Abstract
Background:
Men’s infertility and lack of production of healthy and active sperm are concerns of recent years in most countries. Studies on the preparation of extracellular matrix (ECM) from decellularization of testis tissue and spermatogenesis could provide proper results to solve some of the men’s infertility problems. This study aims to decellularize calf testis by different methods to reach a suitable scaffold and introduce it in spermatogenesis studies.
Materials and Methods:
In this experimental study, calf testis were decellularized by a freeze-de freeze, 1% sodium deoxycholate (SD), 0.1% sodium dodecyl sulfate (SDS), 0.1% SDS-vacuum, 1% SDS, 1% SDS-vacuum, and Triton- X100 methods. The content of DNA, collagen, and glycosaminoglycan (GAG) was analyzed using the kit and staining with Hematoxylin-Eosin, Masson’s trichrome, Alcian blue, and Orcein methods. The morphology of the scaffolds was analyzed with a scanning electron microscope (SEM).
Results:
Methods of 1% SDS, 1% SDS-vacuum, and 1% SD completely removed the cells. The preservation of collagen and GAG was confirmed using the staining kit and methods. The use of a vacuum showed greater porosity in the SEM images. Toxicity and hemolysis were not observed in the scaffolds.
Conclusion:
Testis decellularization with 1% SDS and 1% SD, in addition to cell removal, could maintain the ECM structure to a large extent without having cytotoxic and hemolysis effects.
Keywords: Decellularization, Scaffold, Testis
Introduction
Reproductive disorders are shared all over the world, which causes many social problems and harms the individual economy and society. Today, the number of couples with male infertility factors is increasing due to diet changes, lifestyle, and occupational injuries. Assisted reproductive techniques (ART) offer many efforts to treat reproductive disorders, and they are improving day by day, but in many cases, saving fertility fails (1, 2). Spermatogenesis and germ cell differentiation are complex processes of reproduction that lead to the production of fertile sperm. Spermatogonia stem cells divide inside seminiferous tubules and produce progenitor cells. This process is prompted by hormonal factors, growth factors, cytokines, and extracellular matrix (ECM). Therefore, defects in the function of any of those factors could interfere with spermatogenesis and lead to male infertility (3, 4).
Regenerative medicine and tissue engineering with new approaches to the male fertility subject has provided a unique opportunity to offer practical solutions in this field. Tissue engineering is a science that has taken a big step in tissue repair and treatment by making natural and synthetic scaffolds alone or using cells. Scaffolds of natural origin, such as decellularized tissues, can effectively mimic the body’s natural ECM structure (5). Decellularized scaffolds have the potential to simulate the physical microenvironment that is impacted cell fate and repair. Regenerative medicine and tissue engineering with new approaches to improve fertility have provided a unique opportunity to offer practical solutions in this field. These sciences have taken a big step in treatment by making natural and synthetic scaffolds alone or using cells (6, 7).
Preservation of the ECM is one of the critical cases of the tendency to use this technique in studies. These tissues are obtained from various sources such as corpses and animals and are examined in research works. Due to the decellularization of the tissue, rejection risks of the transplant are almost eliminated, and at the same time, if these tissues are obtained from unusable animal sources, the ethical problems of providing human corpses are eliminated (8).
Due to the increasing male infertility ratio across the world, more attention has been paid to the methods for treating this problem (9). The use of ECM prepared from decellularized tissues is one of the proposed and used methods for the treatment of infertility. In tissue engineering, testicular organoids are cellular groupings that mimic testicular architecture and function. This technology in science opens new horizons for the study and realization of spermatogenesis in vitro. In fact, these scaffolds create conditions similar to the bodies for the growth of sperm. These techniques enable pharmaceutical spermatogenesis research that addresses infertility and reduces animal usage (10). The testis tissue of several species has been decellularized by various methods and introduced for spermatogenesis studies. So far, this process has not been investigated in the decellularized tissue of calf testis. For this purpose, we used the calf testis and vacuum method for the first time in this study. Therefore, the current study aims to take a step towards spermatogenesis research and helping treat male infertility. The results of this study and the preparation of testicular scaffolds derived from ECM to support testicular cells could be valuable for spermatogenesis studies and drug screening.
Materials and Methods
This experimental study was accepted under the management of the Ethics Committee of Kermanshah University of Medical Sciences (IR.KUMS.MED. REC.1401.031).
Calf testis
Preparation of the calf testis
Calf meat is considered one of the food sources. After sacrificing, the testis was removed from the Holstein breed calf and placed in a phosphate-buffered saline (PBS) solution including 2% antibiotics penicillin-streptomycin (P/S) and transferred to the laboratory. Tissues were collected from 5 calf.
Decellularization methods
After transferring the tissue to the laboratory, the tunica albuginea was removed, and the testis was cut into small pieces (about 1 cm3). Seven methods were used to decellularize the testis tissue in this study. In the freeze-de-freeze method, the tissues were placed in liquid nitrogen for 30 seconds (s) and then at room temperature for 10 minutes; this cycle was repeated 5 times. In 1% SD, 0.1% SDS, and 1% SDS methods, the tissues were immersed separately in the above solutions and agitated on a shaker at 90 revolutions per minute (rpm) for 24 hours , then the tissues were shaken in PBS for 12 hours. After the above time, decellularized tissues with 0.1% and 1% SDS are placed under a vacuum for 5 hours. To sterilize the tissues, they put in a PBS solution including 1% P/S for 1 hour, 70% ethanol for 10 minutes, and then exposed to UV radiation for 15 minutes on each side.
