Abstract
Purpose
While computed tomography pulmonary angiography plays an effective role in the diagnosis and prognosis of pulmonary embolism (PE), there are not enough studies regarding ventilation/perfusion (V/Q) scintigraphy. We aimed to evaluate the clinical outcomes of PE patients whose V/Q scintigraphy was reported as high probability for PE.
Method
Demographic data, Simplified Pulmonary Embolism Severity Index (SPESI), radiological findings, V/Q scintigraphy and echocardiographic (ECHO) findings, laboratory data, treatment information and comorbidities of 43 patients whose V/Q scintigraphy was reported as high probability for PE between January 2020 and January 2023 was recorded. Perfusion scintigraphy defects were classified as subsegmental, multiple subsegmental, segmental, and multiple segmental. Those with subsegmental, multiple subsegmental, and segmental perfusion defects were classified as Group 1, and those with multiple segmental defects as Group 2.
Results
The mean age of the patients was 74 years (31–94), being 27 women (62.8 %) and 16 men (37.2 %), and there was no significant difference between the two groups. Multisegmental perfusion defect was detected in 23 (53.5 %) patients. 25 % of patients reported as high-probability PE had a SPESI score of ≥2. There was no significant difference between Groups 1 and 2 in terms of SPESI scoring. Perfusion defect had no significant correlation with SPESI score, D-Dimer, Troponin, pulmonary artery systolic pressure, right ventricular dilatation, and length of hospital stay. The presence of comorbidity was significantly positively correlated only with the SPESI score. There was no difference between the two groups regarding laboratory, radiological, echocardiographic findings, presence of comorbidity, unit of treatment, and duration of hospitalization.
Conclusion
Parameters predicting clinical severity and providing treatment benefits are required in PE patients diagnosed with V/Q scintigraphy.
Keywords: V/Q scintigraphy, Pulmonary embolism, High probability
1. Introduction
Computed tomography pulmonary angiography (CTPA) is preferred more frequently in the diagnosis of pulmonary embolism (PE) since it is easily accessible and is also included in the exclusion of diseases in the differential diagnosis of PE. Right ventricular (RV) dilatation can indicate cardiac involvement by measuring the diameter of the pulmonary artery (PA). While there is a specificity and sensitivity of over 90 % in diagnosing PE in the main pulmonary artery, its lobar and segmental branches, these rates decrease in subsegmental embolisms [1,2]. The diagnostic performance of Ventilation/Perfusion (V/Q) Single-Photon Emission Computed Tomography (SPECT) is comparable to and even exceeding that of CTPA, yet non-diagnostic rates are similar (<5 %) [[3], [4], [5]]. Although there have been significant developments in CT technology to improve image quality and reduce radiation dose, the effective doses of CTPA protocols are still several times higher than those of VQ SPECT, and even the dose absorbed in breast tissue is 18–40 times higher [6]. Therefore, the examination increases the risk of cancer. V/Q scintigraphy is especially preferred in patients with renal failure, and its diagnostic value is higher than CTPA in subsegmental embolisms. Since comorbid pulmonary disorders are less common during pregnancy, V/Q scintigraphy can be interpreted more easily [7].
V/Q scintigraphy is reported as normal, near-normal, low-, intermediate (non-diagnostic), and high probability for PE [8]. In high-probability patients, PE is detected with a probability of more than 85 %. In low or intermediate probability, this rate is around 25 %. If the clinical probability is high, the probability of PE may exceed 40 % in low-probability V/Q scintigraphy [8]. However, the clinical correlations with these probability estimates remain unknown. Furthermore, while the role of CTPA in PE risk classification and treatment strategy is evident, the role of V/Q scintigraphy is unclear. For all these reasons, the present study aimed to evaluate the clinical status of patients with V/Q scintigraphy reported as high probability for PE.
