Abstract
Background
The possible toxicity of natural products must be tested before being used in the market. The present work aimed to evaluate acute, subacute, and subchronic toxicity of an herbal formulation containing Anethum graveolens, Cynara scolymus, Citrus aurantium, Portulaca oleracea, and Silybum marianum.
Material and methods
Acute toxicity (2000 mg/kg, single dose) and sub-acute toxicity (600 and 1200 mg/kg/day, 4 weeks) tests were performed on female and male rats according to OECD 423 and OECD 407 guidelines, respectively. In the subchronic study (12 weeks), the animals were divided into three groups (6 females and 6 males per group): control, low-dose group (food supplemented with 300 mg/kg of the herbal product), and high-dose group (600 mg/kg).
Results
The herbal product at a single dose of 2000 mg/kg did not induce mortality for 14 days. In the sub-acute study, administration of the product for 28 days at 1200 mg/kg/day had no effect on survival, appetite (water and food consumption), body weight, serum biochemical parameters (BUN, creatinine, AST, ALT, ALP, bilirubin, albumin), histology of vital organs (liver, kidney, heart, brain), and hematological markers related to erythrocyte, platelet, and leukocyte. Similarly, in the subchronic study, the product did not induce mortality, change in histology of the vital organs, or alteration in hematological or biochemical parameters (except for an increase in ALP in female rats received 600 mg/kg).
Conclusion
The formulated product shows no signs of toxicity in rats up to 2000 mg/kg, 1200 mg/kg, and 600 mg/kg in acute, subacute, and subchronic phases, respectively. It is suggested to monitor ALP levels in females in case of long-term use of the product.
Keywords: Herbal, Hepatoprotective, Plants, Safety, Toxicity
Graphical Abstract
Highlights
-
•
Toxicity of an herbal formulation containing dill, artichoke, bitter orange, purslane, and milk thistle was tested.
-
•
In acute toxicity test, the product at 2000 mg/kg did not induce mortality for 14 days.
-
•
In sub-acute study (4 weeks, 1200 mg/kg), no change in biochemical and hematological parameters was observed.
-
•
In subchronic study (12 weeks, 600 mg/kg), the product increased ALP but decrease AST and ALT levels in female rats.
-
•
No histopathological alterations were observed in liver, kidney, heart, and brain.
1. Introduction
Today, the market for herbal products used to improve health is very large. Many people around the world use these products for therapeutic and preventive purposes. Also, an increasing number of drugs used in the clinic are derived from plants, examples of which are: paclitaxel isolated from Taxus brevifolia, aspirin from the willow tree, metformin from Galega officinalis, and digoxin from Digitalis purpurea [3], [12], [14], [17]. One of the main reasons for people's growing interest in herbal products is the belief that natural compounds do not cause serious side effects. However, pharmacological and toxicological evidence suggests that this is a wrong belief. There are numerous studies demonstrating that herbal compounds can be toxic and even fatal [5]. Therefore, any herbal product that is newly formulated must be carefully checked for possible toxicity before being used in the market.
In our previous study, we formulated an herbal product for managing non-alcoholic fatty liver disease (NAFLD) and showed that it decreases the grade of the fatty liver and the serum level of hepatic enzymes [22]. This product contains seeds of Anethum graveolens L. (dill, family Apiaceae), leaves of Cynara scolymus L. (artichoke, family Asteraceae), flower of Citrus aurantium L. (bitter orange, family Rutaceae), seeds of Portulaca oleracea L. (purslane, family Portulacaceae), and seeds of Silybum marianum L. (milk thistle, family Asteraceae). Previous studies have shown that each of these plants has antioxidant and/or anti-inflammatory activities [6], [8], [9], [11], [21]. In addition, they showed hepatoprotective [1], [7], [18], [21], nephroprotective [4], [11], [13], and cardioprotective [2], [20] activities in different animal models of the organ damage. However, we cannot ignore the possibility that the combination of these plants in one product may cause side effects in the body.
Therefore, the present work was designed to evaluate the possible toxicity (acute, subacute, and subchronic) of this herbal formulation in rats.
2. Material and methods
2.1. Preparing the herbal formulation
The herbal product was obtained from the Herbal Medicine Division of Imam-Reza Pharmacy (Mashhad, Iran). This product was formulated as a 400 mg capsule containing equal amounts (80 mg) of the powder of each plant (Anethum graveolens seeds, Cynara scolymus leaves, Citrus aurantium flower, Portulaca oleracea seeds, and Silybum marianum seeds). The herbal formulation contains a large amount of polyphenols (106 ± 9 mg gallic acid equivalent per gram of the product) as determined by Folin-Ciocalteu method [10].
To prepare the hydroalcoholic extract, the content of 100 capsules (40 g) was soaked in 70% ethanol (1 L) for 3 days at 40 °C with occasional shaking. The mixture was centrifuged for 5 min at 500 g to sediment the particles. The supernatant extract was collected and its solvent was evaporated in an oven at 40 °C.