Scaffolds characterization
Evaluation of DNA content
The measurement of the remaining DNA content in decellularized testis and native tissue was done with the kit of Sinaclon company, Iran. 100 μl of protease buffer and then 5 μl of protease were added to 30 mg of tissues and kept at 55°C for 3 hours. 100 μl of the sample was mixed with 400 μl of lysis solution and 300 μl of precipitation solution and then centrifuged at 12000 g for 10 minutes. Then, after several steps of washing, pour the washing buffer completely. After the pellet is dried, it is suspended in 50 μl of solvent buffer and then centrifuged for 30 seconds at 12,000 g. The DNA concentration of the supernatant was measured by a NanoDrop spectrophotometer (BioTeK, USA) (11).
Evaluation of glycosaminoglycan content
ECM of the testis is rich in GAG, so its measurement in decellularization methods seems necessary. The experiment was performed according to the protocol of Kiazist Iran. The standard solution (chondroitin sulfate) was prepared in serial dilution according to the kit protocol. 20 mg of tissue was homogenized with enzyme solution and incubated at 65°C for 16 hours. Then it was centrifuged at 6000 g for 15 minutes. 50 μl of precipitating protein was added to the supernatant and centrifuged at 6000 g for 15 minutes. We poured 30 μl of the supernatant into each well of 96 plates and added 200 μl of GAG reagent to them and incubated them for 60 seconds at room temperature. Finally, the absorbance of the wells was read with an ELISA Reader (Stat Fax 2100, USA) at a wavelength of 560 nm (12).
Evaluation of collagen content
Hydroxyproline, a collagen components, plays an important role in maintaining the structure of ECM. Therefore, in this study, the measurement of the amount of collagen was performed using the kit of Kiazist Iran. The standard solution was prepared in the dilution series according to the kit protocol. To 20 mg of tissue, 100 μl of deionized water and then 100 μl of 12 M hydrochloric acid were added and incubated for 3 hours at 120°C. Charcoal was added to each microtube and centrifuged at 12,000 g for 15 minutes. 20 μl of the supernatant was poured into each well of a plate, and oxidation solution (100 μl) and chromogen (100 μl) were added to them and incubated for 60 minutes at of 60°C. In the end, optical absorption was read at a wavelength of 540 nm with an ELISA Reader (Stat Fax 2100, USA) (13).
Histology analysis
The histological evaluation was done after the preparation of the tissues. First, they were fixed, and then extraction and moulding with paraffin were done. Tissues cut with a microtome in diameter of 5 microns. For staining the tissues, each section was deparaffinized with xylene solution, hydrated by ethanol at descending degrees, and then stained with Hematoxylin-Eosin (H-E), Masson’s trichrome, Alcian blue and Orcein for identification of nucleus, collagen, GAG, elastin respectively (14).
Evaluation of cell morphology by scanning electron microscope
A scanning electron microscope (SEM, ZEISS Sigma 300 HV, USA) was used to evaluate the morphology of the tissues after decellularization. The samples were fixed in 4% paraformaldehyde for 24 hours. After washing three times with deionized water in ascending ethanol solutions (40, 50, 60, 70, 80, 90, and 100%), dehydration was done, and then it was dehumidified by a freeze dryer (Christ Alpha 2-4 LDplus) for 1 hour. Scaffolds coated with gold and imaged on their cross-section (15).
Biocompatibility evaluation
Tissue biocompatibility was done using an indirect test (standard ISO 10993-5). Briefly, scaffolds in a 24- well plate were placed containing Dulbecco’s Modified Eagle’s medium (DMEM, Sigma), 7% fetal bovine serum (FBS, Sigma), and 1% P/S (Sigma). They were cultured for 24 hour under suitable incubator conditions (37°C, 5% CO2, 95% humidity). The supernatant was removed and added to human adipose mesenchymal stem cell 1×104 cells in a 96-well plate, and the MTT assay measured the biocompatibility in periods of 48 and 72 hours. 20 μl of 5 mg/ml MTT was added to each well and incubated for 3 hours at 37°C. The supernatant was withdrawn, and 100 μl of dimethyl sulfoxide (DMSO) was added to the wells. After 30 minutes, it was read with an ELISA Reader (Stat Fax 2100, USA) at a wavelength of 570 nm (16). Biocompatibility was calculated using the formula: cellular biocompatibility (%)=sample OD/ control OD×100
Hemocompatibility evaluation
The samples were placed in 4×4 mm dimensions in 2.5 ml microtubes. 2 ml of PBS was added to them and incubated for 30 minutes at 37°C. Two ml of distilled water, and 2 ml of PBS were added to the positive and negative control microtubes, respectively. Then 40 microliters of fresh blood containing anticoagulants were poured into each tube and incubated for 1 hour at 37°C. The supernatant was removed, and the optical absorbance was read at 540 nm (Stat Fax 2100, USA) (17). The following formula calculated the degree of hemolysis (HD). Dn: sample, D0: negative control, D1: positive control. HD (%)=[(Dn-D0)/(D1-D0)]×100%
Statistical analysis
All the results obtained in this study are based on at least 3 repetitions, which were calculated by taking the average and calculating the standard deviation. The results were analyzed using GraphPad Prism software (version 8, GraphPad Software Inc., USA). The normality of the variable and the homogeneity of the variances were checked, and after confirmation, the data were analyzed by one-way ANOVA and Tukey Post hoc test. P<0.05 is considered significant.