2. Material and method
The files of 50 of 304 patients who underwent V/Q scintigraphy at the Nuclear Medicine department between January 2020 and January 2023 and whose result was reported as having a high probability of PE were examined. Seven of these patients were diagnosed with chronic thromboembolic pulmonary hypertension and thus excluded from the study (Fig. 1). The remaining 43 patients were included. Demographic data, Simplified Pulmonary Embolism Severity Index (SPESI) score, radiological findings, V/Q scintigraphy and echocardiographic (ECHO) findings, laboratory data, and treatment information of the patients were recorded. Left ventricular ejection fraction (LVEF), right ventricular dilation and pulmonary artery systolic pressure (PAPs) were noted as ECHO findings.
Fig. 1.
Flowchart for the selection of patients included in the study.
Chest X-ray/computed tomography (CT) findings of the patients were grouped as normal and abnormal (pleural effusion, atelectasis, cardiomegaly, parenchymal abnormalities). D-dimer and Troponin values were recorded from laboratory findings. Comorbid diseases were grouped as cardiovascular diseases (hypertension, heart failure, coronary artery disease), pulmonary diseases (asthma, chronic obstructive pulmonary disease, interstitial lung disease, obstructive sleep apnea syndrome, pneumoconioses), malignancy, and "other diseases" group (e.g., diabetes mellitus, chronic renal failure, hyperthyroidism).
Simplified Pulmonary Embolism Severity Index (SPESI) scoring is calculated by adding 1 point each: being >80 years old, heart rate of ≥110 beats/min, systolic arterial pressure of <100 mmHg, arterial oxygen saturation of <90 %, history of cancer, and history of chronic cardiopulmonary disease. A score of 0 predicts low mortality risk [9].
2.1. Evaluation of ventilation perfusion scintigraphy
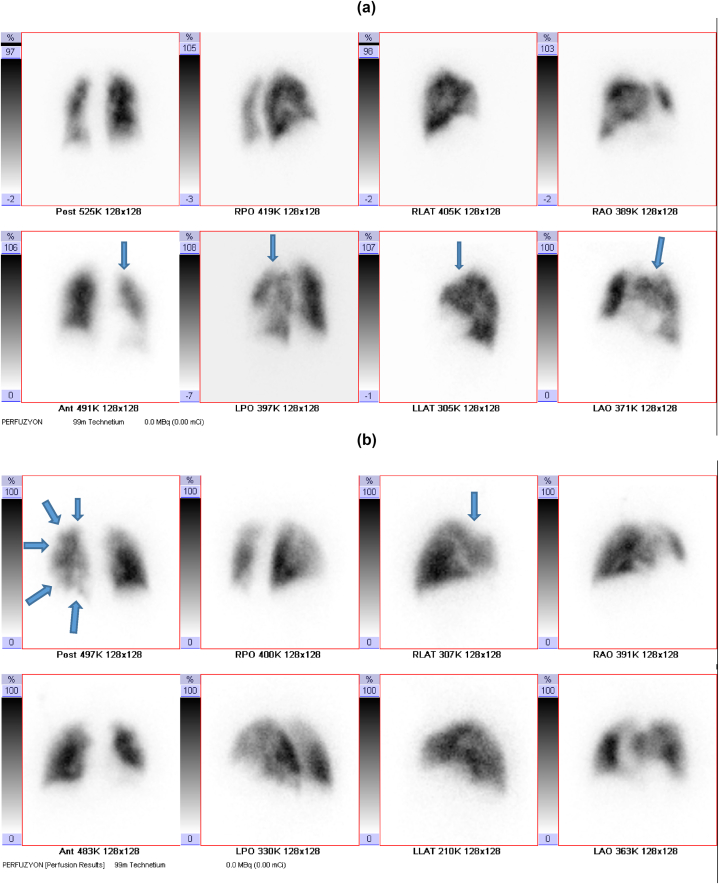
In Lung Perfusion Scintigraphy, anterior, posterior, right and left lateral, oblique planar images are acquired under a Siemens double-headed gamma camera following the intravenous administration of Macro Albumin Aggregate labeled with 5 mCi Technetium 99 m. Moreover, patients undergo SPECT imaging. Patients are classified as normal (no perfusion defects), low, moderate, and high-risk groups according to the probability of PE risks. Patients who do not have perfusion defects in lung perfusion imaging are interpreted as 'normal,' and ventilation scintigraphy is not taken. If abnormal perfusion findings and suspicious hypoperfusion are observed in any segment, lung ventilation scintigraphy imaging is performed on another day. In ventilation scintigraphy, the patient is inhaled with Technegas deep inspiration marked with 10 mCi Technetium 99 m, and then lung ventilation scintigraphy images are obtained. After the images are acquired, perfusion and ventilation images and chest X-ray are evaluated together. If the same defects are observed in the ventilation scintigraphy in hypoperfused segments with impaired lung perfusion, they are interpreted as match defects and evaluated as 'low probability.' If there