2.2. Animals
Female and male albino Wistar rats weighing 180–230 g were obtained from the Laboratory Animals Research Center at Mashhad University of Medical Sciences (Mashhad, Iran). The animals were housed by sex in polypropylene cages under a 12:12 h light/dark cycle at a controlled temperature (22 ± 2 °C) with tap water and food ad libitum. All experiments were carried out under the internationally accepted principles for laboratory animal use and approved by the Ethics Committee of Mashhad University of Medical Sciences (Ethics Committee number: IR.MUMS.MEDICAL.REC.1400.280).
2.3. Acute toxicity test
An acute oral toxicity study was performed according to OECD guideline number 423 [15]. Female rats were randomly divided into three groups: control, intervention-1, and intervention-2. Before starting the test, all animals were fasted overnight. The control group was given saline as a vehicle. Rats in the intervention-1 group were gavaged with a single dose of 2000 mg/kg of the extract of herbal product. In group intervention-2, the herbal product was added to the food (3% w/w, ∼2000 mg/kg/day) for 24 h. The animals were observed individually for mortality and any behavioral signs of toxicity (convulsion, hypo-activity, abdominal rigidity, breathing difficulty, cyanosis, etc.) for 4 h after dosing. Observations were continued periodically for 24 h and then every day for two weeks. Body weights were measured before intervention and at the end of the second week. On the 14th day, the animals were euthanized after deep anesthesia (100 mg/kg of ketamine and 10 mg/kg of xylazine) and a necropsy was done to find apparent changes in the main organs and tissues.
2.4. Sub-acute toxicity test
The experiment was performed following OECD guideline number 407 [16]. Thirty-six rats were randomly divided into three groups of 12 each (6 males and 6 females per group). The control group received the usual food for 28 days. The second group received the food supplemented with the powder of herbal product at a dose of 600 mg/kg/day. The third group received the food supplemented with 1200 mg/kg/day of the product for 28 days. The body weight of the animals, the water intake, and the food consumption were measured before and after the study. During the experiment, animals were monitored for behavioral signs of toxicity as mentioned above. At the end of the study, the animals were fasted and euthanized after deep anesthesia, and blood sampling was performed by cardiac puncture for biochemical and hematologic analyses. Some body organs (heart, kidney, liver, brain, and spleen) were removed and weighed precisely to calculate relative organ weights (organ weight/body weight). Except for the spleen, other organs were fixed in formalin and subjected to routine processing (dehydration, embedding in paraffin, preparing 5 µm sections) for staining with Haematoxylin and eosin and histopathological examination.
2.5. Subchronic toxicity test
In this test, 36 rats were divided into three groups of 12 each (6 females and 6 males per group). Control animals received the usual food for 12 weeks. The animals in the low-dose group received the food supplemented with the powder of herbal product at a dose of 300 mg/kg/day. The rats in the high-dose group received the food supplemented with 600 mg/kg/day of the product for 12 weeks. During the experiment, the body weight, water intake, and food consumption were recorded and doses of the product were adjusted based on the amount of food consumption and body weight. At the end of the study, the rats were fasted, anesthetized (100 mg/kg of ketamine and 10 mg/kg of xylazine), and subjected to blood sampling (by cardiac puncture) for biochemical and hematologic analyses. The body organs were weighed and fixed in formalin for histopathological examination as described above.
2.6. Statistical analysis
Comparison of data between control and treated groups was made by one-way analysis of variance (ANOVA), followed by Tukey HSD post hoc test. The repeated measures two-way ANOVA was used to analyze the change in water intake, food consumption, and body weight during different times of study. The paired t-test was used to compare data before and after the experiment. All results are shown as a mean ± standard error of the mean (SEM) and a P-value of less than 0.05 was considered statistically significant.
3. Results
3.1. Acute toxicity of the herbal product
After administration of a single dose of 2000 mg/kg of the extract or the food supplemented with the herbal product, the animals were observed for 14 days. No behavioral signs of toxicity and no mortality were observed in any groups during the experiment. Also, at necropsy, no macroscopic alterations in the heart, lungs, and visceral organs were observed. As shown in Fig. 1, no significant difference in body weight was found between the control and treated groups.
Fig. 1.
Body weight of female rats treated with a single dose of 2000 mg/kg of the extract (Intervention-1) or with the food supplemented with the herbal product (Intervention-2).