Results
DNA, glycosaminoglycan, and collagen content
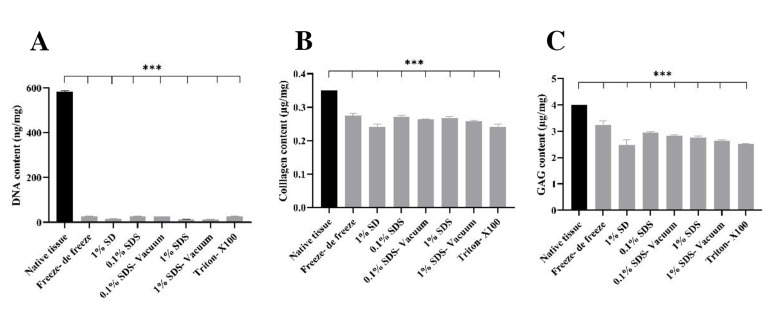
A significant decrease in DNA levels was attained in the methods of freeze- de freeze (27.11 ng/mg), 1% SD (16.5 ng/mg), 0.1% SDS (27.73 ng/mg), 0.1% SDSvacuum (25.66), 1% SDS (14.43), 1% SDS-vacuum (12.91), Triton- X100 (26.92) relative to native tissue (583.33 ng/mg) (Fig .1A). A decrease in collagen content was observed in the decellularized tissue compared to the native tissue (0.35 μg/mg), and this significant difference was reported in all groups (***P<0.001). The greatest reduction occurred in the 1% SD (0.240 μg/mg) method (Fig .1B). The GAG content in the decellularized tissues was significantly reduced compared to the main tissue (4 μg/mg). The highest decrease was reported in 1% SD (2.48 μg/mg) and the lowest in freeze-de freeze (3.23 μg/mg, Fig .1C). The normality of the variable and the homogeneity of the variances were checked, and after confirmation, the data were analyzed by oneway ANOVA and Tukey Post hoc test. A significant comparison of all groups with each other is shown in Table 1.
Fig 1.
DNA, collagen, and GAG content in testis tissue of native and decellularized. The amounts of A. DNA, B. Collagen, and C. GAG in all decellularized tissues are significantly reduced compared to the native tissue. Findings are presented as means ± SD. Analyzed by ANOVA test. ***; P<0.001, GAG; Glycosaminoglycan, SD; Sodium deoxycholate, and SDS; Sodium dodecyl sulfate.
Table 1.
Significant comparison in different groups in DNA, collagen, and GAG content
|
| |||
|---|---|---|---|
| Tukey’s multiple comparisons tests | Significant(DNA) | Significant(Collagen) | Significant(GAG) |
|
| |||
| Freeze-de freeze/1% SD | P<0.001 | P<0.001 | P<0.05 |
| Freeze-de freeze/0.1% SDS | 0.5 | 0.9 | P<0.001 |
| Freeze-de freeze/0.1% SDS-vacuum | 0.06 | 0.2 | P<0.001 |
| Freeze-de freeze/1% SDS | P<0.001 | 0.7 | P<0.001 |
| Freeze-de freeze/1% SDS-vacuum | P<0.001 | P<0.05 | P<0.001 |
| Freeze-de freeze/Triton-X100 | 0.8 | P<0.001 | P<0.001 |
| 1% SD/0.1% SDS | P<0.001 | P<0.001 | P<0.001 |
| 1% SD/0.1% SDS-vacuum | P<0.001 | P<0.01 | P<0.01 |
| 1% SD/1% SDS | 0.8 | P<0.001 | P<0.05 |
| 1% SD/1% SDS-vacuum | 0.8 | P<0.05 | 0.5 |
| 1% SD/Triton- X100 | P<0.001 | 0.9 | 0.9 |
| 0.1% SDS/0.1% SDS-vacuum | 0.9 | 0.5 | 0.6 |
| 0.1% SDS/1% SDS | P<0.001 | 0.9 | 0.2 |
| 0.1% SDS/1% SDS-vacuum | P<0.001 | 0.1 | P<0.01 |
| 0.1% SDS/Triton-X100 | 0.8 | P<0.001 | P<0.001 |
| 0.1% SDS-vacuum/1% SDS | P<0.001 | 0.9 | 0.9 |
| 0.1% SDS-vacuum/1% SDS-vacuum | P<0.001 | 0.9 | 0.2 |
| 0.1% SDS-vacuum/Triton-X100 | 0.8 | P<0.01 | P<0.05 |
| 1% SDS/1% SDS-vacuum | 0.9 | 0.4 | 0.5 |
| 1% SDS/Triton-X100 | P<0.001 | P<0.001 | 0.6 |
| 1% SDS-vacuum/Triton-X100 | P<0.001 | P<0.05 | 0.7 |
|
| |||
GAG; Glycosaminoglycan, SD; Sodium deoxycholate, and SDS; Sodium dodecyl sulfate.