is improvement in ventilation scintigraphy in the hypoperfused segment in one large, two medium, or four small segments in lung perfusion scintigraphy, it is considered a mismatch (incompatible defect) and interpreted as 'high probability' in terms of PE. Patients who do not fit either low or high-probability groups are interpreted as having 'intermediate probability.' Only patients reported as high-probability PE were included in our study. Perfusion scintigraphy defects of these patients were classified as subsegmental, multiple subsegmental, segmental, and multiple segmental. Fig. 2 shows image samples from patients with perfusion defects. Those with subsegmental, multiple subsegmental, and segmental perfusion defects were classified as Group 1, and those with multiple segmental defects as Group 2. Demographic, clinical, radiological, laboratory, and functional data of the two groups were compared. This study, in which routine data for clinical purposes was used, and all patient information was kept confidential, was approved by the Abant Izzet Baysal University Ethics Committee (date: February 21, 2023, approval no: 2023/29).
Fig. 2.
In perfusion scintigraphy, images of cases with a: subsegmental, b: multiple subsegmental, c: segmental, and d: multiple segmental perfusion defects are indicated (perfusion defects are marked with arrows).
Statistical Method.
The analysis of the data obtained as a result of the research was performed in the SPSS 20 statistical package program. Descriptive statistical methods (frequency, arithmetic mean, standard deviation, median, minimum, maximum, and crosstabs) were used. Compliance with normal distribution was evaluated with the Kolmogorov-Smirnov. Independent Sample t-test was used for two independent groups by comparing the arithmetic means of the normally distributed groups. The Mann-Whitney U test examined two independent groups by comparing the medians of the groups that did not show normal distribution. The Chi-Square test evaluated the relationship between categorical variables. The correlation between continuous variables was analyzed with Pearson's or Spearman's correlation coefficient, depending on the suitability of the data. The statistical significance level was accepted as p < 0.05.
3. Results
The mean age of the patients, including 27 women (62.8 %) and 16 men (37.2 %), was 74 years (31–94), and there was no significant difference between the two groups. Furthermore, no significant differences were determined between the two groups regarding laboratory, radiological and echocardiographic findings, presence of comorbidities, unit of treatment, and length of hospital stay. (Table 1). Subsegmental, multiple subsegmental, and segmental perfusion defects were detected in 20 of 43 patients (46.5 %), and Group 1 included these patients. There were multiple segmental perfusion defects in 23 patients (53.5 %), and Group 2 was formed. Of the total 43 patients reported as high-probability PE, 25 % had an sPESI score of ≥2. There was no significant difference between Groups 1 and 2 in terms of SPESI scoring (Table 1).
Table 1.
