The above observations indicated that the improvement in egg performance by CHM might be attributed to maintaining gut homeostasis, and enhancing the gut function in hens.
Some studies have found that dietary intervention has been demonstrated to have a significant impact on regulating the structure and function of the intestinal microbiome community (Leeming et al., 2019). A larger number of microbes live in the intestine, and their composition and richness have been found to correlate with the growth and reproduction of hens (Diaz-Sanchez et al., 2019). Our results demonstrated that different levels of CHMs have good antioxidant and anti-inflammatory capacities and can improve oviduct and intestine health, especially CHM1000. Thus, we further evaluated the structure of the caecal microbiota in the CHM1000 group. The principal component analysis scatterplot showed that CHM1000 remarkably changed the structure of the microbiota, which may be related to gut homeostasis. Besides, the genera Lactobacillus and species Lactobacillus_pontis, Lactobacillus_hamsteri, and Lactobacillus_coleohominis were significantly increased after supplementing with CHM. The class Bacilli, the genera Lactobacillus, and Clostridium were the signature taxa of the CHM group determined using LEfSe (LDA > 2.5). Microorganisms often employed as probiotics in the poultry industry include colonizing Bacillus, Lactobacillus, and Clostridium species. Lactobacillus was considered a beneficial bacterium and could increase the feeding efficiency in chickens (Yan et al., 2017). Microbiota such as Clostridium was known to be important producers of SCFAs (Yao et al., 2020), and their higher relative abundances improve intestine absorption and utilization of nutrients (He et al., 2019). Bacillus sp. has also been shown to have the ability to improve nutrient utilization and intestine morphology (Souza et al., 2021). Furthermore, the chemical components of Chinese herbal medicines, such as saponins and flavonoids, can enhance the beneficial bacteria (Lactobacillus and Clostridium), thereby improving the integrity of the intestinal mucosa and stimulating the intestinal immune system (Bai and Li, 2022).
PICRUSt analysis revealed that the CHM1000 group enriched metabolic pathways of bacterial chemotaxis and butanoate metabolism. Butyric acid is considered to be a crucial energy source in the intestines and plays a significant role in supporting intestinal development and preserving the integrity of intestinal epithelial cells (Sun and O'Riordan, 2013). Butyrate also promotes the production of mucin, consistent with the result that the CHM could increase villus height and up-regulate CLDN-1, OCLN, ZO-1, and MUC-2 expressions. Moreover, spearman's correlation analysis showed Lactobacillus showed a positive correlation with laying rate, TP, E2, IgM, IL-10, and villus height, as well as antioxidant indicators and gut barrier-related genes, and a negative correlation with IL-2, IL-6, and IL-17A of jejunum. The immunomodulatory and antioxidant effects of CHM may be at least partially through modulating of intestinal microbiota. A similar result was found in which Traditional Chinese Herbal Feed Additive maybe relative the abundance of Lactobacillus to promote the antioxidant capacity and immunity of laying hens (Liu et al., 2023). Lactobacillus also can stimulate immune cell activity by producing lactate to stimulate immune cell activity (Wei et al., 2022). Most members of the Ruminococcaceae family produce SCFAs, which play an important role in improving feed efficiency and enhancing host health (Wong et al., 2006; Liu et al., 2022). Our results showed that the Ruminococcaceae_Ruminococcus was positively correlated with villus height, Occludin expression, and Mucin-2 expression. According to the above results, it was inferred that dietary supplementation with CHM could improve production performance and gut health, which was attributed to the increase of potentially beneficial bacteria abundance and enhanced the interaction between bacteria. Therefore, the changes in the composition and diversity of intestinal microbiota following dietary supplementation with CHM could be attributed to the rich content of active ingredients such as polysaccharides, polyphenols, organic acids, and so on, but its specific mechanism needs to be investigated further.
CONCLUSIONS
In conclusion, the results demonstrated that dietary supplementation with supplementation of CHM (Epimedium, Angelica sinensis, Rehmannia glutinosa, Bupleurum chinense, Pericarpium citri reticulatae, Leonurus japonicus, Paeonia lactiflora, Eucommia ulmoides, Lycium barbarum, Codonopsis pilosula, and Astragalus membranaceus) can improve production performance of broiler breeders. These improvements are associated with changes in ovarian follicle development, reproductive hormones, antioxidant capacity, immune function, intestinal morphology, and microbiota. Especially, dietary addition of 1,000 mg/kg (CHM1000) for the Chinese herbal mixture used in this study is recommended for Wenchang broiler breeders.