Histology
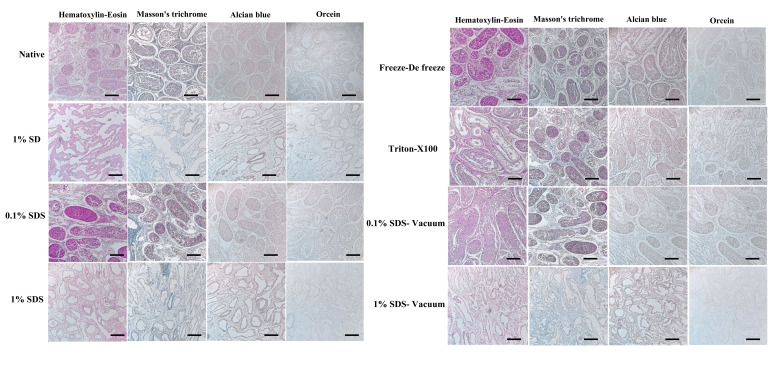
Staining methods of H-E, Masson’s trichrome, Orcein, and Alcian blue were used to study the nucleus, collagen, elastin, and GAG respectively. In the native tissue, the seminiferous tubules and the general structure of the testis tissue can be seen well. In the samples decellularized by the freeze-de freeze, 0.1% SDS, and Triton-X100 methods, the structure of the seminiferous tubules has changed to some extent, and many nuclei can be seen. The structure of collagen, elastin, and GAG are largely preserved. In tissues decellularized with 1% SDS and 1% SD, cells were completely removed. The structure of the seminiferous tubules has changed a lot. Collagen, elastin, and GAG have decreased in tissues. More tissue changes were observed in the samples where a vacuum was used compared to the same groups without a vacuum. These findings were consistent with values obtained from quantitative studies (Fig .2).
Fig 2.
Histological staining (H-E, Masson’s trichrome, Orcein, and Alcian blue) in decellularized tissue. In the native tissue, the structure of the tissue and the seminiferous tubules are well preserved, in the freeze-de freeze, 0.1% SDS, and Triton-X100 methods many nuclei can be seen, but 1% SDS and 1% SD the nuclei are completely removed, collagen, elastin, and GAG have decreased in tissues and the texture architecture has changed the most (magnification 200x, scale bar: 200 μm). GAG; Glycosaminoglycan, SD; Sodium deoxycholate, and SDS; Sodium dodecyl sulfate.
Scanning electron microscope
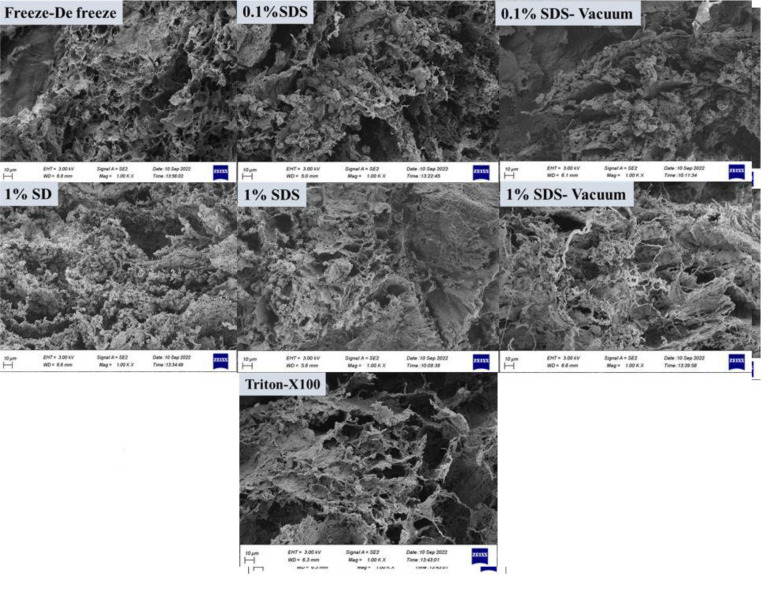
SEM was used to qualitatively assess the morphology structure of the decellularized testis. The cross-section of tissues was utilized to study their three-dimensional structure. In tissue decellularization by freeze-de freeze method, some tissue disintegration was seen. In 0.1% SDS, the collagen fibers are not separated, but in 0.1% SDS-vacuum, tissue disintegration has increased. In the 1% SD method, the collagen strands are separated, and the bundles of the strands are also reduced. In the 1% SDS method, the collagen fibers are separated, but not as much as in the 1% SD method. In the 1% SDS-vacuum method, the break up of these strings is more than 1% SD. In Triton X-100, the cohesion of collagen fibers has decreased, and the strands have separated (Fig .3).
Fig 3.
Evaluation of morphology and cell attachment of testis decellularized tissue by SEM. The yellow arrows show that the structure of the scaffold is mostly preserved as a plate, but the red arrows show the separation of collagen fibers (magnification: 500x, crass section, scale bars: 10 μm). SEM; Scanning electron microscopy, SD; Sodium deoxycholate, and SDS; Sodium dodecyl sulfate.