3.2. Sub-acute toxicity of the herbal product
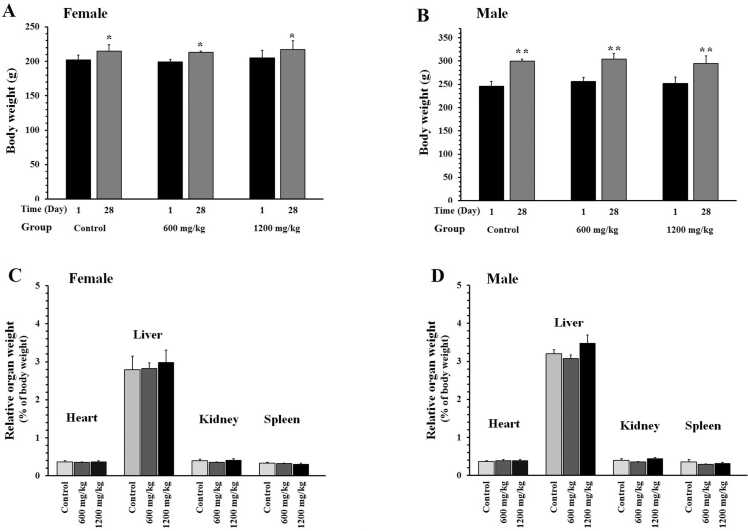
Repeated administration of the herbal product at 600 and 1200 mg/kg did not induce any sign of toxicity in the female and male rats. No mortality was found in any of the groups during the experiment. The body weight of both female and male animals showed a normal trend (Figs. 2A and 2B). Relative organ weight (heart, liver, kidney, and spleen) did not show significant changes between the groups (Figs. 2C and 2D). Food and water consumption in male rats were different before and after the study (Table 1). However, the herbal product had no significant effect on these parameters.
Fig. 2.
Effects of the herbal product on body weight and organ weight of female (A and C) and male (B and D) rats. The animals received the food supplemented with the powder of herbal product at doses of 600 or 1200 mg/kg/day for 28 days. Data are shown as mean ± SEM (n = 6). *P < 0.05 and * *P < 0.01 compared to day 1 in the corresponding group.
Table 1.
Effects of the herbal product on water intake and food consumption of rats in sub-acute toxicity test. The animals received the food supplemented with the powder of herbal product at doses of 600 or 1200 mg/kg/day for 28 days. Data are shown as mean ± SEM (n = 6).
| Parameters | Animal sex | Time | Study group |
||
|---|---|---|---|---|---|
| Control | 600 mg/kg | 1200 mg/kg | |||
| Water intake (mL/24 h) | Female | Day 1 | 32 ± 1 | 30 ± 0.5 | 31 ± 1 |
| Day 28 | 34 ± 1.5 | 32 ± 1 | 33 ± 1.5 | ||
| Male | Day 1 | 44 ± 4 | 42 ± 2 | 38 ± 2 | |
| Day 28 | 48 ± 4** | 46 ± 3** | 41 ± 1.5** | ||
| Food consumption (g/week) | Female | Day 1 | 98 ± 5 | 115 ± 6 | 107 ± 14 |
| Day 28 | 101 ± 5 | 120 ± 7.5 | 111 ± 15 | ||
| Male | Day 1 | 141 ± 8 | 125 ± 2 | 142 ± 6 | |
| Day 28 | 147 ± 10* | 130 ± 3** | 149 ± 6** | ||
P < 0.05 and
P < 0.01 compared to day 1 in the corresponding group.
Table 2 summarizes the serum levels of biochemical parameters of rats in sub-acute toxicity test. No significant alterations among groups were observed in the parameters of kidney function including blood urea nitrogen (BUN) and creatinine. Also, the serum factors of liver activity did not change significantly. These factors include bilirubin, aspartate aminotransferase (AST), alanine transaminase (ALT), alkaline phosphatase (ALP), and albumin. Only a mild, but statistically insignificant, increase was seen in the level of ALP in rats treated with 600 and 1200 mg/kg of the product.
Table 2.
Effects of the herbal product on the serum biochemical parameters of rats in sub-acute toxicity test. The animals received the food supplemented with the powder of herbal product at doses of 600 or 1200 mg/kg/day for 28 days. At the end of the study, blood sampling was performed by cardiac puncture for biochemical analyses. Data are shown as mean ± SEM (n = 6).
| Parameters | Animal sex | Study group |
||
|---|---|---|---|---|
| Control | 600 mg/kg | 1200 mg/kg | ||
| BUN (mg/dL) | Female | 59 ± 3 | 53 ± 3 | 50 ± 2 |
| Male | 57 ± 6 | 56 ± 3 | 55 ± 3 | |
| Creatinine (mg/dL) | Female | 0.93 ± 0.07 | 0.88 ± 0.04 | 0.91 ± 0.02 |
| Male | 0.93 ± 0.07 | 0.94 ± 0.03 | 0.85 ± 0.03 | |
| Total bilirubin (mg/dL) | Female | 0.65 ± 0.02 | 0.64 ± 0.02 | 0.61 ± 0.02 |
| Male | 0.63 ± 0.01 | 0.63 ± 0.01 | 0.59 ± 0.1 | |
| Direct bilirubin (mg/dL) | Female | 0.21 ± 0.04 | 0.22 ± 0.01 | 0.24 ± 0.01 |
| Male | 0.20 ± 0.01 | 0.19 ± 0.02 | 0.19 ± 0.01 | |
| AST (U/L) | Female | 152 ± 21 | 109 ± 4.5 | 170 ± 46 |
| Male | 157 ± 21 | 137 ± 12 | 180 ± 47 | |
| ALT (U/L) | Female | 73 ± 19 | 53 ± 6 | 75 ± 13 |
| Male | 61 ± 4.3 | 58 ± 8.5 | 92 ± 39 | |
| ALP (U/L) | Female | 224 ± 19 | 259 ± 56 | 217 ± 40 |
| Male | 267 ± 32 | 319 ± 44 | 355 ± 31 | |
| Albumin (mg/dL) | Female | 4.6 ± 0.1 | 4.7 ± 0.06 | 4.7 ± 0.1 |
| Male | 4.4 ± 0.1 | 4.6 ± 0.03 | 4.5 ± 0.03 | |
ALP: alkaline phosphatase; ALT: alanine transaminase; AST: aspartate aminotransferase; BUN: blood urea nitrogen
Hematological parameters of the animals were evaluated as shown in Table 3. The levels of these parameters in female and male rats treated with 600 or 1200 mg/kg did not differ significantly from those of control rats.