ACKNOWLEDGMENTS
This work was supported by the National Key Research and Development Program (2022YFD1801103) and Hainan Province's Key Research and Development Project (ZDYF2023XDNY067) and “Quancheng Scholars” Construction Project (2021).
DISCLOSURES
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.psj.2023.103201.
Appendix. Supplementary materials
REFERENCES
- Abbas A.K., Trotta E., S D.R., Marson A., Bluestone J.A. Revisiting IL-2: biology and therapeutic prospects. Sci. Immunol. 2018;3:eaat1482. doi: 10.1126/sciimmunol.aat1482. [DOI] [PubMed] [Google Scholar]
- An X., Bao Q., Di S., Zhao Y., Zhao S., Zhang H., Lian F., Tong X. The interaction between the gut microbiota and herbal medicines. Biomed. Pharmacother. 2019;118 doi: 10.1016/j.biopha.2019.109252. [DOI] [PubMed] [Google Scholar]
- AOAC International . 18th ed. AOAC International; Maryland, USA: 2010. Official Methods of Analysis of AOAC International. [Google Scholar]
- Bag A., Chattopadhyay R.R. Evaluation of synergistic antibacterial and antioxidant efficacy of essential oils of spices and herbs in combination. PLoS One. 2015;10 doi: 10.1371/journal.pone.0131321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai J., Li Y. Effects of Chinese herbal medicines on the productive performance, nutrient retention, egg quality, immune function of laying hens. Biologia (Bratisl) 2022;77:385–393. [Google Scholar]
- Cao S., Guo D., Yin H., Ding X., Bai S., Zeng Q., Liu J., Zhang K., Mao X., Wang J. Improvement in ovarian function following fecal microbiota transplantation from high-laying rate breeders. Poult. Sci. 2023;102 doi: 10.1016/j.psj.2022.102467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz-Sanchez S., Perrotta A.R., Rockafellow I., Alm E.J., Okimoto R., Hawken R., Hanning I. Using fecal microbiota as biomarkers for predictions of performance in the selective breeding process of pedigree broiler breeders. PLoS One. 2019;14 doi: 10.1371/journal.pone.0216080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding X., Cai C., Jia R., Bai S., Zeng Q., Mao X., Xu S., Zhang K., Wang J. Dietary resveratrol improved production performance, egg quality, and intestinal health of laying hens under oxidative stress. Poult. Sci. 2022;101 doi: 10.1016/j.psj.2022.101886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding X.Q., Yuan C.C., Huang Y.B., Jiang L., Qian L.C. Effects of phytosterol supplementation on growth performance, serum lipid, proinflammatory cytokines, intestinal morphology, and meat quality of white feather broilers. Poult. Sci. 2021;100 doi: 10.1016/j.psj.2021.101096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eldin A.B., Ezzat M., Afifi M., Sabry O., Caprioli G. Herbal medicine: the magic way crouching microbial resistance. Nat. Prod. Res. 2023;38:1–10. doi: 10.1080/14786419.2023.2172009. [DOI] [PubMed] [Google Scholar]
- Fu J., Wang Z., Huang L., Zheng S., Wang D., Chen S., Zhang H., Yang S. Review of the botanical characteristics, phytochemistry, and pharmacology of Astragalus membranaceus (Huangqi) Phytother. Res. 2014;28:1275–1283. doi: 10.1002/ptr.5188. [DOI] [PubMed] [Google Scholar]
- Gao Y., Mo S., Cao H., Zhi Y., Ma X., Huang Z., Li B., Wu J., Zhang K., Jin L. The efficacy and mechanism of Angelica sinensis (Oliv.) Diels root aqueous extract based on RNA sequencing and 16S rDNA sequencing in alleviating polycystic ovary syndrome. Phytomedicine. 2023;120 doi: 10.1016/j.phymed.2023.155013. [DOI] [PubMed] [Google Scholar]