Biocompatibility
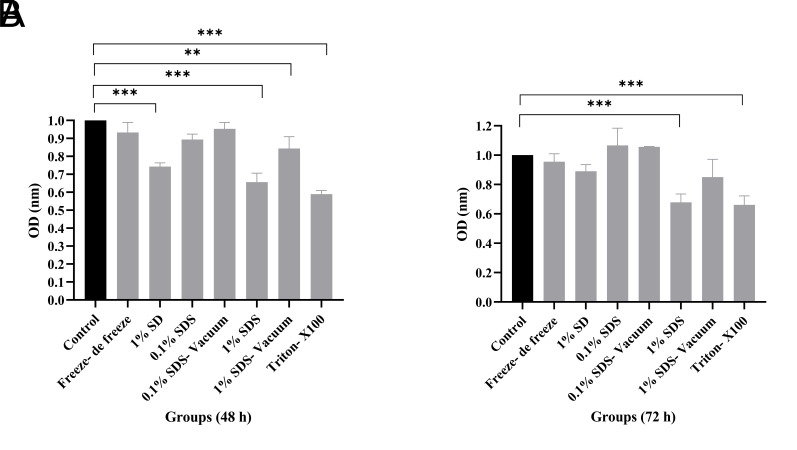
The biocompatibility of decellularized testis tissues was assessed using adipose mesenchymal stem cells by indirect MTT assay at 48 and 72 hours. As can be seen in Figure 4, the optical density (OD) in the control group (cells alone) and all decellularized tissues are compared with each other. Cell proliferation increased in different testicular tissue decellularization methods depending on time so that in 72 hours, the cell growth in all groups was higher than in 48 hours. A significant difference in cell survival was reported in 1% SD, 1% SDS, 15 SDS-vacuum, and Triton groups in 48 hours compared to the control group, while this difference was observed in 72 hours only in 1% SDS and Triton groups (**P<0.01, ***P<0.001). It seems that the use of the type and dose of detergents and the decellularization method have a direct effect on cell proliferation. The normality of the variable and the homogeneity of the variances were checked, and after confirmation, the data were analyzed by one-way ANOVA and Tukey Post hoc test.
Fig 4.
Biocompatibility in testis decellularized tissues by MTT assay. A. Within 48 hours, there was no significant difference in Freeze-de freeze, 0.1% SDS, and 0.1% SDS-vacuum groups compared to the control group, but a significant difference in cell proliferation was reported in 1% SD, 1% SDS, 1% SDS-vacuum, and Triton groups than the control group. In none of these groups cell growth inhibition in IC50 was seen, B. Within 72 hours, cell proliferation in all groups was more than 48 hours. Significant differences were reported in 1% SDS and Triton groups compared to the control group. Findings are presented as means ± SD. Analyzed by ANOVA test. **; P<0.01, ***; P<0.001, SDS; Sodium dodecyl sulfate, SD; Sodium deoxycholate, IC50; The half-maximal inhibitory concentration, and OD; Optical density.
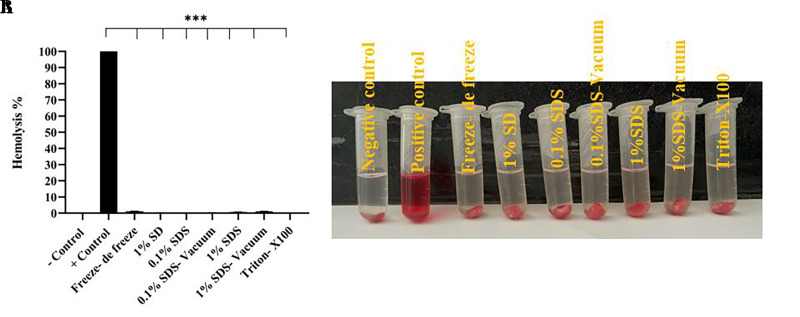
Hemocompatibility
The finding of the hemolytic test is shown in Figure 5. Compatible blood was seen in all tissues decellularized by different methods, which showed a significant difference with the positive control group (***P<0.001). This ratio was reported to be more than 1% in freeze-de freeze (1.23%) and 1% SDS-vacuum (1.1%) methods and less than 1% in other methods. The normality of the variable and the homogeneity of the variances were checked, and after confirmation, the data were analyzed by one-way ANOVA and Tukey Post hoc test.
Fig 5.
Hemocompatibility in testis decellularized tissues by hemolysis test. A. Data were reported as significant in all methods compared to the positive control group. B. The image related to the hemolysis test in different testis tissue decellularization methods, the clear supernatant liquid in the tubes shows hemocompatibility. Findings are presented as means ± SD. Analyzed by ANOVA test. ***; P<0.001, SDS; Sodium dodecyl sulfate, and SD; Sodium deoxycholate.
Discussion
This study subjected calf testis to decellularization using combined chemical-physical methods. Decellularization methods included freeze-de freeze, 1% SD, SDSvacuum (0.1, 1%), and triton-X100. 1% SDS and 1% SD were able to completely remove cells from the tissue. By reducing the percentage of SDS (0.1%) and using the Freeze-de Freeze and triton-X100 methods, the structure of the scaffold remained largely intact, but the removal of cells was not successful. DNA content measurement also quantitatively confirmed the staining findings. Biocompatibility and blood compatibility were observed in all methods. Preservation of collagen and GAG using kits and staining methods showed that these values decrease in 1% SDS (with and without vacuum) and 1% SD methods, more than other methods, and then the tissue architecture changes to some extent. SEM findings also showed the separation of collagen fibers in the above methods. The scaffold was exposed to more porosity in the methods where a vacuum was also present. In our study, the combination of physical and chemical methods caused decellularization of the testicular tissue through the methods of creating turbulence, introducing a vacuum, and using ionic and non-ionic detergents. These methods will work through the mechanism of separation of protein bonds and cause the separation of the cell from the ECM structure. It also results in the separation of many protein bonds such as collagens from the scaffold structure. The simultaneous use of these items will reduce the decellularization time and the percentage of detergents, resulting in better cell removal and preventing the destruction of the scaffold structure.