Table 3.
Effects of the herbal product on hematological parameters of rats in sub-acute toxicity test. The animals received the food supplemented with the powder of herbal product at doses of 600 or 1200 mg/kg/day for 28 days. At the end of the study, blood sampling was performed by cardiac puncture for a complete blood count. Data are shown as mean ± SEM (n = 6).
| Parameters | Animal sex | Study group |
||
|---|---|---|---|---|
| Control | 300 mg/kg | 600 mg/kg | ||
| RBC (×106/μL) | Female | 8.5 ± 0.2 | 8.2 ± 0.16 | 8.5 ± 0.1 |
| Male | 9 ± 0.1 | 8.6 ± 0.1 | 9 ± 0.1 | |
| Hemoglobin (g/dL) | Female | 14.4 ± 0.2 | 13.8 ± 0.1 | 14.2 ± 0.2 |
| Male | 14.8 ± 0.2 | 14.2 ± 0.2 | 14.9 ± 0.2 | |
| Hematocrit (%) | Female | 43.3 ± 1 | 42.4 ± 0.3 | 43.4 ± 1 |
| Male | 45 ± 0.4 | 42 ± 1 | 45 ± 1 | |
| MCV (fL) | Female | 51 ± 1 | 52 ± 1 | 51 ± 1 |
| Male | 49 ± 0.7 | 49 ± 0.6 | 50 ± 0.4 | |
| MCH (pg) | Female | 17 ± 0.1 | 17 ± 0.2 | 16.5 ± 0.1 |
| Male | 16.3 ± 0.3 | 16.5 ± 0.2 | 16.4 ± 0.2 | |
| MCHC (g/dL) | Female | 33 ± 0.2 | 32 ± 0.2 | 33 ± 0.2 |
| Male | 33 ± 0.1 | 33 ± 0.1 | 33 ± 0.2 | |
| Platelets (×103/μL) | Female | 973 ± 81 | 933 ± 58 | 1032 ± 42 |
| Male | 893 ± 51 | 898 ± 46 | 937 ± 23 | |
| WBC (×103/μL) | Female | 3.4 ± 0.5 | 3.7 ± 0.8 | 3.6 ± 0.6 |
| Male | 6 ± 0.5 | 7.7 ± 1.7 | 4.5 ± 1.1 | |
| Neutrophils (%) | Female | 24 ± 1.7 | 22 ± 2.5 | 22 ± 1.8 |
| Male | 26 ± 1.6 | 23 ± 3.5 | 30 ± 4.7 | |
| Lymphocytes (%) | Female | 75 ± 1.8 | 76 ± 2 | 77 ± 1.7 |
| Male | 74 ± 1.7 | 76 ± 3.5 | 70 ± 4.7 | |
| Monocytes (%) | Female | 0.6 ± 0.1 | 2.6 ± 2 | 0.4 ± 0.1 |
| Male | 0.06 ± 0.02 | 0.0 | 0.06 ± 0.02 | |
| Eosinophils (%) | Female | 0.0 | 0.06 ± 0.02 | 0.03 ± 0.02 |
| Male | 0.02 ± 0.02 | 0.02 ± 0.02 | 0.0 | |
| Basophils (%) | Female | 0.1 ± 0.05 | 0.1 ± 0.06 | 0.1 ± 0.06 |
| Male | 0.22 ± 0.1 | 0.3 ± 0.06 | 0.2 ± 0.07 | |
MCH: mean corpuscular hemoglobin; MCHC: mean corpuscular hemoglobin concentration; MCV: mean corpuscular volume; RBC: red blood cell; WBC: white blood cells
Haematoxylin and eosin staining showed no structural alterations in the organs of animals treated with 1200 mg/kg of the product (Supplementary Fig. 1). In the liver, the portal triad, hepatocytes, and sinusoids appeared normal. In the kidney, the Bowman’s capsule, the glomerular capillaries, and the renal tubes did not show pathological alterations. The heart structure showed normal muscle fibers and intercalated discs. Histology of the brain showed no degeneration.