- Giannenas I., Sakkas P., Papadopoulos G.A., Mitsopoulos I., Stylianaki I., Dokou S., Tsiouris V., Papagrigoriou T., Panheleux M., Robert F., Bampidis V.A. The association of Curcuma and Scutellaria plant extracts improves laying hen thermal tolerance and egg oxidative stability and quality under heat stress conditions. Front. Vet. Sci. 2022;9 doi: 10.3389/fvets.2022.957847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Y., Li Y., Zhang S., Wu X., Jiang L., Zhao Q., Xue W., Huo S. The effect of total flavonoids of Epimedium on granulosa cell development in laying hens. Poult. Sci. 2020;99:4598–4606. doi: 10.1016/j.psj.2020.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han C., Wei Y., Wang X., Ba C., Shi W. Protective effect of Salvia miltiorrhiza polysaccharides on liver injury in chickens. Poult. Sci. 2019;98:3496–3503. doi: 10.3382/ps/pez153. [DOI] [PubMed] [Google Scholar]
- Han J., Ulevitch R.J. Limiting inflammatory responses during activation of innate immunity. Nat. Immunol. 2005;6:1198–1205. doi: 10.1038/ni1274. [DOI] [PubMed] [Google Scholar]
- Hanafy A.M., Elnesr S.S. Induction of reproductive activity and egg production by gonadotropin-releasing hormone in non-laying hens. Reprod. Domest. Anim. 2021;56:1184–1191. doi: 10.1111/rda.13972. [DOI] [PubMed] [Google Scholar]
- He J., Guo H., Zheng W., Xue Y., Zhao R., Yao W. Heat stress affects fecal microbial and metabolic alterations of primiparous sows during late gestation. J. Anim. Sci. Biotechnol. 2019;10:84. doi: 10.1186/s40104-019-0391-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He X., Wang J., Li M., Hao D., Yang Y., Zhang C., He R., Tao R. Eucommia ulmoides oliv.: ethnopharmacology, phytochemistry and pharmacology of an important traditional Chinese medicine. J. Ethnopharmacol. 2014;151:78–92. doi: 10.1016/j.jep.2013.11.023. [DOI] [PubMed] [Google Scholar]
- Jian H., Xu Q., Wang X., Liu Y., Miao S., Li Y., Mou T., Dong X., Zou X. Amino acid and fatty acid metabolism disorders trigger oxidative stress and inflammatory response in excessive dietary valine-induced NAFLD of laying hens. Front. Nutr. 2022;9 doi: 10.3389/fnut.2022.849767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang E.Y., Kim H., Jung J., Kim J., Woo T., Choi J., Kim J., Ahn C., Lee H., Go G. Combined extract of Leonurus japonicus Houtt, Eclipta prostrata L., and Pueraria lobata Ohwi improved hot flashes and depression in an ovariectomized rat model of menopause. Foods. 2021;10:180. doi: 10.3390/foods10010180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lan R.X., Park J.W., Lee D.W., Kim I.H. Effects of Astragalus membranaceus, Codonopsis pilosula and allicin mixture on growth performance, nutrient digestibility, faecal microbial shedding, immune response and meat quality in finishing pigs. J. Anim. Physiol. Anim. Nutr. (Berl) 2017;101:1122–1129. doi: 10.1111/jpn.12625. [DOI] [PubMed] [Google Scholar]
- Leeming E.R., Johnson A.J., Spector T.D., Le Roy C.I. Effect of diet on the gut microbiota: rethinking intervention duration. Nutrients. 2019;11:2862. doi: 10.3390/nu11122862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li G.M., Liu L.P., Yin B., Liu Y.Y., Dong W.W., Gong S., Zhang J., Tan J.H. Heat stress decreases egg production of laying hens by inducing apoptosis of follicular cells via activating the FasL/Fas and TNF-alpha systems. Poult. Sci. 2020;99:6084–6093. doi: 10.1016/j.psj.2020.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y.Y., Lin Y.K., Liu X.H., Wang L., Yu M., Li D.J., Zhu Y.Z., Du MR. Leonurine: from gynecologic medicine to pleiotropic agent. Chin. J. Integr. Med. 2020;26:152–160. doi: 10.1007/s11655-019-3453-0. [DOI] [PubMed] [Google Scholar]