The final goal of the decellularization process is to remove the tissue cells using detergents and physical methods and preserve the components and proteins of the ECM. These proteins provide structural and biochemical support to cell proliferation, adhesion, cell-to-cell interaction, and migration. Decellularization of sheep testis with 1% SDS at 24 hours reported results similar to our study (18). However, 1% SDS in the ram testis decellularization within 30 minutes, which was much less than the time of our study (24 hours), showed the complete removal of cells (19). Decellularization of the human testis with SD also indicated cell removal, but the amount of this detergent was 4%, which was reported to be more than the amount used by us (1% SDS). The agitation time was same in the both studies (24 hours) (20). Triton alone failed to remove cells from the testis of the calf, but in studies where it was used in combination with SDS, complete decellularization was achieved (19).
Decellularization with our methods in other tissues was also similar to our results. The use of 1% SDS and vacuum decellularized the pericardial tissue well and caused proper porosity in this tissue (12). Decellularization of cow ovaries with 0.1% SDS showed acceptable results in cell removal (21), while in our study with this detergent and the same percentage, cell removal did not happen completely, which may be due to the difference in the nature of the ovarian and testis tissues. are each other or the difference in the size of the pieces of tissues in these two studies. The tissue pieces of the testis in the present study were 1 cm3 and 500 μm in the cow ovary. The smaller size of the tissue makes the detergent reach the deep areas better and the phenomenon of cell removal occurs.
Less than 50 ng DNA/mg dry weight must remain in the tissue after decellularization to prevent immune reactions (22). In all the methods of current study, the level of DNA was reported lower than this value, but in freeze-de freeze, 0.1% SDS, and Triton methods, DNA removal was less than in other methods. DNA content after pig testis decellularization with different methods was reported in line with our study (23). The DNA content of mouse testis after decellularization with SDS reached 11.37 μg/mg (24), which was largely similar to our decellularization methods with 1% SDS-vacuum method (12.91 μg/mg).
Preservation of the components and structure of the ECM is very important and necessary to create a proper interaction between the cells and the ECM (25). In this study, special dyes and kits were used to evaluate the preservation of ECM components in testicular tissues. Masson’s trichrome, Orcein, and Alcian blue staining quantitative data confirmed the preservation of collagen, elastin, and glycosaminoglycans, respectively. GAG is one of the main elements in cell adhesion, proliferation, and differentiation in ECM. Common detergents in tissue decellularization cause the destruction of this protein structure. However, histological findings (Alcian blue) and quantitative data obtained from this study showed that this protein was not removed, although its amount was reduced compared to the control group. Collagen and elastin fibers are also largely preserved. Other studies in the field of decellularization of testis tissue (19) and other tissues were in line with our study on this topic (26, 27). Collagen decrease in diseases causes testicular atrophy, showing the importance of the ECM in the normal function of the testis because, in case of damage to the ECM of the testicle, the process of spermatogenesis will be disrupted (28).
SEM images represented that the three-dimensional structure of the testis tissue was well preserved in decellularization by freeze-de freeze and 0.1% SDS methods, but as we said, cell removal did not occur completely in these scaffolds. In other methods, the collagen fibers are separated to some extent, which shows their microscopic images. Decellularization of rat testis tissue was also in line with our findings (29).
The biocompatibility of decellularized testis tissues was assessed using human adipose mesenchymal stem cells (cell line) by indirect MTT assay at 48 and 72 hours. Therefore, the scaffolds are biocompatible and can be used for the proliferation and differentiation of spermatogonia cells. Decellularization of rat uterus and bovine ovary tissue with SDS and Triton did not report cytotoxicity similar to our study (30, 31).
Hemocompatibility is a significant property of the scaffold after it is decellularized. The tissue is usually exposed to blood and hurts the erythrocytes to a specific extent. As per the hemolytic index of ASTM F756, scaffolds are believed to be hemolytic when % hemolysis is >5%; slightly hemolytic at % hemolysis is between 2 and 5%, and nonhemolytic for % hemolysis is <2% (32). In our study, all scaffolds were non-hemolytic.
One of the limitations of studies related to testis decellularization is the low filling percentage of seminiferous tubules for injections due to the tubes collapse; the use of the vacuum system, in addition to helping in better decellularization, creates more porosity in the tissue structure and prevents the tubes falling on top of each other. Therefore, options can be suggested by using detergents such as SDS and SD together with the vacuum method for better decellularization of the testis tissue and spermatogenesis studies.
Conclusion
The results showed that the calf testis is successfully decellularized by using 1% SD and 1% SDS (with and without vacuum). The three-dimensional structure and important components of the ECM are preserved to a large extent. The vacuum helps to create more porosity in the tissue and prevents the overlap of seminiferous tubes, therefore suggesting a suitable ECM for spermatogenesis studies.
Acknowledgments
The authors thank the Fertility and Infertility Research Center, Health Technology Institute, Kermanshah University of Medical Sciences, Kermanshah, Iran. This paper originated from an MD dissertation (research code: 4010199) and was funded research deputy of Kermanshah University of Medical Sciences, Kermanshah, Iran. The authors declare no conflicts of interest with respect to the research, authorship, and/or publication of this article.