Comparison of demographic, laboratory, echocardiographic, and clinical data of patients grouped according to a perfusion defect.
| Group 1(n:20) | Group 2 (n:23) | Total (n:43) | p-value | |
|---|---|---|---|---|
| Gender | ||||
| Female | 14(70 %) | 13(56.5 %) | 27 (62.8 %) | 0.362 |
| Male | 6 (30 %) | 10 (43.5 %) | 16 (37.2 %) | |
| Age | 73.5 (39–94) | 74 (31–90) | 74 (31–94) | 0.760 |
| D-Dimer (mg/L, n:23) | 3.72 (0.61–42.9) | 1.90(0.35–15.64) | 3.14 (0.35–42.9) | 0.389 |
| Troponin (ng/L, n:16) | 12 (1–196.3) | 15.9 (1.5–91.1) | 14.75(1–196.3) | 0.315 |
| PAPs (n:27) | 41.8 ± 19.7 | 37.1 ± 14.3 | 39.2 ± 16.7 | 0.472 |
| RV dilatation (n:27) | 1(0.09 %) | 2(0.13 %) | 3(0.11 %) | 0.749 |
| LVEF (n:27) | 60(25–60) | 60(20–65) | 60(20–65) | 0893 |
| Chest X-ray (n:40) | ||||
| Pathological | 17(89.5 %) | 17 (81 %) | 34 (85 %) | 0.451 |
| SPESI (n:32) | ||||
| 0-1 | 10 (71.4 %) | 14 (77.8 %) | 24 (75 %) | 0.681 |
| ≥2 | 4 (28.6) | 4 (22.2 %) | 8 (25 %) | |
| Comorbidity (n:35) | 14(87.5 %) | 18 (94.7 %) | 32 (91.4 %) | 0.446 |
| Cardiovascular | 8(50 %) | 15 (78.9 %) | 23 (65.7 %) | 0.072 |
| Pulmonary | 5 (31.3 %) | 4 (21.1 %) | 9 (25.7) | 0.492 |
| Other | 11 (68.8 %) | 10(52.6 %) | 21 (60 %) | 0.332 |
| Treatment unit (n:33) | ||||
| Outpatient | 7 (50 %) | 14 (73.7 %) | 21 (63.6 %) | 0.176 |
| Ward | 7 (50 %) | 4 (21.1 %) | 11 (33.3 %) | |
| Intensive care | 0 | 1(5.3 %) | 1 (3 %) | |
| Duration of hospitalization (days) (n:14) |
5 (0–7) | 6 (3–120) | 5.5 (0–120) | 0.312 |
SPESI: Simplified Pulmonary Embolism Severity Index, PAPs: pulmonary artery systolic pressure, LVEF: left ventricle ejection fraction, RV: right ventricle.
Perfusion defect had no significant correlation with SPESI score, D-dimer, Troponin, PAPs, RV dilatation, and length of hospital stay. The presence of comorbidity was significantly positively correlated only with the SPESI score (Table 2).
Table 2.
Correlation of the presence of perfusion defect and comorbidity with laboratory, clinical, and echocardiographic parameters.
| Perfusion defect |
Comorbidity |
|||
|---|---|---|---|---|
| r | p | r | p | |
| Troponin | 0.260 | 0.331 | 0.364 | 0.166 |
| D-Dimer | −0.184 | 0.401 | 0.163 | 0.458 |
| SPESI | −0.073 | 0.692 | 0.408 | 0.020 |
| Duration of hospitalization | 0.280 | 0.331 | −0.104 | 0.723 |
| PAPs | −0.144 | 0.472 | −0.128 | 0.533 |
SPESI: Simplified Pulmonary Embolism Severity Index, PAPs: pulmonary artery systolic pressure.
When the patients were examined according to the presence of comorbidity, these patients could not be evaluated further since the comorbidity information of 8 patients could not be reached. Only 3 of 35 patients did not have any comorbidities. All patients without comorbidity had a SPESI score in the range of 0–1, and there was no need for intensive care, but the number was insufficient to perform statistical analysis (Table 3).
Table 3.