3.3. Subchronic toxicity of the herbal product
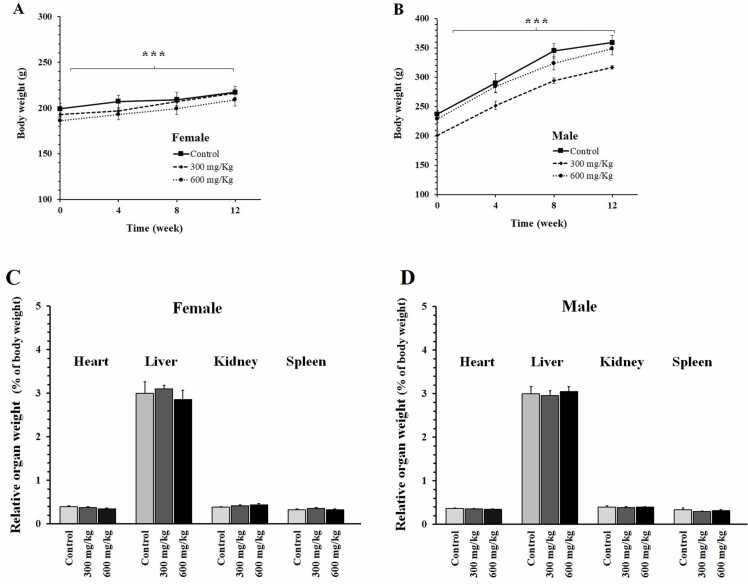
Administration of the herbal product at doses of 300 or 600 mg/kg for 12 weeks did not induce mortality or clinical signs of toxicity. The body weight of both female and male rats showed a regular increase during the experiment (Fig. 3A and B). At the end of the study, no significant difference was found in the weight of the heart, liver, kidney, and spleen between the groups (Figs. 3C and 2D).
Fig. 3.
Effects of the herbal product on body weight and organ weight of female (A and C) and male (B and D) rats. The animals received the food supplemented with the powder of herbal product at doses of 300 or 600 mg/kg/day for 12 weeks. Data are shown as mean ± SEM (n = 6). *** : repeated measures ANOVA (P < 0.001, showing the effect of time as a within-subjects factor on body weight).
Fluctuation was observed in water intake (Fig. 4A and B) and food consumption (Figs. 4C and 4D) in all groups during the experiment. Although there was a significant difference between groups related to water and food intake, the herbal product did not exert a cachexic effect.
Fig. 4.
Effects of the herbal product on water intake and food consumption of rats in sub-chronic toxicity test. The animals received the food supplemented with the powder of herbal product at doses of 300 or 600 mg/kg/day for 12 weeks. Data are shown as mean ± SEM (n = 6). *** : repeated measures ANOVA (P < 0.001, showing the effect of time as a within-subjects factor on food consumption); ## P < 0.01 compared to the control group at week 12.
Biochemical parameters of subchronic oral toxicity are shown in Table 4. No significant differences were observed in the parameters of kidney function (BUN and creatinine) between groups. Also, the levels of bilirubin and albumin did not change significantly. Only a decrease in AST and ALT levels (P < 0.05) and an increase in ALP level (P < 0.01) were seen in female rats treated with 300 mg/kg and 600 mg/kg of the product, respectively.
Table 4.
Effects of the herbal product on the serum biochemical parameters of rats in subchronic toxicity test. The animals received the food supplemented with the powder of herbal product at doses of 300 or 600 mg/kg/day for 12 weeks. At the end of the study, blood sampling was performed by cardiac puncture for biochemical analyses. Data are shown as mean ± SEM (n = 6).
| Parameters | Animal sex | Study group |
||
|---|---|---|---|---|
| Control | 300 mg/kg | 600 mg/kg | ||
| BUN (mg/dL) | Female | 61 ± 2 | 59 ± 4 | 56 ± 3 |
| Male | 50 ± 2.2 | 51 ± 3.5 | 51 ± 4 | |
| Creatinine (mg/dL) | Female | 0.82 ± 0.03 | 0.81 ± 0.03 | 0.84 ± 0.34 |
| Male | 0.83 ± 0.03 | 0.80 ± 0.02 | 0.88 ± 0.04 | |
| Total bilirubin (mg/dL) | Female | 0.65 ± 0.01 | 0.64 ± 0.01 | 0.63 ± 0.01 |
| Male | 0.58 ± 0.02 | 0.62 ± 0.02 | 0.63 ± 0.02 | |
| Direct bilirubin (mg/dL) | Female | 0.20 ± 0.02 | 0.23 ± 0.01 | 0.21 ± 0.01 |
| Male | 0.17 ± 0.01 | 0.24 ± 0.04 | 0.21 ± 0.01 | |
| AST (U/L) | Female | 128 ± 11 | 95 ± 3* | 118 ± 7 |
| Male | 159 ± 15 | 150 ± 7 | 117 ± 12 | |
| ALT (U/L) | Female | 52 ± 3 | 36 ± 1** | 54 ± 4 |
| Male | 55 ± 2.5 | 50 ± 4 | 46 ± 3.5 | |
| ALP (U/L) | Female | 184 ± 14 | 202 ± 16 | 283 ± 26** |
| Male | 270 ± 35 | 248 ± 9 | 223 ± 18 | |
| Albumin (mg/dL) | Female | 4.4 ± 0.08 | 4.5 ± 0.08 | 4.5 ± 0.1 |
| Male | 4.2 ± 0.05 | 4 ± 0.1 | 4.2 ± 0.1 | |
ALP: alkaline phosphatase; ALT: alanine transaminase; AST: aspartate aminotransferase; BUN: blood urea nitrogen;
P < 0.05,
P < 0.01 compared to control group
Hematological biomarkers of the animals treated with the herbal product are presented in Table 5. Administration of the product at doses of 300 and 600 mg/kg/day for 12 weeks had no significant effect on the biomarkers related to erythrocyte, platelet, and leukocyte.