Authors’ Contributions
M.R.K.; Performed the dissection experiments, data measurement, the statistical analysis, and wrote the first draft of the manuscript. Z.A.; Contributed to conception, design of the study, performed the dissection experiments, and revised the manuscript. M.Kh.; Performed data measurement and the statistical analysis. L.R.; Contributed to conception and design of the study. All authors contributed to manuscript revision, read, and approved the submitted version.
References
- 1.Faramarzi A, Aghaz F, Golestan Jahromi M, Bakhtiari M, Khazaei M. Does supplementation of sperm freezing/thawing media with Ceratonia siliqua improve detrimental effect of cryopreservation on sperm parameters and chromatin quality in normozoospermic specimens? Cell Tissue Bank. 2019;20(3):403–409. doi: 10.1007/s10561-019-09779-2. [DOI] [PubMed] [Google Scholar]
- 2.Faramarzi A, Khalili MA, Omidi M, Agha-Rahimi A, Taheri F. Pronuclear pattern does not predict morphokinetics behavior in human embryos. Gynecol Endocrinol. 2018;34(3):248–251. doi: 10.1080/09513590.2017.1388365. [DOI] [PubMed] [Google Scholar]
- 3.Neto FT, Bach PV, Najari BB, Li PS, Goldstein M. Spermatogenesis in humans and its affecting factors. Semin Cell Dev Biol. 2016;59:10–26. doi: 10.1016/j.semcdb.2016.04.009. [DOI] [PubMed] [Google Scholar]
- 4.Kubota H, Brinster RL. Spermatogonial stem cells. Biol Reprod. 2018;99(1):52–74. doi: 10.1093/biolre/ioy077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Khazaei F, Rezakhani L, Alizadeh M, Mahdavian E, Khazaei M. Exosomes and exosome-loaded scaffolds: Characterization and application in modern regenerative medicine. Tissue Cell. 2023;80:102007–102007. doi: 10.1016/j.tice.2022.102007. [DOI] [PubMed] [Google Scholar]
- 6.Sharifi M, Kheradmandi R, Salehi M, Alizadeh M, Ten Hagen TLM, Falahati M. Criteria, challenges, and opportunities for acellularized allogeneic/xenogeneic bone grafts in bone repairing. ACS Biomater Sci Eng. 2022;8(8):3199–3219. doi: 10.1021/acsbiomaterials.2c00194. [DOI] [PubMed] [Google Scholar]
- 7.Rahmati S, Khazaei M, Nadi A, Alizadeh M, Rezakhani L. Exosomeloaded scaffolds for regenerative medicine in hard tissues. Tissue Cell. 2023;82:102102–102102. doi: 10.1016/j.tice.2023.102102. [DOI] [PubMed] [Google Scholar]
- 8.Khazaei M, Khazaei MR, Alizadeh M, Rahmati S, Rezakhani L. Functional survey of decellularized tissues transplantation for infertile females. Cell Tissue Bank. 2022;23(3):407–415. doi: 10.1007/s10561-021-09979-9. [DOI] [PubMed] [Google Scholar]
- 9.Sun H, Gong TT, Jiang YT, Zhang S, Zhao YH, Wu QJ. Global, regional, and national prevalence and disability-adjusted life-years for infertility in 195 countries and territories, 1990-2017: results from a global burden of disease study, 2017. Aging (Albany NY) 2019;11(23):10952–10991. doi: 10.18632/aging.102497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Richer G, Baert Y, Goossens E. In-vitro spermatogenesis through testis modelling: toward the generation of testicular organoids. Andrology. 2020;8(4):879–891. doi: 10.1111/andr.12741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Khavarimehr M, Nejati V, Razi M, Najafi G. Ameliorative effect of omega-3 on spermatogenesis, testicular antioxidant status and preimplantation embryo development in streptozotocin-induced diabetes in rats. Int Urol Nephrol. 2017;49(9):1545–1560. doi: 10.1007/s11255-017-1636-5. [DOI] [PubMed] [Google Scholar]
- 12.Alizadeh M, Rezakhani L, Khodaei M, Soleimannejad M, Alizadeh A. Evaluating the effects of vacuum on the microstructure and biocompatibility of bovine decellularized pericardium. J Tissue Eng Regen Med. 2021;15(2):116–128. doi: 10.1002/term.3150. [DOI] [PubMed] [Google Scholar]
- 13.Rahmati S, Jalili A, Dehkordi MB, Przedborski M. An effective method for decellularization of human foreskin: implications for skin regeneration in small wounds. Cell J. 2022;24(9):506–514. doi: 10.22074/cellj.2022.8005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Asgari F, Asgari HR, Najafi M, Eftekhari BS, Vardiani M. The Decellularized Calf Testis 39 Int J Fertil Steril, Vol 18, No 1, January-March 2024 Gholipourmalekabadi M, et al.Optimization of decellularized human placental macroporous scaffolds for spermatogonial stem cells homing. J Mater Sci Mater Med. 2021;32(5):47–47. doi: 10.1007/s10856-021-06517-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang J, Zhao L, Zhang A, Huang Y, Tavakoli J, Tang Y. Novel bacterial cellulose/gelatin hydrogels as 3D scaffolds for tumor cell culture. Polymers (Basel) 2018;10(6):581–581. doi: 10.3390/polym10060581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Díaz E, Puerto I, Ribeiro S, Lanceros-Mendez S, Barandiarán JM. The influence of copolymer composition on PLGA/nHA scaffolds' cytotoxicity and in vitro