Comparison of demographic, laboratory, echocardiographic, and clinical data of groups with and without coordination.
| With comorbidity (n:32) | Without comorbidity (n:3) | Total (n:35) | p-value | |
|---|---|---|---|---|
| Gender | ||||
| Female | 20(62.5 %) | 3 (100 %) | 23 (65.7 %) | 0.191 |
| Male | 12 (37.5 %) | 0 | 12 ((34.3 %) | |
| Age | 75 (32–94) | 72 (31–74) | 74 (31–94) | 0.226 |
| D-Dimer (mg/L, (N:23) | 3.57 (0.35–42.9) | 1.42 (091–1.94) | 3.14 (035–42.9) | 0.445 |
| Troponin (ng/L, n:16) | 15(1–196.3) | 1.5(1.5–1.5) | 14.75(1–196.3) | 0.159 |
| PAPs (n:26) | 38.3 ± 17.8 | 45 ± 10 | 39.1 ± 17 | 0.533 |
| LVEF (n:27) | 60 (20–65) | 60 (30–65) | 60 (20–65) | 0.623 |
| Chest X-ray (n:34) | . | |||
| Pathological | 26 (83.9 %) | 3 (100 %) | 29 (85.3 %) | 0.451 |
| SPESI (n:32) | ||||
| 0-1 | 21(72.4 %) | 3 (100 %) | 24 (75 %) | 0.294 |
| ≥2 | 8 (27.6) | 0 | 8(25 %) | |
| Treatment unit (n:33) | ||||
| Outpatient | 19 (63.3 %) | 2 (66.7 %) | 21(63:6 %) | 0.949 |
| Ward | 10(33.3 %) | 1 (33.3 %) | 11 (33.3 %) | |
| Intensive care | 1 (3.3 %) | 0 | 1 (3 %) | |
| Duration of hospitalization (n:14) | 5 (0–120) | 6 (6–6) | 5.5 (0–120) | 0.707 |
SPESI: Simplified Pulmonary Embolism Severity Index, PAPs: pulmonary artery systolic pressure, LVEF: left ventricle ejection fraction.
4. Discussion
There are very few studies in the literature regarding the clinical findings and course of patients with a high probability for PE reported on V/Q scintigraphy. Our study is one of the rare studies regarding this subject. In the study of Afsin, V/Q scintigraphy of 95 patients was examined, and normal, low, intermediate, and high probability PE groups were established. It was reported that a high probability of PE did not indicate submassive-massive embolism and that it was not the number of involved segments that determined the clinical presentation. It was emphasized that probability results in V/Q scintigraphy were insufficient to predict clinical findings, and this data should be supported by clinical, radiological, and echocardiographic results [10]. The degree of obstruction by the thrombus in PE and the cardiopulmonary reserve of the patient determine the occurrence of hemodynamic disorder [11]. The resulting hemodynamic impairment and cardiopulmonary reserve also form the basis of SPESI scoring. The sensitivity of the SPESI score in predicting 30-day mortality is high [12]. In our study, only 25 % of patients reported as having a high probability of PE had a SPESI score of ≥2. There was no significant difference between Groups 1 and 2 in terms of SPESI scoring. It was determined that the size and extent of the perfusion defect were not correlated with the SPESI.
When the correlation of perfusion defect with laboratory, SPESI, D-dimer, PAPs, and length of hospital stay was examined, no correlation was observed with any of them, while the presence of comorbidity was significantly positively correlated with the SPESI score. This result is as expected since malignancy, cardiac, and pulmonary diseases from comorbidities are included in the SPESI scoring. In our study, patients without comorbidity were very few, but the specific value of all of these patients was in the range of 0–1, and there was no need for intensive care.
A negative lung perfusion scan obtained on day one after the onset of symptoms excludes the diagnosis of PE, with a negative predictive value close to 100 %. The perfusion defect may be due to bullae, atelectasis, chronic obstructive pulmonary disease (COPD), pneumonia, and other lung diseases, as well as left heart failure. However, what distinguishes PE from other diseases is that the ventilation scintigraphy is normal. The specificity of lung SPECT scintigraphy is 91–96 %, and its negative predictive value is 97–99 % [13]. The specificity of lung perfusion scanning is enhanced by the lobar or segmental shape of the perfusion defect and by negative ventilation scanning. In contrast, one or more non-wedge-shaped perfusion defects, with or without accompanying chest X-ray abnormalities, do not indicate PE as defined in the PISA-PED study [14]. The most valuable finding in lung scintigraphy is the presence of one or more wedge-shaped segmental defects, regardless of whether they suggest concomitant lung pathology [15]. Our study detected multiple segmental perfusion defects in 53.5 % of the patients. There was no significant difference between the two groups regarding pathology in the chest X-ray.