- Liao L., Li J., Li J., Huang Y., Wu Y. Effects of Astragalus polysaccharides on intestinal morphology and intestinal immune cells of Muscovy ducklings infected with Muscovy duck reovirus. Poult. Sci. 2021;100:64–72. doi: 10.1016/j.psj.2020.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu B., Ma R., Yang Q., Yang Y., Fang Y., Sun Z., Song D. Effects of traditional chinese herbal feed additive on production performance, egg quality, antioxidant capacity, immunity and intestinal health of laying hens. Animals (Basel) 2023;13:2510. doi: 10.3390/ani13152510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L., Li H., Tan G., Ma Z. Traditional Chinese herbal medicine in treating amenorrhea caused by antipsychotic drugs: meta-analysis and systematic review. J. Ethnopharmacol. 2022;289 doi: 10.1016/j.jep.2022.115044. [DOI] [PubMed] [Google Scholar]
- Liu L., Yan J., Ge F., Xu X., Lu J., Shi H., Li S., Zhao Y., Zhang C. Saikosaponin‑D improves fear memory deficits in ovariectomized rats via the action of estrogen receptor‑alpha in the hippocampus. Mol. Med. Rep. 2019;20:332–340. doi: 10.3892/mmr.2019.10232. [DOI] [PubMed] [Google Scholar]
- Liu L., Zhou Z., Hong Y., Jiang K., Yu L., Xie X., Mi Y., Zhu S.J., Zhang C., Li J. Transplantion of predominant Lactobacilli from native hens to commercial hens could indirectly regulate their ISC activity by improving intestinal microbiota. Microb. Biotechnol. 2022;15:1235–1252. doi: 10.1111/1751-7915.13917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu M., Zhou J., Li Y., Ding Y., Lian J., Dong Q., Qu Q., Lv W., Guo S. Effects of dietary polyherbal mixtures on growth performance, antioxidant capacity, immune function and jejunal health of yellow-feathered broilers. Poult. Sci. 2023;102 doi: 10.1016/j.psj.2023.102714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X., Lin X., Mi Y., Li J., Zhang C. Grape seed Proanthocyanidin extract prevents ovarian aging by inhibiting oxidative stress in the hens. Oxid. Med. Cell. Longev. 2018;2018 doi: 10.1155/2018/9390810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X., Tang Y., Wu J., Liu J.X., Sun H.Z. Feedomics provides bidirectional omics strategies between genetics and nutrition for improved production in cattle. Anim. Nutr. 2022;9:314–319. doi: 10.1016/j.aninu.2022.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak K.J., Schmittgen T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Luan F., Ji Y., Peng L., Liu Q., Cao H., Yang Y., He X., Zeng N. Extraction, purification, structural characteristics and biological properties of the polysaccharides from Codonopsis pilosula: a review. Carbohydr. Polym. 2021;261 doi: 10.1016/j.carbpol.2021.117863. [DOI] [PubMed] [Google Scholar]
- Luo L.F., Guan P., Qin L.Y., Wang J.X., Wang N., Ji E.S. Astragaloside IV inhibits adriamycin-induced cardiac ferroptosis by enhancing Nrf2 signaling. Mol. Cell. Biochem. 2021;476:2603–2611. doi: 10.1007/s11010-021-04112-6. [DOI] [PubMed] [Google Scholar]
- Ma H., He X., Yang Y., Li M., Hao D., Jia Z. The genus Epimedium: an ethnopharmacological and phytochemical review. J. Ethnopharmacol. 2011;134:519–541. doi: 10.1016/j.jep.2011.01.001. [DOI] [PubMed] [Google Scholar]
- Matur E., Akyazi I., Eraslan E., Ergul E.E., Eseceli H., Keten M., Metiner K., Aktaran B.D. The effects of environmental enrichment and transport stress on the weights of lymphoid organs, cell-mediated immune response, heterophil functions and antibody production in laying hens. Anim. Sci. J. 2016;87:284–292. doi: 10.1111/asj.12411. [DOI] [PubMed] [Google Scholar]
- Miao L., Zhou Q., Peng C., Liu Z., Xiong L. Leonurus japonicus (Chinese motherwort), an excellent traditional medicine for obstetrical and gynecological diseases: a comprehensive overview. Biomed. Pharmacother. 2019;117 doi: 10.1016/j.biopha.2019.109060. [DOI] [PubMed] [Google Scholar]