degradation. Nanomaterials (Basel) 2017;7(7):173–173. doi: 10.3390/nano7070173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sharifi E, Sadati SA, Yousefiasl S, Sartorius R, Zafari M, Rezakhani L, et al. Cell loaded hydrogel containing Ag-doped bioactive glassceramic nanoparticles as skin substitute: Antibacterial properties, immune response, and scarless cutaneous wound regeneration. Bioeng Transl Med. 2022;7(3):e10386–e10386. doi: 10.1002/btm2.10386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ashouri Movassagh S, Ashouri Movassagh S, Banitalebi Dehkordi M, Pourmand G, Gholami K, Talebi A, et al. Isolation, identification and differentiation of human spermatogonial cells on threedimensional decellularized sheep testis. Acta Histochem. 2020;122(8):151623–151623. doi: 10.1016/j.acthis.2020.151623. [DOI] [PubMed] [Google Scholar]
- 19.Bashiri Z, Amiri I, Gholipourmalekabadi M, Falak R, Asgari H, Maki CB, et al. Artificial testis: a testicular tissue extracellular matrix as a potential bio-ink for 3D printing. Biomater Sci. 2021;9(9):3465–3484. doi: 10.1039/d0bm02209h. [DOI] [PubMed] [Google Scholar]
- 20.Murdock MH, David S, Swinehart IT, Reing JE, Tran K, Gassei K, et al. Human testis extracellular matrix enhances human spermatogonial stem cell survival in vitro. Tissue Eng Part A. 2019;25(7-8):663–676. doi: 10.1089/ten.tea.2018.0147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Laronda MM, Jakus AE, Whelan KA, Wertheim JA, Shah RN, Woodruff TK. Initiation of puberty in mice following decellularized ovary transplant. Biomaterials. 2015;50:20–29. doi: 10.1016/j.biomaterials.2015.01.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Isidan A, Liu S, Li P, Lashmet M, Smith LJ, Hara H, et al. Decellularization methods for developing porcine corneal xenografts and future perspectives. Xenotransplantation. 2019;26(6):e12564–e12564. doi: 10.1111/xen.12564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vermeulen M, Del Vento F, de Michele F, Poels J, Wyns C. Development of a cytocompatible scaffold from pig immature testicular tissue allowing human sertoli cell attachment, proliferation and functionality. Int J Mol Sci. 2018;19(1):227–227. doi: 10.3390/ijms19010227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yang Y, Lin Q, Zhou C, Li Q, Li Z, Cao Z, et al. A testis-derived hydrogel as an efficient feeder-free culture platform to promote mouse spermatogonial stem cell proliferation and differentiation. Front Cell Dev Biol. 2020;8:250–250. doi: 10.3389/fcell.2020.00250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Baert Y, Stukenborg JB, Landreh M, De Kock J, Jörnvall H, Söder O, et al. Derivation and characterization of a cytocompatible scaffold from human testis. Hum Reprod. 2015;30(2):256–267. doi: 10.1093/humrep/deu330. [DOI] [PubMed] [Google Scholar]
- 26.Eivazkhani F, Abtahi NS, Tavana S, Mirzaeian L, Abedi F, Ebrahimi B, et al. Evaluating two ovarian decellularization methods in three species. Mater Sci Eng C Mater Biol Appl. 2019;102:670–682. doi: 10.1016/j.msec.2019.04.092. [DOI] [PubMed] [Google Scholar]
- 27.Ventura RD, Padalhin AR, Park CM, Lee BT. Enhanced decellularization technique of porcine dermal ECM for tissue engineering applications. Mater Sci Eng C Mater Biol Appl. 2019;104:109841–109841. doi: 10.1016/j.msec.2019.109841. [DOI] [PubMed] [Google Scholar]
- 28.Cheng CY, Wong EW, Yan HH, Mruk DD. Regulation of spermatogenesis in the microenvironment of the seminiferous epithelium: new insights and advances. Mol Cell Endocrinol. 2010;315(1-2):49–56. doi: 10.1016/j.mce.2009.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kiani M, Movahedin M, Halvaei I, Soleimani M. Formation of organoid-like structures in the decellularized rat testis. Iran J Basic Med Sci. 2021;24(11):1523–1528. doi: 10.22038/IJBMS.2021.58294.12948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Arezoo N, Mohammad H, Malihezaman M. Tissue engineering of mouse uterus using menstrual blood stem cells (MenSCs) and decellularized uterine scaffold. Stem Cell Res Ther. 2021;12(1):475–475. doi: 10.1186/s13287-021-02543-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nikniaz H, Zandieh Z, Nouri M, Daei-Farshbaf N, Aflatoonian R, Gholipourmalekabadi M, et al. Comparing various protocols of human and bovine ovarian tissue decellularization to prepare extracellular matrix-alginate scaffold for better follicle development in vitro. BMC Biotechnol. 2021;21(1):8–8. doi: 10.1186/s12896-020-00658-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Singh H, Purohit SD, Bhaskar R, Yadav I, Bhushan S, Gupta MK, et al. Curcumin in decellularized goat small intestine submucosa for wound healing and skin tissue engineering. J Biomed Mater Res B Appl Biomater. 2022;110(1):210–219. doi: 10.1002/jbm.b.34903. [DOI] [PubMed] [Google Scholar]