The risk of PE increases after the age of 60 [16]. This situation can be explained by the increase in comorbid conditions with age and the decrease in mobilization. In our study, the mean age was 74 (31–94) years, and no significant age difference was determined between the two groups. Although the female gender was more frequent in general patients and both groups, the difference was not statistically significant. These data are also compatible with the literature [17].
D-dimer is produced by the action of plasmin on fibrin, has a high negative predictive value, and is critical to exclude the diagnosis of PE with low or moderate clinical probability [18]. The specificity of D-dimer positivity is low, and it increases in many conditions such as inflammation, necrosis, malignancy, infection, old age, pregnancy, and hospitalization [15]. D-dimer elevation has prognostic significance in PE [19]. Another laboratory prognostic marker is cardiac troponin, as its increase with right ventricular dysfunction indicates a high risk of mortality. The negative predictive value is 0.99, which is higher than the positive predictive value (0.34). Troponin levels peak at least 6–12 h after PE develops. A meta-analysis of 20 studies involving PE patients revealed that any increase in troponins was associated with a five-fold increase in the risk of death and an increased risk of death from PE [20]. In our study, no significant difference was observed in D-dimer and troponin levels between patients with perfusion defects in both groups.
The coexistence of pulmonary hypertension and right ventricular (RV) dilatation in ECHO has a high sensitivity of 93 %, specificity of 81 %, and negative predictive value of 94 % in the diagnosis of PE. RV dysfunction develops in 30–40 % of hemodynamically stable PE patients, as well as in unstable PE patients. Even in hemodynamically stable patients, RV dysfunction is associated with a death rate twice as high. Because up to 20 % of PE patients have normal echocardiographic findings, a normal echocardiogram cannot rule out PE [21]. Only the study of Afsin evaluated the relationship between the PAPs and pulmonary artery diameter measured in CT with PE probability groups, and no correlation was determined [10]. In a case report, postmortem idiopathic pulmonary arterial hypertension was reported in a case with grossly abnormal mismatched defects [22]. Scott reported that significant information about PA pressure could be obtained from radionuclide perfusion scanning with artificial intelligence [23]. Our study revealed no statistically significant correlation between PAPs and RV dilatation with perfusion defects. However, it should not be forgotten that our study sample was small.
In our study, there was no difference between the groups in terms of laboratory, radiological, echocardiological, and clinical severity, and there was no difference in the unit where the patients were treated and the length of hospital stay.
The limitations in our study included being single-centered and retrospective, and our sample group was limited. We would also need patient groups without comorbidities. It is necessary to evaluate with multicenter and large data. However, in this study, issues that had never been investigated before were addressed.
5. Conclusion
Although the present study did not demonstrate a correlation between clinical scoring and cardiac functions, studies examining the size and extent of perfusion defects need to continue with larger samples. While CTPA facilitates disease management by contributing to the prognosis and treatment of PE, parameters that would predict clinical severity and guide treatment are needed in PE patients diagnosed with V/Q scintigraphy.
Ethical approval
This study, in which routine data for clinical purposes was used, and all patient information was kept confidential, was approved by the Abant Izzet Baysal University Ethics Committee. Consent was obtained for the use of participants' internal scan images in the study.
Data availability statement
Data will be made available on request.
CRediT authorship contribution statement
Emine Afsin: Writing – review & editing, Visualization, Software, Methodology, Investigation, Conceptualization. Hamdi Afsin: Writing – original draft, Validation, Supervision, Data curation.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Contributor Information
Emine Afsin, Email: emine.afsin@ibu.edu.tr.