- Nii T. Relationship between mucosal barrier function of the oviduct and intestine in the productivity of laying hens. J. Poult. Sci. 2022;59:105–113. doi: 10.2141/jpsa.0210090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nii T., Bungo T., Isobe N., Yoshimura Y. Intestinal inflammation induced by dextran sodium sulphate causes liver inflammation and lipid metabolism disfunction in laying hens. Poult. Sci. 2020;99:1663–1677. doi: 10.1016/j.psj.2019.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ong M., Cheng J., Jin X., Lao W., Johnson M., Tan Y., Qu X. Paeoniflorin extract reverses dexamethasone-induced testosterone over-secretion through downregulation of cytochrome P450 17A1 expression in primary murine theca cells. J. Ethnopharmacol. 2019;229:97–103. doi: 10.1016/j.jep.2018.09.006. [DOI] [PubMed] [Google Scholar]
- Pan Y., Kong L.D. High fructose diet-induced metabolic syndrome: pathophysiological mechanism and treatment by traditional Chinese medicine. Pharmacol. Res. 2018;130:438–450. doi: 10.1016/j.phrs.2018.02.020. [DOI] [PubMed] [Google Scholar]
- Ploegaert T.C., De Vries R.G., Nieuwland M.G., Lammers A., Savelkoul H.F., Parmentier H.K. Intratracheally administered pathogen-associated molecular patterns affect antibody responses of poultry. Poult. Sci. 2007;86:1667–1676. doi: 10.1093/ps/86.8.1667. [DOI] [PubMed] [Google Scholar]
- Raspoet R., Gantois I., Devloo R., Martel A., Haesebrouck F., Pasmans F., Ducatelle R., Van Immerseel F. Salmonella Enteritidis universal stress protein (usp) gene expression is stimulated by egg white and supports oviduct colonization and egg contamination in laying hens. Vet. Microbiol. 2011;153:186–190. doi: 10.1016/j.vetmic.2011.05.047. [DOI] [PubMed] [Google Scholar]
- Reddy I.J., Mishra A., Mondal S., Awachat V., Kiran G. Endocrine changes in hens (Gallus gallus domesticus) exposed to red spectrum of light. Curr. Res. Poult Sci. 2016;6:13–17. [Google Scholar]
- Saraiva M., O'Garra A. The regulation of IL-10 production by immune cells. Nat. Rev. Immunol. 2010;10:170–181. doi: 10.1038/nri2711. [DOI] [PubMed] [Google Scholar]
- Seidavi A., Tavakoli M., Asroosh F., Scanes C.G., Abd E.M., Naiel M., Taha A.E., Aleya L., El-Tarabily K.A., Swelum A.A. Antioxidant and antimicrobial activities of phytonutrients as antibiotic substitutes in poultry feed. Environ. Sci. Pollut. Res. Int. 2022;29:5006–5031. doi: 10.1007/s11356-021-17401-w. [DOI] [PubMed] [Google Scholar]
- Song W., Zou Z., Chen X., Tan J., Liu L., Wei Q., Xiong P., Song Q., Chen J., Su W., Xu C. Effects of traditional Chinese herbal feed supplement on growth performance, immunity, antioxidant levels, and intestinal health in chickens: a study on Ningdu yellow chickens. Poult. Sci. 2023;102 doi: 10.1016/j.psj.2023.102986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Souza O., Adams C., Rodrigues B., Krause A., Bonamigo R., Zavarize K., Stefanello C. The impact of Bacillus subtilis PB6 and chromium propionate on the performance, egg quality and nutrient metabolizability of layer breeders. Animals (Basel) 2021;11:3084. doi: 10.3390/ani11113084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun W., Shahrajabian M.H., Cheng Q. Health benefits of wolfberry (Gou Qi Zi) on basis of ancient Chinese herbalism and Western modern medicine. Avicenna J. Phytomed. 2021;11:109–119. [PMC free article] [PubMed] [Google Scholar]