Table 5.
Effects of the herbal product on hematological parameters of rats in subchronic toxicity test. The animals received the food supplemented with the powder of herbal product at doses of 300 or 600 mg/kg/day for 12 weeks. At the end of the study, blood sampling was performed by cardiac puncture for a complete blood count. Data are shown as mean ± SEM (n = 6).
| Parameters | Animal sex | Study group |
||
|---|---|---|---|---|
| Control | 300 mg/kg | 600 mg/kg | ||
| RBC (×106/μL) | Female | 8.5 ± 0.2 | 8.3 ± 0.16 | 8.3 ± 0.18 |
| Male | 9.1 ± 0.1 | 8.7 ± 0.2 | 8.7 ± 0.1 | |
| Hemoglobin (g/dL) | Female | 14.4 ± 0.24 | 13.6 ± 0.20 | 13.8 ± 0.28 |
| Male | 14.6 ± 0.15 | 14 ± 0.2 | 14 ± .1 | |
| Hematocrit (%) | Female | 43.3 ± 1 | 41 ± 1 | 42.5 ± 1 |
| Male | 44 ± 0.5 | 42 ± 1 | 42 ± 0.4 | |
| MCV (fL) | Female | 51 ± 1 | 49.3 ± 1 | 51.3 ± 1 |
| Male | 48 ± 0.2 | 48 ± 0.5 | 48 ± 0.2 | |
| MCH (pg) | Female | 17 ± 0.1 | 16.4 ± 0.1 | 17 ± 0.2 |
| Male | 16 ± 0.1 | 16 ± 0.2 | 16 ± 0.1 | |
| MCHC (g/dL) | Female | 33 ± 0.2 | 33 ± 0.1 | 32 ± 0.1 |
| Male | 33 ± 0.2 | 33 ± 0.1 | 33.5 ± 0.1 | |
| Platelets (×103/μL) | Female | 973 ± 81 | 1100 ± 21 | 1029 ± 39 |
| Male | 950 ± 31 | 857 ± 27 | 950 ± 26 | |
| WBC (×103/μL) | Female | 3.7 ± 0.8 | 3 ± 0.4 | 4.7 ± 1.1 |
| Male | 6.7 ± 0.7 | 6.4 ± 1 | 9.4 ± 0.7 | |
| Neutrophils (%) | Female | 24.5 ± 2 | 23 ± 1.2 | 21 ± 2.8 |
| Male | 28 ± 3 | 22.5 ± 3 | 20 ± 1 | |
| Lymphocytes (%) | Female | 75 ± 1.8 | 76 ± 1.3 | 76 ± 1.3 |
| Male | 72 ± 3 | 77 ± 3 | 80 ± 1.3 | |
| Monocytes (%) | Female | 1 ± 0.1 | 1 ± 0.6 | 2.7 ± 2 |
| Male | 0.1 ± 0.03 | 0.4 ± 0.2 | 0.0 | |
| Eosinophils (%) | Female | 0.0 | 0.1 ± 0.06 | 0.08 ± 0.02 |
| Male | 0.06 ± 0.2 | 0.0 | 0.0 | |
| Basophils (%) | Female | 0.1 ± 0.05 | 0.07 ± 0.03 | 0.1 ± 0.05 |
| Male | 0.1 ± 0.03 | 0.1 ± 0.02 | 0.2 ± 0.07 | |
MCH: mean corpuscular hemoglobin; MCHC: mean corpuscular hemoglobin concentration; MCV: mean corpuscular volume; RBC: red blood cell; WBC: white blood cells
Histopathological examination showed no pathological abnormalities in the liver, kidney, heart, and brain of female (Fig. 5) and male (Fig. 6) rats across all the groups.
Fig. 5.
Photomicrographs of histological sections obtained from the liver, kidney, heart, and brain of female rats received the powder of herbal product at doses of 600 mg/kg/day for 12 weeks (original magnification: 100 ×).
Fig. 6.
Photomicrographs of histological sections obtained from the liver, kidney, heart, and brain of male rats received the powder of herbal product at doses of 600 mg/kg/day for 12 weeks (original magnification: 100 ×).