Hamdi Afsin, Email: hamdiafsin@ibu.edu.tr.
References
- 1.Remy-Jardin M., Remy J. Spiral CT angiography of the pulmonary circulation. Radiology. 1999;212(3):615–636. doi: 10.1148/radiology.212.3.r99se02615. [DOI] [PubMed] [Google Scholar]
- 2.Mullins M.D., Becker D.M., Hagspiel K.D., Philbrick J.T. The role of spiral volumetric computed tomography in the diagnosis of pulmonary embolism. Arch. Intern. Med. 2000;160(3):293–298. doi: 10.1001/archinte.160.3.293. [DOI] [PubMed] [Google Scholar]
- 3.Bajc M., Schümichen C., Grüning T., Lindqvist A., Le Roux P.Y., Alatri A., Bauer R.W., Dilic M., Neilly B., Verberne H.J., Delgado Bolton R.C., Jonson B. EANM guideline for ventilation/perfusion single-photon emission computed tomography (SPECT) for diagnosis of pulmonary embolism and beyond. Eur. J. Nucl. Med. Mol. Imag. 2019;46(12):2429–2451. doi: 10.1007/s00259-019-04450-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mortensen J., Gutte H. SPECT/CT and pulmonary embolism. Eur. J. Nucl. Med. Mol. Imag. 2014;41(Suppl 1):S81–S90. doi: 10.1007/s00259-013-2614-5. Suppl 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hess S., Frary E.C., Gerke O., Madsen P.H. State-of-the-Art imaging in pulmonary embolism: ventilation/perfusion single-photon emission computed tomography versus computed tomography angiography - controversies, results, and recommendations from a systematic review. Semin. Thromb. Hemost. 2016;42(8):833–845. doi: 10.1055/s-0036-1593376. [DOI] [PubMed] [Google Scholar]
- 6.Hansen S.L., de Nijs R., Mortensen J., Berg R.M.G. Ventilation-perfusion SPECT versus CTPA in young adult females with suspected pulmonary embolism. Eur. Respir. J. 2020;55(6) doi: 10.1183/13993003.00448-2020. 11 Jun. [DOI] [PubMed] [Google Scholar]
- 7.Scarsbrook A.F., Bradley K.M., Gleeson F.V. Perfusion scintigraphy: diagnostic utility in pregnant women with suspected pulmonary embolic disease. Eur. Radiol. 2007;17(10):2554–2560. doi: 10.1007/s00330-007-0607-0. [DOI] [PubMed] [Google Scholar]
- 8.PIOPED Investigators Value of the ventilation/perfusion scan in acute pulmonary embolism. Results of the prospective investigation of pulmonary embolism diagnosis (PIOPED) JAMA. 1990;263(20):2753–2759. doi: 10.1001/jama.1990.03440200057023. [DOI] [PubMed] [Google Scholar]
- 9.Jiménez D., Aujesky D., Moores L., Gómez V., Lobo J.L., Uresandi F., Otero R., Monreal M., Muriel A., Yusen R.D. RIETE Investigators, “Simplification of the pulmonary embolism severity index for prognostication in patients with acute symptomatic pulmonary embolism.”. Arch. Intern. Med. 2010;170(15):1383–1389. doi: 10.1001/archinternmed.2010.199. [DOI] [PubMed] [Google Scholar]
- 10.Afşin H. 2023. Factors Affectıng the Probabılıty of Pulmonary Embolısm in Ventılatıon/Perfusıon Lung Scıntıgraphy. 10. Internatıonal Gevher Nesibe Medıcal Scıences Conference. February 3-5. Ankara/Turkey. [Google Scholar]
- 11.McIntyre K.M., Sasahara A.A. In: Pulmonary Thromboembolism Year Book Medical Publ. Moser K., Stein M., editors. 1973. Determinants of cardiovascular responses to pulmonary embolism; pp. 144–159. Chicago. [Google Scholar]
- 12.Baloira Villar A., Ruiz Iturriaga L.A. Tromboembolismo pulmonar [Pulmonary thromboembolism] Arch Bronconeumol Oct. 2010;46(Suppl 7):31–37. doi: 10.1016/S0300-2896(10)70044-2. Spanish. [DOI] [PubMed] [Google Scholar]
- 13.Gutte H., Mortensen J., Jensen C.V., von der Recke P., Petersen C.L., Kristoffersen U.S., Kjaer A. Comparison of V/Q SPECT and planar V/Q lung scintigraphy in diagnosing acute pulmonary embolism. Nucl. Med. Commun. 2010;31(1):82–86. doi: 10.1097/MNM.0b013e3283336747. [DOI] [PubMed] [Google Scholar]
- 14.Invasive and noninvasive diagnosis of pulmonary embolism. Preliminary results of the prospective investigative study of acute pulmonary embolism diagnosis (PISA-PED) Chest. 1995;107(1 Suppl):33S–38S. [PubMed] [Google Scholar]
- 15.J. Widimsky. Diagnosis and Treatment of Acute Pulmonary Embolism .http://dx.doi.org/10.1016/j.crvasa.2013.10.001.