- Sun Y., O'Riordan M.X. Regulation of bacterial pathogenesis by intestinal short-chain fatty acids. Adv. Appl. Microbiol. 2013;85:93–118. doi: 10.1016/B978-0-12-407672-3.00003-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan Z., Luo L., Wang X., Wen Q., Zhou L., Wu K. Characterization of the cecal microbiome composition of Wenchang chickens before and after fattening. PLoS One. 2019;14 doi: 10.1371/journal.pone.0225692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanabe Y., Nakamura T., Fujioka K., Doi O. Production and secretion of sex steroid hormones by the testes, the ovary, and the adrenal glands of embryonic and young chickens (Gallus domesticus) Gen. Comp. Endocrinol. 1979;39:26–33. doi: 10.1016/0016-6480(79)90189-8. [DOI] [PubMed] [Google Scholar]
- Uddin T.M., Chakraborty A.J., Khusro A., Zidan B., Mitra S., Emran T.B., Dhama K., Ripon M., Gajdacs M., Sahibzada M., Hossain M.J., Koirala N. Antibiotic resistance in microbes: history, mechanisms, therapeutic strategies and futures prospects. J. Infect. Publ. Health. 2021;14:1750–1766. doi: 10.1016/j.jiph.2021.10.020. [DOI] [PubMed] [Google Scholar]
- Wang J., Tang C., Wang Q., Li R., Chen Z., Han X., Wang J., Xu X. Apoptosis induction and release of inflammatory cytokines in the oviduct of egg-laying hens experimentally infected with H9N2 avian influenza virus. Vet. Microbiol. 2015;177:302–314. doi: 10.1016/j.vetmic.2015.04.005. [DOI] [PubMed] [Google Scholar]
- Wang M., Huang H., Wang L., Yang H., He S., Liu F., Tu Q., He S. Herbal extract mixture modulates intestinal antioxidative capacity and microbiota in weaning piglets. Front. Microbiol. 2021;12 doi: 10.3389/fmicb.2021.706758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Xu L., Sun X., Wan X., Sun G., Jiang R., Li W., Tian Y., Liu X., Kang X. Characteristics of the fecal microbiota of high- and low-yield hens and effects of fecal microbiota transplantation on egg production performance. Res. Vet. Sci. 2020;129:164–173. doi: 10.1016/j.rvsc.2020.01.020. [DOI] [PubMed] [Google Scholar]
- Wei F., Abulahaiti D., Tian C., Chen Y., Jiang S., Lu J., Zhang G. Effects of dietary Astragalus mongholicus, Astragalus polysaccharides and Lactobacillus on growth performance, immunity and antioxidant status in Qingjiaoma finishing broilers. Czech. J. Anim. Sci. 2022;67:275–285. [Google Scholar]
- Wong J.M.W., de Souza R., Kendall C.W.C., Emam A., Jenkins D.J.A. Colonic health: fermentation and short chain fatty acids. J. Clin. Gastroenterol. 2006;40:235–243. doi: 10.1097/00004836-200603000-00015. [DOI] [PubMed] [Google Scholar]
- Wu S. Effect of dietary Astragalus membranaceus polysaccharide on the growth performance and immunity of juvenile broilers. Poult. Sci. 2018;97:3489–3493. doi: 10.3382/ps/pey220. [DOI] [PubMed] [Google Scholar]
- Xia Y., Wang Q., He X.D., Chen Y., JiGe M.T., Zi X.D. Cloning and expression analysis of the follicle-stimulating hormone receptor (FSHR) gene in the reproductive axis of female yaks (Bos grunniens) Domest. Anim. Endocrinol. 2020;70 doi: 10.1016/j.domaniend.2019.07.011. [DOI] [PubMed] [Google Scholar]
- Xie T., Bai S.P., Zhang K.Y., Ding X.M., Wang J.P., Zeng Q.F., Peng H.W., Lu H.Y., Bai J., Xuan Y., Su Z.W. Effects of Lonicera confusa and Astragali Radix extracts supplementation on egg production performance, egg quality, sensory evaluation, and antioxidative parameters of laying hens during the late laying period. Poult. Sci. 2019;98:4838–4847. doi: 10.3382/ps/pez219. [DOI] [PubMed] [Google Scholar]
- Xie Y., Mai C.T., Zheng D.C., He Y.F., Feng S.L., Li Y.Z., Liu C.X., Zhou H., Liu L. Wutou decoction ameliorates experimental rheumatoid arthritis via regulating NF-kB and Nrf2: integrating efficacy-oriented compatibility of traditional Chinese medicine. Phytomedicine. 2021;85 doi: 10.1016/j.phymed.2021.153522. [DOI] [PubMed] [Google Scholar]