4. Discussion
Consumption of herbal products has increased worldwide for nutritional supplements and medicinal purposes. The beneficial therapeutic effects of the product are seen only in the appropriate doses and length of treatment. Serious adverse effects may be observed in case of long-term use in high doses [5]. Therefore, the possible toxicity of formulated herbal products must be tested before being used in the market. The present work aimed to evaluate acute, subacute, and subchronic toxicity of a hepatoprotective herbal formulation.
The acute toxicity study suggests that the herbal product at a single dose of 2000 mg/kg does not induce mortality or severe toxicity. A dose of 2000 mg/kg in rats is approximately equivalent to 324 mg/kg in humans, according to the following formula:
| Human dose = Animal dose × (Animal Km / Human Km) [19] |
Where Km is approximately 6 and 37 for a 0.15 kg rat and a 60 kg human, respectively. Therefore, the median lethal dose (LD50) of the product or its extract appears to be higher than 324 mg/kg for adults (approximately 20 g for a 60 kg subject).
In the sub-acute toxicity test, administration of the product for 28 days at a dose of 1200 mg/kg/day did not affect survival, appetite (water and food consumption), body weight, organs weight, and histology of vital organs (liver, kidney, heart, brain). Also, serum biochemical parameters showed no significant alterations in the function of the kidney (BUN and creatinine) and liver (AST, ALT, ALP, bilirubin, albumin). Biomarkers related to erythrocytes, platelets, and leukocytes did not change after treatment. All these findings suggest that repeated use (28 days) of the product at 1200 mg/kg (approximately 200 mg/kg in humans) does not induce significant toxic effects.
Similarly, in the subchronic study (12 weeks), the product did not induce mortality, clinical signs of toxicity, change in the pathology of the vital organs, or alteration in hematological biomarkers. In biochemical parameters, only an increase in ALP level was observed in female rats treated with 600 mg/kg of the product. On the other hand, the levels of other liver enzymes (AST and ALT) did not increase in male and female rats. Since the level of bilirubin did not increase and liver tissue showed normal histology, the change of ALP seems to be not a sign of hepatotoxicity.
One of the limitations of the sub-acute and sub-chronic experiments is that the herbal product was given to the animals as a mixture with food, not as a gavage. This route of drug administration was chosen to prevent damage to the gastrointestinal tract caused by repeated daily exposure to a gavage needle. The pharmacokinetics of the product when administered daily as a single gavage dose is different from when it is continuously administered with food. Therefore, it cannot be ruled out that the results would have been different if the product had been administered by gavage. Another limitation of the present study is that the duration of the treatment was continued for a maximum of 12 weeks and not longer. Chronic toxicity tests are generally 6–12 months in duration. Therefore, the results of our study cannot guarantee the safety of the product over a considerable part of the lifespan of the species and further studies are required on this issue.
In conclusion, the formulated herbal product shows no signs of toxicity in rats up to 2000 mg/kg, 1200 mg/kg, and 600 mg/kg in acute, subacute, and subchronic phases, respectively. It is suggested to monitor ALP levels in females in case of long-term use of the product.CRediT
CRediT authorship contribution statement
AB took care of the animals, treated them, and performed the measurements. MM, and MSA participated in conducting the animal experiments. STJ conducted the histopathological examination. SAZ suggested the idea of conducting the study and participated in the discussion of results. AG designed and supervised the study, analyzed data, and wrote the manuscript. All authors read the manuscript before submission.
Funding
This study was carried out with the joint financial support of Mashhad University of Medical Sciences (grant No. 992028, 70% support) and Zojaj Darman Toos Drug Company (30% support).
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Handling Editor: Prof. L.H. Lash
Footnotes
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.toxrep.2023.11.002.
Appendix A. Supplementary material
Supplementary material.
.
Data Availability
Data will be made available on request.