- 16.White R.H. The epidemiology of venous thromboembolism. Circulation. 2003;107(23 Suppl 1):I4–I8. doi: 10.1161/01.CIR.0000078468.11849.66. [DOI] [PubMed] [Google Scholar]
- 17.Tapson V.F. Acute pulmonary embolism. N. Engl. J. Med. 2008;358(10):1037–1052. doi: 10.1056/NEJMra072753. [DOI] [PubMed] [Google Scholar]
- 18.Torbicki A., van Beek E.J.R., Charbonnier B., Meyer G., Morpurgo M., Palla A., Perrier A., Galie N., Gorge G., Herold C., Husted S., Kasper W., Kneussi M., Morice A.H., Musset d., Samama M.M., Simonneau G., Sors H., de Swiet M., Turina M., Konik G., Widimsky J. Guidelines on diagnosis and management of acute pulmonary embolism. Eur. Heart J. 2000;21:1301–1336. doi: 10.1053/euhj.2000.2250. [DOI] [PubMed] [Google Scholar]
- 19.Arseven O., Bingöl Z., Çöplü L., Erol S., Oğuzülgen İ.K., Okumuş N.G., Önen Z.P., Öngen H.G., Özgür E.S., Sevinç C., Topçu A.F., Yılmazel Uçar E., Uzun O., Yıldızeli B. Ankara/Trukey; 2021. Türk Toraks Derneği Pulmoner Tromboembolizm Tani Ve Tedavi Uzlaşi Raporu. [Google Scholar]
- 20.Becattini C., Vedovati M.C., Agnelli G. Prognostic value of troponins in acute pulmonary embolism: a meta-analysis. Circulation. 2007;116(4):427–433. doi: 10.1161/CIRCULATIONAHA.106.680421. [DOI] [PubMed] [Google Scholar]
- 21.Authors/Task Force Members. Torbicki A., Perrier A., Konstantinides S., Agnelli G., Galiè N., Vachiery J.L. Guidelines on the diagnosis and manag ement of acute pulmonary embolism: the task force for the diagnosis and management of acute pulmonary embolism of the European society of cardiology (ESC) Eur. Heart J. 2008;29:2276–2315. doi: 10.1093/eurheartj/ehn310. [DOI] [PubMed] [Google Scholar]
- 22.Chan K., Coghlan J.G., Hall M., Haddock J., Bates A., Schreiber B.E. “Grossly abnormal ventilation/perfusion SPECT study in idiopathic pulmonary arterial hypertension without thromboembolism.” heart. lung & circulation. 2018;27(11):e101–e104. doi: 10.1016/j.hlc.2018.01.001. [DOI] [PubMed] [Google Scholar]
- 23.Scott J.A. Pulmonary perfusion patterns and pulmonary arterial pressure. Radiology. 2002;224(2):513–518. doi: 10.1148/radiol.2242011353. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.