- Yan F.F., Liu Y., Liu Y.F., Zhao Y.X. Herba Epimedii water extract elevates estrogen level and improves lipid metabolism in postmenopausal women. Phytother. Res. 2008;22:1224–1228. doi: 10.1002/ptr.2451. [DOI] [PubMed] [Google Scholar]
- Yan W., Sun C., Yuan J., Yang N. Gut metagenomic analysis reveals prominent roles of Lactobacillus and cecal microbiota in chicken feed efficiency. Sci. Rep. 2017;7:45308. doi: 10.1038/srep45308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang C., Wei Q., Kang L., Tang H., Zhu G., Cui X., Chen Y., Jiang Y. Identification and genetic effect of haplotypes in the distal promoter region of chicken CCT6A gene associated with egg production traits. J. Poult. Sci. 2016;53:111–117. doi: 10.2141/jpsa.0140113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L., Liu G., Zhu X., Luo Y., Shang Y., Gu X.L. The anti-inflammatory and antioxidant effects of leonurine hydrochloride after lipopolysaccharide challenge in broiler chicks. Poult. Sci. 2019;98:1648–1657. doi: 10.3382/ps/pey532. [DOI] [PubMed] [Google Scholar]
- Yao Y., Yan L., Chen H., Wu N., Wang W., Wang D. Cyclocarya paliurus polysaccharides alleviate type 2 diabetic symptoms by modulating gut microbiota and short-chain fatty acids. Phytomedicine. 2020;77 doi: 10.1016/j.phymed.2020.153268. [DOI] [PubMed] [Google Scholar]
- Yu A.C., Wang M.A., Chen L., Long C., Guo Y., Sheng X.H., Wang X.G., Xing K., Xiao L.F., Ni H.M., Li J.T., Qi X.L. Effects of dietary pretreated Chinese herbal medicine supplementation on production performance, egg quality, uterine histopathological changes, and antioxidant capacity in late-phase laying hens. Front. Physiol. 2023;14 doi: 10.3389/fphys.2023.1110301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng L., Rana S., Hussain L., Asif M., Mehmood M.H., Imran I., Younas A., Sallam A., Al-Joufi F., Abed S. Polycystic ovary syndrome: a disorder of reproductive age, its pathogenesis, and a discussion on the emerging role of herbal remedies. Front. Pharmacol. 2022;13 doi: 10.3389/fphar.2022.874914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L., Zhong G., Gu W., Yin N., Chen L., Shi S. Dietary supplementation with daidzein and Chinese herbs, independently and combined, improves laying performance, egg quality and plasma hormone levels of post-peak laying hens. Poult. Sci. 2021;100 doi: 10.1016/j.psj.2021.101115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X., Zhou S., Liang Y., Xie G., Zhu M., Wang Z., Qu Q., Long Y., Lv Y., Peng J., Yuan Y., Huang Y., Wang W. Effects of Astragalus, Epimedium, and Fructus Ligustri Lucidi extractive on antioxidant capacity, production performance, and immune mechanism of breeding pigeons under stress. Poult. Sci. 2023;102 doi: 10.1016/j.psj.2022.102350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao H., Shan Y., Ma Z., Yu M., Gong B. A network pharmacology approach to explore active compounds and pharmacological mechanisms of Epimedium for treatment of premature ovarian insufficiency. Drug Des. Devel. Ther. 2019;13:2997–3007. doi: 10.2147/DDDT.S207823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Q., Luan X., Zheng M., Tian X.H., Zhao J., Zhang W.D., Ma B.L. Synergistic mechanisms of constituents in herbal extracts during intestinal absorption: focus on natural occurring nanoparticles. Pharmaceutics. 2020;12:128. doi: 10.3390/pharmaceutics12020128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng J., Liang S., Zhang Y., Sun X., Li Y., Diao J., Dong L., Ni H., Yin Y., Ren J., Yang Y., Zhang Y. Effects of compound chinese herbal medicine additive on growth performance and gut microbiota diversity of Zi goose. Animals (Basel) 2022;12:2942. doi: 10.3390/ani12212942. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.