References
- 1.Almalki W.H. Citrus aurantium flowers essential oil protects liver against ischemia/reperfusion injury. South Afr. J. Bot. 2021;142:325–334. [Google Scholar]
- 2.Ariyanfar H., Matinhomaee H., Hosseini S.A., Ghazalian F. The Effects of Endurance Training and Purslane (Portulaca oleracea) Seed Consumption on Cytochrome-C and Malondialdehyde in the Heart Tissues of Rats Poisoned with H 2 O 2. J. Fasti Health. 2020;8 [Google Scholar]
- 3.Bailey C.J. Metformin: historical overview. Diabetologia. 2017;60:1566–1576. doi: 10.1007/s00125-017-4318-z. [DOI] [PubMed] [Google Scholar]
- 4.Bakry L., El-Hameed A., El-Twab S., Ahmed O., Abel-Moneim A. The preventive effects of Cynara scolymus leaf and flower extracts on diethylnitrosamine/acetylaminoflourene induced nephrotoxicity in male wistar rats. Adv. Anim. Vet. Sci. 2020;8:74–81. [Google Scholar]
- 5.Başaran N., Paslı D., Başaran A.A. Unpredictable adverse effects of herbal products. Food Chem. Toxicol. 2022;159 doi: 10.1016/j.fct.2021.112762. [DOI] [PubMed] [Google Scholar]
- 6.Ben Salem M., Affes H., Athmouni K., Ksouda K., Dhouibi R., et al. Chemicals compositions, antioxidant and anti-inflammatory activity of Cynara scolymus leaves extracts, and analysis of major bioactive polyphenols by HPLC. Evid. -Based Complement. Altern. Med. 2017;2017 doi: 10.1155/2017/4951937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Colak E., Ustuner M.C., Tekin N., Colak E., Burukoglu D., et al. The hepatocurative effects of Cynara scolymus L. leaf extract on carbon tetrachloride-induced oxidative stress and hepatic injury in rats. SpringerPlus. 2016;5:1–9. doi: 10.1186/s40064-016-1894-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Degirmenci H., Erkurt H. Chemical profile and antioxidant potency of Citrus aurantium L. flower extracts with antibacterial effect against foodborne pathogens in rice pudding. LWT. 2020;126 [Google Scholar]
- 9.Hamza L.F. Anethum graveolens: Physicochemical properties, medicinal uses, antimicrobial effects, antioxidant effect, anti-inflammatory and analgesic effects: A review. world. 2017;5 [Google Scholar]
- 10.Hooshmand S., Mahdinezhad M.R., Taraz Jamshidi S., Soukhtanloo M., Mirzavi F., et al. Morus nigra L. extract prolongs survival of rats with hepatocellular carcinoma. Phytother. Res. 2021;35:3365–3376. doi: 10.1002/ptr.7056. [DOI] [PubMed] [Google Scholar]
- 11.Lee A.S., Lee Y.J., Lee S.M., Yoon J.J., Kim J.S., et al. An aqueous extract of Portulaca oleracea ameliorates diabetic nephropathy through suppression of renal fibrosis and inflammation in diabetic db/db mice. Am. J. Chin. Med. 2012;40:495–510. doi: 10.1142/S0192415X12500383. [DOI] [PubMed] [Google Scholar]
- 12.Mahdi J.G. Medicinal potential of willow: A chemical perspective of aspirin discovery. J. Saudi Chem. Soc. 2010;14:317–322. [Google Scholar]
- 13.Malkani N., Naeem A., Ijaz F., Mumtaz S., Ashraf S., Sohail M.I. Silybum marianum (milk thistle) improves vancomycin induced nephrotoxicity by downregulating apoptosis. Mol. Biol. Rep. 2020;47:5451–5459. doi: 10.1007/s11033-020-05635-9. [DOI] [PubMed] [Google Scholar]
- 14.Mamadalieva N., Mamedov N. Taxus brevifolia a high-value medicinal plant, as a source of taxol. Med. Aromat. Plants North Am. 2020:201–218. [Google Scholar]
- 15.OECD (2001) Acute oral toxicity – Acute toxic class method. Available at: 〈https://www.oecd-ilibrary.org/docserver/9789264071001-en.pdf?expires=1697216147&id=id&accname=guest&checksum=21EDC97EBDCAC83B9912EADC3BD354C3〉 (Accessed: 13 October 2023).
- 16.OECD (2008) Repeated dose 28-day oral toxicity study in rodents. Available at: 〈https://www.oecd-ilibrary.org/docserver/9789264070684-en.pdf?expires=1697216518&id=id&accname=guest&checksum=6900FF865D6518DD067FB7AE8BE0F04C〉 (Accessed: 13 October 2023).
- 17.Pal V., Pant M. Exploring Poisonous Plants. CRC Press; 2023. Digitalis purpurea (Foxglove Plant) In; pp. 241–254. [Google Scholar]
- 18.Qiao J.-Y., Li H.-W., Liu F.-G., Li Y.-C., Tian S., et al. Effects of Portulaca oleracea extract on acute alcoholic liver injury of rats. Molecules. 2019;24:2887. doi: 10.3390/molecules24162887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Reagan‐Shaw S., Nihal M., Ahmad N. Dose translation from animal to human studies revisited. FASEB J. 2008;22:659–661. doi: 10.1096/fj.07-9574LSF. [DOI] [PubMed] [Google Scholar]
- 20.Vilahur G., Casaní L., Peña E., Crespo J., Juan-Babot O., et al. Silybum marianum provides cardioprotection and limits adverse remodeling post-myocardial infarction by mitigating oxidative stress and reactive fibrosis. Int. J. Cardiol. 2018;270:28–35. doi: 10.1016/j.ijcard.2018.06.030. [DOI] [PubMed] [Google Scholar]
- 21.Wang X., Zhang Z., Wu S.-C. Health benefits of Silybum marianum: Phytochemistry, pharmacology, and applications. J. Agric. Food Chem. 2020;68:11644–11664. doi: 10.1021/acs.jafc.0c04791. [DOI] [PubMed] [Google Scholar]
- 22.Zojaji S.A., Mozaffari H.M., Ghaderi P., Zojaji F., Hadjzadeh M.-A.-R., et al. Efficacy of an herbal compound in decreasing steatosis and transaminase activities in non-alcoholic fatty liver disease: a randomized clinical trial. Braz. J. Pharm. Sci. 2022;58 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material.
Data Availability Statement
Data will be made available on request.